Simple Summary
People in low- and middle-income countries face a higher risk of developing cancer owing to exposure to harmful environmental and infectious factors. These include polluted air and water, chemicals used in farming and industry, and infections such as HIV, hepatitis, and human papillomavirus. These exposures often occur together and can worsen their effects, especially when healthcare is difficult to access. This article looks at how a person’s total exposure to these harmful influences throughout their life, what scientists call the “exposome”, can help explain why cancer is more common and lethal in poorer countries. This study also explored how these risks affect individuals differently, based on their genes, diets, and living conditions. By understanding the full picture of exposure, this study shows how public health strategies can be improved. These include vaccination, early screening, safe work and home environments, and robust health systems. These findings are especially important for regions such as sub-Saharan Africa and South Asia, where cancer rates are increasing. This research helps point the way toward fairer and more effective methods for preventing and treating cancer worldwide.
Abstract
Cancer disparities in low- and middle-income countries (LMICs) arise from multifaceted interactions between environmental exposures, infectious agents, and systemic inequities, such as limited access to care. The exposome, a framework encompassing the totality of non-genetic exposures throughout life, offers a powerful lens for understanding these disparities. In LMICs, populations are disproportionately affected by air and water pollution, occupational hazards, and oncogenic infections, including human papillomavirus (HPV), hepatitis B virus (HBV), Helicobacter pylori (H. pylori), human immunodeficiency virus (HIV), and neglected tropical diseases, such as schistosomiasis. These infectious agents contribute to increased cancer susceptibility and poor outcomes, particularly in immunocompromised individuals. Moreover, climate change, food insecurity, and barriers to healthcare access exacerbate these risks. This review adopts a population-level exposome approach to explore how environmental and infectious exposures intersect with genetic, epigenetic, and immune mechanisms to influence cancer incidence and progression in LMICs. We highlight the critical pathways linking chronic exposure and inflammation to tumor development and evaluate strategies such as HPV and HBV vaccination, antiretroviral therapy, and environmental regulation. Special attention is given to tools such as exposome-wide association studies (ExWASs), which offer promise for exposure surveillance, early detection, and public health policy. By integrating exposomic insights into national health systems, especially in regions such as sub-Saharan Africa (SSA) and South Asia, LMICs can advance equitable cancer prevention and control strategies. A holistic, exposome-informed strategy is essential for reducing global cancer disparities and improving outcomes in vulnerable populations.
1. Introduction
Cancer remains a leading cause of morbidity and mortality worldwide; however, its impact is not uniformly distributed. Low- and middle-income countries (LMICs) are projected to account for over 70% of the global cancer burden by 2040 [1]. This disparity stems not only from higher incidence rates but also from poorer survival outcomes, driven by challenges such as limited healthcare infrastructure, shortages of medical personnel, delayed diagnosis, and limited access to treatment [2].
Key drivers of cancer in LMICs include a higher prevalence of infectious agents, environmental pollutants, and occupational hazards, often due to insufficient regulatory oversight [3]. For example, hepatitis B virus (HBV) and human papillomavirus (HPV) are widespread in these regions and are major causes of liver and cervical cancer, respectively [4].
Neglected tropical diseases (NTDs), such as Schistosoma haematobium, Opisthorchis viverrini, and Clonorchis sinensis, are also associated with bladder and bile duct cancers [5]. These pathogens promote chronic inflammation and immune modulation, contributing to cancer development in the host [6]. Several helminths have been classified as Group 1 carcinogens by the International Agency for Research on Cancer (IARC), underscoring the role of infectious agents in cancer etiology [6].
In examining these and other environmental contributors to cancer risk, this review adopts the World Bank’s gross national income (GNI)-based classification to define LMICs (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups, accessed on 26 July 2025).
Within this context the exposome, a concept introduced by Christopher Wild in 2005, provides a comprehensive framework for understanding how non-genetic exposures, including environmental, infectious, and behavioral factors, interact with the genome over an individual’s lifetime [7,8]. This framework is particularly relevant for LMICs, where overlapping exposures to pollution, pathogens, and socioeconomic stressors create compounded cancer risks [9].
Although the exposome is traditionally used in individualized exposure science, this review adopts a population-level perspective. This manuscript investigates the cumulative exposures, such as industrial pollutants, occupational hazards, and endemic infectious diseases, that contribute to cancer disparities in LMICs. By evaluating how these exposures shape cancer outcomes, we highlight the relevance and potential of exposome-based methodologies, such as exposome-wide association studies (ExWASs), in advancing cancer research within LMIC contexts. The analysis explores disparities from both a global perspective, comparing LMICs to high-income countries in terms of cancer burden, healthcare access, and survival rates, and a localized view that addresses the geographic, economic, and social inequities within LMICs themselves. Through this dual lens, the manuscript underscores the urgency for context-specific research and policy interventions that incorporate exposomic insights to improve cancer prevention, diagnosis, and treatment in underserved populations.
2. The Exposome and Cancer Disparities
2.1. Definition and Components of the Exposome
The exposome encompasses the totality of environmental exposures that an individual encounters over the course of their life. These include both external factors, such as air and water pollution, occupational hazards, infections, and diet, and internal biological responses, including inflammation, oxidative stress, and metabolic changes (Figure 1) [9].
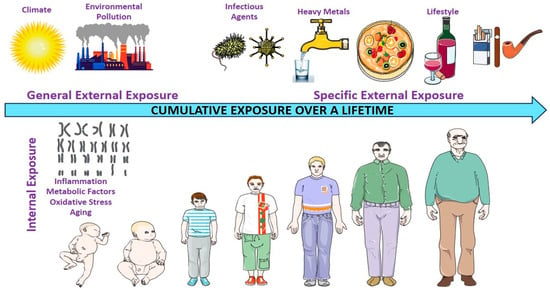
Figure 1.
Environmental exposure experienced by individuals throughout their lifetime. This figure illustrates the exposome framework, comprising three interconnected domains that capture the totality of environmental exposure throughout an individual’s lifespan. The general external domain encompasses broad contextual factors, including climate, urban or rural living environments, socioeconomic status, and ambient pollution (e.g., air quality and industrial emissions). The specific external domain includes more targeted exposures, such as water and air pollutants (e.g., heavy metals), tobacco and alcohol use, infections (e.g., viruses and bacteria), ultraviolet or artificial light exposure, and dietary patterns, all of which are influenced by individual behaviors and local environmental conditions. The internal domain reflects the body’s biological responses to external exposures, including chronic inflammation, oxidative stress, metabolic dysregulation, immune activation, and aging processes. Together, these domains capture the dynamic and cumulative nature of exposures that shape disease susceptibility, including cancer risk, over time.
In the context of cancer, disparities in exposome profiles contribute significantly to the unequal burden of disease between LMICs and high-income countries (HICs). The interactions among environmental pollutants, infectious agents, and physiological stressors form a complex risk landscape that shapes cancer incidence and outcomes in LMICs [7,8].
2.1.1. General External Exposure
In LMICs, widespread environmental pollutants such as fine particulate matter (PM2.5), heavy metals, and pesticides pose significant cancer risks. These exposures often result from unregulated industrial activity, agricultural practices, and weak environmental policies [10]. PM2.5, a common air pollutant, has been consistently associated with an elevated risk of respiratory cancers, particularly lung cancer [11].
Likewise, chronic exposure to heavy metals such as arsenic, lead, and cadmium, which are commonly found in contaminated water and soil, has been associated with an increased risk of lung, bladder, and skin cancers [12].
The use of agricultural pesticides also contributes significantly to environmental carcinogen exposure. Certain classes of pesticides, such as organochlorines and organophosphates, are classified as probable carcinogens and are associated with an increased risk of non-Hodgkin lymphoma and leukemia [10].
Climate change further amplifies these risks by creating environmental conditions that favor the proliferation of aflatoxin-producing fungi, particularly in warmer and more humid regions. Staple crops, such as maize and peanuts, are especially vulnerable. Aflatoxin exposure is a well-established risk factor for liver cancer, with the burden disproportionately affecting regions such as SSA and parts of Asia, where food safety regulations and monitoring are limited [13].
2.1.2. Specific External Exposures
Specific external exposures such as infectious agents and dietary carcinogens are major contributors to cancer risk in LMICs. HPV is the leading cause of cervical cancer, accounting for approximately 70% of the global cases. In SSA, low HPV vaccination coverage and limited cervical cancer screening programs contribute to the disproportionately high incidence rates [14]. Similarly, chronic infections with HBV and Helicobacter pylori (H. pylori) are prevalent in LMICs and significantly elevate the risk of liver and gastric cancers, respectively, by promoting chronic inflammation and cellular damage in target organs [15,16]. Nutritional deficiencies and dietary exposures further exacerbate cancer risk. Economic constraints in LMICs often result in diets lacking essential micronutrients while containing high levels of carcinogens such as nitrosamines, which are formed during the processing of preserved meats. These substances are strongly associated with an increased risk of colorectal cancer [17].
2.1.3. Internal Biological Responses
Internal biological responses, particularly chronic inflammation and metabolic dysregulation, are central mechanisms through which external exposures contribute to cancer development [18]. Chronic inflammation is a hallmark of persistent infection and environmental insults. For example, H. pylori infection induces sustained gastritis, creating a pro-tumorigenic environment that significantly increases the risk of gastric adenocarcinoma and, in some cases, primary gastric lymphoma [19].
In parallel, metabolic alterations, especially those associated with obesity, are emerging as critical risk factors for cancer in LMICs. Urbanization and dietary transitions toward energy-dense, nutrient-poor foods have driven rising obesity rates in these regions [20]. Obesity is associated with an increased risk of hormone-dependent malignancies, such as breast, ovarian, and endometrial cancers. This is largely due to obesity-induced metabolic disruptions, including insulin resistance and elevated circulating estrogen levels, which collectively promote tumor initiation and progression [21].
2.2. Role of the Exposome in Understanding Disparities
2.2.1. Exposure Differences in LMICs Compared with High-Income Countries
LMICs experience a disproportionately high burden of environmental and infectious carcinogenic exposures compared to HICs [3]. A notable example is arsenic contamination in drinking water, particularly in parts of South Asia, where millions are chronically exposed to levels exceeding international safety standards. Prolonged arsenic exposure is associated with an increased risk of skin, lung, and bladder cancers. In contrast, HICs have largely mitigated this risk through stringent water quality regulations and remediation efforts [22].
Occupational exposure also varies significantly across regions. In LMICs, workers in sectors such as mining, construction, and agriculture are frequently exposed to carcinogens such as asbestos and benzene, which are linked to mesothelioma and leukemia, respectively [23]. While regulatory frameworks and occupational safety measures have substantially reduced such risks in HICs, similar protections are often lacking or poorly enforced in LMICs, leaving workers vulnerable to preventable exposures [24].
2.2.2. Cumulative Impact of Environmental and Infectious Agents on Cancer Outcomes
The cumulative interaction between environmental and infectious exposures significantly amplifies cancer risk, particularly in LMICs, where regulatory and healthcare systems are often under-resourced. Co-exposure to aflatoxins and chronic HBV infection is a well-documented example that markedly increases the risk of hepatocellular carcinoma (HCC) due to their synergistic effects on DNA damage and liver inflammation. This phenomenon is especially prevalent in regions with inadequate food safety and storage practices.
Moreover, the convergence of environmental pollutants and oncogenic pathogens accelerates epigenetic alterations, such as aberrant DNA methylation. These changes can activate oncogenes and silence tumor suppressor genes, contributing to malignant transformation [25]. In LMICs, high levels of indoor air pollution primarily from the use of biomass fuel can compound these risks, particularly in the absence of effective healthcare infrastructure and preventive interventions. This interplay between multiple risk factors underscores the necessity of integrated cancer control strategies that go beyond addressing single exposures. Effective interventions must consider the broader environmental and social determinants of health to mitigate the disproportionate cancer burden in these settings [26].
3. Environmental Drivers of Cancer Disparities Within the Exposome Framework
Environmental drivers contributing to cancer disparities in LMICs, particularly across Africa, are complex and shaped by the region’s unique socioeconomic, ecological, and cultural contexts (Figure 2) [27]. The exposome framework, which captures the totality of environmental exposures over a lifetime, offers a valuable lens for understanding these disparities. One major contributor is indoor air pollution resulting from the widespread use of biomass fuels for cooking and heating, which is strongly associated with an increased risk of esophageal squamous cell carcinoma (ESCC) [28]. Occupational exposures to carcinogens, when combined with personal behaviors such as tobacco smoking, further elevate lung cancer risk in urban and industrial settings. Additionally, geographic variations in thyroid cancer prevalence have been observed in countries such as Tanzania, where the incidence appears to be linked to regional differences in dietary iodine levels, highlighting the interaction between environmental and nutritional factors [29]. These examples underscore how environmental risks vary not only between countries but also within them, reinforcing the need for localized interventions. Addressing these disparities requires context-specific public health strategies that incorporate local data on environmental exposure, infrastructure limitations, and sociocultural practices. Such tailored approaches are essential to effectively reduce the cancer burden in African LMICs [30].
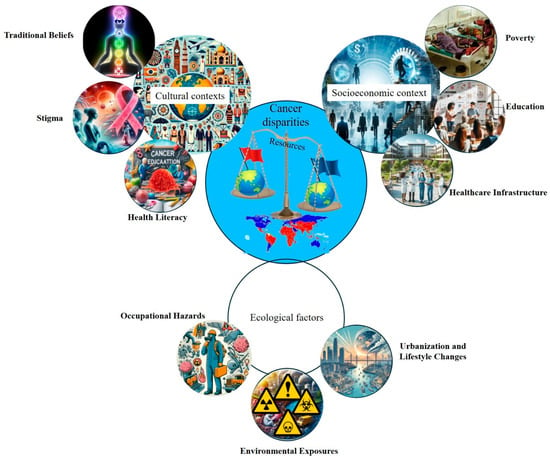
Figure 2.
