Simple Summary
The present systematic review explored how chemotherapy used to treat primary colorectal cancer, especially regimens like FOLFOX, CAPOX, and 5-FU/LV, can cause heart-related side effects. It includes 14 studies from various nations and unveiled that some individuals encountered minor concerns such as changes in heart strain, while others experienced more serious problems such as chest pain, arrhythmias, heart failure, or Takotsubo cardiomyopathy. Echocardiography and biomarkers were useful in detecting these alterations early. Case studies revealed that with careful monitoring and treatment, some patients were able to safely continue chemotherapy after recovering from cardiac toxicity. Overall, the study emphasizes the importance of regular cardiovascular exams before and during cancer therapy to improve patient safety and outcomes.
Abstract
Background/Introduction: Colorectal carcinoma (CRC) belongs to the most commonly diagnosed malignancies to this date, ranking as third across the globe. In addition, CRC remains a leading cause of cancer-related deaths as it is ranked as the second most common cause of mortality. Therapeutic strategies for the management and treatment of CRC have made significant progress in the last two decades, with both adjuvant and neoadjuvant approaches playing critical roles in enhancing favorable outcomes with regimens like FOLFOX, CAPOX, and 5-FU-based therapies demonstrating effectiveness. Nevertheless, growing evidence indicates that these therapies may pose a risk of cardiotoxicity development. A systematic review will be conducted to map the mechanistic pathways of chemotherapy-induced in CRC in order to bridge oncology and cardiology perspectives, highlighting emerging diagnostic tools and long-term surveillance gaps. Purpose: The objective of this study is the investigation of the prevalence and characteristics of cardiovascular problems linked to frequently employed chemotherapy regimens, as well as to evaluate existing diagnostic and therapeutic approaches. Methodology: A thorough search across databases, including PubMed (MEDLINE), Embase, and Cochrane Library, was performed to locate articles published up to 2025. The final studies included in the review underwent quality assessment. Results: Fourteen qualifying studies, comprising both prospective trials and case reports from diverse geographies, were included. Cardiovascular outcomes including myocardial strain, arrhythmias, angina, heart failure, and Takotsubo cardiomyopathy were evaluated. The diagnostic methods assessed comprised echocardiography, cardiac biomarkers, and electrocardiograms. In the reviewed trials, chemotherapy-induced cardiotoxicity varied from asymptomatic ventricular strain to serious cardiac complications. The FOLFOX and 5-FU regimens were predominantly linked to adverse cardiac outcomes. Prompt identification by echocardiographic strain imaging and biomarker monitoring facilitated timely intervention. Case studies revealed that, given proper cardiological support, certain patients could safely recommence chemotherapy following recovery. No standardized cardiac screening protocol was identified among the trials. Conclusions: Chemotherapy for colorectal cancer may present considerable cardiovascular hazards, highlighting the necessity for routine cardiac monitoring prior to and throughout treatment. This systematic review promotes collaborative cardio-oncology strategies to reduce risk and enhance therapeutic safety.
1. Introduction
Colorectal carcinoma (CRC) belongs to the most commonly diagnosed malignancies to this date, ranking as third across the globe [1]. In addition, CRC remains a leading cause of cancer-related deaths as it is ranked as the second most common cause of mortality [1,2]. Epidemiological studies project that by 2035, cases of colon and rectal cancer will increase by 71.5% and 60%, respectively [3]. The incline is mainly attributed to increase in aging of the general population, lifestyle factors, and disparities in access to screening and preventive care.
The majority of CRC cases are sporadic, arising from the accumulation of genetic and environmental factors over time. Around one-third of CRC patients exhibit familial clustering; however, only 5–16% of cases are associated with a germline pathogenic or likely pathogenic variation in a colorectal cancer predisposition gene. Hereditary colorectal cancer (HCRC) encompasses a group of illnesses categorized into two main categories, each exhibiting distinct clinical features: hereditary non-polyposis colorectal cancer (HNPCC) and hereditary polyposis colorectal cancer (HPCC) [1,4,5]. Lynch syndrome and familial adenomatous polyposis (FAP) are defined by germline mutations in DNA mismatch repair genes or changes in the APC gene [6]. Recent advancements in molecular profiling have shown the biological heterogeneity of colorectal cancer (CRC), resulting in its categorization into four consensus molecular subtypes (CMS): CMS1 (MSI-Immune), CMS2 (Canonical), CMS3 (Metabolic), and CMS4 (Mesenchymal). The genomic, epigenetic, and transcriptomic markers of these subtypes are distinct, influencing prognosis, therapeutic response, and tumour behaviour [7].
CRC screening is assessed by the use of fecal occult blood tests or endoscopic procedures, such as sigmoidoscopy or colonoscopy. Diagnosis is achieved by a combination of colonoscopy, biopsy, and imaging studies. Additional diagnostic approaches include biomarkers such carcinoembryonic antigen (CEA) and carbohydrate antigen but, their application is limited to monitoring disease progression rather than its development [8,9]. Multiple screening options are available, but high-quality evidence to indicate the best strategies is limited at the current stage [8].
