Artificial Intelligence in Oncology: A 10-Year ClinicalTrials.gov-Based Analysis Across the Cancer Control Continuum
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Condition/disease,
- (Cancer* OR oncology OR leukemia* OR sarcoma* OR tumor* OR tumour* OR melanoma* OR neoplasm* OR neoplasia OR carcinoma* OR adenocarcinoma* OR lymphoma*),
- AND,
- Other Terms: (“Artificial Intelligence” OR “AI System” OR “Intelligent Systems” OR “Machine Learning” OR “Deep Learning” OR Autoencoder OR “Large Language Model” OR “Natural Language Processing” OR “Neural Network” OR “Convolutional Neural Network” OR “Generative AI” OR “Generative Pre-trained Transformer” OR “ChatGPT”),
- AND,
- (United States),
- AND,
- (Completed Studies),
- AND,
- (Study Start Date ≥ 1 January 2015).
3. Results
4. Discussion
4.1. Trial Characteristics
4.2. AI Applications Across the CCC
4.2.1. Etiology
4.2.2. Prevention
4.2.3. Detection
4.2.4. Diagnosis
4.2.5. Treatment
4.2.6. Survivorship
4.3. Barriers to AI Integration in Oncology
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CCC | Cancer Control Continuum |
| ML | Machine Learning |
| NLP | Natural Language Processing |
| DL | Deep Learning |
| N/A | Not Applicable |
| FIT | Fecal Immunochemical Testing |
| CNN | Convolutional Neural Network |
| EHR | Electronic Health Record |
| CRC | Colorectal Cancer |
| CAD | Computer-Aided Detection |
| BRCA Gist | BReast CAncer Genetics Intelligent Semantic Tutoring |
| PCP | Primary Care Physician |
References
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial Intelligence in Healthcare: Transforming the Practice of Medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef]
- Huhulea, E.N.; Huang, L.; Eng, S.; Sumawi, B.; Huang, A.; Aifuwa, E.; Hirani, R.; Tiwari, R.K.; Etienne, M. Artificial Intelligence Advancements in Oncology: A Review of Current Trends and Future Directions. Biomedicines 2025, 13, 951. [Google Scholar] [CrossRef]
- Kann, B.H.; Hosny, A.; Aerts, H.J.W.L. Artificial Intelligence for Clinical Oncology. Cancer Cell 2021, 39, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Sim, J.Z.T.; Fong, Q.W.; Huang, W.; Tan, C.H. Machine Learning in Medicine: What Clinicians Should Know. Singap. Med. J. 2021, 64, 91–97. [Google Scholar] [CrossRef]
- Aramaki, E.; Wakamiya, S.; Yada, S.; Nakamura, Y. Natural Language Processing: From Bedside to Everywhere. Yearb. Med. Inform. 2022, 31, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A.; Singh, R.P.; Ahmed, M. Computer Vision to Enhance Healthcare Domain: An Overview of Features, Implementation, and Opportunities. Intell. Pharm. 2024, 2, 792–803. [Google Scholar] [CrossRef]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer Heterogeneity: Implications for Targeted Therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Alhejaily, A.-M.G. Artificial Intelligence in Healthcare (Review). Biomed. Rep. 2024, 22, 11. [Google Scholar] [CrossRef]
- Basu, K.; Sinha, R.; Ong, A.; Basu, T. Artificial Intelligence: How Is It Changing Medical Sciences and Its Future? Indian J. Dermatol. 2020, 65, 365–370. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, H.; Mirza, K.M. Pathology in the Artificial Intelligence Era: Guiding Innovation and Implementation to Preserve Human Insight. Acad. Pathol. 2025, 12, 100166. [Google Scholar] [CrossRef]
- El Arab, R.A.; Abu-Mahfouz, M.S.; Abuadas, F.H.; Alzghoul, H.; Almari, M.; Ghannam, A.; Seweid, M.M. Bridging the Gap: From AI Success in Clinical Trials to Real-World Healthcare Implementation—A Narrative Review. Healthcare 2025, 13, 701. [Google Scholar] [CrossRef]
- Plana, D.; Shung, D.L.; Grimshaw, A.A.; Saraf, A.; Sung, J.J.Y.; Kann, B.H. Randomized Clinical Trials of Machine Learning Interventions in Health Care: A Systematic Review. JAMA Netw. Open 2022, 5, e2233946. [Google Scholar] [CrossRef]
- Han, R.; Acosta, J.N.; Shakeri, Z.; Ioannidis, J.P.A.; Topol, E.J.; Rajpurkar, P. Randomised Controlled Trials Evaluating Artificial Intelligence in Clinical Practice: A Scoping Review. Lancet Digit. Health 2024, 6, e367–e373. [Google Scholar] [CrossRef]
- Chopra, H.; Annu; Shin, D.K.; Munjal, K.; Priyanka; Dhama, K.; Emran, T.B. Revolutionizing Clinical Trials: The Role of AI in Accelerating Medical Breakthroughs. Int. J. Surg. 2023, 109, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Hogan, J. We Need More Randomized Clinical Trials of AI. NEJM AI 2024, 1, aie2400881. [Google Scholar] [CrossRef]
- Hutson, M. How AI Is Being Used to Accelerate Clinical Trials. Nature 2024, 627, S2–S5. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Gresham, G.; Meinert, J.L.; Gresham, A.G.; Piantadosi, S.; Meinert, C.L. Update on the Clinical Trial Landscape: Analysis of ClinicalTrials.Gov Registration Data, 2000–2020. Trials 2022, 23, 858. [Google Scholar] [CrossRef]
- Cancer Control Continuum|Division of Cancer Control and Population Sciences (DCCPS). Available online: https://cancercontrol.cancer.gov/about-dccps/about-cc/cancer-control-continuum (accessed on 4 June 2025).
