Review of Related Factors for Persistent Risk of Hepatitis B Virus-Associated Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
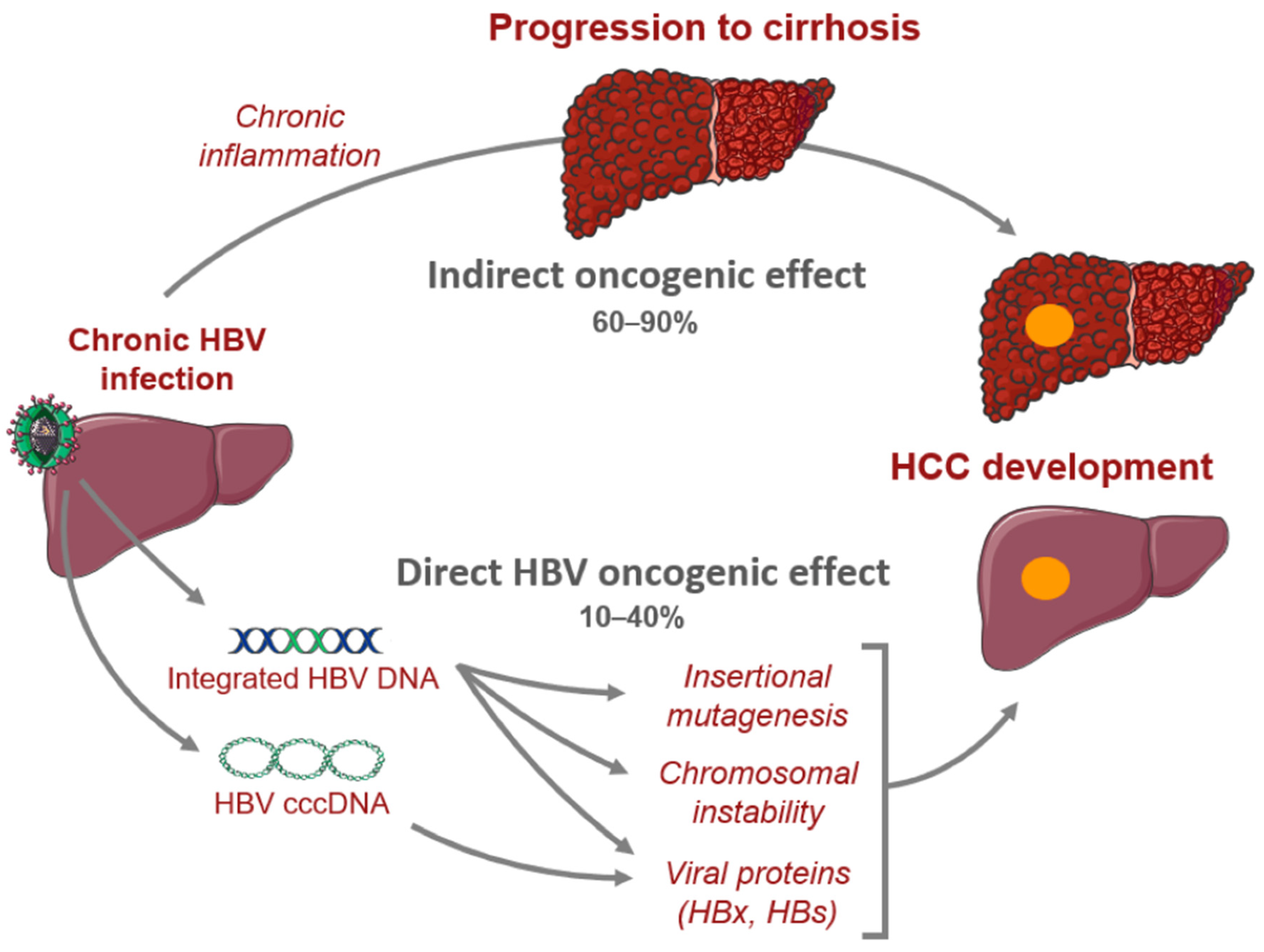
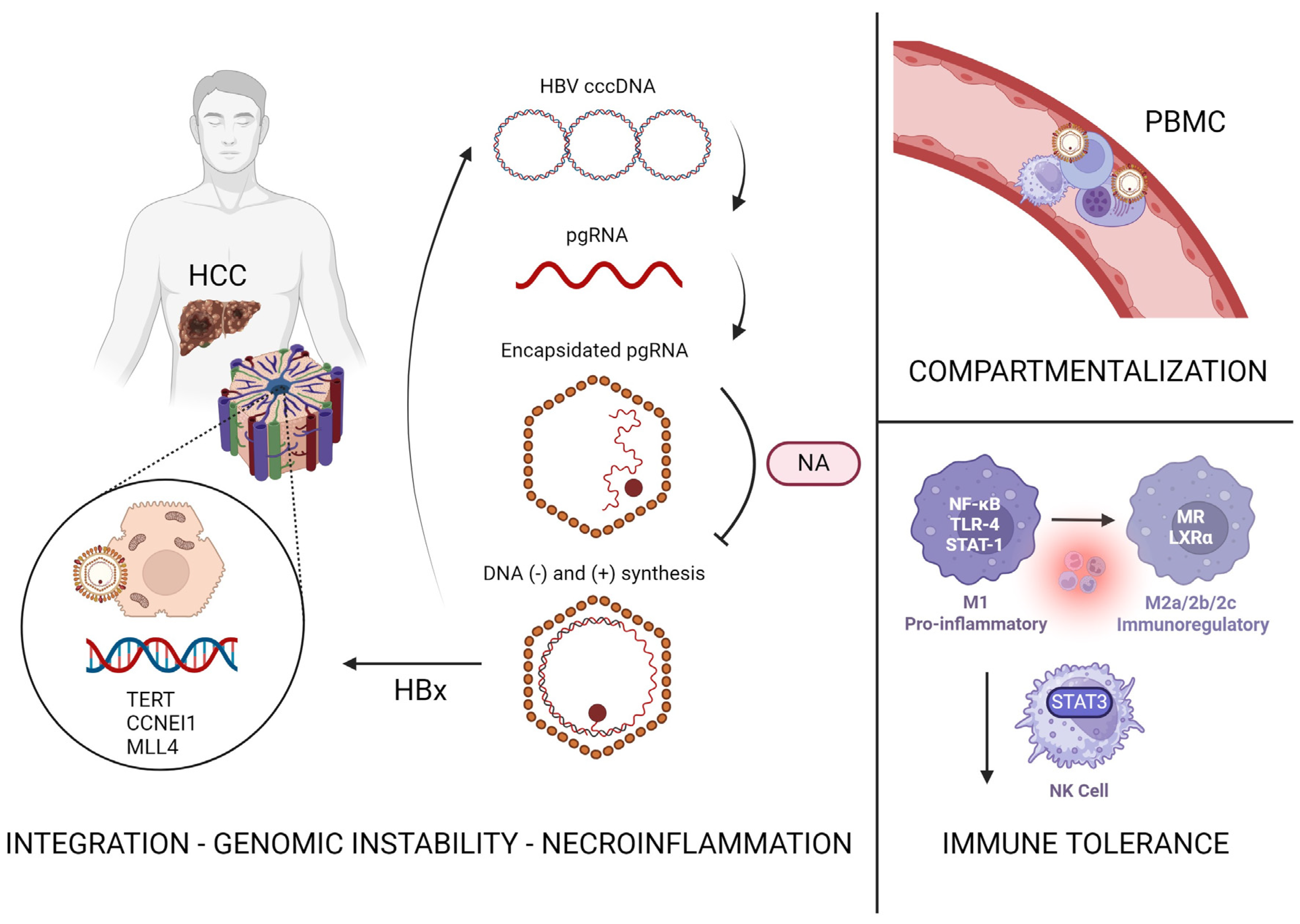
