Simple Summary
Targeting immune system proteins called TLR7 and TLR8 is showing promise in cancer treatment. These proteins help activate a strong immune response that can detect and attack cancer cells. Drugs that stimulate TLR7/8 not only boost immunity but also enhance the effects of traditional treatments like chemotherapy, radiation, and phototherapy-potentially improving therapy outcomes and reducing side effects. TLR7/8 agonists are also being used in cancer vaccines, which train the body to fight cancer more precisely. Early results in lab studies have been encouraging, and clinical trials are now testing their effectiveness in people. This approach offers a powerful combination: it strengthens the body’s natural defenses while working alongside existing therapies to reduce tumor size. Using TLR7/8 agonist-based drugs in personalized cancer treatments may improve effectiveness, reduce side effects, and increase accessibility to more patients.
Abstract
Targeting Toll-like receptors 7 and 8 (TLR7/8) has emerged as a promising strategy in cancer immunotherapy. TLR7/8 agonists activate robust Th1-type immune responses and bridge innate and adaptive immunity. Further, TLR7/8 agonists can serve as valuable adjuncts to conventional therapies, such as chemotherapy and radiotherapy, enhancing efficacy while reducing adverse effects. Their integration into combination regimens for cancer offers a dual advantage: amplifying antitumor immunity and reducing tumor burden. Notably, the incorporation of TLR7/8 agonists into cancer vaccine platforms has yielded encouraging results in preclinical models and is advancing toward clinical application. This review highlights the mechanisms of action, therapeutic potential, and recent progress in the development of TLR7/8 agonist-based strategies for cancer treatment. We also discuss ongoing clinical evaluations and the rationale for combining these agents with existing modalities to enable more effective, personalized, and accessible cancer therapies.
1. Introduction
Cancer remains a leading cause of mortality worldwide, accounting for approximately one in six deaths. Despite advances in conventional treatments such as chemotherapy, radiotherapy, and surgical resection, these approaches are often associated with significant side effects and limited long-term efficacy. As a result, there continues to be a need for more sophisticated and targeted therapeutic strategies to treat cancer.
Cancer immunotherapy has emerged as a transformative approach, harnessing the body’s immune system to recognize and eliminate malignant cells [1,2,3]. This strategy offers several advantages over traditional treatments, including enhanced specificity, potential for long-lasting immune memory [4], broader applicability across cancer types [5], potential for additive or synergistic effects [6], reduced resistance [7], and opportunities for personalized medicine [8]. Notable examples include immune checkpoint inhibitors (e.g., anti-CTLA-4, anti-PD-1/PD-L1 antibodies) [9,10,11], chimeric antigen receptor (CAR) T-cell therapy [12,13,14], and monoclonal antibody-based treatments [15], all of which have significantly improved patient outcomes in various cancers.
Antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages, play a central role in initiating and shaping antitumor immune responses by presenting tumor-associated antigens to T cells [16,17]. The activation of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells is also critical for effective tumor clearance [18,19]. For instance, mature DCs exhibit enhanced immunostimulatory capacity compared to their immature counterparts, contributing to more robust antitumor immunity [20].
Despite these advances, challenges remain in achieving durable responses across all patient populations. One key area of interest is the use of agents that selectively target pattern recognition receptors (PRRs), particularly Toll-like receptors (TLRs), which are expressed on both immune and tumor cells [21]. TLRs serve as a bridge between innate and adaptive immunity and play a pivotal role in immune surveillance and activation [22]. Multiple Toll-like receptors (TLRs) have been explored as immunotherapeutic targets. Among them, TLR7 and TLR8 have attracted particular interest due to their ability to be activated by small molecules. Activation of TLR7/8 stimulates the production of NF-κB-mediated pro-inflammatory cytokines and chemokines [23]. This, in turn, triggers robust innate and adaptive immune responses, enhancing APC function and cytotoxic T cell priming-both essential for effective cancer vaccination. Furthermore, TLR7/8 activation promotes tumor cell apoptosis and strengthens antitumor immunity [24]. TLR7/8 agonists also modulate immunosuppressive myeloid populations and improve the efficacy of immune checkpoint inhibitors, underscoring their translational relevance in cancer immunotherapy. Several small-molecule TLR7/8 agonists, including imiquimod, motolimod, vesatolimod, and DN052, have advanced into clinical investigation, providing a strong foundation of preclinical and clinical evidence for further evaluation.
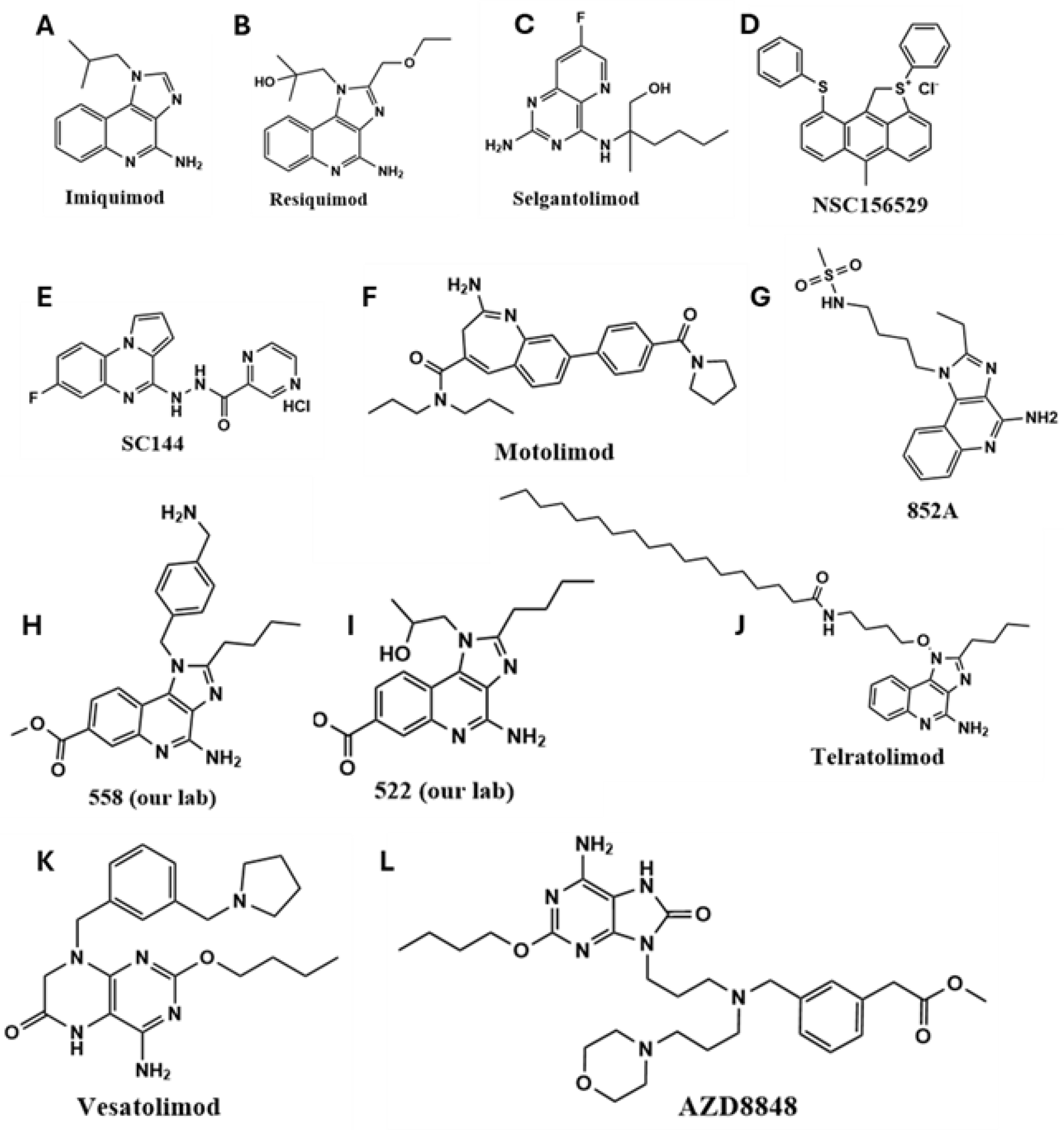
Recent developments have focused on small-molecule and nucleic acid-based TLR7/8 agonists [22,25], which are being evaluated in both preclinical and clinical settings. Pyrido [3,2-d] pyrimidine-derived small molecules have been utilized in the design of TLR agonists [26]. Compounds such as imiquimod (R837; IMQ), the first FDA-approved TLR7 agonist for basal cell carcinoma [27], and its more potent derivative resiquimod (R848), have demonstrated promising immunostimulatory effects [28,29,30]. Other agents, such as 852-A, have shown efficacy in hematological malignancies by enhancing adaptive immune responses [31]. Other TLR7/8 agonists that have been explored for cancer treatment are shown in Figure 1.
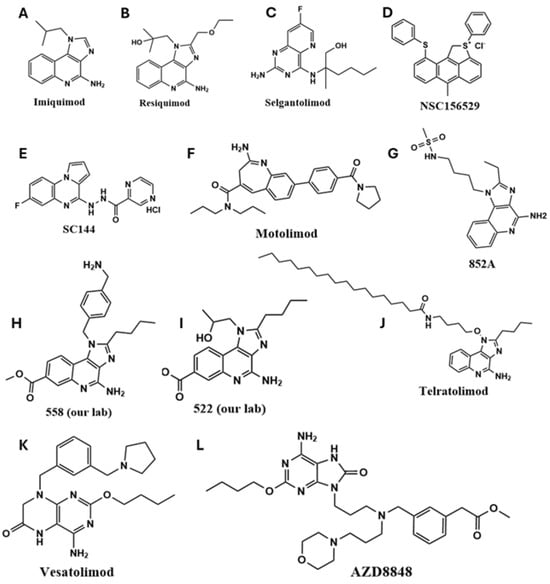
Figure 1.
The chemical structures of synthetic TLR7/8 agonists and their analogs have been used for preclinical and clinical translation. (A) IMQ, (B) Resiquimod, (C) Selgantolimod, (D) NSC156529, (E) SC144, (F) Motolimod, (G) 852A, (H) 558, (I) 522, (J) Telratolimod, (K) Vesatolimod and (L) AZD8848.
Although TLR agonists have demonstrated efficacy as monotherapies, they are increasingly being integrated into combination therapies as immune adjuvants [32]. For example, the combination of oxaliplatin with R848 significantly enhanced antitumor effects in colorectal cancer models [33], underscoring the potential of TLR7/8 agonists to boost the efficacy of conventional treatments. Several therapeutic modalities-including chemotherapy, radiation therapy, and phototherapy-induce tumor cell death but often do not generate durable immune responses. Because these treatments promote the release of tumor antigens, combining them with TLR7/8 agonists can result in the activation of antigen-specific immune response and long-term antitumor immunity. This combinatorial approach may also help overcome tumor immune evasion by enabling the immune system to better recognize and eliminate tumor cells. Ongoing research is focused on optimizing these combination strategies to maximize therapeutic efficacy while minimizing adverse effects.
This review provides a comprehensive overview of TLR7/8 agonist-based strategies in cancer immunotherapy. We discuss their mechanisms of action, structural diversity, and immunological effects, as well as their integration into cancer vaccines and combination therapies. We also highlight recent advances in preclinical and clinical development and address the challenges and safety considerations associated with TLR7/8 agonist-based treatments. While TLR-mediated immunotherapy has shown remarkable promise, further investigation is essential to fully realize its clinical potential and ensure safe, effective application in cancer care.
2. Search Strategy and Selection Criteria
This comprehensive review employed a strategic literature search to ensure broad coverage of studies investigating TLR7/8 agonist-based cancer vaccines and combination therapies. The search was conducted across electronic databases, including PubMed, Scopus, and Web of Science, targeting relevant research and review articles published between 2015 and 2025. Keywords used in the search included: TLR7 agonist, TLR8 agonist, TLR7/8 agonist, TLR7/8-based cancer vaccine, TLR7/8-based cancer immunotherapy, TLR7/8-based combination treatment, and TLR7/8-based clinical trials. Only peer-reviewed articles published in English were selected to highlight recent advancements in this field. Studies were included if they provided preclinical or clinical insights into the immunomodulatory agents, mechanisms of action, delivery strategies, or therapeutic efficacy of TLR7/8 agonists in cancer vaccination or combination approaches. Additional references were identified through cross-referencing the bibliographies of selected research and review articles.
3. Mechanisms of Activation and Downstream Signaling of the TLR Family
The innate immune system serves as the body’s first line of defense, providing a rapid response to injury or pathogen invasion and priming the adaptive immune system for a more specific, long-term response [34]. This early immune activation is mediated by pattern recognition receptors (PRRs), which detect pathogen-associated molecular patterns (PAMPs) and, in some cases, endogenous damage-associated molecular patterns (DAMPs), including tumor-derived antigens [35].
Several classes of PRRs have been identified based on their structural domains and cellular localization. These include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), and absent in melanoma 2 (AIM2)-like receptors (ALRs) [36,37]. Among these, TLRs are particularly important in modulating immune responses and linking innate and adaptive immunity [38]. To date, ten human TLRs have been identified. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are expressed on the plasma membrane, where they detect extracellular signals. In contrast, TLR3, TLR7, TLR8, and TLR9 are localized to intracellular compartments such as endosomes and the endoplasmic reticulum, where they recognize nucleic acid-based ligands [39]. Structurally, TLRs are composed of three main domains: An extracellular domain rich in leucine-rich repeats (LRRs), typically arranged in a horseshoe-like shape, which is responsible for ligand recognition. Each LRR consists of a β-strand and an α-helix connected by loops, spanning 24–29 amino acids. Secondly, a transmembrane domain anchors the receptor in the membrane. And finally, an intracellular Toll/IL-1 receptor (TIR) domain, which initiates downstream signaling upon receptor dimerization [40]. The dimerization is needed to activate TIR and initiate three transcriptional factors: AP-1, NF-kB, and IRF7 [41,42,43].
TLR family members vary across species. Humans possess 10 functional TLRs (TLR1–TLR10), whereas mice have 12 (TLR1–TLR12) [38]. Interestingly, the core structural domains and signaling pathways of TLRs are highly conserved across species [44]. In humans, TLR genes are distributed non-randomly across chromosomes: TLR1, TLR2, TLR3, TLR6, and TLR10 are located on chromosome 4; TLR4 on chromosome 9; TLR5 on chromosome 1; TLR7 and TLR8 on the X chromosome; and TLR9 on chromosome 3. In mice, TLR1, TLR2, and TLR6 are found on chromosome 4; TLR3 and TLR4 on chromosome 5; TLR7 and TLR8 on the X chromosome; TLR9 on chromosome 9; and TLR11–TLR13 on chromosome 14 [45].
3.1. Activation and Signaling of TLR 7/8 Ligand
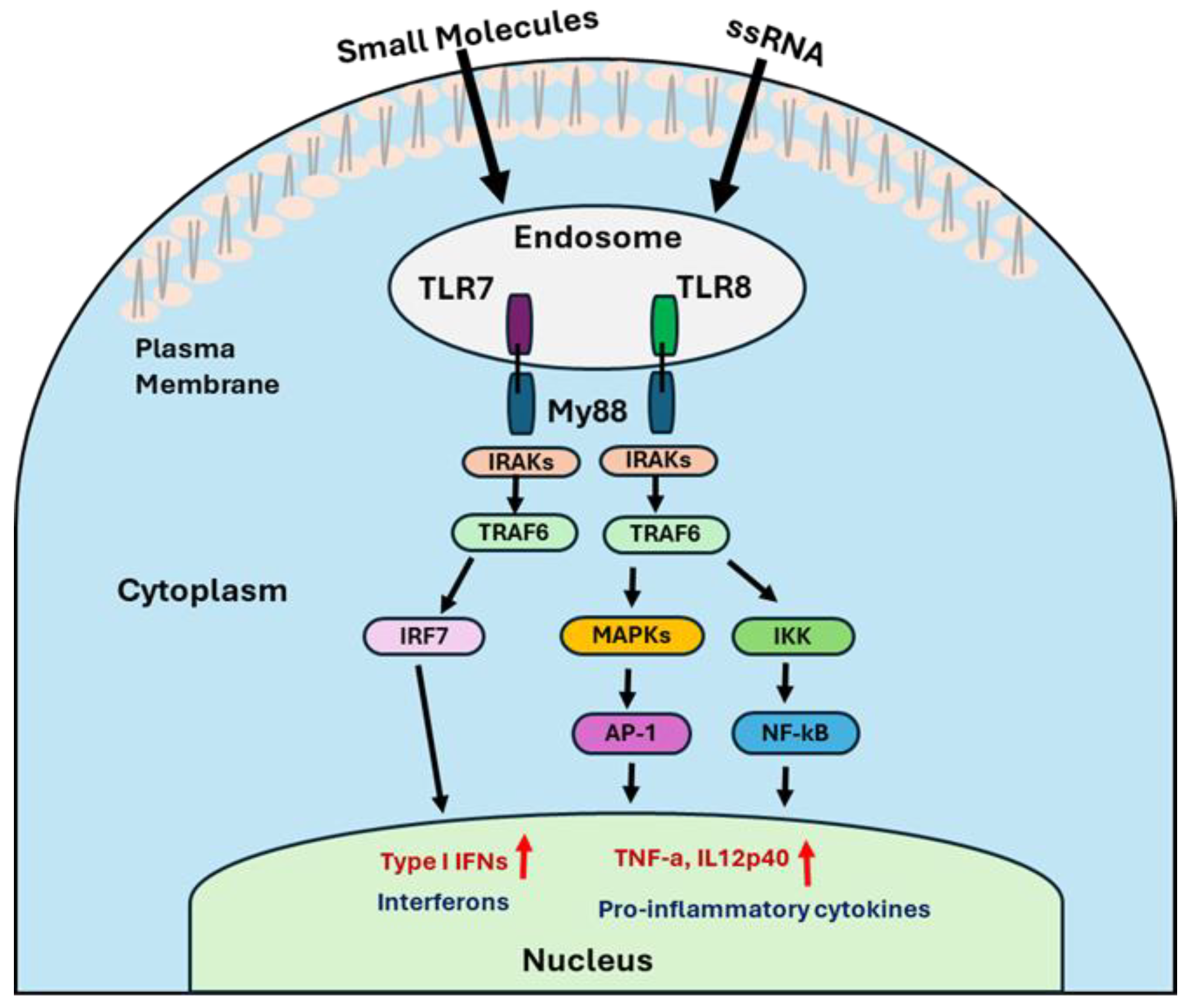
Upon ligand binding, TLR7 and TLR8 undergo conformational changes that promote dimerization and recruitment of the adaptor protein MyD88 (myeloid differentiation primary response gene 88) via its C-terminal TIR domain [46]. The N-terminal death domain of MyD88 then recruits IL-1 receptor-associated kinases (IRAK4 and IRAK1) [47], forming a signaling complex that activates tumor necrosis factor receptor-associated factor 6 (TRAF6) [48]. TRAF6 subsequently activates multiple downstream pathways, including the formation of the IκB kinase (IKK) complex, activation of the mitogen-activated protein kinases (MAPK) family, and recruitment of IFN regulatory factors (IRF7). Then, IKK complex leads to the nuclear translocation of NF-κB, a key transcription factor involved in inflammation and immune activation. Similarly, the MAPK pathway, which activates transcription factors such as cyclic AMP response element-binding protein (CREB) and activator protein 1 (AP-1), promotes the production of pro-inflammatory cytokines like IL-6, IL-12p70, and TNF-α. Activation of the IRF7 pathway leads to the expression of type I interferons, including IFN-α and IFN-β (Figure 2) [22,49,50].