- Nagendran, M.; Chen, Y.; Lovejoy, C.A.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; Ioannidis, J.P.A.; Collins, G.S.; Maruthappu, M. Artificial Intelligence versus Clinicians: Systematic Review of Design, Reporting Standards, and Claims of Deep Learning Studies. BMJ 2020, 368, m689. [Google Scholar] [CrossRef]
- Liu, G.; Li, N.; Chen, L.; Yang, Y.; Zhang, Y. Registered Trials on Artificial Intelligence Conducted in Emergency Department and Intensive Care Unit: A Cross-Sectional Study on ClinicalTrials.Gov. Front. Med. 2021, 8, 634197. [Google Scholar] [CrossRef]
- Turner, B.; Rajeshuni, N.; Tran, E.M.; Ludwig, C.A.; Tauqeer, Z.; Weeks, B.; Kinde, B.; Pershing, S. Characteristics of Ophthalmology Trials Registered in ClinicalTrials.Gov, 2007–2018. Am. J. Ophthalmol. 2020, 211, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Pediatric Trials Run in India: An Analysis of Clinical Trials.Gov 2006–2015. Available online: https://pubmed.ncbi.nlm.nih.gov/30648225/ (accessed on 5 June 2025).
- Dong, J.; Geng, Y.; Lu, D.; Li, B.; Tian, L.; Lin, D.; Zhang, Y. Clinical Trials for Artificial Intelligence in Cancer Diagnosis: A Cross-Sectional Study of Registered Trials in ClinicalTrials.Gov. Front. Oncol. 2020, 10, 1629. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xiu, X.; Liu, S.; Qian, Q.; Wu, S. Characteristics of Artificial Intelligence Clinical Trials in the Field of Healthcare: A Cross-Sectional Study on ClinicalTrials.Gov. Int. J. Environ. Res. Public Health 2022, 19, 13691. [Google Scholar] [CrossRef] [PubMed]
- Saady, M.; Eissa, M.; Yacoub, A.S.; Hamed, A.B.; Azzazy, H.M.E.-S. Implementation of Artificial Intelligence Approaches in Oncology Clinical Trials: A Systematic Review. Artif. Intell. Med. 2025, 161, 103066. [Google Scholar] [CrossRef]
- Hachache, R.; Yahyaouy, A.; Riffi, J.; Tairi, H.; Abibou, S.; Adoui, M.E.; Benjelloun, M. Advancing Personalized Oncology: A Systematic Review on the Integration of Artificial Intelligence in Monitoring Neoadjuvant Treatment for Breast Cancer Patients. BMC Cancer 2024, 24, 1300. [Google Scholar] [CrossRef]
- Macheka, S.; Ng, P.Y.; Ginsburg, O.; Hope, A.; Sullivan, R.; Aggarwal, A. Prospective Evaluation of Artificial Intelligence (AI) Applications for Use in Cancer Pathways Following Diagnosis: A Systematic Review. BMJ Oncol. 2024, 3, e000255. [Google Scholar] [CrossRef]
- Luchini, C.; Pea, A.; Scarpa, A. Artificial Intelligence in Oncology: Current Applications and Future Perspectives. Br. J. Cancer 2022, 126, 4–9. [Google Scholar] [CrossRef]
- Home|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 5 June 2025).
- Home—MeSH—NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh (accessed on 12 June 2025).