Cancer disparities in LMICs. This conceptual diagram highlights the diverse and interconnected cultural, socioeconomic, and environmental determinants of cancer disparities in LMICs. Using the exposome framework, it illustrates how cumulative exposure to environmental pollutants, infectious agents, lifestyle behaviors, and healthcare access barriers interact with systemic inequities to influence cancer incidence, progression, and outcomes. This figure underscores the need for integrated, context-specific strategies to address the multifactorial nature of cancer risk in resource-limited settings.
3.1. Air and Water Pollution and Its Association with Cancer in LMICs
Air and water pollution are major environmental health challenges in LMICs, particularly in rapidly urbanizing regions of Africa (Figure 3). In cities such as Lagos (Nigeria) and Johannesburg (South Africa), rising industrial activity and traffic emissions have significantly degraded air quality. Common pollutants such as fine particulate matter (PM2.5), nitrogen dioxide (NO2), and volatile organic compounds (VOCs) are strongly associated with respiratory and urinary tract cancers, notably lung and bladder cancer [31].
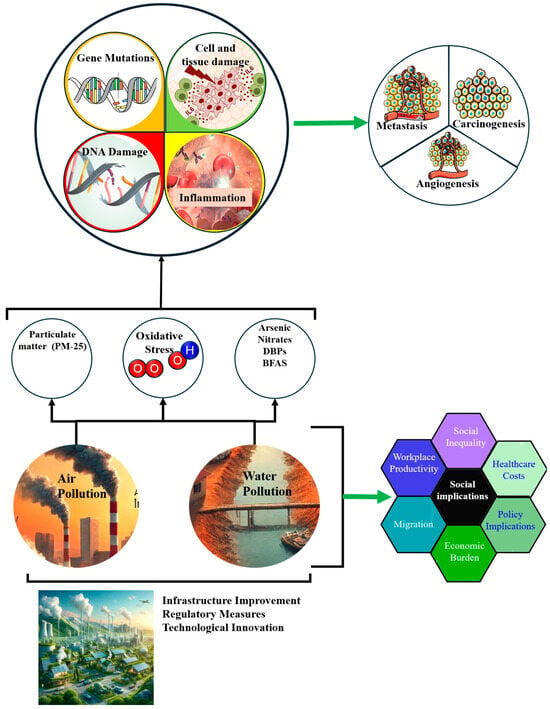
Figure 3.
Environmental Health Issues in African LMICs. The diagram demonstrates the complex pathways through which air and water pollution (PM-25, arsenic, nitrates, DBPs, and BFAS) leads to oxidative stress, resulting in gene mutations, DNA damage, inflammation, and subsequent health issues such as metastasis, carcinogenesis, and angiogenesis. Social implications include increased healthcare costs, social inequality, and decreased workplace productivity. Efforts to mitigate these impacts include infrastructure improvements, regulatory measures, and technological innovations.
Chronic NO2 exposure, in particular, correlates with elevated lung cancer incidence, although efforts to accurately assess this risk are often constrained by limited localized data [32]. In response, mitigation strategies such as the adoption of clean energy technologies, improved air quality monitoring, and sustainable urban planning are critical to reducing exposure and associated health risks [33].
Water pollution also contributes significantly to cancer risk, particularly in areas with poor sanitation and weak industrial waste regulations. In the Niger Delta, chronic oil pollution exposes communities to polycyclic aromatic hydrocarbons (PAHs) and heavy metals, such as arsenic and cadmium [34]. These contaminants induce oxidative stress and DNA damage, contributing to the development of liver, bladder, and gastric cancers (Figure 3). Addressing this requires investment in water quality monitoring, affordable filtration technologies, and stricter enforcement of environmental protection laws [34].
The Niger Delta exemplifies the complex link between environmental degradation, pollution-related exposure, and cancer risk, particularly where socioeconomic vulnerabilities amplify health impacts [35].
3.2. Occupational Exposure and Industrial Waste
Occupational exposure to carcinogens plays a significant role in cancer disparities across Africa, particularly in high-risk sectors such as mining and agriculture (Figure 4). In South Africa, gold miners face an elevated risk of lung cancer and mesothelioma due to their long-term exposure to silica dust and asbestos. The incidence of silicosis and related diseases, including lung cancer and tuberculosis, remains high among these workers [36].
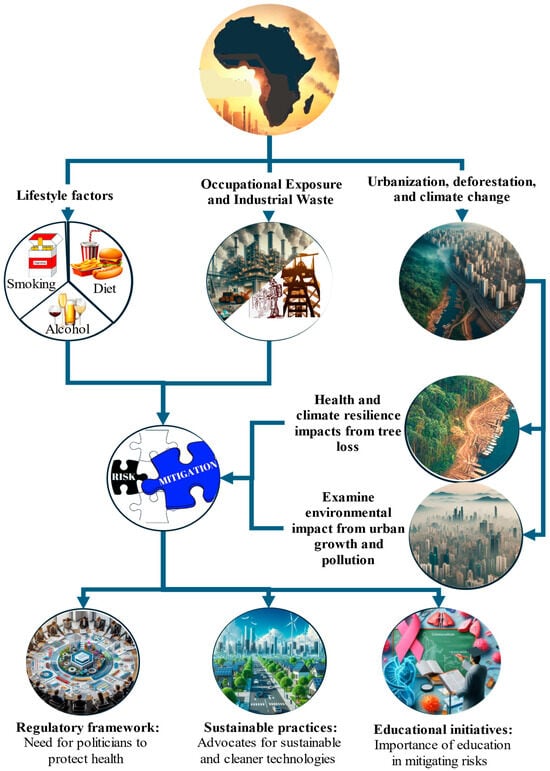
Figure 4.
Occupational exposure and industrial waste in Africa. This figure highlights occupational risks, industrial waste management, regulatory frameworks, and educational initiatives, emphasizing their roles in mitigating health impacts and addressing cancer. This figure further highlights sustainable practices, the impacts of urbanization, and the effects of deforestation, emphasizing their roles in air pollution, climate resilience, and public health in African LMICs.
Informal or artisanal mining further increases vulnerability due to poor safety measures and a lack of protective equipment [37]. Improper industrial waste management in rapidly urbanizing cities, such as Nairobi and Accra, exacerbates community-level exposure to environmental carcinogens [37]. The unregulated disposal of hazardous materials contaminates the air, water, and soil, increasing the risk of respiratory and gastrointestinal cancers. In South Africa, the legacy of asbestos mining continues to threaten surrounding communities with long-term exposure to asbestos-related diseases (ARDs) [37].
In the agricultural sector, the widespread use of pesticides, especially those containing carcinogenic compounds, has been linked to various hematologic malignancies. Limited regulation and poor training on safe pesticide use mean that many farm workers operate without protective equipment, increasing their cancer risk [37].
Addressing these occupational and environmental threats requires integrated policy approaches that strengthen workplace safety, enforce hazardous waste regulations, and promote sustainable industrial and agricultural practices [38].
3.3. Urbanization and Deforestation
Urbanization across African countries has introduced major public health challenges, particularly in terms of worsening air quality. In many low-income urban areas, reliance on biomass fuels such as charcoal and firewood for cooking, especially in poorly ventilated homes, contributes significantly to indoor air pollution, a known risk factor for respiratory diseases [39]. The promotion of cleaner cooking technologies and public awareness campaigns has begun to reduce this health burden [39]. Transitioning to cleaner fuels offers substantial public health benefits by lowering the incidence of pollution-related diseases [39].
Deforestation, which is largely driven by agricultural expansion and urban sprawl, undermines environmental and health systems. Forest loss contributes to greenhouse gas emissions and erodes natural buffers that are critical for climate regulation and food security [40]. These ecological disruptions increase drought frequency and food insecurity, indirectly weakening immune health and increasing disease vulnerability. Moreover, unplanned urban expansion often pushes informal settlements into industrial zones, where residents are exposed to air and water pollutants [41].
This overlap between environmental degradation and urban poverty amplifies the risk of cancer and other chronic diseases. The interlinked effects of urbanization, deforestation, and climate change pose a growing threat to public health in Africa. With urban areas expected to house over 50% of the continent’s population by 2050, there is an urgent need to address inefficient biomass fuel use, especially charcoal, which is four to six times less efficient than wood fuel. Community-based forest management and REDD+ initiatives offer viable solutions for reducing emissions while supporting sustainable livelihoods [41].
3.4. Lifestyle Factors Compounded by External Exposure
In African populations, lifestyle factors, including tobacco use, alcohol consumption, and changing dietary patterns, interact with environmental exposures to increase cancer risk. Tobacco smoking remains prevalent in many regions and significantly compounds the carcinogenic effects of air pollution. Combined exposure to smoking and airborne pollutants contributes to higher rates of lung, bladder, and oral cancers [42].
Alcohol consumption also increases the risk of cancer, particularly when combined with exposure to aflatoxins or infectious agents. A growing body of research links alcohol intake to breast cancer, with increasing consumption observed among women and youth in sub-Saharan Africa (SSA) due to shifting cultural norms and Western influence [43].
Urbanization has also altered dietary behaviors, leading to an increased intake of ultra-processed foods and red meat, which are associated with colorectal and obesity-related cancers. In contrast, traditional African diets rich in grains, legumes, and vegetables offer protective effects [44]. Promoting traditional diets through public health campaigns and urban agriculture may help counter rising cancer trends. However, in informal settlements, limited access to healthy food and persistent environmental exposure remain significant barriers. Specific regional dietary staples have also been associated with the risk of cancer. Mabusela et al. [45] identified a link between maize meal consumption and endemic esophageal squamous cell carcinoma. The authors found that esterified linoleic acid in maize meal degrades during storage, increasing prostaglandin E2 (PGE2) production and promoting low-acid reflux, which is a risk factor for esophageal cancer. Similarly, in the Eastern Cape, traditional beer consumption has been associated with esophageal cancer [46].
4. Infectious Agents as a Component of the Exposome
4.1. Disproportionate Prevalence of Oncogenic Infectious Agents in LMICs
4.1.1. HPV and Cervical Cancer
HPV is the primary etiological agent of cervical cancer globally, with LMICs experiencing the highest disease burden. SSA, in particular, records some of the highest cervical cancer incidence and mortality rates due to limited access to HPV vaccination and organized screening programs [47]. Persistent infection with high-risk HPV genotypes, most notably HPV-16 and HPV-18, is strongly associated with the progression from cervical intraepithelial neoplasia to invasive cervical cancer [48]. Structural barriers such as inadequate healthcare infrastructure, logistical challenges in vaccine distribution, and cultural resistance to vaccination exacerbate the burden in LMICs [49]. Moreover, the prevalence of HPV and co-infection with multiple HPV types is significantly elevated in HIV-positive women, compounding cervical cancer risk in immunocompromised populations [50]. To address this, integrating HPV vaccination into national immunization schedules and school-based health programs has shown promise in improving vaccine uptake. Additionally, expanding access to affordable screening tools, such as HPV DNA testing and visual inspection with acetic acid (VIA), is essential [51]. The World Health Organization’s (WHO) global strategy to eliminate cervical cancer underscores the importance of achieving high HPV vaccination coverage, aiming to significantly reduce cervical cancer incidence and mortality in LMICs over the coming decades [52].
4.1.2. HBV and HCV in Liver Cancer
Chronic infections with HBV and hepatitis C virus (HCV) are major drivers of hepatocellular carcinoma (HCC), especially in Africa and Asia. HBV often becomes chronic through perinatal transmission or early childhood exposure, significantly increasing the lifetime risk of liver cancer [53]. HCV is predominantly transmitted through blood exposure and poses a heightened risk in regions with inadequate blood screening and where intravenous drug use is common [54]. The combined exposure to viral hepatitis and aflatoxins, such as the naturally occurring mycotoxins found in improperly stored grains, further elevates HCC risk, particularly in LMICs [54]. This synergistic interaction highlights the need for integrated prevention strategies. Expanding neonatal HBV vaccination programs is a proven and cost-effective intervention that dramatically reduces the incidence of chronic HBV infection and subsequent HCC [55]. In addition, promoting food safety and proper grain storage is essential to limit exposure to aflatoxins. The advent of direct-acting antivirals (DAAs) has revolutionized HCV treatment, resulting in significant reductions in viral load, liver damage, and liver cancer incidence in treated populations [56].
4.1.3. Role of HIV in Increasing Cancer Susceptibility
Human immunodeficiency virus (HIV) increases cancer susceptibility through immune suppression, which facilitates the persistence and proliferation of oncogenic viruses such as HPV, Epstein–Barr virus (EBV), and Kaposi’s sarcoma-associated herpesvirus (KSHV). Consequently, individuals with chronic HIV infection are at a heightened risk for AIDS-defining cancers, including Kaposi’s sarcoma, non-Hodgkin lymphoma, and cervical cancer, especially in high-prevalence regions such as Southern Africa [57]. While antiretroviral therapy (ART) has improved life expectancy and offers opportunities for integrated cancer care, access to ART and screening programs remains uneven across LMICs [58]. HIV-positive individuals often experience impaired cancer immune surveillance, contributing to delayed diagnoses and more advanced disease at presentation [59]. For instance, women living with HIV are less likely to undergo regular cervical cancer screening, exacerbating their risk [60]. Improving outcomes in HIV-affected populations requires better integration of HIV care with cancer screening and prevention services [61]. Moreover, the increased longevity of individuals on ART has been accompanied by a rise in non-AIDS-defining cancers (NADCs). This trend is driven by co-infection with oncogenic viruses and higher prevalence of other risk factors, such as smoking and alcohol use, among people living with HIV [62]. These patterns underscore the need for tailored cancer surveillance and early detection strategies in long-term ART recipients.