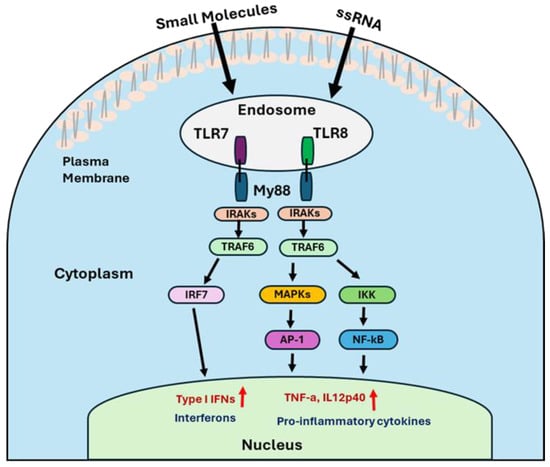
Figure 2.
The illustration represents the mechanistic signaling pathways of TLR7/8 agonists.
These signaling cascades collectively lead to the production of inflammatory cytokines and type I interferons, thereby enhancing both innate and adaptive immune responses. The ability of TLR7/8 to activate multiple immune pathways makes them attractive targets for immunotherapy, particularly in the context of cancer, where robust immune activation is essential for effective tumor clearance. Interestingly, we have identified that our lab-synthesized TLR7/8 agonist, 558, could produce type II IFN, in addition to type I IFNs. Global proteomics analysis was performed to investigate the mechanisms underlying the production of type II IFNs. Moreover, this study demonstrated that 558 synergistically activated STING and inflammasome pathways to its effect on TLR7/8 signaling on DC maturation [51].
3.2. Biomarkers for TLR7/8-Based Therapies
Identifying predictive biomarkers within the tumor microenvironment is essential for guiding cancer immunotherapy and improving patient outcomes. PD-L1 expression on tumor cells, for example, has been correlated with enhanced responses to anti-PD-1/PD-L1 therapies, although its predictive accuracy remains limited due to modest sensitivity and specificity [52,53]. Similarly, elevated tumor mutational burden (TMB) and inflammatory gene-expression profiles (GEPs) have shown promise as predictive indicators. However, a meta-analysis revealed that multiplex immunohistochemistry/immunofluorescence (mIHC/IF) outperformed these individual markers in predicting treatment response [54].
Tumor-infiltrating lymphocytes (TILs), particularly their density and phenotypic characteristics, have emerged as valuable biomarkers across various cancer types [55,56]. Myeloid cell signatures are also gaining attention for their potential to predict responses to immune checkpoint inhibitors, though their clinical application remains challenging [57]. Recently, TLRs, especially TLR4 and TLR7, have been investigated as prognostic biomarkers in the context of vaccine-based therapies. Meta-analyses suggest that high expression of TLR4 or TLR7 may be associated with poorer survival across multiple cancers [58,59]. Interestingly, one study reported that elevated TLR expression combined with high TIL density predicted improved outcomes in colorectal cancer [60].
Despite these promising findings, the clinical utility of these biomarkers in vaccine-based immunotherapy remains constrained by the lack of standardized thresholds, multicenter validation, and robust prospective data. Continued research is needed to refine biomarker selection and validation, enabling more personalized and effective vaccine strategies in cancer treatment.
4. Challenges Associated with TLR7/8 Agonists
A major challenge with the use of TLR7/8 agonists is the variability in patient response. Identifying individuals who are most likely to benefit from TLR-based therapies is critical. For example, two independent studies found that combining the TLR8 agonist motolimod with chemotherapeutic drugs such as 5-FU, DOX, and platinum did not improve overall survival across all patients. However, a predefined subpopulation exhibited significantly better progression-free and overall survival outcomes [61,62]. This highlights the importance of stratifying patients based on gene expression profiles, biomarker levels, and local immune responses to optimize treatment efficacy.
Systemic administration of TLR7/8 agonists can also lead to excessive cytokine release, resulting in adverse effects such as fever, hypotension, and, in severe cases, cytokine release syndrome. Natalie et al. reported that systemic delivery of R848 in mice caused brain swelling [63]. Another study showed that R848 induced acute sickness behaviors, including hypophagia, weight loss, and reduced locomotor activity, along with elevated pro-inflammatory gene expression in the central nervous system [64].
In addition to systemic toxicity, poor tumor penetration can limit therapeutic efficacy. To address this, novel delivery systems are being developed to enhance tumor targeting and minimize off-target effects. For instance, the lipophilic TLR7/8 agonist MEDI9197 demonstrated improved retention at the injection site, underscoring the challenges of systemic transport and tumor localization [65]. Another concern is immune desensitization following repeated exposure to TLR agonists. Prolonged treatment with R848 induces a refractory state in microglia, characterized by diminished pro-inflammatory responses and reduced sickness behavior in mice [64]. However, Bourquin et al. demonstrated that adjusting the dosing schedule, using repeated low-dose R848 injections spaced five days apart, significantly reduced tumor burden compared to a single high-dose regimen. This suggests that optimizing dosage and timing may help overcome TLR tolerance [66].
5. Combination Therapies Involving TLR 7/8 Agonists
To further enhance therapeutic outcomes and prevent immune escape, TLR7/8 agonists are increasingly being combined with other treatment modalities, including immune checkpoint inhibitors, chemotherapy, radiation therapy, and phototherapy. These combinatorial strategies aim to synergistically activate the immune system while directly targeting tumor cells, offering a more comprehensive and durable approach to cancer treatment.
5.1. TLR7/8 Agonist Combined with Other Immunostimulators
Various tumor-driven mechanisms can impair CD8+ T cells’ activation and function, limiting the effectiveness of TLR7/8 agonists. To enhance therapeutic outcomes, various immunostimulatory agents have been co-administered with TLR7/8 agonists.
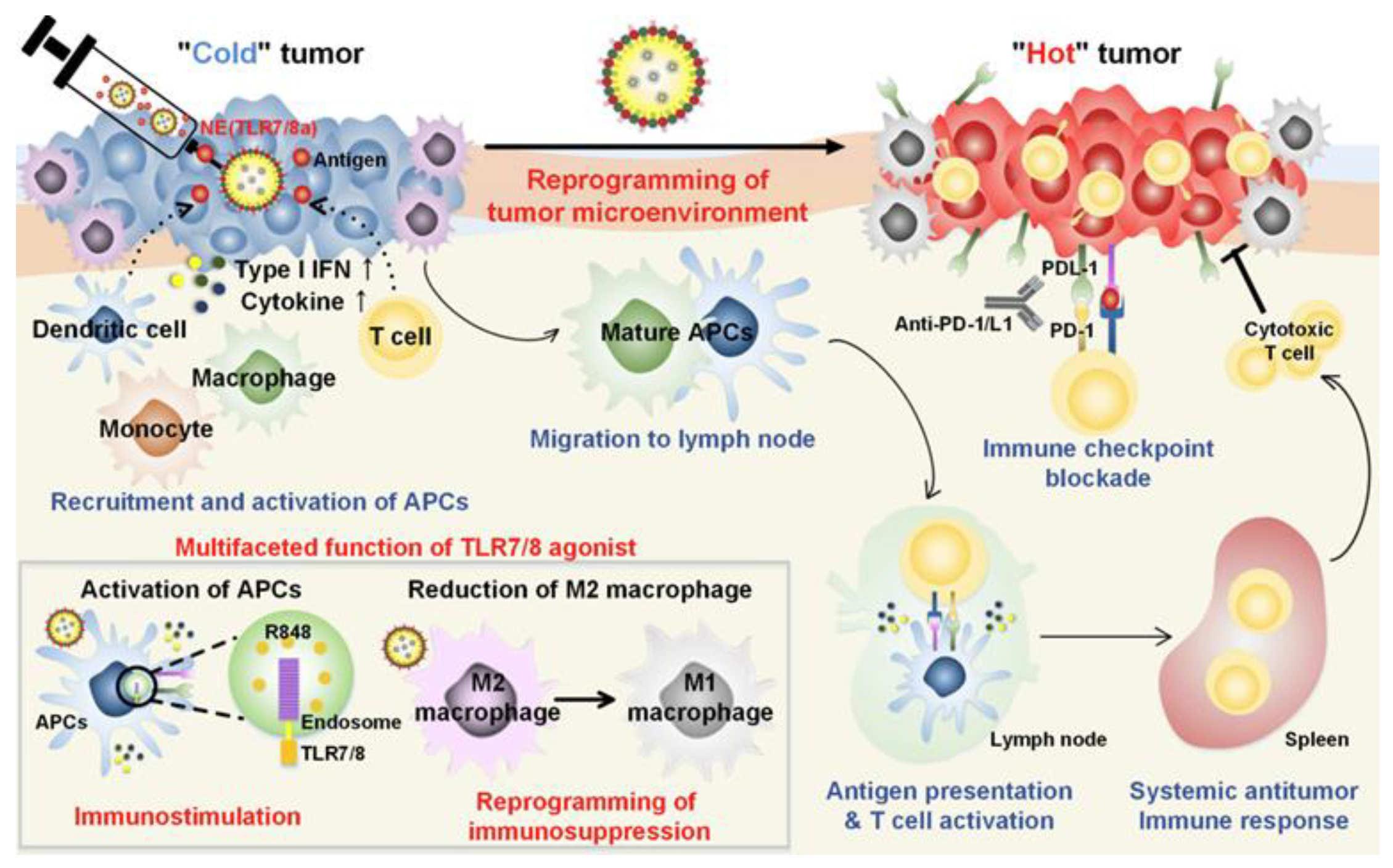
For instance, Kim et al. demonstrated that combining a tyrosine kinase inhibitor (sunitinib) with a TLR7/8 agonist (compound 522) in a nanoparticle-based vaccine significantly reduced the presence of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in tumors [67]. The addition of anti-PD-L1 antibodies further alleviated CD8+ T cell exhaustion. This triple combination therapy not only reduced immunosuppressive cell populations but also activated CD8+ T cells and promoted memory responses. In another study, a nanoemulsion (NE) formulation of R848 was used to overcome tumor-induced immunosuppression and stimulate T cell-mediated responses. Mice treated with NE(R848) showed significantly reduced tumor progression and improved survival compared to those receiving free drug. When NE(R848) was combined with ovalbumin (OVA) and anti-PD-L1, the treatment led to enhanced T cell infiltration, elevated IFN-γ secretion, and superior tumor control. A tumor rechallenge experiment confirmed the induction of long-term antitumor memory. The simplicity of NE(R848) fabrication, its lyophilization capability, and the use of clinically safe components make it a promising platform for cancer immunotherapy and infectious disease management (Figure 3) [68].
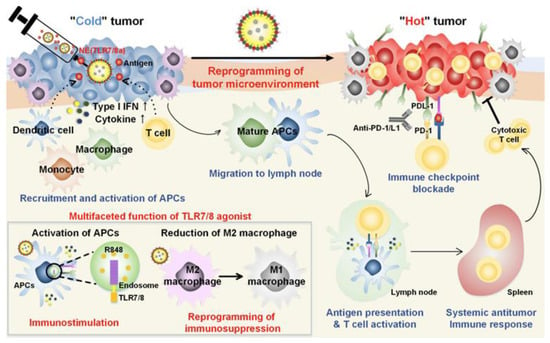
Figure 3.
Schematic representation of the mechanisms of combining TLR7/8 agonist and checkpoint inhibitor for cancer immunotherapy. Adapted with permission from [68]. Copyright © 2019, American Chemical Society. IFN-I: type I IFNs, APCs: antigen-presenting cells, R848: an immune adjuvant resiquimod.
Ni et al. developed a nanoscale metal–organic framework (nMOF) system, Hf-DBP-modified to co-deliver a hydrophilic anti-CD47 antibody (αCD47) and a hydrophobic TLR7/8 agonist (IMD) [69]. This platform enabled radiotherapy–radiodynamic therapy and reprogrammed the tumor microenvironment. IMD repolarized M2 macrophages to the pro-inflammatory M1 phenotype, while αCD47 promoted phagocytosis by blocking the “don’t eat me” signal. When combined with anti-PD-L1, this strategy eliminated both primary and distant tumors in a bilateral colorectal cancer model, highlighting the potential of MOFs for delivering immune adjuvants in macrophage-targeted therapies.
Moreover, Jiang et al. developed cancer cell membrane-coated MOFs to deliver 3M-052 (M) and epigenetic inhibitors, such as BRD4, for the treatment of TNBC [70]. This treatment modality resulted in significant recruitment of DCs with long-term antitumor immune response. Furthermore, this study demonstrated increased lymphocyte infiltration and ICD along with reduced Tregs. To address systemic toxicity associated with TLR agonists, Smith et al. developed PEG–PLA nanoparticles functionalized with TLR7/8 agonists [71]. These self-assembling nanoparticles improved pharmacokinetics, reduced systemic exposure, and enhanced tumor-specific delivery of the agonists. In vivo studies showed elevated serum IFN-α levels and improved therapeutic efficacy when combined with anti-PD-L1, resulting in reduced tumor growth and extended survival in a murine colon adenocarcinoma model.
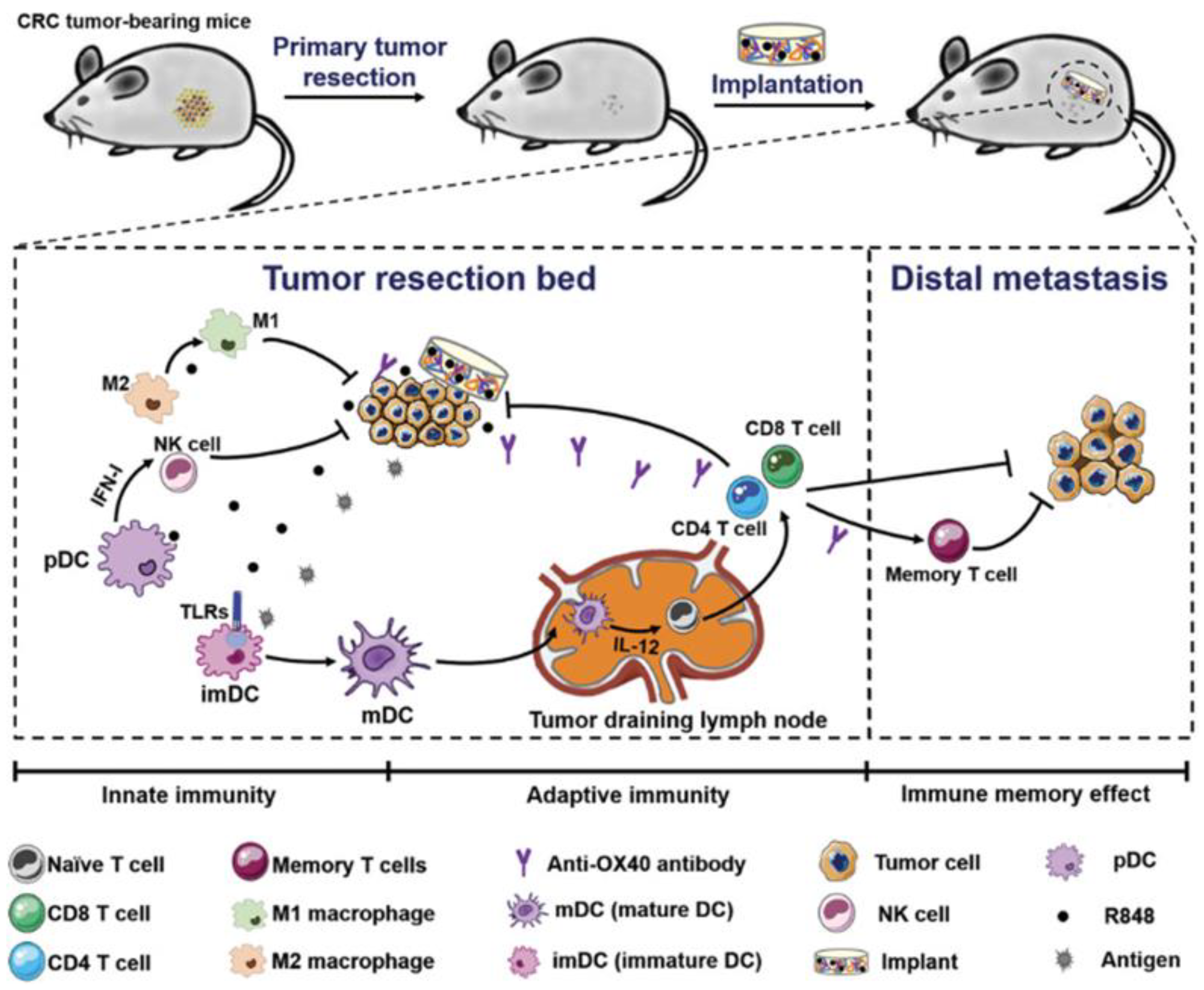
Postoperative tumor recurrence and metastasis remain major clinical challenges. Ji et al. addressed this by designing a biopolymer implant composed of 4-arm PEG-NH2 and oxidized dextran (ODEX) to co-deliver R848 and anti-OX40 antibodies following colorectal cancer surgery. This implant eradicated residual tumors for up to 150 days, inhibited distant tumor growth, and induced durable immune memory. Early immune responses included increased NK cell infiltration and DC activation, followed by robust T cell infiltration (Figure 4) [72].
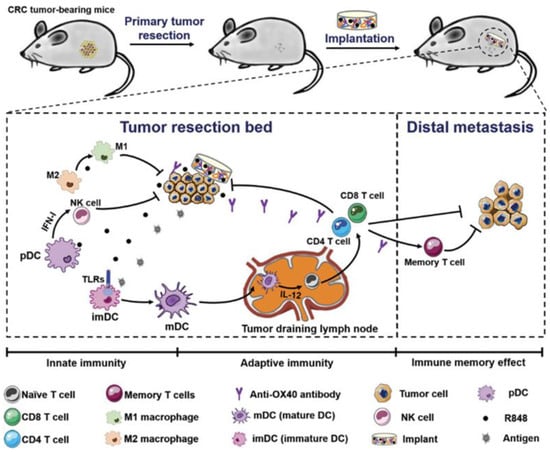
Figure 4.
Schematic presentation of treatment strategy and implementation of biopolymers co-encapsulating R848 and anti-OX40 antibody, and possible mechanisms in immune responses after the treatments in colorectal cancer. Reprinted with permission from [72]. Copyright © 2020, John Wiley and Sons. TLRs: Toll-like receptors, IFN-I: type I IFNs, imDC: immature dendritic cells, mDC: mature dendritic cells, R848: an immune adjuvant resiquimod.
Another delivery strategy was implemented by Kang et al. to treat ovarian cancer. The group developed large anionic liposomes (RSQLP) for intraperitoneal delivery of R848. This system specifically targeted tumor-associated macrophages (TAMs), repolarizing them to the M1 phenotype. It also increased tumor-infiltrating CD8+ and CD4+ T cells. When combined with anti-PD-L1, the treatment led to complete tumor rejection for up to 250 days, indicating long-term antitumor immunity [73].
Huang et al. designed ROS-responsive mesoporous nanoparticles (MSN@TheraVac) conjugated with anti-PD-L1 to co-deliver nucleosome-binding protein 1 (HMGN1) and R848 for colon cancer treatment [74]. This system enabled sequential release: rapid αPD-L1 to prevent T cell exhaustion, followed by HMGN1 and R848 to mature DCs. The treatment eliminated tumors in CT26-bearing mice and enhanced tumor-specific immune responses in draining lymph nodes.
