Simple Summary
WW domain-containing oxidoreductase (WWOX), a tumor suppressor, is downregulated in various tumor tissues. It is correlated with tumorigenesis, progression, treatment resistance, and clinical prognosis. For instance, the downregulation of WWOX expression is associated with increased tumor grade and aggressiveness, along with lower recurrence-free and overall survival rates in patients diagnosed with Basal-like and Triple-Negative Breast Cancer. This review provides a comprehensive overview of the structure, function, and role of WWOX in tumors, emphasizing its critical regulatory effects on cancer. Additionally, it discusses the potential applications and future prospects of WWOX as a tumor biomarker and therapeutic target.
Abstract
Discovered and cloned in 2000, the WW domain-containing oxidoreductase (WWOX) gene serves as a crucial tumor suppressor gene. Its expression is frequently downregulated in a wide spectrum of human malignancies, and this reduction is strongly correlated with accelerated tumor progression and poor patient prognosis. WWOX exerts its tumor-suppressive effects through direct physical interactions with numerous key signaling proteins. However, much of the current research remains in its early stages, particularly studies focusing on WWOX as a biomarker and WWOX-targeting therapies. Furthermore, there is a notable deficiency in related clinical validation, leading to uncertainties regarding clinical translation. This review specifically focuses on elucidating the significant contributions of WWOX in modulating critical oncogenic traits within cancer cells. We detail its impact on uncontrolled proliferation, invasive potential, metastatic spread, metabolic reprogramming that favors tumor growth, interactions with the immune response, and the maintenance of genetic stability. Following this exploration of WWOX’s diverse mechanistic roles in cancer biology, the review further discusses the emerging translational potential of targeting WWOX pathways, including its application as a prognostic biomarker and the development of strategies that exploit WWOX function or restoration for novel cancer therapeutics.
1. Introduction
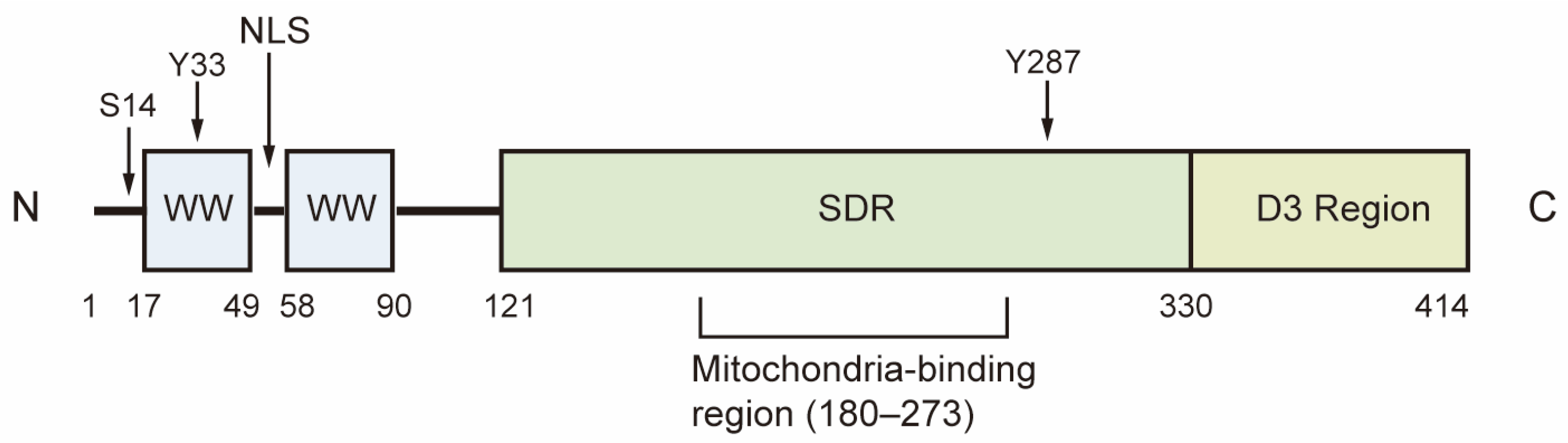
WW domain-containing oxidoreductase (WWOX) is a highly conserved candidate tumor suppressor, with a homology of 93.9% between human and mouse WWOX proteins. The WWOX gene is located in the region of human chromosome 16q23.3-24.1, also known as FRA16D, which is recognized as the second most common chromosomal fragile site. This region is particularly susceptible to chromosomal breakage and rearrangement, leading to copy number variations in both germ and somatic cells [1,2]. The WWOX gene encodes a 46 kDa protein composed of 414 amino acids, featuring two WW domains at the N-terminal, a nuclear localization signal situated between the WW domains, and a short-chain dehydrogenase/reductase (SDR) domain at the C-terminal [1]. The WW1 domain interacts with proteins that contain the PPxY or LPxY motif, while the WW2 domain functions as a chaperone protein, enhancing the binding affinity of WW1. The SDR domain, characterized by its NSYK motif for binding both estrogen and androgen, demonstrates oxidoreductase activity and plays a crucial role in sex-steroid metabolism (Figure 1) [1,3].
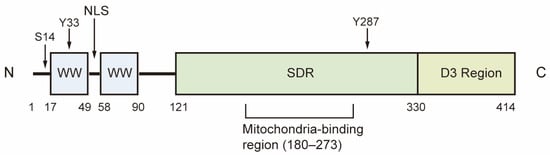
Figure 1.
Structure of WWOX. The first WW domain of WWOX is responsible for its physical interactions with proteins. The SDR domain demonstrates redox activity, while the D3 region at the C-terminal is associated with pro-apoptotic activity. Ser14, Tyr33 and Tyr287 are the phosphorylation sites of WWOX. S14: Ser14; Y33: Tyr33; Y287: Tyr287; NLS: Nuclear localization signal; SDR, short-chain dehydrogenase/reductase.
WWOX is expressed at varying levels across different tissues and cell types, with particularly high expression observed in epithelial and nerve cells [4,5,6]. A decrease in WWOX expression has been linked to tumorigenesis and disease progression. Human germline biallelic mutations in WWOX are associated with spinocerebellar ataxia, autosomal recessive-12 (SCAR12), and WWOX-related epileptic encephalopathy (WOREE) syndrome [7,8,9]. These two debilitating neurological disorders typically present as severe epileptic encephalopathy and significant global developmental delay. Moreover, Wwox-null mice display similar intractable epilepsy, ataxia, hypomyelination, growth retardation, and premature death [8,10,11]. These mice also exhibit severe metabolic defects, including hypoglycemia, hypoalbuminemia, and abnormalities in steroid synthesis and bone metabolism, leading to mortality at approximately four weeks of age due to developmental delays and metabolic disorders [8,12,13,14]. Additionally, Wwox−/− mice develop spontaneous tumors, with osteosarcoma detected histologically at postnatal days 3, 5, and 2.5 weeks, even in the absence of carcinogenic treatment. At an average age of 15 ± 1.5 months, Wwox+/− mice show a significantly higher tumor burden and incidence compared to wild-type (WT) mice. Furthermore, treatment with ethyl nitrosourea (ENU), a chemical mutagen, markedly increases the incidence of lung cancer and lymphoma in Wwox+/− mice [14]. Following ENU treatment, these mice develop tumors earlier than WT mice, with a significantly greater number of tumors observed per individual. Notably, WWOX exhibits loss of heterozygosity or reduced expression in tumors, which correlates with genomic instability, tumor progression, treatment resistance, and poor prognosis [2,14,15,16,17]. However, Watanabe et al. discovered that the levels of WWOX protein are elevated in non-invasive breast cancer and gastric cancer tissues, challenging the notion that WWOX functions as a classical tumor suppressor [5]. In contrast, WWOX is significantly downregulated during metastasis in these tissues. This tissue-specific heterogeneity in WWOX expression may be related to its functional roles across different types and subtypes of tumors or at various stages of tumor development, underscoring the necessity for further investigation. This article reviews the structure, function, and role of WWOX in tumors, while also discussing its potential applications and future prospects as a tumor biomarker and therapeutic target. Specifically, we summarize the effectors regulated by WWOX and their biological processes in tumors (Figure 2 and Table 1).
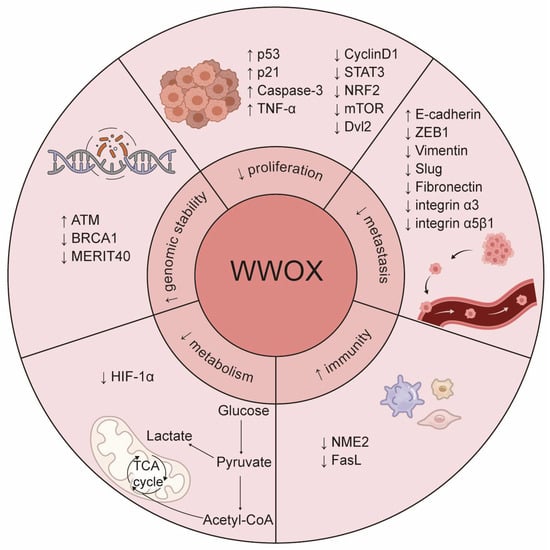
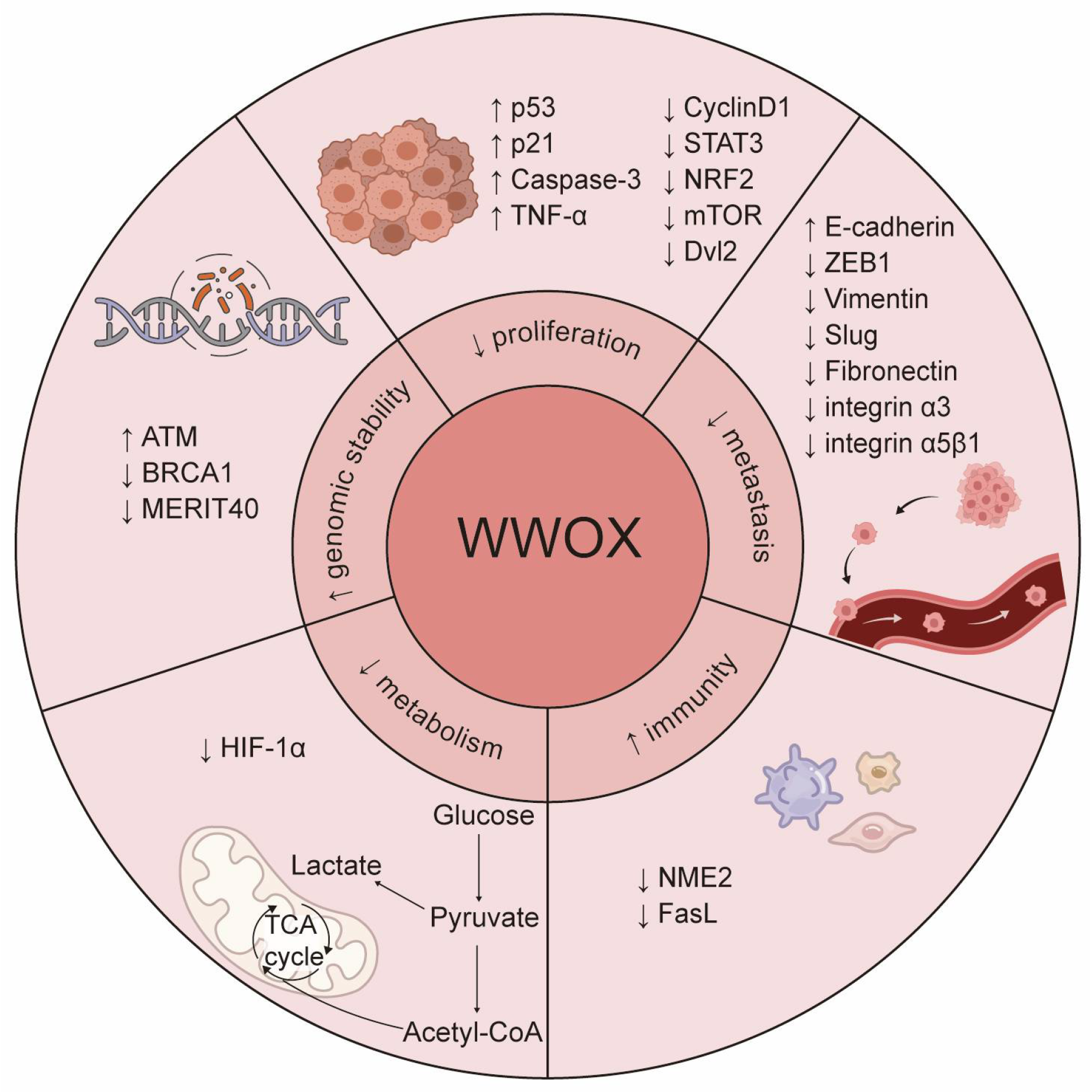
Figure 2.
