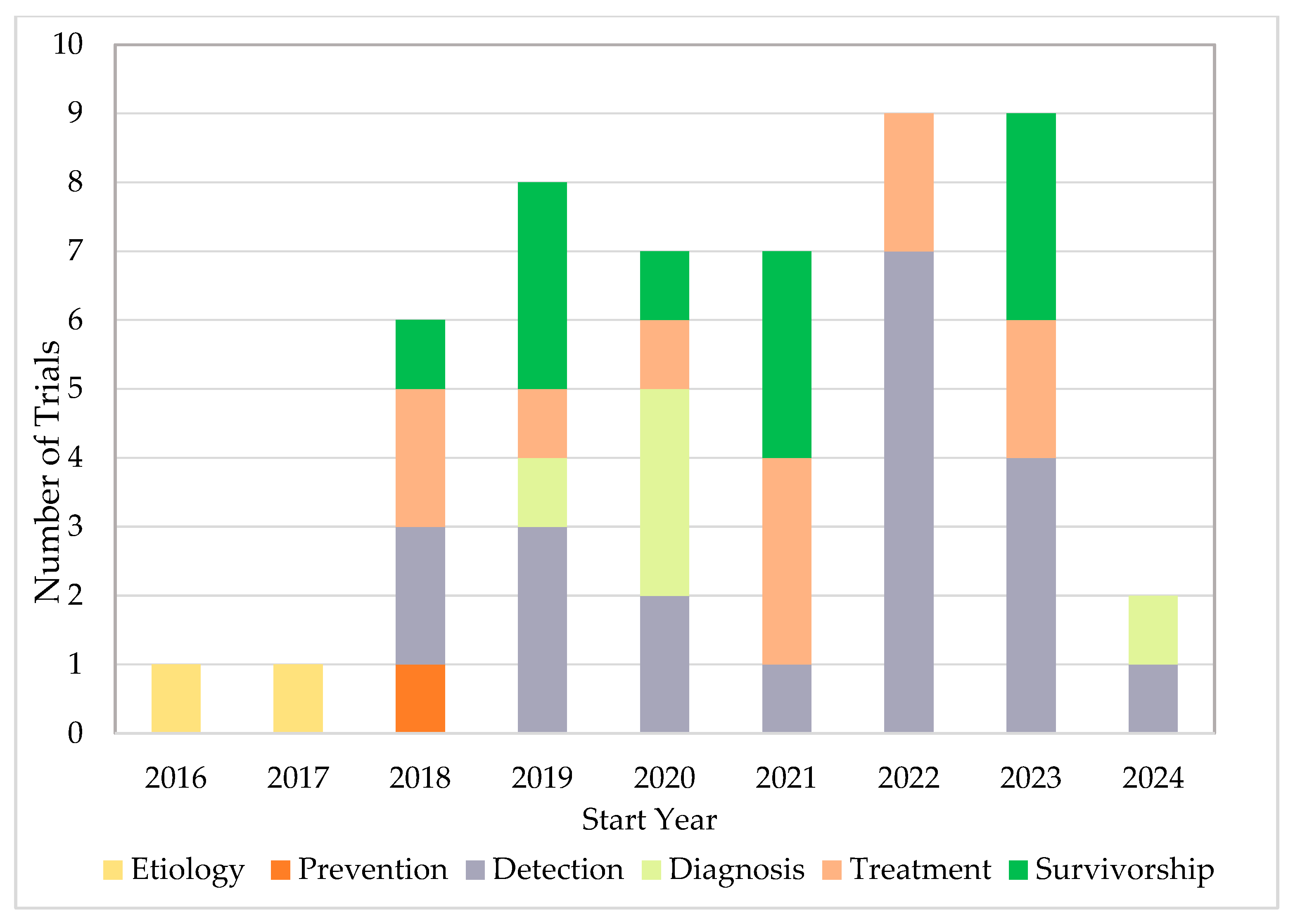Simple Summary
Artificial intelligence (AI) is increasingly being integrated into healthcare, enhancing the accuracy and efficiency of diagnosis and treatment. In oncology, where personalized care approaches are essential, AI offers unique opportunities to support clinicians and patients. In this study, we conducted a cross-sectional analysis of completed U.S.-based oncology clinical trials that involved AI technologies registered on ClinicalTrials.gov over the past decade. We identified 50 completed trials, most of which were interventional (66%). Machine Learning was the most frequently applied AI application, and most trials focused on cancer detection, particularly for colorectal cancer and unspecified cancer types. By mapping these studies across the Cancer Control Continuum, this study illustrates current progress and diverse applications of AI in oncology. It underscores the need for more transparent evaluation to ensure the clinical validity and the impact of AI tools on improving patient care.
Abstract
Background/Objectives: Artificial Intelligence (AI) is rapidly advancing in medicine, facilitating personalized care by leveraging complex clinical data, imaging, and patient monitoring. This study characterizes current practices in AI use within oncology clinical trials by analyzing completed U.S. trials within the Cancer Control Continuum (CCC), a framework that spans the stages of cancer etiology, prevention, detection, diagnosis, treatment, and survivorship. Methods: This cross-sectional study analyzed U.S.-based oncology trials registered on ClinicalTrials.gov between January 2015 and April 2025. Using AI-related MeSH terms, we identified trials addressing stages of the CCC. Results: Fifty completed oncology trials involving AI were identified; 66% were interventional and 34% observational. Machine Learning was the most common AI application, though specific algorithm details were often lacking. Other AI domains included Natural Language Processing, Computer Vision, and Integrated Systems. Most trials were single-center with limited participant enrollment. Few published results or reported outcomes, indicating notable reporting gaps. Conclusions: This analysis of ClinicalTrials.gov reveals a dynamic and innovative landscape of AI applications transforming oncology care, from cutting-edge Machine Learning models enhancing early cancer detection to intelligent chatbots supporting treatment adherence and personalized survivorship interventions. These trials highlight AI’s growing role in improving outcomes across the CCC in advancing personalized cancer care. Standardized reporting and enhanced data sharing will be important for facilitating the broader application of trial findings, accelerating the development and clinical integration of reliable AI tools to advance cancer care.
1. Introduction
Over the past decade, the use of Artificial Intelligence (AI) in medicine has surged, ushering in a new era of rapid innovation in healthcare [1,2]. These AI advancements are fueled by the growing availability of multi-dimensional clinical data and its ongoing digitization [3,4]. Sophisticated AI technologies, including Machine Learning (ML), Natural Language Processing (NLP), Deep Learning (DL), and Computer Vision Systems, can now integrate and synthesize these data to support individualized patient care [5,6,7,8]. Oncology is well-poised to benefit from AI due to the heterogeneous nature of tumor presentation and cancer outcomes, positioning AI to support targeted cancer therapeutics [3,9]. Thus far, AI has been utilized to optimize cancer research, streamline clinical workflows, and enhance understanding of tumor biology [10,11,12,13]. Radiology and pathology have already integrated AI for image classification, nodule detection, tumor characterization, and surveillance of cancerous lesions [10,12,14,15,16].
Despite the rapid development of diverse AI technologies, the thorough evaluation of their performance in real-world medical settings remains limited [17,18,19]. To bridge the gap between technical success and practical applications, particularly on patient outcomes and treatment decisions, AI has increasingly carved out a niche within clinical trials [20,21]. These trials provide a structured setting to validate AI technologies prior to widespread adoption, exploring applications ranging from improving diagnostic accuracy to revolutionizing the clinical trials process itself [2,20,22,23]. ClinicalTrials.gov, the largest trial registry [24], was used in the present study to investigate AI applications in oncology clinical trials.