- European Commission; Joint Research Centre. AI Watch: Defining Artificial Intelligence: Towards an Operational Definition and Taxonomy of Artificial Intelligence; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Amir, O.; Grosz, B.J.; Gajos, K.Z.; Swenson, S.M.; Sanders, L.M. AI Support of Teamwork for Coordinated Care of Children with Complex Conditions; AI Access Foundation: San Francisco, CA, USA, 2014. [Google Scholar]
- Heinlein, L.; Maron, R.C.; Hekler, A.; Haggenmüller, S.; Wies, C.; Utikal, J.S.; Meier, F.; Hobelsberger, S.; Gellrich, F.F.; Sergon, M.; et al. Prospective Multicenter Study Using Artificial Intelligence to Improve Dermoscopic Melanoma Diagnosis in Patient Care. Commun. Med. 2024, 4, 177. [Google Scholar] [CrossRef]
- Kolbinger, F.R.; Veldhuizen, G.P.; Zhu, J.; Truhn, D.; Kather, J.N. Reporting Guidelines in Medical Artificial Intelligence: A Systematic Review and Meta-Analysis. Commun. Med. 2024, 4, 71. [Google Scholar] [CrossRef]
- Collins, G.S.; Dhiman, P.; Andaur Navarro, C.L.; Ma, J.; Hooft, L.; Reitsma, J.B.; Logullo, P.; Beam, A.L.; Peng, L.; Van Calster, B.; et al. Protocol for Development of a Reporting Guideline (TRIPOD-AI) and Risk of Bias Tool (PROBAST-AI) for Diagnostic and Prognostic Prediction Model Studies Based on Artificial Intelligence. BMJ Open 2021, 11, e048008. [Google Scholar] [CrossRef]
- Sounderajah, V.; Ashrafian, H.; Rose, S.; Shah, N.H.; Ghassemi, M.; Golub, R.; Kahn, C.E.; Esteva, A.; Karthikesalingam, A.; Mateen, B.; et al. A Quality Assessment Tool for Artificial Intelligence-Centered Diagnostic Test Accuracy Studies: QUADAS-AI. Nat. Med. 2021, 27, 1663–1665. [Google Scholar] [CrossRef]
- Wolfe, C.R.; Reyna, V.F.; Widmer, C.L.; Cedillos, E.M.; Fisher, C.R.; Brust-Renck, P.G.; Weil, A.M. Efficacy of a Web-Based Intelligent Tutoring System for Communicating Genetic Risk of Breast Cancer: A Fuzzy-Trace Theory Approach. Med. Decis. Mak. 2015, 35, 46–59. [Google Scholar] [CrossRef]
- MD Anderson Cancer Center. A Randomized, Double-Blind, Placebo-Controlled Study of 4-Hydroxytamoxifen Topical Gel in Women with Mammographically Dense Breast; MD Anderson Cancer Center: Houston, TX, USA, 2024.
- Glissen Brown, J.R.; Mansour, N.M.; Wang, P.; Chuchuca, M.A.; Minchenberg, S.B.; Chandnani, M.; Liu, L.; Gross, S.A.; Sengupta, N.; Berzin, T.M. Deep Learning Computer-Aided Polyp Detection Reduces Adenoma Miss Rate: A United States Multi-Center Randomized Tandem Colonoscopy Study (CADeT-CS Trial). Clin. Gastroenterol. Hepatol. 2022, 20, 1499–1507.e4. [Google Scholar] [CrossRef]
- Kim, H.J.; Parsa, N.; Byrne, M.F. The Role of Artificial Intelligence in Colonoscopy. Semin. Colon Rectal Surg. 2024, 35, 101007. [Google Scholar] [CrossRef]
- Patel, V.; Khan, M.N.; Shrivastava, A.; Sadiq, K.; Ali, S.A.; Moore, S.R.; Brown, D.E.; Syed, S. Artificial Intelligence Applied to Gastrointestinal Diagnostics: A Review. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 4–11. [Google Scholar] [CrossRef]
- TaiHao Medical Inc. Evaluation of TaiHao Breast Ultrasound Diagnosis Software; TaiHao Medical Inc.: Taipei, Taiwan, 2022. [Google Scholar]
- Brown, A.E.; Najmi, M.; Duke, T.; Grabell, D.A.; Koshelev, M.V.; Nelson, K.C. Skin Cancer Education Interventions for Primary Care Providers: A Scoping Review. J. Gen. Intern. Med. 2022, 37, 2267–2279. [Google Scholar] [CrossRef]
- Saraiva, M.M.; Ribeiro, T.; Ferreira, J.P.S.; Boas, F.V.; Afonso, J.; Santos, A.L.; Parente, M.P.L.; Jorge, R.N.; Pereira, P.; Macedo, G. Artificial Intelligence for Automatic Diagnosis of Biliary Stricture Malignancy Status in Single-Operator Cholangioscopy: A Pilot Study. Gastrointest. Endosc. 2022, 95, 339–348. [Google Scholar] [CrossRef]
- Penn Medicine Uses AI Chatbot “Penny” to Improve Cancer Care. Available online: https://www.healthcareitnews.com/news/penn-medicine-uses-ai-chatbot-penny-improve-cancer-care (accessed on 5 June 2025).