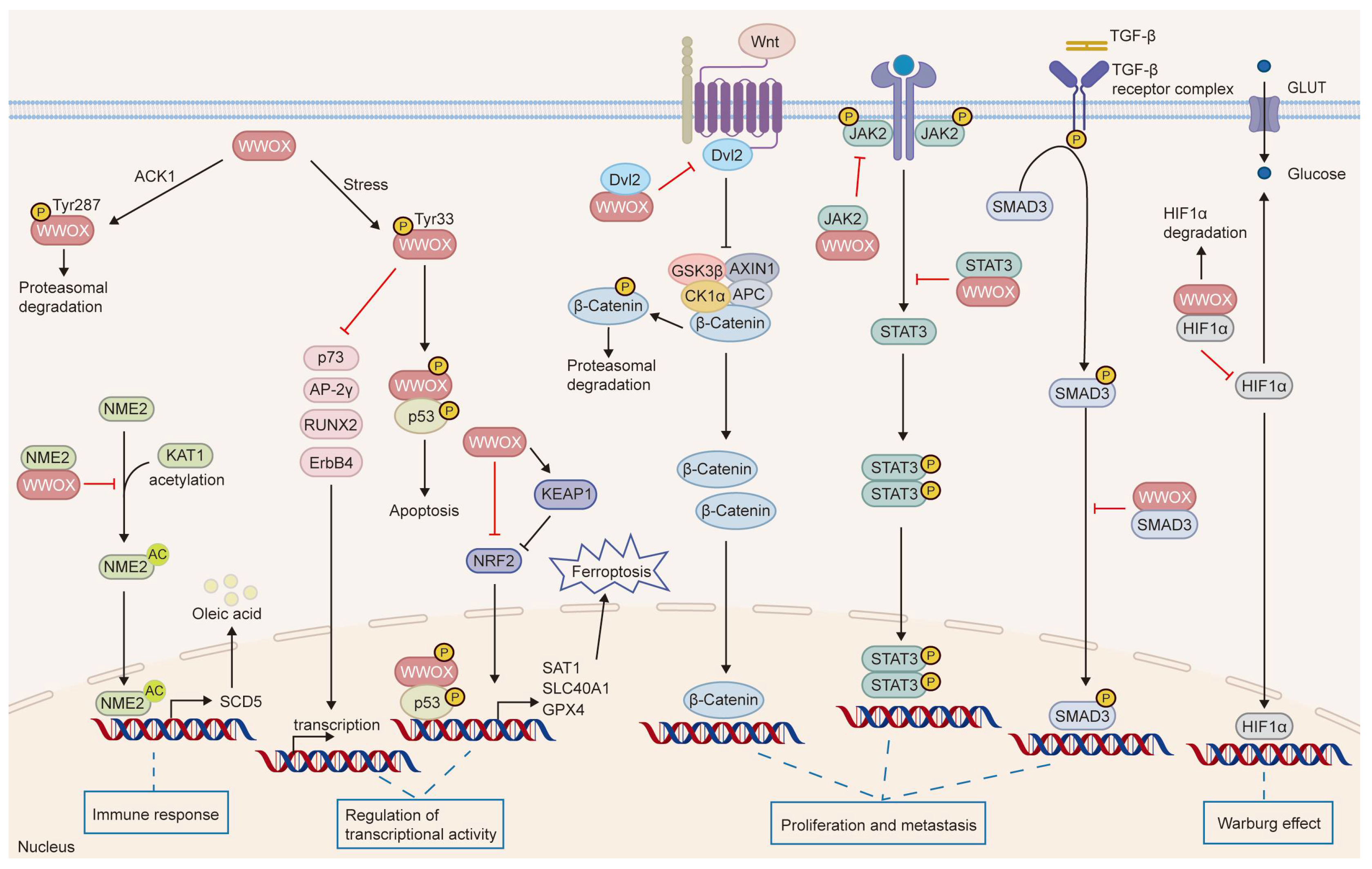
The roles of WWOX in cancer. WWOX functions as a tumor suppressor and critically regulates several cancer hallmarks, including the inhibition of proliferation and metastasis, prevention of immune evasion, regulation of metabolism, and maintenance of genomic stability. Specifically, WWOX modulates cell proliferation and survival by inhibiting the Wnt/β-catenin and JAK2/STAT3 signaling pathways. It activates the caspase cascade to induce apoptosis and inhibits metastasis by suppressing EMT. Furthermore, WWOX affects cell adhesion and migration by altering the expression of cell adhesion molecules, such as integrin α3 and integrin α5β1. Additionally, WWOX regulates the tumor microenvironment and supports the survival of immune cells, thereby preventing cancer cells from evading the immune system. It also regulates DNA repair pathways, contributing to genomic stability. Lastly, WWOX regulates tumor cell metabolism by modulating the metabolic reprogramming mediated by HIF-1α. The upward arrow signifies positive regulation, whereas the downward arrow denotes negative regulation.

Table 1.
Downstream effectors of WWOX.
2. Epigenetic Regulation and Post-Translational Modification of WWOX
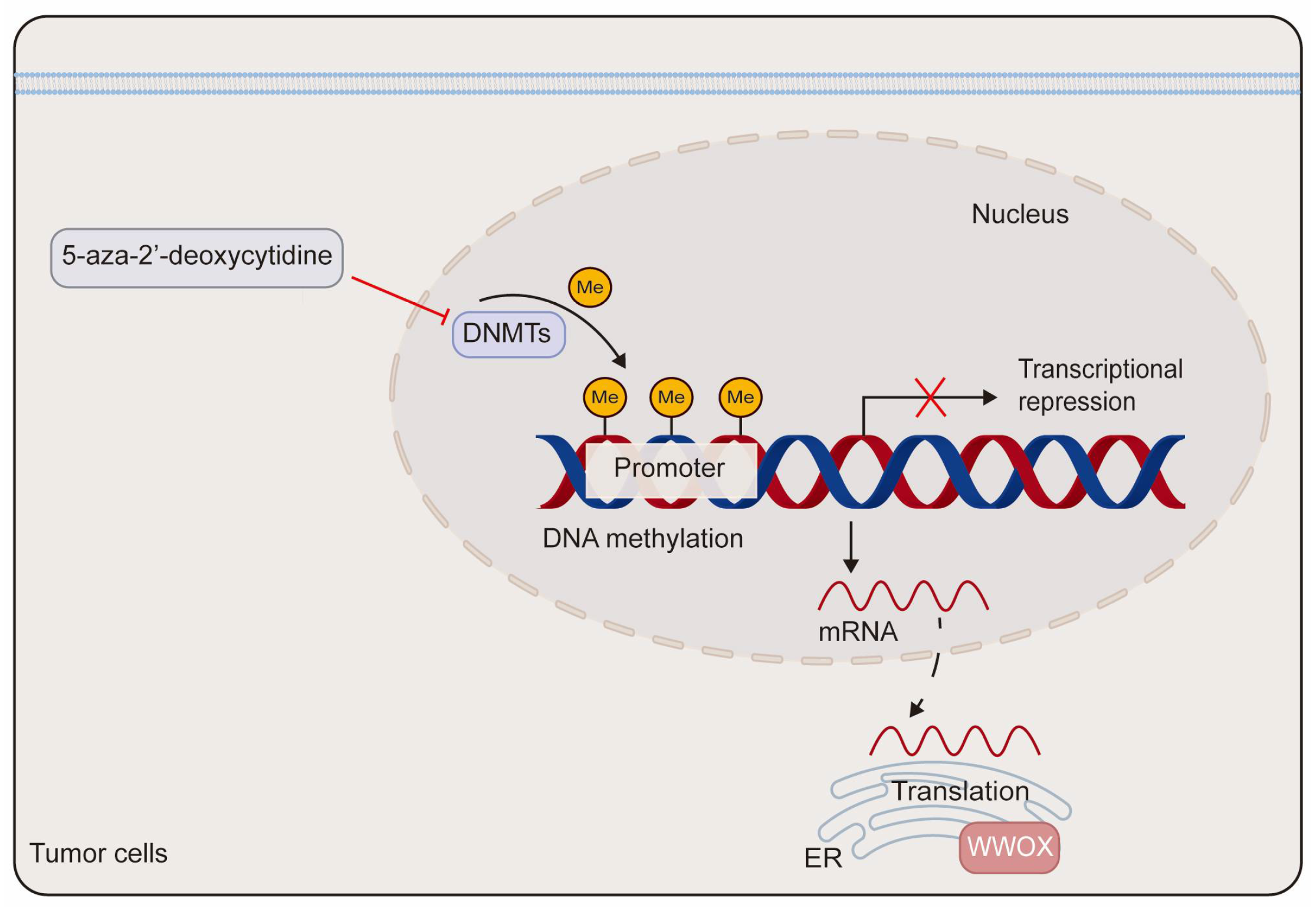
DNA methylation has been implicated in the transcriptional silencing of WWOX in tumors. Research indicates that site-specific promoter hypermethylation of the WWOX gene is observed in 2 out of 15 (13%) primary pancreatic adenocarcinoma patients and in 2 out of 9 (22%) pancreatic cancer cell lines. Notably, WWOX expression is significantly elevated in the pancreatic cancer cell line Hs766T following treatment with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine [54]. In comparison to adjacent non-neoplastic tissues, both the promoter and exon 1 regions of the WWOX gene exhibit hypermethylation in lung and breast cancers, which correlates with reduced WWOX expression [55]. Additionally, in prostate cancer-derived cells, DNA hypermethylation within the WWOX regulatory region contributes to the downregulation of WWOX expression. Treatment with either 5-aza-2′-deoxycytidine or trichostatin A, a histone deacetylase inhibitor, markedly enhances the expression of WWOX mRNA and protein in prostate cancer-derived cells (Figure 3) [23]. Furthermore, aberrant splicing of the WWOX gene contributes to the production of abnormal transcripts found in various cancers. This is supported by the identification of smaller spliced variants in breast and ovarian cancer cells, as well as by the high incidence of loss of heterozygosity and aberrant transcripts in esophageal, lung, and breast cancers [56,57].
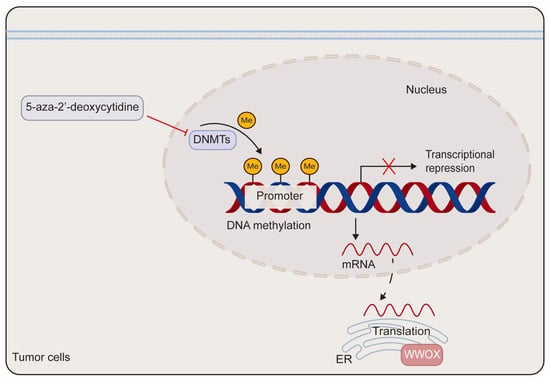
Figure 3.
The schematic diagram of the epigenetic regulation of WWOX. The methylation of the WWOX promoter leads to the silencing of WWOX expression. This transcriptional silencing can be reversed by the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine. DNMTs refer to DNA methyltransferases.
The subcellular localization of WWOX varies depending on cell types and exogenous stimuli. Endogenous WWOX is predominantly found in the mitochondria and nucleus across various cell lines, while ectopic WWOX has also been detected in the Golgi apparatus [2,5,6,58,59]. The phosphorylation of WWOX influences its subcellular localization and functional roles. Stress or apoptotic stimuli induce the phosphorylation of WWOX at Tyr33, leading to its nuclear translocation and the subsequent induction of cell death [20,21,58,59]. Additionally, WWOX binds to estrogen and androgen through its SDR domain. Sex hormones trigger the phosphorylation of WWOX at Tyr33 and its nuclear translocation, independent of estrogen and androgen receptors [6]. Notably, the phosphorylation of WWOX at Tyr33 is essential for promoting cell death. When WWOX transitions from Tyr33 phosphorylation to Ser14 phosphorylation, it facilitates tumor progression and the development of Alzheimer’s disease [60]. The zinc finger-like protein that regulates apoptosis (Zfra) is a natural protein and one of WWOX’s binding partners. The artificially synthesized polypeptides Zfra1-31 and Zfra4-10 inhibit the growth of various tumors [60,61]. The Zfra peptide suppresses pulmonary metastasis of U87-MG glioblastoma cells by inhibiting WWOX Ser14 phosphorylation [61]. Treatment with the Zfra peptide significantly reduces the expression of pS14-WWOX in the lungs and brains of mice, thereby preventing the pulmonary metastasis of B16F10 melanoma cells and mitigating melanoma-induced neurodegeneration in the hippocampus, as well as the formation of cortical plaques. However, the Zfra peptide does not inhibit WWOX Tyr33 phosphorylation [60]. Furthermore, the pS14-WWOX7-21 peptide, which mimics the functional characteristics of endogenous pS14-WWOX, promotes melanoma growth in vivo and significantly inhibits the proliferation and death of 4T1 breast cancer cell spheroids mediated by ceritinib in vitro [62].