4.1.4. Helicobacter Pylori Infection and Gastric Cancer
H. pylori is classified as a Group I carcinogen by the World Health Organization due to its strong association with chronic gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma. The burden of H. pylori-associated gastric cancer is particularly high in North Africa, driven by poor sanitation, limited access to clean water, and dietary exposure to carcinogens such as nitrosamines [63,64]. Epidemiological studies estimate that approximately 80–90% of gastric cancer cases globally are linked to H. pylori infection, underscoring its pivotal role in gastric carcinogenesis [65]. The bacterium’s ability to induce chronic inflammation and alter gastric epithelial cell biology through virulence factors, such as CagA and VacA, contributes to this elevated cancer risk. Effective public health interventions, including improved sanitation, population-level screening, and access to eradication therapies, are essential for reducing the incidence of H. pylori-related gastric cancer [66]. Additionally, dietary factors, particularly the intake of antioxidants such as vitamins C and E, have been shown to modulate infection risk and disease progression, emphasizing the importance of integrative strategies that address both environmental and nutritional determinants [67].
4.2. Neglected Tropical Diseases (NTDSs) and Cancer
Neglected tropical diseases (NTDs) primarily affect the poorest and most marginalized communities, specifically those without access to clean water, adequate sanitation, and proper housing. Poor hygiene and overcrowded conditions increase the vulnerability to infection, especially in the tropical and subtropical regions. Globally, approximately 1.5 billion people (24% of the world’s population) are infected with soil-transmitted helminths, with the highest prevalence in SSA, China, South America, and Southeast Asia [51].
Environmental conditions, such as warm climates, poor sanitation, and limited access to clean water, facilitate the continuous transmission of these infections. Even after treatment, reinfection is common owing to persistent environmental exposure [68]. Helminths have evolved sophisticated mechanisms to evade the immune system, including immune modulation, which inadvertently impairs the host’s ability to combat other pathogens [69]. This immunomodulation has been implicated in decreased cancer immune surveillance and the promotion of chronic inflammation, both of which increase oncogenic risk [70,71,72].
Infection with O. viverrini and C. sinensis is strongly linked to cholangiocarcinoma (bile duct cancer) [73], whereas S. haematobium is responsible for 46–75% of bladder cancers in endemic regions [74]. In addition, Schistosoma japonicum has been associated with colorectal cancer due to chronic inflammation caused by egg deposition [74]. Similarly, Schistosoma mansoni activates hepatocellular carcinoma-associated transcription factors (c-Jun and STAT3) in liver cells, promoting hepatocarcinogenesis [75]. Even protozoa previously considered benign, such as Blastocystis hominis, have been associated with colorectal cancer [76]. These findings underscore the strong etiological link between NTDs and cancer, particularly in LMICs, where both conditions are highly endemic and often underdiagnosed.
4.3. Interaction of Infectious Agents with Other Environmental Exposures
The interplay between infectious agents and environmental exposure is a critical contributor to cancer development, particularly in LMICs. When combined, these factors often amplify each other’s carcinogenic potential, increasing the risk of cancer through synergistic mechanisms of action. A well-documented example is co-exposure to HBV and aflatoxins, which significantly elevates the risk of HCC. Aflatoxins promote DNA adduct formation, whereas HBV suppresses immune surveillance, creating a permissive environment for cellular transformation [77]. Similarly, exposure to heavy metals can intensify the oxidative stress and DNA damage caused by H. pylori [78]. This combination leads to chronic inflammation, a hallmark of carcinogenesis, and increases the risk of gastric cancer [79].
HIV-induced immunosuppression also increases cancer susceptibility by facilitating persistent infections with oncogenic viruses such as HPV and Epstein–Barr virus. This immune compromise contributes to higher rates of cervical cancer and Kaposi’s sarcoma [80]. Environmental pollutants, including particulate matter and waterborne toxins such as arsenic, can further impair DNA repair mechanisms, thereby increasing the cancer risk in individuals already infected with oncogenic pathogens.
To mitigate these cumulative risks, public health interventions must address both infectious and environmental factors. Priorities include expanding HBV vaccination, improving grain storage to prevent aflatoxin contamination, implementing H. pylori eradication strategies, and enforcing industrial regulations to reduce air and water pollution. Enhanced environmental surveillance is crucial for monitoring and managing these overlapping exposures (Figure 5).
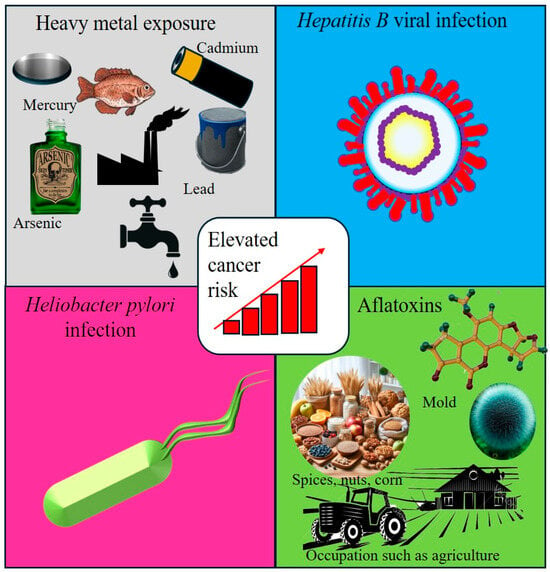
Figure 5.
Interactions between infectious agents and environmental exposure. This figure illustrates the interplay between infectious agents and environmental exposure, both of which contribute to the risk of cancer. It highlights key carcinogenic factors, including heavy metals, Hepatitis B Virus (HBV), Helicobacter pylori (H. pylori), and aflatoxins, emphasizing their synergistic effects in promoting carcinogenesis through chronic inflammation, DNA damage, and immune modulation.
4.4. Case Studies: Regions with a High Burden of Infection-Associated Cancer
4.4.1. East Africa
Cervical cancer remains a major public health concern in East Africa, particularly in countries such as Uganda and Kenya, where it is the leading cause of cancer-related deaths among women. The region faces alarmingly low HPV vaccination coverage, with rates reported to be less than 30%. This, combined with limited access to cervical cancer screening, contributes to persistently high mortality rates [81].
Recent studies have indicated that vaccine uptake is especially low in urban and peri-urban settings, with coverage as low as 8.6% in Kampala and 19.6% in Lira City, Northern Uganda [82]. Contributing factors include inadequate awareness of HPV and cervical cancer, widespread cultural misconceptions, and systemic barriers to vaccine distribution [83]. Integrating HPV vaccination into school-based health programs is a promising strategy to overcome these challenges. Such programs have been associated with improved vaccine acceptance and increased coverage among adolescent girls, helping reduce both the incidence and mortality of cervical cancer [84].
Moreover, expanding access to cost-effective diagnostic methods, such as HPV DNA testing, is essential for scaling up cervical cancer screening. The WHO advocates a comprehensive three-pronged approach that includes HPV vaccination, routine screening, and prompt treatment of precancerous lesions (Figure 6) [85].
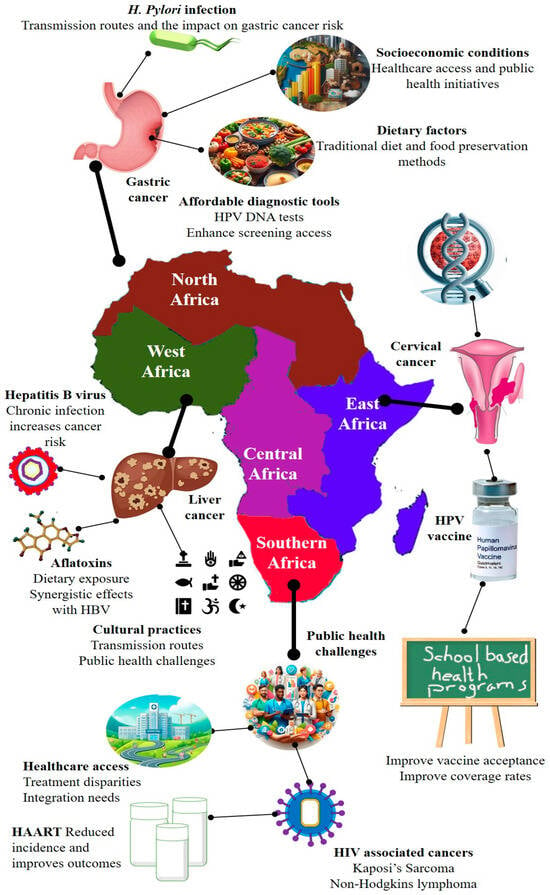
Figure 6.
Regional cancer burden and associated risk factors in Africa. This map highlights the geographic distribution of predominant cancer types and their associated risk factors across African regions. In North Africa, the high prevalence of gastric cancer is primarily driven by Helicobacter pylori infection, dietary habits, and socioeconomic disparities. West and Central Africa bear a significant burden of liver cancer, largely attributed to chronic hepatitis B virus (HBV) infection and dietary aflatoxin exposure. In East Africa, cervical cancer is the most common malignancy among women and is closely linked to persistent human papillomavirus (HPV) infection and limited access to screening services. Southern Africa exhibits a high incidence of HIV-associated cancers, particularly Kaposi’s sarcoma and non-Hodgkin lymphoma, exacerbated by limited healthcare access and uneven antiretroviral therapy (ART) coverage. This figure underscores the urgent need for affordable diagnostics (e.g., HPV DNA testing), expanded vaccine coverage, and culturally sensitive, school-based health education to reduce cancer disparities across the African continent.
4.4.2. West Africa
In West Africa, particularly in Nigeria and Ghana, liver cancer remains a major public health concern, largely driven by chronic HBV infection and environmental exposure to aflatoxins (Figure 6). Aflatoxin B1 (AFB1), a toxin produced by molds that commonly contaminate food crops such as maize and ground nuts, is strongly associated with HCC, especially in individuals co-infected with HBV [86]. A systematic review by Liu et al. (2012) confirmed that the synergistic effect of AFB1 and chronic HBV infection significantly elevates the risk of developing liver cancer in regions where both exposures are prevalent [87]. Cultural practices such as the use of shared razors and traditional scarification also contribute to HBV transmission, highlighting the importance of culturally sensitive community education [88]. To address these multifactorial risks, public health strategies should focus on improving neonatal HBV vaccination coverage, which has been proven to reduce transmission and the subsequent development of liver disease. Comprehensive interventions that combine vaccination with educational campaigns on food safety and infection prevention are essential to reduce the liver cancer burden in this region [88].
4.4.3. Southern Africa
Southern Africa faces a substantial public health burden driven by the intersection of high HIV prevalence and AIDS-defining cancers (ADCs), particularly Kaposi’s sarcoma (KS) and non-Hodgkin lymphoma (NHL) (Figure 6). These cancers are significantly more prevalent in regions with high HIV infection rates. The introduction of highly active antiretroviral therapy (HAART) has led to a decline in the incidence and mortality rates of KS and NHL [89]. Persistent inequities in access to antiretroviral therapy (ART) continue to negatively impact cancer outcomes in the region [90].
Integrating cancer care into existing HIV treatment frameworks is a critical strategy for improving outcomes, enabling concurrent management of both conditions [91]. Additionally, HIV-positive women are at an increased risk of cervical cancer, necessitating the incorporation of cervical cancer screening into HIV care protocols. Evidence suggests that such integrated models improve screening uptake and facilitate earlier diagnosis, which is key to effective treatment [92].
4.4.4. North Africa
In North Africa, particularly Egypt, the prevalence of gastric cancer is closely linked to high rates of H. pylori infection and associated dietary risk factors (Figure 6). H. pylori is a well-established etiological agent in gastric carcinogenesis, contributing to chronic gastritis, peptic ulcers, and ultimately gastric adenocarcinoma [93]. The World Health Organization classifies H. pylori as a Group 1 carcinogen due to its strong association with gastric cancer [94]. Genetic susceptibility further exacerbates this risk. For example, individuals with the GSTM1 null genotype are more vulnerable to gastric cancer when infected with H. pylori [95].
Inadequate sanitation and limited access to effective H. pylori eradication therapies in the region exacerbate this public health challenge. Many affected individuals remain undiagnosed and untreated, increasing the risk of advanced disease progression. To address this issue, national efforts should focus on improving sanitation infrastructure and expanding access to affordable diagnostic and treatment options. Implementing population-wide H. pylori screening and eradication programs could enable the early detection and prevention of gastric cancer, thereby reducing the disease burden across the region [96].
5. Mechanistic Pathways Linking the Exposome to Cancer
5.1. Synergistic Effects of Environmental Toxins and Infectious Agents
The exposome plays a pivotal role in determining cancer risk [7]. Understanding the mechanistic pathways through which these exposures exert their carcinogenic effects is crucial for identifying prevention strategies (Figure 7), particularly in LMICs, where the burden of environmental toxins and infectious diseases is often higher than that in HICs. This interplay between these two factors underscores the complex, multifactorial etiology of cancer, with environmental and infectious agents acting together to amplify the risk and progression of cancer. Viral infections inhibit DNA repair mechanisms, facilitating the integration of viral genomes into the host cell’s DNA. This integration disrupts cellular homeostasis and inhibits apoptosis, allowing the persistence of chronic infections. Over time, these chronic infections give rise to precancerous cells characterized by compromised tumor suppressor gene function and dysregulation of cell cycle genes. These alterations lead to aberrant epigenetic modifications that further exacerbate genomic instability. The resulting genomic instability and persistent inflammation create a microenvironment favorable for tumor development and progression, ultimately culminating in cancer (Figure 7).