2. HBV Pathophysiology and Hepatocarcinogenesis
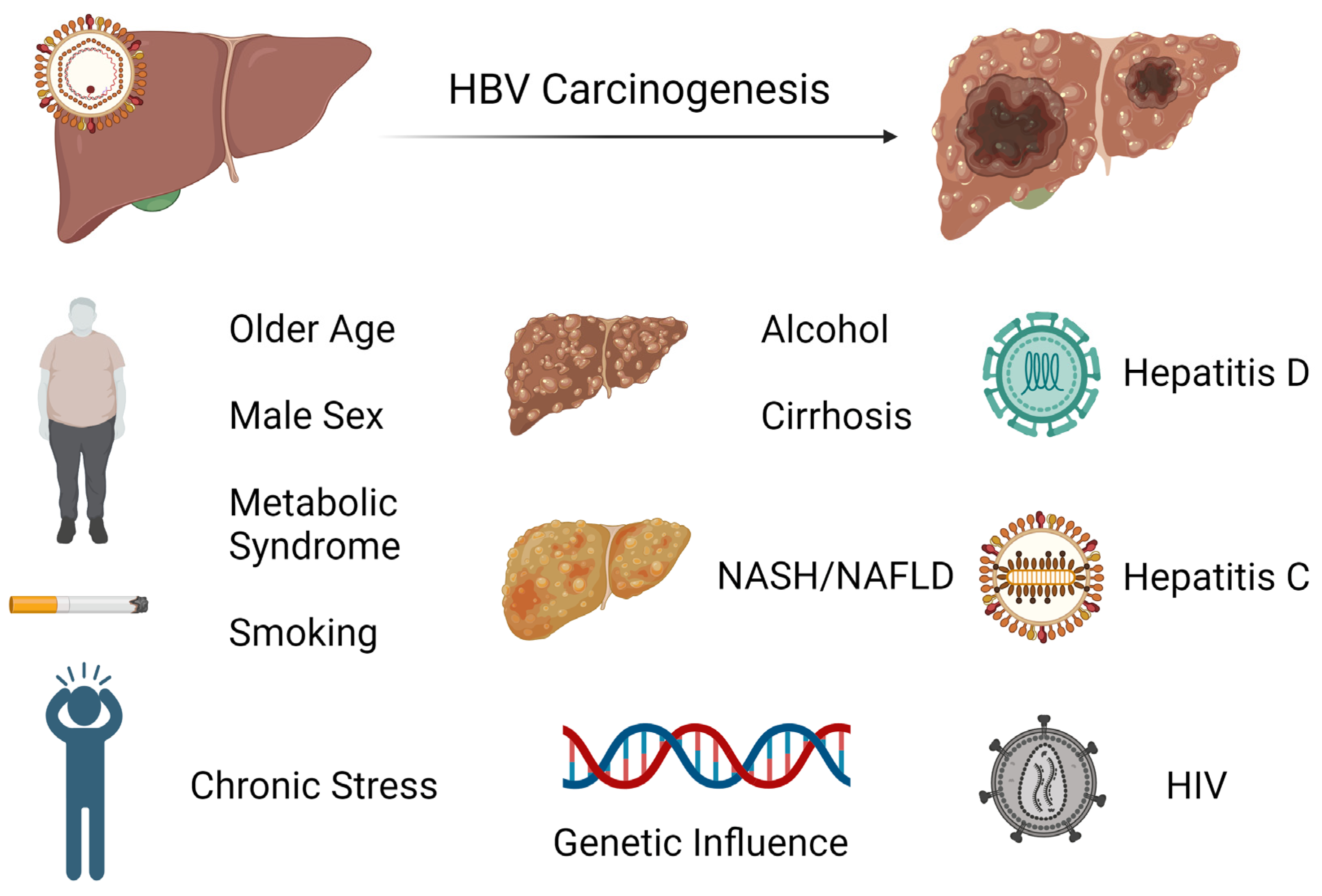
3. HCC Risk Factors and Surveillance
4. Incidence of HCC in Response to Treatment of HBV
5. Persistent Risk of HCC in HBV Infection
