Safety of Post-Transplant Cyclophosphamide-Based Prophylaxis in AML Patients with Pre-Existing Cardiac Morbidity Undergoing Allogeneic Hematopoietic Cell Transplantation
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Prior Cardiac Morbidity, Pre-Transplant Cardiac Evaluations and Main Definitions
2.3. Allo-HCT Information and Main Definitions
2.4. Endpoint Definition
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics and Allo-HCT Information
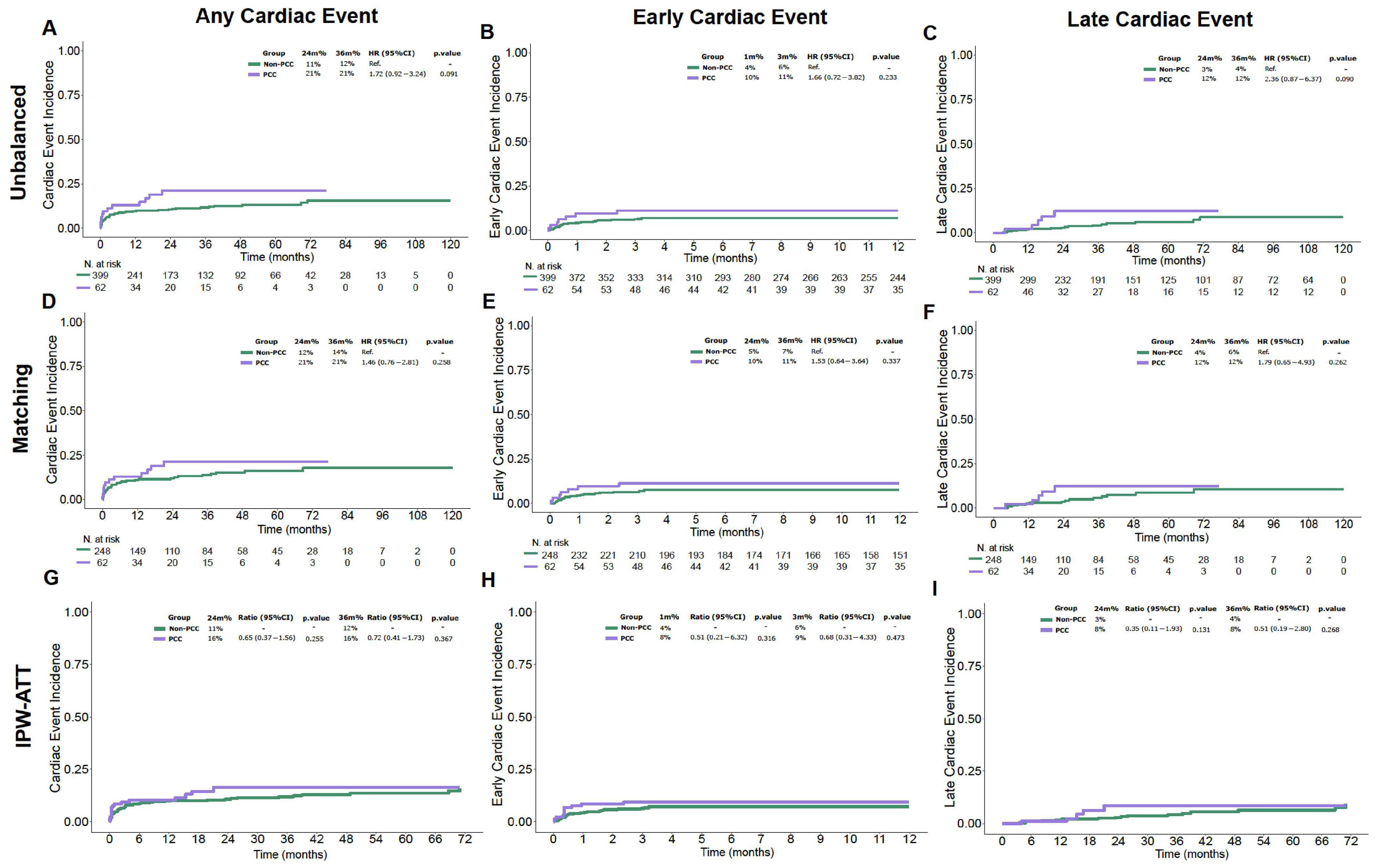
3.2. Cumulative Incidence of Early and Late Cardiac Events
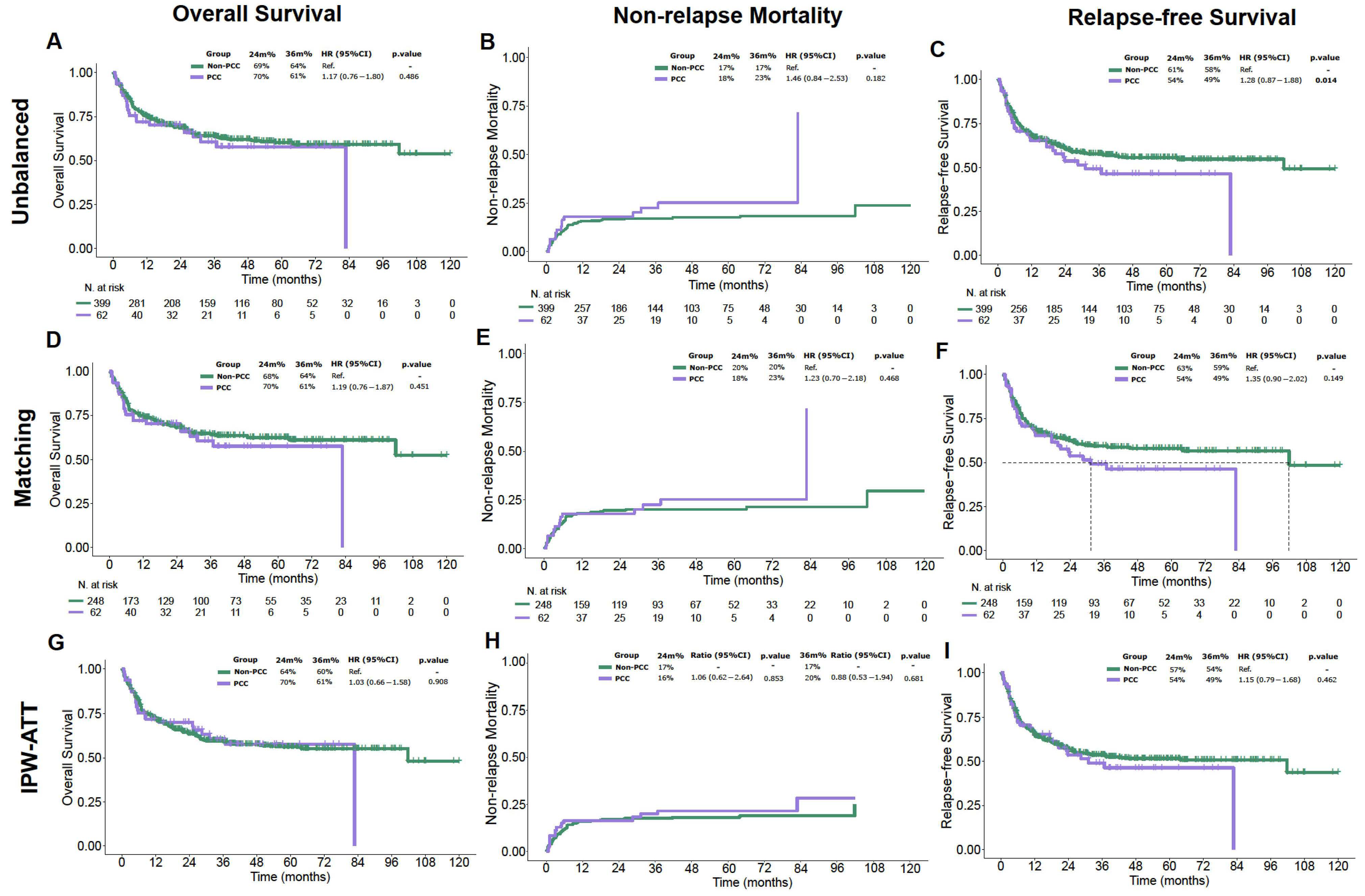
3.3. Efficacy Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luznik, L.; O’DOnnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow Transpl. 2008, 14, 641–650. [Google Scholar] [CrossRef]
- Bolaños-Meade, J.; Hamadani, M.; Wu, J.; Al Malki, M.M.; Martens, M.J.; Runaas, L.; Elmariah, H.; Rezvani, A.R.; Gooptu, M.; Larkin, K.T.; et al. Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis. N. Engl. J. Med. 2023, 388, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Broers, A.E.C.; de Jong, C.N.; Bakunina, K.; Hazenberg, M.D.; Kooy, M.v.M.; de Groot, M.R.; van Gelder, M.; Kuball, J.; van der Holt, B.; Meijer, E.; et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: Results of the prospective randomized HOVON-96 trial. Blood Adv. 2022, 6, 3378–3385. [Google Scholar] [CrossRef]
- Curtis, D.J.; Patil, S.S.; Reynolds, J.; Purtill, D.; Lewis, C.; Ritchie, D.S.; Gottlieb, D.J.; Yeung, D.T.; Wong, E.; Tey, S.-K.; et al. Graft-versus-Host Disease Prophylaxis with Cyclophosphamide and Cyclosporin. N. Engl. J. Med. 2025, 393, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Duléry, R.; Mohty, R.; Labopin, M.; Sestili, S.; Malard, F.; Brissot, E.; Battipaglia, G.; Médiavilla, C.; Banet, A.; Van de Wyngaert, Z.; et al. Early Cardiac Toxicity Associated with Post-Transplant Cyclophosphamide in Allogeneic Stem Cell Transplantation. JACC Cardio Oncol. 2021, 3, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.C.; Whited, L.K.; Saliba, R.M.; Rondon, G.; Banchs, J.; Shpall, E.J.; Champlin, R.E.; Popat, U.R. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021, 5, 5599–5607. [Google Scholar] [CrossRef]