The intracellular tyrosine kinase, activated Cdc42-associated kinase (ACK1), promotes tumor progression in prostate cancer and hepatocellular carcinoma (HCC) by negatively regulating WWOX [63,64]. Specifically, activated ACK1 phosphorylates WWOX at Tyr287, which facilitates the polyubiquitination of WWOX and its subsequent degradation. While it is established that ACK1 is crucial for the degradation of WWOX, the specific ubiquitination site responsible for WWOX degradation and the ubiquitin E3 ligase involved in this process remain unclear [63].
Through mass spectrometry and protein interaction analysis, it has been demonstrated that WWOX physically binds to the ubiquitin E3 ligase ITCH via the WW1 domain. ITCH mediates the Lys63 linked ubiquitination of WWOX, which stabilizes WWOX and promotes its nuclear translocation, thereby enhancing cell death [65]. In addition, ITCH mediates the ubiquitination of WWOX at Lys274, facilitating WWOX’s nuclear translocation and its involvement in DNA damage repair [15].
3. WWOX in Cell Proliferation and Survival
WWOX inhibits cell proliferation and promotes apoptosis through various pathways. In prostate cancer, WWOX mediates G1 cell cycle arrest by downregulating cyclin D1 expression, which in turn suppresses the proliferation of 22Rv1 prostate cancer cells and inhibits tumor growth in nude mice [18]. Furthermore, in ovarian cancer, WWOX influences the cell cycle of ovarian cancer stem cells and inhibits their proliferation by downregulating the expression levels of cyclins, specifically cyclin E-CDK2 and cyclin D1-CDK4 [66]. Similarly, the overexpression of WWOX in osteosarcoma significantly enhances the expression of p53 and p21 while inhibiting cyclin D1 expression [19].
In addition, WWOX functions as a pro-apoptotic protein that promotes tumor cell death. Research has shown that the ectopic expression of WWOX triggers apoptosis via a caspase-dependent pathway and inhibits the growth of prostate, lung, breast, cervical cancer, and pancreatic cancer both in vitro and in vivo [23,24,25,26,27,28]. Moreover, the forced expression of WWOX in SNU387 cells not only induces spontaneous apoptosis but also suppresses cell proliferation. The JNK inhibitor SP600129 enhances WWOX-induced apoptosis and works synergistically to inhibit HCC growth alongside WWOX [67]. Additionally, the assembly of the WWOX/TIAF1/p53 ternary complex is crucial for the suppression of tumor progression. This complex functions by inhibiting metastasis and anchorage-independent growth, blocking Smad4-dependent SMAD promoter transactivation, and inducing apoptosis [68,69].
Stress stimuli, including tumor necrosis factor, ultraviolet light, and staurosporine, induce the conserved phosphorylation of Tyr33 in the WW1 domain of WWOX (denoted as pY33-WWOX). This phosphorylated form of WWOX interacts with various proteins independently of polyproline [20]. Notably, pY33-WWOX binds to Ser46-phosphorylated p53, and this interaction synergistically stabilizes p53, promoting apoptosis [20,21]. Additionally, the tyrosine kinase SRC phosphorylates WWOX at Tyr33, which facilitates its binding to the PPPY487 motif of p73, thereby enhancing WWOX-mediated apoptosis [29].
WWOX binds proteins through its WW1 domain, regulating their localization, stability, and transactivation functions, and participates in various signaling pathways, modulating cellular and biological processes such as tumor suppression, metabolism, and growth and development [20,35,47,70,71]. Notably, WWOX serves as a novel negative regulator of the Wnt/β-catenin pathway. It physically interacts with dishevelled2 (Dvl2), sequestering Dvl2 in the cytoplasm, which inhibits its function in stabilizing β-catenin and diminishes the transcriptional activity of the Wnt/β-catenin pathway [35,36]. Furthermore, BCL9-2 functions as a coactivator of β-catenin. In the nucleus, WWOX binds to BCL9-2, thereby inhibiting the Wnt/β-catenin signaling pathway [39]. In the context of triple-negative breast cancer, WWOX inhibits STAT3-dependent tumor cell growth and metastasis by modulating the IL-6/JAK2/STAT3 signaling pathway, leading to the downregulation of p-STAT3 target genes [33]. Co-immunoprecipitation studies have demonstrated that the WW1 domain of WWOX directly targets JAK2, inhibiting STAT3 activation by preventing JAK2 phosphorylation and its binding to STAT3. Furthermore, the WW1 domain of WWOX can specifically recognize and interact with STAT3, thereby inhibiting STAT3-mediated transcriptional activity of the IL-6 promoter, which results in reduced IL-6 mRNA expression and production [33]. Similarly, in hepatocellular carcinoma (HCC), toosendanin (TSN), a novel agonist of WWOX, dose-dependently increases WWOX mRNA and protein expression and enhances its interaction with STAT3 and Dvl2, thereby inhibiting both the JAK2/STAT3 and Wnt/β-catenin signaling pathways [34]. Given the interplay between WWOX and oncogenic signaling pathways, the application of Wnt/β-catenin inhibitors in WWOX-deficient contexts may demonstrate preclinical efficacy. Moreover, the combination of FDA-approved JAK/STAT inhibitors with strategies aimed at restoring WWOX function presents a promising direction for translational research. Furthermore, a notable association between WWOX and COTE1 has been observed. Overexpression of COTE1 in Focus HCC cells significantly reduces the expression of Tyr33 WWOX and Ser46 p53, thereby suppressing Tyr33 WWOX/Ser46 p53-mediated cell death [72]. Moreover, WWOX induces ferroptosis in HCC by mediating p53 activation and inhibiting NF-E2-related factor 2 (NRF2) through protein–protein interactions [22].
WWOX functions as a tumor suppressor by interacting with transcription factors and sequestering them in the cytoplasm, thereby inhibiting their transactivation functions. Aqeilan et al. demonstrated that WWOX physically binds to the PPPYFPPPY64 motif of Activator Protein-2γ (AP-2γ) and the PPPY64 motif of c-Jun through its first WW domain, leading to its sequestration in the cytoplasm and subsequent inhibition of its transcriptional activity and transactivation function [40,41]. Furthermore, they found that the WW1 domain of WWOX competes with Yes-associated protein (YAP) for binding to the PPIY1037 and PPPY1285 motifs of Erythroblastic Leukemia Viral Oncogene Homolog 4 (ErbB-4), thereby inhibiting YAP-mediated transactivation of ErbB-4 [42]. In osteosarcoma, WWOX binds to RUNX2 via the WW1 domain, thereby inhibiting its transactivation ability [43,44], whereas in breast cancer, the same domain facilitates the cytoplasmic sequestration of SMAD3, which suppresses its transcriptional activity [45]. Additionally, studies have shown that WWOX engages with SMAD3 and BMP2 to regulate TGFβ/BMP signaling, suppressing epithelial–mesenchymal transition (EMT) and cancer stem cell phenotypes, thereby inhibiting the progression of pancreatic ductal adenocarcinoma [46].
4. WWOX in Cell Invasion and Migration
During tumor progression and metastasis, the abnormal activation of EMT facilitates tumor cell migration and invasion, increases tumor stemness, and enhances resistance to chemotherapy and immunotherapy [73]. Key transcription factors, including SNAIL1/2, ZEB1/2, and TWIST1/2, are responsible for inducing EMT. Following EMT activation, the expression of the epithelial marker E-cadherin is suppressed, while mesenchymal markers such as N-cadherin, vimentin, and fibronectin are upregulated [73].
WWOX has been shown to regulate cell phenotype and inhibit metastasis by modulating epidermal and mesenchymal markers. The overexpression of WWOX in MG63 osteosarcoma cells significantly increases E-cadherin levels while reducing the expression of ZEB1, vimentin, and Slug, ultimately leading to the inhibition of cell proliferation, invasion, and migration [19]. Conversely, the loss of WWOX in HCC70 breast cancer cells results in decreased E-cadherin levels and increased fibronectin expression [37]. In MDA-MB-435s, HCC1806, and MDA-MB-231 cells, the restoration of WWOX elevates miR-146a expression, which directly targets the 3′-untranslated region (UTR) of fibronectin to inhibit its expression. The overexpression of WWOX in MDA-MB-231 cells significantly suppresses lung metastasis and colonization in immunocompromised mice, while also inhibiting fibronectin expression in vivo. Similarly, analysis of clinical data from The Cancer Genome Atlas-Breast Invasive Carcinoma (TCGA-BRCA) cohort reveals a positive correlation between WWOX expression and E-cadherin levels, alongside a negative trend with fibronectin levels in TNBC samples. Moreover, in MDA-MB-231 cells, doxycycline-induced WWOX expression leads to decreased levels of MYC and fibronectin. WWOX inhibits MYC activity by binding to MYC on chromatin, mitigating MYC’s suppression of miR-146a, which in turn inhibits fibronectin expression, EMT, tumor invasion, and metastasis [37]. Furthermore, miR-146a targets the 3′-UTR of SMAD3, resulting in reduced SMAD3 expression and enhancing WWOX-mediated suppression of metastasis [36]. The classical Wnt signaling pathway orchestrates EMT, with Dvls serving as key molecules within this pathway. Phosphorylated Dvls recruit Axin to dismantle the β-catenin degradation complex, facilitating β-catenin stabilization and subsequent nuclear translocation. WWOX inhibits the Wnt/β-catenin signaling pathway and suppresses EMT by physically interacting with Dvl2 [36].
WWOX inhibits the tumorigenicity and metastasis of tumor cells by regulating their interaction with the extracellular matrix. As adhesion molecules, integrins facilitate the local peritoneal dissemination of ovarian cancer. Fibronectin, a primary ligand for integrin family members, is an extracellular matrix component that facilitates peritoneal metastasis. WWOX transfection significantly decreases the expression of membranous integrin α3 in PEO1 cells, as well as membranous integrin α3 and integrin α5β1 in SKOV3 cells, thereby inhibiting the adhesion of ovarian cancer cells to fibronectin and their migration [38]. Furthermore, WWOX expression promotes apoptosis in ovarian cancer cells cultured in suspension in vitro [38]. Additionally, WWOX regulates the migration of breast cancer and melanoma cells in vitro and inhibits metastasis in vivo by targeting p-STAT3 [33].
5. WWOX in the Immune Response
HCC is characterized by a high recurrence rate and poor prognosis. Immune checkpoint inhibitors (ICIs) have emerged as a significant treatment option for HCC. Research has demonstrated that WWOX deficiency induces an immunosuppressive tumor microenvironment, resulting in HCC resistance to PD-1 treatment. Liu et al. evaluated eight HCC tissues using cytometry by time-of-flight (CyTOF) analysis. The results shows that M2 macrophage markers, such as CD68 and CD204, are elevated in CD45+ cells of the WWOXlow subgroup, whereas no significant differences are observed in CD8+ T-cell associated markers, such as PD-L1, Granzyme B and PD-1. Notably, increased macrophage infiltration and decreased CD8+ T-cell infiltration have been observed in HCC tissues from patients deficient in WWOX [53]. Furthermore, higher levels of WWOX have been detected in the serum of patients who responded favorably to PD-1 antibody treatment. Immunohistochemical (IHC) results from 176 HCC patients indicate that WWOX is significantly upregulated in immune-responsive HCC tissues, with WWOX expression negatively correlated with CD68 and CD206 expression [53]. Prognostic analysis reveals that HCC patients with WWOXlowCD68high and WWOXlowCD206high tumors exhibit the poorest OS, and WWOXlowCD206high is an independent prognostic factor. In a WWOXlow humanized orthotopic HCC mouse model, PD-1 treatment significantly inhibited tumor growth in control mice, but not in the Huh7-shWWOX group. Additionally, CD8+ T-cell infiltration is reduced in the tumor tissues of Huh7-shWWOX mice [53]. Co-culturing WWOXlow HCC cells (HCCLM3-Vector and Huh7-shWWOX) with macrophages enhances macrophage recruitment, leading to significant upregulation of M2-like macrophage markers such as CD163, MRC1, IL-10, TGF-β, and ARG-1, along with increased CD206 protein expression. Oleic acid (OA), a product of fatty acid synthesis, promotes tumor progression by inducing the polarization of M2-like macrophages [53]. SCD5, a key enzyme in OA metabolism, shows upregulated protein and mRNA levels in Huh7-shWWOX cells, accompanied by increased OA concentration in the cell culture supernatant. Mechanistically, WWOX and KAT1 competitively interact with NME2 to inhibit its acetylation, thereby promoting NME2 binding to the SCD5 promoter and inhibiting SCD5 transcription, which in turn suppresses M2 macrophage polarization mediated by the SCD5-OA axis [53].
In addition, it was observed that the overexpression of WWOX in U251 human glioma cells significantly reduces the levels of FasL and TGF-β, promotes the proliferation of co-cultured Jurkat T cells, and decreases Jurkat T cell apoptosis. Conversely, WWOX deficiency impairs immune responses by inducing apoptotic signals mediated by Fas/FasL [30].