The National Cancer Institute’s (NCI) cancer control continuum (CCC), which delineates the various stages of cancer from its etiology to survivorship [25], was utilized as a framework to categorize these AI applications. NCI subcategories were also determined for each trial to provide further application specificity. Investigating trials along the CCC is especially valuable at this stage, as the comparative effectiveness of narrow versus general AI applications in healthcare remains an area of active investigation. Given that AI integration in healthcare is still evolving, oncology may currently benefit most from narrow AI approaches that target CCC touchpoints [3,26]. Previous studies have investigated AI applications registered on ClinicalTrials.gov in the fields of critical care [27], ophthalmology [28], pediatrics [29], cancer diagnosis [30], and general healthcare [31]. Additionally, systematic [32] and narrative reviews [3] have explored cancer-specific AI applications [33], post-diagnostic AI use [34], and broader implementation challenges [35]. However, this study is the first comprehensive analysis of completed, US-based AI-related oncology clinical trials registered on ClinicalTrials.gov over the past decade, evaluated using the CCC framework [25]. By leveraging ClinicalTrials.gov, we characterize study design, cancer types, and intended AI applications, offering insights into how AI is being planned and evaluated in clinical research.
2. Materials and Methods
This cross-sectional study analyzed data from ClinicalTrials.gov [36], to identify AI-related oncology clinical trials. The search strategy included terms for the following concepts: (1) Medical Subject Headings (MeSH) terms related to cancer; (2) AI-related MeSH terms sourced from the National Library of Medicine [37]; (3) location: United States; (4) trial period from 1 January 2015 to 18 April 2025; (5) Completed studies to ensure consistent, complete data for meaningful comparison. Trials with a recruitment status of “recruiting”, “not yet recruiting”, “active, not recruiting”, “unknown status”, “enrolling by initiation”, “terminated,” or “withdrawn” were excluded. Studies were also excluded if they did not focus explicitly on both cancer and AI. The ClinicalTrials.gov search query syntax is presented below, with further details provided in the Supplementary Materials.
- Condition/disease,
- (Cancer* OR oncology OR leukemia* OR sarcoma* OR tumor* OR tumour* OR melanoma* OR neoplasm* OR neoplasia OR carcinoma* OR adenocarcinoma* OR lymphoma*),
- AND,
- Other Terms: (“Artificial Intelligence” OR “AI System” OR “Intelligent Systems” OR “Machine Learning” OR “Deep Learning” OR Autoencoder OR “Large Language Model” OR “Natural Language Processing” OR “Neural Network” OR “Convolutional Neural Network” OR “Generative AI” OR “Generative Pre-trained Transformer” OR “ChatGPT”),
- AND,
- (United States),
- AND,
- (Completed Studies),
- AND,
- (Study Start Date ≥ 1 January 2015).
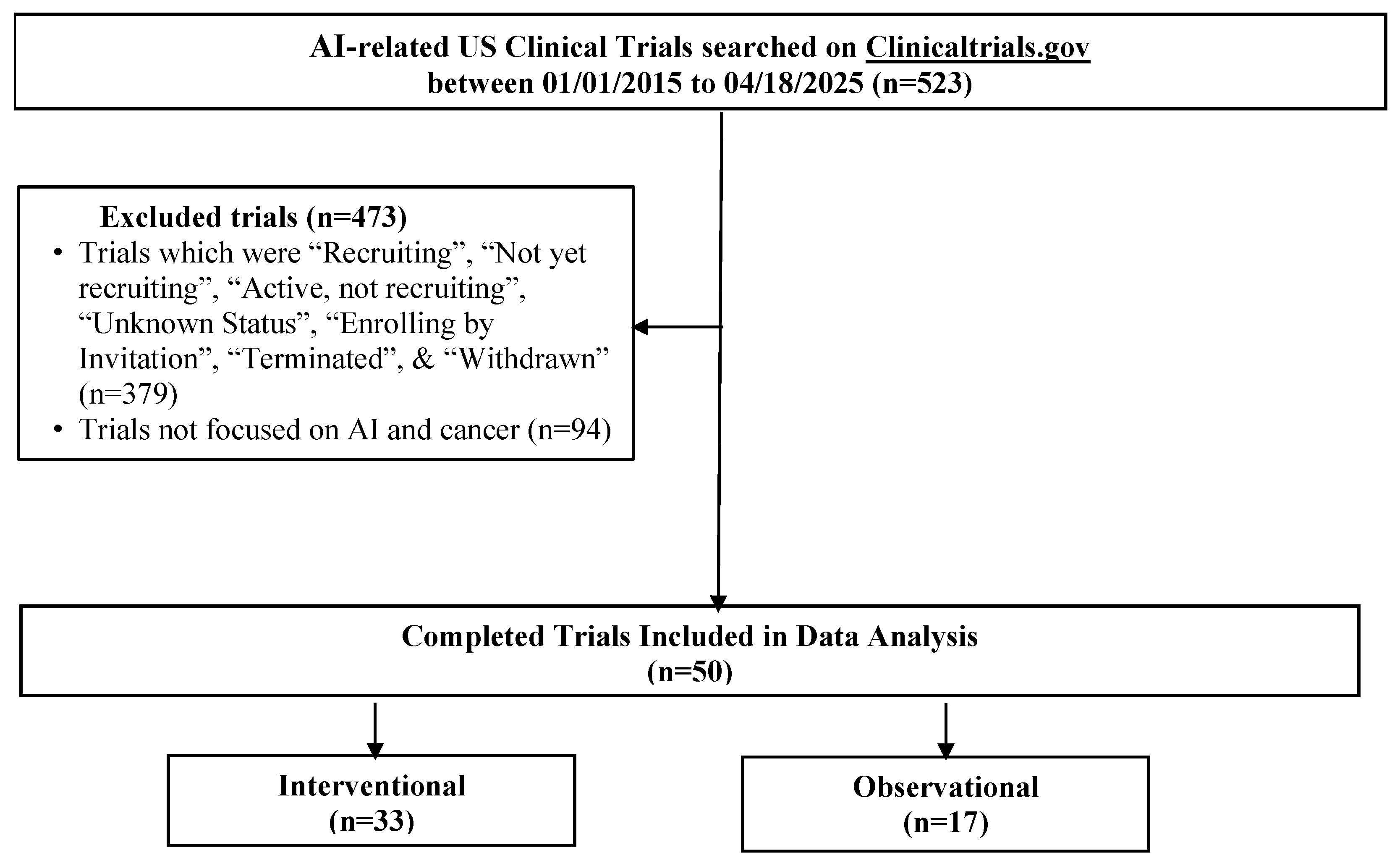
Demographic and trial-level data, including age, sex, cancer type, AI term(s), study design, and trial characteristics, were manually abstracted from ClinicalTrials.gov by one author (HV) and independently verified by two authors (KV and SK) for accuracy. Fifty trials met the inclusion criteria (see Figure 1 and the full search query provided in the Supplementary Materials for the full list of terms).
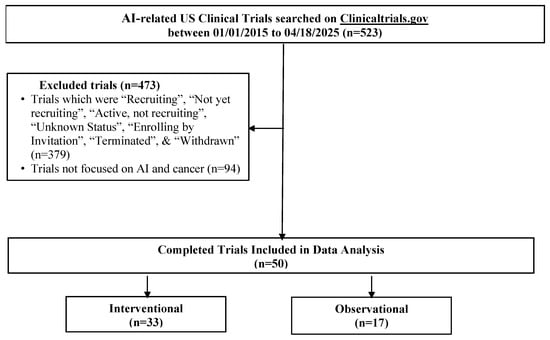
Figure 1.
Flow diagram of AI-related oncology clinical trials identified from ClinicalTrials.gov.