Finally, Bhatnagar et al. implemented a multi-adjuvant strategy combining a TLR7/8 agonist with a STING agonist (DMXAA) and OVA antigen [75]. This combination matured DCs in tumors, spleen, and lymph nodes, and significantly increased antigen-specific CD8+ T cell and NK cell responses. The triple therapy reduced tumor burden and improved survival compared to other treatment groups.
Antibody–drug conjugates (ADCs) represent a targeted therapeutic strategy designed to deliver drugs directly to tumor sites, minimizing systemic toxicity. Among these, TLR7/8 agonist–antibody conjugates have shown promising results in preclinical models, including enhanced production of pro-inflammatory cytokines and improved therapeutic efficacy in mice [76,77,78,79]. However, translation into clinical trials has encountered significant challenges, particularly related to neuroinflammation and cytokine release syndrome, which compromise both safety and therapeutic effectiveness. To address these issues, several studies have investigated species-specific differences that may affect the translatability of preclinical findings to human applications [80,81].
For instance, Sega et al. employed mouse-derived antibodies conjugated with a TLR7 agonist to treat the CT26 syngeneic colon carcinoma model [82]. Their findings revealed significantly higher accumulation of the TLR7 agonist at the tumor site compared to free drug administration. The ADCs also demonstrated consistent processing via antibody-dependent cellular cytotoxicity (ADCC), resulting in sustained activation of myeloid cells within the tumor microenvironment (TME). In another study, a tumor-targeting LIV1 antibody was conjugated with TLR7/8 agonists to assess antitumor responses [83]. This approach enhanced inflammation and T cell recruitment in the TME more effectively than free drug treatment, leading to improved myeloid cell activation, antitumor efficacy, and tolerability. Additionally, a vaccine platform incorporating neoantigen–TLR7/8 agonist conjugates was developed to stimulate anticancer T cell responses. These constructs significantly boosted T cell activity against tumor antigens and offered a versatile framework for co-delivering peptides and adjuvants in cancer immunotherapy [84].
Various combination strategies involving antibodies, inhibitors, or STING agonists alongside TLR7/8 agonists have been employed to enhance therapeutic efficacy. These combinations have been delivered using platforms such as liposomes, nanoemulsions, metal–organic frameworks (MOFs), or as free drugs. Studies have demonstrated that these approaches elicit stronger antitumor immune responses compared to treatment with TLR7/8 agonists alone. While the addition of antibodies has shown promising therapeutic outcomes, a thorough evaluation of antibody-associated toxicities is essential before advancing to clinical applications. Moreover, testing these combinations across a broader range of cancer cell lines—rather than limiting evaluations to one or two—could help identify cancer-specific requirements and improve the translatability of these therapies to clinical settings.
5.2. Combination with Chemotherapy
Chemotherapy remains a cornerstone of cancer treatment, with drugs such as paclitaxel, doxorubicin, and cisplatin widely used due to their ability to induce immunogenic cell death (ICD) [70,85,86,87]. Combining chemotherapeutic agents with TLR7/8 agonists has shown enhanced therapeutic efficacy by simultaneously promoting tumor cell death and immune activation [88]. For example, co-administration of oxaliplatin with liposome-encapsulated R848 significantly increased CD8+ T cell infiltration and markedly reduced CT26 murine colorectal tumor growth compared to free drug treatments. This combination demonstrated the potential of liposomal R848 to enhance immune activation in colorectal cancer [89].
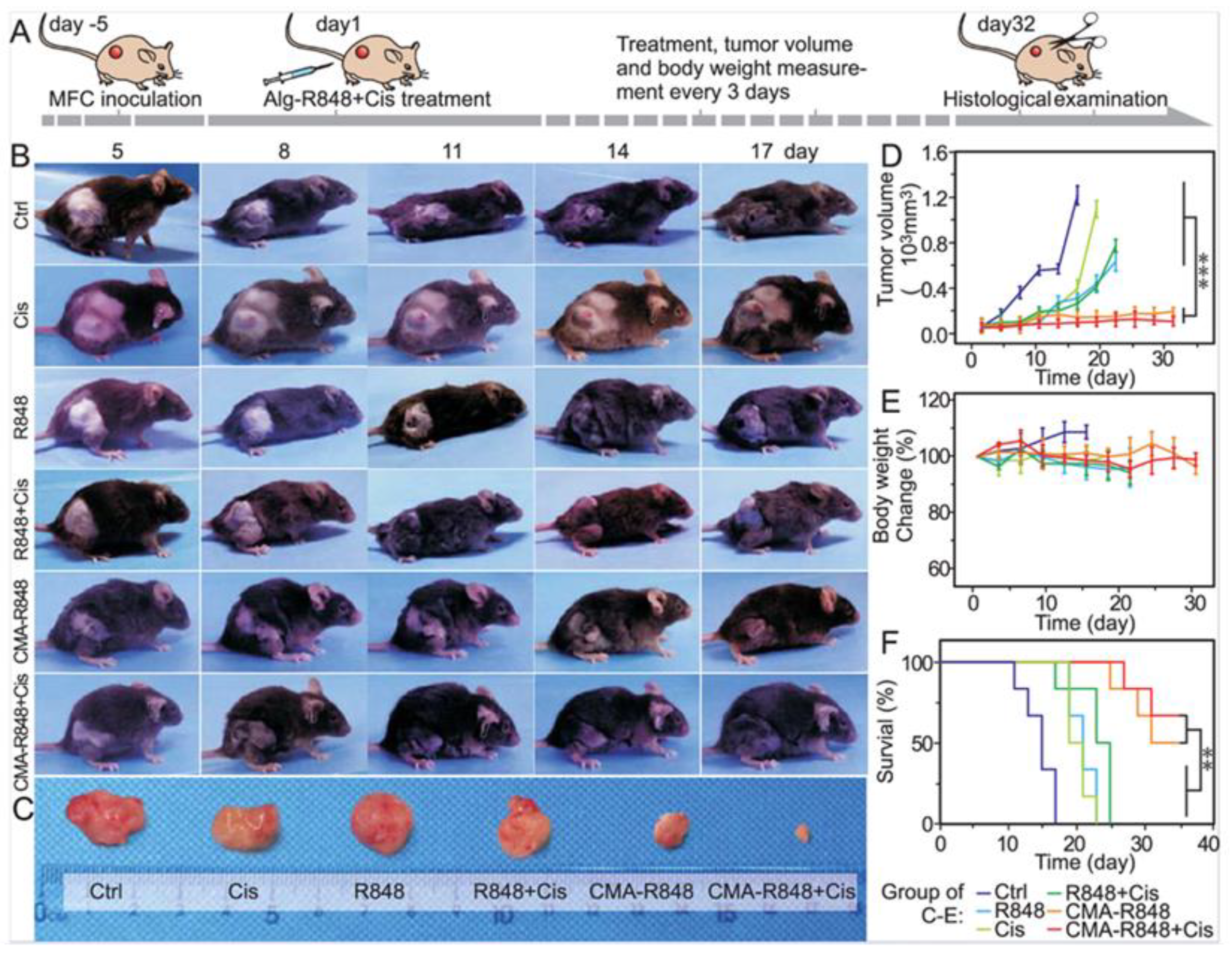
In another study, micelles were engineered by conjugating hydrophilic carboxymethylated alginate with hydrophobic R848, forming CMA-R848 for gastric cancer therapy. To further improve efficacy, cisplatin was co-administered with the R848-loaded micelles. Upon reaching the mildly acidic tumor microenvironment, ester bonds in the micelles cleaved, releasing R848 locally. CMA-R848 treatment activated bone marrow-derived dendritic cells (BMDCs) and elevated IL-6 and TNF-α levels. Additionally, M2 macrophages were repolarized to the M1 phenotype, and IL-10 levels decreased while IL-12 increased. The combination of CMA-R848 and cisplatin significantly reduced tumor burden and enhanced CD4+ and CD8+ T cell populations, suggesting a shift from a “cold” to a “hot” tumor microenvironment (Figure 5) [90].
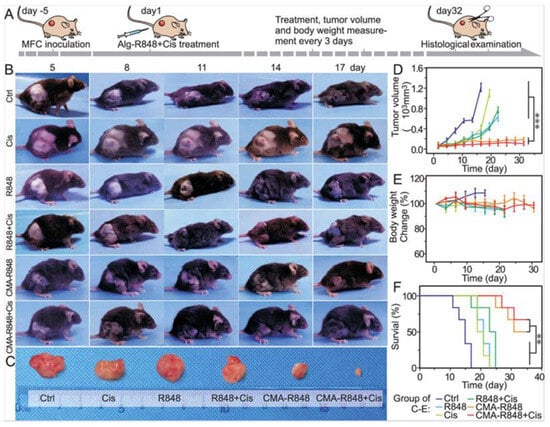
Figure 5.
Evaluation of antitumor efficacy of CMA-R848 and cisplatin treatment in vivo studies on GC model mice. (A) The illustration of tumor establishment in the mouse model and the schedule for subsequent treatments with therapeutics. (B,C) The images of the Tumor volume following various treatments. (D,E) Tumor volume changes with various treatments. (F) Survival rate of the mice with individual treatments. n = 6, The statistical analysis was determined by one-way ANOVA test, ** p < 0.01, *** p < 0.001. Adapted with permission from [90]. Copyright © 2023, American Chemical Society. CMA: carboxymethylated alginate, R848: an immune adjuvant resiquimod.
Jianqin et al. developed matrix metalloproteinase-2 (MMP-2)-sensitive hydrogels for chemo-immunotherapy in metastatic breast cancer [91]. These hydrogels incorporated nuclear-targeted tetrahedral DNA nanostructures and disulfide-crosslinked polyethylenimine, loaded with doxorubicin (DOX) and R837. The nanoparticles effectively penetrated tumor tissues and promoted tumor-associated antigen (TAA) release. In vitro, the combination therapy significantly increased secretion of IL-6, IL-12p70, and TNF-α. In vivo, CD8+ T cell populations rose to 5.52%, indicating a strong cytotoxic immune response.
Another study utilized a thermoresponsive hydrogel vaccine for breast cancer. Pluronic F127 polymers formed the hydrogel matrix, embedding polydopamine nanoparticles coated with hyaluronic acid. IQ (a TLR7 agonist) was combined with DOX to enhance tumor-specific immune activation through memory T cell proliferation and dendritic cell (DC) maturation. This injectable hydrogel platform significantly slowed tumor growth and eliminated tumors within 7–21 days. Treated mice exhibited elevated memory T cell and DC responses and extended survival to two months, compared to one month in untreated controls [92].
For aggressive melanoma, injectable hydrogels composed of polyethylene glycol thiol and poly(ethylene glycol) diacrylate were used to co-deliver R837 and DOX. This strategy minimized systemic toxicity and improved therapeutic outcomes. The combination treatment suppressed melanoma growth both in vitro and in vivo, induced ICD, activated DCs, and promoted M1 macrophage polarization. Cytokines such as IFN-γ and TNF-α were significantly elevated in the spleen and tumor microenvironment, supporting the potential of this approach for targeted cancer therapy [93].
Although the combination of TLR7/8 agonists with chemotherapeutic agents has yielded significantly enhanced therapeutic outcomes, further optimization of dose–response parameters is essential for successful clinical translation. Establishing appropriate experimental conditions to accurately assess drug retention time and deep tissue penetration within tumors is critical. These insights will help define precise pharmacokinetic and pharmacodynamic (PK/PD) profiles, thereby improving therapeutic efficacy. Additionally, conducting preclinical studies using patient-derived xenograft (PDX) tumor models in humanized mice may strengthen the translational potential of these combination strategies for clinical applications.
5.3. Combination with Phototherapy
Phototherapy, encompassing photothermal therapy (PTT) and photodynamic therapy (PDT), is a widely used non-invasive strategy for ablating primary tumor masses. Despite its promise, phototherapy alone often fails to achieve complete tumor eradication, necessitating combination approaches to enhance therapeutic efficacy.
Recent studies have explored the integration of phototherapy with immunostimulatory agents and inhibitors to boost antitumor responses. For example, virus-like nanoparticles (VLPs) derived from bacteria, yeast, plants, and mammalian cells-known for their biocompatibility and scalability-have been investigated as delivery vehicles for cancer therapy [94]. In a proof-of-concept study, Christian Isalomboto Nkanga et al. utilized tobacco mosaic virus (TMV) particles, chemically modified with polydopamine (PDA) for photothermal conversion, and loaded with the TLR7 agonist 1V209. This formulation significantly improved survival in C57BL/6 mice with B16F10 dermal melanoma (60% vs. 20% in controls) and enhanced systemic antitumor immunity via increased tumor-specific T cells [95]. Yosothamani et al. developed polyaniline (PANi)-based nanoparticles targeting estrogen receptor-positive (ER+) breast cancer cells. These nanoparticles were functionalized with endoxifen (END) and loaded with the TLR7 agonist R837. Combined with anti-PD-L1 therapy and NIR laser irradiation, the treatment elevated CD8+ and CD4+ T cells, with the most pronounced immune activation observed in the group receiving all three modalities [96].
Qin et al. fabricated an NIR-activated hydrogel using agarose and the photothermal agent M-4, co-delivering R837 and doxorubicin (DOX). This synergistic approach enhanced DC maturation and activated T cells in both primary and secondary tumors, demonstrating robust systemic immune responses and reduced tumor burden [97]. Mei and colleagues employed naturally occurring polymers such as alginate and collagen to create self-assembling hydrogels encapsulating R837 and methylene blue (MB). These hydrogels exhibited shear-thinning and self-healing properties, enabling sustained drug release and localization, and showed promising photothermal-induced antitumor immune responses [98].
Yue et al. designed a core–shell nanoplatform composed of zinc porphyrin and mesoporous nanoparticles (MPSNs@R837), integrating R837 and anti-PD-L1 for enhanced photoimmunotherapy. The combination of PTT, PDT, and immunotherapy significantly inhibited tumor growth and promoted DC maturation and T cell activation, outperforming monotherapies [99]. Jiang et al. developed a photoactivable polymeric nanoagonist (APNA) using an NIR-II absorbing semiconducting polymer functionalized with R848 via a thermos-responsive linker. Under NIR-II irradiation, APNA induced potent cell death and activated DCs, leading to elevated CD8+ and CD4+ T cells in distant tumors and metastatic sites, including the lungs and liver. This strategy demonstrated strong potential for nanovaccine development [100].
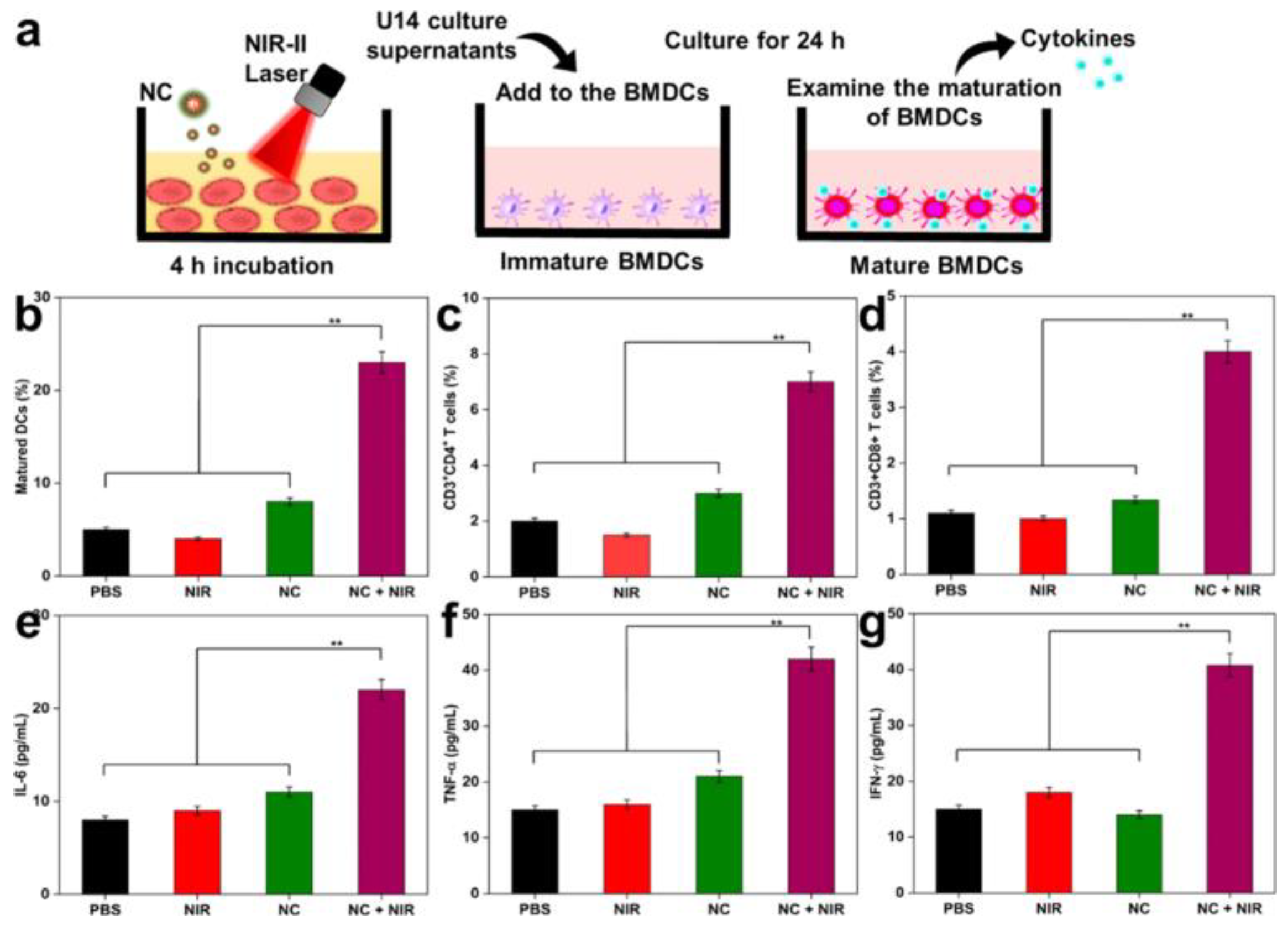
Further advancing this concept, another group synthesized NIR-II responsive nanocomposites (NCs) from polypyrrole, functionalized with TAT peptides and hyaluronic acid (HA) for nuclear targeting and R848 delivery. These NCs exhibited excellent photothermal stability and induced nucleus-specific cell death under NIR-II irradiation. Immune activation markers such as calreticulin and HMGB1 were upregulated, alongside increased DC maturation and pro-inflammatory cytokines (IL-6, TNF-α, IFN-γ), confirming the efficacy of this nanovaccine platform (Figure 6) [101].
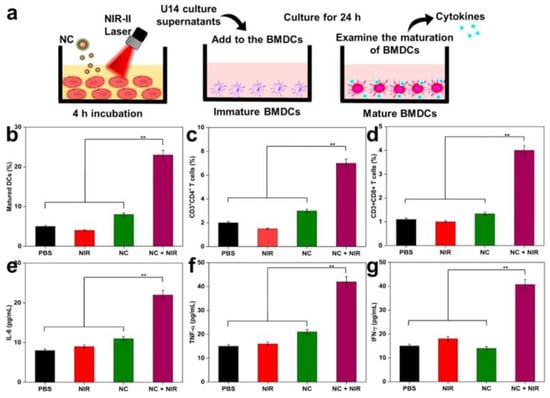
Figure 6.