6. WWOX in Metabolism
WWOX is a key regulator of glucose metabolism. Studies in Drosophila have shown that WWOX binds to various proteins involved in metabolic processes, including isocitrate dehydrogenase, malate dehydrogenase, and Cu-Zn superoxide dismutase [74]. Notably, WWOX is not only crucial for glucose metabolism regulation but also exhibits expression changes in response to metabolic fluctuations. During the transition from glycolysis to oxidative phosphorylation, there is an increase in the steady-state levels of WWOX mRNA; conversely, under hypoxic conditions where cellular metabolism relies predominantly on glycolysis, WWOX mRNA levels decrease [75,76]. Metabolic reprogramming is a hallmark of cancer, with aerobic glycolysis representing the classical phenotype associated with this process. This phenotype is characterized by heightened glucose uptake and increased lactate production [77]. HIF-1α facilitates glycolysis by upregulating glucose transporter 1 (GLUT1) and transactivating glycolytic genes such as pyruvate kinase M2 (PKM2) and hexokinase 2 (HK2), thereby enhancing glucose uptake and utilization by cells [78].
Under normoxic conditions, mouse embryonic fibroblasts (MEFs) with a specific knockout of Wwox exhibit increased glucose uptake, elevated expression of HIF-1α, enhanced transcription of glycolytic genes, inhibition of the tricarboxylic acid cycle, decreased mitochondrial respiration, and increased glycolysis [47]. H-RAS transformed Wwox KO MEFs demonstrate greater tumorigenicity, characterized by higher levels of the HIF-1α target gene GLUT1 and augmented aerobic glycolysis compared to WT MEFs. The knockdown of WWOX in MCF7 breast cancer cells leads to the upregulation of HIF-1α glycolysis genes. Furthermore, the overexpression of WWOX in WWOX-depleted MCF7 cells suppresses the expression of HIF-1α target genes. The direct binding of the WWOX WW1 domain to HIF-1α impairs its transcriptional activity, consequently inhibiting its transactivation function. Additionally, WWOX facilitates HIF-1α hydroxylation and promotes its degradation. Wwox expression is downregulated in Wwox WT MEFs under hypoxic conditions or upon treatment with CoCl2. Overexpression of WWOX in Wwox WT MEFs under hypoxia leads to reduced expression of HIF-1α glycolysis target genes. Inhibition of HIF-1α expression using digoxin in Wwox KO MEFs reverses the abnormal increase in glucose uptake and suppresses the expression of HIF-1α target genes [47].
In addition, hepatocyte-specific Wwox knockout (WwoxΔHep) mice exhibit elevated levels of Ki67 and hepatoma-related proliferation genes, including c-Myc, c-Jun, c-Fos and Axin in liver tissues following N-nitrosodiethylamine (DEN) treatment [48]. WwoxΔHep mice demonstrate a higher incidence of HCC after DEN treatment compared to control mice. After 10 months of DEN treatment, WwoxΔHep mice show a significant increase in HIF-1α protein levels and activity, alongside notable upregulation of PKM2 and GLUT1 protein expression [48]. Digoxin has been shown to inhibit HIF-1α transactivation, thereby suppressing tumor growth [79,80]. Following 6 months of DEN treatment, WwoxΔHep mice were administered digoxin for an additional 8 months. Post-digoxin treatment, the expression of HIF-1α glycolysis target genes is downregulated, and liver tumor growth is significantly inhibited in DEN-WwoxΔHep mice compared to those treated with saline [48]. These findings suggest that WWOX regulates HIF-1α-mediated metabolic reprogramming, thus suppressing tumor cell survival and growth. Importantly, either genetic or pharmacological depletion of HIF-1α can reverse the phenotypes associated with WWOX deficiency. The use of metabolic inhibitors, including HIF-1α inhibitors, digoxin, or GLUT1 inhibitors, may offer potential therapeutic strategies for treating WWOX-deficient tumors.
7. WWOX in Genomic Stability
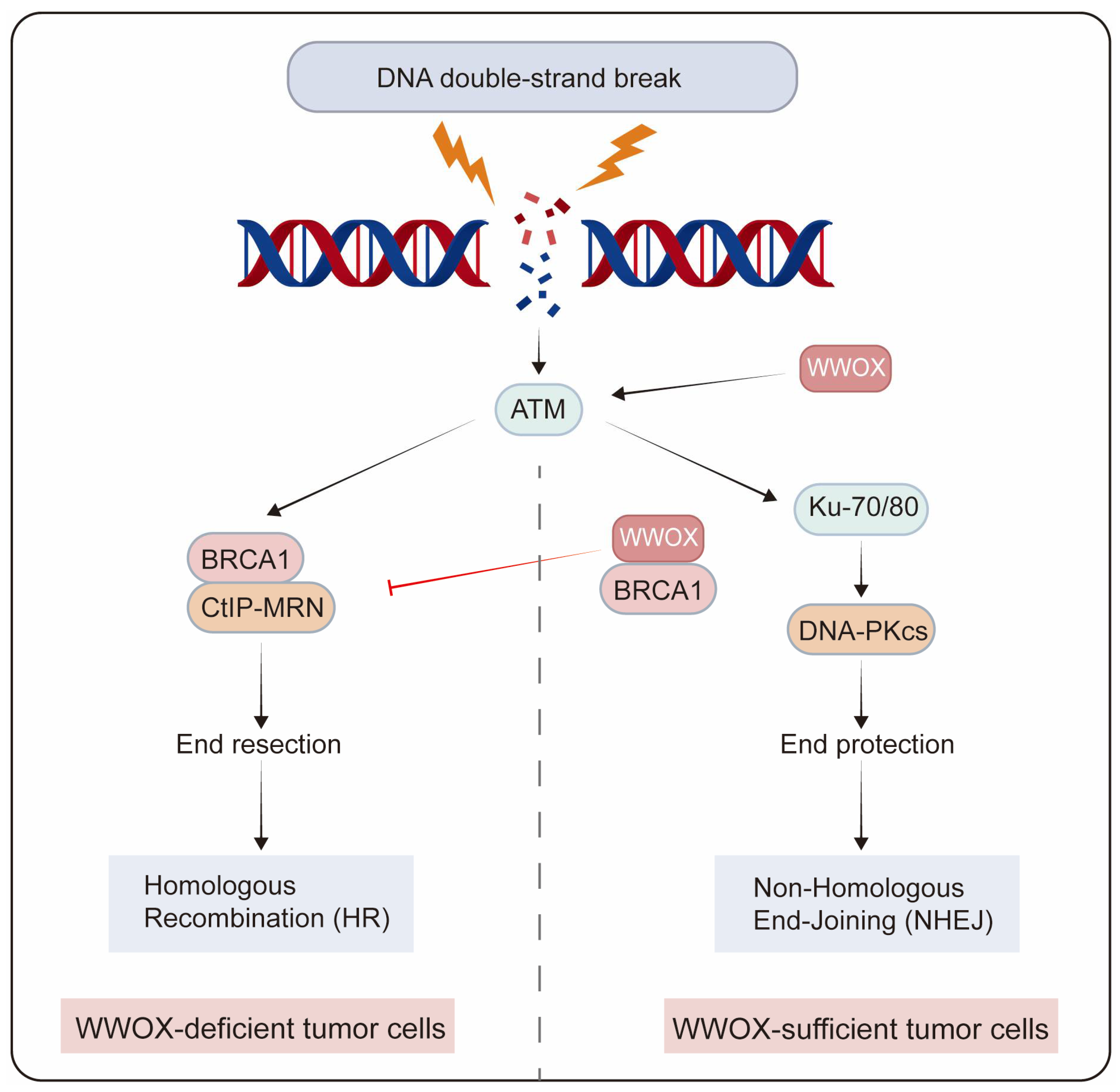
Genome integrity is crucial for normal cellular physiology. Following DNA damage, the activated DNA damage response plays a crucial role in maintaining genomic stability during replication by preventing potentially harmful mutations. DNA double-strand breaks (DSBs) represent one of the most severe types of DNA damage, which can result in chromosomal abnormalities. The two primary pathways for DSB repair are non-homologous end joining (NHEJ) and homologous recombination (HR) [81]. NHEJ directly rejoins the ends of DSBs, independent of sequence homology, while HR predominantly occurs during the S and G2 phases of the cell cycle, utilizing sister chromatids as templates for DSB repair. In the MCF7 breast cancer cell line, depletion of WWOX leads to an increase in DSBs following DNA damage induction, and the loss of the WWOX gene product results in genomic instability post-DNA damage. The activation of the DNA damage checkpoint kinase ataxia telangiectasia-mutated (ATM) is delayed in WWOX-deficient cells after DNA damage [15]. Moreover, WWOX-deficient breast cancer cells exhibit enhanced HR and reduced NHEJ repair, resulting in resistance to cisplatin and radiotherapy. BRCA1 is a pivotal factor in DNA end resection, a major determinant of HR. WWOX inhibits HR by interacting with Brca1 and promotes NHEJ repair [49]. Premature resection at DSBs causes dysregulated HR repair that is not confined to the S/G2 phases of the cell cycle, thereby increasing genomic mutations in cell populations resistant to IR and platinum treatment. Conversely, in MDA-MB231 cells that overexpress WWOX, the interaction between WWOX and BRCA1 predominantly facilitates the repair of DSBs through the NHEJ pathway [50]. Additionally, WWOX overexpression enhances NHEJ repair in BRCA1 wild-type TNBC cells (Figure 4) [51].
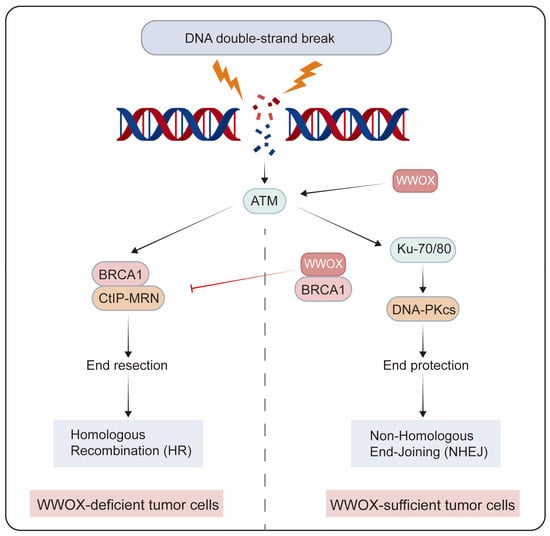
Figure 4.
The role of WWOX in determining the choice of DSB repair pathways in tumor cells. WWOX-deficient breast cancer cells exhibit enhanced HR and reduced NHEJ repair. In contrast, in breast cancer cells with sufficient WWOX, the interaction between WWOX and BRCA1 inhibits HR while promoting NHEJ repair.
Low expression of WWOX is associated with poor response and shorter overall survival in cancer patients undergoing DNA damage induction therapy, such as radiotherapy or platinum-based treatments. WWOX expression may serve as a predictive biomarker for the efficacy of DNA damage induction therapy. The Mediator of RAP80 Interaction and Targeting 40 kDa protein (MERIT40) acts as an activator of HR and plays a crucial role in the DNA damage response (DDR). Hyperactivity of HR can lead to genomic instability, which is a contributing factor to cancer development. WWOX mitigates the excessive HR activity induced by MERIT40 overexpression by binding to the TBM2 motif of MERIT40, thereby hindering its interaction with Tankyrase [52]. In addition, WWOX promotes cellular senescence and maintains genome integrity in mouse embryonic fibroblasts (MEFs) by inhibiting the excessive production of reactive oxygen species (ROS). Wwox knockout MEFs exhibit reduced expression of p16Ink4a and p21Cip1/Waf1 during serial passage in culture, leading to an escape from cellular senescence and increased genomic instability [82]. In summary, WWOX is pivotal in the selection of DNA repair pathways and the maintenance of genomic stability.
8. Therapeutic Potential of WWOX in Cancer
8.1. WWOX as a Potential Tumor Biomarker
WWOX genetic variants may serve as independent predictors of survival across multiple cancers. Low expression or deletion of WWOX, as well as copy number variation, have been associated with increased invasiveness and poor prognosis in various tumors, including lung cancer [83,84], breast cancer [33,85,86], HCC [87], gastric cancer [88], ovarian cancer [89], intrahepatic cholangiocarcinoma [90] and clear cell renal cell carcinoma [91] (Table 2). Research indicates that the depletion of Wwox and p53 in mouse osteoblast progenitors accelerates osteosarcoma progression, resulting in a more aggressive form of the disease compared to p53 knockout alone [92]. The loss of WWOX during the early stages of tumorigenesis promotes osteosarcomagenesis. Bone marrow cells from young non-osteosarcoma mice with a combined knockout of Wwox and Trp53 exhibit significantly upregulated expression of Myc and its target MCM7, compared to bone marrow cells with Trp53 knockout alone, demonstrating tumorigenicity both in vivo and in vitro [93].

