Simple Summary
Blood coagulation and cancer are intrinsically related; hyper-thrombotic complications are often observed in certain types of tumors. On the other hand, coagulation proteases are shown to promote the progression of cancer in a variety of unique mechanisms. The present review highlights how coagulation protease-induced signaling contributes to the immune evasion of different cancers, leading to the development and progression of cancer. Understanding the mechanistic insights will aid developing novel therapeutics against coagulation protease-driven response, which could be used in combination with conventional immune checkpoint inhibitors to increase the efficacy of cancer treatment and patient survival.
Abstract
Blood coagulation and cancer are intrinsically connected, hypercoagulation-associated thrombotic complications are commonly observed in certain types of cancer, often leading to decreased survival in cancer patients. Apart from the common role in coagulation, coagulation proteases often trigger intracellular signaling in various cancers via the activation of a G protein-coupled receptor superfamily protease: protease-activated receptors (PARs). Although the role of PARs is well-established in the development and progression of certain types of cancer, their impact on cancer immune response is only just emerging. The present review highlights how coagulation protease-driven PAR signaling plays a key role in modulating innate and adaptive immune responses. This is followed by a detailed discussion on the contribution of coagulation protease-induced signaling in cancer immune evasion, thereby supporting the growth and development of certain tumors. A special section of the review demonstrates the role of coagulation proteases, thrombin, factor VIIa, and factor Xa in cancer immune evasion. Targeting coagulation protease-induced signaling might be a potential therapeutic strategy to boost the immune surveillance mechanism of a host fighting against cancer, thereby augmenting the clinical consequences of targeted immunotherapeutic regimens.
1. Introduction
Blood coagulation is a tightly regulated biological process that prevents excessive bleeding upon injury to a blood vessel [1]. The process is facilitated by the conversion of the liquid form of blood into a semisolid mass, thereby forming a clot [2]. Vessel injury triggers the activation of the blood coagulation cascade, in which several clotting proteases function in orchestration to cease profuse bleeding [3]. Apart from their common role in coagulation, clotting proteases also induce cellular signaling via the activation of a G protein-coupled receptor (GPCR) family of proteases called protease-activated receptors (PARs) [4,5]. Blood coagulation and cancer are intrinsically related; enhanced blood coagulation is often observed in certain cancer patients, known as cancer-associated thrombosis (CAT), which is considered to be the second leading cause of mortality in cancer patients [6,7]. Moreover, the expression of PARs is shown to be significantly up-regulated in different types of solid tumors [8,9], and coagulation protease-driven PAR signaling often plays a crucial role in the progression of cancer [10,11]. PAR signaling not only supports tumor growth [12] but also promotes cancer metastasis [11]. Furthermore, the activation of PARs has also been shown to promote tumor angiogenesis [13]. Additional studies indicate that PAR activation induces epithelial-to-mesenchymal transition (EMT) in certain types of cancer, facilitating tumor metastasis [14]. EMT is characterized by an up-regulation of immune-suppressing mechanisms, leading to a futile anti-tumor immune response, ultimately contributing to the immune evasion of cancer [15]. Although the contribution of coagulation proteases and their receptors are well-established in tumor growth, metastasis, and angiogenesis, their role in cancer immune evasion occurs only at the initial stages. The present review thoroughly summarizes how the coagulation protease-mediated activation of PARs influences cancer immune evasion. The first part of the review highlights how different coagulation proteases activate different PAR isoforms. This is followed by a brief understanding of the role of PARs in tumor progression. We also briefly focus on the immune evasion mechanisms facilitating tumor survival and progression. The latter part of the review highlights how different coagulation proteases contribute to the immune evasion mechanisms of certain cancers. Finally, we discuss the recent immunotherapy-based clinical trials for different types of cancer.
2. PAR Activation by Different Coagulation Proteases
Mammalian cells express four PAR isoforms: PAR1, PAR2, PAR3, and PAR4. In 1991, PAR1, the prototypical receptor that is encoded by the F2R gene [16,17], was discovered on human platelets as the receptor of coagulation protease thrombin. Thereafter, other PAR family members, PAR2, PAR3, and PAR4, which are encoded by F2RL1 [18], F2RL2 [19], and F2RL3 [20], respectively, were discovered through homology screening [20,21,22,23,24]. An irreversible proteolytic mechanism triggers the activation of PARs. Coagulation proteases bind to and cleave PARs at the N-terminal end, exposing a new N-terminal peptide that serves as a tether ligand and promotes PAR activation upon binding to a conserved region of extracellular loop 2 (ECL2) on the receptor (Figure 1) [16,25]. Therefore, synthetic peptides mimicking the first six amino acids of the newly generated N-terminus of PARs can activate the receptor in the absence of coagulation proteases [26,27,28]. However, an exception exists: PAR3 shows unresponsiveness towards synthetic agonist peptides [29]. The ligand activation of PARs induces a conformational change in the receptor, resulting in the affinity of the receptor to intracellular G proteins being altered, thereby triggering the signaling response [30]. Occasionally, PAR activation also triggers the phosphorylation of the receptor itself by G protein-coupled receptor kinase (GRK), aiding in the recruitment of β-arrestin, and GRK transduces signaling through it while preventing G protein-associated signaling [31,32]. However, the same PAR can be cleaved at different N-terminal sites by different coagulation proteases, leading to different conformational settings and associated signaling responses. This multiple, site-specific cleavage of a PAR by different coagulation proteases justifies how different signaling events are mediated by the activation of the same PAR [33,34,35]. The different coagulation protease-driven PAR signaling, and their specific cleavage sites are summarized in Table 1.
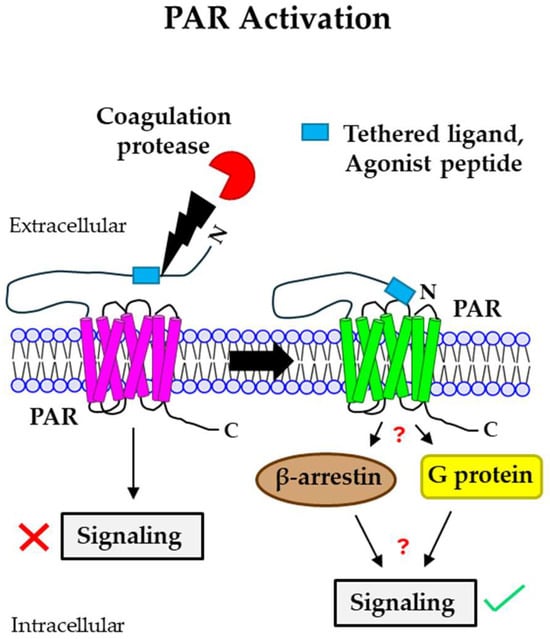
Figure 1.
Mechanism of PAR activation by coagulation proteases. Coagulation proteases cleave PARs at the N-terminal extracellular domain, leading to the generation of a new N-terminus end. This newly formed N-termini acts as a tethered ligand that binds to the ECL2 region of the receptor itself, resulting in the activation of the receptor. Agonist peptides often activate PARs by directly binding to the receptor and do not require PAR cleavage. PAR activation mediated by different coagulation proteases triggers either intracellular G protein- or β-arrestin-induced signaling, leading to different cellular responses. PAR: protease-activated receptor; ECL2: extracellular loop 2.

Table 1.
Different PARs, activated by various coagulation proteases and their specific cleavage sites.
2.1. PAR1 Signaling via Coagulation Proteases
Coagulation protease thrombin binds to the N-terminal extracellular domain (exodomain) of PAR1 and cleaves the Arg41-Ser42 (R41-S42) peptide bond [36,37]. In addition to this, thrombin also binds to the acidic hirudin-like sequence of PAR1 at the C-terminus end [36,44]. The C-terminal end binding of PAR1 imparts the target specificity of thrombin. Thrombin-induced PAR1 cleavage promotes the interaction of the PAR1 C-terminal loop with Gq and G12/13 subfamilies of intracellular G-protein, leading to the signaling response [45,46,47]. Coagulation protease-activated protein C (aPC) activates PAR1 [48] via cleavage at the Arg46 (R46) residue of the exodomain [38,39]. Unlike thrombin, aPC does not interact with the hirudin-like domain of PAR1 [49], but it essentially requires binding with endothelial cell protein C receptor (EPCR) for PAR1 cleavage [50,51,52]. Instead of G proteins, aPC-driven PAR1 signaling involves intracellular β-arrestins, specifically β-arrestin-2 [53]. Clotting protease-activated factor VII (FVIIa) [54,55], the structural analog of aPC, also cleaves PAR1 depending on EPCR binding [56,57,58,59]. However, unlike aPC, FVIIa cleaves PAR1 at the R41 site and signals through β-arrestin-1 [35,60,61]. Like thrombin, activated factor X (FXa)-mediated PAR1 signaling requires cleavage at the canonical site, R41 [34,40], and is believed to be associated with intracellular G proteins [34,62]. However, the requirement of EPCR in FXa-mediated PAR1 signaling remains controversial. Some studies suggest the involvement of EPCR [63], whereas others indicate the EPCR-independent activation of PAR1 by FXa [64]. In contrast, other studies indicate that the FXa-driven activation of PAR1 is dependent on binding with a unique protein: annexin 2 [65]. Another coagulation-associated protease, plasmin, is shown to cleave PAR1 at multiple sites, K32-A33, R41-S42, R70-L71, K76-S77, and K82-Q83 [41]; however, the mechanism of plasmin action through PAR1 is yet to be established. Kallikrein-14, another coagulation-related serine protease, is also reported to activate PAR1 [66], probably via cleavage at the R46-N47 site [5].