(a) In vitro approach to mature BMDC. (b) Maturation of DCs in tumor-draining lymph nodes with various treatments. (c,d) Quantification of CD4+ T-cells and CD8+ T-cells (gated on CD3+) in tumors after 10 days of various treatments (analyzed by Flow Cytometry). (e–g) ELISA analysis of IL-6, TNF-α, and IFN-γ of mice after various treatments. Laser treatment was 1064 nm of 1 W/cm2 for 5 min. The statistical significance of the experimental data was assessed via the mean ± SE and Student’s t-test. Data are presented as the mean ± SD (n = 5); and ** p < 0.01. Adapted with permission from [101]. Copyright © 2023, American Chemical Society. NIR-II: second Near-Infrared, BMDCs: bone marrow-derived dendritic cells.
In another study, PANi nanoparticles were coated with poly(vinylpyrrolidone) (PVP) to prevent aggregation and modified with RGD peptides for targeted delivery to cancer cells. R837 was encapsulated to enhance antitumor immune responses. Flow cytometry analysis revealed significantly higher populations of CD4+ and CD8+ T cells compared to other treatment groups. This synergistic strategy also promoted DC activation and reduced tumor recurrence relative to NIR alone and untreated controls, suggesting its potential as an effective nanovaccine platform [102].
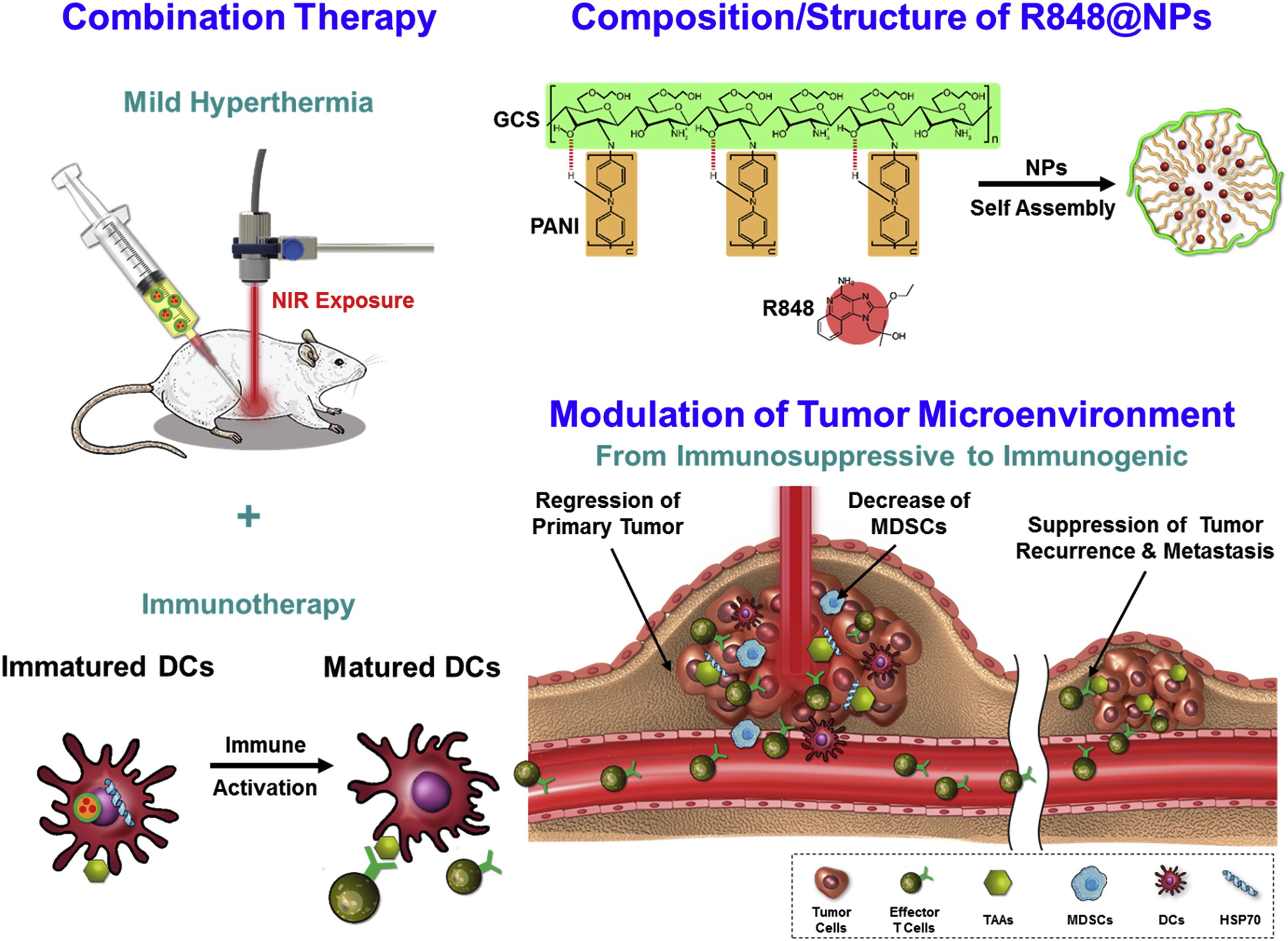
Chen et al. proposed that low-temperature PTT may be more effective and safer than high-temperature PTT, which can damage surrounding healthy tissues and immune cells. To address the limitations of deep tissue penetration and heat generation, they designed R848-loaded PANi-conjugated glycol-chitosan nanoparticles (R848@NPs). These acted as in situ low-temperature cancer vaccines. The treatment significantly upregulated CD80 and CD86 expression on bone marrow-derived DCs, and increased IL-6 and TNF-α levels, indicating effective immune activation. In vivo studies using Balb/c mice exposed to NIR laser at 0.9 W/cm2 and 2.0 W/cm2 showed that low-temperature treatment with R848@NPs most effectively inhibited tumor growth and enhanced effector T cell responses. This strategy elicited systemic antitumor immunity and holds promise for clinical translation (Figure 7) [103].
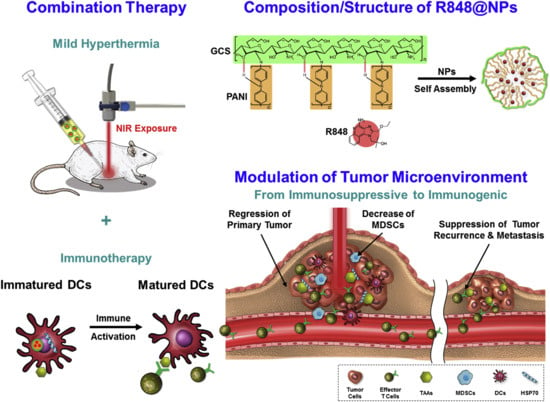
Figure 7.
Schematic diagram of treatment strategy and mechanisms of immune responses and fabrication of self-assembled nanoparticles of PANI-GCS loaded with R848. Reprinted with permission from [103]. Copyright © 2019, Elsevier Ltd. PANI: polyaniline, GCS: glycol-chitosan, R848: an immune adjuvant resiquimod.
Moreover, Meng and colleagues demonstrated that mild hyperthermia therapy could significantly inhibit tumor growth, metastasis, and enhance antitumor immune responses in melanoma. They fabricated a thermoresponsive hydrogel system (R837@PDA@CGP) by embedding R837 nanocrystals coated with PDA into chitosan hydrogel. Nanocrystals were chosen for their superior drug loading capacity [104]. In vivo studies using B16F10 melanoma models in C57BL/6 mice showed that a single dose of R837@PDA@CGP followed by NIR irradiation led to significant tumor reduction and enhanced DC maturation. CD8+ T cell populations increased to 15.90 ± 2.88%, and IFN-γ levels were elevated, confirming the formulation’s ability to boost antitumor immunity [105].
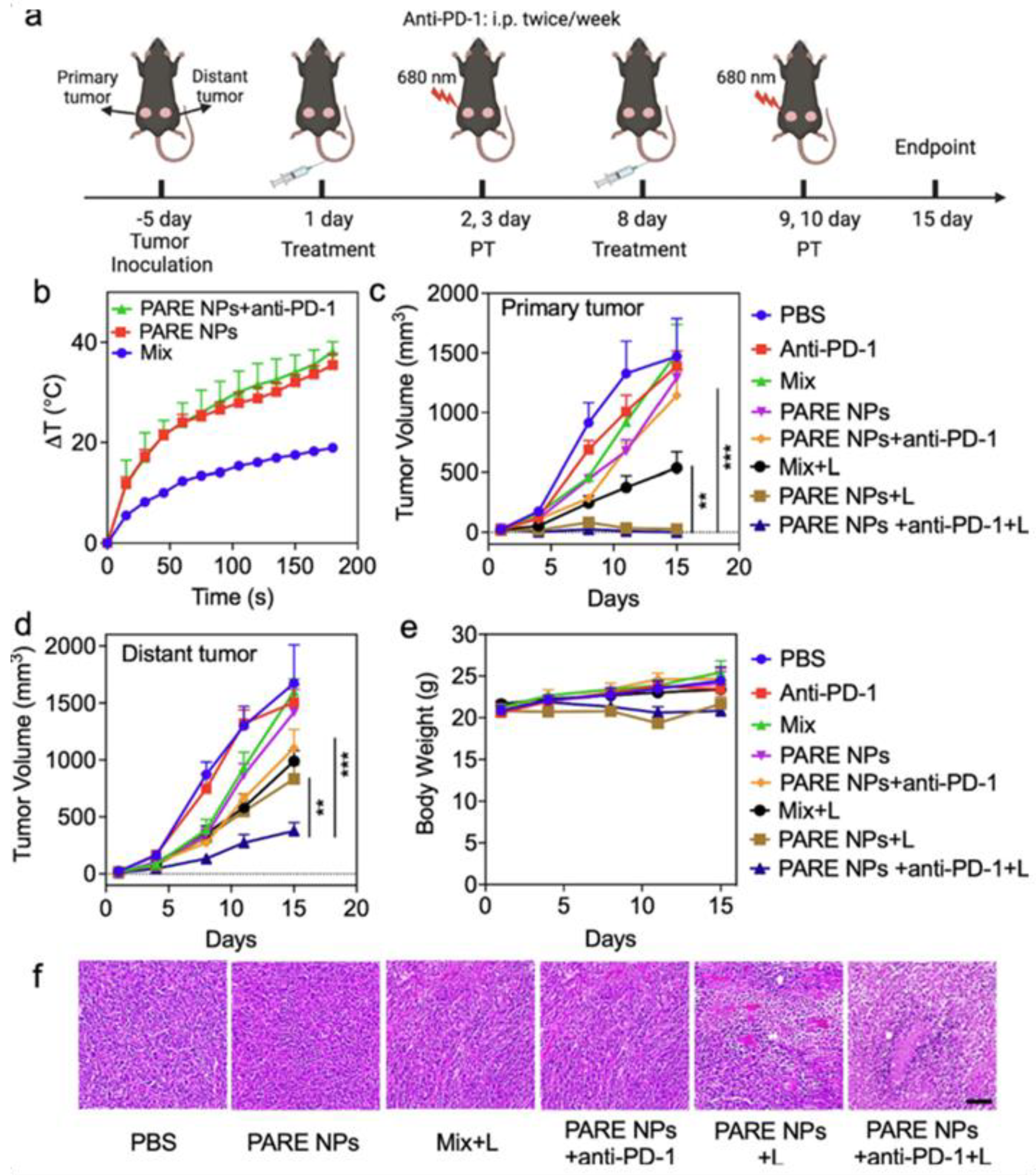
Qu et al. developed prodrug-based nanoparticles (PARE NPs) by conjugating pyropheophorbide-A (PA) and R848. These self-assembling NPs generated ROS and photothermal effects, enhancing immune responses. In SCC-7 tumor models, laser treatment combined with anti-PD-1 therapy significantly reduced both primary and distant tumors. CD4+ T cell populations increased to >60%, and DC maturation in lymph nodes was markedly improved, demonstrating systemic immunity and potential for treating head and neck cancers (Figure 8) [106].
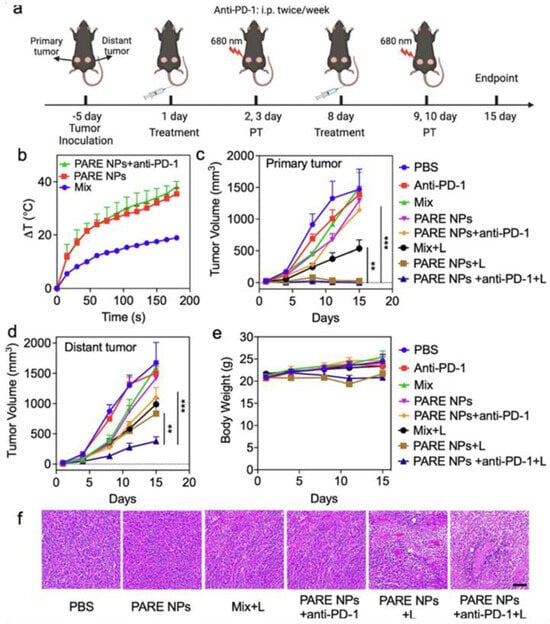
Figure 8.
Evaluation of antitumor efficacies of developed NPs in in vivo studies. (a) The illustration of tumor establishment in HNSCC tumor mouse model and the schedule for subsequent treatments with both laser and therapeutics. The laser was treated with 0.5 W/cm2 for 3 min. (b) The photothermal ablation effects of the tumor after the treatments of Mix, PARE NPs alone, and triple combination. (c,d) The changes in tumor volume of both primary tumors and the distal tumor. (e) Assessment of Body weight changes in tumor-bearing mice. (f) Assessment of H&E staining of the tumor tissue after various treatments. The scale bar is 100 μm. Statistical analysis was performed by Student’s t-test for two groups, and one-way ANOVA for multiple groups. Adapted with permission from [106]. Copyright © 2023, Elsevier B.V. PARE NPs: Self-assembled nanoparticles of prodrug derived from pyropheophorbide-A, PA, a photosensitizer, and R848, an immune adjuvant resiquimod.
Wang et al. reported acidic-responsive polymeric nanoparticles (ARNPs) engineered with R848 and PA to activate in tumor microenvironments. Under 671 nm laser irradiation, ARNPs produced ROS, triggered immunogenic cell death (ICD), and released R848 to enhance antigen presentation. In CT-26 colorectal tumor models, ARNPs combined with laser and checkpoint blockade therapy led to tumor regression and extended survival (50% on day 32). Increased CTL infiltration and reduced Tregs confirmed the efficacy of this combinatory approach [107].
Another promising strategy involved a liposomal formulation (Lipo@IR808@Loxo) combining NIR-responsive IR808 and loxoribine. This system enhanced PTT efficacy and antigen release under NIR irradiation, while loxoribine activated APCs. In 4T1 tumor-bearing mice, Lipo@IR808@Loxo + Laser treatment completely eradicated tumors and induced CRT expression, confirming ICD. Anti-PD-1 therapy further boosted immune responses, leading to the elimination of bilateral tumors [108].
Revuri et al. introduced BAGEL-R848, an injectable thermosensitive hydrogel composed of MnO2 NPs, R848, and hyaluronic acid/pluronic F127. Designed for localized delivery, BAGEL-R848 showed high photothermal conversion efficiency and significantly reduced both primary and distant tumors in 4T1 models. Elevated levels of TNF-α, IL-6, IFN-γ, and IL-12p70 confirmed strong immune activation, highlighting its potential as a cancer treatment platform [109].
An extensive study reported that PDT alone is insufficient for complete cancer treatment. Researchers demonstrated that combining PDT with immunostimulatory agents-such as poly(I:C), R848, and macrophage inflammatory protein 3 (MIP3), enhanced antitumor immunity while minimizing systemic side effects. PLGA nanoparticles were used to deliver these agents locally. In MC38, CT26, and TC-1 tumor models, combination therapy significantly delayed tumor growth compared to monotherapies. Splenocytes from treated mice showed elevated CD8+ T cells producing IFN-γ and TNF-α. In TC-1 models, HPV-E7-specific CD8+ T cells were significantly increased, confirming robust systemic immunity [110].
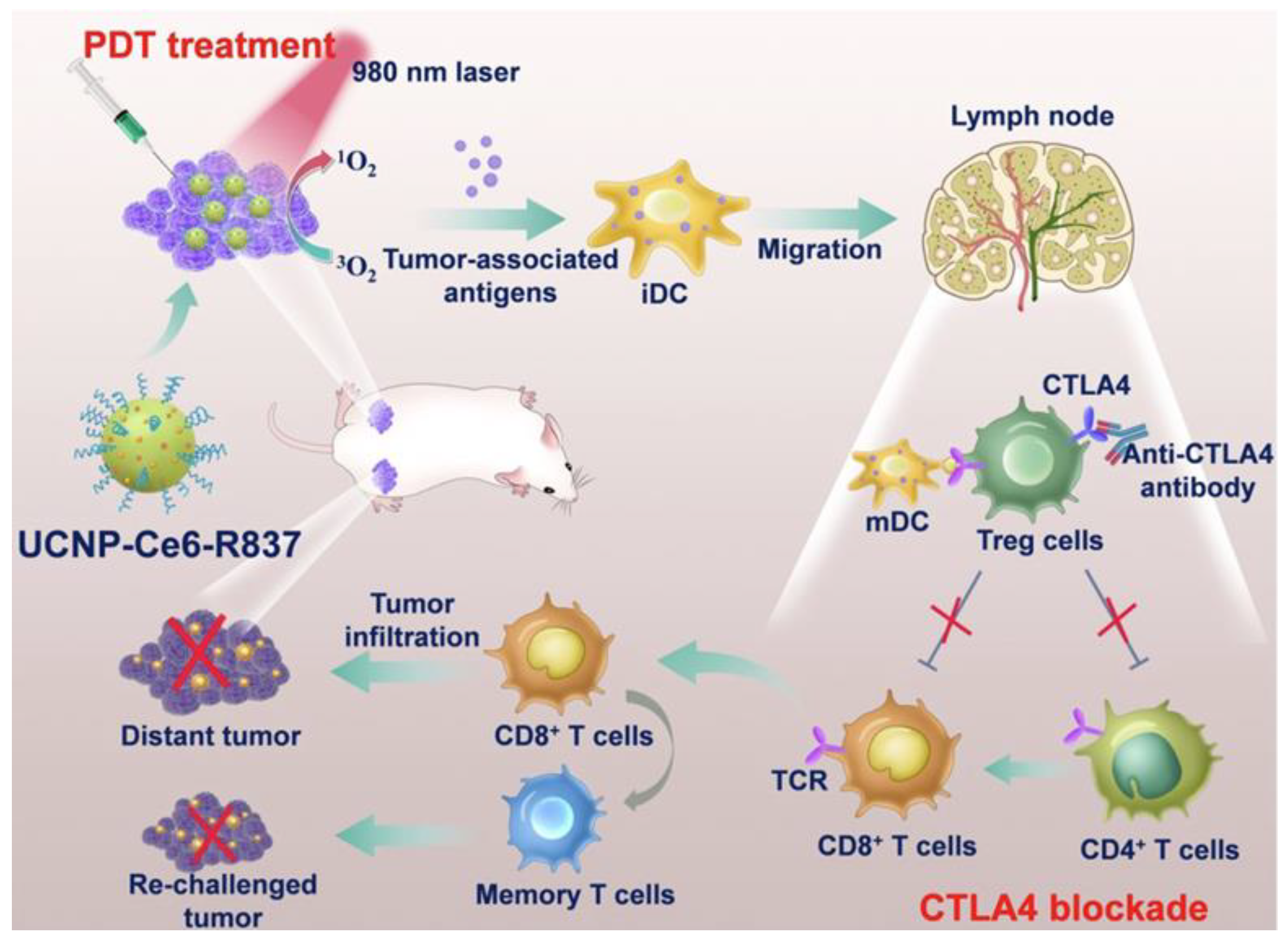
Xu et al. developed upconversion nanoparticles (UCNPs) with deep tissue penetration capabilities, co-encapsulating R837 and chlorin e6 (Ce6) into PEG-coated UCNPs (UCNP-Ce6-R837). This system enhanced DC maturation and cytokine production (TNF-α, IL-12), bridging innate and adaptive immunity. When combined with CTLA-4 checkpoint blockade, UCNP-Ce6-R837 treatment effectively eliminated tumors and prevented distant tumor growth, as shown in Figure 9 [111].