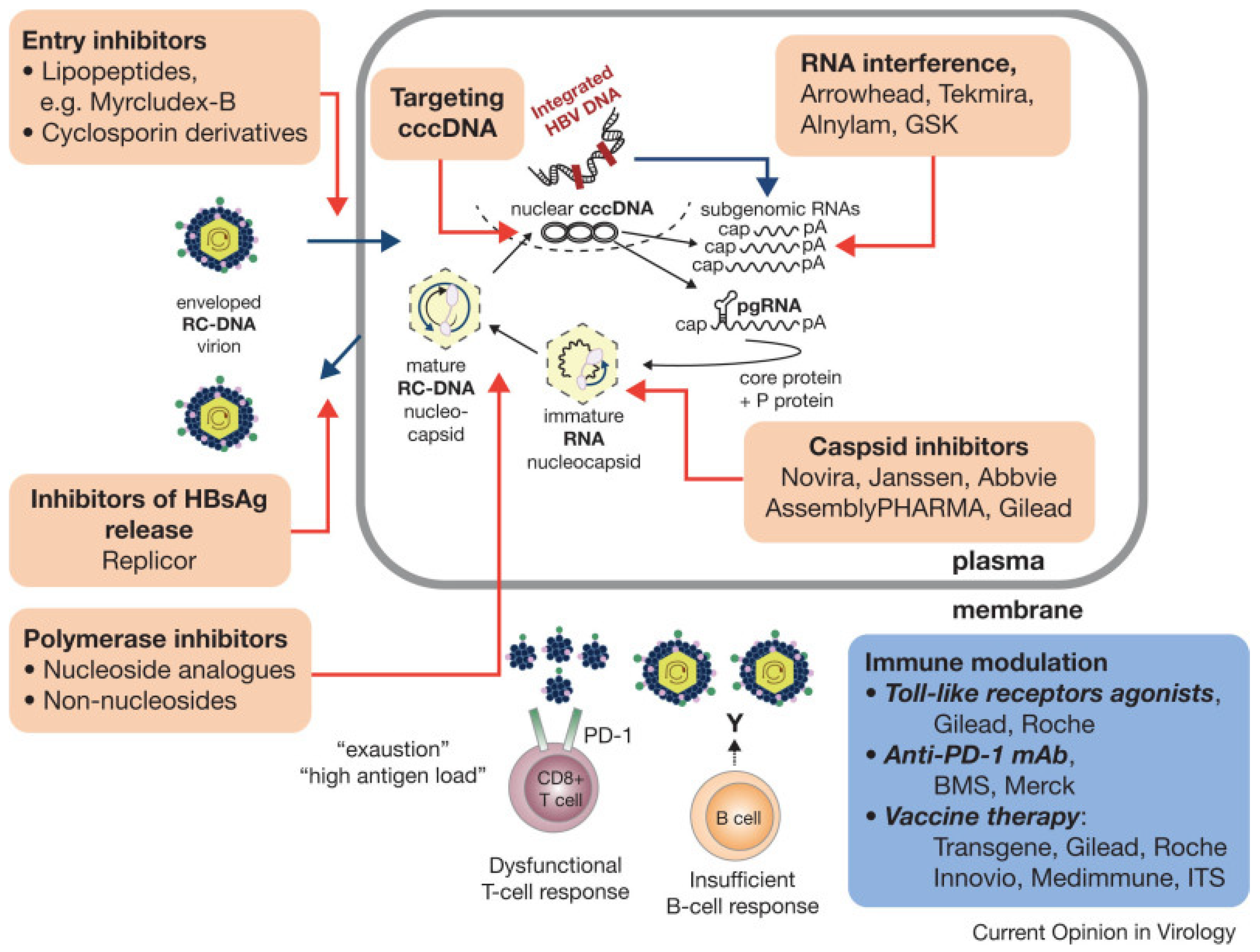
6. The Road to HBV Cure
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Liu, Z.; Gu, F. Epidemiology and Prevention of Hepatitis B Virus Infection. Int. J. Med. Sci. 2005, 2, 50–57. [Google Scholar] [CrossRef]
- Bashir Hamidu, R.; Hann, R.R.; Hann, H.-W. Chronicles of HBV and the Road to HBV Cure. Livers 2023, 3, 232–239. [Google Scholar] [CrossRef]
- Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 21 December 2023).