Table 2.
WWOX is a biomarker for prognostication and predicting drug sensitivity in various types of cancer.
Studies have demonstrated that breast cancer patients exhibiting low levels of WWOX expression tend to have a higher tumor grade, an increased risk of distant metastasis, and shorter recurrence-free survival and overall survival rates. This suggests that WWOX expression levels may serve as a predictive marker for the malignant progression of breast cancer [33]. Furthermore, while WWOX expression is generally low in HCC, it is significantly upregulated in the serum and tissues of HCC patients who respond immunologically to PD-1 treatment. WWOX deficiency promotes an immunosuppressive tumor microenvironment in HCC, thereby contributing to resistance to ICIs. Consequently, WWOX holds promise as a predictive biomarker for the efficacy of ICIs [53].
In addition, WWOX polymorphisms are associated with postoperative recurrence and tumor aggressiveness in HCC and prostate cancer [94,95]. Specifically, WWOX rs11644322 serves as a primary predictor for pancreatic cancer treated with gemcitabine, where the G > A single nucleotide polymorphism (SNP) in WWOX rs11644322 correlates with a poorer prognosis in pancreatic cancer patients [96]. Recently, Cheng et al. discovered that the G > T change at rs9922483 is associated with an elevated risk of mortality in gastric cancer patients, as evidenced by a genome-wide association study involving 796 patients in the eastern Chinese population [97]. The rs9922483 locus may interact long-range with the WWOX promoter, and the G > T alteration inhibits the transcriptional activity of this promoter. The T allele decreases the binding affinity of NR3C1, resulting in reduced WWOX expression, which subsequently lowers the survival rate of gastric cancer patients [97].
8.2. WWOX in Chemoresistance
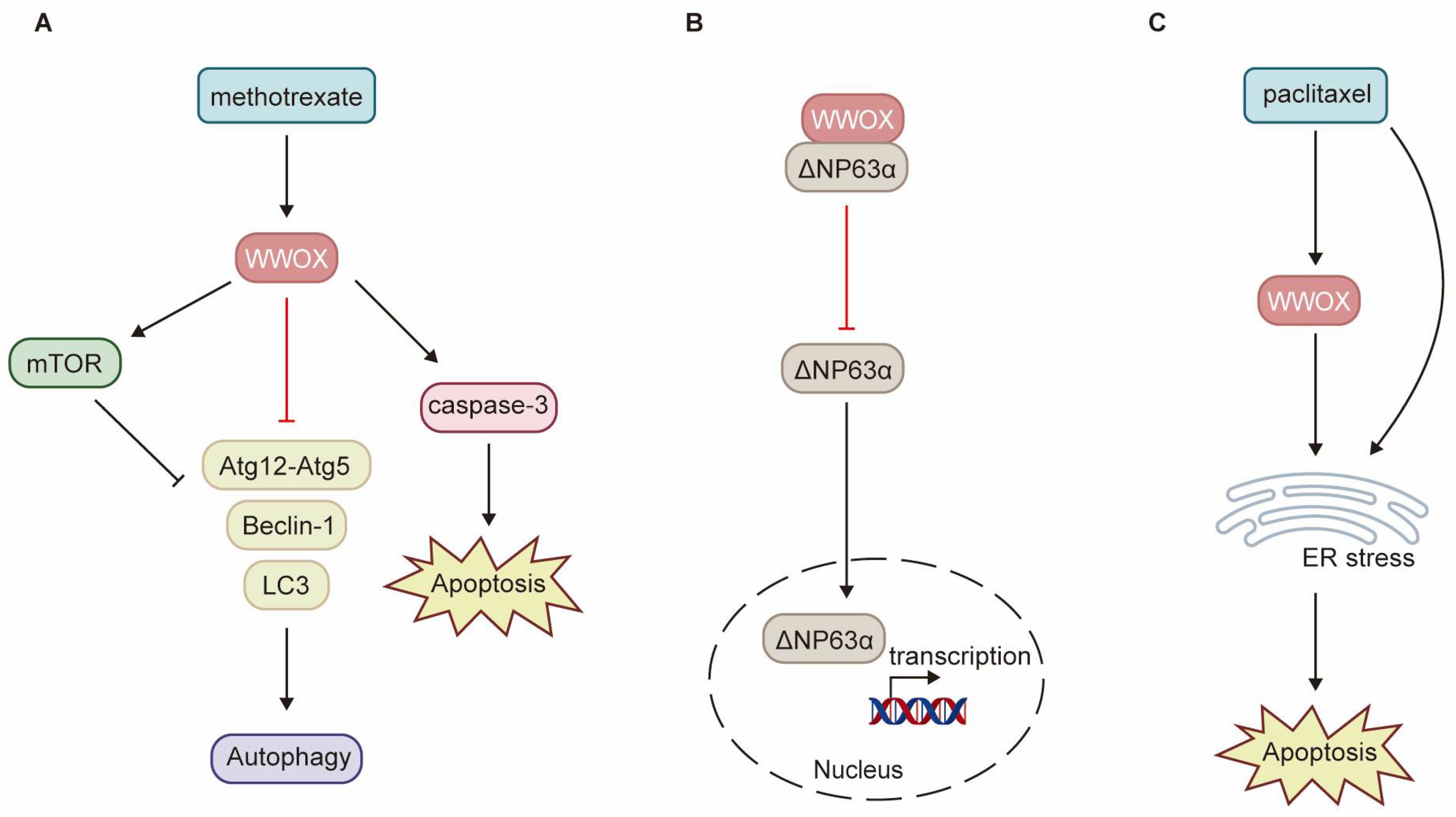
WWOX enhances the sensitivity of cancer cells to chemotherapy through various pathways. Low levels of WWOX expression are frequently associated with tumor chemoresistance. Research has demonstrated that methotrexate (MTX) significantly upregulates WWOX protein expression, activates caspase-3, and induces apoptosis in squamous cell carcinoma (SCC) tumor tissues and SCC-4 and SCC-15 cell lines [32]. The induction of WWOX expression by MTX treatment is crucial for determining the sensitivity of SCC to MTX-induced apoptosis. Consequently, SCC-9 cells exhibit resistance to MTX due to the inability of MTX treatment to induce WWOX expression. By interacting with mTOR, WWOX enhances the MTX-induced phosphorylation of mTOR and p70S6K, inhibits the expression of autophagy-related proteins such as Beclin-1, Atg12-Atg5, and LC3-Ⅱ, and promotes MTX-induced apoptosis (Figure 5A) [32]. ΔNp63α, the most prevalent isoform of p63, can induce chemoresistance in tumor cells through multiple pathways. WWOX inhibits ITCH-mediated ubiquitination and degradation of ΔNp63α, thereby increasing its protein stability. Additionally, WWOX partners with ΔNp63α via the WW1 domain, sequestering ΔNp63α in the cytoplasm and inhibiting its transactivation function. Overexpression of WWOX has been shown to inhibit ΔNp63α-mediated resistance to cisplatin in SaOS2 osteosarcoma cells (Figure 5B) [98]. Paclitaxel exerts its antitumor effects by disrupting mitosis and activating endoplasmic reticulum (ER) stress. Notably, WWOX expression significantly enhances paclitaxel-mediated cell death in epithelial ovarian cancer (EOC) cells [16]. WWOX sensitizes EOC to paclitaxel through ER stress-induced apoptosis (Figure 5C). Furthermore, high WWOX expression is associated with longer overall survival and progression-free survival in ovarian cancer patients treated with paclitaxel [16]. However, there is currently a notable absence of clinical trials to validate these findings.
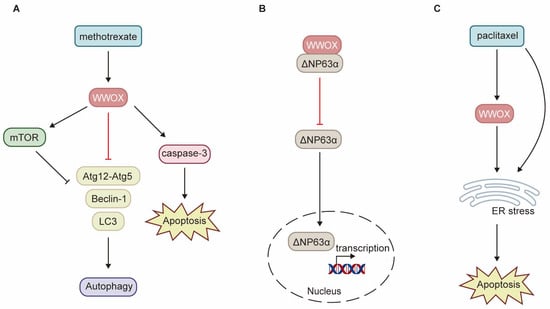
Figure 5.
Schematic diagram illustrating the role of WWOX in chemoresistance. (A) In SCC, WWOX enhances the inhibition of autophagy mediated by mTOR, as well as apoptosis induced by methotrexate. (B) In osteosarcoma, WWOX mitigates chemoresistance mediated by ΔNp63α through direct interaction with ΔNp63α, effectively sequestering it within the cytoplasm. (C) WWOX facilitates apoptosis induced by ER stress, thereby augmenting cell death mediated by paclitaxel. ER refers to the endoplasmic reticulum.
8.3. Compounds Related to WWOX
Given the high costs and elevated failure rates associated with new drug development, drug repurposing and the identification of new indications for existing drugs have emerged as promising strategies in the field of anti-tumor drug development. Recent studies on colorectal cancer have demonstrated that aspirin exerts an anti-tumor metastatic effect by inhibiting the TXA2-ARHGEF1 signaling pathway and enhancing T cell anti-tumor immunity [99]. Similarly, albendazole (ABZ), an anthelmintic agent, has been shown to dose-dependently upregulate WWOX expression and strengthen its interaction with p53 and C-MYC in HCC. This interaction leads to cell cycle arrest, induces apoptosis, and inhibits cell migration, indicating that ABZ may serve as a potential WWOX-targeted therapeutic agent for HCC [100].
Evodiamine and Toosendanin (TSN), both traditional Chinese medicinal compounds, have been identified as potential targeted agonists of WWOX. These compounds exert anti-tumor effects by dose-dependently upregulating the expression of WWOX [34,101,102]. Research indicates that TSN exhibits a strong binding affinity for WWOX. Both TSN alone and in combination with regorafenib can significantly enhance WWOX levels in vitro and in vivo, thereby suppressing the proliferation and migration of HCC cells [34,102].
In addition, WWOX regulates the ferroptosis induced by TSN. The activation of spermidine/spermine N1-acetyltransferase 1 (SAT1) facilitates ferroptosis by enhancing polyamine metabolism [103]. TSN markedly upregulates the protein expression of phosphorylated WWOX (p-WWOX) and phosphorylated p53 (p-p53) in MHCC-97L and HepG2 cells, enhancing the interaction between p-WWOX and p-p53 within the nucleus. This interaction leads to the upregulation of SAT1 gene expression while downregulating SLC40A1 gene expression [22]. Additionally, NF-E2-related factor 2 (NRF2) serves as the primary antioxidant transcription factor in the cellular response to oxidative stress, promoting the transcription of various protective target genes, including FPN1 and glutathione peroxidase 4 (GPX4) [104,105]. TSN enhances the interaction between WWOX and NRF2 as well as KEAP1 in MHCC-97L and HepG2 cells. The overexpression of WWOX leads to an increase in KEAP1 expression, inhibition of NRF2 expression and transcriptional activity, and subsequent downregulation of its downstream targets, FPN1 and GPX4 [22]. In summary, TSN modulates the transcriptional activity of p53 and NRF2 by regulating their interactions with WWOX, thereby influencing the expression of downstream ferroptosis-related target genes.
8.4. Other WWOX Modulators
The hydrogel drug delivery system exhibits excellent biocompatibility, stability, and sustained release capabilities, making it suitable for long-term and targeted treatment of tumors. Liu et al. transfected AY-27 bladder cancer cells with lentivirus (LV) to overexpress WWOX (LV-WWOX) and subsequently encapsulated this construct in gelatin hydrogel to create LV-WWOX-hydrogel (H-LV-WWOX) [31]. Research has demonstrated that lentivirus-mediated overexpression of WWOX significantly inhibits bladder tumor growth both in vitro and in vivo [31,106]. AY-27 cells treated with H-LV-WWOX and LV-WWOX show a marked reduction in cell viability, a significant increase in caspase-3 activity and expression levels, and elevated TNF-α expression [31]. In a rat orthotopic bladder cancer model (F344/AY-27), intravesical instillation of H-LV-WWOX promotes the generation of ROS, enhances caspase-3 activity, and increases TNF-α-mediated cell death [31]. The gene therapy mediated by the H-LV-WWOX drug delivery system achieves sustained release, high transduction efficiency, and reduced systemic toxicity, thereby offering a more effective and targeted therapeutic strategy for WWOX-based tumor treatment.
Chang et al. synthesized the WWOX7-21 and WWOX7-11 peptides to replicate the tumor-suppressive role of endogenous WWOX by mimicking its three-dimensional structures and generating specific antibodies [61,62]. Tail vein injections of either the WWOX7-21 or WWOX7-11 peptide significantly inhibited the growth and metastasis of melanoma cells in BALB/c mice. Furthermore, the WWOX7-21 peptide markedly enhanced ceritinib-mediated death in 4T1 breast cancer stem cell spheroids [62]. This study further validated the critical role of WWOX in inhibiting tumor growth and metastasis, indicating that both the WWOX7-21 and WWOX7-11 peptides hold promise for tumor inhibition in vivo. However, these studies are still in the early preclinical phase. The development of peptide-based drugs encounters several practical challenges, including poor stability, short half-life, and a protracted clinical development process. Transitioning from laboratory research to clinical application continues to pose significant challenges.