- Hong, J.C.; Eclov, N.C.W.; Dalal, N.H.; Thomas, S.M.; Stephens, S.J.; Malicki, M.; Shields, S.; Cobb, A.; Mowery, Y.M.; Niedzwiecki, D.; et al. System for High-Intensity Evaluation During Radiation Therapy (SHIELD-RT): A Prospective Randomized Study of Machine Learning-Directed Clinical Evaluations During Radiation and Chemoradiation. J. Clin. Oncol. 2020, 38, 3652–3661. [Google Scholar] [CrossRef]
- Manz, C.R.; Parikh, R.B.; Evans, C.N.; Chivers, C.; Regli, S.H.; Bekelman, J.E.; Small, D.; Rareshide, C.A.L.; O’Connor, N.; Schuchter, L.M.; et al. Integrating Machine-Generated Mortality Estimates and Behavioral Nudges to Promote Serious Illness Conversations for Cancer Patients: Design and Methods for a Stepped-Wedge Cluster Randomized Controlled Trial. Contemp. Clin. Trials 2020, 90, 105951. [Google Scholar] [CrossRef] [PubMed]
- Manz, C.R.; Parikh, R.B.; Small, D.S.; Evans, C.N.; Chivers, C.; Regli, S.H.; Hanson, C.W.; Bekelman, J.E.; Rareshide, C.A.L.; O’Connor, N.; et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Li, Y.-L.; Wei, M.-Y.; Li, G.-Y. Innovation and Challenges of Artificial Intelligence Technology in Personalized Healthcare. Sci. Rep. 2024, 14, 18994. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Fatima, H.; Qureshi, A.; Kumar, S.; Hanan, A.; Hussain, J.; Abdullah, S. Drawbacks of Artificial Intelligence and Their Potential Solutions in the Healthcare Sector. Biomed. Mater. Devices 2023, 1, 731–738. [Google Scholar] [CrossRef]
- Cunha Reis, T. The Roadblocks to AI Adoption in Surgery: Data, Real-Time Applications and Ethics. Med. Adv. 2024, 2, 380–383. [Google Scholar] [CrossRef]
- Daneshjou, R.; Vodrahalli, K.; Novoa, R.A.; Jenkins, M.; Liang, W.; Rotemberg, V.; Ko, J.; Swetter, S.M.; Bailey, E.E.; Gevaert, O.; et al. Disparities in Dermatology AI Performance on a Diverse, Curated Clinical Image Set. Sci. Adv. 2022, 8, eabq6147. [Google Scholar] [CrossRef]
- Raj, G.M.; Dananjayan, S.; Gudivada, K.K. Applications of Artificial Intelligence and Machine Learning in Clinical Medicine: What Lies Ahead? Med. Adv. 2024, 2, 202–204. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Hanna, M.; Pantanowitz, J.; Lennerz, J.; Henricks, W.H.; Shen, P.; Quinn, B.; Bennet, S.; Rashidi, H.H. Regulatory Aspects of Artificial Intelligence and Machine Learning. Mod. Pathol. 2024, 37, 100609. [Google Scholar] [CrossRef] [PubMed]

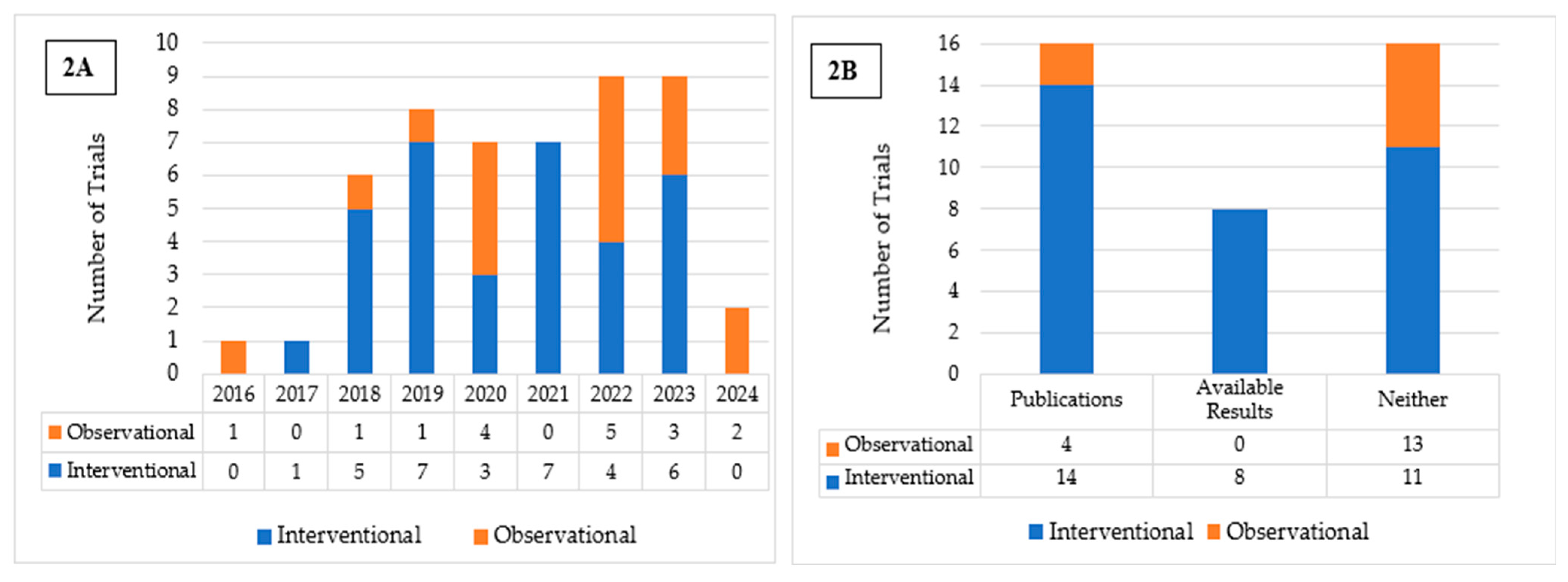
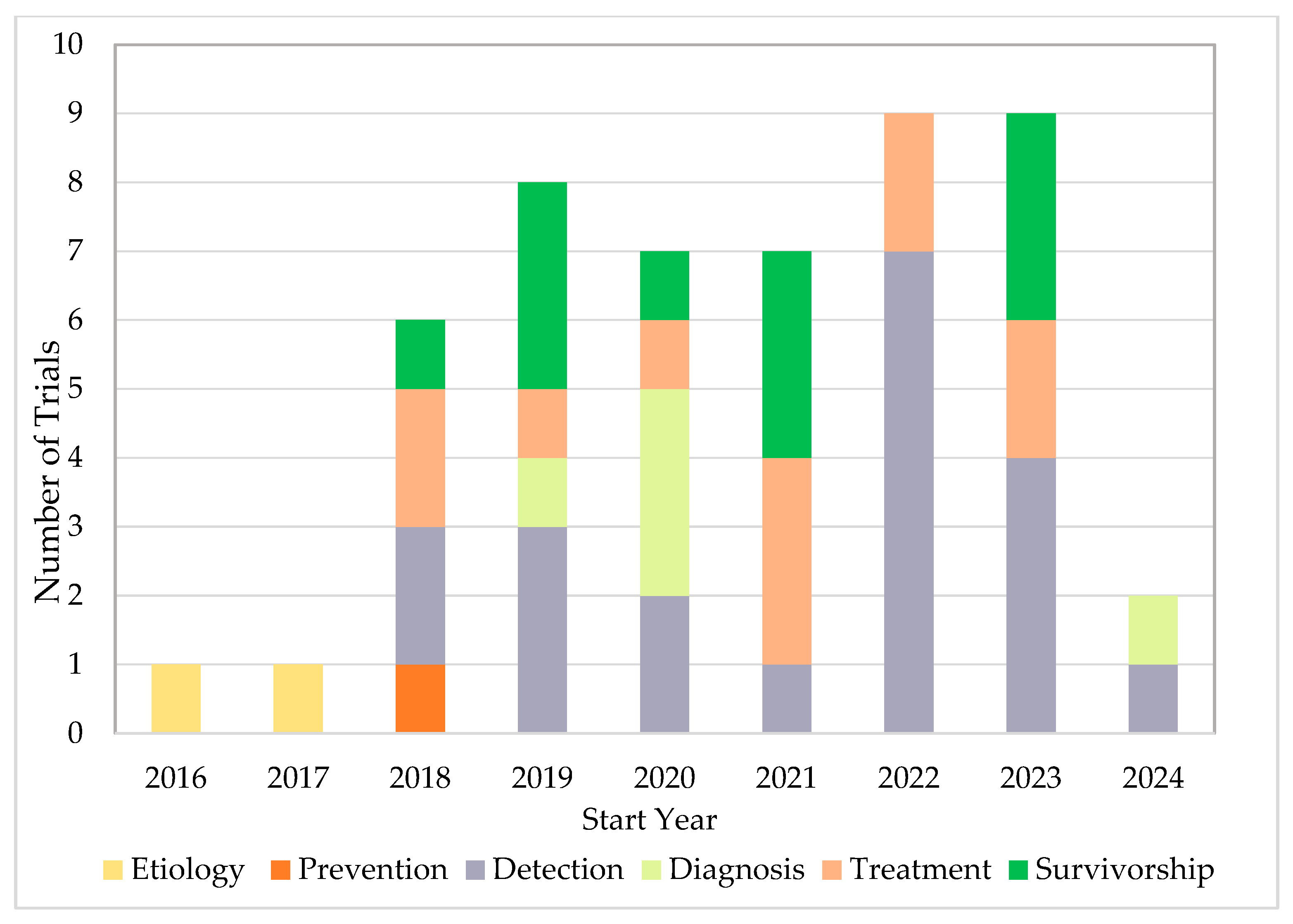

| Study Characteristics | All n = 50 | Interventional n = 33 | Observational n = 17 |
|---|---|---|---|
| Results | |||
| Results Available No Results Available | 8 42 | 8 25 | 0 17 |
| Published Studies Available No Published Studies Available | 18 32 | 14 19 | 4 13 |
| Participant Enrollment | |||
| ≤100 100–500 500–1000 >1000 | 17 18 3 12 | 11 15 1 6 | 6 3 2 6 |
| Median | 198 | 194 | 355 |
| Age group | |||
| Adults and older adults Children, adults, and older adults Older adults (>65) | 44 5 1 | 32 0 1 | 12 5 0 |
| Gender | |||
| Female only Male only Both | 7 2 41 | 6 0 27 | 1 2 14 |
| Center | |||
| Single center Multi-center | 31 19 | 21 12 | 10 7 |
| Funder Type | |||
| NIH Industry Other * | 3 15 32 | 1 8 24 | 2 7 8 |