- Pithawa, A.K. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, diagnosis, management. Med. J. Armed Forces India 2007, 63, 205. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000, 118, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014, 20, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, L.G.; Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 2006, 1, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S. Chronic hepatitis B. N. Engl. J. Med. 2002, 346, 1682–1683. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Pisani, P.; Ferlay, J. Global cancer statistics. CA Cancer J. Clin. 1999, 49, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G. Natural history and prognosis of hepatitis B. Semin. Liver Dis. 2003, 23, 47–58. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef]
- Beasley, R.P.; Hwang, L.Y.; Lin, C.C.; Leu, M.L.; Stevens, C.E.; Szmuness, W.; Chen, K.P. Incidence of hepatitis B virus infections in preschool children in Taiwan. J. Infect. Dis. 1982, 146, 198–204. [Google Scholar] [CrossRef]
- Alter, M.J.; Hadler, S.C.; Margolis, H.S.; Alexander, W.J.; Hu, P.Y.; Judson, F.N.; Mares, A.; Miller, J.K.; Moyer, L.A. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA 1990, 263, 1218–1222. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Tsai, K.N.; Ou, J.J. Pathogenicity and virulence of Hepatitis B virus. Virulence 2022, 13, 258–296. [Google Scholar] [CrossRef]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection–Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef]
- Khanam, A.; Chua, J.V.; Kottilil, S. Immunopathology of Chronic Hepatitis B Infection: Role of Innate and Adaptive Immune Response in Disease Progression. Int. J. Mol. Sci. 2021, 22, 5497. [Google Scholar] [CrossRef]
- Noverati, N.; Bashir-Hamidu, R.; Halegoua-DeMarzio, D.; Hann, H.W. Hepatitis B Virus-Associated Hepatocellular Carcinoma and Chronic Stress. Int. J. Mol. Sci. 2022, 23, 3917. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Tsai, S.L.; Sheen, I.S.; Chao, M.; Yeh, C.T.; Hsieh, S.Y.; Chu, C.M. Clinical and virological course of chronic hepatitis B virus infection with hepatitis C and D virus markers. Am. J. Gastroenterol. 1998, 93, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.; Thimme, R.; Purcell, R.H.; Chisari, F.V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Chisari, F.V.; Isogawa, M.; Wieland, S.F. Pathogenesis of hepatitis B virus infection. Pathol. Biol. 2010, 58, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Mousa, O.Y. Hepatitis B. In StatPearls; Statpearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Croagh, C.M.; Lubel, J.S. Natural history of chronic hepatitis B: Phases in a complex relationship. World J. Gastroenterol. 2014, 20, 10395–10404. [Google Scholar] [CrossRef]
- Takashima, H.; Araki, K.; Miyazaki, J.; Yamamura, K.; Kimoto, M. Characterization of T-cell tolerance to hepatitis B virus (HBV) antigen in transgenic mice. Immunology 1992, 75, 398–405. [Google Scholar]
- Shi, Y.H.; Shi, C.H. Molecular characteristics and stages of chronic hepatitis B virus infection. World J. Gastroenterol. 2009, 15, 3099–3105. [Google Scholar] [CrossRef]
- Wong, G.L. Management of chronic hepatitis B patients in immunetolerant phase: What latest guidelines recommend. Clin. Mol. Hepatol. 2018, 24, 108–113. [Google Scholar] [CrossRef]
- Hui, C.K.; Leung, N.; Yuen, S.T.; Zhang, H.Y.; Leung, K.W.; Lu, L.; Cheung, S.K.; Wong, W.M.; Lau, G.K.; Hong Kong Liver Fibrosis Study, G. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology 2007, 46, 395–401. [Google Scholar] [CrossRef]