2.2. Coagulation Protease-Driven PAR2 Signaling
Coagulation protease FVIIa is well-known for the cleavage and subsequent activation of PAR2. FVIIa binds to its primary receptor, tissue factor (TF), and transduces signaling via PAR2 [67,68], thereby exerting various cellular responses [69]. Similar to FVIIa, FXa also triggers PAR2 signaling [67,68]. Thrombin, on the other hand, at a very high concentration (100–500 nM), is shown to cleave PAR2, thereby activating the receptor [70]. Kallikrein-14 induces Ca2+ signaling in human embryonic kidney cells via the activation of PAR2 [66]. All these proteases preferentially cleave the peptide bond of PAR2 at R36-S37 [5,42,43].
2.3. PAR3 Signaling by Coagulation Proteases
A low thrombin concentration is shown to activate PAR3 [29]; however, thrombin-mediated PAR3 cleavage essentially requires the presence of PAR4 [29]. aPC-driven signaling in various cell types is also reported to be mediated by PAR3 [71,72,73]. Like aPC, FXa also triggers PAR3 activation and promotes signal transduction [74]. aPC and FXa preferentially cleave PAR3 at the R41-G42 site [5,39], whereas thrombin-induced PAR3 activation occurs via cleavage at K38-T39 [22].
2.4. PAR4 Activation via Coagulation Proteases
At a relatively high concentration, thrombin cleaves PAR4 at R47-G48 [30], thereby activating the receptor [75]. In some instances, Kallikrein-14 is also shown to induce signaling via the proteolytic cleavage of PAR4. However, the cleavage site for Kallikrein-14 remains unknown.
3. Coagulation Protease-Driven PAR Signaling in Cancer
Coagulation protease-mediated PAR signaling often plays a key role in the growth, development, and metastasis of tumors. The present section briefly highlights the contribution of different PARs to cancer progression. Figure 2 and Table 2 briefly summarize the role of coagulation protease-driven PAR signaling in cancer pathogenesis.
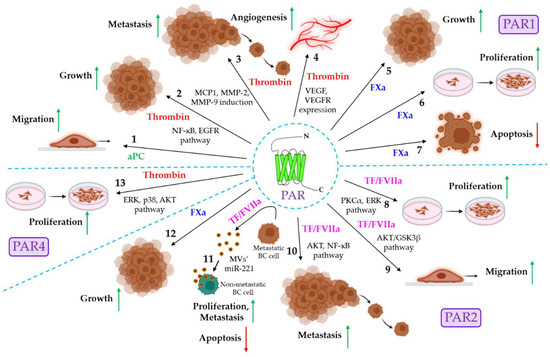
Figure 2.
The role of coagulation protease-driven PAR signaling in cancer. PAR1, PAR2, and PAR4 are shown to be associated with cancer progression. PAR1 signaling in cancer. 1. aPC promotes cancer cell migration via PAR1 activation. Thrombin triggers cancer progression in multiple ways. 2. Thrombin stimulates the PAR1-dependent activation of the C and EGFR pathways, resulting in tumor growth. 3. Thrombin/PAR1 signaling also induces MCP1, MMP-2, and MMP-9 expression, triggering tumor metastasis. 4. Thrombin/PAR1 signaling also promotes tumor angiogenesis via the induction of VEGF and VEGFR. 5–7. The FXa-mediated activation of PAR1 is shown to promote tumor growth and proliferation while down-regulating apoptosis. The role of PAR2 in cancer. 8. TF/FVIIa/PAR2 signaling promotes cancer proliferation via the activation of PKCα and the ERK pathway. 9. The TF/FVIIa-dependent activation of the AKT/GSK3β pathway induces the migration of cancer cells. 10. TF/FVIIa/PAR2 signaling also induces tumor metastasis via the AKT/NF-ĸB pathway. 11. The TF/FVIIa-mediated activation of PAR2 promotes the release of miR-221-laden MVs from metastatic breast cancer (BC) cells, which deliver miR-221 to non-metastatic BC cells, thereby inducing proliferation, metastasis, and anti-apoptosis to MV-fused recipient cells. 12. FXa also stimulates PAR2 to promote tumor growth. PAR4 signaling in cancer. 13. Thrombin stimulates PAR4 to induce cancer cell proliferation via the activation of the ERK, p38, and AKT signaling pathways. The green upward arrows indicate up-regulation; the red downward arrows indicate down-regulation. PAR: protease-activated receptor: aPC: activated protein C: NF-ĸB: nuclear factor kappa-light-chain-enhancer of activated B cells: EGFR: extracellular growth factor receptor, MCP1: monocyte chemoattractant protein-1; MMP: matrix metalloproteinase; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor; FXa: activated factor X; TF: tissue factor; FVIIa: activated factor VII; PKCα: protein kinase Cα; ERK: extracellular signal-regulated kinase; GSK3β: glycogen synthase kinase 3β; miR: microRNA.

Table 2.
The role of coagulation protease-mediated PAR signaling in cancer.
3.1. Coagulation Proteases and PAR1 Signaling in Cancer
The expression of PAR1 is shown to be significantly elevated in various cancers such as breast, lung, pancreatic, colon, gastric, prostate, liver, renal cancer, etc. [88]. On numerous occasions, the coagulation protease-driven activation of PAR1 is shown to promote tumor progression in various ways. The coagulation proteases that are known for promoting tumor progression via PAR1 are aPC, thrombin, and FXa. aPC triggers human breast cancer migration via the EPCR-dependent activation of PAR1 [76]. In gastric cancer, thrombin-induced PAR1 activation triggers tumor growth and invasion via the activation of the NF-ĸB and EGFR pathways [10]. In pancreatic ductal adenocarcinoma (PDAC), thrombin-stimulated PAR1 activation is shown to stimulate the ERK-dependent induction of macrophage chemokine MCP1 and matrix metalloproteinase MMP-9, resulting in PDAC growth and metastasis [77]. In nasopharyngeal carcinoma, the thrombin-induced activation of PAR1 leads to the induction of MMP-2 and MMP-9, triggering tumor metastasis by facilitating extracellular matrix (ECM) degradation and the disruption of the basement membrane [78]. In another study, thrombin-triggered PAR1 activation is observed to stimulate EGFR-dependent p21-activated kinase (Pak1) activity, which promotes the invasiveness of inflammatory breast cancer [79]. Thrombin/PAR1 signaling is also shown to influence tumor angiogenesis in an indirect mechanism. The increased expression of thrombin is observed in the tumor microenvironment (TME) [89] and PAR1 is ubiquitously expressed in various cells in the TME, such as endothelial cells, platelets, macrophages, fibroblasts, etc. [90]. Thrombin-induced PAR1 activation in fibroblasts and endothelial cells augments the expression of pro-angiogenic factors, vascular endothelial growth factor (VEGF), and its receptor, VEGFR2, which could trigger tumor angiogenesis [80]. Thrombin also enhances the endothelial expression of PAF, IL-6, and IL-8 [91], which could promote endothelial proliferation and angiogenesis. Like thrombin, FXa also promotes melanoma growth, which is believed to be dependent on PAR1, as PAR1 agonists show similar effects to that of FXa [81], and FXa is well-known for the activation of PAR1 [34,40]. The perturbation of FXa-driven PAR1 signaling in mice by the administration of FXa-specific inhibitors, rivaroxaban or edoxaban, is also shown to suppress the proliferation of colorectal tumors while accelerating tumor apoptosis [82].
3.2. Coagulation Protease-Driven PAR2 Signaling in Cancer
Similar to PAR1, PAR2 is also widely expressed in various cancers [92]. Coagulation protease FVIIa is often shown to promote cancer progression in various ways upon binding to its principal receptor, TF, and the subsequent activation of PAR2. For example, in colon cancer, TF/FVIIa/PAR2 signaling stimulates the proliferation and migration of cancer cells via the PKCα- and ERK-dependent transcriptional activation of c-Jun/AP-1 [83]. In breast cancer also, the TF/FVIIa-mediated activation of PAR2 is shown to promote cancer invasiveness via the AKT/GSK3β-driven nuclear accumulation of β-catenin and the induction of EMT [84]. In another study, the same group reported the contribution of the AKT/NF-ĸB signaling axis to the TF/FVIIa/PAR2-dependent metastasis of human breast cancer via the induction of MMP-2 [11]. PAR2-mediated human breast cancer progression also occurs through indirect mechanisms. TF/FVIIa/PAR2 signaling induces the release of nano-sized microvesicles (MVs) from human highly metastatic breast cancer cells [93], which are enriched with microRNA221 (miR-221), and the MV-mediated transfer of miR-221 confers proliferative, metastatic, and anti-apoptotic potential to non-metastatic breast cancer cells via the induction of EMT [85,94]. PAR2 activation via another protease, trypsin, also induces pro-metastatic MV generation from metastatic breast cancer cells, imparting metastatic potential to non-metastatic breast cancer cells [95]. Like FVIIa, FXa is also shown to stimulate colon cancer growth via the activation of PAR2 and the subsequent intracellular activation of ERK, p38, and AKT [86].
Although the role of PAR1 and PAR2 is well-established in cancer, the contribution of other PARs (PAR3 and PAR4) to tumor pathogenesis remains to be completely understood. However, indirect evidence indicates the crucial role of PAR4 in the thrombin-induced proliferation of human colon cancer cells [87].