- Pérez-Valencia, A.I.; Cascos, E.; Carbonell-Ordeig, S.; Charry, P.; Gómez-Hernando, M.; Rodríguez-Lobato, L.G.; Suárez-Lledó, M.; Martínez-Cibrian, N.; Antelo, M.G.; Solano, M.T.; et al. Incidence, risk factors, and impact of early cardiac toxicity after allogeneic hematopoietic cell transplant. Blood Adv. 2023, 7, 2018–2031. [Google Scholar] [CrossRef]
- Ishida, S.; Doki, N.; Shingai, N.; Yoshioka, K.; Kakihana, K.; Sakamaki, H.; Ohashi, K. The clinical features of fatal cyclophosphamide-induced cardiotoxicity in a conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Ann. Hematol. 2016, 95, 1145–1150. [Google Scholar] [CrossRef]
- Braverman, A.C.; Antin, J.H.; Plappert, M.T.; Cook, E.F.; Lee, R.T. Cyclophosphamide cardiotoxicity in bone marrow transplantation: A prospective evaluation of new dosing regimens. J. Clin. Oncol. 1991, 9, 1215–1223. [Google Scholar] [CrossRef]
- Lin, C.; Vader, J.M.; Slade, M.; DiPersio, J.F.; Westervelt, P.; Romee, R. Cardiomyopathy in patients after posttransplant cyclophosphamide–based hematopoietic cell transplantation. Cancer 2017, 123, 1800–1809. [Google Scholar] [CrossRef]
- Salas, M.Q.; Cascos, E.; López-García, A.; Pérez, E.; Baile-González, M.; Rodríguez, C.M.; Cascón, M.J.P.; Luque, M.; Esquirol, A.; Fernando, I.H.; et al. Cardiac events after allo-HCT in patients with acute myeloid leukemia. Blood Adv. 2024, 8, 5497–5509. [Google Scholar] [CrossRef]
- Pinto, F.R.; Cascos, E.; Pérez-López, E.; Baile-González, M.; Rodríguez, C.M.; Cascón, M.J.P.; Luque, M.; Esquirol, A.; Calvo, C.M.; Peña-Muñóz, F.; et al. Early cardiac events after haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. subanalysis exploring cardiac toxicity conducted on behalf of GETH-TC. Front. Immunol. 2025, 16, 1571678. [Google Scholar] [CrossRef]
- Hayek, S.S.; Zaha, V.G.; Bogle, C.; Deswal, A.; Langston, A.; Rotz, S.; Vasbinder, A.; Yang, E.; Okwuosa, T.; American Heart Association Cardio-Oncology Committee of the Council on Clinical Cardiology and Council on Genomic and Precision Medicine; et al. Cardiovascular Management of Patients Undergoing Hematopoietic Stem Cell Transplantation: From Pretransplantation to Survivorship: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1113–e1127. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef]