Each study was classified as either an interventional or an observational study. For interventional trials, additional variables abstracted included allocation method (Randomized, non-Randomized, and not applicable [N/A]), interventional model assignment (single group, crossover, parallel, factorial), and masking (non-masked, single, double, triple). For observational trials, we recorded time perspective (prospective, retrospective) and observational model (cohort, case–control, family-based, and other). Finally, each trial’s AI application was categorized by the CCC framework domains: etiology, prevention, detection, diagnosis, treatment, and survivorship. Trials with multiple NCI subcategories were counted in each relevant domain. To reflect AI’s evolving role in oncology, we adapted the original CCC framework [25] by expanding definitions and incorporating terms relevant to contemporary study design and AI use. For example, we included imaging and fecal immunochemical testing (FIT) under detection in AI-enhanced screening and risk stratification. These modifications were based on iterative reviews of trial objectives, interventions, and clinical relevance. Additionally, the number of AI technologies in each trial type was characterized using a previously published AI taxonomy [38]. No institutional review board approval was necessary for this analysis of deidentified, publicly available data.
3. Results
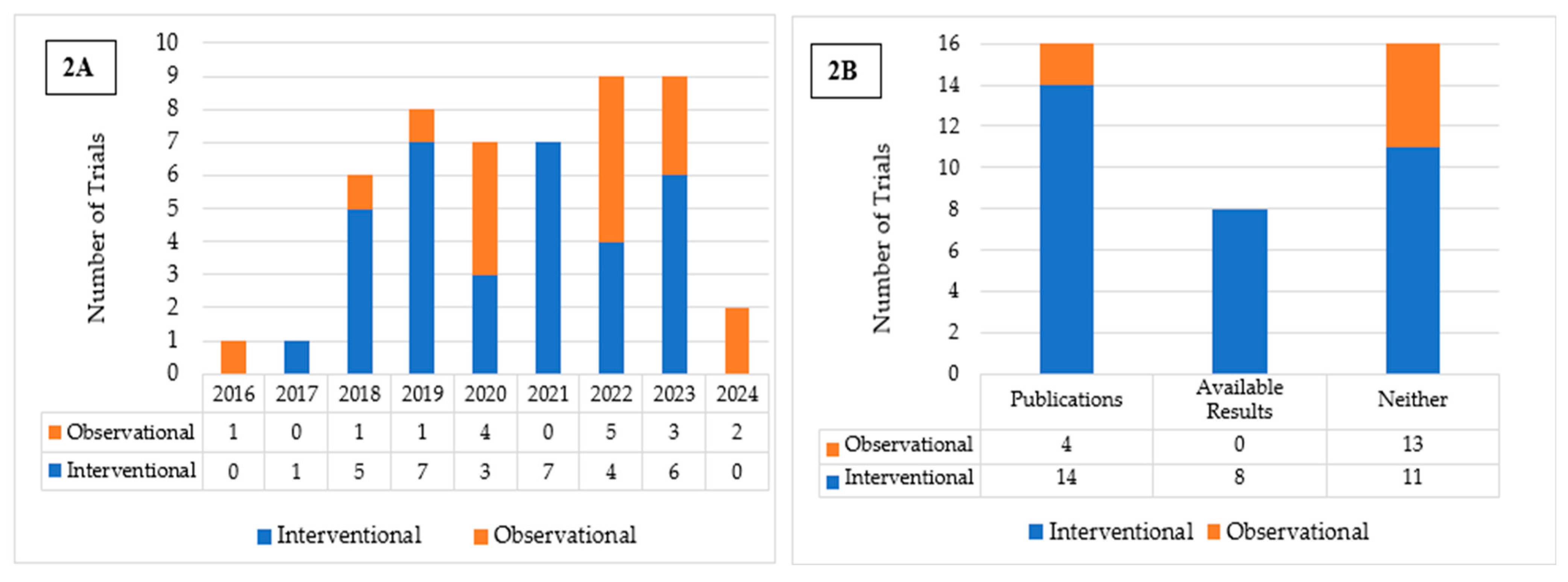
Fifty completed U.S.-based clinical trials focusing on AI applications in oncology during the 10-year search period from 2015 to 2025 were identified (Figure 1). 33 (66.0%) were interventional and 17 (34.0%) were observational (Table 1). As illustrated in Figure 2A, the number of registered clinical trials increased from 2016, with interventional trials peaking in 2019 (n = 7), 2021 (n = 7), and 2023 (n = 6), and observational trials peaking in 2020 (n = 4) and 2022 (n = 5). Trial numbers declined in 2024, and no eligible trials were identified in 2025, likely reflecting the time required for trial completion and result reporting. Figure 3 illustrates the distribution of AI-related oncology trials across the CCC stages, with a peak in trial initiations between 2019 and 2023. Early in the decade, trials were etiology-focused, while post-2017 saw a marked surge in detection, treatment, and survivorship trials, reflecting a shift toward clinically applied AI research.

Table 1.
Characteristics of completed AI-related oncology clinical trials from Clinicaltrials.gov.
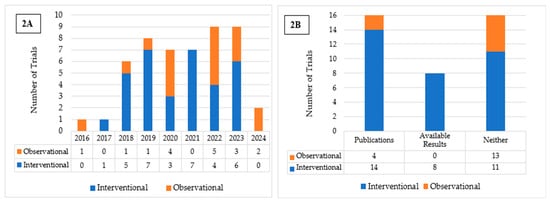
Figure 2.
(A) Distribution of Interventional and Observational Trials by Year of Registration. (B) Publication and Results Reporting Status of Interventional and Observational Trials.
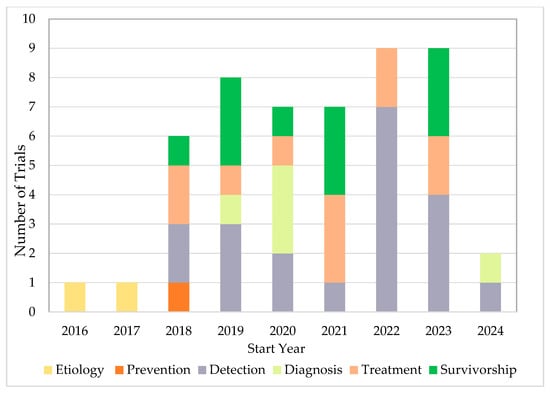
Figure 3.
AI-Related Oncology Trials Initiations Across the CCC Stages.
Among the interventional trials, eight (24.2%) had available results and 14 (42.4%) had associated publications listed on ClinicalTrials.gov (Table 1; Figure 2B). In contrast, none of the observational trials presented available results, and only four (23.5%) listed associated publications (Table 1; Figure 2B). Interventional trials predominantly enrolled fewer than 100 (33.3%) participants or between 100 and 500 (45.5%), with a median enrollment of 194. Observational trials frequently enrolled either fewer than 100 participants (35.3%) or more than 1000 (35.3%), resulting in a higher median enrollment of 355. Participants in both trial types were predominantly adults and older adults (97.0% and 70.6%, respectively), had similarly high rates of unrestricted gender recruitment (81.8% and 82.4%, respectively), and were mostly single-center (63.6% and 58.8%, respectively). For both trial types, a substantial proportion of studies were funded by sources other than the NIH and Industry, such as universities, individuals, and organizations, comprising 72.7% of interventional and 47.1% of observational trials. More interventional trials employed FDA-regulated devices (n = 15) than observational trials (n = 3). Eight interventional and all three observational trials involved unapproved/uncleared devices. Among interventional trials, the most reported cancer category included either multiple or unspecified cancer types (33.3%), followed by colorectal (30.3%) and breast cancer (12.1%). Similarly, in observational trials, multiple cancer types or unspecified (29.4%) were most common, followed by colorectal (17.6%), lung (11.8%), prostate (11.8%), and skin cancer (11.8%) (Table 1).