- McMahon, B.J. The natural history of chronic hepatitis B virus infection. Hepatology 2009, 49, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.J.; Sheen, I.S.; Liaw, Y.F. Hepatitis B virus DNA level predicts hepatic decompensation in patients with acute exacerbation of chronic hepatitis B. Clin. Gastroenterol. Hepatol. 2010, 8, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chu, C.M.; Liaw, Y.F. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology 2010, 51, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Lu, S.N.; Liaw, Y.F.; You, S.L.; Sun, C.A.; Wang, L.Y.; Hsiao, C.K.; Chen, P.J.; Chen, D.S.; Chen, C.J.; et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N. Engl. J. Med. 2002, 347, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bonino, F.; Rosina, F.; Rizzetto, M.; Rizzi, R.; Chiaberge, E.; Tardanico, R.; Callea, F.; Verme, G. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology 1986, 90, 1268–1273. [Google Scholar] [CrossRef]
- Lok, A.S.; Hadziyannis, S.J.; Weller, I.V.; Karvountzis, M.G.; Monjardino, J.; Karayiannis, P.; Montano, L.; Thomas, H.C. Contribution of low level HBV replication to continuing inflammatory activity in patients with anti-HBe positive chronic hepatitis B virus infection. Gut 1984, 25, 1283–1287. [Google Scholar] [CrossRef]
- Bixler, D.; Zhong, Y.; Ly, K.N.; Moorman, A.C.; Spradling, P.R.; Teshale, E.H.; Rupp, L.B.; Gordon, S.C.; Boscarino, J.A.; Schmidt, M.A.; et al. Mortality Among Patients With Chronic Hepatitis B Infection: The Chronic Hepatitis Cohort Study (CHeCS). Clin. Infect. Dis. 2019, 68, 956–963. [Google Scholar] [CrossRef]
- Brechot, C.; Pourcel, C.; Louise, A.; Rain, B.; Tiollais, P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature 1980, 286, 533–535. [Google Scholar] [CrossRef]
- Chemin, I.; Zoulim, F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. 2009, 286, 52–59. [Google Scholar] [CrossRef] [PubMed]
- De Mitri, M.S.; Cassini, R.; Bernardi, M. Hepatitis B virus-related hepatocarcinogenesis: Molecular oncogenic potential of clear or occult infections. Eur. J. Cancer 2010, 46, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Neuveut, C.; Wei, Y.; Buendia, M.A. Mechanisms of HBV-related hepatocarcinogenesis. J. Hepatol. 2010, 52, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Block, P.D.; Shinn, B.; Kim, J.H.; Hann, H.W. Hepatitis B-related hepatocellular carcinoma and stress: Untangling the host immune response from clinical outcomes. Hepat. Oncol. 2020, 8, HEP35. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Peneau, C.; Zucman-Rossi, J.; Nault, J.C. Genomics of Viral Hepatitis-Associated Liver Tumors. J. Clin. Med. 2021, 10, 1827. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Yiu, K.K.; Lai, J.W.; Chan, V.K.; Cheung, K.K.; Wong, E.W.; Wong, V.W. Meta-analysis: The association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2013, 37, 517–526. [Google Scholar] [CrossRef]
- Yang, H.I.; Yeh, S.H.; Chen, P.J.; Iloeje, U.H.; Jen, C.L.; Su, J.; Wang, L.Y.; Lu, S.N.; You, S.L.; Chen, D.S.; et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J. Natl. Cancer Inst. 2008, 100, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Clinical and virological aspects of blood donors infected with hepatitis B virus genotypes B and C. J. Clin. Microbiol. 2002, 40, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Lampertico, P.; Manolakopoulos, S.; Lok, A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. J. Hepatol. 2010, 53, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Yang, H.I.; Yang, W.S.; Liu, C.J.; Chen, P.J.; You, S.L.; Wang, L.Y.; Sun, C.A.; Lu, S.N.; Chen, D.S.; et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology 2008, 135, 111–121. [Google Scholar] [CrossRef]