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT−NIH−CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018, 53, 1401–1415. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transpl. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transpl. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef] [PubMed]
- Denz, R.; Klaaßen-Mielke, R.; Timmesfeld, N. A comparison of different methods to adjust survival curves for confounders. Stat. Med. 2023, 42, 1461–1479. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Penack, O.; Marchetti, M.; Aljurf, M.; Arat, M.; Bonifazi, F.; Duarte, R.F.; Giebel, S.; Greinix, H.; Hazenberg, M.D.; Kröger, N.; et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024, 11, e147–e159. [Google Scholar] [CrossRef]
- Alizadehasl, A.; Shahrami, B.; Rahbarghazi, R.; Aliabadi, A.Y.; Jebelli, S.F.H.; Zonooz, Y.A.; Hakimian, H.; Fathi, F.; Forati, S.; Rezabakhsh, A. Post-transplant cyclophosphamide-induced cardiotoxicity: A comprehensive review. J. Cardiovasc. Thorac. Res. 2024, 16, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Marumo, A.; Omori, I.; Tara, S.; Otsuka, Y.; Konuma, R.; Adachi, H.; Wada, A.; Kishida, Y.; Konishi, T.; Nagata, A.; et al. Cyclophosphamide-induced cardiotoxicity at conditioning for allogeneic hematopoietic stem cell transplantation would occur among the patients treated with 120 mg/kg or less. Asia-Pac. J. Clin. Oncol. 2022, 18, E507–E514. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.Q.; Cascos, E.; López-García, A.; Pérez-López, E.; Baile-González, M.; López-Corral, L.; Cascón, M.J.P.; Luque, M.; Esquirol, A.; Fernando, I.H.; et al. Cardiac events occurring after allogeneic hematopoietic cell transplantation with post-transplant cyclophosphamide. Study conducted on behalf of the GETH-TC. Bone Marrow Transpl. 2024, 59, 1694–1703. [Google Scholar] [CrossRef]
- Palomo, M.; Moreno-Castaño, A.B.; Salas, M.Q.; Escribano-Serrat, S.; Rovira, M.; Guillen-Olmos, E.; Fernandez, S.; Ventosa-Capell, H.; Youssef, L.; Crispi, F.; et al. Endothelial activation and damage as a common pathological substrate in different pathologies and cell therapy complications. Front. Med. 2023, 10, 1285898. [Google Scholar] [CrossRef] [PubMed]