Among interventional trials, 22 (66.7%) trials had randomized allocation, one (3.0%) had non-randomized allocation, and ten (30.3%) did not fall into either category (Table 2). The majority employed a parallel assignment (n = 19, 57.6%); ten (30.3%) used single group assignment, while two each (6.1%) used crossover or factorial assignments. Most trials had no masking (n = 20, 60.6%), while the remainder included single (n = 6, 18.2%), double (n = 4, 12.1%), or triple (n = 3, 9.1%) masking.

Table 2.
Characteristics of interventional and observational AI-related oncology trials from ClinicalTrials.gov (2015–2025).
Within the observational trials group, nine (52.9%) were cohort studies, three (17.6%) were case–control, one (5.9%) was family-based, and four (23.5%) fell into other types of studies. Regarding time perspective, the majority were prospective studies (n = 9, 52.9%), followed by retrospective studies (n = 5, 29.4%), cross-sectional (n = 1, 5.9%), and two classified as other (11.8%).
The most common AI subdomain across both trial types was ML (Table 3). Among the interventional trials, specific ML algorithms consisted of Convolutional Neural Networks (CNN; n = 1) and DL (n = 4), while seven trials did not specify the ML algorithm used. In observational trials, the specific ML algorithms included CNN (n = 1), Deep Ensemble (n = 1), and Deep Neural Network (n = 1), whereas 12 trials did not specify the ML algorithm used.

Table 3.
Applications of artificial intelligence in oncology clinical trials.
The next most frequently applied AI subdomain was NLP (Table 3). Three of the interventional trials listed specific NLP applications, including a Chatbot (n = 1) and Sentiment Analysis (n = 2). None of the observational trials specified the NLP application used. One interventional trial used Computer Vision with an application in Intelligent Real-Time Image Segmentation. Two interventional trials also reported the use of an integrated AI system with the application of an Intelligent System. Most trials did not specify the AI subdomain (17 interventional trials and eight observational trials).
Both interventional and observational trials targeted multiple stages of the CCC. The corresponding NCI subcategories and examples of specific study applications in each stage are summarized in Table 4.

Table 4.
Summary.
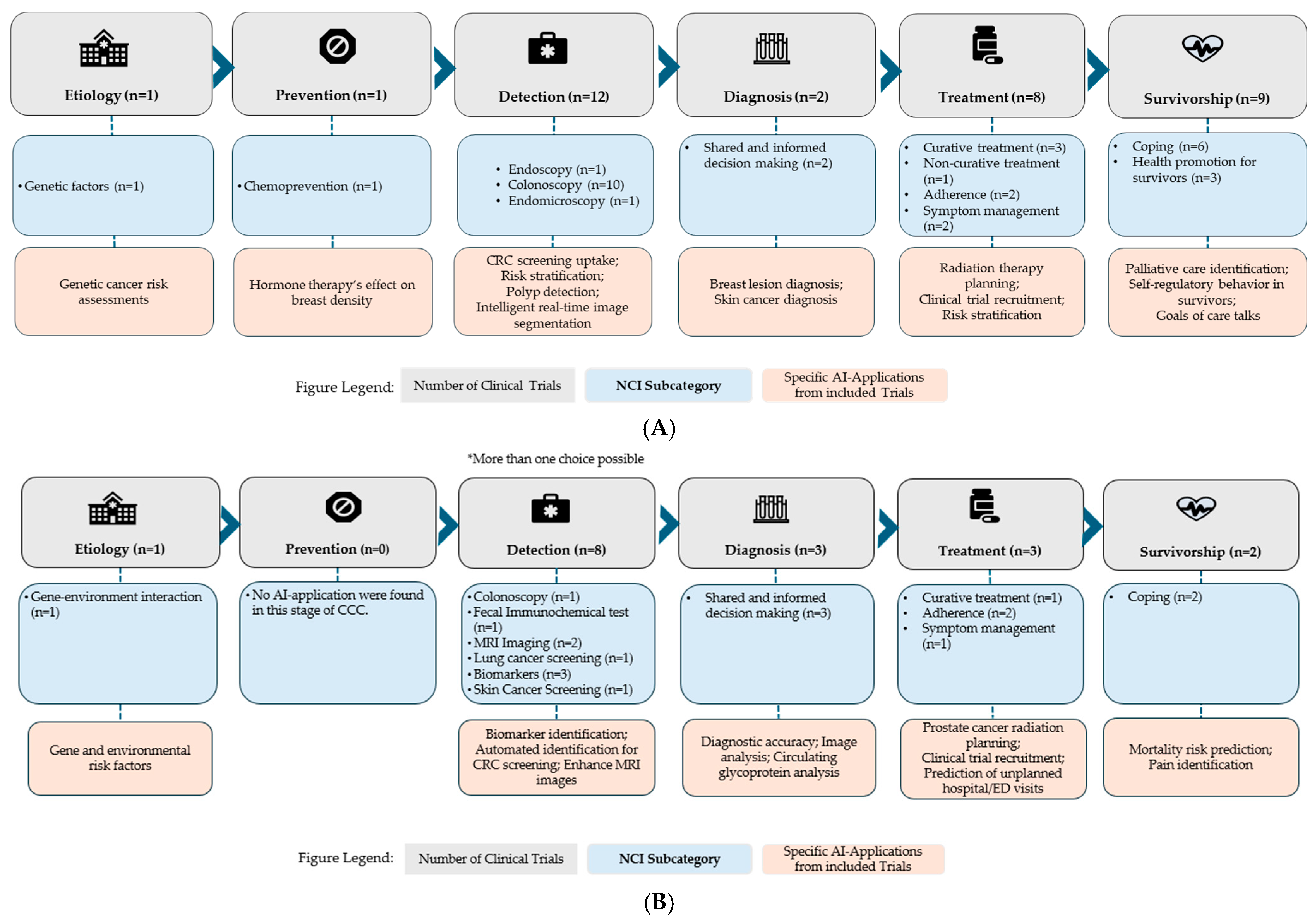
Across the CCC, interventional trials targeted various phases. (Figure 4A). One interventional trial focused on etiology, using AI to study genetic factors, and another targeted chemoprevention. Detection was the most targeted CCC component, with 12 trials applying AI in colonoscopy (n = 10), endoscopy (n = 1), and endomicroscopy (n = 1). Two trials focused on diagnosis, using AI to support shared and informed decision-making, while eight trials targeted treatment, including curative treatment (n = 3), adherence (n = 2), symptom management (n = 2), and non-curative treatment (n = 1). Nine trials targeted survivorship, including coping (n = 6) and health promotion (n = 3).
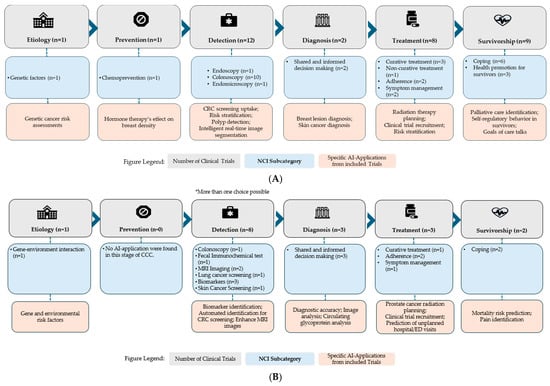
Figure 4.
(A) Number of Interventional clinical trials across the Cancer Control Continuum stages. (B) Number of Observational clinical trials across the Cancer Control Continuum stages. * More than one choice possible indicates that some trials were classified under multiple CCC stages if their AI applications spanned more than one domain.
Observational trials similarly covered the CCC, with a more limited scope. One focused on etiology to investigate gene-environment interactions (Figure 4B). None targeted prevention. Detection was again the most frequently targeted, with eight trials focusing on AI applications in colonoscopy (n = 1), FIT (n = 1), MRI imaging (n = 2), lung cancer screening (n = 1), biomarkers (n = 3), and skin cancer screening (n = 1). Three trials focused on diagnosis, utilizing AI to support shared and informed decision-making. Three trials targeted curative treatment (n = 1), adherence (n = 2), and symptom management (n = 1). Finally, two trials focused on survivorship, both involving coping. AI Applications of all included trials are provided in Table 5 with additional information in Supplementary Table S1.