| FDA Regulation Status | |||
| Regulated Device Regulated + Unapproved/Uncleared | 18 11 | 15 8 | 3 3 |
| Cancer type | |||
| Bile Duct | 1 | 0 | 1 |
| Blood | 1 | 1 | 0 |
| Breast | 4 | 4 | 0 |
| Colorectal | 13 | 10 | 3 |
| Esophagus | 2 | 1 | 1 |
| Gastrointestinal | 2 | 2 | 0 |
| Gynecologic | 3 | 2 | 1 |
| Head and Neck | 1 | 1 | 0 |
| Lung | 2 | 0 | 2 |
| Prostate | 2 | 0 | 2 |
| Skin | 3 | 1 | 2 |
| Multiple or Unspecified | 16 | 11 | 5 |
| Characteristics | Number of Trials |
|---|---|
| Interventional (n = 33) | |
| Allocation | |
| Randomized | 22 |
| Non-randomized | 1 |
| N/A * | 10 |
| Intervention model | |
| Single group assignment | 10 |
| Crossover assignment | 2 |
| Parallel assignment | 19 |
| Factorial assignment | 2 |
| Masking | |
| Non-masked | 20 |
| Masked | 13 |
| Single | 6 |
| Double | 4 |
| Triple | 3 |
| Observational (n = 17) | |
| Observational model | |
| Cohort | 9 |
| Case–Control | 3 |
| Family-Based | 1 |
| Other | 4 |
| Time perspective | |
| Cross sectional | 1 |
| Prospective | 9 |
| Retrospective | 5 |
| Other | 2 |
| AI Domain (n) * | Subdomain (n) * | Keyword (n) | |
|---|---|---|---|
| Interventional Trials | |||
| Learning (n = 12) | Machine Learning (n = 12) NCT03984773, NCT06888089, NCT06621225, NCT04551105, NCT04867850, NCT04535414, NCT03746392, NCT04277650, NCT05611151, NCT04458168, NCT04195646, NCT03063619 | Convolutional Neural Network (n = 1) NCT06621225 | |
| Deep Learning (n = 4) NCT06621225, NCT03925337, NCT04551105, NCT04195646 | |||
| Communication (n = 5) | Natural Language Processing (n = 5) NCT06888089, NCT05113264, NCT03371147, NCT05069519, NCT03746392 | Chatbot (n = 1) NCT05113264 | |
| Sentiment Analysis (n = 2) NCT03371147, NCT05069519 | |||
| Perception (n = 1) | Computer Vision (n = 1) NCT03814824 | Intelligent Real Time Image Segmentation (n = 1) NCT03814824 | |
| Other (n = 19) | Integrated AI System (n = 2) NCT03511690, NCT03999177 | Intelligent System (n = 2) NCT03511690, NCT03999177 | |
| Artificial Intelligence not specified (n = 17) NCT06182332, NCT05096286, NCT06305364, NCT05963724, NCT06888089, NCT05826288, NCT03925337, NCT03954548, NCT05113927, NCT03953976, NCT05030454, NCT05611151, NCT04411810, NCT05275556, NCT03620071, NCT04754347, NCT03867409 | |||
| Observational Trials | |||
| Learning (n = 15) | Machine Learning (n = 15) NCT04441775, NCT05147389, NCT06463860, NCT06463977, NCT03688906, NCT04442425, NCT05385718, NCT03837327, NCT03174574, NCT04369053, NCT06381583, NCT05383976, NCT05122247, NCT06576232, NCT05126173 | Convolutional Neural Network (n = 1) NCT05147389 | |
| Deep Ensemble (n = 1) NCT05126173 | |||
| Deep Neural Network (n = 1) NCT06463860 | |||
| Communication (n = 1) | Natural Language Processing (n = 1) NCT04442425 | ||
| Other (n = 8) | Artificial Intelligence not specified (n = 8) NCT04441775, NCT05147389, NCT03837327, NCT04369053, NCT06561217, NCT06576232, NCT05126173, NCT05872503 | ||
| CCC Domain | NCI Subcategory |
|---|---|
| Etiology | Environmental factors Genetic factors Gene–environment interactions Medication/pharmaceutical exposure Infectious agents Health behaviors |
| Prevention | Tobacco control Diet Physical activity Sun protection HPV vaccine Limited alcohol use Chemoprevention |