- Tolosa-Ridao, C.; Cascos, E.; Rodríguez-Lobato, L.G.; Pedraza, A.; Suárez-Lledó, M.; Charry, P.; Solano, M.T.; Martinez-Sanchez, J.; Cid, J.; Lozano, M.; et al. EASIX and cardiac adverse events after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2024, 59, 974–982. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef]
- Saadh, M.J.; Muhammad, F.A.; Albadr, R.J.; Sanghvi, G.; Jyothi, S.R.; Kundlas, M.; Joshi, K.K.; Rakhmatullaev, A.; Taher, W.M.; Alwan, M.; et al. Inflammasomes and Cardiovascular Disease: Linking Inflammation to Cardiovascular Pathophysiology. Scand. J. Immunol. 2025, 101, e70020. [Google Scholar] [CrossRef]
- Rotz, S.J.; Collier, P.; Hamilton, B.K. Post-Transplantation Cyclophosphamide: An Old Nemesis to a New Transplant Paradigm? JACC Cardio Oncol. 2021, 3, 260–262. [Google Scholar] [CrossRef]
- Gent, D.G.; Saif, M.; Dobson, R.; Wright, D.J. Cardiovascular Disease After Hematopoietic Stem Cell Transplantation in Adults: JACC: CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2024, 6, 475–495. [Google Scholar] [CrossRef]
- Tonorezos, E.S.; Stillwell, E.E.; Calloway, J.J.; Glew, T.; Wessler, J.D.; Rebolledo, B.J.; Pham, A.; Steingart, R.M.; Lazarus, H.; Gale, R.P.; et al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transpl. 2015, 50, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Qazilbash, M.H.; Amjad, A.I.; Qureshi, S.; Qureshi, S.R.; Saliba, R.M.; Khan, Z.U.; Hosing, C.; Giralt, S.A.; De Lima, M.J.; Popat, U.R.; et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients with low left ventricular ejection fraction. Biol. Blood Marrow Transpl. 2009, 15, 1265–1270. [Google Scholar] [CrossRef]
- Kanate, A.S.; Perales, M.-A.; Hamadani, M. Eligibility Criteria for Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. J. Natl. Compr. Cancer Netw. 2020, 18, 635–643. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, T.; Xiong, X.; Liu, N.; Pang, B.; Ruan, Y.; Gao, Y.; Shang, H.; Xing, Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104577. [Google Scholar] [CrossRef]