Therapeutic strategies for the management and treatment of CRC have made significant progress in the last two decades, with both adjuvant and neoadjuvant approaches playing critical roles in enhancing favorable outcomes. Adjuvant therapy, which is typically administered in post-surgical resection, is designed for the elimination of any residual microscopic disease and decrease recurrence, rates with a particular emphasis on stage III and high-risk stage II CRC. This goal is reached by chemotherapy regimens including as FOLFOX (Folinic acid (also known as leucovorin), Fluorouracil (5-FU), and Oxaliplatin) or XELOX (CAPOX) (capecitabine and oxaliplatin combined). Moertel et al. proposed for the first time in the literature that mortality rates of stage III lymph node-positive colon cancer patients decreased by 33% after the administration of a 12-month regimen of 5-fluorouracil (5-FU) and levamisole [10]. In both stage III and high-risk stage II CRC, adjuvant chemotherapy with FOLFOX (fluorouracil, leucovorin, and oxaliplatin) regimen has been proved to reduce recurrence rates and enhance disease-free survival (DFS) [11]. In stage III patients, FOLFOX substantially increases DFS in comparison to 5-FU/LV alone, and it also exhibits a trend toward improved overall survival (OS). In high-risk stage II patients, FOLFOX, particularly when combined with oxaliplatin, exhibits an improvement in DFS and a reduction in recurrence risk [11].
On the other hand, neoadjuvant therapy, is particularly advantageous in the treatment of locally advanced rectal carcinoma. Chemoradiotherapy or total neoadjuvant therapy can enhance local management, improve tumour resectability, and occasionally support organ-preserving strategies. New evidence suggests that immunotherapy has great potential in the neoadjuvant setting for tumours with elevated microsatellite instability or mismatch repair deficiency [12,13]. A meta-analysis that was conducted by Gosavi R et al. (2021) suggested that neoadjuvant chemotherapy is a safe treatment option for the management of locally advanced colon cancer (LACC), indicating that tumour downstaging and increase in the rate of R0 resection have an oncological benefit. Consequently, neoadjuvant chemotherapy can be recommended as an alternative treatment option prior to surgery for clinically staged advanced colon malignancies (T4b), particularly when a clear resection margin is uncertain [14]. Even, with the therapeutic advancements present from the treatment options available, several factors must be taken into consideration of which adverse effects are included into the spectrum. Most notably, chemotherapy-induced cardiotoxicity is of great warning, which poses new challenges in the long-term management of CRC patients.
Chemotherapy-induced cardiotoxicity is a term referring to the spectrum of cardiac damage caused by cancer treatments and in particular from chemotherapeutic agents. It encircles both structural and functional cardiac impairments, ranging from asymptomatic changes in left ventricular ejection fraction (LVEF) to overt heart failure, arrhythmias, ischemia, and pericardial or valvular disease. A commonly used clinical definition includes a symptomatic drop in LVEF of ≥5% to below 55%, or an asymptomatic decline of ≥10% to below 55% [15]. The pathophysiology varies by drug class but often involves oxidative stress, mitochondrial dysfunction, and direct injury to cardiomyocytes. Anthracyclines like doxorubicin are known for dose-dependent, irreversible cardiotoxicity, while agents such as trastuzumab may cause reversible cardiac dysfunction [16,17,18,19,20]. Additionally, 5-FU is the second most common chemotherapeutic drug associated with cardiotoxicity after anthracyclines. It can manifest as chest pain, acute coronary syndrome/myocardial infarction or death [21]. The risk is influenced by cumulative dose, treatment duration, patient age, pre-existing cardiovascular conditions, and concurrent use of other cardiotoxic therapies. Early detection and monitoring are crucial, as cardiotoxicity can significantly impact both cancer prognosis and long-term cardiovascular health [15,22] (Table 1) (Figure 1).

Table 1.
Overview of chemotherapy-related cardiotoxicity.
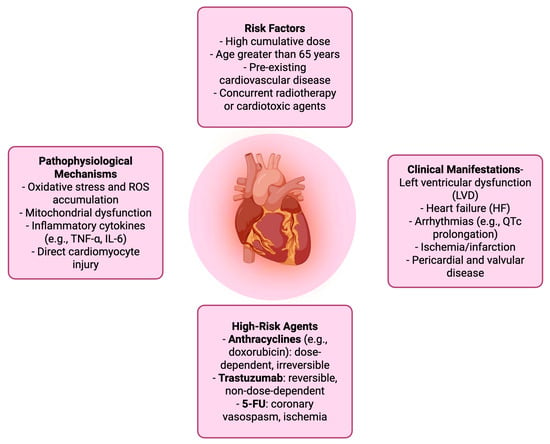
Figure 1.
Chemotherapy-Related cardiotoxicity.
Despite increasing recognition of chemotherapy-induced cardiotoxicity, its mechanistic underpinnings in the context of primary colorectal cancer remain poorly delineated. This systematic comprehensively synthesizes mechanistic evidence across diverse chemotherapy regimens specific to CRC, integrating molecular, imaging, and biomarker-based insights. By bridging oncology and cardiology perspectives, this work identifies critical gaps in early detection, long-term surveillance, and risk stratification laying the groundwork for future cardio-oncology integration and precision medicine approaches.
Objective
The primary objective of the current systematic review is to investigate and summarize the current evidence present concerning the mechanisms and prevalence of cardiotoxicity arising from chemotherapy regimens that are used to treat primary CRC. This review endeavors to identify at-risk populations, evaluate early detection and prevention strategies, and delineate pharmacologic and pathophysiologic pathways that contribute to cardiac dysfunction in CRC patients undergoing chemotherapy. The ultimate objective is to enhance clinical outcomes.