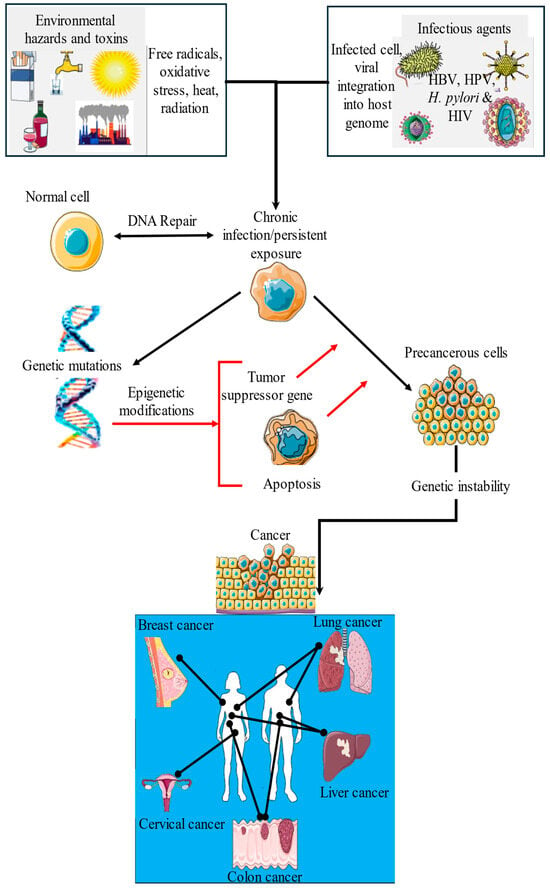
Figure 7.
Synergistic interactions between environmental toxins and infectious agents in cancer development. This schematic illustrates how environmental exposures such as air pollutants, tobacco smoke, unhealthy diet, and alcohol consumption interact with infectious agents, such as HBV, HPV, H. pylori, and HIV, to drive the onset and progression of various cancers (e.g., lung, breast, colon, liver, and cervical). These combined exposures lead to genetic and epigenetic alterations, such as DNA damage, gene mutations, and disruption of gene expression. These molecular changes impair key cellular processes, including apoptosis, cell cycle control, and immune surveillance, thereby promoting cancer development. This figure highlights the complex and multifactorial etiology of cancer, particularly in high-exposure settings that are common in LMICs.
5.1.1. Epigenetic Changes Driven by Chronic Exposure
Epigenetic modifications, such as DNA methylation, histone changes, and altered non-coding RNA expression, are key mechanisms through which environmental toxins and infectious agents contribute to cancer development [97].
These heritable changes do not alter the DNA sequence but influence gene expression, often silencing tumor suppressor genes or activating oncogenes, thereby promoting tumorigenesis [98]. Chronic exposure to environmental toxins, including heavy metals, pesticides, and air pollutants, induces epigenetic changes. For instance, long-term As exposure is linked to skin, lung, and bladder cancers through hypermethylation of tumor suppressor genes [98]. These modifications disrupt normal cellular regulation and facilitate malignant transformation [99].
Infectious agents such as HPV and HBV also play a prominent role in reshaping the epigenetic landscape. Persistent infection with high-risk HPV strains leads to viral DNA integration and deregulation of cell cycle genes via epigenetic changes [100]. Similarly, HBV infection, particularly in endemic areas such as SSA and East Asia, has been associated with abnormal DNA methylation of cancer-related genes, such as cyclin-dependent kinase inhibitor 2A (CDKN2A), commonly known as p16INK4a, and Ras association domain family member 1 (RASSF1A), both of which play critical roles in liver carcinogenesis [101].
Importantly, co-exposure to environmental carcinogens and infectious agents may have synergistic effects, enhancing epigenetic disruption and amplifying cancer risk [9]. This convergence illustrates the complex multilevel interactions between the exposome and cancer pathways in vulnerable populations.
5.1.2. Oxidative Stress and DNA Damage Pathways
Reactive oxygen species (ROS), which are natural byproducts of cellular metabolism, can damage DNA, promote mutations, and alter the signaling pathways that drive tumorigenesis. Chronic exposure to environmental pollutants such as fine particulate matter (PM2.5), benzene, and pesticides elevates ROS production, leading to oxidative DNA damage, genomic instability, and ultimately, cancer [102]. For instance, benzene exposure is strongly associated with increased ROS generation, resulting in DNA strand breaks and mutations that contribute to leukemia [103]. Infections also play a significant role: H. pylori causes chronic gastric inflammation and promotes the release of ROS and reactive nitrogen species that damage DNA and contribute to gastric carcinogenesis [19]. Similarly, persistent HPV infection activates oxidative stress pathways that, in conjunction with viral oncogene expression and pre-existing genetic alterations, contribute to cervical cancer development [104]. The synergistic effect of environmental pollutants and infectious agents further amplifies oxidative stress and causes DNA damage. This dual burden is particularly pronounced in LMICs, where co-exposure to both sources is common and significantly elevates the risk of cancer.
5.2. Immune System Dysregulation and Chronic Inflammation
Persistent Infection and Cancer Risks
Persistent infection with high-risk strains of HPV is a major cause of cervical cancer. It triggers chronic inflammation in the cervical epithelium, characterized by sustained immune activation and the release of pro-inflammatory cytokines by macrophages and T lymphocytes. This inflammatory microenvironment promotes cellular transformation and tumor development [86]. Similarly, chronic hepatitis B virus (HBV) infection induces long-term hepatic inflammation. Continuous immune cell activation leads to elevated levels of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which support the survival and proliferation of infected hepatocytes [105]. These cytokines also contribute to genomic instability, which is a key mechanism of HCC development. Tumor-promoting inflammation caused by chronic infections facilitates immune evasion and angiogenesis, further enhancing cancer progression (Figure 7) [105].
5.3. Molecular and Genetic Susceptibilities Influenced by Cumulative Exposures
5.3.1. Genetic Polymorphisms and Susceptibility to Environmental Toxins
Genetic polymorphisms significantly influence an individual’s ability to metabolize and detoxify environmental toxins, thereby modifying their susceptibility to environmentally driven cancers [106]. Variants in genes involved in xenobiotic metabolism and DNA repair can either mitigate or exacerbate the carcinogenic effects of exposures. Cytochrome P450 enzymes (CYPs), particularly CYP1A1, play a central role in the metabolism of polycyclic aromatic hydrocarbons (PAHs) found in tobacco smoke and industrial pollutants. Polymorphisms in CYP1A1 are associated with increased lung cancer risk, especially among smokers [107]. Similarly, deletions or functional variants in GSTM1 and GSTT1, which encode glutathione S-transferase enzymes essential for detoxifying reactive intermediates, are associated with an elevated risk of bladder and lung cancers in individuals exposed to environmental toxins [108]. Variants in NQO1, an enzyme involved in quinone detoxification, are also correlated with a heightened cancer risk in settings of high air pollution [109]. In addition to metabolic genes, emerging evidence implicates polymorphisms in circadian rhythm genes such as ARNTL, NPAS2, and RORA in cancer susceptibility. Disruptions in circadian cycles due to night shift work are increasingly recognized as carcinogenic factors. Individuals with specific variants in these genes show increased risk for aggressive prostate cancer and potentially other malignancies, particularly when exposed to chronic circadian disruption [110]. These findings underscore the critical role of gene–environment interactions in the development of cancer. Identifying high-risk genetic profiles could support more personalized cancer prevention strategies, particularly in populations facing sustained environmental exposures.
5.3.2. Gene–Environment Interactions in Infectious-Related Cancer Risk
Gene–environment interactions play a pivotal role in modulating cancer risk associated with chronic infections, particularly in regions where oncogenic pathogens such as HPV, HBV, and H. pylori are highly prevalent [111]. Host genetic variations that influence immune response, inflammation, and infection clearance can significantly modify cancer susceptibility in infected individuals [108].
Polymorphisms in the human leukocyte antigen (HLA) system, particularly in HLA-DQ alleles, have been linked to an increased risk of cervical cancer in women with persistent HPV infection [112]. These HLA variants may compromise antigen presentation and impair immune-mediated viral clearance, allowing for prolonged HPV persistence and increasing the likelihood of progression to malignancy [113]. Similarly, single nucleotide polymorphisms (SNPs) in immune-regulatory genes such as IL-10 have been associated with elevated HCC risk in individuals with chronic HBV infection [114]. Reduced IL-10 expression may result in insufficient control of hepatic inflammation, promoting fibrosis and carcinogenesis. In the context of H. pylori infection, polymorphisms in pro-inflammatory cytokine genes, including IL-1β and TNF-α, can intensify mucosal inflammation, leading to chronic gastritis and heightened risk of gastric cancer [16]. Collectively, these examples highlight how genetic predispositions, especially those affecting immune and inflammatory pathways, can magnify the oncogenic potential of chronic infections. Understanding these interactions is essential for developing targeted prevention and screening strategies in high-risk populations.
5.3.3. Cumulative Exposures and Their Impact on Genetic Stability
Chronic exposure to environmental toxins such as tobacco smoke, air pollution, and industrial chemicals induces a range of DNA lesions, including base modifications, strand breaks, and crosslinks. If unrepaired, these lesions can accumulate and initiate carcinogenesis [115]. Persistent exposure can overwhelm cellular DNA repair systems, resulting in genomic instability and an increased mutation burden. The situation is exacerbated when combined with chronic infections like HPV or HBV, which further compromise genomic integrity through viral DNA integration into the host genome [113]. This cumulative DNA damage significantly increases the risk of malignant transformation. Moreover, genetic predispositions that affect DNA repair efficiency can amplify these risks. Polymorphisms in key repair genes, such as TP53 and BRCA1/BRCA2, impair DNA damage correction, facilitating mutation accumulation. For instance, individuals with BRCA1 mutations, which are critical for repairing double-strand breaks, are at a markedly elevated risk of breast and ovarian cancers [116].
5.4. Cultural Practices and Cancer Risk in LMICs
Cultural traditions and social behaviors significantly influence cancer risk in LMICs. Practices embedded in daily life—shaped by socioeconomic conditions, traditional beliefs, and limited health education—contribute to the burden of cancers, especially those affecting the lungs, esophagus, and liver [117]. Although ethnicity, population origin, and regional diversity affect cancer patterns in SSA, the role of cultural attitudes and traditional medicine in cancer prevention and care remains underexplored [117].
One notable practice is the use of traditional tobacco pipes, particularly by women in Southern Africa. Unlike filtered cigarettes, pipe tobacco involves deep inhalation of unfiltered smoke, increasing exposure to carcinogens and elevating the risk of lung, oral, and esophageal cancers [118]. Cultural ceremonies in some Nguni communities also incorporate different types of tobacco, complicating efforts to introduce anti-smoking campaigns without addressing deeply rooted traditions [119]. Alcohol consumption, especially of unregulated sorghum-brewed beverages commonly used in community celebrations, has also been linked to an increased incidence of esophageal and liver cancers. These homemade brews may contain harmful contaminants that exacerbate their carcinogenic potential [120].
Urbanization has driven shifts in lifestyle, including increased sedentary behavior and dietary change. The replacement of traditional, high-fiber, plant-based diets with ultra-processed foods has contributed to rising obesity rates, which are associated with breast, colorectal, and endometrial cancers [121]. Additionally, rural and informal communities often lack access to health education, leading to misconceptions regarding cancer etiology and symptoms. Cultural norms, gender roles, and daily hardships—such as walking long distances for water or food—can delay health-seeking behavior, often resulting in late-stage diagnoses [122].
Culturally tailored public health strategies are essential for addressing these challenges. Interventions that engage community leaders, respect local customs, and provide accessible education can encourage healthier behavior. Integrating cancer prevention into culturally relevant platforms and improving community health literacy are critical steps toward reducing culturally mediated cancer risk.
6. Mitigating Cancer Disparities Through an Exposome-Informed Approach
6.1. Prevention Strategies
6.1.1. Vaccination Campaigns
Vaccination remains one of the most effective strategies for preventing cancers linked to infectious agents, particularly HPV and HBV. These viruses are major contributors to cervical and liver cancers, respectively, and are disproportionately prevalent in LMICs, exacerbating cancer disparities [123]. The global burden of cervical cancer, primarily driven by persistent HPV infection, is heavily concentrated in LMICs, where HPV vaccination coverage is low. However, there have been notable successes. For example, Rwanda’s national HPV vaccination program has achieved over 90% coverage among adolescent girls, resulting in a measurable decline in cervical cancer incidence [124].
Despite this potential, the widespread implementation of vaccination programs in LMICs faces multiple barriers. These include high vaccine costs, limited infrastructure for delivery, logistical challenges, and cultural resistance to immunization, often requiring community-specific education and outreach strategies [125]. Similarly, Ghana’s introduction of a national HBV vaccination program in the early 2000s has contributed to a decline in chronic HBV infection, a leading cause of liver cancer in the region [126]. Globally, universal HBV vaccination has been significantly successful in reducing liver cancer incidence, as observed in Taiwan and China. However, in many LMICs, the HBV vaccination coverage remains suboptimal. Strengthening vaccine supply chains, improving public awareness of HBV’s oncogenic potential, and addressing socioeconomic barriers are essential steps to expanding vaccine uptake in high-risk regions [127].
6.1.2. Environmental Policy Reforms
Environmental exposures such as air pollution, heavy metals, and contaminated water are major contributors to cancer risk in LMICs. Implementing robust environmental policies to reduce these exposures is crucial for cancer prevention, particularly in urban and industrial regions [3].
Air pollution, especially fine PM2.5, is a well-established carcinogen linked to lung cancer, cardiovascular disease, and respiratory illnesses. The Global Burden of Disease Study attributes a substantial portion of cancer-related deaths in LMICs to PM2.5 exposure [128]. Cities like Lagos (Nigeria) and Cairo (Egypt) experience high levels of pollution due to industrial emissions, vehicular exhaust, and unregulated waste burning [129]. To address this, governments must enforce stricter air quality standards, invest in clean energy solutions, and incentivize the adoption of low-emission technologies. The African Development Bank’s initiatives to support clean energy and green infrastructure represent an important step toward mitigating environmental carcinogens [130].
Water contamination is a pressing concern, especially in countries such as Nigeria, Ghana, and Burkina Faso, where high levels of arsenic in groundwater pose a serious public health threat, increasing the risk of skin, lung, and bladder cancers [131]. In response, both national governments and international partners are investing in solutions such as improved purification systems, community-based water filtration, and affordable water testing technologies to ensure access to safe drinking water [132].