- Vasbinder, A.; Catalan, T.; Anderson, E.; Chu, C.; Kotzin, M.; Murphy, D.; Cheplowitz, H.; Diaz, K.M.; Bitterman, B.; Pizzo, I.; et al. Cardiovascular Risk Stratification of Patients Undergoing Hematopoietic Stem Cell Transplantation: The CARE-BMT Risk Score. J. Am. Heart Assoc. 2024, 13, e033599. [Google Scholar] [CrossRef]
- Simela, C.; Walker, J.M.; Ghosh, A.K.; Chen, D.H. SGLT2 inhibitors for prevention and management of cancer treatment-related cardiovascular toxicity: A review of potential mechanisms and clinical insights. Cardio-Oncology 2025, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Paccone, A.; Maurea, C.; Scherillo, M.; Merola, A.; Giordano, V.; Palma, G.; et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents ejection fraction reduction, reduces myocardial and renal NF-κB expression and systemic pro-inflammatory biomarkers in models of short-term doxorubicin cardiotoxicity. Front. Cardiovasc. Med. 2024, 11, 1289663. [Google Scholar] [CrossRef] [PubMed]
| Overall N = 461 (%) | PCC N = 62 (%) | Non-PCC N = 399 (%) | p Value | |
|---|---|---|---|---|
| Median Age | 55 (43–63) | 60 (48–66) | 54 (42–63) | 0.016 |
| Sex | 0.12 | |||
| Male | 236 (51) | 38 (61) | 198 (50) | |
| Female | 225 (49) | 24 (39) | 201 (50) | |
| Cardiovascular Risk Factors | ||||
| HTA | 399 (98) | 19 (31) | 76 (19) | 0.05 |
| Dyslipidemia | 60 (13) | 9 (15) | 51 (13) | 0.9 |
| Mellitus Diabetes | 36 (8) | 7 (11) | 29 (7) | 0.4 |
| Obesity | 27 (6) | 5 (8) | 29 (7.3) | >0.9 |
| HCT-CI > 3 | 73 (17) | 15 (25) | 58 (15) | 0.2 |
| Missing | 23 | 1 | 22 | |
| Disease Status | 0.3 | |||
| Complete Remission 1 | 345 (75) | 42 (68) | 303 (76) | |
| Complete Remission 2 | 69 (15) | 13 (21) | 56 (14) | |
| Refractory/Relapsed | 47 (11) | 7 (12) | 40 (10) | |
| Conditioning Intensity | 0.029 | |||
| MAC | 249 (54) | 25 (40) | 224 (56) | |
| RIC | 212 (46) | 37 (60) | 175 (44) | |
| Donor Type | 0.8 | |||
| Matched Sibling Donor | 86 (19) | 10 (16) | 76 (19) | |
| 10/10 MUD | 77 (17) | 9 (15) | 68 (17) | |
| 7/8 MMUD | 30 (6.5) | 5 (8) | 25 (6.3) | |
| Haploidentical | 268 (58) | 38 (61) | 230 (58) | |
| Day + 100 Cumulative Incidence of ECE | - | 11% (4.9–21%) | 7% (4.8–9.9%) | 0.53 |
| 2-year Cumulative Incidence of LCE | - | 12% (4.4–25%) | 2.9% (1.4–5.3%) | 0.09 |
| Main Outcomes: | ||||
| 2-year Overall Survival, % (IC95) | - | 70% (60–83%) | 69% (64–73%) | 0.5 |