2. Materials and Methods
In order to guarantee methodological transparency and rigour, this systematic review was implemented in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines, as detailed in Supplementary Table S1. The review was not registered. To clarify the research query of this systematic review, a PICO (Population, Intervention, Comparator, Outcome) framework was employed, as detailed in Table 2.

Table 2.
PICO table.
2.1. Search Strategy
A strategic literature search was performed across multiple scientific databases, including PubMed (MEDLINE), Embase, and Cochrane Library, for the identification of the relevant studies published to this date. Keywords and MeSH terms related to CRC, chemotherapy regimens and cardiotoxicity were used for the creation of a Boolean string found in the Supplementary data Table S2. References were reviewed for identification of any additional relevant articles.
2.2. Study Selection—Eligibility Criteria
Inclusion and exclusion criteria were applied to ensure that only the most relevant and targeted articles were included during the screening process.
2.3. Inclusion Criteria
- Studies focusing on patients aged older than 19 years old diagnosed with primary CRC undergoing chemotherapy.
- Eligible interventions comprise commonly used chemotherapeutic agents such as fluoropyrimidines (5-fluorouracil and capecitabine), oxaliplatin, irinotecan, and regimens like FOLFOX, CAPEOX, FOLFIRI, and FOLFOXIRI.
- Studies must report cardiotoxicity outcomes including cardiac dysfunction, arrhythmias, myocarditis, QT prolongation, heart failure, or ischemic events in patients without a prior history of cardiovascular disease.
- Only clinical trials, cohort studies, case reports, cased series, case–control designs, and mechanistic studies published up to 2025 in English, with full-text availability, are considered for evaluation.
2.4. Exclusion Criteria
- Studies involving non-adult patients (under the age of 19 years old).
- Patients with metastatic CRC, other gastrointestinal malignancies, disease recurrence or non-cancer diagnoses.
- Interventions limited to radiotherapy or immunotherapy alone, or chemotherapy unrelated to CRC.
- Studies not reporting cardiovascular outcomes, or those involving patients with known cardiovascular disease.
- Additional filters must remove non-English studies without translation.
- Editorials, commentaries, abstracts, literature reviews and non-human or in vitro studies.
2.5. Screening Process
For the screening process a double-blind implementation was used for the elimination of decision influence between the two reviewers (S.T. and I.K.). The initial screening involved a systematic review of titles and abstracts to determine eligibility based on predefined inclusion and exclusion criteria. Any studies that referred to chemotherapy regimens for primary CRC and reported cardiotoxicity outcomes were shortlisted for the second round of screening. This stage excluded any publication that clearly involved pediatric patients, metastatic disease, radiotherapy or immunotherapy alone, or cardiovascular comorbidities.
Moving on, full-text articles were retrieved for all potentially relevant studies. Each article underwent a second round of evaluation to confirm compliance with study design, publication date, language, and outcome specifications. Discrepancies in selection were resolved by consultation with a third investigator (M.P.) when necessary. The PRISMA flow diagram in Figure 2 outlines clearly the screening process taken place. From the final studies the relevant data were extracted and summarized into tables (Table 3, Table 4 and Table 5).
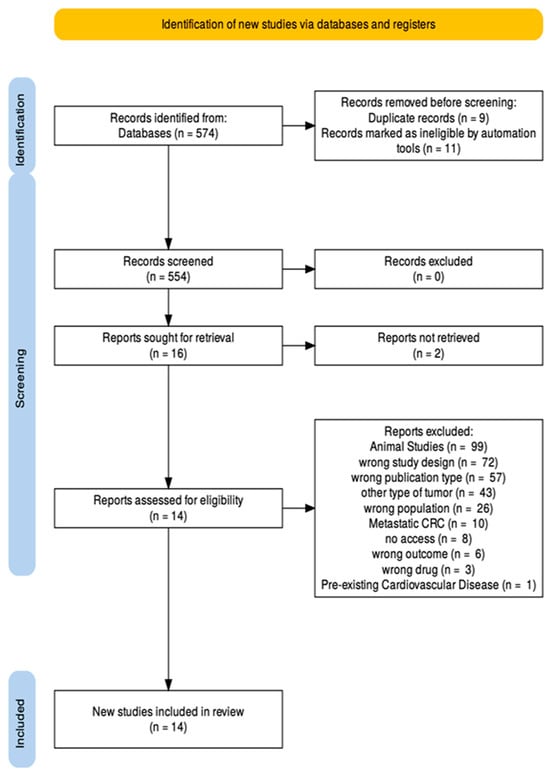
Figure 2.
PRISMA flow diagram.

Table 3.
Summary of included studies evaluating chemotherapy-induced cardiotoxicity in primary colorectal cancer patients. Abbreviations: Increase, ↑; Decrease, ↓.

Table 4.
Overview of included studies investigating chemotherapy-associated cardiotoxicity in primary colorectal cancer patients. Abbreviations: Increase, ↑; Decrease, ↓.

Table 5.