| Detection | Pap/HPV testing Mammography Fecal occult blood test Colonoscopy Lung cancer screening |
| Diagnosis | Shared and informed decision making |
| Treatment | Curative treatment Non-curative treatment Adherence Symptom management |
| Survivorship | Coping Health Promotion for Survivors |
| NCT # (Start Year, N) | Cancer Type | Cancer Control Continuum Term | AI Application and Implementation |
|---|---|---|---|
| Interventional | |||
| NCT03511690 (2017, n = 95) | Multiple or Unspecified | Etiology (Genetic factors) | AI-driven avatar-based tutoring system. |
| NCT03063619 (2018, n = 194) | Breast | Prevention (Chemoprevention) | ML-assisted volumetric breast density measurement. |
| NCT03371147 (2018, n = 30) | Multiple or Unspecified | Treatment (Symptom Management) | NLP (sentiment analysis)-based digital platform (CancerLife) collects patient-reported symptoms and psychosocial data. |
| NCT03746392 (2018, n = 150) | Multiple or Unspecified | Survivorship (Coping) | ML and NLP analyze Electronic Health Records (EHRs) to prompt goals-of-care conversations. |
| NCT03867409 (2018, n = 2105) | Colorectal | Detection (Colonoscopy) | Culturally tailored messages delivered through AI-based virtual technology to increase Colorectal (CRC) screening. |
| NCT04277650 (2018, n = 311) | Multiple or Unspecified | Treatment (Symptom Management) | ML identifies high-risk individuals undergoing outpatient radiation or chemoradiation for targeted evaluations. |
| NCT03620071 (2019, n = 67) | Multiple or Unspecified | Survivorship (Coping) | GoalKeeper (Intelligent Information Sharing) [39] leverages AI to coordinate care via a mobile health platform. |
| NCT03814824 (2019, 148) | Esophagus | Detection (Endomicroscopy) | Intelligent Real-time AI image segmentation improves Volumetric Laser Endomicroscopy by flagging dysplastic regions. |
| NCT03925337 (2019, n = 234) | Colorectal | Detection (Colonoscopy) | Computer Aided Detection (CAD) DL software processes colonoscopy video in real-time. |
| NCT03953976 (2019, n = 67) | Head and Neck | Treatment (Curative Treatment) | AI-driven radiomic analysis of imaging scans guides precise targeting of involved lymph nodes. |
| NCT03984773 (2019, n = 78) | Multiple or Unspecified | Survivorship (Coping) | ML predicts patients at high risk of short-term mortality, prompting serious illness conversations. |
| NCT03999177 (2019, n = 30) | Breast | Survivorship (Health promotion for survivors) | Integrated AI-driven motion sensing guides lymphatic exercises. |
| NCT04195646 (2019, n = 300) | Colorectal | Detection (Colonoscopy) | EndoVigilant CAD processes colonoscopy video in real-time. |
| NCT03954548 (2020, n = 249) | Colorectal | Detection (Colonoscopy) | CNN based CAD device (GI-Genius) analyzes real-time colonoscopy videos. |
| NCT04411810 (2020, n = 149) | Skin | Diagnosis (Shared and Informed Decision Making) | AI-based Nevisense measures electrical impedance of skin lesions to assess malignancy. |
| NCT04551105 (2020, n = 16) | Breast | Diagnosis (Shared and Informed Decision Making) | DL analyzes multi-site breast ultrasound images. |