| 2-year Non-Relapse mortality, % | - | 17% (13–21%) | 18% (9.5–29%) | 0.2 |
| Unbalanced | IPW-ATT Balanced | PSM Balanced | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Non-PCC | PCC | SMD | Non-PCC | PCC | SMD | Non-PCC | PCC | SMD |
| N = 399 | N = 62 | Weights Sum: 62 | Weights Sum: 62 | N = 248 | N = 62 | ||||
| HTN | 0.3 | 0.01 | 0.1 | ||||||
| No | 323 (81%) | 43 (69%) | 43 (70%) | 43 (69%) | 178 (72%) | 43 (69%) | |||
| Yes | 76 (19%) | 19 (31%) | 19 (30%) | 19 (31%) | 70 (28%) | 19 (31%) | |||
| Age | 54 (42, 63) | 60 (48, 66) | 59 (49, 65) | 60 (48, 66) | 0.00 | 59 (50, 65) | 60 (48, 66) | ||
| Conditioning intensity | 0.3 | 0.00 | 0.1 | ||||||
| MAC | 224 (56%) | 25 (40%) | 25 (41%) | 25 (40%) | 107 (43%) | 25 (40%) | |||
| RIC | 175 (44%) | 37 (60%) | 37 (59%) | 37 (60%) | 141 (57%) | 37 (60%) | |||
| Disease status | 0 | 0.01 | 0 | ||||||
| RC | 359 (90%) | 55 (89%) | 55 (89%) | 55 (89%) | 223 (90%) | 55 (89%) | |||
| R/R LMA | 40 (10%) | 7 (11%) | 7 (11%) | 7 (11%) | 25 (10%) | 7 (11%) | |||
| Donor | 0.1 | 0.02 | 0.1 | ||||||
| MSD | 76 (19%) | 10 (16%) | 10 (16%) | 10 (16%) | 45 (18%) | 10 (16%) | |||
| MUD | 68 (17%) | 9 (15%) | 9 (15%) | 9 (15%) | 40 (16%) | 9 (15%) | |||
| MMUD | 25 (6.3%) | 5 (8.1%) | 5 (8.6%) | 5 (8.1%) | 20 (8.1%) | 5 (8.1%) | |||
| HAPLO | 230 (58%) | 38 (61%) | 37 (61%) | 38 (61%) | 143 (58%) | 38 (61%) | |||
| HCT > 3 | 0.2 | 0.01 | 0.1 | ||||||
| No | 333 (83%) | 47 (76%) | 47 (76%) | 47 (76%) | 193 (78%) | 47 (76%) | |||
| Yes | 66 (17%) | 15 (24%) | 15 (24%) | 15 (24%) | 55 (22%) | 15 (24%) | |||
| DLP | 0.1 | 0.01 | 0 | ||||||
| No | 348 (87%) | 53 (85%) | 53 (86%) | 53 (85%) | 210 (85%) | 53 (85%) | |||
| Yes | 51 (13%) | 9 (15%) | 9 (14%) | 9 (15%) | 38 (15%) | 9 (15%) | |||
| Early Cardiac Events (ECE) | Total Events N = 35 | PCC N = 7 | Non-PCC N = 28 | p Value |
|---|---|---|---|---|
| ECE grade (CTCAE), n (%) | ||||
| Grade I–II | 9 (54.3) | 3 (32.9) | 16 (57.2) | 0.064 |
| Grade III–IV | 12 (34.3) | 2 (28.6) | 10 (35.7) | |
| Grade V | 4 (11.4) | 2 (28.6) | 2 (7.1) | |
| ECE, n patients (%) | ||||
| Arrythmia | 11 (31.4) | 3 (42.9) | 8 (28.6) | 0.528 |
| Heart Failure | 9 (25.7) | 3 (42.9) | 6 (21.4) | |