Table 5.
Characteristics of AI-related interventional and observational oncology trials.
4. Discussion
This cross-sectional analysis of trials registered on ClinicalTrials.gov [36], over the past decade, highlights the applications of AI in interventional and observational oncology trials. To our knowledge, this is the first study to provide a comprehensive assessment of completed AI-related oncology clinical trials across the CCC.
4.1. Trial Characteristics
This analysis highlights the growing number of AI-related oncology trials, indicating increased interest in integrating AI across the CCC. These trials investigated a variety of interventions, including FDA-regulated devices, some of which were not yet approved or cleared. Study designs included parallel, single-group, crossover, and factorial designs. Other designs, such as family-based and case–control studies, as well as a small subset that did not fit traditional categories, illustrate the use of non-standard methodologies. Single-center studies were common, while multicenter trials were less frequent [40].
Despite the growing number of trials, few reported results or had associated publications, highlighting a need for improved transparency. Robust reporting is essential to achieve a comprehensive understanding of AI algorithms, including their parameter configurations, foundational theories, and procedural implementations. Moreover, to evaluate clinical efficacy, it is critical that results related to patient outcomes, such as diagnostic accuracy and treatment efficacy, are systematically reported on ClinicalTrials.gov. Standardized reporting frameworks, such as CONSORT-AI for RCTs and SPIRIT-AI for interventional protocols [41], along with PROBAST-AI [42] for bias assessment, and QUADAS-AI [43], offer guidance to improve methodological rigor, reduce bias, and support reproducibility. Greater adherence to these guidelines will be essential for advancing trustworthy AI integration in oncology.
4.2. AI Applications Across the CCC
Most trials targeted multiple or unspecified cancer types, reflecting the broad applicability of AI in oncology. Among site-specific cancers, colorectal cancer (CRC) was the most frequently studied, likely due to its prevalence and availability of conducive data for AI-driven approaches. To illustrate the range of AI applications, we highlight representative trials for each CCC stage below, demonstrating how AI has been applied across the cancer care continuum.
4.2.1. Etiology
A limited number of trials focused on etiology highlights a potential gap in the application of AI across the CCC. Despite AI’s potential to help uncover genetic and environmental contributors to cancer, its application in etiology-related cancer trials remains limited. One interventional trial (NCT03511690) evaluated the use of an intelligent tutoring system, “BReast CAncer Genetics Intelligent Semantic Tutoring” (BRCA Gist), designed to promote participation in genetic cancer risk assessment among underserved Latina and Black women at hereditary risk of ovarian and breast cancer. The intervention uses AI-supported avatars to deliver personalized tutoring on breast cancer, metastasis, risk factors, genetic testing, and its implications. The avatars incorporate text, graphics, and video and are available in multiple ethnicities to provide culturally tailored education. The system employs Latent Semantic Analysis to interpret user responses and generate personalized feedback. A related publication reported that participants using BRCA Gist demonstrated improved understanding of breast cancer genetics and made more informed decisions about genetic testing compared to controls [44]. This trial illustrates how NLP and adaptive AI technologies can enhance shared decision making and support genetic risk counseling. The observational trial (NCT03174574) applied ML to survey-based data to identify risk factors for pancreatic and melanoma cancers in individuals with hereditary predispositions. The study demonstrated the utility of AI in analyzing gene-environment interactions to advance etiological understanding in high-risk populations. Both trials underscore the untapped potential of AI to inform cancer prevention and risk assessment through enhanced etiologic insights.
4.2.2. Prevention
Like the limited number of trials focused on etiology, cancer prevention remains an underrepresented area for AI-driven interventions, with only one interventional trial identified in this domain. This randomized trial (NCT03063619) evaluated afimoxifene, a hormone therapy, to reduce breast cancer risk in women with dense breast tissue. A secondary objective compared two breast density measurement tools: Cumulus, a semi-automated software using reader-defined thresholds, and Volpara, which applies X-ray physics and ML to generate volumetric breast measurements for breast cancer prevention. The results reported that Volpara produced more consistent and less variable measurements, while Cumulus was more sensitive to larger changes in breast density [45]. This trial illustrates the potential of ML-based tools to enhance chemoprevention strategies in high-risk populations.
4.2.3. Detection
Detection was the most frequently targeted touchpoint of the CCC, with 12 interventional and 8 observational trials focused on this domain. Most interventional trials applied AI to enhance CRC detection. For example, one trial (NCT06621225) evaluated the performance of a CNN-based computer-aided detection (CAD) system called “GI Genius”, which analyzes real-time endoscopic images to better detect polyps. Another trial (NCT03925337) assessed a DL-based research software developed using a database of polyp images and operation videos to detect polyps and adenomas during colonoscopy procedures. A related publication reported that AI-assisted colonoscopy significantly reduced the adenoma miss rate compared to standard procedures [46]. Another trial (NCT03867409) explored the use of virtual human technology to promote CRC screening by delivering culturally tailored messages to at-risk communities. The high number of AI-related colonoscopy trials highlights AI’s increasing influence on gastrointestinal endoscopy [47]. This field is well-suited for AI integration due to its reliance on real-time image and video analysis [48] as well as its potential to utilize virtual platforms that enhance patient engagement and screening uptake.
Among observational trials, two observational trials also focused on improving CRC detection. One validated Freenome, an ML-based blood test for CRC biomarkers (NCT04369053), while the NCT05383976 trial used ML to risk-stratify patients who had not undergone colonoscopy and provided navigator support to increase screening uptake. A third observational trial (NCT05126173) focused on skin cancer detection, clinically validating “DERM”, a Deep Ensemble algorithm for identifying malignancies from lesion images. Together, these trials underscore the diverse applications of AI in oncology, ranging from biomarker discovery and risk stratification to image-based detection, and demonstrate its potential across multiple cancer types and care pathways. Many trials focused on CRC, likely because colonoscopy is well-suited for AI integration, particularly in real-time image analysis, due to the availability of large annotated datasets, standardized screening protocols, and the clinical importance of early polyp detection, all of which make CRC an ideal candidate for AI integration.
4.2.4. Diagnosis
Diagnosis was a less frequently targeted component of the CCC. An innovative approach involved a DL-based computer-assisted detection (NCT04551105) and diagnosis system for the interpretation of breast ultrasound images of cancer lesions, exploring how an AI-assisted device may enhance diagnostic accuracy and reduce reading time compared to non-AI image reading [49].
Recent efforts in observational research have explored tools like DermDx (NCT06463860), a DL-based reading aid designed to support primary care physicians in skin cancer diagnosis, which demonstrates AI’s potential to serve as the first point of contact in cancer detection [50]. Another trial (NCT05147389) sought to clinically validate a CNN-based model trained on cholangioscopy images to distinguish malignant from benign bile duct lesions. An associated publication reported that the model achieved statistically high accuracy in differentiating lesion types [51]. These trials demonstrate that current AI applications in diagnosis are leveraging image-based data to target various cancers. They also highlight AI’s potential to improve diagnostic accuracy, reduce clinical burden, and expand access to expert-level diagnostic support.