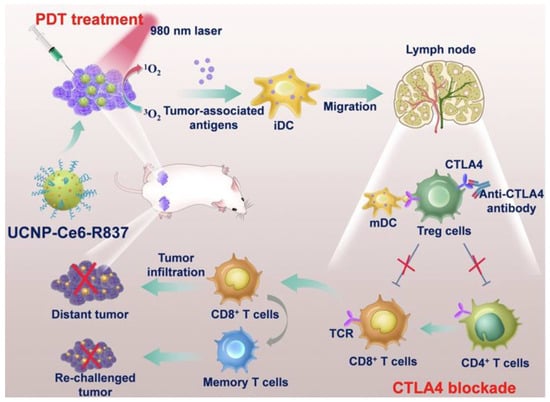
Figure 9.
Schematic illustration of the mechanisms of the combination of NIR-mediated PDT with TLR7 agonist and checkpoint inhibitor for cancer immunotherapy. Under the NIR laser treatment, UCNP-Ce6-R837 nanoparticles initiate tumor destruction. In combination with adjuvant and checkpoint inhibitors, tumor-associated antigens promoted strong antitumor immune responses to eliminate both primary and distal tumors as well as prevent tumor recurrence. Adapted with permission from [111]. Copyright © 2017, American Chemical Society. PDT: photodynamic therapy, iDC: immature dendritic cell, iDC: mature dendritic cell, UCNP: upconversion nanoparticles, Ce6: Photosensitizer, CTLA4: critical inhibitory receptor on T cells, R837: immune adjuvant IMQ.
To address limitations in topical drug penetration for skin cancer, PDT was combined with IMQ cream in treating cutaneous squamous cell carcinoma (cSCC). Using 5-aminolevulinic acid (ALA) as a photosensitizer, patients with invasive cSCC on the lips and feet received PDT at two-week intervals, alongside daily application of 5% IMQ cream. This regimen led to complete lesion removal after several treatment cycles [112]. Collectively, these studies underscore the potential of combining phototherapy with immunomodulatory strategies to achieve synergistic antitumor effects, offering promising avenues for translational cancer immunotherapy.
These studies utilized a range of organic and inorganic photosensitizers to enhance therapeutic efficacy. Careful selection of these agents is essential to minimize the risk of adverse toxicity. Additionally, experimental designs should aim to reduce collateral damage to surrounding healthy tissues, thereby improving the safety profile of photosensitizer-based treatments.
5.4. Combination with Radiation Therapy and Sonodynamic Therapy
Radiation therapy has long been a cornerstone of conventional cancer treatment, with extensive clinical applications [113,114]. However, its use is often limited by adverse effects on healthy tissues, particularly when radioisotopes are administered systemically. To mitigate these side effects, radiation therapy is increasingly applied in a localized manner. For example, Dewan et al. investigated the topical application of IMQ in combination with radiation therapy (RT) in a TSA mouse model. The treatment significantly reduced tumor growth and increased infiltration of CD11c+ DCs, CD4+ T cells, and CD8+ T cells. The addition of RT further enhanced antitumor responses compared to IMQ alone. Moreover, pre-treatment with low-dose cyclophosphamide amplified the therapeutic effect and reduced tumor recurrence, suggesting a promising strategy for breast cancer metastasis [115]. Building on this, Adlard et al. explored systemic administration of the TLR7 agonist DSR-6434 in combination with ionizing radiation for non-dermatological tumors. In CT26 and KHT tumor-bearing mice, this approach achieved 55% complete tumor regression and improved survival. Lung metastases were significantly reduced compared to radiation alone, and CD8+ T cell populations were notably elevated, demonstrating enhanced antitumor immunity [116]. Cho et al. further demonstrated synergistic antitumor and anti-metastatic effects in melanoma using IMQ and ionizing radiation. The combination induced cell death via autophagy and increased autophagosome formation. Incorporating 3-methyladenine (3-MA) into the treatment regimen enhanced CD4+ and CD8+ T cell populations while reducing regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), indicating potential for treating radio-resistant melanoma [117]. Dovedi et al. showed that systemic administration of R848 in combination with RT significantly improved therapeutic outcomes. Weekly intravenous doses of R848 enhanced CD8+ T cell-mediated responses and led to complete tumor rejection in 100% of mice rechallenged with EG7 or EL4 cells. This strategy demonstrated strong potential for treating B and T cell malignancies [118]. Ye et al. applied a similar approach in a pancreatic ductal adenocarcinoma (PDAC) model, combining R848 with stereotactic body radiotherapy (SBRT). While SBRT alone was insufficient to generate tumor antigens, the combination treatment activated CD8+ T cells and modulated cytokine profiles-decreasing IL-4, IL-6, and IL-10, while increasing IFN-γ, granzyme B, and CCL5. In a hepatic metastasis model, similar immunomodulatory effects were observed, supporting the potential of this strategy for metastatic PDAC [28]. Ota et al. investigated DSP-0509, another TLR7 agonist, in combination with RT in CT26 tumor-bearing mice. Systemic delivery of DSP-0509 with RT led to T cell-dependent immune activation and complete tumor elimination in 30% of treated mice—an outcome not achieved with cisplatin and RT. This approach may be particularly beneficial for tumors with poor immunogenicity or resistance to checkpoint inhibitors, warranting further clinical evaluation [119].
Sonodynamic therapy (SDT) offers a promising alternative to light-based treatments, particularly for deep-seated or inaccessible tumors [120,121]. SDT is non-invasive, safe, and capable of precise spatial targeting due to its superior tissue penetration [122,123]. Unlike phototherapy, SDT can trigger immunogenic cell death (ICD), releasing tumor-associated antigens (TAAs), inducing calreticulin (CRT) surface expression, and HMGB1 [124]. However, SDT alone is often insufficient to elicit robust immune responses [125], necessitating combination strategies. Yue et al. demonstrated that combining SDT with checkpoint blockade immunotherapy significantly enhances antitumor efficacy. They developed nanosensitizers composed of liposomes loaded with hematoporphyrin monomethyl ether (HMME) and R837 (HMME/R837@Lip). Compared to HMME@Lip alone, the combination promoted greater DC maturation and elevated cytokine levels (TNF-α, IL-6). When combined with anti-PD-L1 antibodies, this strategy suppressed both primary and distant tumors in 4T1 and CT26 models and prevented lung metastasis, providing a compelling proof-of-concept for integrating immunotherapy with non-invasive tumor treatments [126].
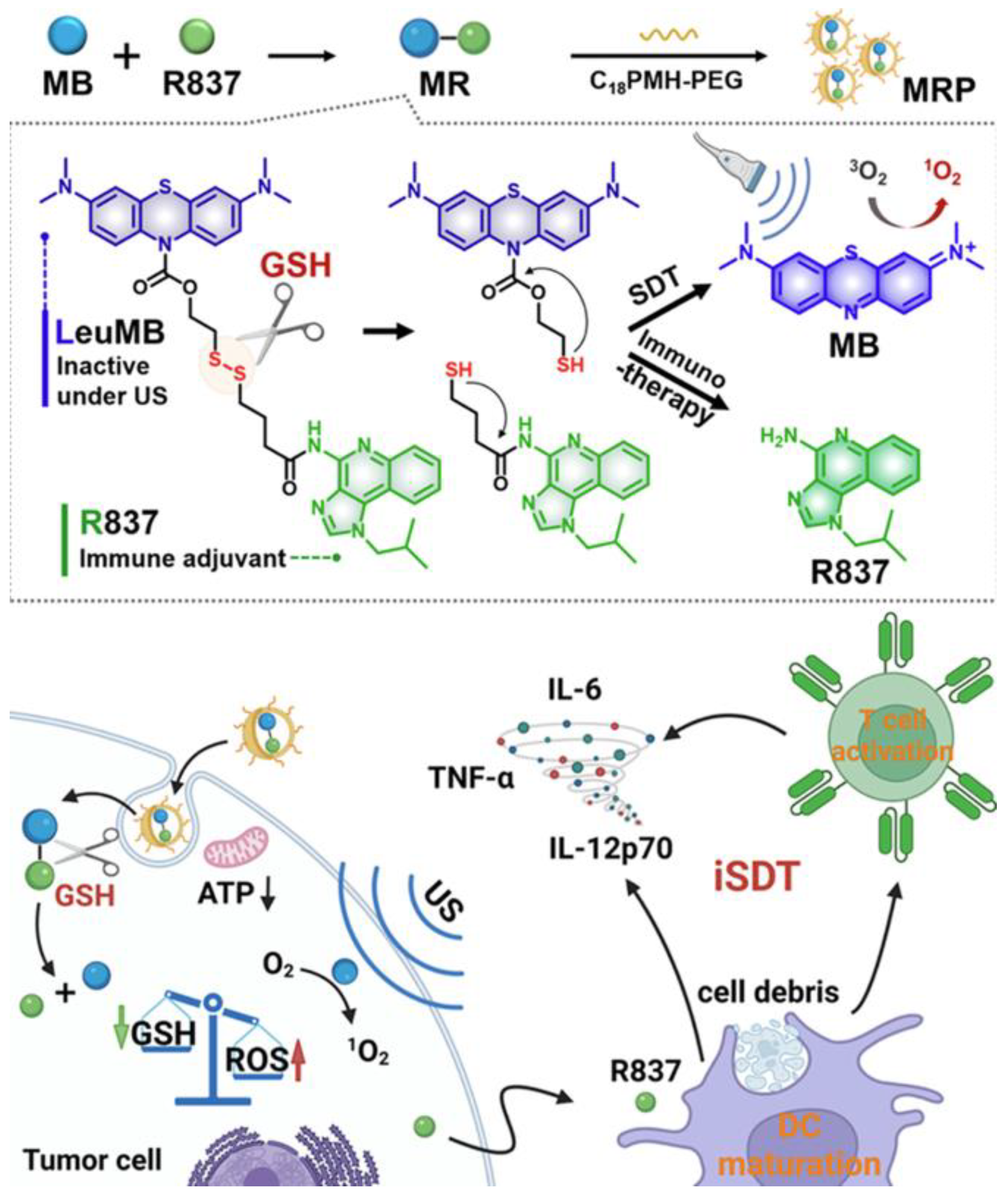
Recent investigations have focused on tumor-specific conditions within the tumor microenvironment (TME) to guide treatment design [127,128]. Prodrug-based strategies have garnered increasing attention due to their tumor-selective activation and reduced off-target toxicity [129,130]. For example, Lei and colleagues developed a glutathione-responsive prodrug composed of a reduced form of methylene blue and the TLR7 agonist R837. These components were encapsulated within amphiphilic polymers (C18PMH-PEG) to create drug-loaded nanoparticles, referred to as MRP. The MRP system exhibited selective activation and potent therapeutic effects at tumor sites, leading to significantly reduced tumor growth compared to individual treatments. To further enhance efficacy, anti-PD-L1 antibodies were incorporated, resulting in improved immune memory and reduced tumor recurrence (Figure 10) [131].
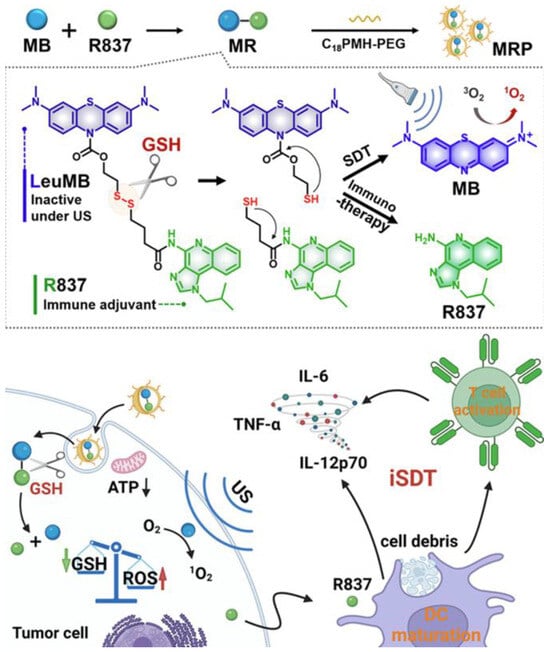
Figure 10.
Fabrication of MB-R837-PEG (MRP) nanoparticles for glutathione-mediated immunosonodynamic treatment (iSDT). Adapted with permission from [131]. Copyright © 2022, American Chemical Society. GSH: glutathione, ATP: adenosine triphosphate, ROS: reactive oxygen species, US: ultrasound, MB: methylene blue, R837: immune adjuvant IMQ.
To overcome limitations associated with anti-PD-1/PD-L1 antibodies-such as low tumor accumulation and immunogenicity, another group developed nanoparticles via self-assembly containing Ce6, R848, and the PD-L1 inhibitor JQ1 (termed CRJ NPs). CRJ NPs combined with SDT significantly inhibited tumor growth, whereas monotherapies showed minimal effect. Cytokine analysis confirmed elevated levels of antitumor immune mediators, supporting the therapeutic potential of this strategy [132].
Although the combination of TLR7/8 agonists with sonodynamic therapy (SDT) has shown notable therapeutic enhancement, further studies are necessary to assess potential damage to healthy tissues. Particular attention should be given to evaluating whether the generation of reactive oxygen and nitrogen species (ROS/RNS) contributes to off-target toxicity during treatment. Conversely, radiation therapy remains a widely used and effective modality for solid tumors; however, its clinical application is often limited by off-target adverse effects. Incorporating radioisotopes into hydrogel-based delivery systems presents a promising strategy to minimize collateral damage to healthy tissues and improve the safety profile of radiotherapy.
Additionally, we summarize the combination of TLR7/8 agonists with other treatment modalities in Table 1.

Table 1.
Summarization of combining TLR 7/8 agonists with other treatment modalities.
6. Development of TLR7/8 Agonist-Based Cancer Vaccines and Their Clinical Translation
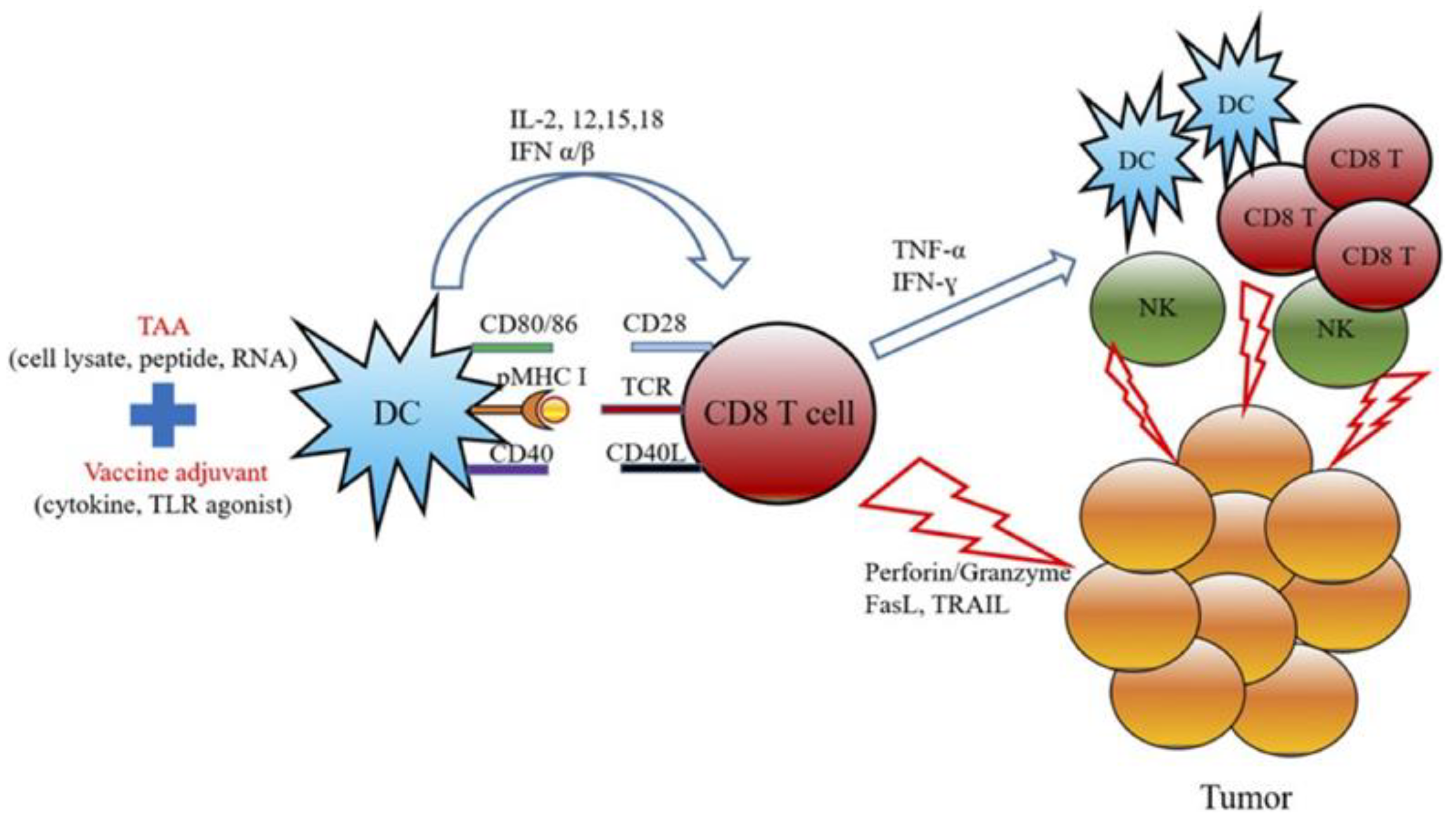
Cancer vaccine development typically involves combining tumor-associated antigens (TAAs) with adjuvants to stimulate cytotoxic CD8+ T cells capable of targeting cancer cells. These components initiate DC maturation, which subsequently enhances tumor-specific T cell responses [136]. Mature DCs express co-stimulatory molecules and release pro-inflammatory cytokines, presenting TAAs to activate cytotoxic T cells and NK cells [137]. This cascade further amplifies immune activation across DCs, T cells, and NK cells. A mechanistic illustration of cancer vaccine pathways is presented here (Figure 11) [138].
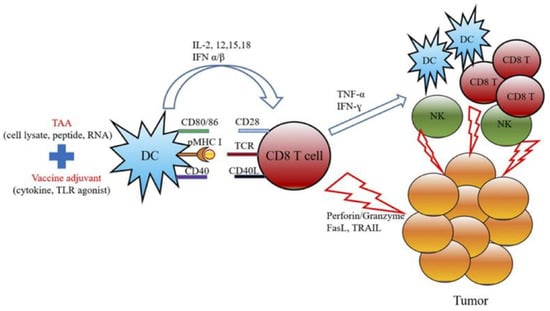
Figure 11.