| Stroke/Ischemia | 3 (8.6) | 0 | 3 (10.7) | |
| Pericarditis/Pericardial Effusion | 11 (31.4) | 1 (14.3) | 10 (35.7) | |
| Other (Cardiomiopathy) | 1 (2.9) | 0 | 1 (3.6) | |
| Days to ECE, median (range) | 15 (8–49) | 7 (3–29) | 15 (8–51) | 0.933 |
| Death due to CE | 4 (11.4) | 5 (71.4) | 2 (7.1) | 0.171 |
| Death during follow-up | 17 (48.6) | 2 (28.6) | 12 (42.9) | 0.228 |
| Day +30 Mortality Rate | 4 (11.4) | 2 (28.6) | 2 (7.1) | 0.214 |
| Day +60 Mortality Rate | 6 (17.1) | 2 (28.6) | 4 (14.2) | 0.889 |
| Main Outcomes: | ||||
| 2-year Overall Survival, % (IC95) | 53.4 (35.6–68.3) | 28.6 (4.1–61.2) | 59.9 (39.3–75.4) | 0.164 |
| 2-year Non-Relapse mortality, % | 37.1 (21.3–53.0) | 57.1 (12.1–86.2) | 32.1 (15.8–49.8) | 0.255 |
| Late Cardiac events (LCE) | Total events N = 26 | PCC N = 5 | Non-PCC N = 21 | p value |
| LCE grade (CTCAE), n (%) | 0.230 | |||
| Grade I–II | 17 (65.4) | 5 (20.0) | 12 (57.2) | |
| Grade III–IV | 6 (23.1) | 0 | 6 (28.59 | |
| Grade V | 2 (7.7) | 0 | 2 (9.5) | |
| LCE, n patients (%) | ||||
| Arrythmia | 5 (19.2) | 0 | 5 (23.8) | 0.391 |
| Heart Failure | 10 (38.5) | 3 (60.0) | 7 (33.5) | |
| Stroke/Ischemia | 4 (15.4) | 1 (20.0) | 4 (19.0) | |
| Pericarditis/Pericardial Effusion | 5 (19.2) | 0 | 4 (19.0) | |
| Other | 2 (7.7) | 1 (20.0) | 1 (4.8) | |
| Days to LCE, median (range) | 437 (183–850) | 469 (264–574) | 383 (172–1087) | <0.001 |
| Death due to CE | 3 (11.5) | 1 (20.0) | 2 (9.5) | 0.937 |
| Death during follow-up | 10 (38.5) | 2 (40.0) | 8 (38.1) | 0.488 |
| Day + 30 Mortality Rate | 5 (19.2) | 0 | 5 | 0.073 |
| Day + 60 Mortality Rate | 5 (19.2) | 0 | 5 | 0.073 |
| Main Outcomes: | ||||
| 2-year Overall Survival, % (IC95) | 76.2 (54.2–88.5) | 100 | 70.2 (45.1–85.4) | 0.887 |
| 2-year Non-Relapse mortality, % | 3.8 (0.3–16.8) | 0 | 4.8 (0.3–20.3) | 0.149 |
| ECE N = 7 | LCE N = 5 | p Value | |
|---|---|---|---|
| Prior Cardiac Morbidity | 0.76 | ||
| Arrythmia | 3 (42.8) | 1 (20.0) | |
| Heart Failure (insuf ventric) | 2 (28.5) | 0 | |
| Isquemia | 0 | 1 (20.0) | |
| Pericardiac Disease | 1 (14.3) | 0 | |
| Moderate/Server Valvopathy | 1 (14.3) | 3 (60.0) | |
| Other | 0 | 0 | |
| CE, n patients (%) | 0.76 | ||
| Arrythmia | 3 (42.9) | 0 | |