4. Cancer and Immune Evasion Mechanisms
Immune evasion is a mechanism by which normal cells within the body overcome the attack of the body’s own immune system. Cancer cells are antigenic by nature and are, therefore, vulnerable to recognition by the immune system under normal circumstances. However, cancer cells possess some extraordinary features by which they bypass the immune attack of the body, aiding in their survival. Cancer cells evade host immune response by various mechanisms. The present section briefly discusses the known mechanisms by which cancer cells evade immune attack in the body (Figure 3). In the process of killing cancer cells [96,97,98,99,100], neoantigens released during the death of cancer cells are taken up by the dendritic cells (DCs), which present the antigens to the T-cells through major histocompatibility complex (MHC) molecules, MHC class I and II. Effector T-cells recognize these antigens and get activated. These activated T-cells then infiltrate into the tumor and bind to the cancer cells, resulting in the killing of cancer cells via granule/exocytosis mechanism or apoptotic ligand/receptor interaction. However, defects in the above-mentioned mechanisms often result in bypassing the host’s immune attack against the cancer, leading to the successful development of tumors. Cancer cells evoke several mechanisms to avoid host immune attack (Table 3), as discussed below:
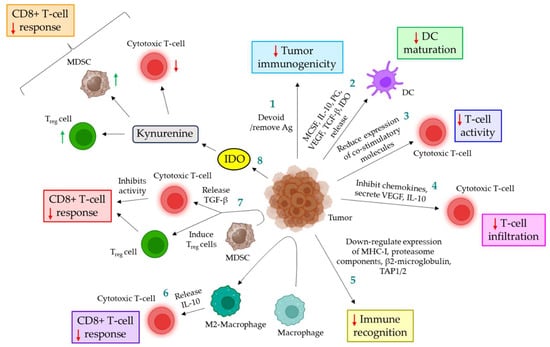
Figure 3.
Different types of cancer immune evasion mechanisms. 1. Cancer cells are either devoid of antigens or remove antigens, thereby down-regulating tumor immunogenicity. 2. Tumor cells inhibit DC maturation via the release of MCSF, IL-10, prostaglandin, VEGF, TGF-β, and IDO. 3. In the TME, cells exhibit lower expression of co-stimulatory molecules, leading to decreased T-cell activity. 4. Cancer cells inhibit chemokines and secrete VEGF and IL-10, which together down-regulate the infiltration of T-cells. 5. Tumor cells exhibit a reduced expression of MHC-I, proteasome components, β2-microglobulin, and TAP1/2, thereby avoiding immune recognition. 6. In the TME, macrophages are converted to M2-macrophages, which promote the release of IL-10, triggering the down-regulation of the CD8+ T-cell response. 7. In the TME, MDSCs release TGF-β, which down-regulates CD8+ T-cell activity. MDSC also induces Treg cells. Together, these perturb the CD8+ T-cell response. 8. Tumor cells release IDO, which is converted to kynurenine; this further reduces the activity of cytotoxic T-cells, induces MDSC and Treg cells, and, ultimately, leads to the down-regulation of the CD8+ T-cell response. The Red down arrows indicate down-regulation; the green up arrows indicate up-regulation. MCSF: macrophage colony-stimulating factor; IL: interleukin; VEGF: vascular endothelial growth factor; TGF-β: transforming growth factor β; IDO: indoleamine 2:3 dioxygenase; DC: dendritic cell; VEGF: vascular endothelial growth factor; IL: interleukin; MHC-I: major histocompatibility complex I; TAP: transporter associated with antigen processing; MDSC: myeloid-derived suppressor cells; Treg cells: regulatory T-cells.

Table 3.
Various immune evasion mechanisms exerted by the tumor cells.
4.1. Down-Regulating Immunogenicity of Tumor
On numerous occasions, it has been shown that cancer cells are devoid of immunogenic antigens or typically remove the immunogenic antigens to bypass the immunosurveillance of the host [98,101,102].
4.2. Interfering Maturation of Dendritic Cells
Cancer cells often down-regulate the maturation of DC via the release of macrophage colony-stimulating factor (MCSF) [103], IL-10 [104], prostaglandin [105], VEGF [106], TGF-β [107], and indoleamine 2,3 dioxygenase (IDO) [108].
4.3. Down-Regulating the Activity of T-Cells
Besides antigen recognition, the complete activity of T-cells essentially requires co-stimulatory interactions between T-cells and DCs, such as CD70:CD27, B7.1/B7.2: CD28, OX40L:OX40, GITRL:GITR, and 4-1BBL:4-1BB. Cells in the tumor microenvironment are shown to express reduced levels of co-stimulatory molecules. T-cell activation without co-stimulation leads to the expression of negative modulating factors, rendering T-cells unresponsive [109,110,111,112].
4.4. Perturbation of T-Cell Infiltration
Cancer cells inhibit the infiltration of T-cells in several ways. During T-cell activation and in response to IFN-γ, T-cells express surface chemokine receptors such as CXCR3 [122]. The ligands for the receptor, such as CXCL9, -10, and -11, are expressed in cancer cells, and this receptor-ligand interaction facilitates the infiltration of T-cells into the TME. Cancer cells often bypass this mechanism by either reducing the expression of the ligands, decomposing the ligands, or inducing post-translational modifications, limiting the infiltration of T-cells into the TME [113]. Moreover, tumor cells release VEGF, which targets endothelial cells, leading to the reduced expression of adherent factors, ultimately perturbing T-cell adhesion on vascular endothelium, essential for the infiltration of T-cells [114,115]. Furthermore, tumor cells are shown to secrete IL-10 and prostaglandin E2 (PGE2), which induce the expression of death mediator Fas ligand (FasL) in endothelial cells, thereby leading to CD8+ T-cell apoptosis [116]. In addition, cancer-associated fibroblasts (CAFs) are believed to produce ECM components, such as collagen, which are deposited in the tumor substrate, further inhibiting the migration of T-cells towards the cancer cells [117,118].
4.5. Inhibition of Immune Recognition
Cancer cells often modulate certain self-molecules that are essential for immune recognition, thereby evading immune response. For example, the expression of surface antigens, MHC-I, proteasome components, β2-microglobulin, TAP1/2, etc., are shown to be down-regulated in cancer cells via the modulation of gene expression, contributing to immune evasion [119].
4.6. Up-Regulating the Function of Immunosuppressive Cells
In TME, macrophages are differentiated into M2-phenotypes, which suppress CD8+ T-cell response via the release of IL-10 [120]. Myeloid-derived suppressor cells (MDSCs) in the TME are shown to up-regulate the function of regulatory T-cells (Treg cells) while down-regulating cytotoxic T-cell response. MDSC-released TGF-β suppresses cytotoxic T-cell activity via down-regulating the expression of perforin and granzyme [120]. On the other hand, the population of MDSC-induced Treg cells in the TME is shown to be significantly up-regulated, which inhibits the CD8+ T-cell response, leading to tumor progression [120]. Cancer cell-expressed IDO induces kynurenine, which suppresses the function of cytotoxic T-cells while inducing Treg cells and MDSCs [121].
5. Coagulation Protease-Driven Cancer Immune Evasion
In the previous section, we demonstrated how coagulation proteases influence the progression of cancer in various ways. However, the role of coagulation proteases in cancer immune evasion contributing to disease progression is only just emerging. A few studies indicate that clotting protease thrombin, FVIIa, and FXa contribute to cancer immune evasion via unique mechanisms. The present section briefly illustrates how different clotting proteases influence the progression of tumors by evading a host’s immune responses.
5.1. The Role of Thrombin in Cancer Immune Evasion
TGF-β1 plays a crucial role in the immunosuppressive responses of cancer. It suppresses the cancer immune response by not only inhibiting the clonal expansion of cytotoxic T-cells but also reducing their cytotoxicity [123]. TGF-β1 is found to be associated with glycoprotein A repetitions predominant (GARP) on platelets in a latent form (LTGF-β1). The level of thrombin is shown to be significantly higher in patients suffering from cancer-associated thrombosis. Thrombin is shown to cleave GARP on platelets, leading to the release of active TGF-β1, which could promote immunosuppressive functions [124]. Thrombin-induced PAR1 signaling is also observed to suppress the anti-tumor immunity of pancreatic ductal adenocarcinoma, leading to tumor growth and progression [125]. The thrombin-mediated activation of PAR1 in pancreatic ductal adenocarcinoma also triggers the evasion of cytotoxic T-cells via the induction of immunosuppressive genes Csf2 and Ptgs2 [126]. The thrombin-induced release of IL-6 from different cells in the TME [127] down-regulates the differentiation, maturation, and antigen-presenting abilities of DC through STAT3 signaling, thereby evading tumor immune response [128]. Thrombin also triggers the release of TNF-α from monocytes, adipose cells, vascular smooth muscle cells, and monocyte-derived macrophages [127], which facilitates tumor immune evasion in multiple ways: it (1) promotes the accumulation and activity of the negative regulatory cells of the tumor immune response, such as Treg cells [129], MDSCs [130], and regulatory B-cells (Breg cells) [131], (2) interferes with the tumor infiltration of cytotoxic T-cells [132,133], and (3) induces the activation-triggered death of cytotoxic T-cells [134]. Different cells in the TME also release MCP-1 in response to thrombin [127], which plays immunosuppressive roles via inducing macrophage polarization into M2 phenotypes, leading to tumor immune evasion [135]. Figure 4 illustrates how thrombin triggers the immune evasion of cancer in different mechanisms.
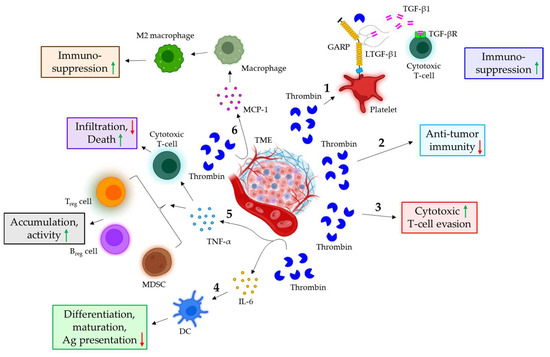
Figure 4.