6.2. Advancing Exposomic Research in LMICs
6.2.1. Affordable and Accessible Technologies for Exposure Assessment
Accurate and affordable exposure assessment tools are critical for understanding the health impacts of environmental hazards in LMICs [133]. The growing availability of low-cost sensors has enabled real-time monitoring of air pollutants such as PM2.5, NO2, and sulfur dioxide (SO2). These tools support policymakers in identifying high-risk areas and implementing targeted interventions [134]. Air quality monitoring is gaining attention in African cities in response to increasing urbanization and industrial growth. Organizations such as the African Network for the Prevention of Occupational and Environmental Cancer (ANPOEC) have promoted the deployment of low-cost sensors to monitor air pollution and particulate matter, helping local governments develop data-driven policies to reduce exposure to carcinogens [135].
Water safety is another priority in many LMICs, where centralized infrastructure is often absent. Portable water-testing kits allow for the rapid detection of contaminants, such as arsenic, fluoride, and pathogens, in drinking water. These low-cost tools have empowered local health workers and communities to identify waterborne carcinogens and mitigate exposure risks [136].
Mobile phone applications and wearable devices are increasingly used in exposomic research and public health. These technologies can monitor individual exposure to pollutants in real time, thereby enabling informed health decisions. A study in India demonstrated the utility of mobile apps for tracking PM2.5 and educating users on pollution risks, highlighting the potential for similar tools in African settings [137].
6.2.2. Integrating Exposomic Data into Public Health Interventions
To reduce cancer disparities, the systematic incorporation of exposomic data into public health strategies is essential. In African contexts where environmental, dietary, and infectious exposures intersect, this integration is essential for designing effective, context-specific cancer control programs [138]. For example, in Kenya, researchers have linked high liver cancer rates to overlapping HBV infection and aflatoxin-contaminated maize. The integration of such data has enabled coordinated interventions, including HBV vaccination and improved food safety [139]. In South Africa, combining air pollution data with cancer incidence rates revealed urban hotspots for lung cancer, informing the development of targeted screening and prevention efforts [140].
Beyond targeted interventions, the integration of exposomic data into public health frameworks can contribute to the overall strengthening of healthcare systems. By systematically monitoring environmental exposures across regions and populations, health authorities can identify emergent risks early, allocate resources more efficiently, and enhance surveillance capacity. Exposomic approaches also support a shift toward precision public health, where prevention, diagnostics, and treatment strategies are adapted to specific population-level exposure profiles. This is especially critical in resource-limited settings, where optimizing health system responses can maximize impact with limited infrastructure. Moreover, the inclusion of exposome-informed tools in health information systems promotes interdisciplinary collaboration between environmental scientists, epidemiologists, clinicians, and policymakers, fostering a more integrated and proactive approach to health system governance. These examples illustrate how exposomic insights can guide the development of more precise, evidence-based public health policies in LMICs.
6.3. Strengthening Healthcare Systems
Addressing Healthcare Access Inequities in LMICs
Access to cancer care in many African countries remains limited, particularly in rural and underserved regions of the continent. A major contributing factor is the shortage of cancer treatment facilities and diagnostic services. Strengthening local healthcare infrastructure through the establishment of regional cancer centers and the expansion of affordable diagnostic tools is a crucial step toward equitable care delivery [2]. The African Cancer Organization has called for broader access to cancer treatment services, emphasizing affordability and outreach to marginalized communities (African Cancer Organization, n.d.). In addition, international partnerships such as the collaboration between the Global Health Initiative (GHI) and the African Union (AU) have played a critical role in providing technical support, resources, and capacity-building programs to improve cancer care across the continent. These efforts have contributed to the development of regional cancer centers and enhanced the ability of local healthcare providers to deliver timely and effective treatment [141].
6.4. Policy and Global Collaboration
6.4.1. International Partnerships for Exposome-Informed Cancer Control
International partnerships are pivotal in advancing exposome-informed cancer control in Africa. Collaborations with institutions such as the International Agency for Research on Cancer (IARC) have enhanced cancer surveillance, research infrastructure, and national control programs across the continent [119]. In countries such as Uganda and Kenya, joint efforts with the IARC have enabled the integration of exposomic research into public health strategies. Studies on environmental carcinogens, including aflatoxins and air pollution, have yielded critical insights into region-specific cancer risks in SSA [142]. These partnerships not only strengthen technical capacity but also promote data-driven interventions tailored to the environmental and infectious disease profiles of African nations.
6.4.2. Funding and Capacity-Building for LMIC Research Infrastructure
Sustainable funding is critical for strengthening cancer research infrastructure in LMICs, particularly in Africa, where environmental exposures significantly contribute to cancer disparities [2]. International organizations, including the Wellcome Trust and the Bill & Melinda Gates Foundation, have supported research initiatives aimed at elucidating the links between environmental risk factors and cancer outcomes [143]. Simultaneously, capacity-building efforts have focused on training local scientists, healthcare professionals, and public health officials to conduct exposomic and molecular epidemiology research. Initiatives like the African Cancer Research Consortium (ACRC) provide funding, technical resources, and collaborative networks to empower regional research institutions [144]. These investments are essential for enabling context-specific cancer control strategies that incorporate environmental, infectious, and social determinants of health.
7. Case Studies and Success Stories
7.1. Community-Driven Cancer Prevention Programs Integrate Environmental and Infectious Risk Mitigation
Community-led cancer prevention initiatives have been substantially successful in reducing cancer risk, particularly in settings burdened by both environmental and infectious exposures. In Nigeria, farmer cooperatives have adopted hermetic grain storage methods to reduce aflatoxin contamination in staple crops, significantly lowering liver cancer risk associated with chronic aflatoxin exposure [145]. Community health workers (CHWs) play a vital role in raising awareness and promoting preventive behavior. In Uganda, CHWs have improved cervical cancer screening uptake through culturally tailored educational outreach [146]. In Cambodia, participatory health workshops that integrate clean water access with H. pylori education have improved sanitation practices and reduced gastric cancer incidence [147].
In urban Kenya, community leaders have launched grassroots campaigns to tackle pollution by establishing local recycling centers and clean water kiosks. These programs not only reduce environmental hazards but also strengthen community awareness of the links between pollution and cancer risk [148]. These examples underscore the effectiveness of community-based cancer prevention strategies. By leveraging local knowledge and engagement, such programs can simultaneously address environmental and infectious risks while fostering sustainable public health outcomes [149].
7.2. Success of Vaccination and Environmental Cleanup Efforts to Reduce Cancer Burden
Vaccination campaigns and environmental remediation efforts have significantly reduced the cancer burden in LMICs. In Rwanda, a national HPV vaccination program achieving over 90% coverage among adolescent girls has been instrumental in decreasing cervical cancer incidence, especially when paired with routine screening initiatives [150]. Similarly, Ghana’s infant HBV vaccination program has led to a marked decline in chronic HBV infections, a major driver of liver cancer in the region [49]. These programs have been further bolstered by culturally sensitive public education efforts that improve vaccine uptake by dispelling misinformation and promoting trust [151].
Environmental cleanup initiatives complement public health gains. In Nigeria’s Niger Delta, community-driven efforts to remediate oil-contaminated sites have reduced population-level exposure to carcinogenic PAHs [152]. In South Africa, asbestos decontamination projects in former mining communities have substantially lowered the incidence of mesothelioma [153]. Together, these cases underscore the power of integrated approaches combining vaccination, environmental restoration, and public education to address the root causes of cancer disparities in LMICs.
8. Future Directions and Research Priorities
8.1. Development of Exposome-Wide Association Studies (ExWASs) in LMICs
Exposome-wide association studies (ExWASs) provide a comprehensive framework for evaluating the cumulative effects of environmental, genetic, and infectious factors on cancer risk [9]. In LMICs, where the cancer burden is shaped by unique exposure profiles and socioeconomic constraints, ExWAS methodologies offer a critical path toward contextualized cancer prevention strategies. However, the implementation of ExWASs in these settings is hindered by the limited infrastructure for large-scale environmental exposure monitoring. While initiatives like the African Genome Variation Project have advanced genetic research, the integration of genomic data with high-resolution environmental exposure tracking remains insufficient [154]. A major research priority is the investigation of region-specific co-exposure. For instance, aflatoxin exposure prevalent in SSA due to poor grain storage synergizes with chronic HBV infection to significantly increase HCC risk [155]. Similarly, the interplay between urban air pollution, changing diets, and HPV infection warrants investigation for its role in cervical and colorectal cancer risk, particularly in rapidly urbanizing LMIC contexts [156].
To make ExWASs feasible and impactful in LMICs, capacity building is essential. Training local researchers in molecular epidemiology, biostatistics, and bioinformatics will enable context-relevant study design and data interpretation. International collaborations can support these efforts through resource sharing, mentorship, and co-development of research agendas aligned with regional cancer priorities (Figure 8) [157].

Figure 8.
Integrated framework for reducing cancer disparities in low- and middle-income countries (LMICs) through exposome research and digital innovation. This schematic presents a multidisciplinary strategy to address cancer disparities in LMICs by combining exposome-wide association studies (ExWASs), digital health tools, and precision public health interventions. Core components include the identification of environmental and infectious risk factors through ExWASs, the use of artificial intelligence (AI) and big data analytics for population-level exposure mapping and risk prediction, and the development of localized capacity for implementation. The model emphasizes equitable access, community-driven data collection, and the integration of digital surveillance with targeted cancer prevention and control programs.
8.2. AI and Big Data Are Leveraged to Map Cumulative Exposures and Predict Cancer Risk
Artificial intelligence (AI) and big data analytics are transforming cancer research by enabling the integration of large, heterogeneous datasets to assess cumulative exposures and predict cancer risk [158]. AI-driven tools can analyze satellite imagery and environmental monitoring data to identify pollution hotspots, such as areas with elevated air or water contamination, that contribute to cancer incidence [159]. Machine learning algorithms, trained on population-specific data, can forecast cancer risk patterns, helping to guide targeted public health interventions [160].
Beyond risk prediction, AI has shown promise in community-based surveillance. AI-powered mobile health platforms enable real-time reporting of environmental exposures and symptoms by community members, thereby enhancing data collection and awareness [161]. These technologies support timely, locally tailored responses and strengthen community engagement in cancer prevention efforts [162].
However, infrastructure limitations and data scarcity, particularly in LMICs, remain significant barriers. Centralized, African-specific data repositories are urgently needed to support algorithm development and reduce bias from non-representative training datasets (Figure 8) [163]. Establishing standardized benchmarking systems is essential to ensure AI tools are both effective and equitable across diverse populations [160].
9. Conclusions
The exposome, a comprehensive measure of all environmental exposures across an individual’s lifetime, offers a powerful framework for understanding cancer disparities, particularly in LMICs [9]. In these regions, the convergence of environmental pollutants, infectious diseases, inadequate healthcare infrastructure, and socioeconomic inequalities intensifies cancer risk. By adopting an exposomic perspective, cancer research can move beyond isolated genetic or behavioral risk factors to embrace a more integrated view of disease causation. This holistic lens is especially critical for SSA and other LMICs, where exposures such as air and water pollution, aflatoxin-contaminated food, and persistent infections like HPV and HBV contribute significantly to the cancer burden [164]. Incorporating exposomic data into public health systems has the potential to enhance cancer prevention, early detection, and treatment, particularly in contexts where late-stage diagnoses are common and healthcare resources are limited [144]. Strengthening HPV and HBV vaccination programs can substantially reduce the incidence of cervical and liver cancers, while policies targeting environmental carcinogens could further alleviate preventable cancer cases [165]. Success depends on robust infrastructure, data integration, and equitable access to preventive services.
Realizing the promise of exposomics requires global collaboration, sustained investment, and capacity building in LMICs. Integrating exposomic research into national cancer control strategies can drive more equitable health outcomes. Ultimately, a comprehensive, exposome-informed approach can reshape global cancer prevention, making it more inclusive, context-specific, and effective across all populations.
Author Contributions
Conceptualization: Z.D., M.A. and T.M. (Tebogo Marutha). Methodology: Z.D., R.M. and R.H. Formal analysis: M.A., Z.M. and B.P. Writing—original draft: M.A., T.M. (Tebogo Marutha) and R.H. Writing—review and editing: All authors. Supervision: Z.D., R.H. and B.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was made possible by the South African Medical Research Council (SAMRC) Grant Number 23108, the National Research Foundation (NRF) Grant Number 138139, the SAMRC Division of Research Capacity Development, the South African Medical Research Council Clinician Researcher Program (SAMRC-CRP) Project Code: 57029, and the Cancer Grand Challenges through the Cancer Research UK (CRUK).
Informed Consent Statement
This review article synthesizes existing published data and does not involve original human or animal research conducted by the authors. As such, ethics approval was not required in accordance with the Cancer Letters guidelines for review articles. All referenced studies included in this analysis complied with ethical standards as confirmed by their original methodologies.
Data Availability Statement
Data sharing is not applicable to this review, as no new datasets were generated or analyzed.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sources had no role in the study design, data interpretation, or publication decision.