| NCT04458168 (2021, n = 120) | Gynecologic | Survivorship (Coping) | ML-based App, Purposeful, customizes app resources. |
| NCT04754347 (2021, n = 1472) | Colorectal | Detection (Colonoscopy) | CAD device (Skout) analyses colonoscopy video. |
| NCT04867850 (2021, n = 4450) | Multiple or Unspecified | Survivorship (Coping) | ML predicts 6-month mortality risk. |
| NCT05030454 (2021, n = 10) | Multiple or Unspecified | Treatment (Curative Treatment) | AI-powered ETHOS system integrates CT-guided adaptive radiotherapy and optical surface imaging. |
| NCT05069519 (2021, n = 126) | Multiple or Unspecified | Survivorship (Health promotion for survivors) | NLP sentiment analysis guides personalized exercise and social support from Fitbit and Facebook. |
| NCT05113264 (2021, n = 60) | Gastrointestinal | Treatment (Adherence) | NLP-based chatbot “Penny” triages patient-reported symptoms. |
| NCT05113927 (2021, n = 482) | Breast | Treatment (Curative treatment) | AI (SELENE) system provides real-time intraoperative imaging of lumpectomy margins. |
| NCT05096286 (2022, n = 10) | Multiple or Unspecified | Treatment (Non-curative treatment) | AI ETHOS system automates MRI image processing. |
| NCT05275556 (2022, n = 1410) | Colorectal | Detection (Colonoscopy) | AI processes White Light Endoscopy colonoscopy video. |
| NCT05963724 (2022, n = 1100) | Colorectal | Detection (Colonoscopy) | AI (GI-Genius) analyzes colonoscopy video. |
| NCT06621225 (2022, n = 264) | Colorectal | Detection (Colonoscopy) | ML (CNN) analyzes real-time colonoscopy images. |
| NCT04535414 (2023, n = 195) | Gastrointestinal | Detection (Endoscopy) | ML assists detection of early signet ring cell carcinoma. |
| NCT05611151 (2023, n = 830) | Colorectal | Detection (Colonoscopy) | ML-based CAD system, WISE VISION, processes white light colonoscopy images in real time. |
| NCT05826288 (2023, n = 11) | Blood | Survivorship (Health promotion for survivors) | AI monitoring supports post-BMT and CAR-T home care. |
| NCT06182332 (2023, n = 221) | Gynecologic | Survivorship (Coping) | AI analyzes EHR to identify advanced gynecologic cancer patients for outpatient palliative care. |
| NCT06305364 (2023, n = 426) | Colorectal | Detection (Colonoscopy) | Gixam device uses AI-analyzed tongue images to predict colorectal adenomas. |
| NCT06888089 (2023, n = 20,707) | Multiple or Unspecified | Treatment (Adherence) | ML combines genomic, clinical data, and NLP of imaging reports to identify trial-eligible patients. |
| Observational | |||
| NCT03174574 (2016, n = 3) | Multiple or Unspecified | Etiology (Gene-environment interactions) | ML analyzes genetic mutation data. |
| NCT03688906 (2018, n = 3275) | Colorectal | Detection (Biomarkers) | ML analyzes multi-omics blood biomarkers to develop a non-invasive assay for early CRC detection. |
| NCT03837327 (2019, n = 1025) | Gynecologic | Diagnosis (Shared and Informed Decision Making) | ML analyzes glycoprotein patterns to distinguish benign from malignant adnexal masses. |
| NCT04441775 (2020, n = 5) | Prostate | Treatment (Curative) | ML generates standardized radiation treatment plans. |
| NCT05147389 (2020, n = 170) | Bile Duct | Diagnosis (Shared and Informed Decision Making) | ML (CNN) processes live cholangioscopy video footage. |