RNA-based therapies represent a novel approach to tumor treatment. Certain endogenous non-coding RNAs (ncRNAs) are capable of regulating the expression of tumor-related genes and are implicated in tumorigenesis and progression. Targeting these ncRNAs for the development of anti-tumor drugs shows significant promise [107]. Research has indicated that exosomal miR-625-3p, secreted by cancer-associated fibroblasts, promotes the proliferation, invasion, and EMT of CRC cells, inhibits apoptosis, and enhances chemotherapy resistance by downregulating CELF2/WWOX. Inhibition of miR-625-3p expression markedly reduces the malignant behaviors of CRC cells [108]. Moreover, miR-134 is significantly upregulated in head and neck squamous cell carcinoma (HNSCC) tissues, and its expression is negatively correlated with WWOX levels [109]. Reporter assays have demonstrated that in OECM-1 and SAS HNSCC cell lines, ectopically expressed miR-134 directly targets the 3′-UTR of WWOX, thereby inhibiting WWOX reporter activity and promoting cell proliferation, migration, and invasion. Furthermore, ectopic expression of WWOX significantly suppresses the invasion induced by miR-134 in HNSCC cells [109]. Additionally, long non-coding RNA WWOX-AS1 is downregulated in HCC and osteosarcoma patient tumor tissues and cell lines, with its overexpression significantly inhibiting the progression of both HCC and osteosarcoma [110,111]. Furthermore, WWOX-AS1 overexpression notably inhibits the proliferation, migration, and EMT of HepG2 and Huh7 cells, acting as an RNA sponge for miR-20b-5p, which in turn upregulates WWOX expression by sequestering miR-20b-5p [110]. Therefore, ncRNA-based therapies present promising strategies for tumor treatment by directly or indirectly enhancing WWOX expression, thereby inhibiting tumor progression.
9. Conclusions and Perspectives
WWOX is downregulated in various tumor tissues and is associated with tumorigenesis, progression, treatment resistance, and clinical prognosis, indicating its potential as a novel biomarker. As a tumor suppressor, WWOX regulates several critical processes in cancer, including cell proliferation, apoptosis, invasion, migration, genomic instability, metabolic reprogramming, and tumor immunity (Figure 6). Furthermore, WWOX is recognized as a systemic transcriptional regulator that engages in multiple cellular signaling pathways through protein–protein interactions. Notably, alterations in WWOX phosphorylation sites contribute to its diverse roles in tumors. Therefore, further investigation into the mechanisms by which WWOX operates in different tumor cell types is essential.
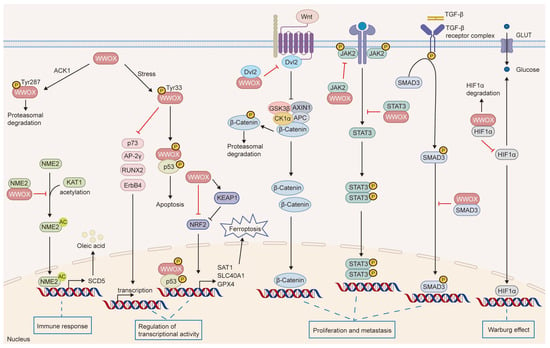
Figure 6.
The signaling pathways influenced by WWOX. WWOX modulates several cellular pathways, including JAK2/STAT3, Wnt/β-catenin, TGFβ, and KEAP1/NRF2. It interacts with HIF-1α to inhibit its transactivation function. Furthermore, WWOX binds to p73, AP-2γ, RUNX2, and ErbB4, sequestering them in the cytoplasm, which inhibits their transcriptional and transactivation functions. Additionally, WWOX binds to NME2, inhibiting its acetylation and the transcription of SCD5, thereby suppressing M2 macrophage polarization mediated by the SCD5-OA axis. Moreover, the interaction between phosphorylated WWOX and phosphorylated p53 induces apoptosis.
Future research should prioritize a comprehensive validation of WWOX as a biomarker in clinical data, which holds significant implications for tumor diagnosis and prognosis assessment. Restoring WWOX expression in tumors has been shown to effectively inhibit tumor growth, thereby positioning WWOX as a promising target for anti-tumor therapeutic strategies, despite the inherent challenges. Current animal experiments have investigated various WWOX-targeting drugs, including small molecule compounds, peptides, and RNA-based therapies. However, further clinical trials are essential to evaluate their safety and efficacy. Additionally, the discovery and design of WWOX activators could facilitate the development of more effective anti-tumor therapies, although the development and testing process is often lengthy and costly. Furthermore, WWOX may exhibit varying expression levels across different types and subtypes of tumors or at distinct stages of tumor development, necessitating further investigation into the tissue-specific differences in WWOX function.
Author Contributions
Conceptualization, H.Y.; formal analysis, H.Y. and B.L.; supervision, B.L.; investigation, J.Z.; resources, J.Z.; writing—original draft preparation, H.Y.; funding acquisition, Y.L.; project administration, Y.L.; writing—review and editing, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32470971), Funding for Chongqing Young and Middle-Aged Medical Excellence Team, the Research Capacity Enhancement Project of Chongqing University Cancer Hospital (Y2024003), the National Outstanding Youth Reserve Talent Training Project, the Major project of joint medical research of science and health in Chongqing (2025DBXM006), and the Chongqing Oncology (Specialty) Development.
Conflicts of Interest
The authors declare no competing financial interests.
Abbreviations
| WWOX | WW domain-containing oxidoreductase |
| SDR | Short-chain dehydrogenase/reductase |
| SCAR12 | Spinocerebellar ataxia, autosomal recessive-12 |
| WOREE | WWOX-related epileptic encephalopathy |
| WT | Wild-type |
| ENU | Ethyl nitrosourea |
| Zfra | Zinc finger-like protein that regulates apoptosis |
| ACK1 | Activated Cdc42-associated kinase |
| HCC | Hepatocellular carcinoma |
| Dvl2 | Dishevelled2 |
| TSN | Toosendanin |
| NRF2 | NF-E2-related factor 2 |
| AP-2γ | Activator Protein-2γ |
| YAP | Yes-associated protein |
| ErbB-4 | Erythroblastic Leukemia Viral Oncogene Homolog 4 |
| EMT | Epithelial–mesenchymal transition |
| ICIs | Immune checkpoint inhibitors |
| IHC | Immunohistochemical |
| GLUT1 | Glucose transporter 1 |
| PKM2 | Pyruvate kinase M2 |
| MEFs | Mouse embryonic fibroblasts |
| DEN | N-nitrosodiethylamine |
| DSBs | DNA double-strand breaks |
| NHEJ | Non-homologous end joining |
| HR | Homologous recombination |
| ATM | Ataxia telangiectasia-mutated |
| MERIT40 | Mediator of RAP80 Interaction and Targeting 40 kDa protein |
| ROS | Reactive oxygen species |
| MTX | Methotrexate |
| SCC | Squamous cell carcinoma |
| ER | Endoplasmic reticulum |
| EOC | Epithelial ovarian cancer |
| ABZ | Albendazole |
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 |
| FPN1 | Ferroportin 1 |
| GPX4 | Glutathione peroxidase 4 |
| HNSCC | Head and neck squamous cell carcinoma |
References
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar] [PubMed]
- Bednarek, A.K.; Keck-Waggoner, C.L.; Daniel, R.L.; Laflin, K.J.; Bergsagel, P.L.; Kiguchi, K.; Brenner, A.J.; Aldaz, C.M. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001, 61, 8068–8073. [Google Scholar] [PubMed]
- Ludes-Meyers, J.H.; Kil, H.; Bednarek, A.K.; Drake, J.; Bedford, M.T.; Aldaz, C.M. WWOX binds the specific proline-rich ligand PPXY: Identification of candidate interacting proteins. Oncogene 2004, 23, 5049–5055. [Google Scholar] [CrossRef]
- Nunez, M.I.; Ludes-Meyers, J.; Aldaz, C.M. WWOX protein expression in normal human tissues. J. Mol. Histol. 2006, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Hippo, Y.; Taniguchi, H.; Iwanari, H.; Yashiro, M.; Hirakawa, K.; Kodama, T.; Aburatani, H. An opposing view on WWOX protein function as a tumor suppressor. Cancer Res. 2003, 63, 8629–8633. [Google Scholar] [PubMed]
- Chang, N.S.; Schultz, L.; Hsu, L.J.; Lewis, J.; Su, M.; Sze, C.I. 17beta-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: Potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 2005, 24, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Piard, J.; Hawkes, L.; Milh, M.; Villard, L.; Borgatti, R.; Romaniello, R.; Fradin, M.; Capri, Y.; Héron, D.; Nougues, M.C.; et al. The phenotypic spectrum of WWOX-related disorders: 20 additional cases of WOREE syndrome and review of the literature. Genet. Med. 2019, 21, 1308–1318. [Google Scholar] [CrossRef]
- Mallaret, M.; Synofzik, M.; Lee, J.; Sagum, C.A.; Mahajnah, M.; Sharkia, R.; Drouot, N.; Renaud, M.; Klein, F.A.; Anheim, M.; et al. The tumour suppressor gene WWOX is mutated in autosomal recessive cerebellar ataxia with epilepsy and mental retardation. Brain 2014, 137, 411–419. [Google Scholar] [CrossRef]
- Oliver, K.L.; Trivisano, M.; Mandelstam, S.A.; De Dominicis, A.; Francis, D.I.; Green, T.E.; Muir, A.M.; Chowdhary, A.; Hertzberg, C.; Goldhahn, K.; et al. WWOX developmental and epileptic encephalopathy: Understanding the epileptology and the mortality risk. Epilepsia 2023, 64, 1351–1367. [Google Scholar] [CrossRef]
- Repudi, S.; Kustanovich, I.; Abu-Swai, S.; Stern, S.; Aqeilan, R.I. Neonatal neuronal WWOX gene therapy rescues Wwox null phenotypes. EMBO Mol. Med. 2021, 13, e14599. [Google Scholar] [CrossRef]
- Repudi, S.; Steinberg, D.J.; Elazar, N.; Breton, V.L.; Aquilino, M.S.; Saleem, A.; Abu-Swai, S.; Vainshtein, A.; Eshed-Eisenbach, Y.; Vijayaragavan, B.; et al. Neuronal deletion of Wwox, associated with WOREE syndrome, causes epilepsy and myelin defects. Brain 2021, 144, 3061–3077. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Hagan, J.P.; de Bruin, A.; Rawahneh, M.; Salah, Z.; Gaudio, E.; Siddiqui, H.; Volinia, S.; Alder, H.; Lian, J.B.; et al. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology 2009, 150, 1530–1535. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Hassan, M.Q.; de Bruin, A.; Hagan, J.P.; Volinia, S.; Palumbo, T.; Hussain, S.; Lee, S.H.; Gaur, T.; Stein, G.S.; et al. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J. Biol. Chem. 2008, 283, 21629–21639. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Trapasso, F.; Hussain, S.; Costinean, S.; Marshall, D.; Pekarsky, Y.; Hagan, J.P.; Zanesi, N.; Kaou, M.; Stein, G.S.; et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc. Natl. Acad. Sci. USA 2007, 104, 3949–3954. [Google Scholar] [CrossRef]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef] [PubMed]
- Janczar, S.; Nautiyal, J.; Xiao, Y.; Curry, E.; Sun, M.; Zanini, E.; Paige, A.J.; Gabra, H. WWOX sensitises ovarian cancer cells to paclitaxel via modulation of the ER stress response. Cell Death Dis. 2017, 8, e2955. [Google Scholar] [CrossRef]
- Abdeen, S.K.; Salah, Z.; Maly, B.; Smith, Y.; Tufail, R.; Abu-Odeh, M.; Zanesi, N.; Croce, C.M.; Nawaz, Z.; Aqeilan, R.I. Wwox inactivation enhances mammary tumorigenesis. Oncogene 2011, 30, 3900–3906. [Google Scholar] [CrossRef]
- Lin, J.T.; Li, H.Y.; Chang, N.S.; Lin, C.H.; Chen, Y.C.; Lu, P.J. WWOX suppresses prostate cancer cell progression through cyclin D1-mediated cell cycle arrest in the G1 phase. Cell Cycle 2015, 14, 408–416. [Google Scholar] [CrossRef]