Quantitative summary of reported cardiotoxicity risks stratified by chemotherapy regimen in primary colorectal cancer patients. 1 Regimen abbreviations are defined in the Abbreviations list. 2 Total sample size is the sum of participants across studies reporting the regimen. 3 Incidence/risk values are as reported in the original studies; ranges reflect variability in definitions and endpoints. 4 References correspond to those in the main reference list. Abbreviations: GLS, global longitudinal strain; LVtw, left ventricular twist; LVEF, left ventricular ejection fraction; HF, heart failure; AMI, acute myocardial infarction; ACS, acute coronary syndrome; MACE, major adverse cardiovascular events, ↑, Increase; ↓, Decrease.
2.6. Quality Assessment
For the evaluation of the methodological strength and clinical relevance of the included studies, two validated appraisal tools were utilized: the Newcastle–Ottawa Scale (NOS) for cohort and observational studies, and the JBI Checklist for Case Reports and Case Series for descriptive accounts.
3. Results
3.1. Study Design and Geographical Scope of Included Research
The current study integrated data from a total of 14 studies conducted in nine different countries, including China, Italy, Poland, Hong Kong, Taiwan, Denmark, Greece, Canada and the United States, with the total sample size reaching 61,614 participants. The included studies compromised of a wide range of methodological types, including six prospective observational cohorts [23,24,25,26,27,28] to provide insights into cardiovascular outcomes among patients undergoing CRC chemotherapy. Four studies were retrospective cohort analyses [29,30,31,32], while Lee et al. (2022) [33] presented a population-based cohort analysis involving CRC patients with matched controls. Płońska-Gościniak et al. (2017) [25] was the only multicentre prospective cohort. Additional contributions include a single case series by McAndrew et al. (2021) [34] and two thorough case reports [35,36] which improve overall understanding of individual patient trajectories and unusual cardiovascular problems associated with treatment.
3.2. Quality Assessment—Newcastle-Ottawa Scale (NOS)
Three out of twelve cohort studies included achieved full scores using the Newcastle-Ottawa Scale (NOS), i.e., 9/9: Wong et al. (2025), Lee et al. (2022), and Huang et al. (2022) [29,32,33]. The high score of these studies can be largely attributed to their population-based designs made, matched control groups, long-term outcome tracking, and advanced statistical adjustments. Moving on, five studies that were assessed scored 7/9, including Wang (2021), Sonaglioni (2020), Gościniak (2017), Visvikis (2020), and Dyhl-Polk (2021), [23,24,25,26,27]. The reason for not reaching full score is due to lack of control cohorts being included but the studies overall retained strong internal validity and longitudinal outcome measurement. When it comes to Liu (2024) and Wang (2023) [30,31] they scored 8/9, that is c supported by retrospective designs and multivariate modeling for cardiotoxicity risk prediction. Cardiovascular outcomes reported showed increased heterogeneity, with some detecting subclinical cardiac dysfunction via speckle-tracking echocardiography and tissue Doppler, while others reported increased vascular burden post-chemotherapy [27] or elevated biomarkers signaling silent ischemia [26]. Large-scale cohort studies [29,32,33] found modest but statistically significant increases in cardiac events, particularly in older patients with comorbidities, and helped establish independent predictors of fluoropyrimidine-induced cardiotoxicity. A detailed overview of the NOS assessment criteria and scoring methodology is shown in Supplementary data Tables S3–S5.
3.3. Quality Assessment—JBI Checklist
All four case reports [Ben-Yakov (2017) [28], McAndrew (2021) [34], Vargo (2015) [36], and Sami (2025) [35]]; attained a perfect score (14/14) according to the critical evaluation criteria derived from the JBI checklist. Each case distinctly reported patient demographics, delineated a historical clinical history, and used precise diagnostic techniques such as echocardiography, angiography and cardiac MRI, when applicable. Interventions were accurately outlined and customized to address the specific cardiotoxic pathophysiological mechanisms, including vasospasm management, heart failure treatment, and inpatient chemotherapy rechallenge regimens. Outcomes pre and post chemotherapy were well presented, suggesting symptom relief and cardiac recovery in every instance. Cardiac adverse events, such as coronary vasospasm, cardiogenic shock, and Takotsubo cardiomyopathy, were examined unambiguously, with each case providing significant clinical insights that enhance best clinical practice for 5-FU rechallenge and cardio-oncology monitoring. These articles collectively provide methodologically robust and meaningful clinical observations that strengthen the evidence base for the management of fluoropyrimidine-induced cardiotoxicity. A detailed overview of the JBI checklist can be accessed in Supplementary data Table S6.
4. Discussion
The intersection of oncology and cardiology is gaining traction, especially in light of new data that chemotherapy-induced cardiotoxicity may be more common and complicated than previously thought. In this analysis, we analyzed data from a wide range of international studies to demonstrate the complexities and diversity of cardiovascular outcomes related to CRC treatment (Figure 3).
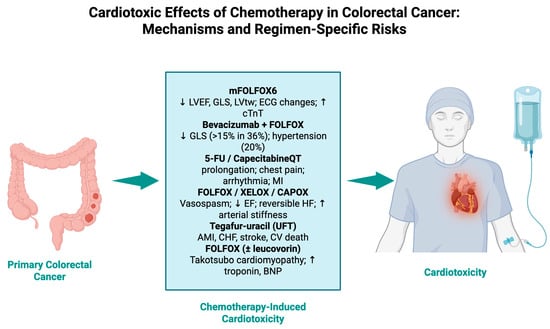
Figure 3.