The schematic diagram represents the mechanism of actions for cancer vaccine development by combining the treatment of TAAs and cancer adjuvants. Adapted with permission from [138]. Copyright © 2019, Elsevier.
Figure 11.
The schematic diagram represents the mechanism of actions for cancer vaccine development by combining the treatment of TAAs and cancer adjuvants. Adapted with permission from [138]. Copyright © 2019, Elsevier.
Although autologous tumor cell-based vaccines have shown efficacy, their clinical application is limited by the need for patient-specific tumor tissue. As an alternative, peptide- or protein-based vaccines derived from tumor cells have been extensively explored. However, these approaches have generally failed to elicit robust T cell responses [139], highlighting the need for additional immune stimulants. Among these, Toll-like receptor (TLR) agonists have been widely investigated as adjuvants to enhance cancer vaccine efficacy. TLR7/8 agonists, in particular, can directly activate the innate immune system, promote antigen presentation by DCs, and stimulate strong T cell responses [140]. Despite their promise, FDA-approved cancer vaccines incorporating TLR7/8 agonists remain limited.
To address this gap, Geoffrey et al. proposed polymer-based particulates functionalized with TLR7/8 agonists to improve vaccine efficacy. Their study demonstrated enhanced pharmacokinetic profiles and prolonged accumulation in draining lymph nodes (dLNs), along with broad antibody responses and robust T cell activation [141]. In another study, Shubhmita et al. employed a multi-adjuvant strategy combining ovalbumin with DMXAA and compound 522, which significantly enhanced CD8+ T cell and NK cell responses, leading to tumor suppression and improved survival compared to controls [75]. R848-loaded nanoemulsions combined with anti-PD-1 antibodies have also shown synergistic antitumor effects. This formulation promoted DC maturation and T cell proliferation, highlighting its potential as a cancer vaccine platform [142]. Kim et al. developed a sustained-release liposomal formulation of R848, chemically conjugated with cholesterol and cationic lipids. Compared to the commercial adjuvant AS01, this nanovaccine demonstrated superior antitumor efficacy and tumor inhibition [143].
Due to their immunomodulatory properties, small-molecule TLR7/8 agonists have progressed into clinical evaluation following promising preclinical results. For example, SHR2150 (TLR7 agonist) was combined with chemotherapy and PD-1 or CD47 antibodies in patients with metastatic solid tumors (NCT04588324). RO7119929 was orally administered to assess safety, PK/PD, and antitumor activity in patients with hepatic metastases (NCT04338685), involving 55 participants in a Phase I study. IMQ was evaluated for breast and skin metastases via topical application over eight weeks (NCT00899574), with 10 patients enrolled. The treatment was well tolerated, and two patients showed localized cytokine production [144]. IQ was also combined with local radiation therapy and cyclophosphamide (CTX) to assess systemic responses in skin metastases (NCT01421017), involving 31 patients in a Phase I/II trial. The study demonstrated safety and systemic antitumor immunity [145]. IQ combined with laser therapy was tested in patients with stage III or IV melanoma and skin metastases (NCT00453050), enrolling 70 patients. 852A, administered subcutaneously, showed antitumor effects in breast, ovarian, and cervical cancer patients (NCT00319748) [146]. One patient completed 24 doses with an additional 17 doses, and the study reported sustained tolerance and moderate antitumor activity. Another report indicated that two-thirds of patients with resistant metastatic melanoma treated with 852A experienced disease stabilization without further tumor growth or significant side effects [147].
Additional clinical trials include DSP-0509, tested in patients with advanced solid tumors either alone or with pembrolizumab (NCT03416335), enrolling 36 patients. BNT411, evaluated in patients with extensive-stage small cell lung cancer (ES-SCLC) as monotherapy or in combination with atezolizumab, carboplatin, and etoposide (NCT04101357). No severe adverse events or dose-limiting toxicities were observed, and one patient responded positively among the 11 enrolled. DN1508052-01, administered subcutaneously in patients with solid tumors (NCT03934359), involved 19 participants. The study assessed safety, maximum tolerated dose, and PK, revealing a favorable PK profile lasting up to two months.
VTX-2337, a TLR8 agonist, has been evaluated in multiple trials, such as Trials for B-cell lymphomas were terminated (NCT01289210, NCT02650635), but studies in HNSCC (NCT03906526, NCT01334177, NCT01836029), ovarian cancer (NCT01666444, NCT01294293, NCT02431559), and advanced solid tumors (NCT02650635) were completed. Combination with cetuximab showed no dose-limiting toxicities and led to increased plasma cytokine levels and NK cell activation (NCT01836029) [148]. Gregory et al. reported elevated biomarkers (IL-6, G-CSF, MCP-1, MIP1-β) in late-stage cancer patients and healthy volunteers, consistent with preclinical PK/PD profiles [149]. A trial combining VTX-2337 with nivolumab in patients with SCCHN is currently recruiting (NCT03906526).
MEDI9197, another TLR7/8 agonist, was tested in patients with advanced solid tumors. It was combined with durvalumab or palliative radiation therapy to evaluate antitumor activity. The study showed increased intratumoral CD8+ and PD-L1+ cells following treatment (NCT02556463) [150].
In another study, three DC vaccine formulations—tumor lysate + DCs, tumor lysate + DCs + 0.2% resiquimod, and tumor lysate + DCs + polyICLC—were evaluated in patients with glioma to determine the most effective approach (NCT01204684). This Phase II trial enrolled 24 patients.
Topical application of resiquimod gel at two concentrations was tested in patients with cutaneous T cell lymphomas to assess safety and antitumor activity (NCT01676831). Thirteen patients participated in Phase I and II trials. Two additional trials (NCT00470379, NCT01748747) evaluated the efficacy of peptide vaccines combined with resiquimod in patients with stage II–IV melanoma following surgical tumor removal.
BM201, administered intratumorally in 17 patients with refractory or metastatic solid tumors (NCT06368960), demonstrated a sustained plasma PK profile and significant tumor reduction in most patients. Notably, 29.4% of subjects exhibited potent abscopal effects. The study showed a favorable safety profile and recommended further evaluation of dose-dependent toxicity.
BDB001 was administered intravenously as monotherapy or in combination with pembrolizumab (NCT03486301) or atezolizumab (NCT04196530) in Phase I trials. The primary objective was to assess safety and tolerability, while secondary endpoints included efficacy and PK/PD. Approximately 29% of subjects receiving dose levels 3 and 4 showed sustained and prominent clinical responses. The treatment was well tolerated and supported robust systemic immune activation, prompting recommendations for dose escalation trials [151]. An ongoing trial is evaluating BDB001 in combination with atezolizumab and radiation therapy in patients with solid tumors (NCT03915678). A related compound, BDB018, is being tested as monotherapy or in combination with atezolizumab in a Phase I trial for solid tumors (NCT04840394).
BDC-1001, another TLR7/8 agonist, is under investigation as a single agent or in combination with nivolumab for HER2-expressing advanced malignancies (NCT04278144). This Phase I/II trial enrolled 75 patients and includes four parts: dose determination (Part 1), dose escalation with nivolumab (Part 2), efficacy evaluation of monotherapy (Part 3), and combination efficacy assessment (Part 4).
LHC165 is being evaluated as monotherapy or in combination with PDR001 for advanced malignancies (NCT03301896). This trial is active but not currently recruiting.
Finally, NKTR-262, administered intratumorally in combination with nivolumab, was tested in patients with solid and metastatic tumors. The study was terminated after Phase I due to results (NCT03138733).
Together, these studies highlight the translational potential of TLR7/8 agonists in cancer vaccine development. Continued innovation in formulation and delivery strategies will be critical to overcoming current limitations and advancing these platforms toward broader clinical use. We also summarize ongoing and completed trials involving TLR7/8 agonists in Table 2 [22,49]. Due to the limited publicly available clinical data for many of these studies, we have strived to include patient participation, cancer-specific treatments, trial status, and study outcomes where available.

Table 2.
List of TLR 7/8 agonists applied for clinical settings.
7. Conclusions and Future Directions
TLR7/8 agonists have emerged as promising candidates in cancer immunotherapy due to their ability to activate innate immunity and stimulate robust antitumor responses. These agonists have been widely explored not only for cancer treatment but also for combating infectious diseases. Synthetic small-molecule TLR ligands have demonstrated potent immunostimulatory effects in both preclinical and clinical settings, and their role as vaccine adjuvants continues to gain traction.
Despite encouraging results, the limited therapeutic efficacy of TLR7/8 agonists has prompted deeper investigation into their molecular mechanisms of action. One major challenge is systemic toxicity, which can hinder clinical outcomes. Advances in structural biology and structure–activity relationship (SAR) analyses have helped elucidate the molecular interactions of these agonists, paving the way for more targeted and safer designs.
However, challenges remain. Optimal dosing schedules and timing intervals are critical to avoid adverse effects, including the risk of autoimmunity due to repeated dosing and TLR tolerance. Extensive research is ongoing to fine-tune these parameters and ensure sustained pro-inflammatory immune activation [64,152,153,154]. An emerging area of interest in immunotherapy research is the impact of circadian biology on Toll-like receptor (TLR) expression and agonist efficacy. Circadian rhythm-dependent regulation of TLR expression and activation presents a significant challenge, as TLR-mediated immune responses fluctuate throughout the 24 h cycle. These temporal variations in receptor availability and signaling efficiency can influence cytokine production and antigen presentation, resulting in inconsistent immunological outcomes. Such variability undermines experimental reproducibility and complicates the optimization of dosing schedules for TLR-based vaccines and immunotherapies. Consequently, a deeper understanding of circadian influences on TLR signaling is essential for achieving reliable and effective immune activation [155,156].
Further investigation is warranted to elucidate the mechanisms underlying the development of innate immune-targeted therapies. Pathways such as autophagy and inflammasome activation may influence pro-tumor or antitumor responses depending on cancer type, tumor microenvironment, and disease stage. Consequently, targeting macrophages or inflammasome components presents a promising anticancer strategy [157,158]. Numerous studies have explored the blockade of inhibitory immune checkpoints, including anti-CTLA-4 and anti-PD-1/PD-L1 antibodies, which can alleviate immunosuppressive effects. However, these therapies have shown significant clinical responses only in a limited subset of patients [159]. In this context, TLR7/8 agonists—either as monotherapies or in combination with other immunostimulatory agents—have demonstrated the ability to suppress tumor growth and enhance immune activation. Notably, combining TLR agonists with immune checkpoint inhibitors such as PD-1/PD-L1, CTLA-4, TIGIT, and LAG3 has yielded synergistic effects and improved therapeutic outcomes across various cancer models [160,161,162].
In addition to combining multiple immunostimulatory agents and multiple treatment modalities, the development of optimized formulation strategies is essential to minimize systemic toxicity. The design of advanced delivery systems plays a critical role in achieving sustained, targeted, and curative cancer vaccine formulations. Continued progress in the development of TLR7/8 agonists—either as monotherapies or in combination with other therapeutic approaches—holds promise for improving treatment outcomes and enhancing survival rates across diverse patient populations.
Author Contributions
J.M. and J.P. wrote and edited the manuscript; S.P., T.S.G. and D.F. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
National Institute of Health (CA260825 to JP).
Data Availability Statement
This review article does not contain any new data.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TLRs | Toll-like receptors |
| PRRs | pattern recognition receptors |
| APCs | Antigen-presenting cells |
| DCs | dendritic cells |
| CTLs | cytotoxic T lymphocytes |
| NK | natural killer |
| IMQ | imiquimod |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| MyD88 | Myeloid Differentiation Primary Response Gene 88 |
| MDSCs | myeloid-derived suppressor cells |
| Tregs | regulatory T cells |
| NE | nanoemulsion |
| MOF | metal–organic framework |
| ICD | immunogenic cell death |
| BMDCs | bone marrow-derived dendritic cells |
| DOX | doxorubicin |
| TAA | tumor-associated antigen |
| NCs | nanocomposites |
| R848@NPs | R848-loaded PANi-conjugated glycol-chitosan nanoparticles |
| PARE NPs | prodrug-based nanoparticles |
| ARNPs | acidic-responsive polymeric nanoparticles |
| UCNPs | upconversion nanoparticles |
| PDT | Photodynamic therapy |
| RT | radiation therapy |
| SDT | Sonodynamic therapy |
| dLNs | draining lymph nodes |
References
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Akunne, O.Z.; Anulugwo, O.E.; Azu, M.G. Emerging Strategies in Cancer Immunotherapy: Expanding Horizons and Future Perspectives. Int. J. Mol. Immuno Oncol. 2024, 9, 77–99. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef]
- Silva, D.J.; Mesquita, A. Complete and Long-Lasting Response to Immunotherapy. Medicine 2022, 101, e28940. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Cancer Immunotherapy, Part 3: Challenges and Future Trends. Pharm. Ther. 2017, 42, 514–521. [Google Scholar]
- Sanmamed, M.F.; Berraondo, P.; Rodriguez-Ruiz, M.E.; Melero, I. Charting Roadmaps towards Novel and Safe Synergistic Immunotherapy Combinations. Nat. Cancer 2022, 3, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Maciejko, L.; Smalley, M.; Goldman, A. Cancer Immunotherapy and Personalized Medicine: Emerging Technologies and Biomarker Based Approaches. J. Mol. Biomark. Diagn. 2017, 08, 350. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of Immune Checkpoint Inhibitors: Insights into the Regulation of Circular RNAS Involved in Cancer Hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.-C. CAR T-Cell-Based Gene Therapy for Cancers: New Perspectives, Challenges, and Clinical Developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.-T.; et al. Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef]
- Feins, S.; Kong, W.; Williams, E.F.; Milone, M.C.; Fraietta, J.A. An Introduction to Chimeric Antigen Receptor (CAR) T-cell Immunotherapy for Human Cancer. Am. J. Hematol. 2019, 94, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.V.; Nino-Castro, A.C.; Schultze, J.L. Regulatory Dendritic Cells: There Is More than Just Immune Activation. Front. Immunol. 2012, 3, 274. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.; Macri, C.; Mintern, J.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers 2019, 11, 521. [Google Scholar] [CrossRef]
- Escors, D. Tumour Immunogenicity, Antigen Presentation, and Immunological Barriers in Cancer Immunotherapy. New J. Sci. 2014, 2014, 734515. [Google Scholar] [CrossRef]
- Kawarada, Y.; Ganss, R.; Garbi, N.; Sacher, T.; Arnold, B.; Hämmerling, G.J. NK- and CD8+ T Cell-Mediated Eradication of Established Tumors by Peritumoral Injection of CpG-Containing Oligodeoxynucleotides. J. Immunol. 2001, 167, 5247–5253. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Huang, H.; Jiang, J.; Zhang, T.; Ni, C. Mature Dendritic Cells Enriched in Immunoregulatory Molecules (MregDCs): A Novel Population in the Tumour Microenvironment and Immunotherapy Target. Clin. Transl. Med. 2023, 13, 1199. [Google Scholar] [CrossRef]