References
- Bray, F.; Parkin, D.M.; Gnangnon, F.; Tshisimogo, G.; Peko, J.-F.; Adoubi, I.; Assefa, M.; Bojang, L.; Awuah, B.; Koulibaly, M.; et al. Cancer in sub-Saharan Africa in 2020: A review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol. 2022, 23, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, O.; Teibo, J.O.; Atiba, F.A.; Oladimeji, T.; Paimo, O.K.; Ataya, F.S.; Batiha, G.E.-S.; Alexiou, A. Addressing cancer care inequities in sub-Saharan Africa: Current challenges and proposed solutions. Int. J. Equity Health 2023, 22, 189. [Google Scholar] [CrossRef]
- Larsen, K.; Rydz, E.; Peters, C.E. Inequalities in Environmental Cancer Risk and Carcinogen Exposures: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 5718. [Google Scholar] [CrossRef]
- Guzha, B.T.; Matubu, A.; Nyandoro, G.; Mubata, H.O.; Moyo, E.; Murewanhema, G.; Chirenje, Z.M. The impact of DNA tumor viruses in low-to-middle income countries (LMICS): A literature review. Tumour Virus Res. 2024, 18, 200289. [Google Scholar] [CrossRef]
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Neglected Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef]
- Gouveia, M.J.; Pakharukova, M.Y.; Rinaldi, G.; Mordvinov, V.A.; Brindley, P.J.; Gärtner, F.; Vale, N. Helminth infection-induced carcinogenesis: Spectrometric insights from the liver flukes, Opisthorchis and Fasciola. Exp. Results 2020, 1, e40. [Google Scholar] [CrossRef]
- Vineis, P.; Dagnino, S. An evolutionary perspective for the exposome. Exposome 2024, 4, osae008. [Google Scholar] [CrossRef]
- Wright, R.J.; Hanson, H.A. A tipping point in cancer epidemiology: Embracing a life course exposomic framework. Trends Cancer 2022, 8, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Gerken, J.; Vincent, G.T.; Zapata, D.; Barron, I.G.; Zapata, I. Comprehensive assessment of pesticide use patterns and increased cancer risk. Front. Cancer Control Soc. 2024, 2, 1368086. [Google Scholar] [CrossRef]
- Pope, C.A.; Coleman, N.; Pond, Z.A.; Burnett, R.T. Corrigendum to “fine particulate air pollution and human mortality: 25+ years of cohort studies” [environ. Res. 183 (2020) 108924]. Environ. Res. 2020, 191, 109974. [Google Scholar] [CrossRef] [PubMed]
- Radfard, M.; Hashemi, H.; Baghapour, M.A.; Samaei, M.R.; Yunesian, M.; Soleimani, H.; Azhdarpoor, A. Prediction of human health risk and disability-adjusted life years induced by heavy metals exposure through drinking water in fars province, Iran. Sci. Rep. 2023, 13, 19080. [Google Scholar] [CrossRef] [PubMed]
- Meijer, N.; Kleter, G.; de Nijs, M.; Rau, M.L.; Derkx, R.; van der Fels-Klerx, H.J. The aflatoxin situation in Africa: Systematic literature review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2286–2304. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.; et al. Cervical cancer in low and middle-income countries (Review). Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Rizzo, G.E.M.; Cabibbo, G.; Craxì, A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses 2022, 14, 986. [Google Scholar] [CrossRef]
- Shirani, M.; Pakzad, R.; Haddadi, M.H.; Akrami, S.; Asadi, A.; Kazemian, H.; Moradi, M.; Kaviar, V.H.; Zomorodi, A.R.; Khoshnood, S.; et al. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 543. [Google Scholar] [CrossRef]
- Ruiz-Saavedra, S.; Pietilä, T.K.; Zapico, A.; de los Reyes-Gavilán, C.G.; Pajari, A.-M.; González, S. Dietary Nitrosamines from Processed Meat Intake as Drivers of the Fecal Excretion of Nitrosocompounds. J. Agric. Food Chem. 2024, 72, 17588–17598. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Emmanuel, B.N.; Peter, D.A.; Peter, M.O.; Adedayo, I.S.; Olaifa, K. Helicobacter pylori infection in Africa: Comprehensive insight into its pathogenesis, management, and future perspectives. J. Umm Al-Qura Univ. Appl. Sci. 2024, 11, 378–401. [Google Scholar] [CrossRef]
- Ramaboli, M.C.; Ocvirk, S.; Khan Mirzaei, M.; Eberhart, B.L.; Valdivia-Garcia, M.; Metwaly, A.; Neuhaus, K.; Barker, G.; Ru, J.; Nesengani, L.T.; et al. Diet changes due to urbanization in South Africa are linked to microbiome and metabolome signatures of Westernization and colorectal cancer. Nat. Commun. 2024, 15, 3379. [Google Scholar] [CrossRef]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Wan Ibrahim, W.N.; Mohd Faudzi, S.M. Arsenic contamination in rice and drinking water: An insight on human cognitive function. J. Hazard. Mater. Adv. 2025, 17, 100543. [Google Scholar] [CrossRef]
- Quintero Santofimio, V.; Amaral, A.F.S.; Feary, J. Occupational exposures in low- and middle-income countries: A scoping review. PLOS Glob. Public Health 2024, 4, e0003888. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; El-Zaemey, S.; Dorji, N.; Rai, B.D.; Fritschi, L. Exposure to Occupational Hazards Among Health Care Workers in Low- and Middle-Income Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 2603. [Google Scholar] [CrossRef]
- Herceg, Z. Epigenetic Mechanisms as an Interface Between the Environment and Genome. Adv. Exp. Med. Biol. 2016, 903, 3–15. [Google Scholar]
- Avis, W.; Bartington, S. Monitoring Air Quality in Low- Income and Lower Middle-Income Countries; The Institute of Development Studies and Partner Organisations: Brighton, UK, 2020. [Google Scholar]
- Kimanya, M.E.; Routledge, M.N.; Mpolya, E.; Ezekiel, C.N.; Shirima, C.P.; Gong, Y.Y. Estimating the risk of aflatoxin-induced liver cancer in Tanzania based on biomarker data. PLoS ONE 2021, 16, e0247281. [Google Scholar] [CrossRef]
- Okello, S.; Akello, S.J.; Dwomoh, E.; Byaruhanga, E.; Opio, C.K.; Zhang, R.; Corey, K.E.; Muyindike, W.R.; Ocama, P.; Christiani, D.D. Biomass fuel as a risk factor for esophageal squamous cell carcinoma: A systematic review and meta-analysis. Environ. Health 2019, 18, 60. [Google Scholar] [CrossRef]
- Sakafu, L.L.; Mselle, T.F.; Mwaiselage, J.D.; Maunda, K.K.; Eddin, B.S.; Zafereo, M.E. Thyroid Cancer and Iodine Deficiency Status: A 10-Year Review at a Single Cancer Center in Tanzania. OTO Open 2018, 2, 2473974X18777238. [Google Scholar] [CrossRef]
- Sikarwar, A.; Golaz, V. Substantial increase in population exposure to multiple environmental burdens in sub-Saharan Africa (2000–2019). Environ. Res. Lett. 2024, 19, 044068. [Google Scholar] [CrossRef]
- Echendu, A.J.; Okafor, H.F.; Iyiola, O. Air Pollution, Climate Change and Ecosystem Health in the Niger Delta. Soc. Sci. 2022, 11, 525. [Google Scholar] [CrossRef]
- Nriagu, J.; Udofia, E.; Ekong, I.; Ebuk, G. Health Risks Associated with Oil Pollution in the Niger Delta, Nigeria. Int. J. Environ. Res. Public Health 2016, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Marais, E.A.; Wiedinmyer, C. Air Quality Impact of Diffuse and Inefficient Combustion Emissions in Africa (DICE-Africa). Environ. Sci. Technol. 2016, 50, 10739–10745. [Google Scholar] [CrossRef]
- Oghenetega, O.B.; Okunlola, M.A.; Ana, G.R.E.E.; Morhason-Bello, O.; Ojengbede, O.A. Exposure to oil pollution and maternal outcomes: The Niger Delta prospective cohort study. PLoS ONE 2022, 17, e0263495. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, O. Addressing Environmental Health Problems in Ogoniland Through Implementation of United Nations Environment Program Recommendations: Environmental Management Strategies. Environments 2017, 4, 28. [Google Scholar] [CrossRef]
- Nelson, G. Occupational respiratory diseases in the South African mining industry. Glob. Health Action 2013, 6, 19520. [Google Scholar] [CrossRef]
- Schapira, J.S.; Bolhar, R.; Master, S.; Wilson, A.H. Mineralogical, Petrological and Geochemical Characterisation of Chrysotile, Amosite and Crocidolite Asbestos Mine Waste from Southern Africa in Context of Risk Assessment and Rehabilitation. Minerals 2023, 13, 1352. [Google Scholar] [CrossRef]
- Harikannan, N.; Vinodh, S.; Antony, J. Analysis of the relationship among industry 4.0 technologies, sustainable manufacturing practices and organizational sustainable performance using structural equation modelling. TQM J. 2023, 37, 42–72. [Google Scholar] [CrossRef]
- Khan, M.N.; Nurs, C.Z.B.; Mofizul Islam, M.; Islam, M.R.; Rahman, M.M. Household air pollution from cooking and risk of adverse health and birth outcomes in Bangladesh: A nationwide population-based study. Environ. Health 2017, 16, 57. [Google Scholar] [CrossRef]
- Longobardi, P.; Montenegro, A.; Beltrami, H.; Eby, M. Deforestation Induced Climate Change: Effects of Spatial Scale. PLoS ONE 2016, 11, e0153357. [Google Scholar] [CrossRef]
- Bamwesigye, D.; Doli, A.; Hlavackova, P. REDD+: An Analysis of Initiatives in East Africa Amidst Increasing Deforestation. Eur. J. Sustain. Dev. 2020, 9, 224–237. [Google Scholar] [CrossRef]
- Mukong, A.K.; Van Walbeek, C.; Ross, H. Lifestyle and Income-Related Inequality in Health in South Africa. Int. J. Equity Health 2017, 16, 103. [Google Scholar] [CrossRef]
- Qian, F.; Ogundiran, T.; Hou, N.; Ndom, P.; Gakwaya, A.; Jombwe, J.; Morhason-Bello, I.; Adebamowo, C.; Ademola, A.; Ojengbede, O.; et al. Alcohol Consumption and Breast Cancer Risk Among Women in Three Sub-Saharan African Countries. PLoS ONE 2014, 9, e106908. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Salamanca-Fernández, E.; Garcia-Villanova, B.; Sánchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef]
- Sammon, A.M.; Iputo, J.E. Maize meal predisposes to endemic squamous cancer of the oesophagus in Africa: Breakdown of esterified linoleic acid to the free form in stored meal leads to increased intragastric pge2 production and a low-acid reflux. Med. Hypotheses 2006, 67, 1431–1436. [Google Scholar] [CrossRef]
- Weiler, A.M.; Hergesheimer, C.; Brisbois, B.; Wittman, H.; Yassi, A.; Spiegel, J.M. Food sovereignty, food security and health equity: A meta-narrative mapping exercise. Health Policy Plan. 2014, 30, 1078–1092. [Google Scholar] [CrossRef]
- Guillaume, D.; Waheed, D.-e.-N.; Schleiff, M.; Muralidharan, K.K.; Vorsters, A.; Limaye, R.J. Global perspectives of determinants influencing HPV vaccine introduction and scale-up in low- and middle-income countries. PLoS ONE 2024, 19, e0291990. [Google Scholar] [CrossRef] [PubMed]
- Rabiu, K.; Alausa, T.; Akinlusi, F.; Davies, N.; Shittu, K.; Akinola, O. Parental acceptance of human papillomavirus vaccination for adolescent girls in Lagos, Nigeria. J. Fam. Med. Prim. Care 2020, 9, 2950. [Google Scholar] [CrossRef] [PubMed]
- Tsu, V.D.; LaMontagne, D.S.; Atuhebwe, P.; Bloem, P.N.; Ndiaye, C. National implementation of HPV vaccination programs in low-resource countries: Lessons, challenges, and future prospects. Prev. Med. 2021, 144, 106335. [Google Scholar] [CrossRef]
- Okoye, J.O.; Ofodile, C.A.; Adeleke, O.K.; Obioma, O. Prevalence of high-risk HPV genotypes in sub-Saharan Africa according to HIV status: A 20-year systematic review. Epidemiol. Health 2021, 43, e2021039. [Google Scholar] [CrossRef]
- Barnes, K. Soil-Transmitted Helminth Infections: Travelers Beware; Iowa State University: Ames, IA, USA, 2022. [Google Scholar]
- Barnabas, R.V.; Brown, E.R.; Onono, M.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Donnell, D.; Galloway, D.; Cherne, S.; Heller, K.; et al. Single-dose HPV vaccination efficacy among adolescent girls and young women in Kenya (the KEN SHE study): Study protocol for a randomized controlled trial. Trials 2021, 22, 661. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.S.; Park, S.-H. Immune-Mediated Liver Injury in Hepatitis B Virus Infection. Immune Netw. 2015, 15, 191. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Chen, D.S. Prevention of Hepatitis B. Cold Spring Harb. Perspect. Med. 2015, 5, a021493. [Google Scholar] [CrossRef]
- Lin, L.; Yan, L.; Liu, Y.; Qu, C.; Ni, J.; Li, H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer 2020, 9, 563–582. [Google Scholar] [CrossRef]
- Jaquet, A.; Odutola, M.; Ekouevi, D.K.; Tanon, A.; Oga, E.; Akakpo, J.; Charurat, M.; Zannou, M.D.; Eholie, S.P.; Sasco, A.J.; et al. Cancer and HIV infection in referral hospitals from four West African countries. Cancer Epidemiol. 2015, 39, 1060–1065. [Google Scholar] [CrossRef]
- Humphrey, J.; Nagel, E.; Carlucci, J.G.; Edmonds, A.; Kinikar, A.; Anderson, K.; Leroy, V.; Machado, D.; Yin, D.E.; Tulio Luque, M.; et al. Integration of HIV care into maternal and child health services in the global IeDEA consortium. Front. Glob. Women’S Health 2023, 4, 1066297. [Google Scholar] [CrossRef]