- Gao, K.; Yin, J.; Dong, J. Deregulated WWOX is involved in a negative feedback loop with microRNA-214-3p in osteosarcoma. Int. J. Mol. Med. 2016, 38, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S.; Doherty, J.; Ensign, A.; Schultz, L.; Hsu, L.J.; Hong, Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J. Biol. Chem. 2005, 280, 43100–43108. [Google Scholar] [CrossRef]
- Chang, N.S.; Doherty, J.; Ensign, A.; Lewis, J.; Heath, J.; Schultz, L.; Chen, S.T.; Oppermann, U. Molecular mechanisms underlying WOX1 activation during apoptotic and stress responses. Biochem. Pharmacol. 2003, 66, 1347–1354. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Nie, K.; Peng, X.; Huo, J.; Fu, X.; Zhang, Y. WWOX-mediated p53/SAT1 and NRF2/FPN1 axis contribute to toosendanin-induced ferroptosis in hepatocellular carcinoma. Biochem. Pharmacol. 2025, 233, 116790. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.R.; Iliopoulos, D.; Semba, S.; Fabbri, M.; Druck, T.; Volinia, S.; Croce, C.M.; Morrison, C.D.; Klein, R.D.; Huebner, K. A role for the WWOX gene in prostate cancer. Cancer Res. 2006, 66, 6477–6481. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Iliopoulos, D.; Trapasso, F.; Aqeilan, R.I.; Cimmino, A.; Zanesi, N.; Yendamuri, S.; Han, S.Y.; Amadori, D.; Huebner, K.; et al. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 15611–15616. [Google Scholar] [CrossRef]
- Zhou, Y.; Shou, F.; Zhang, H.; You, Q. Adenovirus-delivered wwox inhibited lung cancer growth in vivo in a mouse model. Cancer Gene Ther. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Fabbri, M.; Druck, T.; Qin, H.R.; Han, S.Y.; Huebner, K. Inhibition of breast cancer cell growth in vitro and in vivo: Effect of restoration of Wwox expression. Clin. Cancer Res. 2007, 13, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, W.; Li, B.; Lu, C.; Wan, X. WWOX induces apoptosis and inhibits proliferation in cervical cancer and cell lines. Int. J. Mol. Med. 2013, 31, 1139–1147. [Google Scholar] [CrossRef]
- Nakayama, S.; Semba, S.; Maeda, N.; Aqeilan, R.I.; Huebner, K.; Yokozaki, H. Role of the WWOX gene, encompassing fragile region FRA16D, in suppression of pancreatic carcinoma cells. Cancer Sci. 2008, 99, 1370–1376. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.Y.; Melino, G.; Huebner, K.; et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Liu, Y. Upregulation of tumor suppressor WWOX promotes immune response in glioma. Cell Immunol. 2013, 285, 1–5. [Google Scholar] [CrossRef]
- Liu, C.W.; Chen, P.H.; Lin, K.J.; Cheng, Y.T.; Chang, L.C. Novel Hydrogel-Mediated Lentiviral Gene Delivery via Intravesical Administration for Bladder Cancer Treatment. Pharmaceutics 2025, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Lai, F.J.; Sheu, H.M.; Lin, Y.S.; Chang, T.H.; Jan, M.S.; Chen, S.M.; Hsu, P.C.; Huang, T.T.; Huang, T.C.; et al. WWOX suppresses autophagy for inducing apoptosis in methotrexate-treated human squamous cell carcinoma. Cell Death Dis. 2013, 4, e792. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Song, L.; Xu, Y.; Wu, Y.; Dai, C.; Wang, X.; Sun, X.; Hou, Y.; Li, W.; Zhan, X.; et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat. Commun. 2018, 9, 3486. [Google Scholar] [CrossRef]
- Yang, T.; Xu, R.; Huo, J.; Wang, B.; Du, X.; Dai, B.; Zhu, M.; Zhan, Y.; Zhang, D.; Zhang, Y. WWOX activation by toosendanin suppresses hepatocellular carcinoma metastasis through JAK2/Stat3 and Wnt/β-catenin signaling. Cancer Lett. 2021, 513, 50–62. [Google Scholar] [CrossRef]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009, 28, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Khawaled, S.; Nigita, G.; Distefano, R.; Oster, S.; Suh, S.S.; Smith, Y.; Khalaileh, A.; Peng, Y.; Croce, C.M.; Geiger, T.; et al. Pleiotropic tumor suppressor functions of WWOX antagonize metastasis. Signal. Transduct. Target. Ther. 2020, 5, 43. [Google Scholar] [CrossRef]
- Khawaled, S.; Suh, S.S.; Abdeen, S.K.; Monin, J.; Distefano, R.; Nigita, G.; Croce, C.M.; Aqeilan, R.I. WWOX Inhibits Metastasis of Triple-Negative Breast Cancer Cells via Modulation of miRNAs. Cancer Res. 2019, 79, 1784–1798. [Google Scholar] [CrossRef]
- Gourley, C.; Paige, A.J.; Taylor, K.J.; Ward, C.; Kuske, B.; Zhang, J.; Sun, M.; Janczar, S.; Harrison, D.J.; Muir, M.; et al. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res. 2009, 69, 4835–4842. [Google Scholar] [CrossRef]
- El-Hage, P.; Petitalot, A.; Monsoro-Burq, A.H.; Maczkowiak, F.; Driouch, K.; Formstecher, E.; Camonis, J.; Sabbah, M.; Bièche, I.; Lidereau, R.; et al. The Tumor-Suppressor WWOX and HDAC3 Inhibit the Transcriptional Activity of the β-Catenin Coactivator BCL9-2 in Breast Cancer Cells. Mol. Cancer Res. 2015, 13, 902–912. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Palamarchuk, A.; Weigel, R.J.; Herrero, J.J.; Pekarsky, Y.; Croce, C.M. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res. 2004, 64, 8256–8261. [Google Scholar] [CrossRef]
- Gaudio, E.; Palamarchuk, A.; Palumbo, T.; Trapasso, F.; Pekarsky, Y.; Croce, C.M.; Aqeilan, R.I. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res. 2006, 66, 11585–11589. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Donati, V.; Palamarchuk, A.; Trapasso, F.; Kaou, M.; Pekarsky, Y.; Sudol, M.; Croce, C.M. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005, 65, 6764–6772. [Google Scholar] [CrossRef]
- Kurek, K.C.; Del Mare, S.; Salah, Z.; Abdeen, S.; Sadiq, H.; Lee, S.H.; Gaudio, E.; Zanesi, N.; Jones, K.B.; DeYoung, B.; et al. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res. 2010, 70, 5577–5586. [Google Scholar] [CrossRef]
- Del Mare, S.; Aqeilan, R.I. Tumor Suppressor WWOX inhibits osteosarcoma metastasis by modulating RUNX2 function. Sci. Rep. 2015, 5, 12959. [Google Scholar] [CrossRef]
- Ferguson, B.W.; Gao, X.; Zelazowski, M.J.; Lee, J.; Jeter, C.R.; Abba, M.C.; Aldaz, C.M. The cancer gene WWOX behaves as an inhibitor of SMAD3 transcriptional activity via direct binding. BMC Cancer 2013, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Husanie, H.; Abu-Remaileh, M.; Maroun, K.; Abu-Tair, L.; Safadi, H.; Atlan, K.; Golan, T.; Aqeilan, R.I. Loss of tumor suppressor WWOX accelerates pancreatic cancer development through promotion of TGFβ/BMP2 signaling. Cell Death Dis. 2022, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor suppressor WWOX regulates glucose metabolism via HIF1α modulation. Cell Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Khalaileh, A.; Pikarsky, E.; Aqeilan, R.I. WWOX controls hepatic HIF1α to suppress hepatocyte proliferation and neoplasia. Cell Death Dis. 2018, 9, 511. [Google Scholar] [CrossRef]
- Schrock, M.S.; Batar, B.; Lee, J.; Druck, T.; Ferguson, B.; Cho, J.H.; Akakpo, K.; Hagrass, H.; Heerema, N.A.; Xia, F.; et al. Wwox-Brca1 interaction: Role in DNA repair pathway choice. Oncogene 2017, 36, 2215–2227. [Google Scholar] [CrossRef]
- Park, D.; Gharghabi, M.; Schrock, M.S.; Plow, R.; Druck, T.; Yungvirt, C.; Aldaz, C.M.; Huebner, K. Interaction of Wwox with Brca1 and associated complex proteins prevents premature resection at double-strand breaks and aberrant homologous recombination. DNA Repair 2022, 110, 103264. [Google Scholar] [CrossRef]
- Bidany-Mizrahi, T.; Shweiki, A.; Maroun, K.; Abu-Tair, L.; Mali, B.; Aqeilan, R.I. Unveiling the relationship between WWOX and BRCA1 in mammary tumorigenicity and in DNA repair pathway selection. Cell Death Discov. 2024, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Taouis, K.; Vacher, S.; Guirouilh-Barbat, J.; Camonis, J.; Formstecher, E.; Popova, T.; Hamy, A.S.; Petitalot, A.; Lidereau, R.; Caputo, S.M.; et al. WWOX binds MERIT40 and modulates its function in homologous recombination, implications in breast cancer. Cancer Gene Ther. 2023, 30, 1144–1155. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Xu, M.; Zhou, Q.; Weng, J.; Hu, Z.; Xu, M.; Xu, W.; Yi, Y.; Shi, Y.; et al. WWOX tuning of oleic acid signaling orchestrates immunosuppressive macrophage polarization and sensitizes hepatocellular carcinoma to immunotherapy. J. Immunother. Cancer 2024, 12, e010422. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, T.; Yendamuri, S.; Trapasso, F.; Matsuyama, A.; Aqeilan, R.I.; Alder, H.; Rattan, S.; Cesari, R.; Nolli, M.L.; Williams, N.N.; et al. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin. Cancer Res. 2004, 10, 2459–2465. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Guler, G.; Han, S.Y.; Johnston, D.; Druck, T.; McCorkell, K.A.; Palazzo, J.; McCue, P.A.; Baffa, R.; Huebner, K. Fragile genes as biomarkers: Epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene 2005, 24, 1625–1633. [Google Scholar] [CrossRef]
- Paige, A.J.; Taylor, K.J.; Taylor, C.; Hillier, S.G.; Farrington, S.; Scott, D.; Porteous, D.J.; Smyth, J.F.; Gabra, H.; Watson, J.E. WWOX: A candidate tumor suppressor gene involved in multiple tumor types. Proc. Natl. Acad. Sci. USA 2001, 98, 11417–11422. [Google Scholar] [CrossRef]
- Chang, N.S.; Hsu, L.J.; Lin, Y.S.; Lai, F.J.; Sheu, H.M. WW domain-containing oxidoreductase: A candidate tumor suppressor. Trends Mol. Med. 2007, 13, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.J.; Cheng, C.L.; Chen, S.T.; Wu, C.H.; Hsu, L.J.; Lee, J.Y.; Chao, S.C.; Sheen, M.C.; Shen, C.L.; Chang, N.S.; et al. WOX1 is essential for UVB irradiation-induced apoptosis and down-regulated via translational blockade in UVB-induced cutaneous squamous cell carcinoma in vivo. Clin. Cancer Res. 2005, 11, 5769–5777. [Google Scholar] [CrossRef]
- Chang, N.S.; Pratt, N.; Heath, J.; Schultz, L.; Sleve, D.; Carey, G.B.; Zevotek, N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J. Biol. Chem. 2001, 276, 3361–3370. [Google Scholar] [CrossRef]
- Lee, M.H.; Shih, Y.H.; Lin, S.R.; Chang, J.Y.; Lin, Y.H.; Sze, C.I.; Kuo, Y.M.; Chang, N.S. Zfra restores memory deficits in Alzheimer’s disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 189–204. [Google Scholar] [CrossRef]
- Su, W.-P.; Wang, W.-J.; Chang, J.-Y.; Ho, P.-C.; Liu, T.-Y.; Wen, K.-Y.; Kuo, H.-L.; Chen, Y.-J.; Huang, S.-S.; Subhan, D.; et al. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs that Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Ho, P.-C.; Nagarajan, G.; Chen, Y.-A.; Kuo, H.-L.; Subhan, D.; Su, W.-P.; Chang, J.-Y.; Lu, C.-Y.; Chang, K.T.; et al. WWOX Possesses N-Terminal Cell Surface-Exposed Epitopes WWOX7-21 and WWOX7-11 for Signaling Cancer Growth Suppression and Prevention In Vivo. Cancers 2019, 11, 1818. [Google Scholar] [CrossRef]
- Mahajan, N.P.; Whang, Y.E.; Mohler, J.L.; Earp, H.S. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: Role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res. 2005, 65, 10514–10523. [Google Scholar] [CrossRef]