4.2.5. Treatment
AI applications in cancer treatment focused on curative and non-curative care, treatment adherence, and symptom management. An example includes “CancerLife” (NCT03371147), a mobile application that supports cancer patients and caregivers by collecting patient-reported symptoms and analyzing emotional tone using IBM Watson’s NLP-based Sentiment Analysis. While “MatchMiner” (NCT06888089), uses an ML and NLP-powered platform to integrate genomic and clinical data to match patients to biomarker-driven therapeutic trials, aiming to optimize treatment decisions and clinical trial enrollment. “Penny” (NCT05113264), an NLP-assisted chatbot, reminds patients to promote oral chemotherapy adherence and symptom management. This intervention reflects an interactive approach to promote chemotherapy adherence through direct patient engagement [52]. “Ethos” (NCT05030454), an AI-powered device that adapts radiation treatment plans in response to anatomical changes between therapy sessions, represents a promising avenue to enhance the precision and safety of radiotherapy delivery.
Observational trials in cancer treatment are applying AI in various ways, including symptom monitoring, treatment adherence, personalized planning, and clinical trial matching (e.g., NCT04441775, NCT06561217). The “SHIELD-RT” trial (NCT05122247) evaluated a previously validated ML model to predict unplanned hospital admissions and emergency department visits among cancer patients receiving systemic therapy [53]. This model supports personalized care planning and timely clinical interventions by identifying high-risk patients. Such applications reflect a shift toward more precise and efficient patient-centered care.
4.2.6. Survivorship
AI applications in survivorship trials addressed emotional coping and health promotion among cancer survivors. ML algorithms can identify oncology patients at high risk of six-month mortality (e.g., NCT03984773) [54]. Patients identified as high-risk triggered behavioral nudges, prompting oncologists to initiate serious illness conversations. An associated publication reported significantly more conversations in the intervention group than in the control group, illustrating how AI can influence clinician behavior and promote goal-concordant end-of-life care [55]. The NCT05069519 trial aimed to promote healthy behaviors among cancer survivors through personalized exercise regimens. The study incorporated Fitbits for activity tracking and a private Facebook group for health education. NLP and text mining were used to analyze posts, assess sentiment and engagement, and deliver weekly personalized feedback, demonstrating the potential of combining AI and social media to support behavioral change in survivorship care. NCT04458168 evaluated the feasibility and acceptability of “Purposeful”, a mobile application that supports behavior change and prompts self-reflection among survivors. The app uses ML to personalize content based on user interactions, displaying how AI can be tailored to individual needs in survivorship care.
Among observational trials, NCT04442425 explored coping among cancer patients by evaluating facial recognition technology for pain monitoring. Participants submitted audio-visual pain reports via a mobile application, and researchers used ML and NLP to analyze facial expressions and speech related to pain. This approach highlights a novel use of AI for real-time pain monitoring. These trials demonstrate the expanding use of AI in survivorship, particularly in promoting behavior change, to support advanced care planning, and monitoring pain to provide personalized long-term cancer care.
4.3. Barriers to AI Integration in Oncology
Despite the transformative potential of AI in cancer care, several barriers hinder its adoption in clinical practice. AI models require large, diverse, and high-quality datasets for effective training. However, obtaining such data can be challenging due to concerns regarding data confidentiality, ownership, and institutional data silos [56,57]. These data challenges are further compounded in surgical contexts where variation in operative techniques, the substantial time burden associated with annotating surgical imaging and video data, and hesitancy to record and distribute such data limit the creation of comprehensive datasets [58]. Furthermore, ML technologies rely on continuous data input to improve performance, which can be difficult to maintain in real-world clinical settings [57]. Data security and patient privacy are additional concerns as AI applications handle sensitive information, necessitating informed consent and strict data protection measures [56,57,58].
Algorithmic bias is a major limitation in AI applications. Bias can arise when training datasets fail to generalize to large, diverse patient populations [56]. For example, one study found that an AI algorithm diagnosed skin cancer more accurately in lighter skin tones than in darker ones, emphasizing the need for representative datasets in model development [59]. Another key barrier to clinical adoption is the lack of transparency in AI model decision making. Often described as “black boxes”, these models produce outputs that are difficult to interpret or validate, complicating accountability in cases of clinical error, which poses an ethical and legal challenge in patient care [57,60]. Regulatory challenges, such as FDA approval for adaptive AI algorithms [61], further hinder integration due to difficulties in validating evolving models. However, most included trials did not involve applications currently seeking approval, reflecting their early-stage development.
It is clear that careful attention to AI-related concerns is essential. This study highlights that publicly available trials [36] should provide detailed information on training datasets and the specific models or algorithms employed to enhance transparency and reproducibility. Moreover, rigorous testing through prospective randomized trials is essential for clinical validation; however, this study design was notably underrepresented among the included trials. Transparent reporting and robust study design remain critical for the safe and effective integration of AI into oncological practice.
4.4. Limitations
This study had several limitations. First, the MeSH terms used were extracted from the National Library of Medicine’s defined list for AI and cancer. Although we sought to ensure this list was comprehensive, more specific or emerging AI and cancer terms may not have been included, potentially leading to an underestimation of the AI-related trials registered on ClinicalTrials.gov. Second, the analysis was limited to ClinicalTrials.gov, which, although one of the most used clinical trial registries, does not represent the global scope of oncology trials. Moreover, we further restricted our analysis to studies conducted in the United States. Consequently, AI-related oncology trials conducted in other countries or registered in international trial databases were not included, which may limit the generalizability of our findings and underrepresent global efforts in this area. Future studies should incorporate multiple international trial registries to provide a more comprehensive overview of global AI applications in oncology. Additionally, inconsistent reporting of trial outcomes such as clinical efficacy, safety, and adherence restricted our ability to assess the implementation readiness of AI tools in oncology trials. Finally, this study focused solely on extracting data from trials marked as complete to obtain the most detailed information available.
5. Conclusions
This study is the first to provide a comprehensive overview of US-based AI-related oncology clinical trials registered as complete on ClinicalTrials.gov between 2015 and 2025. Although ClinicalTrials.gov is publicly accessible, relatively few trials post results or have peer-reviewed publications, which limits understanding of how these tools perform in practice. As a result, a gap remains between AI-based predictive modeling tools and the real-world practice of clinical oncology care. Addressing this gap will require more rigorous, multicenter trial designs, broader dissemination of findings, and standardized, transparent reporting of AI methodology in accordance with existing reporting frameworks, such as the CONSORT-AI and SPIRIT-AI guidelines. The current trial landscape reflects a rapidly evolving and innovative field with significant potential to transform cancer care across the continuum. However, to enhance the generalizability and global relevance of these findings, the focus should shift to diverse populations and global settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17213537/s1. Table S1. Characteristics of AI-related interventional and observational oncology trials.
Author Contributions
Conceptualization, S.D.K.; methodology, L.A. and S.D.K.; data curation, H.V., S.M., K.V.J. and S.D.K.; formal analysis, H.V. and S.D.K.; visualization, H.V.; writing—original draft preparation, H.V.; writing—review and editing, H.V., S.M., K.V.J., P.S., M.L., A.F., A.Z., D.B. and S.D.K.; funding acquisition, S.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study is funded internally by the UF Health Cancer Institute and additionally supported by the National Institutes of Health, National Institute of Dental and Craniofacial Research (NIH/NIDCR)—funded Research Project Grant (R56DE034781).
Data Availability Statement
No new data were created or analyzed in this study. The data supporting the findings of this work are derived from publicly available clinical trial records accessible at ClinicalTrials.gov (https://clinicaltrials.gov).