Cardiotoxic effects of chemotherapy in colorectal cancer: mechanisms and regimen-specific risks.
4.1. Chemotherapy Regimens and Treatment Combinations
The examined chemotherapy regimens primarily involved fluoropyrimidine-based treatments, namely 5-FU, capecitabine, and tegafur-uracil (UFT) either alone or in combination with agents such as oxaliplatin, leucovorin, cisplatin, or bevacizumab. Notable combinations included FOLFOX [23,24,27], CAPOX [34], XELOX [27] and mFOLFOX6 [23]. The diversity in regimens allowed for comparisons of cardiotoxic risk across both monotherapies and multidrug protocols, as evidenced by analyses [25,29,31,33,37]. Tegafur-uracil (UFT) was specifically examined in Huang et al. (2022) [32], allowing for a unique comparison to non-UFT protocols. Furthermore, pharmacological drugs such as leucovorin and bevacizumab, particularly in combination forms such as FOLFOX plus Bevacizumab [24,31] and cisplatin [26] were included, which contributed to risk stratification across regimens. Several case reports also identified specific adverse cardiac events associated with 5-FU-based therapy [28,35,36].
4.2. Advanced Imaging Techniques for Detecting Chemotherapy-Induced Cardiotoxicity
Cardiotoxicity outcomes ranged from minor subclinical alterations to major cardiac events. Several studies have shown that sophisticated imaging techniques such as 3D and 2D speckle-tracking echocardiography (STE) can detect early cardiac damage. Wang et al. (2021) [23] and Sonaglioni et al. (2020) [24] found reductions in global longitudinal strain (GLS) and left ventricular twist (LVtw) despite preserved left ventricular ejection fraction (LVEF), implying that strain-based imaging may be more sensitive for detecting early cardiotoxicity. Płońska-Gościniak et al. (2017) [25] utilized tissue Doppler echocardiography to assess transitory QT interval prolongation and small changes in cardiac velocities 12 months post-chemotherapy.
4.3. Clinical Manifestations and Cardiotoxic Sequelae
Chemotherapy-induced cardiotoxicity in CRC patients presents a wide clinical spectrum, ranging from transient, asymptomatic cardiovascular changes to severe and potentially life-threatening cardiac events. Commonly addressed clinical presentations include chest pain, hypertension, arrhythmias, and myocardial ischemia. Dyhl-Polk et al. (2021) [26] reported silent ischemia in 14.1% of CRC patients receiving their first 5-FU infusion. Notably, elevations in copeptin levels were more reliable than troponin as markers of ischemic stress, suggesting copeptin’s role as a promising biomarker for early cardiovascular adverse events.
Acute, but rare, manifestations, underscored mainly in case studies, include reversible cardiomyopathy, cardiogenic shock, and stress-induced cardiomyopathy, which are typically triggered by capecitabine- or 5-FU-based regimens. McAndrew et al., 2021 [34], and Sami et al., 2025 [35], reported complete recovery of cardiac function in affected patients, followed by successful chemotherapy rechallenge under monitored conditions. Such evidence highlights the feasibility of continuing chemotherapy safely with appropriate cardiovascular surveillance and interdisciplinary management.
Population-based research in Hong Kong, Taiwan, and China found strong evidence of long-term vascular risks. Wong et al. (2025) [29] found a 1.06% incidence of major adverse cardiovascular events (MACE), with no increased risk compared to matched controls or between 5-FU and capecitabine. Lee et al. (2022) [33] and Huang et al. (2022) [32] found significantly higher rates of stroke, heart failure, and ischemic heart disease in older patients and those treated with UFT, particularly in stage III CRC. These risks were heightened by prevalent cardiovascular comorbidities such as diabetes, hypertension, and dyslipidemia.
Biomarkers and risk-stratification methods proved useful in predicting and monitoring cardiotoxicity. Increased cardiac troponins and copeptin levels have been correlated with ischemic events, whereas Liu et al. (2024) [30] reported that the systemic immune-inflammation index (SII) was a good predictor of cardiotoxicity in patients with low monocyte cell count. Wang et al. (2023) [31] developed a risk nomogram that includes characteristics like age ≥ 60, BMI ≥ 22.97 kg/m2, ≤3 chemotherapy cycles, and concurrent bevacizumab usage, which are all independently related to elevated cardiotoxicity risk.
These findings line up with a wider revolution in oncological care that prioritizes increased survival rates and quality of life, and the reduction in long-term toxicities. As treatment methods become increasingly precise and survival rates rise, understanding the concealed costs of therapy, particularly those impacting cardiac health, has become essential. Our findings highlight a crucial necessity to reconsider current monitoring systems and risk assessment methodologies.
4.4. Nanotechnology-Driven Innovations in Cardiotoxicity Prevention and Monitoring
In addition, nanotechnology is set to play a transformative role in the early identification, prevention, and mitigation of chemotherapy-related cardiotoxicity. Nanoparticle-mediated drug delivery systems possess the capability to specifically target tumour cells while preserving cardiac tissue, therefore minimizing off-target harm [38,39,40]. Engineered nanocarriers can be formulated to co-deliver chemotherapeutic drugs with cardioprotective substances, enhancing therapeutic efficacy and safety. Therapeutic drugs can be given directly to tumour areas while preserving healthy tissues, reducing off-target effects, by functionalizing nanoparticles (NPs) with ligands or antibodies that attach to receptors on cancer cells [41]. Moreover, nanosensors and nanobiosensors could provide real-time monitoring of cardiac biomarkers at the cellular level, hence enabling early identification of subclinical myocardial injury. Future investigations should examine the incorporation of nanotechnology into cardio-oncology treatments, focusing on biocompatibility, long-term safety, and translational viability [41,42,43,44].