- Xie, B.; Zen, Q.; Wang, X.; He, X.; Xie, Y.; Zhang, Z.; Li, H. ACK1 promotes hepatocellular carcinoma progression via downregulating WWOX and activating AKT signaling. Int. J. Oncol. 2015, 46, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Bar-Mag, T.; Huang, H.; Kim, T.; Salah, Z.; Abdeen, S.K.; Sudol, M.; Reichmann, D.; Sidhu, S.; Kim, P.M.; et al. Characterizing WW domain interactions of tumor suppressor WWOX reveals its association with multiprotein networks. J. Biol. Chem. 2014, 289, 8865–8880. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tong, J.; Lin, X.; Han, Q.; Huang, H. Effect of the WWOX gene on the regulation of the cell cycle and apoptosis in human ovarian cancer stem cells. Mol. Med. Rep. 2015, 12, 1783–1788. [Google Scholar] [CrossRef]
- Aderca, I.; Moser, C.D.; Veerasamy, M.; Bani-Hani, A.H.; Bonilla-Guerrero, R.; Ahmed, K.; Shire, A.; Cazanave, S.C.; Montoya, D.P.; Mettler, T.A.; et al. The JNK inhibitor SP600129 enhances apoptosis of HCC cells induced by the tumor suppressor WWOX. J. Hepatol. 2008, 49, 373–383. [Google Scholar] [CrossRef][Green Version]
- Chou, P.Y.; Lin, S.R.; Lee, M.H.; Schultz, L.; Sze, C.I.; Chang, N.S. A p53/TIAF1/WWOX triad exerts cancer suppression but may cause brain protein aggregation due to p53/WWOX functional antagonism. Cell Commun. Signal. 2019, 17, 76. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chiang, M.F.; Lin, S.R.; Lee, M.H.; He, H.; Chou, P.Y.; Chen, S.J.; Chen, Y.A.; Yang, L.Y.; Lai, F.J.; et al. TIAF1 self-aggregation in peritumor capsule formation, spontaneous activation of SMAD-responsive promoter in p53-deficient environment, and cell death. Cell Death Dis. 2012, 3, e302. [Google Scholar] [CrossRef]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef]
- Hsu, L.J.; Schultz, L.; Hong, Q.; Van Moer, K.; Heath, J.; Li, M.Y.; Lai, F.J.; Lin, S.R.; Lee, M.H.; Lo, C.P.; et al. Transforming growth factor beta1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J. Biol. Chem. 2009, 284, 16049–16059. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Shen, J.; Wang, Y.; Xu, Y.; Wang, Y.; Han, Z.; Li, X. Upregulation of the putative oncogene COTE1 contributes to human hepatocarcinogenesis through modulation of WWOX signaling. Int. J. Oncol. 2014, 45, 719–731. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, L.V.; Colella, A.; Dayan, S.; Chen, Q.; Choo, A.; Jacob, R.; Price, G.; Venter, D.; Richards, R.I. Drosophila orthologue of WWOX, the chromosomal fragile site FRA16D tumour suppressor gene, functions in aerobic metabolism and regulates reactive oxygen species. Hum. Mol. Genet. 2011, 20, 497–509. [Google Scholar] [CrossRef]
- Richards, R.I.; Choo, A.; Lee, C.S.; Dayan, S.; O’Keefe, L. WWOX, the chromosomal fragile site FRA16D spanning gene: Its role in metabolism and contribution to cancer. Exp. Biol. Med. 2015, 240, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dayan, S.; O’Keefe, L.V.; Choo, A.; Richards, R.I. Common chromosomal fragile site FRA16D tumor suppressor WWOX gene expression and metabolic reprograming in cells. Genes Chromosomes Cancer 2013, 52, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 2022, 86, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Reinfeld, B.I.; Rathmell, W.K.; Kim, T.K.; Rathmell, J.C. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell. Mol. Immunol. 2022, 19, 46–58. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R.; et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar] [CrossRef]
- Ouyang, X.; Han, S.N.; Zhang, J.Y.; Dioletis, E.; Nemeth, B.T.; Pacher, P.; Feng, D.; Bataller, R.; Cabezas, J.; Stärkel, P.; et al. Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1α Transactivation in Steatohepatitis. Cell Metab. 2018, 27, 339–350.e333. [Google Scholar] [CrossRef]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell. Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Cheng, H.C.; Huang, P.H.; Lai, F.J.; Jan, M.S.; Chen, Y.L.; Chen, S.Y.; Chen, W.L.; Hsu, C.K.; Huang, W.; Hsu, L.J. Loss of fragile WWOX gene leads to senescence escape and genome instability. Cell Mol. Life Sci. 2023, 80, 338. [Google Scholar] [CrossRef]
- Donati, V.; Fontanini, G.; Dell’Omodarme, M.; Prati, M.C.; Nuti, S.; Lucchi, M.; Mussi, A.; Fabbri, M.; Basolo, F.; Croce, C.M.; et al. WWOX expression in different histologic types and subtypes of non-small cell lung cancer. Clin. Cancer Res. 2007, 13, 884–891. [Google Scholar] [CrossRef]
- Becker, S.; Markova, B.; Wiewrodt, R.; Hoffarth, S.; Hähnel, P.S.; Pleiner, S.; Schmidt, L.H.; Breitenbuecher, F.; Schuler, M. Functional and clinical characterization of the putative tumor suppressor WWOX in non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.K.; Ben-David, U.; Shweiki, A.; Maly, B.; Aqeilan, R.I. Somatic loss of WWOX is associated with TP53 perturbation in basal-like breast cancer. Cell Death Dis. 2018, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chao, L.; Ma, G.; Chen, L.; Zang, Y.; Sun, J. The prognostic significance of WWOX expression in patients with breast cancer and its association with the basal-like phenotype. J. Cancer Res. Clin. Oncol. 2011, 137, 271–278. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, W.; Sun, J.; Atyah, M.; Yin, Y.; Zhang, W.; Guo, L.; Ye, Q.; Dong, Q.; Shi, Y.; et al. Low expression of WW domain-containing oxidoreductase associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Cancer Med. 2018, 7, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhou, L.; Song, W.; Wu, S.; Zhu, B.; Wang, D. Expression of ORAOV1, CD133 and WWOX correlate with metastasis and prognosis in gastric adenocarcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 8916–8924. [Google Scholar] [PubMed]
- Paige, A.J.; Zucknick, M.; Janczar, S.; Paul, J.; Mein, C.A.; Taylor, K.J.; Stewart, M.; Gourley, C.; Richardson, S.; Perren, T.; et al. WWOX tumour suppressor gene polymorphisms and ovarian cancer pathology and prognosis. Eur. J. Cancer 2010, 46, 818–825. [Google Scholar] [CrossRef]
- Huang, C.; Tian, Y.; Peng, R.; Zhang, C.; Wang, D.; Han, S.; Jiao, C.; Wang, X.; Zhang, H.; Wang, Y.; et al. Association of downregulation of WWOX with poor prognosis in patients with intrahepatic cholangiocarcinoma after curative resection. J. Gastroenterol. Hepatol. 2015, 30, 421–433. [Google Scholar] [CrossRef]
- Lin, J.T.; Tzai, T.S.; Liao, C.Y.; Wang, J.S.; Wu, T.T.; Wang, H.Y.; Wu, C.H.; Yu, C.C.; Lu, P.J. WWOX protein expression varies among RCC histotypes and downregulation of WWOX protein correlates with less-favorable prognosis in clear RCC. Ann. Surg. Oncol. 2013, 20, 193–199. [Google Scholar] [CrossRef]
- Del Mare, S.; Husanie, H.; Iancu, O.; Abu-Odeh, M.; Evangelou, K.; Lovat, F.; Volinia, S.; Gordon, J.; Amir, G.; Stein, J.; et al. WWOX and p53 Dysregulation Synergize to Drive the Development of Osteosarcoma. Cancer Res. 2016, 76, 6107–6117. [Google Scholar] [CrossRef]
- Akkawi, R.; Hidmi, O.; Haj-Yahia, A.; Monin, J.; Diment, J.; Drier, Y.; Stein, G.S.; Aqeilan, R.I. WWOX promotes osteosarcoma development via upregulation of Myc. Cell Death Dis. 2024, 15, 13. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, C.; Zhang, W.; Atyah, M.; Yin, Y.; Guo, L.; Tang, W.; Dong, Q.; Ye, Q.; Ren, N. Association of WWOX rs9926344 polymorphism with poor prognosis of hepatocellular carcinoma. J. Cancer 2018, 9, 1239–1247. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, C.L.; Wang, S.S.; Yang, C.K.; Li, J.R.; Chen, C.S.; Hung, S.C.; Chiu, K.Y.; Cheng, C.L.; Ou, Y.C.; et al. WWOX Polymorphisms as Predictors of the Biochemical Recurrence of Localized Prostate Cancer after Radical Prostatectomy. Int. J. Med. Sci. 2023, 20, 969–975. [Google Scholar] [CrossRef]
- Schirmer, M.A.; Lüske, C.M.; Roppel, S.; Schaudinn, A.; Zimmer, C.; Pflüger, R.; Haubrock, M.; Rapp, J.; Güngör, C.; Bockhorn, M.; et al. Relevance of Sp Binding Site Polymorphism in WWOX for Treatment Outcome in Pancreatic Cancer. J. Natl. Cancer Inst. 2016, 108, djv387. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chang, Y.; Xia, Z.; Liu, Y.; Liu, X.; Xiong, L.; Liu, C.; Zhu, X.; Wang, M.; Qiu, L. Remote modulation of WWOX by an intronic variant associated with survival of Chinese gastric cancer patients. Int. J. Cancer 2024, 154, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Salah, Z.; Bar-mag, T.; Kohn, Y.; Pichiorri, F.; Palumbo, T.; Melino, G.; Aqeilan, R.I. Tumor suppressor WWOX binds to ΔNp63α and sensitizes cancer cells to chemotherapy. Cell Death Dis. 2013, 4, e480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Yamashita-Kanemaru, Y.; Morris, B.I.; Contursi, A.; Trajkovski, D.; Xu, J.; Patrascan, I.; Benson, J.; Evans, A.C.; Conti, A.G.; et al. Aspirin prevents metastasis by limiting platelet TXA(2) suppression of T cell immunity. Nature 2025, 640, 1052–1061. [Google Scholar] [CrossRef]
- Yang, T.; Cheng, C.; Xu, R.; Huo, J.; Peng, X.; Chen, Y.; Liang, Y.; Su, Z.; Zhang, Y. Albendazole exerts an anti-hepatocellular carcinoma effect through a WWOX-dependent pathway. Life Sci. 2022, 310, 121086. [Google Scholar] [CrossRef]
- Hu, C.Y.; Wu, H.T.; Su, Y.C.; Lin, C.H.; Chang, C.J.; Wu, C.L. Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway. Molecules 2017, 22, 1175. [Google Scholar] [CrossRef]
- Yang, T.; Huo, J.; Xu, R.; Zhang, Y. Synergistic effect of toosendanin and regorafenib against cell proliferation and migration by regulating WWOX signaling pathway in hepatocellular carcinoma. Phytother. Res. 2021, 35, 4567–4578. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiong, Y.; Zhang, Y.; Leng, Y.; Tao, J.; Li, L.; Qiu, Z.; Xia, Z. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 2021, 27, 149–164. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Y.; Yang, N.; Zhang, H.; Zhang, X.; Geng, Y.; Zhao, J.; Chen, Z.; Dong, C.; Lin, L.; et al. Apoptotic Bodies Derived from Fibroblast-Like Cells in Subcutaneous Connective Tissue Inhibit Ferroptosis in Ischaemic Flaps via the miR-339-5p/KEAP1/Nrf2 Axis. Adv. Sci. 2024, 11, e2307238. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Chen, P.H.; Yu, T.J.; Lin, K.J.; Chang, L.C. WWOX Modulates ROS-Dependent Senescence in Bladder Cancer. Molecules 2022, 27, 7388. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Wei, C.; Xia, S.; Qiao, Z.; Zhang, X.W.; Yu, B.; Zhou, J.; Wang, R. Exosomal miR-625-3p secreted by cancer-associated fibroblasts in colorectal cancer promotes EMT and chemotherapeutic resistance by blocking the CELF2/WWOX pathway. Pharmacol. Res. 2022, 186, 106534. [Google Scholar] [CrossRef]
- Liu, C.J.; Shen, W.G.; Peng, S.Y.; Cheng, H.W.; Kao, S.Y.; Lin, S.C.; Chang, K.W. miR-134 induces oncogenicity and metastasis in head and neck carcinoma through targeting WWOX gene. Int. J. Cancer 2014, 134, 811–821. [Google Scholar] [CrossRef]
- Xu, D.; Liu, X.; Wu, J.; Wang, Y.; Zhou, K.; Chen, W.; Chen, J.; Chen, C.; Chen, L. LncRNA WWOX-AS1 sponges miR-20b-5p in hepatocellular carcinoma and represses its progression by upregulating WWOX. Cancer Biol. Ther. 2020, 21, 927–936. [Google Scholar] [CrossRef]
- Qu, G.; Ma, Z.; Tong, W.; Yang, J. LncRNA WWOX-AS1 inhibits the proliferation, migration and invasion of osteosarcoma cells. Mol. Med. Rep. 2018, 18, 779–788. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).