- Shekarian, T.; Valsesia-Wittmann, S.; Brody, J.; Michallet, M.C.; Depil, S.; Caux, C.; Marabelle, A. Pattern Recognition Receptors: Immune Targets to Enhance Cancer Immunotherapy. Ann. Oncol. 2017, 28, 1756–1766. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Zhang, P.; Xing, H.; Zhao, S.; Song, Y.; Wan, D.; Yu, J. Targeting Toll-like Receptor 7/8 for Immunotherapy: Recent Advances and Prospectives. Biomark. Res. 2022, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Gorden, K.B.; Gorski, K.S.; Gibson, S.J.; Kedl, R.M.; Kieper, W.C.; Qiu, X.; Tomai, M.A.; Alkan, S.S.; Vasilakos, J.P. Synthetic TLR Agonists Reveal Functional Differences between Human TLR7 and TLR8. J. Immunol. 2005, 174, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, H.; Barrat, F.J.; Hessel, E.M.; Coffman, R.L. Therapeutic Targeting of Innate Immunity with Toll-like Receptor Agonists and Antagonists. Nat. Med. 2007, 13, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Sakaniwa, K.; Shimizu, T. Targeting the Innate Immune Receptor TLR8 Using Small-Molecule Agents. Acta Crystallogr. Sect. D Struct. Biol. 2020, 76, 621–629. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Y.; He, L.; Sun, S.; Xia, T.; Hu, L.; Yao, L.; Wang, L.; Li, D.; Shi, H.; et al. Structure-Based Design of Highly Potent Toll-like Receptor 7/8 Dual Agonists for Cancer Immunotherapy. J. Med. Chem. 2021, 64, 7507–7532. [Google Scholar] [CrossRef]
- Ryu, J.; Yang, F.C. A Review of Topical Imiquimod in the Management of Basal Cell Carcinoma, Actinic Keratoses, and Other Skin Lesions. Clin. Med. Ther. 2009, 1, 1557–1575. [Google Scholar] [CrossRef]
- Ye, J.; Mills, B.N.; Qin, S.S.; Garrett-Larsen, J.; Murphy, J.D.; Uccello, T.P.; Han, B.J.; Vrooman, T.G.; Johnston, C.J.; Lord, E.M.; et al. Toll-like Receptor 7/8 Agonist R848 Alters the Immune Tumor Microenvironment and Enhances SBRT-Induced Antitumor Efficacy in Murine Models of Pancreatic Cancer. J. Immunother. Cancer 2022, 10, e004784. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Norgard, M.A.; Zhu, X.; Levasseur, P.R.; Sivagnanam, S.; Liudahl, S.M.; Burfeind, K.G.; Olson, B.; Pelz, K.R.; Angeles Ramos, D.M.; et al. The TLR7/8 Agonist R848 Remodels Tumor and Host Responses to Promote Survival in Pancreatic Cancer. Nat. Commun. 2019, 10, 4682. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.; Wang, G.; Mei, T.; Yang, H.; Liu, Y. The TLR7/8 Agonist R848 Optimizes Host and Tumor Immunity to Improve Therapeutic Efficacy in Murine Lung Cancer. Int. J. Oncol. 2022, 61, 81. [Google Scholar] [CrossRef]
- Inglefield, J.R.; Dumitru, C.D.; Alkan, S.S.; Gibson, S.J.; Lipson, K.E.; Tomai, M.A.; Larson, C.J.; Vasilakos, J.P. TLR7 Agonist 852A Inhibition of Tumor Cell Proliferation Is Dependent on Plasmacytoid Dendritic Cells and Type I IFN. J. Interf. Cytokine Res. 2008, 28, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Vasilakos, J.P.; Tomai, M.A. The Use of Toll-like Receptor 7/8 Agonists as Vaccine Adjuvants. Expert. Rev. Vaccines 2013, 12, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Y.; Xiong, Y.; Liu, S.; Qiu, C.; Zhu, Z.; Mao, H.; Yu, M.; Wang, X. TLR 7/8 Agonist Reverses Oxaliplatin Resistance in Colorectal Cancer via Directing the Myeloid-Derived Suppressor Cells to Tumoricidal M1-Macrophages. Cancer Lett. 2020, 469, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Travis, J. On the Origin of The Immune System. Science 2009, 324, 580–582. [Google Scholar] [CrossRef]
- Medzhitov, R. Pattern Recognition Theory and the Launch of Modern Innate Immunity. J. Immunol. 2013, 191, 4473–4474. [Google Scholar] [CrossRef]
- Chen, R.; Zou, J.; Chen, J.; Zhong, X.; Kang, R.; Tang, D. Pattern Recognition Receptors: Function, Regulation and Therapeutic Potential. Signal Transduct. Target. Ther. 2025, 10, 216. [Google Scholar] [CrossRef]
- McKernan, D.P. Pattern Recognition Receptors as Potential Drug Targets in Inflammatory Disorders. Adv. Protein Chem. Struct. Biol. 2020, 119, 65–109. [Google Scholar]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Pahlavanneshan, S.; Sayadmanesh, A.; Ebrahimiyan, H.; Basiri, M. Toll-Like Receptor-Based Strategies for Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 9912188. [Google Scholar] [CrossRef]
- Leulier, F.; Lemaitre, B. Toll-like Receptors—Taking an Evolutionary Approach. Nat. Rev. Genet. 2008, 9, 165–178. [Google Scholar] [CrossRef]
- Satoh, T.; Akira, S. Toll-Like Receptor Signaling and Its Inducible Proteins. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Brennan, J.J.; Gilmore, T.D. Evolutionary Origins of Toll-like Receptor Signaling. Mol. Biol. Evol. 2018, 35, 1576–1587. [Google Scholar] [CrossRef]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Armant, M.A.; Fenton, M.J. Toll-like Receptors: A Family of Pattern-Recognition Receptors in Mammals. Genome Biol. 2002, 3, reviews3011.1. [Google Scholar] [CrossRef]
- Janssens, S.; Beyaert, R. A Universal Role for MyD88 in TLR/IL-1R-Mediated Signaling. Trends Biochem. Sci. 2002, 27, 474–482. [Google Scholar] [CrossRef]
- Kawagoe, T.; Sato, S.; Jung, A.; Yamamoto, M.; Matsui, K.; Kato, H.; Uematsu, S.; Takeuchi, O.; Akira, S. Essential Role of IRAK-4 Protein and Its Kinase Activity in Toll-like Receptor–Mediated Immune Responses but Not in TCR Signaling. J. Exp. Med. 2007, 204, 1013–1024. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR Signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like Receptor 7/8 Agonist Therapeutics and Their Delivery Approaches: From Antiviral Formulations to Vaccine Adjuvants. Adv. Drug Deliv. Rev. 2021, 175, 113803. [Google Scholar] [CrossRef]
- Leśniak, M.; Lipniarska, J.; Majka, P.; Kopyt, W.; Lejman, M.; Zawitkowska, J. The Role of TRL7/8 Agonists in Cancer Therapy, with Special Emphasis on Hematologic Malignancies. Vaccines 2023, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Revuri, V.; Merali, C.; Wang, B.; Schultz, J.R.; Larson, P.; Yu, D.; Prabha, S.; Griffith, T.S.; Ferguson, D.; et al. A Novel Imidazoquinoline With TLR 7/8, STING, and Inflammasome Activity Demonstrates Antitumor Efficacy in Mouse Melanoma and Neu-Driven Mammary Adenocarcinoma. J. Immunother. 2025. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Piao, M.; Xun, Z.; Wang, Y.; Ning, C.; Zhang, X.; Zhang, L.; Wang, Y.; Wang, S.; et al. Biomarkers and Prognostic Factors of PD-1/PD-L1 Inhibitor-Based Therapy in Patients with Advanced Hepatocellular Carcinoma. Biomark. Res. 2024, 12, 26. [Google Scholar] [CrossRef]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade. JAMA Oncol. 2019, 5, 1195. [Google Scholar] [CrossRef]
- Ballot, E.; Ladoire, S.; Routy, B.; Truntzer, C.; Ghiringhelli, F. Tumor Infiltrating Lymphocytes Signature as a New Pan-Cancer Predictive Biomarker of Anti PD-1/PD-L1 Efficacy. Cancers 2020, 12, 2418. [Google Scholar] [CrossRef]
- Stenzel, P.J.; Schindeldecker, M.; Tagscherer, K.E.; Foersch, S.; Herpel, E.; Hohenfellner, M.; Hatiboglu, G.; Alt, J.; Thomas, C.; Haferkamp, A.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Leukocytes and of Immune Checkpoint Molecules PD1 and PDL1 in Clear Cell Renal Cell Carcinoma. Transl. Oncol. 2020, 13, 336–345. [Google Scholar] [CrossRef]
- Peranzoni, E.; Ingangi, V.; Masetto, E.; Pinton, L.; Marigo, I. Myeloid Cells as Clinical Biomarkers for Immune Checkpoint Blockade. Front. Immunol. 2020, 11, 1590. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Xiao, T.; Pan, C.; Liu, X.; Zhao, Y. Prognostic Role of Toll-like Receptors in Cancer: A Meta-Analysis. Ther. Clin. Risk Manag. 2018, 14, 1323–1330. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Cheng, R.Y.S.; Switzer, C.H.; Heinecke, J.L.; Ambs, S.; Glynn, S.; Young, H.A.; Trinchieri, G.; Wink, D.A. Molecular Pathways: Toll-like Receptors in the Tumor Microenvironment—Poor Prognosis or New Therapeutic Opportunity. Clin. Cancer Res. 2013, 19, 1340–1346. [Google Scholar] [CrossRef]
- Beilmann-Lehtonen, I.; Kasurinen, J.; Hagström, J.; Kaprio, T.; Böckelman, C.; Haglund, C. High Tissue Expression of TLRs Combined with High Density of Tumor Infiltrating Lymphocytes Predicts a Better Prognosis in Colorectal Cancer Patients. PLoS ONE 2023, 18, e0280085. [Google Scholar] [CrossRef]
- Monk, B.J.; Brady, M.F.; Aghajanian, C.; Lankes, H.A.; Rizack, T.; Leach, J.; Fowler, J.M.; Higgins, R.; Hanjani, P.; Morgan, M.; et al. A Phase 2, Randomized, Double-Blind, Placebo- Controlled Study of Chemo-Immunotherapy Combination Using Motolimod with Pegylated Liposomal Doxorubicin in Recurrent or Persistent Ovarian Cancer: A Gynecologic Oncology Group Partners Study. Ann. Oncol. 2017, 28, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck. JAMA Oncol. 2018, 4, 1583. [Google Scholar] [CrossRef] [PubMed]
- Zahr, N.M.; Zhao, Q.; Goodcase, R.; Pfefferbaum, A. Systemic Administration of the TLR7/8 Agonist Resiquimod (R848) to Mice Is Associated with Transient, In Vivo-Detectable Brain Swelling. Biology 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, K.A.; Norgard, M.A.; Levasseur, P.R.; Olson, B.; Burfeind, K.G.; Buenafe, A.C.; Zhu, X.; Jeng, S.; McWeeney, S.K.; Marks, D.L. Persistent Toll-like Receptor 7 Stimulation Induces Behavioral and Molecular Innate Immune Tolerance. Brain. Behav. Immun. 2019, 82, 338–353. [Google Scholar] [CrossRef]
- Mullins, S.R.; Vasilakos, J.P.; Deschler, K.; Grigsby, I.; Gillis, P.; John, J.; Elder, M.J.; Swales, J.; Timosenko, E.; Cooper, Z.; et al. Intratumoral Immunotherapy with TLR7/8 Agonist MEDI9197 Modulates the Tumor Microenvironment Leading to Enhanced Activity When Combined with Other Immunotherapies. J. Immunother. Cancer 2019, 7, 244. [Google Scholar] [CrossRef]
- Bourquin, C.; Hotz, C.; Noerenberg, D.; Voelkl, A.; Heidegger, S.; Roetzer, L.C.; Storch, B.; Sandholzer, N.; Wurzenberger, C.; Anz, D.; et al. Systemic Cancer Therapy with a Small Molecule Agonist of Toll-like Receptor 7 Can Be Improved by Circumventing TLR Tolerance. Cancer Res. 2011, 71, 5123–5133. [Google Scholar] [CrossRef]
- Kim, H.; Khanna, V.; Kucaba, T.A.; Zhang, W.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Combination of Sunitinib and PD-L1 Blockade Enhances Anticancer Efficacy of TLR7/8 Agonist-Based Nanovaccine. Mol. Pharm. 2019, 16, 1200–1210. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.; Kim, J.-E.; Lee, S.N.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Noh, Y.-W.; Kang, Y.J.; Kim, Y.S.; et al. Lyophilizable and Multifaceted Toll-like Receptor 7/8 Agonist-Loaded Nanoemulsion for the Reprogramming of Tumor Microenvironments and Enhanced Cancer Immunotherapy. ACS Nano 2019, 13, 12671–12686. [Google Scholar] [CrossRef]
- Ni, K.; Luo, T.; Culbert, A.; Kaufmann, M.; Jiang, X.; Lin, W. Nanoscale Metal–Organic Framework Co-Delivers TLR-7 Agonists and Anti-CD47 Antibodies to Modulate Macrophages and Orchestrate Cancer Immunotherapy. J. Am. Chem. Soc. 2020, 142, 12579–12584. [Google Scholar] [CrossRef]
- Jiang, Z.; Cai, G.; Liu, H.; Liu, L.; Huang, R.; Nie, X.; Gui, R.; Li, J.; Ma, J.; Cao, K.; et al. A Combination of a TLR7/8 Agonist and an Epigenetic Inhibitor Suppresses Triple-Negative Breast Cancer through Triggering Anti-Tumor Immune. J. Nanobiotechnol. 2024, 22, 296. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.A.; Gale, E.C.; Roth, G.A.; Maikawa, C.L.; Correa, S.; Yu, A.C.; Appel, E.A. Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment. Biomacromolecules 2020, 21, 3704–3712. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Si, X.; Yao, H.; Ma, S.; Xu, Y.; Zhao, J.; Ma, C.; He, C.; Tang, Z.; et al. Biopolymer Immune Implants’ Sequential Activation of Innate and Adaptive Immunity for Colorectal Cancer Postoperative Immunotherapy. Adv. Mater. 2021, 33, e2004559. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Flores, L.; Ngai, H.W.; Cornejo, Y.R.; Haber, T.; McDonald, M.; Moreira, D.F.; Gonzaga, J.M.; Abidi, W.; Zhang, Y.; et al. Large, Anionic Liposomes Enable Targeted Intraperitoneal Delivery of a TLR 7/8 Agonist To Repolarize Ovarian Tumors’ Microenvironment. Bioconjug. Chem. 2021, 32, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nahar, S.; Alam, M.M.; Hu, S.; McVicar, D.W.; Yang, D. Reactive Oxygen Species-Sensitive Biodegradable Mesoporous Silica Nanoparticles Harboring TheraVac Elicit Tumor-Specific Immunity for Colon Tumor Treatment. ACS Nano 2023, 17, 19740–19752. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Revuri, V.; Shah, M.; Larson, P.; Shao, Z.; Yu, D.; Prabha, S.; Griffith, T.S.; Ferguson, D.; Panyam, J. Combination of STING and TLR 7/8 Agonists as Vaccine Adjuvants for Cancer Immunotherapy. Cancers 2022, 14, 6091. [Google Scholar] [CrossRef]
- Gadd, A.J.R.; Greco, F.; Cobb, A.J.A.; Edwards, A.D. Targeted Activation of Toll-Like Receptors: Conjugation of a Toll-Like Receptor 7 Agonist to a Monoclonal Antibody Maintains Antigen Binding and Specificity. Bioconjug. Chem. 2015, 26, 1743–1752. [Google Scholar] [CrossRef]
- Ackerman, S.E.; Pearson, C.I.; Gregorio, J.D.; Gonzalez, J.C.; Kenkel, J.A.; Hartmann, F.J.; Luo, A.; Ho, P.Y.; LeBlanc, H.; Blum, L.K.; et al. Immune-Stimulating Antibody Conjugates Elicit Robust Myeloid Activation and Durable Antitumor Immunity. Nat. Cancer 2020, 2, 18–33. [Google Scholar] [CrossRef]
- Janku, F.; Han, S.-W.; Doi, T.; Amatu, A.; Ajani, J.A.; Kuboki, Y.; Cortez, A.; Cellitti, S.E.; Mahling, P.C.; Subramanian, K.; et al. Preclinical Characterization and Phase I Study of an Anti–HER2-TLR7 Immune-Stimulator Antibody Conjugate in Patients with HER2+ Malignancies. Cancer Immunol. Res. 2022, 10, 1441–1461. [Google Scholar] [CrossRef]
- Metz, H.; Childs, M.; Brevik, J.; Winship, D.; Brender, T.; Comeau, M.; Moyes, K.; Chang, J.; Adamo, J.; Setter, B.; et al. SBT6050, a HER2-Directed TLR8 Therapeutic, as a Systemically Administered, Tumor-Targeted Human Myeloid Cell Agonist. J. Clin. Oncol. 2020, 38, 3110. [Google Scholar] [CrossRef]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of Gene Expression Profiles between Human and Mouse Monocyte Subsets. Blood 2010, 115, e10–e19. [Google Scholar] [CrossRef] [PubMed]
- Shay, T.; Jojic, V.; Zuk, O.; Rothamel, K.; Puyraimond-Zemmour, D.; Feng, T.; Wakamatsu, E.; Benoist, C.; Koller, D.; Regev, A. Conservation and Divergence in the Transcriptional Programs of the Human and Mouse Immune Systems. Proc. Natl. Acad. Sci. USA 2013, 110, 2946–2951. [Google Scholar] [CrossRef] [PubMed]
- Sega, E.; Kotapati, S.; Poudel, Y.B.; Cheng, Q.; Sadanala, K.; Schneider, B.; Chekler, E.P.; Rao, C.; Gangwar, S.; Sproul, T.; et al. Targeted Delivery of TLR7 Agonists to the Tumor Microenvironment Enhances Tumor Immunity via Activation of Tumor-Resident Myeloid Cells. Bioconjug. Chem. 2025, 36, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, S.; Poudel, Y.; Sega, E.; Mukhopadhyay, A.; Ramakrishnan, R.; Ukairo, O.; Liu, S.; Akter, R.; Sadanala, K.; Que, K.; et al. Discovery and Characterization of a First-in-Class LIV1-TLR7/8 Immunomodulatory Conjugate with Robust Myeloid Activation and Antitumor Activity. J. Med. Chem. 2025, 68, 11322–11339. [Google Scholar] [CrossRef]
- Lynn, G.M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R.A.; Coble, V.L.; Tobin, K.; Nichols, S.R.; Itzkowitz, Y.; Zaidi, N.; et al. Peptide–TLR-7/8a Conjugate Vaccines Chemically Programmed for Nanoparticle Self-Assembly Enhance CD8 T-Cell Immunity to Tumor Antigens. Nat. Biotechnol. 2020, 38, 320–332. [Google Scholar] [CrossRef]
- Jiang, M.; Zeng, J.; Zhao, L.; Zhang, M.; Ma, J.; Guan, X.; Zhang, W. Chemotherapeutic Drug-Induced Immunogenic Cell Death for Nanomedicine-Based Cancer Chemo–Immunotherapy. Nanoscale 2021, 13, 17218–17235. [Google Scholar] [CrossRef]
- Cerbelli, B.; Pernazza, A.; Botticelli, A.; Fortunato, L.; Monti, M.; Sciattella, P.; Campagna, D.; Mazzuca, F.; Mauri, M.; Naso, G.; et al. PD-L1 Expression in TNBC: A Predictive Biomarker of Response to Neoadjuvant Chemotherapy? Biomed. Res. Int. 2017, 2017, 1750925. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-Dependent Immunogenicity of Doxorubicin-Induced Tumor Cell Death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Martínez-Pérez, A.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Chemo-Immunotherapy: A New Trend in Cancer Treatment. Cancers 2023, 15, 2912. [Google Scholar] [CrossRef]
- Pauli, G.; Chao, P.-H.; Qin, Z.; Böttger, R.; Lee, S.E.; Li, S.-D. Liposomal Resiquimod for Enhanced Immunotherapy of Peritoneal Metastases of Colorectal Cancer. Pharmaceutics 2021, 13, 1696. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhao, S.; Chen, H.; Lu, T.; Wang, J.; Han, J.; Wu, W.; Shen, X.; Li, C. Carboxymethylated Alginate-Resiquimod Micelles Reverse the Immunosuppressive Tumor Microenvironment and Synergistically Enhance the Chemotherapy and Immunotherapy for Gastric Cancer. ACS Appl. Mater. Interfaces 2023, 15, 35999–36012. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Zhan, X.; Chen, K.; Pu, Y.; Liang, Y.; He, B. In Situ Injection of Dual-Delivery PEG Based MMP-2 Sensitive Hydrogels for Enhanced Tumor Penetration and Chemo-Immune Combination Therapy. Nanoscale 2021, 13, 9577–9589. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Xu, L.; Zhuang, Z.; Liu, J.; Liu, S.; Wu, Y.; Gong, A.; Zhang, M.; Du, F. NIR Responsive Tumor Vaccine in Situ for Photothermal Ablation and Chemotherapy to Trigger Robust Antitumor Immune Responses. J. Nanobiotechnol. 2021, 19, 142. [Google Scholar] [CrossRef]