4.5. Limitations
This systematic review is restricted by several inherent limitations that necessitate caution in interpretation. The diversity in study designs from observational cohorts to descriptive case reports introduces heterogeneity in diagnostic protocols, cardiotoxicity definitions and outcome reporting. Lack of randomized controlled trials (RCTs) weakens causal inference, while limited access to baseline cardiovascular data in some cohorts may lead to underestimation of potential subclinical toxicities. Geographic clustering in East Asia and North America decreases the pertinence of findings to global populations, particularly in underserved regions. Moreover, shorter follow-up durations hinder comprehensive understanding of long-term cardiovascular risk, and the publication bias intrinsic in case reports may skew observed outcomes towards more dramatic or successful events.
4.6. Prospective Research Directions
Future research should prioritize the implementation of extensive, multicenter RCTs to conclusively associate chemotherapy methods with cardiotoxic effects in colorectal cancer patients. Implementing systematic pre-treatment cardiac evaluations, including baseline echocardiogram, full biomarker panels, and genetic screening, would improve the precision of patient risk classification. Advanced diagnostic modalities such as 3-D speckle-tracking echocardiography and novel serum biomarkers such as copeptin necessitate systematic validation as early indicators of myocardial injury. Moreover, prospective longitudinal cohorts drawn from diverse, representative populations with follow-up periods extending beyond five to ten years are essential to elucidate the chronic cardiotoxic risk and overall survival. The integration of oncologic and cardiologic care through multidisciplinary cardio-oncology programs, which combine treatment decision-making with continuous cardiac surveillance, has the potential to refine safety thresholds and inform evidence-based rechallenge strategies. Thus, looking forward, future aspects of research should also explore AI and machine learning (ML) algorithms as predictive models for the risk of cardiotoxicity development, integrating clinical, imaging, and genomic data. Also, pharmacogenomic profiling is a good addition for the identification of patient-specific susceptibilities and guide safer chemotherapy selection. The development of cardioprotective agents and targeted interventions for the mitigation of myocardial injury without compromising oncologic efficacy will provide beneficial results as well as the incorporation of real-time wearable cardiac monitoring technologies to detect early functional changes during chemotherapy administration. Together medical societies can create a global harmonization of cardio-oncology guidelines, by enabling standardized risk assessment and surveillance protocols across healthcare systems. These future directions will aim to transform cardiotoxicity management from reactive to proactive, ensuring safer, more personalized care for colorectal cancer patients undergoing chemotherapy.
5. Conclusions
The findings of this systematic review demonstrate that fluoropyrimidine-based chemotherapy regimens, including 5-fluorouracil, capecitabine, and tegafur-uracil, either alone or in combination with oxaliplatin (FOLFOX, CAPOX/XELOX), carry a measurable risk of cardiotoxicity in patients with primary CRC. Cardiac clinical manifestations span subclinical myocardial strain abnormalities diagnosed by speckle-tracking and tissue Doppler echocardiography to overt clinical adverse events, such as coronary vasospasm, arrhythmias, ischemia, reversible heart failure, and stress-induced cardiomyopathy. Serum biomarkers, such as hs-Troponin-I, N-terminal pro-B-type natriuretic peptide and copeptin, and advanced imaging modalities permit earlier identification and stratification of myocardial injury, while population-based cohorts and retrospective analyses underscore that older age, baseline cardiovascular comorbidities, and specific therapeutic combinations, such as concurrent bevacizumab or limited chemotherapy cycles, significantly elevate the risk. Case reports further establish that, under meticulous cardio-oncology surveillance, many patients can safely resume to potentially curative chemotherapy regimens after an acute cardiotoxic event.
To alleviate these risks and improve patient outcomes, integration of standard cardiac monitoring protocols, including baseline and serial echocardiographic strain imaging, targeted serum biomarker surveillance, and individualized risk-stratification nomograms, should become fundamental to CRC care. Prospective, multicenter studies with extended follow-up periods are required to further validate early detection diagnostic methods, define long-term cardiovascular sequelae, and evaluate cardioprotective interventions. Establishing multidisciplinary cardio-oncology pathways will be crucial to balance chemotherapeutic efficacy with cardiovascular safety, ultimately enhancing both survival rates and quality of life for patients undergoing treatment for primary colorectal malignancies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17193129/s1, Table S1: PRISMA 2020 Checklist [45]; Table S2: Boolean String; Table S3: Criteria; Table S4: Quality Assesment Scale—Newcastle-Ottawa Scale (NOS); Table S5: Quality Assessment Scale—Newcastle-Ottawa Scale (NOS); Table S6: Quality Assesment Scale—JBI checklist.