The role of thrombin in cancer immune evasion. 1. In the TME, thrombin triggers the cleavage of GARP on platelets, leading to the release of TGF-β1 from the GARP-LTGF-β1 complex. The released TGF-β1 binds to the receptor on cytotoxic T-cells, thereby conferring immunosuppressive functions. 2. Thrombin also suppresses anti-tumor immunity. 3. Furthermore, thrombin is shown to promote the evasion of cytotoxic T-cells. 4. Thrombin stimulates the release of IL-6 from various cells in the TME, which down-regulates the differentiation, maturation, and antigen-presenting ability of DC. 5. In the TME, thrombin is also shown to induce the release of TNF-α, which not only increases the accumulation and activity of Treg cells, Breg cells, and MDSCs but also evades the cytotoxic T-cell response via down-regulating infiltration and up-regulating apoptosis. 6. The thrombin-mediated release of MCP-1 in the TME also triggers macrophage differentiation into M2 phenotypes, leading to immunosuppressive responses. The green upward arrows indicate induction; the red downward arrows indicate inhibition. GARP: glycoprotein A repetitions predominant; LTGF-β1: latent TGF-β1; IL: interleukin; DC: dendritic cell; Ag: antigen; TNF-α: tumor necrosis factor α; MDSC: myeloid-derived suppressor cells; Treg cells: regulatory T-cells; Breg cells: regulatory B-cells; MCP-1: monocyte chemoattractant protein 1.
5.2. FVIIa in Cancer Immune Evasion
Although the role of FVIIa is well established in cancer growth and metastasis, its contribution to cancer immune evasion remains understudied. In a recent study, Paul et al., for the first time, showed that the TF/FVIIa-dependent activation of PAR2 triggers the immune evasion of breast cancer via the induction of programmed death-ligand 1 (PD-L1) expression and its stability [136]. PD-L1 is shown to be expressed in various cancer cells, and its receptor, PD-1, is found in the tumor-infiltrating lymphocytes. The interaction of PD-L1/PD-1 not only triggers the down-regulation of lymphocyte proliferation but also induces lymphocyte apoptosis, leading to tumor immune evasion [137,138]. In their study, Paul et al. demonstrated that the TF/FVIIa-mediated activation of PAR2 promotes the expression of PD-L1 in human triple-negative breast cancer (TNBC) cells, leading to a down-regulation of CD8+ T-cell activity [136]. The treatment of cells with PAR2 activation peptide shows a similar induction of PD-L1 expression to that of FVIIa and PAR2 knock-down, which significantly down-regulates FVIIa-driven PD-L1 expression [136]. Mechanistically, the authors indicate that TF/FVIIa/PAR2 signaling triggers the inactivation of LATS1, thereby resulting in the loss of YAP/TAZ phosphorylation, leading to their subsequent localization into the nucleus [136]. The Knock-down of PAR2 or blocking cell surface TF dramatically reduced FVIIa-mediated LATS1 inactivation and the nuclear translocation of YAP/TAZ [136]. The perturbation of this signaling pathway manifests a lower expression of PD-L1 by FVIIa, thereby increasing CD8+ T-cell activity [136]. In addition, the authors also demonstrate that besides PD-L1 expression, TF/FVIIa-driven PAR2 activation promotes PD-L1 stability via glycosylation through N-glycosyltransferases, STT3A, and STT3B [136]. In earlier studies, the same group reported that TF/FVIIa/PAR2 signaling promotes the AKT/GSK3β-driven nuclear accumulation of β-catenin, thereby triggering TNBC metastasis [84]. In the present study, Paul et al. delineate that the TF/FVIIa/PAR2-mediated nuclear translocation of β-catenin further promotes the expression of STT3A and STT3B, contributing to PD-L1 stability and the associated TNBC immune evasion [136]. The knock-down of STT3A or- B is shown to reduce PD-L1 stability, thereby increasing the activity of CD8+ T-cells [136]. In vivo, mice bearing TF knock-out tumors showed reduced tumor growth and enhanced CD8+ T-cell population in tumors, which also promotes the efficacy of anti-PD-1 therapy [136]. Figure 5 highlights the mechanism of the FVIIa-mediated immune evasion of human TNBC.
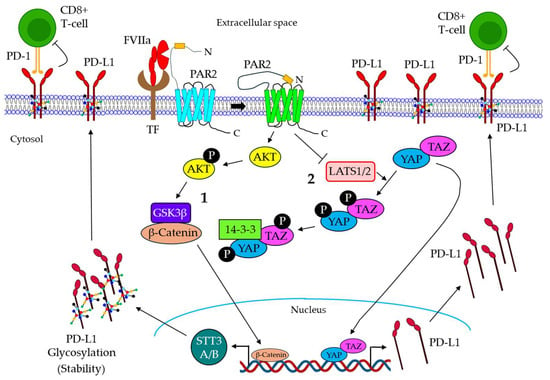
Figure 5.
Mechanism of FVIIa-mediated cancer immune evasion. 1. The TF/FVIIa-mediated activation of PAR2 promotes AKT phosphorylation, which triggers the GSK3β-dependent accumulation of β-catenin into the nucleus, resulting in the induction of STT3A/B expression in TNBC cells. The induced expression of STT3A/B promotes PD-L1 glycosylation, leading to the stability of PD-L1. 2. TF/FVIIa/PAR2 signaling also promotes the inactivation of LATS1/2, which is essential for YAP/TAP phosphorylation and their retention in the cytosol. LATS1/2 inactivation results in the nuclear translocation of YAP/TAZ, leading to the induction of PD-L1. PD-L1 is translocated on the surface of the cancer cells, which further binds the PD-1 receptor of CD8+ T-cells, leading to T-cell inhibition. FVIIa: active factor VII; TF: tissue factor; GSK3β: glycogen synthase kinase 3 beta; PD-L1: programmed death-ligand 1; PD-1: programmed cell death protein 1; CD: cluster of differentiation; LATS1/2: large tumor suppressor kinase ½; YAP: yes-associated protein; TAZ: Tafazzin.
5.3. The Role of FXa in Cancer Immune Evasion
Only a few studies identify FXa as an important contributor to tumor immune evasion. In a study by Graf et al., FXa-driven PAR2 signaling is shown to promote tumor immune evasion [139]. In this study, the authors indicated that FXa, originating from myeloid cells, induces the immune evasion of tumors via signaling through PAR2, and FXa-specific inhibitor, rivaroxaban, shows a synergistic effect with anti-PD-L1 therapy in improving anti-tumor immunity [139]. Consistent with these findings, Haist et al. also showed the improved therapeutic efficacy of immune checkpoint inhibitors while administered with FXa inhibitors in patients suffering from metastatic malignant melanoma [140]. Additionally, FXa/PAR2 signaling in tumor-associated macrophages is discussed in the secretion of immunosuppressive mediators, and FXa inhibition is shown to increase the population of cytotoxic T-cells with tumor-killing ability while reducing the Infiltration by immunosuppressive cells, MDSCs, and Treg in the TME [141]. Figure 6 illustrates how FXa promotes immune evasion via the activation of PAR2.
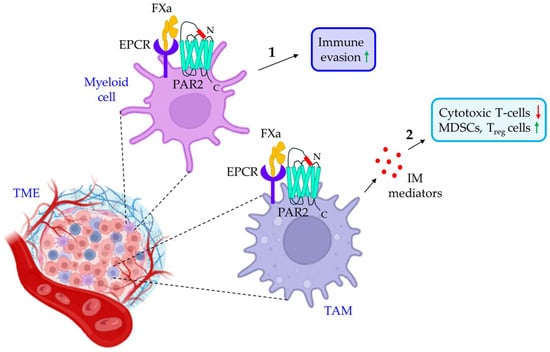
Figure 6.
The role of FXa/PAR2 signaling in cancer immune evasion. 1. FXa in the TME myeloid cells triggers PAR2 to promote tumor immune evasion. 2. TAMs in the TME also express PAR2, which is activated by FXa, leading to the release of IM mediators. IM mediators promote tumor immune evasion by either reducing the population of cytotoxic T-cells or up-regulating MDSCs and Treg cells. The green upward arrows indicate up-regulation; the red downwards arrows indicate down-regulation. TME: tumor microenvironment; EPCR: endothelial cell protein C receptor; FXa: activated factor X; PAR2: protease-activated receptor 2; TAM: tumor-associated macrophage; IM: immunomodulatory; MDSCs: myeloid-derived suppressor cells; Treg cells: regulatory T-cells.
6. Biomarkers for Thrombosis Associated with Immune Checkpoint Inhibitors
A biomarker is defined as a material for which the presence in an organism indicates abnormalities, such as infection or disease [142]. The dynamics of C-reactive proteins (CRPs) are primarily used as a biomarker to determine the treatment responses of immune checkpoint inhibitors associated with venous thromboembolism [143] (VTE) [144]. The treatment of patients with immune checkpoint inhibitors results in an immediate burst of CRP levels within a month, which drops beyond the baseline within 3 months [145,146]. The levels of MDSCs, granulocyte-macrophage colony-stimulating factor (GM-CSF), soluble vascular cell adhesion molecule 1 (sVCAM-1), IL-8, and IL-1 receptor against are shown to be significantly elevated in cancer patients suffering from VTE post immune checkpoint inhibitor challenge [147]. This serves as a predictive biomarker for cancer-associated thrombosis after treatment with immune checkpoint inhibitors. A high level of troponin T (TnT; ≥14 ng/L) in cancer patients’ blood following immune checkpoint inhibitors treatment marks the risk of arterial thrombosis-associated cardiovascular anomalies such as stroke, ischemic attack, pulmonary embolism, heart failure, cardiovascular death, etc. [148]. In another retrospective study, troponin I (TnI) was shown to significantly increase (≥50 ng/L) in the plasma of metastatic cancer patients following treatment with the immune checkpoint inhibitor pembrolizumab, which is an indicator of major adverse cardiac events such as VTE, heart failure, acute coronary syndrome, myocarditis, etc. [149]. Therefore, cardiac troponin level is often considered as a predictive biomarker for cardiac anomalies in cancer after treatment with immune checkpoint inhibitors. Table 4 briefly summarizes the predictive biomarkers in cancer patients associated with thrombosis following immune checkpoint inhibitors treatment.