- Camandaroba, M.P.G.; Araujo, R.L.C.d.; Silva, V.S.e.; Mello, C.A.L.d.; Riechelmann, R.P. Treatment outcomes of patients with localized anal squamous cell carcinoma according to HIV infection: Systematic review and meta-analysis. J. Gastrointest. Oncol. 2018, 10, 48–60. [Google Scholar] [CrossRef]
- Ibrahim Khalil, A.; Mpunga, T.; Wei, F.; Baussano, I.; de Martel, C.; Bray, F.; Stelzle, D.; Dryden-Peterson, S.; Jaquet, A.; Horner, M.J.; et al. Age-specific burden of cervical cancer associated with HIV: A global analysis with a focus on sub-Saharan Africa. Int. J. Cancer 2021, 150, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; Bütikofer, L.; Schmidlin, K.; Sengayi, M.; Maskew, M.; Giddy, J.; Taghavi, K.; Moore, R.D.; Goedert, J.J.; Gill, M.J.; et al. Cervical cancer risk in women living with HIV across four continents: A multicohort study. Int. J. Cancer 2019, 146, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, A.M.; Mazzitelli, M.; Presa, N.; Cozzolino, C.; Sasset, L.; Leoni, D.; Bragato, B.; Scaglione, V.; Baldo, V.; Parisi, S.G. Changing Prevalence of AIDS and Non-AIDS-Defining Cancers in an Incident Cohort of People Living with HIV over 28 Years. Cancers 2023, 16, 70. [Google Scholar] [CrossRef]
- Kesicioglu, T.; Sengul, D. Histopathological helicobacter pylori verification and related regimen in the presence of esophageal strictures secondary to surgical procedures for the esophageal cancer. Cancer Rep. Rev. 2018, 2, 1000167. [Google Scholar] [CrossRef]
- Risch, H.A. Pancreatic cancer: Helicobacter pylori Colonization, N-Nitrosamine exposures, and ABO blood group. Mol. Carcinog. 2011, 51, 109–118. [Google Scholar] [CrossRef]
- Wang, T.; Cai, H.; Sasazuki, S.; Tsugane, S.; Zheng, W.; Cho, E.R.; Jee, S.H.; Michel, A.; Pawlita, M.; Xiang, Y.-B.; et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: A pooled analysis of prospective studies in china, japan, and korea. Int. J. Cancer 2016, 140, 591–599. [Google Scholar] [CrossRef]
- Srinarong, C.; Siramolpiwat, S.; Wongcha-um, A.; Mahachai, V.; Vilaichone, R.-K. Improved Eradication Rate of Standard Triple Therapy by Adding Bismuth and Probiotic Supplement for Helicobacter pylori Treatment in Thailand. Asian Pac. J. Cancer Prev. 2014, 15, 9909–9913. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Masoodi, M.; Aslani, Z.; Naghshi, S.; Khalighi Sikaroudi, M.; Shidfar, F. Association between dietary antioxidant index and risk of Helicobacter pylori infection among adults: A case–control study. BMC Gastroenterol. 2022, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.-W.; Melville, S.; Utzinger, J.; King, C.H.; Zhou, X.-N. Soil-Transmitted Helminth Reinfection after Drug Treatment: A Systematic Review and Meta-Analysis. PLoS Neglected Trop. Dis. 2012, 6, e1621. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Balic, A.; Gomez-Escobar, N.; Nair, M.; Taylor, M.D.; Allen, J.E. Helminth parasites—Masters of regulation. Immunol. Rev. 2004, 201, 89–116. [Google Scholar] [CrossRef]
- Dlamini, Z.; Molefi, T.; Khanyile, R.; Mkhabele, M.; Damane, B.; Kokoua, A.; Bida, M.; Saini, K.S.; Chauke-Malinga, N.; Luvhengo, T.E.; et al. From Incidence to Intervention: A Comprehensive Look at Breast Cancer in South Africa. Oncol. Ther. 2023, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pastille, E.; Frede, A.; McSorley, H.J.; Gräb, J.; Adamczyk, A.; Kollenda, S.; Hansen, W.; Epple, M.; Buer, J.; Maizels, R.M.; et al. Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer. PLoS Pathog. 2017, 13, e1006649. [Google Scholar] [CrossRef]
- Zender, L.; Spector, M.S.; Xue, W.; Flemming, P.; Cordon-Cardo, C.; Silke, J.; Fan, S.-T.; Luk, J.M.; Wigler, M.; Hannon, G.J.; et al. Identification and Validation of Oncogenes in Liver Cancer Using an Integrative Oncogenomic Approach. Cell 2006, 125, 1253–1267. [Google Scholar] [CrossRef]
- O’Rourke, A. A systematic review of the effects of hepatitis B and C virus on the progression of liver fluke infection to liver cancer. Trop. Dis. Travel Med. Vaccines 2024, 10, 6. [Google Scholar] [CrossRef]
- von Bülow, V.; Lichtenberger, J.; Grevelding, C.G.; Falcone, F.H.; Roeb, E.; Roderfeld, M. Does Schistosoma Mansoni Facilitate Carcinogenesis? Cells 2021, 10, 1982. [Google Scholar] [CrossRef]
- Roderfeld, M.; Padem, S.; Lichtenberger, J.; Quack, T.; Weiskirchen, R.; Longerich, T.; Schramm, G.; Churin, Y.; Irungbam, K.; Tschuschner, A.; et al. Schistosoma mansoni Egg–Secreted Antigens Activate Hepatocellular Carcinoma–Associated Transcription Factors c-Jun and STAT3 in Hamster and Human Hepatocytes. Hepatology 2019, 72, 626–641. [Google Scholar] [CrossRef]
- Sulżyc-Bielicka, V.; Kołodziejczyk, L.; Adamska, M.; Skotarczak, B.; Jaczewska, S.; Safranow, K.; Bielicki, P.; Kładny, J.; Bielicki, D. Colorectal cancer and Blastocystis sp. infection. Parasites Vectors 2021, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2020, 52, 23–30. [Google Scholar] [CrossRef]
- Engin, A.B.; Karahalil, B.; Karakaya, A.E.; Engin, A. Exposure to Helicobacter pylori and Serum Kynurenine to Tryptophan Ratio in Patients with Gastric Cancer. Pteridines 2010, 21, 110–120. [Google Scholar] [CrossRef]
- Singh, E.; Naidu, G.; Davies, M.-A.; Bohlius, J. Hiv-associated malignancies in children. Curr. Opin. HIV AIDS 2017, 12, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.G.; Kim, J.J.; Castle, P.E.; Ortendahl, J.D.; O’Shea, M.; Diaz, M.; Goldie, S.J. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int. J. Cancer 2011, 130, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Bitariho, G.K.; Tuhebwe, D.; Tigaiza, A.; Nalugya, A.; Ssekamatte, T.; Kiwanuka, S.N. Knowledge, perceptions and uptake of human papilloma virus vaccine among adolescent girls in Kampala, Uganda; a mixed-methods school-based study. BMC Pediatr. 2023, 23, 368. [Google Scholar] [CrossRef] [PubMed]
- Deignan, C.; Swartz, A.; Cooper, S.; Colvin, C.J. Stakeholders’ Understandings of Human Papillomavirus (HPV) Vaccination in Sub-Saharan Africa: A Rapid Qualitative Systematic Review. Vaccines 2021, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Mugisha, E.; LaMontagne, D.S.; Katahoire, A.R.; Murokora, D.; Kumakech, E.; Seruyange, R.; Tsu, V.D. Feasibility of delivering HPV vaccine to girls aged 10 to 15 years in Uganda. Afr. Health Sci. 2015, 15, 33. [Google Scholar] [CrossRef]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human Papillomavirus Vaccination in South Africa: Programmatic Challenges and Opportunities for Integration with Other Adolescent Health Services? Front. Public Health 2022, 10, 799984. [Google Scholar] [CrossRef]
- Luo, C.; Yu, S.; Zhang, J.; Wu, X.; Dou, Z.; Li, Z.; Yang, E.; Zhang, L. Hepatitis B or C viral infection and the risk of cervical cancer. Infect. Agents Cancer 2022, 17, 54. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, C.-C.H.; Marsh, G.M.; Wu, F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Adekanle, O.; Ndububa, D.A.; Olowookere, S.A.; Ijarotimi, O.; Ijadunola, K.T. Knowledge of Hepatitis B Virus Infection, Immunization with Hepatitis B Vaccine, Risk Perception, and Challenges to Control Hepatitis Among Hospital Workers in a Nigerian Tertiary Hospital. Hepat. Res. Treat. 2015, 2015, 439867. [Google Scholar] [CrossRef]
- Shiels, M.S.; Engels, E.A. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS 2017, 12, 6–11. [Google Scholar] [CrossRef]
- Sasco, A.J.; Jaquet, A.; Boidin, E.; Ekouevi, D.K.; Thouillot, F.; LeMabec, T.; Forstin, M.-A.; Renaudier, P.; N’Dom, P.; Malvy, D.; et al. The Challenge of AIDS-Related Malignancies in Sub-Saharan Africa. PLoS ONE 2010, 5, e8621. [Google Scholar] [CrossRef]
- Wanyenze, R.K.; Bwanika, J.B.; Beyeza-Kashesya, J.; Mugerwa, S.; Arinaitwe, J.; Matovu, J.K.B.; Gwokyalya, V.; Kasozi, D.; Bukenya, J.; Makumbi, F. Uptake and correlates of cervical cancer screening among HIV-infected women attending HIV care in Uganda. Glob. Health Action 2017, 10, 1380361. [Google Scholar] [CrossRef]
- Emru, K.; Abebaw, T.-A.; Abera, A. Role of awareness on cervical cancer screening uptake among HIV positive women in Addis Ababa, Ethiopia: A cross-sectional study. Women’s Health 2021, 17, 17455065211017041. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Kalali, B.; Mejías-Luque, R.; Javaheri, A.; Gerhard, M.H. pylori Virulence Factors: Influence on Immune System and Pathology. Mediat. Inflamm. 2014, 2014, 426309. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.; Peng, Q.; Lu, Y.; Li, S.; Qin, X.; Chen, Z.; Chen, J. Glutathione s-transferase gene gstm1, gene-gene interaction, and gastric cancer susceptibility: Evidence from an updated meta-analysis. Cancer Cell Int. 2014, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Canzian, F.; Rizzato, C.; Stein, A.; Flores-Luna, L.; Camorlinga-Ponce, M.; Mendez-Tenorio, A.; Chen, W.; Kasamatsu, E.; Mercedes Bravo, M.; Torres, J.; et al. Phylogenetic origin of Helicobacter pylori pathogenicity island and risk of stomach cancer and high-grade premalignant gastric lesions. Eur. J. Cancer Prev. 2023, 32, 301–304. [Google Scholar] [CrossRef]
- Emileva Krasteva, M. Environmental Stress, Epigenetic Modifications, Adaptation, and Disease: A Fine Interplay. In Genetics; IntechOpen: London, UK, 2024. [Google Scholar]
- Islam, R.; Zhao, L.; Wang, Y.; Lu-Yao, G.; Liu, L.-Z. Epigenetic Dysregulations in Arsenic-Induced Carcinogenesis. Cancers 2022, 14, 4502. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Rusyn, I. Environmental Toxicants, Epigenetics, and Cancer. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; pp. 215–232. [Google Scholar]
- Da Silva, M.; De Albuquerque, B.; Allyrio, T.; De Almeida, V.; Cobucci, R.; Bezerra, F.; Andrade, V.; Lanza, D.; De Azevedo, J.; De Araújo, J.; et al. The role of HPV-induced epigenetic changes in cervical carcinogenesis (Review). Biomed. Rep. 2021, 15, 60. [Google Scholar] [CrossRef]
- Inokawa, Y.; Inaoka, K.; Sonohara, F.; Hayashi, M.; Kanda, M.; Nomoto, S. Molecular alterations in the carcinogenesis and progression of hepatocellular carcinoma: Tumor factors and background liver factors. Oncol. Lett. 2016, 12, 3662–3668. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, G.-D. Particulate Matter-Induced Emerging Health Effects Associated with Oxidative Stress and Inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef]
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2011, 33, 240–252. [Google Scholar] [CrossRef]
- El Hajj, H.; Hemida, R.; Taumberger, N.; Friko, I.; Gassama, O.; Ramogola-Masire, D.; Gubena, F.; Ikpeze, O.; Nakazzi, C.B.; Gultekin, M. Advancing cervical cancer prevention in Africa: ESGO’S strategic initiatives and collaborative efforts. Int. J. Gynecol. Cancer 2025, 101841. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, T.; Tang, L.; Li, Y. Cytokines and chemokines in HBV Infection. Front. Mol. Biosci. 2021, 8, 805625. [Google Scholar] [CrossRef] [PubMed]
- Aronica, L.; Ordovas, J.M.; Volkov, A.; Lamb, J.J.; Stone, P.M.; Minich, D.; Leary, M.; Class, M.; Metti, D.; Larson, I.A.; et al. Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine. Nutrients 2022, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome p450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Hequn, C.; Longfei, L.; Long, W.; Zhi, C.; Feng, Z.; Jinbo, C.; Chao, L.; Xiongbing, Z. GSTM1 and GSTT1 polymorphisms are associated with increased bladder cancer risk: Evidence from updated meta-analysis. Oncotarget 2016, 8, 3246–3258. [Google Scholar] [CrossRef]
- Gorący, J.; Bogacz, A.; Uzar, I.; Wolek, M.; Łochyńska, M.; Ziętek, P.; Czerny, B.; Cymbaluk-Płoska, A.; Modliborski, P.; Kamiński, A. The Analysis of NADPH Quinone Reductase 1 (NQO1) Polymorphism in Polish Patients with Colorectal Cancer. Biomolecules 2021, 11, 1024. [Google Scholar] [CrossRef]
- Wendeu-Foyet, M.G.; Cénée, S.; Koudou, Y.; Trétarre, B.; Rébillard, X.; Cancel-Tassin, G.; Cussenot, O.; Boland, A.; Olaso, R.; Deleuze, J.F.; et al. Circadian genes polymorphisms, night work and prostate cancer risk: Findings from the EPICAP study. Int. J. Cancer 2020, 147, 3119–3129. [Google Scholar] [CrossRef]