- Li, J.; Luo, G.; Zhang, C.; Long, S.; Guo, L.; Yang, G.; Wang, F.; Zhang, L.; Shi, L.; Fu, Y.; et al. In Situ Injectable Hydrogel-Loaded Drugs Induce Anti-Tumor Immune Responses in Melanoma Immunochemotherapy. Mater. Today Bio 2022, 14, 100238. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Ortega-Rivera, O.A.; Steinmetz, N.F. Photothermal Immunotherapy of Melanoma Using TLR-7 Agonist Laden Tobacco Mosaic Virus with Polydopamine Coat. Nanomed. Nanotechnol. Biol. Med. 2022, 44, 102573. [Google Scholar] [CrossRef] [PubMed]
- Yasothamani, V.; Vivek, R. Targeted NIR-Responsive Theranostic Immuno-Nanomedicine Combined TLR7 Agonist with Immune Checkpoint Blockade for Effective Cancer Photothermal Immunotherapy. J. Mater. Chem. B 2022, 10, 6392–6403. [Google Scholar] [CrossRef]
- Qin, T.; Li, R.; Jin, H.; Wang, Y.; Feng, L. Injectable Thermosensitive Hydrogel to Enhance the Photothermal Ablation and Systemic Immunotherapy of Breast Tumors. Biomater. Sci. 2022, 10, 6003–6012. [Google Scholar] [CrossRef]
- Mei, E.; Li, S.; Song, J.; Xing, R.; Li, Z.; Yan, X. Self-Assembling Collagen/Alginate Hybrid Hydrogels for Combinatorial Photothermal and Immuno Tumor Therapy. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 570–575. [Google Scholar] [CrossRef]
- Yue, J.; Mei, Q.; Wang, P.; Miao, P.; Dong, W.-F.; Li, L. Light-Triggered Multifunctional Nanoplatform for Efficient Cancer Photo-Immunotherapy. J. Nanobiotechnol. 2022, 20, 181. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Xu, C.; Pu, K. Activatable Polymer Nanoagonist for Second Near-Infrared Photothermal Immunotherapy of Cancer. Nat. Commun. 2021, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, L.; Yasothamani, V.; Haldorai, Y.; Selvan Christyraj, J.R.S.; Vivek, R. TLR-7/8 Agonist-Loaded Polypyrrole-Based Theranostic Nanovaccine for Second Near-Infrared Photothermal Immunotherapy of Cancer. ACS Appl. Nano Mater. 2023, 6, 6279–6291. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Rithisa, B.; Vivek, R. The Dynamic Therapeutic Effect of a Targeted Photothermal Nanovaccine Incorporating Toll-like Receptor 7 Agonist Enhanced Cancer Immunotherapy. J. Mater. Chem. B 2023, 11, 9005–9018. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-M.; Pan, W.-Y.; Wu, C.-Y.; Yeh, C.-Y.; Korupalli, C.; Luo, P.-K.; Chou, C.-J.; Chia, W.-T.; Sung, H.-W. Modulation of Tumor Microenvironment Using a TLR-7/8 Agonist-Loaded Nanoparticle System That Exerts Low-Temperature Hyperthermia and Immunotherapy for in Situ Cancer Vaccination. Biomaterials 2020, 230, 119629. [Google Scholar] [CrossRef]
- Müller, R. Junghanns Nanocrystal Technology, Drug Delivery and Clinical Applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef]
- Meng, Z.; Fang, X.; Fu, B.; Qian, C.; Yang, Z.; Bai, Y.; Tao, X.; Huang, H.; Ma, C.; Miao, W.; et al. Tumor Immunotherapy Boosted by R837 Nanocrystals through Combining Chemotherapy and Mild Hyperthermia. J. Control. Release 2022, 350, 841–856. [Google Scholar] [CrossRef]
- Qu, H.; Li, L.; Chen, H.; Tang, M.; Cheng, W.; Lin, T.; Li, L.; Li, B.; Xue, X. Drug-Drug Conjugates Self-Assembled Nanomedicines Triggered Photo−/Immuno- Therapy for Synergistic Cancer Treatments. J. Control. Release 2023, 363, 361–375. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Jin, Y.; Gao, J.; Li, J.; Zheng, X.; Gao, W.; Saeed, M.; Sheng, W.; Yu, H. Self-Immolated Nanoadjuvant for In Situ Vaccination Immunotherapy of Colorectal Cancer. Adv. Healthc. Mater. 2023, 12, e2300524. [Google Scholar] [CrossRef]
- Chen, B.; Huang, R.; Zeng, W.; Wang, W.; Min, Y. Nanocodelivery of an NIR Photothermal Agent and an Acid-Responsive TLR7 Agonist Prodrug to Enhance Cancer Photothermal Immunotherapy and the Abscopal Effect. Biomaterials 2024, 305, 122434. [Google Scholar] [CrossRef]
- Revuri, V.; Rajendrakumar, S.K.; Park, M.; Mohapatra, A.; Uthaman, S.; Mondal, J.; Bae, W.K.; Park, I.; Lee, Y. Heat-Confined Tumor-Docking Reversible Thermogel Potentiates Systemic Antitumor Immune Response During Near-Infrared Photothermal Ablation in Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2021, 10, e2100907. [Google Scholar] [CrossRef]
- Huis in ‘t Veld, R.V.; Da Silva, C.G.; Jager, M.J.; Cruz, L.J.; Ossendorp, F. Combining Photodynamic Therapy with Immunostimulatory Nanoparticles Elicits Effective Anti-Tumor Immune Responses in Preclinical Murine Models. Pharmaceutics 2021, 13, 1470. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, A.K.; Wang, P.; Keyal, U.; Zhao, Z.; Ji, J.; Zhu, L.; Wang, X.; Zhang, G. Therapeutic Effect of Imiquimod Enhanced ALA-PDT on Cutaneous Squamous Cell Carcinoma. Photodiagnosis Photodyn. Ther. 2018, 23, 273–280. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Thompson, M.K.; Poortmans, P.; Chalmers, A.J.; Faivre-Finn, C.; Hall, E.; Huddart, R.A.; Lievens, Y.; Sebag-Montefiore, D.; Coles, C.E. Practice-Changing Radiation Therapy Trials for the Treatment of Cancer: Where Are We 150 Years after the Birth of Marie Curie? Br. J. Cancer 2018, 119, 389–407. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Vanpouille-Box, C.; Kawashima, N.; DiNapoli, S.; Babb, J.S.; Formenti, S.C.; Adams, S.; Demaria, S. Synergy of Topical Toll-like Receptor 7 Agonist with Radiation and Low-Dose Cyclophosphamide in a Mouse Model of Cutaneous Breast Cancer. Clin. Cancer Res. 2012, 18, 6668–6678. [Google Scholar] [CrossRef]
- Adlard, A.L.; Dovedi, S.J.; Telfer, B.A.; Koga-Yamakawa, E.; Pollard, C.; Honeychurch, J.; Illidge, T.M.; Murata, M.; Robinson, D.T.; Jewsbury, P.J.; et al. A Novel Systemically Administered Toll-like Receptor 7 Agonist Potentiates the Effect of Ionizing Radiation in Murine Solid Tumor Models. Int. J. Cancer 2014, 135, 820–829. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, H.-J.; Ko, H.-J.; Yoon, B.-I.; Choe, J.; Kim, K.-C.; Hahn, T.-W.; Han, J.A.; Choi, S.S.; Jung, Y.M.; et al. The TLR7 Agonist Imiquimod Induces Anti-Cancer Effects via Autophagic Cell Death and Enhances Anti-Tumoral and Systemic Immunity during Radiotherapy for Melanoma. Oncotarget 2017, 8, 24932–24948. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Melis, M.H.M.; Wilkinson, R.W.; Adlard, A.L.; Stratford, I.J.; Honeychurch, J.; Illidge, T.M. Systemic Delivery of a TLR7 Agonist in Combination with Radiation Primes Durable Antitumor Immune Responses in Mouse Models of Lymphoma. Blood 2013, 121, 251–259. [Google Scholar] [CrossRef]
- Ota, Y.; Inagaki, R.; Nagai, Y.; Hirose, Y.; Murata, M.; Yamamoto, S. TLR7 Agonist, DSP-0509, with Radiation Combination Therapy Enhances Anti-Tumor Activity and Modulates T Cell Dependent Immune Activation. BMC Immunol. 2024, 25, 48. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, R.; Zhao, L.; Kong, R.; Yang, N.; Liu, Y.; Chen, A.; Wang, C.; Cheng, H.; Li, S. Photodynamic Therapy Initiated Immunotherapy of Self-Delivery Re-Educator by Inducing Immunogenic Cell Death and Macrophage Polarization. Chem. Eng. J. 2022, 435, 134783. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, R.; Cai, H.; Yang, N.; Chen, Z.; Zhao, L.; Huang, Y.; Li, P.; Cheng, H.; Chen, A.; et al. Coordination-Driven Self-Assembly of Biomedicine to Enhance Photodynamic Therapy by Inhibiting Proteasome and Bcl-2. Adv. Healthc. Mater. 2023, 12, e2300711. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Hu, J.; Li, Y.; Feng, S.; Pan, D.; Feng, L.; Shen, L. Near-Infrared Phosphorescent Carbon Dots for Sonodynamic Precision Tumor Therapy. Nat. Commun. 2022, 13, 5735. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, H.; Li, B.; Feng, C.; Dai, Y. An Ultrasound-Triggered STING Pathway Nanoagonist for Enhanced Chemotherapy-Induced Immunogenic Cell Death. Small 2024, 20, e2309850. [Google Scholar] [CrossRef]
- Yuan, M.; Liang, S.; Zhou, Y.; Xiao, X.; Liu, B.; Yang, C.; Ma, P.; Cheng, Z.; Lin, J. A Robust Oxygen-Carrying Hemoglobin-Based Natural Sonosensitizer for Sonodynamic Cancer Therapy. Nano Lett. 2021, 21, 6042–6050. [Google Scholar] [CrossRef]
- Yu, N.; Li, M.; Zhang, Y.; Wang, F.; Yu, X.; Cai, R.; Li, J. Dual-Modulation of Immunosuppressive Pathways Using Sono-Activatable Semiconducting Polymer Nanofeedbacks for Cancer Immunotherapy. Nano Today 2023, 52, 101944. [Google Scholar] [CrossRef]
- Yue, W.; Chen, L.; Yu, L.; Zhou, B.; Yin, H.; Ren, W.; Liu, C.; Guo, L.; Zhang, Y.; Sun, L.; et al. Checkpoint Blockade and Nanosonosensitizer-Augmented Noninvasive Sonodynamic Therapy Combination Reduces Tumour Growth and Metastases in Mice. Nat. Commun. 2019, 10, 2025. [Google Scholar] [CrossRef]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the Tumor Microenvironment: Potential Strategy for Cancer Therapeutics. Biochim. Biophys. Acta-Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; You, Q.; Zhang, X. Prodrug Strategy for Cancer Cell-Specific Targeting: A Recent Overview. Eur. J. Med. Chem. 2017, 139, 542–563. [Google Scholar] [CrossRef]
- Giang, I.; Boland, E.L.; Poon, G.M.K. Prodrug Applications for Targeted Cancer Therapy. AAPS J. 2014, 16, 899–913. [Google Scholar] [CrossRef]
- Lei, H.; Kim, J.H.; Son, S.; Chen, L.; Pei, Z.; Yang, Y.; Liu, Z.; Cheng, L.; Kim, J.S. Immunosonodynamic Therapy Designed with Activatable Sonosensitizer and Immune Stimulant Imiquimod. ACS Nano 2022, 16, 10979–10993. [Google Scholar] [CrossRef]
- Hu, H.; Yan, J.; Zhu, H.; Wang, X.; Zhao, Y.; Li, S.; Ma, Z.; Xu, F.; Xia, M.; Jiao, J.; et al. Self-Delivered Sonodynamic Nanomedicine for Enhanced Tumor Immunotherapy by Simultaneously Reversing the Immunosuppression and Immune Resistance. Chem. Eng. J. 2024, 501, 157580. [Google Scholar] [CrossRef]
- Nuhn, L.; De Koker, S.; Van Lint, S.; Zhong, Z.; Catani, J.P.; Combes, F.; Deswarte, K.; Li, Y.; Lambrecht, B.N.; Lienenklaus, S.; et al. Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses. Adv. Mater. 2018, 30, e1803397. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Ota, Y.; Murata, M.; Sugaru, E.; Koga-Yamakawa, E.; Eguchi, K.; Hirose, Y.; Yamamoto, S.; Umehara, H.; et al. Intravenous Administration of the Selective Toll-like Receptor 7 Agonist DSR-29133 Leads to Anti-Tumor Efficacy in Murine Solid Tumor Models Which Can Be Potentiated by Combination with Fractionated Radiotherapy. Oncotarget 2016, 7, 17035–17046. [Google Scholar] [CrossRef] [PubMed]
- Schölch, S.; Rauber, C.; Tietz, A.; Rahbari, N.N.; Bork, U.; Schmidt, T.; Kahlert, C.; Haberkorn, U.; Tomai, M.A.; Lipson, K.E.; et al. Radiotherapy Combined with TLR7/8 Activation Induces Strong Immune Responses against Gastrointestinal Tumors. Oncotarget 2015, 6, 4663–4676. [Google Scholar] [CrossRef]
- Lanzavecchia, A.; Sallusto, F. Regulation of T Cell Immunity by Dendritic Cells. Cell 2001, 106, 263–266. [Google Scholar] [CrossRef]
- Shang, N.; Figini, M.; Shangguan, J.; Wang, B.; Sun, C.; Pan, L.; Ma, Q.; Zhang, Z. Dendritic Cells Based Immunotherapy. Am. J. Cancer Res. 2017, 7, 2091–2102. [Google Scholar]
- Kim, H.; Griffith, T.S.; Panyam, J. Poly(d,l-Lactide-Co-Glycolide) Nanoparticles as Delivery Platforms for TLR7/8 Agonist-Based Cancer Vaccine. J. Pharmacol. Exp. Ther. 2019, 370, 715–724. [Google Scholar] [CrossRef]
- Pullen, A.M.; Kappler, J.W.; Marrack, P. Tolerance to Self Antigens Shapes the T-Cell Repertoire. Immunol. Rev. 1989, 107, 125–140. [Google Scholar] [CrossRef]
- Dubensky, T.W.; Reed, S.G. Adjuvants for Cancer Vaccines. Semin. Immunol. 2010, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In Vivo Characterization of the Physicochemical Properties of Polymer-Linked TLR Agonists That Enhance Vaccine Immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Kim, S.; Lee, S.N.; Kim, S.-Y.; Kim, J.-E.; Lee, K.Y.; Kim, M.S.; Heo, J.Y.; Park, Y.M.; Ku, B.M.; et al. Therapeutic Efficacy of Cancer Vaccine Adjuvanted with Nanoemulsion Loaded with TLR7/8 Agonist in Lung Cancer Model. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102415. [Google Scholar] [CrossRef]
- Kim, S.; Park, Y.; Kim, J.; Kim, S.; Choi, K.; Kang, T.; Lee, I.; Lim, Y.T.; Um, S.H.; Kim, C. ProLonged Liposomal Delivery of TLR7/8 Agonist for Enhanced Cancer Vaccine. Vaccines 2023, 11, 1503. [Google Scholar] [CrossRef]
- Adams, S.; Kozhaya, L.; Martiniuk, F.; Meng, T.-C.; Chiriboga, L.; Liebes, L.; Hochman, T.; Shuman, N.; Axelrod, D.; Speyer, J.; et al. Topical TLR7 Agonist Imiquimod Can Induce Immune-Mediated Rejection of Skin Metastases in Patients with Breast Cancer. Clin. Cancer Res. 2012, 18, 6748–6757. [Google Scholar] [CrossRef]
- Adams, S.; Demaria, S.; Rinchai, D.; Wang, E.; Novik, Y.; Oratz, R.; Fenton-Kerimian, M.; Levine, P.G.; Li, X.; Marincola, F.; et al. Topical TLR7 Agonist and Radiotherapy in Patients with Metastatic Breast Cancer. J. Immunother. Cancer 2025, 13, e011173. [Google Scholar] [CrossRef]
- Geller, M.A.; Cooley, S.; Argenta, P.A.; Downs, L.S.; Carson, L.F.; Judson, P.L.; Ghebre, R.; Weigel, B.; Panoskaltsis-Mortari, A.; Curtsinger, J.; et al. Toll-like Receptor-7 Agonist Administered Subcutaneously in a Prolonged Dosing Schedule in Heavily Pretreated Recurrent Breast, Ovarian, and Cervix Cancers. Cancer Immunol. Immunother. 2010, 59, 1877–1884. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Becker, J.C.; Grob, J.-J.; Schadendorf, D.; Tebbs, V.; Skalsky, J.; Kaehler, K.C.; Moosbauer, S.; Clark, R.; et al. An Exploratory Study of Systemic Administration of the Toll-like Receptor-7 Agonist 852A in Patients with Refractory Metastatic Melanoma. Clin. Cancer Res. 2008, 14, 856–864. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Morishima, C.; Eaton, K.D.; Baik, C.S.; Goulart, B.H.; Anderson, L.N.; Manjarrez, K.L.; Dietsch, G.N.; Bryan, J.K.; Hershberg, R.M.; et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017, 23, 2442–2450. [Google Scholar] [CrossRef]
- Dietsch, G.N.; Randall, T.D.; Gottardo, R.; Northfelt, D.W.; Ramanathan, R.K.; Cohen, P.A.; Manjarrez, K.L.; Newkirk, M.; Bryan, J.K.; Hershberg, R.M. Late-Stage Cancer Patients Remain Highly Responsive to Immune Activation by the Selective TLR8 Agonist Motolimod (VTX-2337). Clin. Cancer Res. 2015, 21, 5445–5452. [Google Scholar] [CrossRef]
- Siu, L.; Brody, J.; Gupta, S.; Marabelle, A.; Jimeno, A.; Munster, P.; Grilley-Olson, J.; Rook, A.H.; Hollebecque, A.; Wong, R.K.S.; et al. Safety and Clinical Activity of Intratumoral MEDI9197 Alone and in Combination with Durvalumab and/or Palliative Radiation Therapy in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2020, 8, e001095. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Tolcher, A.W.; Rasco, D.W.; Johnson, M.L.; Alistar, A.T.; Li, L.; Chung, A.H.; Andtbacka, R.H.I. BDB001, an Intravenously Administered Toll-like Receptor 7 and 8 (TLR7/8) Agonist, in Combination with Pembrolizumab in Advanced Solid Tumors: Phase 1 Safety and Efficacy Results. J. Clin. Oncol. 2021, 39, 2512. [Google Scholar] [CrossRef]
- Shi, Y.; White, D.; He, L.; Miller, R.L.; Spaner, D.E. Toll-like Receptor-7 Tolerizes Malignant B Cells and Enhances Killing by Cytotoxic Agents. Cancer Res. 2007, 67, 1823–1831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koga-Yamakawa, E.; Murata, M.; Dovedi, S.J.; Wilkinson, R.W.; Ota, Y.; Umehara, H.; Sugaru, E.; Hirose, Y.; Harada, H.; Jewsbury, P.J.; et al. TLR7 Tolerance Is Independent of the Type I IFN Pathway and Leads to Loss of Anti-Tumor Efficacy in Mice. Cancer Immunol. Immunother. 2015, 64, 1229–1239. [Google Scholar] [CrossRef]
- Hayashi, T.; Gray, C.S.; Chan, M.; Tawatao, R.I.; Ronacher, L.; McGargill, M.A.; Datta, S.K.; Carson, D.A.; Corr, M. Prevention of Autoimmune Disease by Induction of Tolerance to Toll-like Receptor 7. Proc. Natl. Acad. Sci. USA 2009, 106, 2764–2769. [Google Scholar] [CrossRef]
- Greenberg, E.N.; Marshall, M.E.; Jin, S.; Venkatesh, S.; Dragan, M.; Tsoi, L.C.; Gudjonsson, J.E.; Nie, Q.; Takahashi, J.S.; Andersen, B. Circadian Control of Interferon-Sensitive Gene Expression in Murine Skin. Proc. Natl. Acad. Sci. USA 2020, 117, 5761–5771. [Google Scholar] [CrossRef]
- Silver, A.C.; Buckley, S.M.; Hughes, M.E.; Hastings, A.K.; Nitabach, M.N.; Fikrig, E. Daily Oscillations in Expression and Responsiveness of Toll-like Receptors in Splenic Immune Cells. Heliyon 2018, 4, e00579. [Google Scholar] [CrossRef]
- Chung, C.; Seo, W.; Silwal, P.; Jo, E.-K. Crosstalks between Inflammasome and Autophagy in Cancer. J. Hematol. Oncol. 2020, 13, 100. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Guo, R.; Bian, Z.; Song, Y. Targeting Macrophages in Hematological Malignancies: Recent Advances and Future Directions. J. Hematol. Oncol. 2022, 15, 110. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Manochakian, R.; Zhao, Y.; Lou, Y. Immunotherapies Targeting Stimulatory Pathways and Beyond. J. Hematol. Oncol. 2021, 14, 78. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ribatti, D. Update in TIGIT Immune-Checkpoint Role in Cancer. Front. Oncol. 2022, 12, 871085. [Google Scholar] [CrossRef]
- Chiang, E.Y.; Mellman, I. TIGIT-CD226-PVR Axis: Advancing Immune Checkpoint Blockade for Cancer Immunotherapy. J. Immunother. Cancer 2022, 10, e004711. [Google Scholar] [CrossRef]
- Andrews, L.P.; Cillo, A.R.; Karapetyan, L.; Kirkwood, J.M.; Workman, C.J.; Vignali, D.A.A. Molecular Pathways and Mechanisms of LAG3 in Cancer Therapy. Clin. Cancer Res. 2022, 28, 5030–5039. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).