Table 4.
Biomarkers for thrombosis, associated with immune checkpoint inhibitors.
7. Immune Regulators in Clinical Trials: The Translational Significance in Cancer-Associated Thrombosis
More often, the results observed in preclinical studies may not be reflected in humans, and in several instances, a drug showing promising results in preclinical studies proves to be unsafe in humans or becomes ineffective. Therefore, before releasing a drug into the market, clinical trials on human subjects become indispensable. Clinical trials basically comprise four phases: (1) phase I usually involves a small group of patients (15–50 individuals) to determine the dose, toxicity, and side effects of the drug [150]; (2) phase II involves a fairly large number of patients (100–300 individuals), determining the efficacy, optimum dose, and safety measures [151,152]; (3) phase III involves a huge number of patients (thousands of individuals), further confirming the efficacy and safety profiles [153,154], based on which the drug is approved and released into the market; (4) phase IV involves post-marketing analysis [155]. Although several immune checkpoint inhibition-based therapies exist in clinical trials, the US clinical trial database (https://clinicaltrials.gov/; accessed on 8 February 2024) currently lists only two clinical trial studies that involve cancer-associated thrombosis and the immune response. The clinical trial ‘Exploring Cancer-Associated Thromboembolism Prognosis Biomarkers and Polymorphisms (CAT_PB)’ (NCT number: NCT06065592) aims to assess the biomarkers and related polymorphisms in cancer-associated thrombosis and their interaction with immune systems. It also analyzes the effectiveness of combination therapy using targeted inhibitors, such as Palbociclib, along with anticoagulants, such as rivaroxaban. The second clinical trial, ‘Neoadjuvant Pembrolizumab and Axitinib in Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus (NEOPAX)’ (NCT Number: NCT05969496) primarily focused on evaluating the combined effect of Pembrolizumab and Axitinib in altering inferior vena cava tumor thrombus burden, thereby decreasing surgical complications, leading to improved survival in patients. The details of the clinical trials (both recruiting and non-recruiting with interventional and observational) involving cancer-associated thrombosis and immune response are shown in Table 5.

Table 5.
Current clinical trials, according to the US clinical trial database (https://clinicaltrials.gov/; accessed on 8 February 2024), that involve cancer-associated thrombosis and immune response (both recruiting and non-recruiting with interventional and observational).
8. Conclusions and Future Directions
Coagulation and cancer are intrinsically related; hypercoagulation-associated thrombotic complications are frequently observed in numerous cancers. The coagulation protease-driven activation of PAR signaling plays a major role in cancer progression via the induction of cancer growth, proliferation, metastasis, and angiogenesis while down-regulating apoptosis. Emerging evidence indicates that cancer cells evoke unique mechanisms by which they evade host immune attack, thereby contributing to tumor pathogenesis. Although the role of coagulation protease-mediated PAR signaling is well documented in tumor progression through various mechanisms, their contribution to conferring cancer immune evasion is only just emerging. The present review highlights the recent findings to understand how coagulation proteases, such as thrombin, FVIIa, and FXa, promote the immune evasion of various tumors. Thrombin produces immunosuppressive functions by interfering with the infiltration and activity of cytotoxic T-cells and the maturation and differentiation of DCs, inducing the accumulation and activity of MDSC as well as regulatory T- or B-cells. FVIIa, on the other hand, confers immune evasion by down-regulating the activity and survival of cytotoxic T-cells. The immunosuppressive mechanisms of FXa include the down-regulation of cytotoxic T-cells and the up-regulation of MDSCs and Treg cells. The conventional immunotherapeutic mechanisms that have shown promising results in clinical trials against various solid tumors are the use of several immune checkpoint inhibitors that target CTLA-4, PD-L1, and/or PD-1. However, it has been shown that cancer cells often surpass these therapeutic mechanisms by various means, contributing to decreased survival in patients. Therefore, the application of combination therapy involving immune checkpoint inhibitors along with other important targets would serve therapeutic outcomes better. As clotting protease-driven PAR signaling has been recently discovered to evade hosts’ immunosurveillance mechanisms quite efficiently, potential inhibitors of coagulation proteases or PAR, along with immune checkpoint inhibitors in combination, would result in boosting a host’s immune response to fight against cancer. This combination therapy may open a new therapeutic window on the treatment of cancer besides conventional therapeutic means.
Author Contributions
S.P. contributed to the literature search, experimental data collection, and preparation of the manuscript. T.M. contributed to the literature search and experimental data collection. K.D. contributed to the conceptualization, literature search, experimental data collection, study design, critical review, and preparation of the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
K.D. received the Ramalingaswami Re-entry Fellowship (Ref: BT/HRD/35/02/2006) from Department of Biotechnology, Government of India.
Acknowledgments
All the images in the manuscript were created with BioRender.com. We acknowledge that Bio Render provided us with the platform for the preparation of images.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ag | antigen |
| AP-1 | activator protein 1 |
| aPC | activated protein C |
| Breg | regulatory B-cell |
| CAF | cancer-associated fibroblast |
| CAT | cancer-associated thrombosis |
| CD | cluster of differentiation |
| CRP | C-reactive protein |
| Csf2 | colony stimulating factor 2 gene |
| CTLA-4 | cytotoxic T-lymphocyte associated protein 4 |
| CXCL | C-X-C Motif Chemokine Ligand |
| CXCR | C-X-C chemokine receptor |
| DC | dendritic cell |
| ECL2 | extracellular loop 2 |
| ECM | extracellular matrix |
| EGFR | extracellular growth factor receptor |
| EMT | epithelial to mesenchymal transition |
| EPCR | endothelial cell protein C receptor |
| ERK | extracellular signal-regulated kinase |
| FasL | Fas ligand |
| FVIIa | activated factor VII |
| FXa | activated factor X |
| GARP | glycoprotein A repetitions predominant |
| GITR | glucocorticoid-induced tumor necrosis factor receptor (TNFR)-related protein |
| GITRL | GITR ligand |
| GM-CSF | granulocyte-macrophage CSF |
| GSK3β | glycogen synthase kinase 3β |
| GPCR | G protein-coupled receptor |
| GRK | G protein-coupled receptor kinase |
| IDO | indoleamine 2,3 dioxygenase |
| IFN-γ | interferon γ |
| IL | interleukin |
| LATS | large tumor suppressor kinase |
| LTGF-β1 | latent TGF-β1 |
| MCP1 | monocyte chemoattractant protein 1 |
| MCSF | macrophage colony stimulating factor |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility complex |
| miR | microRNA |
| MMP | matrix metalloproteinase |
| MV | microvesicle |
| NCT | National Clinical Trial |
| NF-ĸB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NSCLC | non-small cell lung cancer |
| PAF | platelet-activating factor |
| Pak1 | p21-activated kinase |
| PAR | protease-activated receptor |
| PDAC | pancreatic ductal adenocarcinoma |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| PGE2 | prostaglandin E2 |
| PKCα | protein kinase Cα |
| Ptgs2 | prostaglandin-endoperoxide synthase 2 gene |
| SNP | single nucleotide polymorphism |
| STAT3 | signal transducer and activator of transcription 3 |
| sVCAM-1 | soluble vascular cell adhesion molecule 1 |
| TAP | transporter associated with antigen processing |
| TAZ | tafazzin |
| TF | tissue factor |
| TGF-β | transforming growth factor β |
| TME | tumor microenvironment |
| Tn | troponin |
| TNBC | triple-negative breast cancer |
| Treg | regulatory T-cell |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | VEGF receptor 2 |
| VTE | venous thromboembolism |
| YAP | yes-associated protein |
References
- Hemker, H.C.; Kahn, M.J. Reaction sequence of blood coagulation. Nature 1967, 215, 1201–1202. [Google Scholar] [CrossRef]
- Dahlback, B. Blood coagulation. Lancet 2000, 355, 1627–1632. [Google Scholar] [CrossRef]
- Walsh, P.N.; Ahmad, S.S. Proteases in blood clotting. Essays Biochem. 2002, 38, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.R. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb. Haemost. 2014, 112, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Falanga, A.; Piccioli, A. Cancer and venous thromboembolism. Lancet Oncol. 2005, 6, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.; Pasi, J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br. J. Cancer 2010, 102 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Cisowski, J.; Nguyen, N.; O’Callaghan, K.; Xu, J.; Agarwal, A.; Kuliopulos, A.; Covic, L. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene 2016, 35, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chou, Y.T.; Fu, H.W. Protease-activated receptor 2 induces migration and promotes Slug-mediated epithelial-mesenchymal transition in lung adenocarcinoma cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 486–503. [Google Scholar] [CrossRef]