Acknowledgments
We would like to thank Joel Divaker and Meghann Wheeler for their assistance with the initial screening of studies for this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| CCC | Cancer Control Continuum |
| ML | Machine Learning |
| NLP | Natural Language Processing |
| DL | Deep Learning |
| N/A | Not Applicable |
| FIT | Fecal Immunochemical Testing |
| CNN | Convolutional Neural Network |
| EHR | Electronic Health Record |
| CRC | Colorectal Cancer |
| CAD | Computer-Aided Detection |
| BRCA Gist | BReast CAncer Genetics Intelligent Semantic Tutoring |
| PCP | Primary Care Physician |
References
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial Intelligence in Healthcare: Transforming the Practice of Medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef]
- Huhulea, E.N.; Huang, L.; Eng, S.; Sumawi, B.; Huang, A.; Aifuwa, E.; Hirani, R.; Tiwari, R.K.; Etienne, M. Artificial Intelligence Advancements in Oncology: A Review of Current Trends and Future Directions. Biomedicines 2025, 13, 951. [Google Scholar] [CrossRef]
- Kann, B.H.; Hosny, A.; Aerts, H.J.W.L. Artificial Intelligence for Clinical Oncology. Cancer Cell 2021, 39, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Sim, J.Z.T.; Fong, Q.W.; Huang, W.; Tan, C.H. Machine Learning in Medicine: What Clinicians Should Know. Singap. Med. J. 2021, 64, 91–97. [Google Scholar] [CrossRef]
- Aramaki, E.; Wakamiya, S.; Yada, S.; Nakamura, Y. Natural Language Processing: From Bedside to Everywhere. Yearb. Med. Inform. 2022, 31, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A.; Singh, R.P.; Ahmed, M. Computer Vision to Enhance Healthcare Domain: An Overview of Features, Implementation, and Opportunities. Intell. Pharm. 2024, 2, 792–803. [Google Scholar] [CrossRef]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer Heterogeneity: Implications for Targeted Therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Alhejaily, A.-M.G. Artificial Intelligence in Healthcare (Review). Biomed. Rep. 2024, 22, 11. [Google Scholar] [CrossRef]
- Basu, K.; Sinha, R.; Ong, A.; Basu, T. Artificial Intelligence: How Is It Changing Medical Sciences and Its Future? Indian J. Dermatol. 2020, 65, 365–370. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, H.; Mirza, K.M. Pathology in the Artificial Intelligence Era: Guiding Innovation and Implementation to Preserve Human Insight. Acad. Pathol. 2025, 12, 100166. [Google Scholar] [CrossRef]
- El Arab, R.A.; Abu-Mahfouz, M.S.; Abuadas, F.H.; Alzghoul, H.; Almari, M.; Ghannam, A.; Seweid, M.M. Bridging the Gap: From AI Success in Clinical Trials to Real-World Healthcare Implementation—A Narrative Review. Healthcare 2025, 13, 701. [Google Scholar] [CrossRef]
- Plana, D.; Shung, D.L.; Grimshaw, A.A.; Saraf, A.; Sung, J.J.Y.; Kann, B.H. Randomized Clinical Trials of Machine Learning Interventions in Health Care: A Systematic Review. JAMA Netw. Open 2022, 5, e2233946. [Google Scholar] [CrossRef]
- Han, R.; Acosta, J.N.; Shakeri, Z.; Ioannidis, J.P.A.; Topol, E.J.; Rajpurkar, P. Randomised Controlled Trials Evaluating Artificial Intelligence in Clinical Practice: A Scoping Review. Lancet Digit. Health 2024, 6, e367–e373. [Google Scholar] [CrossRef]
- Chopra, H.; Annu; Shin, D.K.; Munjal, K.; Priyanka; Dhama, K.; Emran, T.B. Revolutionizing Clinical Trials: The Role of AI in Accelerating Medical Breakthroughs. Int. J. Surg. 2023, 109, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Hogan, J. We Need More Randomized Clinical Trials of AI. NEJM AI 2024, 1, aie2400881. [Google Scholar] [CrossRef]
- Hutson, M. How AI Is Being Used to Accelerate Clinical Trials. Nature 2024, 627, S2–S5. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Gresham, G.; Meinert, J.L.; Gresham, A.G.; Piantadosi, S.; Meinert, C.L. Update on the Clinical Trial Landscape: Analysis of ClinicalTrials.Gov Registration Data, 2000–2020. Trials 2022, 23, 858. [Google Scholar] [CrossRef]
- Cancer Control Continuum|Division of Cancer Control and Population Sciences (DCCPS). Available online: https://cancercontrol.cancer.gov/about-dccps/about-cc/cancer-control-continuum (accessed on 4 June 2025).
- Nagendran, M.; Chen, Y.; Lovejoy, C.A.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; Ioannidis, J.P.A.; Collins, G.S.; Maruthappu, M. Artificial Intelligence versus Clinicians: Systematic Review of Design, Reporting Standards, and Claims of Deep Learning Studies. BMJ 2020, 368, m689. [Google Scholar] [CrossRef]
- Liu, G.; Li, N.; Chen, L.; Yang, Y.; Zhang, Y. Registered Trials on Artificial Intelligence Conducted in Emergency Department and Intensive Care Unit: A Cross-Sectional Study on ClinicalTrials.Gov. Front. Med. 2021, 8, 634197. [Google Scholar] [CrossRef]
- Turner, B.; Rajeshuni, N.; Tran, E.M.; Ludwig, C.A.; Tauqeer, Z.; Weeks, B.; Kinde, B.; Pershing, S. Characteristics of Ophthalmology Trials Registered in ClinicalTrials.Gov, 2007–2018. Am. J. Ophthalmol. 2020, 211, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Pediatric Trials Run in India: An Analysis of Clinical Trials.Gov 2006–2015. Available online: https://pubmed.ncbi.nlm.nih.gov/30648225/ (accessed on 5 June 2025).
- Dong, J.; Geng, Y.; Lu, D.; Li, B.; Tian, L.; Lin, D.; Zhang, Y. Clinical Trials for Artificial Intelligence in Cancer Diagnosis: A Cross-Sectional Study of Registered Trials in ClinicalTrials.Gov. Front. Oncol. 2020, 10, 1629. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xiu, X.; Liu, S.; Qian, Q.; Wu, S. Characteristics of Artificial Intelligence Clinical Trials in the Field of Healthcare: A Cross-Sectional Study on ClinicalTrials.Gov. Int. J. Environ. Res. Public Health 2022, 19, 13691. [Google Scholar] [CrossRef] [PubMed]
- Saady, M.; Eissa, M.; Yacoub, A.S.; Hamed, A.B.; Azzazy, H.M.E.-S. Implementation of Artificial Intelligence Approaches in Oncology Clinical Trials: A Systematic Review. Artif. Intell. Med. 2025, 161, 103066. [Google Scholar] [CrossRef]
- Hachache, R.; Yahyaouy, A.; Riffi, J.; Tairi, H.; Abibou, S.; Adoui, M.E.; Benjelloun, M. Advancing Personalized Oncology: A Systematic Review on the Integration of Artificial Intelligence in Monitoring Neoadjuvant Treatment for Breast Cancer Patients. BMC Cancer 2024, 24, 1300. [Google Scholar] [CrossRef]
- Macheka, S.; Ng, P.Y.; Ginsburg, O.; Hope, A.; Sullivan, R.; Aggarwal, A. Prospective Evaluation of Artificial Intelligence (AI) Applications for Use in Cancer Pathways Following Diagnosis: A Systematic Review. BMJ Oncol. 2024, 3, e000255. [Google Scholar] [CrossRef]
- Luchini, C.; Pea, A.; Scarpa, A. Artificial Intelligence in Oncology: Current Applications and Future Perspectives. Br. J. Cancer 2022, 126, 4–9. [Google Scholar] [CrossRef]
- Home|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 5 June 2025).
- Home—MeSH—NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh (accessed on 12 June 2025).