Author Contributions
Conceptualization, S.T., I.K. and A.G.; methodology, S.T. and I.K.; software, S.T. and I.K.; validation, P.C., S.T. and I.K.; formal analysis, D.G., E.T., S.T. and I.K.; investigation, S.T., I.K. and M.P.; resources, S.T. and I.K.; data curation, S.T. and I.K.; writing—original draft preparation, S.T., I.K., P.C. and M.P.; writing—review and editing, T.P., V.P., D.G. and A.G. visualization, S.T., I.K. and A.G. supervision, T.P., V.P. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef]
- Rebuzzi, F.; Ulivi, P.; Tedaldi, G. Genetic Predisposition to Colorectal Cancer: How Many and Which Genes to Test? Int. J. Mol. Sci. 2023, 24, 2137. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.D.; Arends, M.J. Molecular pathological classification of colorectal cancer—An update. Virchows Arch. 2024, 484, 273–285. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and Fluorouracil for Adjuvant Therapy of Resected Colon Carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- Assed Bastos, D.; Coelho Ribeiro, S.; de Freitas, D.; Hoff, P.M. Review: Combination therapy in high-risk stage II or stage III colon cancer: Current practice and future prospects. Ther. Adv. Med. Oncol. 2010, 2, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Yang, A.S.H.; Fichera, A.; Tsai, M.-H.; Wu, Y.-H.; Yeh, Y.-M.; Shyr, Y.; Lai, E.C.-C.; Lai, C.-H. Neoadjuvant Radiotherapy vs Up-Front Surgery for Resectable Locally Advanced Rectal Cancer. JAMA Netw Open 2025, 8, e259049. [Google Scholar] [CrossRef]
- Body, A.; Prenen, H.; Latham, S.; Lam, M.; Tipping-Smith, S.; Raghunath, A.; Segelov, E. The Role of Neoadjuvant Chemotherapy in Locally Advanced Colon Cancer. Cancer Manag. Res. 2021, 13, 2567–2579. [Google Scholar] [CrossRef]
- Gosavi, R.; Chia, C.; Michael, M.; Heriot, A.G.; Warrier, S.K.; Kong, J.C. Neoadjuvant chemotherapy in locally advanced colon cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 2063–2070. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An Inter-national Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Eaton, H.; Timm, K.N. Mechanisms of trastuzumab induced cardiotoxicity—Is exercise a potential treatment? Cardio-Oncology 2023, 9, 22. [Google Scholar] [CrossRef]
- Zeglinski Bsc, M.; Ludke Msc, A.; Jassal, D.S.; Singal, P.K. Trastuzumab-induced cardiac dysfunction: A “dual-hit”. Exp. Clin. Cardiol. 2011, 16, 70. [Google Scholar]
- Ling, G.; Ge, F.; Li, W.; Wei, Y.; Guo, S.; Zhang, Y.; Li, Y.; Zhang, Y.; Liu, H.; Wu, Y.; et al. Anthracycline-induced cardiotoxicity: Emerging mechanisms and therapies. Med. Plus 2025, 2, 100074. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Bhutani, V.; Varzideh, F.; Wilson, S.; Kansakar, U.; Jankauskas, S.; Santulli, G. Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update. J. Cardiovasc. Dev. Dis. 2025, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Al-Hussaniy, H.A.; Alburghaif, A.H.; Alkhafaje, Z.; Al-Zobaidy, M.A.-H.J.; Alkuraishy, H.M.; Mostafa-Hedeab, G.; Azam, F.; Al-Samydai, A.M.; Al-Tameemi, Z.S.; Naji, M.A. Chemotherapy-induced cardiotoxicity: A new perspective on the role of Digoxin, ATG7 activators, Resveratrol, and herbal drugs. J. Med. Life 2023, 16, 491–500. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, W.; Zhai, Z.; Huang, L.; Feng, J.; Guo, X.; Liu, K.; Zhang, C.; Wang, Z.; Lu, G.; et al. Use of spectral tracking technique to evaluate the changes in left ventricular function in patients undergoing chemotherapy for colorectal cancer. Int. J. Cardiovasc. Imaging 2021, 37, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef]
- Płońska-Gościniak, E.; Różewicz, M.; Kasprzak, J.; Wojtarowicz, A.; Mizia-Stec, K.; Hryniewiecki, T.; Pysz, P.; Kułach, A.; Bodys, A.; Sulżyc, V.; et al. Tissue Doppler echocardiography detects subclinical left ventricular dysfunction in patients undergoing chemotherapy for Colon Cancer: Insights from ONCOECHO multicentre study. Kardiol. Pol. 2017, 75, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dyhl-Polk, A.; Schou, M.; Vistisen, K.K.; Sillesen, A.-S.; Serup-Hansen, E.; Faber, J.; Klausen, T.W.; Bojesen, S.E.; Vaage-Nilsen, M.; Nielsen, D.L. Myocardial Ischemia Induced by 5-Fluorouracil: A Prospective Electrocardiographic and Cardiac Biomarker Study. Oncologist 2021, 26, e403–e413. [Google Scholar] [CrossRef]