- Fujimoto, D.; Hirono, Y.; Goi, T.; Katayama, K.; Matsukawa, S.; Yamaguchi, A. The activation of Proteinase-Activated Receptor-1 (PAR1) mediates gastric cancer cell proliferation and invasion. BMC Cancer 2010, 10, 443. [Google Scholar] [CrossRef]
- Das, K.; Prasad, R.; Ansari, S.A.; Roy, A.; Mukherjee, A.; Sen, P. Matrix metalloproteinase-2: A key regulator in coagulation proteases mediated human breast cancer progression through autocrine signaling. Biomed. Pharmacother. 2018, 105, 395–406. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, R.; Chen, X.; Ying, X.; Chen, G.; Li, M.; Dong, C. Twist-mediated PAR1 induction is required for breast cancer progression and metastasis by inhibiting Hippo pathway. Cell Death Dis. 2020, 11, 520. [Google Scholar] [CrossRef]
- Yin, Y.J.; Salah, Z.; Maoz, M.; Even Ram, S.C.; Ochayon, S.; Neufeld, G.; Katzav, S.; Bar-Shavit, R. Oncogenic transformation induces tumor angiogenesis: A role for PAR1 activation. FASEB J. 2003, 17, 163–174. [Google Scholar] [CrossRef]
- Chang, L.H.; Chen, C.H.; Huang, D.Y.; Pai, H.C.; Pan, S.L.; Teng, C.M. Thrombin induces expression of twist and cell motility via the hypoxia-inducible factor-1alpha translational pathway in colorectal cancer cells. J. Cell. Physiol. 2011, 226, 1060–1068. [Google Scholar] [CrossRef]
- Datar, I.; Schalper, K.A. Epithelial-Mesenchymal Transition and Immune Evasion during Lung Cancer Progression: The Chicken or the Egg? Clin. Cancer Res. 2016, 22, 3422–3424. [Google Scholar] [CrossRef]
- Vu, T.K.; Hung, D.T.; Wheaton, V.I.; Coughlin, S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64, 1057–1068. [Google Scholar] [CrossRef]
- Vu, T.K.; Wheaton, V.I.; Hung, D.T.; Charo, I.; Coughlin, S.R. Domains specifying thrombin-receptor interaction. Nature 1991, 353, 674–677. [Google Scholar] [CrossRef]
- Nystedt, S.; Emilsson, K.; Wahlestedt, C.; Sundelin, J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9208–9212. [Google Scholar] [CrossRef]
- Schmidt, V.A.; Nierman, W.C.; Maglott, D.R.; Cupit, L.D.; Moskowitz, K.A.; Wainer, J.A.; Bahou, W.F. The human proteinase-activated receptor-3 (PAR-3) gene. Identification within a Par gene cluster and characterization in vascular endothelial cells and platelets. J. Biol. Chem. 1998, 273, 15061–15068. [Google Scholar] [CrossRef]
- Kahn, M.L.; Zheng, Y.W.; Huang, W.; Bigornia, V.; Zeng, D.; Moff, S.; Farese, R.V., Jr.; Tam, C.; Coughlin, S.R. A dual thrombin receptor system for platelet activation. Nature 1998, 394, 690–694. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Molino, M.; Kahn, M.; Brass, L.F. Protease activated receptors: Theme and variations. Oncogene 2001, 20, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Connolly, A.J.; Zeng, D.; Kahn, M.L.; Zheng, Y.W.; Timmons, C.; Tram, T.; Coughlin, S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 1997, 386, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Nystedt, S.; Emilsson, K.; Larsson, A.K.; Strombeck, B.; Sundelin, J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur. J. Biochem. 1995, 232, 84–89. [Google Scholar] [CrossRef]
- Xu, W.F.; Andersen, H.; Whitmore, T.E.; Presnell, S.R.; Yee, D.P.; Ching, A.; Gilbert, T.; Davie, E.W.; Foster, D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. USA 1998, 95, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Chen, J.; Ishii, M.; Ishii, K.; Wang, L.; Nanevicz, T.; Turck, C.W.; Vu, T.K.; Coughlin, S.R. Specificity of the thrombin receptor for agonist peptide is defined by its extracellular surface. Nature 1994, 368, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, R.M.; Naughton, M.A.; Teng, W.; Hung, D.T.; Rose, J.; Vu, T.K.; Wheaton, V.I.; Turck, C.W.; Coughlin, S.R. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J. Biol. Chem. 1992, 267, 13146–13149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ishii, M.; Wang, L.; Ishii, K.; Coughlin, S.R. Thrombin receptor activation. Confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J. Biol. Chem. 1994, 269, 16041–16045. [Google Scholar] [CrossRef]
- Lerner, D.J.; Chen, M.; Tram, T.; Coughlin, S.R. Agonist recognition by proteinase-activated receptor 2 and thrombin receptor. Importance of extracellular loop interactions for receptor function. J. Biol. Chem. 1996, 271, 13943–13947. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Matsui, M.; Zheng, Y.W.; Sulciner, D.J.; Weiss, E.J.; Ludeman, M.J.; Coughlin, S.R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature 2000, 404, 609–613. [Google Scholar] [CrossRef]
- Macfarlane, S.R.; Seatter, M.J.; Kanke, T.; Hunter, G.D.; Plevin, R. Proteinase-activated receptors. Pharmacol. Rev. 2001, 53, 245–282. [Google Scholar]
- Lefkowitz, R.J.; Rajagopal, K.; Whalen, E.J. New roles for beta-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol. Cell 2006, 24, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Xiao, K.; Lefkowitz, R.J. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 2011, 36, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Soh, U.J.; Paing, M.M.; Arora, P.; Trejo, J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc. Natl. Acad. Sci. USA 2009, 106, 6393–6397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Metcalf, M.; Bunnett, N.W. Biased signaling of protease-activated receptors. Front. Endocrinol. 2014, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Kondreddy, V.; Pendurthi, U.R.; Xu, X.; Griffin, J.H.; Rao, L.V.M. FVIIa (Factor VIIa) Induces Biased Cytoprotective Signaling in Mice through the Cleavage of PAR (Protease-Activated Receptor)-1 at Canonical Arg41 (Arginine41) Site. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Sinha, R.K.; Burnier, L.; Bouwens, E.A.; Griffin, J.H. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 2012, 120, 5237–5246. [Google Scholar] [CrossRef]
- Schuepbach, R.A.; Madon, J.; Ender, M.; Galli, P.; Riewald, M. Protease-activated receptor-1 cleaved at R46 mediates cytoprotective effects. J. Thromb. Haemost. 2012, 10, 1675–1684. [Google Scholar] [CrossRef]
- Pompili, E.; De Franchis, V.; Giampietri, C.; Leone, S.; De Santis, E.; Fornai, F.; Fumagalli, L.; Fabrizi, C. Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves. Biomolecules 2021, 11, 1668. [Google Scholar] [CrossRef]
- Kuliopulos, A.; Covic, L.; Seeley, S.K.; Sheridan, P.J.; Helin, J.; Costello, C.E. Plasmin desensitization of the PAR1 thrombin receptor: Kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry 1999, 38, 4572–4585. [Google Scholar] [CrossRef]
- Camerer, E.; Huang, W.; Coughlin, S.R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. USA 2000, 97, 5255–5260. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Franchini, A.G.; Madon, J.; Schuepbach, R.A. Thrombin cleaves and activates the protease-activated receptor 2 dependent on thrombomodulin co-receptor availability. Thromb. Res. 2019, 177, 91–101. [Google Scholar] [CrossRef]
- Lane, D.A.; Philippou, H.; Huntington, J.A. Directing thrombin. Blood 2005, 106, 2605–2612. [Google Scholar] [CrossRef]
- McLaughlin, J.N.; Shen, L.; Holinstat, M.; Brooks, J.D.; Dibenedetto, E.; Hamm, H.E. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J. Biol. Chem. 2005, 280, 25048–25059. [Google Scholar] [CrossRef] [PubMed]
- Camerer, E.; Coughlin, S.R. APC signaling: Tickling PAR1 for barrier protection? Blood 2005, 105, 3004–3005. [Google Scholar] [CrossRef]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-activated receptors: Contribution to physiology and disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Keshava, S.; Mukherjee, T.; Rao, L.V.M. Activated protein C-released endothelial extracellular vesicles: A potential mechanism for their cytoprotective effects. Blood 2024, 143, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Ludeman, M.J.; Kataoka, H.; Srinivasan, Y.; Esmon, N.L.; Esmon, C.T.; Coughlin, S.R. PAR1 cleavage and signaling in response to activated protein C and thrombin. J. Biol. Chem. 2005, 280, 13122–13128. [Google Scholar] [CrossRef]
- Riewald, M.; Petrovan, R.J.; Donner, A.; Mueller, B.M.; Ruf, W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002, 296, 1880–1882. [Google Scholar] [CrossRef]
- Regan, L.M.; Mollica, J.S.; Rezaie, A.R.; Esmon, C.T. The interaction between the endothelial cell protein C receptor and protein C is dictated by the gamma-carboxyglutamic acid domain of protein C. J. Biol. Chem. 1997, 272, 26279–26284. [Google Scholar] [CrossRef] [PubMed]
- Fukudome, K.; Esmon, C.T. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J. Biol. Chem. 1994, 269, 26486–26491. [Google Scholar] [CrossRef] [PubMed]
- Soh, U.J.; Trejo, J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc. Natl. Acad. Sci. USA 2011, 108, E1372–E1380. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Pendurthi, U.R.; Manco-Johnson, M.; Martin, E.J.; Brophy, D.F.; Rao, L.V.M. Factor VIIa treatment increases circulating extracellular vesicles in hemophilia patients: Implications for the therapeutic hemostatic effect of FVIIa. J. Thromb. Haemost. 2022, 20, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pendurthi, U.R.; Steinoe, A.; Esmon, C.T.; Rao, L.V. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J. Biol. Chem. 2007, 282, 11849–11857. [Google Scholar] [CrossRef] [PubMed]