- European Commission; Joint Research Centre. AI Watch: Defining Artificial Intelligence: Towards an Operational Definition and Taxonomy of Artificial Intelligence; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Amir, O.; Grosz, B.J.; Gajos, K.Z.; Swenson, S.M.; Sanders, L.M. AI Support of Teamwork for Coordinated Care of Children with Complex Conditions; AI Access Foundation: San Francisco, CA, USA, 2014. [Google Scholar]
- Heinlein, L.; Maron, R.C.; Hekler, A.; Haggenmüller, S.; Wies, C.; Utikal, J.S.; Meier, F.; Hobelsberger, S.; Gellrich, F.F.; Sergon, M.; et al. Prospective Multicenter Study Using Artificial Intelligence to Improve Dermoscopic Melanoma Diagnosis in Patient Care. Commun. Med. 2024, 4, 177. [Google Scholar] [CrossRef]
- Kolbinger, F.R.; Veldhuizen, G.P.; Zhu, J.; Truhn, D.; Kather, J.N. Reporting Guidelines in Medical Artificial Intelligence: A Systematic Review and Meta-Analysis. Commun. Med. 2024, 4, 71. [Google Scholar] [CrossRef]
- Collins, G.S.; Dhiman, P.; Andaur Navarro, C.L.; Ma, J.; Hooft, L.; Reitsma, J.B.; Logullo, P.; Beam, A.L.; Peng, L.; Van Calster, B.; et al. Protocol for Development of a Reporting Guideline (TRIPOD-AI) and Risk of Bias Tool (PROBAST-AI) for Diagnostic and Prognostic Prediction Model Studies Based on Artificial Intelligence. BMJ Open 2021, 11, e048008. [Google Scholar] [CrossRef]
- Sounderajah, V.; Ashrafian, H.; Rose, S.; Shah, N.H.; Ghassemi, M.; Golub, R.; Kahn, C.E.; Esteva, A.; Karthikesalingam, A.; Mateen, B.; et al. A Quality Assessment Tool for Artificial Intelligence-Centered Diagnostic Test Accuracy Studies: QUADAS-AI. Nat. Med. 2021, 27, 1663–1665. [Google Scholar] [CrossRef]
- Wolfe, C.R.; Reyna, V.F.; Widmer, C.L.; Cedillos, E.M.; Fisher, C.R.; Brust-Renck, P.G.; Weil, A.M. Efficacy of a Web-Based Intelligent Tutoring System for Communicating Genetic Risk of Breast Cancer: A Fuzzy-Trace Theory Approach. Med. Decis. Mak. 2015, 35, 46–59. [Google Scholar] [CrossRef]
- MD Anderson Cancer Center. A Randomized, Double-Blind, Placebo-Controlled Study of 4-Hydroxytamoxifen Topical Gel in Women with Mammographically Dense Breast; MD Anderson Cancer Center: Houston, TX, USA, 2024.
- Glissen Brown, J.R.; Mansour, N.M.; Wang, P.; Chuchuca, M.A.; Minchenberg, S.B.; Chandnani, M.; Liu, L.; Gross, S.A.; Sengupta, N.; Berzin, T.M. Deep Learning Computer-Aided Polyp Detection Reduces Adenoma Miss Rate: A United States Multi-Center Randomized Tandem Colonoscopy Study (CADeT-CS Trial). Clin. Gastroenterol. Hepatol. 2022, 20, 1499–1507.e4. [Google Scholar] [CrossRef]
- Kim, H.J.; Parsa, N.; Byrne, M.F. The Role of Artificial Intelligence in Colonoscopy. Semin. Colon Rectal Surg. 2024, 35, 101007. [Google Scholar] [CrossRef]
- Patel, V.; Khan, M.N.; Shrivastava, A.; Sadiq, K.; Ali, S.A.; Moore, S.R.; Brown, D.E.; Syed, S. Artificial Intelligence Applied to Gastrointestinal Diagnostics: A Review. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 4–11. [Google Scholar] [CrossRef]
- TaiHao Medical Inc. Evaluation of TaiHao Breast Ultrasound Diagnosis Software; TaiHao Medical Inc.: Taipei, Taiwan, 2022. [Google Scholar]
- Brown, A.E.; Najmi, M.; Duke, T.; Grabell, D.A.; Koshelev, M.V.; Nelson, K.C. Skin Cancer Education Interventions for Primary Care Providers: A Scoping Review. J. Gen. Intern. Med. 2022, 37, 2267–2279. [Google Scholar] [CrossRef]
- Saraiva, M.M.; Ribeiro, T.; Ferreira, J.P.S.; Boas, F.V.; Afonso, J.; Santos, A.L.; Parente, M.P.L.; Jorge, R.N.; Pereira, P.; Macedo, G. Artificial Intelligence for Automatic Diagnosis of Biliary Stricture Malignancy Status in Single-Operator Cholangioscopy: A Pilot Study. Gastrointest. Endosc. 2022, 95, 339–348. [Google Scholar] [CrossRef]
- Penn Medicine Uses AI Chatbot “Penny” to Improve Cancer Care. Available online: https://www.healthcareitnews.com/news/penn-medicine-uses-ai-chatbot-penny-improve-cancer-care (accessed on 5 June 2025).
- Hong, J.C.; Eclov, N.C.W.; Dalal, N.H.; Thomas, S.M.; Stephens, S.J.; Malicki, M.; Shields, S.; Cobb, A.; Mowery, Y.M.; Niedzwiecki, D.; et al. System for High-Intensity Evaluation During Radiation Therapy (SHIELD-RT): A Prospective Randomized Study of Machine Learning-Directed Clinical Evaluations During Radiation and Chemoradiation. J. Clin. Oncol. 2020, 38, 3652–3661. [Google Scholar] [CrossRef]
- Manz, C.R.; Parikh, R.B.; Evans, C.N.; Chivers, C.; Regli, S.H.; Bekelman, J.E.; Small, D.; Rareshide, C.A.L.; O’Connor, N.; Schuchter, L.M.; et al. Integrating Machine-Generated Mortality Estimates and Behavioral Nudges to Promote Serious Illness Conversations for Cancer Patients: Design and Methods for a Stepped-Wedge Cluster Randomized Controlled Trial. Contemp. Clin. Trials 2020, 90, 105951. [Google Scholar] [CrossRef] [PubMed]
- Manz, C.R.; Parikh, R.B.; Small, D.S.; Evans, C.N.; Chivers, C.; Regli, S.H.; Hanson, C.W.; Bekelman, J.E.; Rareshide, C.A.L.; O’Connor, N.; et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Li, Y.-L.; Wei, M.-Y.; Li, G.-Y. Innovation and Challenges of Artificial Intelligence Technology in Personalized Healthcare. Sci. Rep. 2024, 14, 18994. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Fatima, H.; Qureshi, A.; Kumar, S.; Hanan, A.; Hussain, J.; Abdullah, S. Drawbacks of Artificial Intelligence and Their Potential Solutions in the Healthcare Sector. Biomed. Mater. Devices 2023, 1, 731–738. [Google Scholar] [CrossRef]
- Cunha Reis, T. The Roadblocks to AI Adoption in Surgery: Data, Real-Time Applications and Ethics. Med. Adv. 2024, 2, 380–383. [Google Scholar] [CrossRef]
- Daneshjou, R.; Vodrahalli, K.; Novoa, R.A.; Jenkins, M.; Liang, W.; Rotemberg, V.; Ko, J.; Swetter, S.M.; Bailey, E.E.; Gevaert, O.; et al. Disparities in Dermatology AI Performance on a Diverse, Curated Clinical Image Set. Sci. Adv. 2022, 8, eabq6147. [Google Scholar] [CrossRef]
- Raj, G.M.; Dananjayan, S.; Gudivada, K.K. Applications of Artificial Intelligence and Machine Learning in Clinical Medicine: What Lies Ahead? Med. Adv. 2024, 2, 202–204. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Hanna, M.; Pantanowitz, J.; Lennerz, J.; Henricks, W.H.; Shen, P.; Quinn, B.; Bennet, S.; Rashidi, H.H. Regulatory Aspects of Artificial Intelligence and Machine Learning. Mod. Pathol. 2024, 37, 100609. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).