| Heart Failure | 3 (42.9) | 3 (60.0) | |
| Stroke/Ischemia | 0 | 1 (20.0) | |
| Pericarditis/Pericardial Effusion | 1 (14.3) | 0 | |
| Other (Cardiomiopathy) | 0 | 1 (20.0) | |
| Newly diagnosed CE, n patients (%) | 4 (57.1) | 5 (100) | 0.20 |
| Descompensation, n patients (%) | 3 (42.9) | 0 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrent-Rodríguez, A.; Cascos, E.; Garcés, V.N.; Pérez-López, E.; Baile-González, M.; Rodríguez, C.M.; Cascón, M.J.P.; Luque, M.; Esquirol, A.; Calvo, C.M.; et al. Safety of Post-Transplant Cyclophosphamide-Based Prophylaxis in AML Patients with Pre-Existing Cardiac Morbidity Undergoing Allogeneic Hematopoietic Cell Transplantation. Cancers 2025, 17, 3128. https://doi.org/10.3390/cancers17193128
Torrent-Rodríguez A, Cascos E, Garcés VN, Pérez-López E, Baile-González M, Rodríguez CM, Cascón MJP, Luque M, Esquirol A, Calvo CM, et al. Safety of Post-Transplant Cyclophosphamide-Based Prophylaxis in AML Patients with Pre-Existing Cardiac Morbidity Undergoing Allogeneic Hematopoietic Cell Transplantation. Cancers. 2025; 17(19):3128. https://doi.org/10.3390/cancers17193128
Chicago/Turabian StyleTorrent-Rodríguez, Arnau, Enric Cascos, Víctor Navarro Garcés, Estefanía Pérez-López, Mónica Baile-González, Carlos Martín Rodríguez, María Jesús Pascual Cascón, Marta Luque, Albert Esquirol, Carmen Martín Calvo, and et al. 2025. "Safety of Post-Transplant Cyclophosphamide-Based Prophylaxis in AML Patients with Pre-Existing Cardiac Morbidity Undergoing Allogeneic Hematopoietic Cell Transplantation" Cancers 17, no. 19: 3128. https://doi.org/10.3390/cancers17193128
APA StyleTorrent-Rodríguez, A., Cascos, E., Garcés, V. N., Pérez-López, E., Baile-González, M., Rodríguez, C. M., Cascón, M. J. P., Luque, M., Esquirol, A., Calvo, C. M., Peña-Muñoz, F., Fernando, I. H., Ormtegi, I. O., Marín, A. J. S., Fernández-Luis, S., Domínguez-García, J. J., Fernández, S. V., Lorenzo, J. L. L., de Sanmamed Girón, M. F., ... Salas, M. Q., on behalf of the GETH-TC. (2025). Safety of Post-Transplant Cyclophosphamide-Based Prophylaxis in AML Patients with Pre-Existing Cardiac Morbidity Undergoing Allogeneic Hematopoietic Cell Transplantation. Cancers, 17(19), 3128. https://doi.org/10.3390/cancers17193128