- Pendurthi, U.R.; Rao, L.V. Factor VIIa interaction with endothelial cells and endothelial cell protein C receptor. Thromb. Res. 2010, 125 (Suppl. S1), S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Keshava, S.; Ansari, S.A.; Kondreddy, V.; Esmon, C.T.; Griffin, J.H.; Pendurthi, U.R.; Rao, L.V.M. Factor VIIa induces extracellular vesicles from the endothelium: A potential mechanism for its hemostatic effect. Blood 2021, 137, 3428–3442. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Keshava, S.; Kolesnick, R.; Pendurthi, U.R.; Rao, L.V.M. MicroRNA-10a enrichment in factor VIIa-released endothelial extracellular vesicles: Potential mechanisms. J. Thromb. Haemost. 2024, 22, 441–454. [Google Scholar] [CrossRef]
- Das, K.; Keshava, S.; Mukherjee, T.; Wang, J.; Magisetty, J.; Kolesnick, R.; Pendurthi, U.R.; Rao, L.V.M. Factor VIIa releases phosphatidylserine-enriched extracellular vesicles from endothelial cells by activating acid sphingomyelinase. J. Thromb. Haemost. 2023, 21, 3414–3431. [Google Scholar] [CrossRef]
- Das, K.; Keshava, S.; Pendurthi, U.R.; Rao, L.V.M. Factor VIIa suppresses inflammation and barrier disruption through the release of EEVs and transfer of microRNA 10a. Blood 2022, 139, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Brude, O.P.; Archer, F.; Leoni, P.; Derian, C.; Bolsover, S.; Laurent, G.J.; Chambers, R.C. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp. Cell Res. 2005, 304, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, R.A.; Riewald, M. Coagulation factor Xa cleaves protease-activated receptor-1 and mediates signaling dependent on binding to the endothelial protein C receptor. J. Thromb. Haemost. 2010, 8, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Nayak, R.; Clark, C.A.; Gopalakrishnan, R.; Esmon, C.T.; Pendurthi, U.R.; Rao, L.V. Factor X binding to endothelial cell protein C receptor: Comparison with factor VIIa and activated protein C. Blood 2011, 118, 2635–2636. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, G.; Ahamed, J.; Pawlinski, R.; Liu, C.; Mackman, N.; Ruf, W.; Edgington, T.S. Factor Xa binding to annexin 2 mediates signal transduction via protease-activated receptor 1. Circ. Res. 2008, 102, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulou, K.; Hansen, K.K.; Saifeddine, M.; Vergnolle, N.; Tea, I.; Blaber, M.; Blaber, S.I.; Scarisbrick, I.; Diamandis, E.P.; Hollenberg, M.D. Kallikrein-mediated cell signalling: Targeting proteinase-activated receptors (PARs). Biol. Chem. 2006, 387, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Ruf, W.; Dorfleutner, A.; Riewald, M. Specificity of coagulation factor signaling. J. Thromb. Haemost. 2003, 1, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, V.; Mandal, S.K.; Papanna, V.; Rao, L.V.; Pendurthi, U.R. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1447–1455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Petersen, H.H.; Ahamed, J.; Felding-Habermann, B.; Takada, Y.; Mueller, B.M.; Ruf, W. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008, 111, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Mihara, K.; Ramachandran, R.; Saifeddine, M.; Hansen, K.K.; Renaux, B.; Polley, D.; Gibson, S.; Vanderboor, C.; Hollenberg, M.D. Thrombin-Mediated Direct Activation of Proteinase-Activated Receptor-2: Another Target for Thrombin Signaling. Mol. Pharmacol. 2016, 89, 606–614. [Google Scholar] [CrossRef]
- Guo, H.; Liu, D.; Gelbard, H.; Cheng, T.; Insalaco, R.; Fernandez, J.A.; Griffin, J.H.; Zlokovic, B.V. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 2004, 41, 563–572. [Google Scholar] [CrossRef]
- Bock, F.; Shahzad, K.; Wang, H.; Stoyanov, S.; Wolter, J.; Dong, W.; Pelicci, P.G.; Kashif, M.; Ranjan, S.; Schmidt, S.; et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc. Natl. Acad. Sci. USA 2013, 110, 648–653. [Google Scholar] [CrossRef]
- Burnier, L.; Mosnier, L.O. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood 2013, 122, 807–816. [Google Scholar] [CrossRef]
- Stavenuiter, F.; Mosnier, L.O. Noncanonical PAR3 activation by factor Xa identifies a novel pathway for Tie2 activation and stabilization of vascular integrity. Blood 2014, 124, 3480–3489. [Google Scholar] [CrossRef]
- Adam, F.; Verbeuren, T.J.; Fauchere, J.L.; Guillin, M.C.; Jandrot-Perrus, M. Thrombin-induced platelet PAR4 activation: Role of glycoprotein Ib and ADP. J. Thromb. Haemost. 2003, 1, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.M.; Church, F.C. Activated protein C promotes breast cancer cell migration through interactions with EPCR and PAR-1. Exp. Cell Res. 2007, 313, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Flick, M.J.; Rewerts, C.; Cruz, C.; Palumbo, J.S.; Luyendyk, J.P.; Yang, Y.; Konieczny, S.F. Tumor Cell Thrombin/PAR-1 Signaling Drives Pancreatic Ductal Adenocarcinoma Growth and Dissemination. Blood 2015, 126, 1070. [Google Scholar] [CrossRef]
- Zhu, Q.; Luo, J.; Wang, T.; Ren, J.; Hu, K.; Wu, G. The activation of protease-activated receptor 1 mediates proliferation and invasion of nasopharyngeal carcinoma cells. Oncol. Rep. 2012, 28, 255–261. [Google Scholar] [CrossRef]
- Ohshiro, K.; Bui-Nguyen, T.M.; Divijendra Natha, R.S.; Schwartz, A.M.; Levine, P.; Kumar, R. Thrombin stimulation of inflammatory breast cancer cells leads to aggressiveness via the EGFR-PAR1-Pak1 pathway. Int. J. Biol. Markers 2012, 27, e305–e313. [Google Scholar] [CrossRef] [PubMed]
- Nierodzik, M.L.; Chen, K.; Takeshita, K.; Li, J.J.; Huang, Y.Q.; Feng, X.S.; D’Andrea, M.R.; Andrade-Gordon, P.; Karpatkin, S. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood 1998, 92, 3694–3700. [Google Scholar] [CrossRef]
- Arce, M.; Pinto, M.P.; Galleguillos, M.; Munoz, C.; Lange, S.; Ramirez, C.; Erices, R.; Gonzalez, P.; Velasquez, E.; Tempio, F.; et al. Coagulation Factor Xa Promotes Solid Tumor Growth, Experimental Metastasis and Endothelial Cell Activation. Cancers 2019, 11, 1103. [Google Scholar] [CrossRef]
- Hiramoto, K.; Akita, N.; Nishioka, J.; Suzuki, K. Edoxaban, a Factor Xa-Specific Direct Oral Anticoagulant, Significantly Suppresses Tumor Growth in Colorectal Cancer Colon26-Inoculated BALB/c Mice. TH Open 2023, 7, e1–e13. [Google Scholar] [CrossRef]
- Hu, L.; Xia, L.; Zhou, H.; Wu, B.; Mu, Y.; Wu, Y.; Yan, J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCalpha and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumour Biol. 2013, 34, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Ansari, S.A.; Das, K.; Prasad, R.; Bhattacharya, A.; Mallik, S.; Mukherjee, A.; Sen, P. Coagulation factor VIIa-mediated protease-activated receptor 2 activation leads to beta-catenin accumulation via the AKT/GSK3beta pathway and contributes to breast cancer progression. J. Biol. Chem. 2017, 292, 13688–13701. [Google Scholar] [CrossRef]
- Das, K.; Paul, S.; Singh, A.; Ghosh, A.; Roy, A.; Ansari, S.A.; Prasad, R.; Mukherjee, A.; Sen, P. Triple-negative breast cancer-derived microvesicles transfer microRNA221 to the recipient cells and thereby promote epithelial-to-mesenchymal transition. J. Biol. Chem. 2019, 294, 13681–13696. [Google Scholar] [CrossRef]
- Meyer, U.; Polster, S.; Roennpagel, V.; Grammbauer, S.; Dombrowski, F.; Ritter, C.; Rauch, B. Effects of the activated coagulation factor X (FXa) and its protease-activated receptor-2 (PAR2) on colon cancer cell growth in vitro and in vivo. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655.3281. [Google Scholar] [CrossRef]
- Han, N.; Jin, K.; He, K.; Cao, J.; Teng, L. Protease-activated receptors in cancer: A systematic review. Oncol. Lett. 2011, 2, 599–608. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Song, S.; Yue, X.; Li, Q. Protease-activated receptor-1 (PAR-1): A promising molecular target for cancer. Oncotarget 2017, 8, 107334–107345. [Google Scholar] [CrossRef]
- Zigler, M.; Kamiya, T.; Brantley, E.C.; Villares, G.J.; Bar-Eli, M. PAR-1 and thrombin: The ties that bind the microenvironment to melanoma metastasis. Cancer Res. 2011, 71, 6561–6566. [Google Scholar] [CrossRef][Green Version]
- Garcia-Lopez, M.T.; Gutierrez-Rodriguez, M.; Herranz, R. Thrombin-activated receptors: Promising targets for cancer therapy? Curr. Med. Chem. 2010, 17, 109–128. [Google Scholar] [CrossRef]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ettelaie, C.; Collier, M.E.; Featherby, S.; Benelhaj, N.E.; Greenman, J.; Maraveyas, A. Analysis of the potential of cancer cell lines to release tissue factor-containing microvesicles: Correlation with tissue factor and PAR2 expression. Thromb. J. 2016, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Prasad, R.; Singh, A.; Bhattacharya, A.; Roy, A.; Mallik, S.; Mukherjee, A.; Sen, P. Protease-activated receptor 2 promotes actomyosin dependent transforming microvesicles generation from human breast cancer. Mol. Carcinog. 2018, 57, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Paul, S.; Mukherjee, T.; Ghosh, A.; Sharma, A.; Shankar, P.; Gupta, S.; Keshava, S.; Parashar, D. Beyond Macromolecules: Extracellular Vesicles as Regulators of Inflammatory Diseases. Cells 2023, 12, 1963. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Prasad, R.; Roy, S.; Mukherjee, A.; Sen, P. The Protease Activated Receptor2 Promotes Rab5a Mediated Generation of Pro-metastatic Microvesicles. Sci. Rep. 2018, 8, 7357. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Coukos, G. Deciphering and reversing tumor immune suppression. Immunity 2013, 39, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Chen, D.S. Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann. Oncol. 2016, 27, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, Y.; Huang, M.; Kusmartsev, S.; Bhattacharya, R.; Cheng, P.; Salup, R.; Jove, R.; Gabrilovich, D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 2004, 172, 464–474. [Google Scholar] [CrossRef]