- Visvikis, A.; Kyvelou, S.; Pietri, P.; Georgakopoulos, C.; Manousou, K.; Tousoulis, D.; Stefanadis, C.; Vlachopoulos, C.; Pektasides, D. Cardiotoxic profile and arterial stiffness of adjuvant chemotherapy for colorectal cancer. Cancer Manag. Res. 2020, 12, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yakov, M.; Mattu, A.; Brady, W.J.; Dubbs, S.B. Prinzmetal angina (Coronary vasospasm) associated with 5-fluorouracil chemotherapy. Am. J. Emerg. Med. 2017, 35, 1038.e3–1038.e5. [Google Scholar] [CrossRef]
- Wong, C.-K.; Ho, I.; Choo, A.; Lau, R.; Ma, T.-F.; Chiu, A.C.H.-O.; Lam, T.-H.; Lin, M.; Leung, R.W.-H.; Tam, F.C.-C.; et al. Cardiovascular safety of 5-fluorouracil and capecitabine in colorectal cancer patients: Real-world evidence. Cardio-Oncology 2025, 11, 3. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Wang, W.; Dong, H.; Wang, G.; Chen, W.; Chen, J.; Chen, W. The association between systemic immune-inflammation index and cardi-otoxicity related to 5-Fluorouracil in colorectal cancer. BMC Cancer 2024, 24, 782. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Dong, H.; Wang, G.; Chen, W.; Chen, J.; Chen, W. Risk factors for fluoropyrimidine-induced cardiotoxicity in colorectal cancer: A retrospective cohort study and establishment of a prediction nomogram for 5-FU induced cardiotoxicity. Front. Oncol. 2023, 13, 1017237. [Google Scholar] [CrossRef]
- Huang, W.-K.; Ho, W.-P.; Hsu, H.-C.; Chang, S.-H.; Chen, D.-Y.; Chou, W.-C.; Chang, P.-H.; Chen, J.-S.; Yang, T.-S.; See, L.-C. Risk of cardiovascular disease among different fluoropyrimidine-based chemotherapy regimens as adjuvant treatment for resected colorectal cancer. Front. Cardiovasc. Med. 2022, 9, 880956. [Google Scholar] [CrossRef]
- Lee, S.F.; Yip, P.L.; Vellayappan, B.A.; Chee, C.E.; Wong, L.C.; Wan, E.Y.-F.; Chan, E.W.-Y.; Lee, C.-F.; Lee, F.A.-S.; Luque-Fernandez, M.A. Incident Cardiovascular Diseases among Survivors of High-Risk Stage II–III Colorectal Cancer: A Cluster-Wide Cohort Study. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 1125–1133. [Google Scholar] [CrossRef]
- McAndrew, E.N.; Jassal, D.S.; Goldenberg, B.A.; Kim, C.A. Capecitabine-mediated heart failure in colorectal cancer: A case series. Eur. Heart J. Case Rep. 2021, 5, ytab079. [Google Scholar] [CrossRef]
- Sami, N.; Raizada, A.; Phillip, G.J.; Rana, D.; Alishetti, S. Takotsubo Cardiomyopathy in a Patient Undergoing 5-Fluorouracil Infusion: Considerations for Folinic Acid, 5-Fluorouracil, and Oxaliplatin (FOLFOX) Cardiotox-icity and Rechallenge. Cureus 2025, 17, e82145. [Google Scholar] [CrossRef]
- Vargo, C.A.; Blazer, M.; Reardon, J.; Gulati, M.; Bekaii-Saab, T. Successful completion of adjuvant chemotherapy in a patient with colon cancer experiencing 5-fluorouracil-induced cardiac vasospasm. Clin. Color. Cancer 2016, 15, e61–e63. [Google Scholar] [CrossRef]
- Liu, X.-X.; Su, J.; Long, Y.-Y.; He, M.; Zhu, Z.-Q. Perioperative risk factors for survival outcomes in elective colorectal cancer surgery: A retrospective cohort study. BMC Gastroenterol. 2021, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Farani, M.R.; Lak, M.; Cho, W.C.; Kang, H.; Azarian, M.; Yazdian, F.; Harirchi, S.; Khoshmaram, K.; Alipourfard, I.; Hushmandi, K.; et al. Carbon nanomaterials: A promising avenue in colorectal cancer treatment. Carbon Lett. 2024, 34, 2035–2053. [Google Scholar] [CrossRef]
- Narayana, S.; Gowda, B.J.; Hani, U.; Shimu, S.S.; Paul, K.; Das, A.; Ashique, S.; Ahmed, M.G.; Tarighat, M.A.; Abdi, G. Inorganic nanoparticle-based treatment approaches for colorectal cancer: Recent advancements and challenges. J. Nanobiotechnol. 2024, 22, 427. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Y.; Li, M.; Yu, Q. Will nanomedicine become a good solution for the cardiotoxicity of chemotherapy drugs? Front. Pharmacol. 2023, 14, 1143361. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.M.; Ali, R.; Elaziz, N.A.A.; Habib, H.; Abbas, F.M.; Yassin, M.T.; Maniah, K.; Abdelaziz, R. Nanotechnology in oncology: Advances in biosynthesis, drug delivery, and theranostics. Discov. Oncol. 2025, 16, 1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Wan, W.; Yang, H.; Zhao, J. Advances in nanotechnological approaches for the detection of early markers associated with severe cardiac ailments. Nanomedicine 2024, 19, 1487–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Zhang, Y.; Fang, F.; Liu, J.; Xia, Y.; Liu, Y. Cardiac Biomarkers for the Detection and Management of Cancer Thera-py-Related Cardiovascular Toxicity. J. Cardiovasc. Dev. Dis. 2022, 9, 372. [Google Scholar] [CrossRef]
- Yu, A.F.; Ky, B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart 2016, 102, 425–430. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).