- Oyesola, O.O.; Souza, C.O.S.; Loke, P.n. The Influence of Genetic and Environmental Factors and Their Interactions on Immune Response to Helminth Infections. Front. Immunol. 2022, 13, 869163. [Google Scholar] [CrossRef]
- Cai, H.; Feng, Y.; Fan, P.; Guo, Y.; Kuerban, G.; Chang, C.; Yao, X.; Peng, Y.; Wang, R. HPV16 E6-specific T cell response and HLA-A alleles are related to the prognosis of patients with cervical cancer. Infect. Agents Cancer 2021, 16, 61. [Google Scholar] [CrossRef]
- Seifert, F.; Eisenblätter, R.; Beckmann, J.; Schürmann, P.; Hanel, P.; Jentschke, M.; Böhmer, G.; Strauß, H.-G.; Hirchenhain, C.; Schmidmayr, M.; et al. Association of two genomic variants with HPV type-specific risk of cervical cancer. Tumour Virus Res. 2023, 16, 200269. [Google Scholar] [CrossRef]
- Mei, T.T.Y.; Aung, H.H.; Tung, W.S.; Naing, C. Association between IL-10 gene polymorphisms (− 1082 A/G, -819 T/C, -592 A/C) and hepatocellular carcinoma: A meta-analysis and trial sequential analysis. BMC Cancer 2023, 23, 842. [Google Scholar] [CrossRef]
- Kojima, Y.; Machida, Y.J. DNA–protein crosslinks from environmental exposure: Mechanisms of formation and repair. Environ. Mol. Mutagen. 2020, 61, 716–729. [Google Scholar] [CrossRef]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, R.A.; Breen, N. The Social Determinants of Cancer. Am. J. Prev. Med. 2008, 35, S141–S150. [Google Scholar] [CrossRef]
- Romero, Y. World cancer day 2021: Prevention, awareness and accountability in tobacco control. Tob. Prev. Cessat. 2021, 7, 8. [Google Scholar] [CrossRef]
- Veljkovikj, I.; Ilbawi, A.M.; Roitberg, F.; Luciani, S.; Barango, P.; Corbex, M.; Dorji, G.; Gunawardena, N.; Johnson, S.; Juric, A.; et al. Evolution of the joint International Atomic Energy Agency (IAEA), International Agency for Research on Cancer (IARC), and WHO cancer control assessments (impact reviews). Lancet Oncol. 2022, 23, e459–e468. [Google Scholar] [CrossRef]
- Middleton, D.R.S.; Mmbaga, B.T.; Menya, D.; Dzamalala, C.; Nyakunga-Maro, G.; Finch, P.; Mlombe, Y.; Schüz, J.; McCormack, V.; Kigen, N.; et al. Alcohol consumption and oesophageal squamous cell cancer risk in east Africa: Findings from the large multicentre ESCCAPE case-control study in Kenya, Tanzania, and Malawi. Lancet Glob. Health 2022, 10, e236–e245. [Google Scholar] [CrossRef]
- Kagawa-Singer, M. Providing resources for Asian Americans and Pacific Islanders. Cancer Pract. 2001, 9, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Mwaka, A.D. Sociocultural Influences on Cancer Care in Sub-Saharan Africa. In Global Perspectives in Cancer Care; Oxford University Press: Oxford, UK, 2022; pp. 249–260. [Google Scholar]
- Enokida, T.; Moreira, A.; Bhardwaj, N. Vaccines for immunoprevention of cancer. J. Clin. Investig. 2021, 131, e146956. [Google Scholar] [CrossRef]
- Sayinzoga, F.; Umulisa, M.C.; Sibomana, H.; Tenet, V.; Baussano, I.; Clifford, G.M. Human papillomavirus vaccine coverage in Rwanda: A population-level analysis by birth cohort. Vaccine 2020, 38, 4001–4005. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; Reynolds, H.W.; Waziri, H.; Attahiru, A.; Olowo-okere, A.; Kamateeka, M.; Waziri, N.E.; Garba, A.M.; Corrêa, G.C.; Garba, R.; et al. Exploring the landscape of routine immunization in Nigeria: A scoping review of barriers and facilitators. Vaccine X 2024, 20, 100563. [Google Scholar] [CrossRef]
- Abesig, J.; Chen, Y.; Wang, H.; Sompo, F.M.; Wu, I.X.Y. Prevalence of viral hepatitis B in Ghana between 2015 and 2019: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0234348. [Google Scholar] [CrossRef] [PubMed]
- Al-Busafi, S.A.; Alwassief, A. Global Perspectives on the Hepatitis B vaccination: Challenges, Achievements, and the Road to Elimination by 2030. Vaccines 2024, 12, 288. [Google Scholar] [CrossRef]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, X.; Yue, J.; Gao, Y.; Tillotson, M.R.; Zhao, X. Nasal filter reveal exposure risks of inhalable particulates and heavy metals in urban women. Environ. Int. 2024, 188, 108743. [Google Scholar] [CrossRef] [PubMed]
- Bank, A.D. Delivering Inclusive and Sustainable Infrastructure: Featured Infrastructure Projects and Initiatives; Inter-American Development Bank: Washington, DC, USA, 2017. [Google Scholar]
- Ahoulé, D.G.; Lalanne, F.; Mendret, J.; Brosillon, S.; Maïga, A.H. Arsenic in African Waters: A Review. Water Air Soil Pollut. 2015, 226, 302. [Google Scholar] [CrossRef]
- Irunde, R.; Ijumulana, J.; Ligate, F.; Maity, J.P.; Ahmad, A.; Mtamba, J.; Mtalo, F.; Bhattacharya, P. Arsenic in Africa: Potential sources, spatial variability, and the state of the art for arsenic removal using locally available materials. Groundw. Sustain. Dev. 2022, 18, 100746. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Tran, N.K.S.; Nguyen, H.A.; Chon, N.M.; Trinh, K.T.L.; Lee, N.Y. Recent advances in portable devices for environmental monitoring applications. Biomicrofluidics 2024, 18, 051501. [Google Scholar] [CrossRef]
- Singh, D.; Dahiya, M.; Kumar, R.; Nanda, C. Sensors and systems for air quality assessment monitoring and management: A review. J. Environ. Manag. 2021, 289, 112510. [Google Scholar] [CrossRef]
- Wernecke, B.; Wright, C. Commentary: Opportunities for the application of low-cost sensors in epidemiological studies to advance evidence of air pollution impacts on human health. Clean Air J. 2021, 31, 1–4. [Google Scholar] [CrossRef]
- Luvhimbi, N.; Tshitangano, T.G.; Mabunda, J.T.; Olaniyi, F.C.; Edokpayi, J.N. Water quality assessment and evaluation of human health risk of drinking water from source to point of use at Thulamela municipality, Limpopo Province. Sci. Rep. 2022, 12, 6059. [Google Scholar] [CrossRef]
- Motlagh, N.H.; Zaidan, M.A.; Fung, P.L.; Lagerspetz, E.; Aula, K.; Varjonen, S.; Siekkinen, M.; Rebeiro-Hargrave, A.; Petäjä, T.; Matsumi, Y.; et al. Transit pollution exposure monitoring using low-cost wearable sensors. Transp. Res. Part D Transp. Environ. 2021, 98, 102981. [Google Scholar] [CrossRef]
- Matus, P.; Urquidi, C.; Cárcamo, M.; Vidal, V. Integrating the exposome and one health approach to national health surveillance: An opportunity for Latin American countries in health preventive management. Front. Public Health 2024, 12, 1376609. [Google Scholar] [CrossRef]
- Sirma, A.; Makita, K.; Grace Randolph, D.; Senerwa, D.; Lindahl, J. Aflatoxin Exposure from Milk in Rural Kenya and the Contribution to the Risk of Liver Cancer. Toxins 2019, 11, 469. [Google Scholar] [CrossRef]
- Okello, N.O.; Okello, T.W.; Zunckel, M. Changes in health risk associated with air pollution and policy response effectiveness, Richards Bay, South Africa. Clean Air J. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Mwisongo, A.; Nabyonga-Orem, J. Global health initiatives in Africa—Governance, priorities, harmonisation and alignment. BMC Health Serv. Res. 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; Berhane, K.; Chevrier, J.; Collman, G.; Eskenazi, B.; Fobil, J.; Hoyo, C.; John, C.C.; Kumie, A.; Nicol, M.; et al. Integrating environmental health and genomics research in Africa: Challenges and opportunities identified during a human heredity and health in africa (h3africa) consortium workshop. AAS Open Res. 2019, 2, 159. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.J.; Salicrup, L.A. Funding for Cancer Research and Clinical Studies in Low- and Middle-Income Countries. In Cancer Research and Clinical Trials in Developing Countries; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 137–189. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Friedman, D.B.; Young, V.M.; Freedman, D.A.; Adams, S.A.; Brandt, H.M.; Xirasagar, S.; Felder, T.M.; Ureda, J.R.; Hurley, T.; Khang, L.; et al. Reducing Cancer Disparities Through Innovative Partnerships: A Collaboration of the South Carolina Cancer Prevention and Control Research Network and Federally Qualified Health Centers. J. Cancer Educ. 2011, 27, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Kabanda, R.; Bikaitwoha Maniple, E.; Baluku, J.B.; Kiconco, A. Utilisation of Community Audio Towers in Health Education for Prevention of Cervical Cancer by Health Workers in Kyotera District, Uganda: A Cross-Sectional Study. Risk Manag. Healthc. Policy 2021, 14, 3667–3673. [Google Scholar] [CrossRef]
- Jatho, A.; Mugisha, N.M.; Kafeero, J.; Holoya, G.; Okuku, F.; Niyonzima, N.; Orem, J. Capacity building for cancer prevention and early detection in the Ugandan primary healthcare facilities: Working toward reducing the unmet needs of cancer control services. Cancer Med. 2020, 10, 745–756. [Google Scholar] [CrossRef]
- Rosser, J.I.; Njoroge, B.; Huchko, M.J. Changing knowledge, attitudes, and behaviors regarding cervical cancer screening: The effects of an educational intervention in rural Kenya. Patient Educ. Couns. 2015, 98, 884–889. [Google Scholar] [CrossRef]
- Mbugua, R.G.; Karanja, S.; Oluchina, S. Effectiveness of a Community Health Worker-Led Intervention on Knowledge, Perception, and Prostate Cancer Screening Among Men in Rural Kenya. Adv. Prev. Med. 2022, 2022, 4621446. [Google Scholar] [CrossRef] [PubMed]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.-C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef]
- Milondzo, T.; Meyer, J.C.; Dochez, C.; Burnett, R.J. Human Papillomavirus Vaccine Hesitancy Highly Evident Among Caregivers of Girls Attending South African Private Schools. Vaccines 2022, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Adoga, A.A.; Kokong, D.D.; Nimkur, T.L.; Ma’an, N.D. Environmental and Life-Style Related Risk Factors for Sinonasal and Nasopharyngeal Malignancies Among a Prospective Cohort in Jos, Nigeria. Int. J. Otolaryngol. 2018, 2018, 8524861. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef]
- Long, X.-D.; Deng, Y.; Huang, X.-Y.; Yao, J.-G.; Su, Q.-Y.; Wu, X.-M.; Wang, J.; Xu, Q.-Q.; Zhu, X.-Y.; Wang, C.; et al. Molecular Mechanisms of Hepatocellular Carcinoma Related to Aflatoxins: An Update. In Liver Research and Clinical Management; InTech: London, UK, 2018. [Google Scholar]
- Kristanto, Y.; Hartono, A.R. Dietary management in colorectal cancer prevention: A review. Intisari Sains Medis 2021, 12, 801–804. [Google Scholar] [CrossRef]
- Moy, K.A.; Fan, Y.; Wang, R.; Gao, Y.-T.; Yu, M.C.; Yuan, J.-M. Alcohol and Tobacco Use in Relation to Gastric Cancer: A Prospective Study of Men in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2287–2297. [Google Scholar] [CrossRef]
- Riaz, I.B.; Khan, M.A.; Haddad, T.C. Potential application of artificial intelligence in cancer therapy. Curr. Opin. Oncol. 2024, 36, 437–448. [Google Scholar] [CrossRef]
- Jabbari, H.; Bigdeli, N. The Role and Application of Artificial Intelligence (AI) in Leveraging Big Data in the Healthcare Domain. Health Nexus 2023, 1, 83–86. [Google Scholar] [CrossRef]
- Cirillo, D.; Núñez-Carpintero, I.; Valencia, A. Artificial intelligence in cancer research: Learning at different levels of data granularity. Mol. Oncol. 2021, 15, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M. Integrating Artificial Intelligence and Big Data into Smart Healthcare Systems: A Comprehensive Review of Current Practices and Future Directions. Artif. Intell. Evol. 2023, 133–153. [Google Scholar] [CrossRef]
- Senkomago, V.; Joseph, R.; Sierra, M.; Van Dyne, E.; Endeshaw, M.; Duran, D.; Sugerman, D.E.; Saraiya, M. CDC Activities to Enhance Training in Cancer Prevention and Control in Field Epidemiology Training Programs in Low- and Middle-Income Countries. J. Glob. Oncol. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Vogel, A.L.; Morgan, C.; Duncan, K.; Williams, M.J. Subsequent Cancer Prevention and Control Activities Among Low- and Middle-Income Country Participants in the US National Cancer Institute’S Summer Curriculum in Cancer Prevention. J. Glob. Oncol. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Sauvaget, C.; Ramadas, K.; Ngoma, T.; Teguete, I.; Muwonge, R.; Naud, P.; Nessa, A.; Kuhaprema, T.; Qiao, Y. Clinical trials of cancer screening in the developing world and their impact on cancer healthcare. Ann. Oncol. 2011, 22, vii20–vii28. [Google Scholar] [CrossRef]
- Ndububa, D.A. Liver cancer surveillance in people with hepatitis b in africa. Lancet Gastroenterol. Hepatol. 2024, 9, 491–492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).