- Williams, L.M.; Ricchetti, G.; Sarma, U.; Smallie, T.; Foxwell, B.M. Interleukin-10 suppression of myeloid cell activation—A continuing puzzle. Immunology 2004, 113, 281–292. [Google Scholar] [CrossRef]
- Sa-Nunes, A.; Bafica, A.; Lucas, D.A.; Conrads, T.P.; Veenstra, T.D.; Andersen, J.F.; Mather, T.N.; Ribeiro, J.M.; Francischetti, I.M. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol. 2007, 179, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef]
- Zong, J.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Tumor-derived factors modulating dendritic cell function. Cancer Immunol. Immunother. 2016, 65, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef]
- Gimmi, C.D.; Freeman, G.J.; Gribben, J.G.; Gray, G.; Nadler, L.M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc. Natl. Acad. Sci. USA 1993, 90, 6586–6590. [Google Scholar] [CrossRef]
- Macian, F.; Garcia-Cozar, F.; Im, S.H.; Horton, H.F.; Byrne, M.C.; Rao, A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 2002, 109, 719–731. [Google Scholar] [CrossRef]
- Williams, M.A.; Tyznik, A.J.; Bevan, M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006, 441, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Qin, R.Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Zarychta, E.; Ruszkowska-Ciastek, B. Cooperation between Angiogenesis, Vasculogenesis, Chemotaxis, and Coagulation in Breast Cancer Metastases Development: Pathophysiological Point of View. Biomedicines 2022, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef]
- Taylor, B.C.; Balko, J.M. Mechanisms of MHC-I Downregulation and Role in Immunotherapy Response. Front. Immunol. 2022, 13, 844866. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.T.; Zeng, Z.; Salim, N.; Mattarollo, S.; Wells, J.W.; Leggatt, G.R. The Role of CXCR3 and Its Chemokine Ligands in Skin Disease and Cancer. Front. Med. 2018, 5, 271. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, L.; Flavell, R.A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat. Med. 2001, 7, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Metelli, A.; Wu, B.X.; Riesenberg, B.; Guglietta, S.; Huck, J.D.; Mills, C.; Li, A.; Rachidi, S.; Krieg, C.; Rubinstein, M.P.; et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-beta. Sci. Transl. Med. 2020, 12, eaay4860. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Stang, A.; Schweickert, P.G.; Lanman, N.A.; Paul, E.N.; Monia, B.P.; Revenko, A.S.; Palumbo, J.S.; Mullins, E.S.; Elzey, B.D.; et al. Thrombin Signaling Promotes Pancreatic Adenocarcinoma through PAR-1-Dependent Immune Evasion. Cancer Res. 2019, 79, 3417–3430. [Google Scholar] [CrossRef]
- Schweickert, P.G.; Yang, Y.; White, E.E.; Cresswell, G.M.; Elzey, B.D.; Ratliff, T.L.; Arumugam, P.; Antoniak, S.; Mackman, N.; Flick, M.J.; et al. Thrombin-PAR1 signaling in pancreatic cancer promotes an immunosuppressive microenvironment. J. Thromb. Haemost. 2021, 19, 161–172. [Google Scholar] [CrossRef]
- Alexander, E.T.; Gilmour, S.K. Immunomodulatory role of thrombin in cancer progression. Mol. Carcinog. 2022, 61, 527–536. [Google Scholar] [CrossRef]
- Rebe, C.; Ghiringhelli, F. STAT3, a Master Regulator of Anti-Tumor Immune Response. Cancers 2019, 11, 1280. [Google Scholar] [CrossRef]
- Chen, X.; Baumel, M.; Mannel, D.N.; Howard, O.M.; Oppenheim, J.J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol. 2007, 179, 154–161. [Google Scholar] [CrossRef]
- Zhao, X.; Rong, L.; Zhao, X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef]
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667. [Google Scholar] [CrossRef]
- Bertrand, F.; Rochotte, J.; Colacios, C.; Montfort, A.; Andrieu-Abadie, N.; Levade, T.; Benoist, H.; Segui, B. Targeting TNF alpha as a novel strategy to enhance CD8+ T cell-dependent immune response in melanoma? Oncoimmunology 2016, 5, e1068495. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Rochotte, J.; Colacios, C.; Montfort, A.; Tilkin-Mariame, A.F.; Touriol, C.; Rochaix, P.; Lajoie-Mazenc, I.; Andrieu-Abadie, N.; Levade, T.; et al. Blocking Tumor Necrosis Factor alpha Enhances CD8 T-cell-Dependent Immunity in Experimental Melanoma. Cancer Res. 2015, 75, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Fisher, G.; Miller, R.E.; Peschon, J.; Lynch, D.H.; Lenardo, M.J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 1995, 377, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Das, K.; Ghosh, A.; Chatterjee, A.; Bhoumick, A.; Basu, A.; Sen, P. Coagulation factor VIIa enhances programmed death-ligand 1 expression and its stability in breast cancer cells to promote breast cancer immune evasion. J. Thromb. Haemost. 2023, 21, 3522–3538. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Paul, S.; Ghosh, A.; Gupta, S.; Mukherjee, T.; Shankar, P.; Sharma, A.; Keshava, S.; Chauhan, S.C.; Kashyap, V.K.; et al. Extracellular Vesicles in Triple-Negative Breast Cancer: Immune Regulation, Biomarkers, and Immunotherapeutic Potential. Cancers 2023, 15, 4879. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Wilgenbus, P.; Pagel, S.; Pott, J.; Marini, F.; Reyda, S.; Kitano, M.; Macher-Goppinger, S.; Weiler, H.; Ruf, W. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci. Immunol. 2019, 4, eaaw8405. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Pemler, S.; Heinz, J.; Fleischer, M.I.; Graf, C.; Ruf, W.; Loquai, C.; Grabbe, S. Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors-A Retrospective, Real-World Cohort Study. Cancers 2021, 13, 5103. [Google Scholar] [CrossRef]
- Ruf, W.; Graf, C. Coagulation signaling and cancer immunotherapy. Thromb. Res. 2020, 191 (Suppl. S1), S106–S111. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Mukherjee, T.; Shankar, P. The Role of Extracellular Vesicles in the Pathogenesis of Hematological Malignancies: Interaction with Tumor Microenvironment; a Potential Biomarker and Targeted Therapy. Biomolecules 2023, 13, 897. [Google Scholar] [CrossRef]
- Kondreddy, V.; Keshava, S.; Das, K.; Magisetty, J.; Rao, L.V.M.; Pendurthi, U.R. The Gab2-MALT1 axis regulates thromboinflammation and deep vein thrombosis. Blood 2022, 140, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Riedl, J.; Barth, D.; Chan, W.-S.E.; Wiedemann, S.; Höller, C.; Fuereder, T.; Jost, P.; Pabinger, I.; Preusser, M.; et al. Early Dynamics of C-Reactive Protein Predict Risk of Venous Thromboembolism in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Blood 2022, 140 (Suppl. S1), 1250–1251. [Google Scholar] [CrossRef]
- Fukuda, S.; Saito, K.; Yasuda, Y.; Kijima, T.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Kageyama, Y.; Fujii, Y. Impact of C-reactive protein flare-response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J. Immunother. Cancer 2021, 9, e001564. [Google Scholar] [CrossRef] [PubMed]
- Klumper, N.; Saal, J.; Berner, F.; Lichtensteiger, C.; Wyss, N.; Heine, A.; Bauernfeind, F.G.; Ellinger, J.; Brossart, P.; Diem, S.; et al. C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J. Immunother. Cancer 2022, 10, e004024. [Google Scholar] [CrossRef] [PubMed]
- Roopkumar, J.; Swaidani, S.; Kim, A.S.; Thapa, B.; Gervaso, L.; Hobbs, B.P.; Wei, W.; Alban, T.J.; Funchain, P.; Kundu, S.; et al. Increased Incidence of Venous Thromboembolism with Cancer Immunotherapy. Med 2021, 2, 423–434.e3. [Google Scholar] [CrossRef]
- Petricciuolo, S.; Delle Donne, M.G.; Aimo, A.; Chella, A.; De Caterina, R. Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur. J. Clin. Investig. 2021, 51, e13400. [Google Scholar] [CrossRef]
- Waissengein, B.; Abu Ata, B.; Merimsky, O.; Shamai, S.; Wolf, I.; Arnold, J.H.; Bar-On, T.; Banai, S.; Khoury, S.; Laufer-Perl, M. The predictive value of high sensitivity troponin measurements in patients treated with immune checkpoint inhibitors. Clin. Res. Cardiol. 2023, 112, 409–418. [Google Scholar] [CrossRef]
- Ivy, S.P.; Siu, L.L.; Garrett-Mayer, E.; Rubinstein, L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: A report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin. Cancer Res. 2010, 16, 1726–1736. [Google Scholar] [CrossRef]
- Stallard, N. Optimal sample sizes for phase II clinical trials and pilot studies. Stat. Med. 2012, 31, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H.; Thall, P.F. New designs for phase 2 clinical trials. Blood 2003, 102, 442–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahajan, R.; Gupta, K. Adaptive design clinical trials: Methodology, challenges and prospect. Indian J. Pharmacol. 2010, 42, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, P.R.; Castro, M.G. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail? Curr. Gene Ther. 2009, 9, 368–374. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ye, X.; Guo, X.; Zhang, T.; He, J. Overview of phase IV clinical trials for postmarket drug safety surveillance: A status report from the ClinicalTrials.gov registry. BMJ Open 2016, 6, e010643. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).