| NCT04442425 (2020, n = 83) | Multiple or Unspecified | Survivorship (Coping) | ML and NLP analyze facial, voice, and language cues to classify cancer-related pain. |
| NCT04369053 (2020, n = 48,995) | Colorectal | Detection (Biomarkers) | AI analyzes multi-omic patterns of cell-free biomarkers to develop a non-invasive blood assay for CRC detection. |
| NCT05385718 (2022, n = 694) | Multiple or Unspecified | Detection (MRI Imaging) | ML enhances MRI images for cancer detection. |
| NCT05383976 (2022, n = 201) | Colorectal | Detection (Colonoscopy; Fecal Immunochemical Test) | ML risk-stratifies patients in poverty-affected zip codes to increase CRC screening. |
| NCT05122247 (2022, n = 12,000) | Multiple or Unspecified | Treatment (Adherence; Symptom Management) | ML analyzes clinical data to predict unplanned hospital admissions and emergency visits. |
| NCT06576232 (2022, n = 1147) | Lung | Detection (Lung Cancer Screening) | ML analyzes low-dose CT images to support lung cancer screening. |
| NCT05126173 (2022, n = 1111) | Skin | Detection (Skin Cancer Screening) | Deep ensemble analyzes dermoscopic images. |
| NCT06463977 (2023, n = 52) | Lung | Survivorship (Coping) | ML generates individualized mortality risk predictions based on clinical data. |
| NCT06381583 (2023, n = 658) | Esophagus | Detection (Biomarkers) | ML identifies blood microRNA biomarkers for esophageal disease staging. |
| NCT06561217 (2023, n = 355) | Multiple or Unspecified | Treatment (Adherence) | Mendel uses AI to identify patients for clinical trials. |
| NCT06463860 (2024, n = 81) | Skin | Diagnosis (Shared and Informed Decision Making) | DermDx uses DL to analyze dermoscopic images. |
| NCT05872503 (2024, n = 3) | Prostate | Detection (MRI Imaging) | AI processes MRI scans to detect prostate cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, H.; Mistry, S.; Jayam, K.V.; Shrestha, P.; Adkins, L.; Liang, M.; Fares, A.; Zarrinpar, A.; Braithwaite, D.; Karanth, S.D. Artificial Intelligence in Oncology: A 10-Year ClinicalTrials.gov-Based Analysis Across the Cancer Control Continuum. Cancers 2025, 17, 3537. https://doi.org/10.3390/cancers17213537
Verma H, Mistry S, Jayam KV, Shrestha P, Adkins L, Liang M, Fares A, Zarrinpar A, Braithwaite D, Karanth SD. Artificial Intelligence in Oncology: A 10-Year ClinicalTrials.gov-Based Analysis Across the Cancer Control Continuum. Cancers. 2025; 17(21):3537. https://doi.org/10.3390/cancers17213537
Chicago/Turabian StyleVerma, Himanshi, Shilpi Mistry, Krishna Vamsi Jayam, Pratibha Shrestha, Lauren Adkins, Muxuan Liang, Aline Fares, Ali Zarrinpar, Dejana Braithwaite, and Shama D. Karanth. 2025. "Artificial Intelligence in Oncology: A 10-Year ClinicalTrials.gov-Based Analysis Across the Cancer Control Continuum" Cancers 17, no. 21: 3537. https://doi.org/10.3390/cancers17213537
APA StyleVerma, H., Mistry, S., Jayam, K. V., Shrestha, P., Adkins, L., Liang, M., Fares, A., Zarrinpar, A., Braithwaite, D., & Karanth, S. D. (2025). Artificial Intelligence in Oncology: A 10-Year ClinicalTrials.gov-Based Analysis Across the Cancer Control Continuum. Cancers, 17(21), 3537. https://doi.org/10.3390/cancers17213537