- Cheuk-Fung Yip, T.; Wai-Sun Wong, V.; Lik-Yuen Chan, H.; Tse, Y.K.; Pik-Shan Kong, A.; Long-Yan Lam, K.; Chung-Yan Lui, G.; Lai-Hung Wong, G. Effects of Diabetes and Glycemic Control on Risk of Hepatocellular Carcinoma After Seroclearance of Hepatitis B Surface Antigen. Clin. Gastroenterol. Hepatol. 2018, 16, 765–773.e762. [Google Scholar] [CrossRef] [PubMed]
- Orito, E.; Hasebe, C.; Kurosaki, M.; Osaki, Y.; Joko, K.; Watanabe, H.; Kimura, H.; Nishijima, N.; Kusakabe, A.; Izumi, N.; et al. Risk of hepatocellular carcinoma in cirrhotic hepatitis B virus patients during nucleoside/nucleotide analog therapy. Hepatol. Res. 2015, 45, 872–879. [Google Scholar] [CrossRef]
- Song, C.; Zhu, J.; Ge, Z.; Yu, C.; Tian, T.; Wang, H.; Han, J.; Shen, H.; Dai, J.; Lu, J.; et al. Spontaneous Seroclearance of Hepatitis B Surface Antigen and Risk of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 1204–1206. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Zhang, W.; Tang, L.; Yang, H.; Yan, K.; Jiang, L.; Yang, J.; Li, C.; Yang, J.; et al. The Influence of Metabolic Syndrome on the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Infection in Mainland China. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 2038–2046. [Google Scholar] [CrossRef]
- Motta, B.M.; Masarone, M.; Torre, P.; Persico, M. From Non-Alcoholic Steatohepatitis (NASH) to Hepatocellular Carcinoma (HCC): Epidemiology, Incidence, Predictions, Risk Factors, and Prevention. Cancers 2023, 15, 5458. [Google Scholar] [CrossRef]
- Ramai, D.; Singh, J.; Lester, J.; Khan, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef]
- Yeung, P.; Wong, D.K.; Lai, C.L.; Fung, J.; Seto, W.K.; Yuen, M.F. Association of hepatitis B virus pre-S deletions with the development of hepatocellular carcinoma in chronic hepatitis B. J. Infect. Dis. 2011, 203, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Tanaka, Y.; Shinkai, N.; Poon, R.T.; But, D.Y.; Fong, D.Y.; Fung, J.; Wong, D.K.; Yuen, J.C.; Mizokami, M.; et al. Risk for hepatocellular carcinoma with respect to hepatitis B virus genotypes B/C, specific mutations of enhancer II/core promoter/precore regions and HBV DNA levels. Gut 2008, 57, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Keng, V.W.; Largaespada, D.A.; Villanueva, A. Why men are at higher risk for hepatocellular carcinoma? J. Hepatol. 2012, 57, 453–454. [Google Scholar] [CrossRef]
- Jang, T.Y.; Wei, Y.J.; Liu, T.W.; Yeh, M.L.; Liu, S.F.; Hsu, C.T.; Hsu, P.Y.; Lin, Y.H.; Liang, P.C.; Hsieh, M.H.; et al. Role of hepatitis D virus infection in development of hepatocellular carcinoma among chronic hepatitis B patients treated with nucleotide/nucleoside analogues. Sci. Rep. 2021, 11, 8184. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, K.; Luo, J. HIV-HBV and HIV-HCV Coinfection and Liver Cancer Development. Cancer Treat. Res. 2019, 177, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Wu, L.W.; Tseng, T.C.; Chen, C.L.; Yang, H.C.; Su, T.H.; Wang, C.C.; Kuo, S.F.; Liu, C.H.; Chen, P.J.; et al. Hepatitis B Surface Antigen Loss and Hepatocellular Carcinoma Development in Patients With Dual Hepatitis B and C Infection. Medicine 2016, 95, e2995. [Google Scholar] [CrossRef] [PubMed]
- Clinical Practice Guidelines for Hepatocellular Carcinoma-The Japan Society of Hepatology 2009 update. Hepatol. Res. 2010, 40 (Suppl. S1), 2–144. [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Block, P.; Shinn, B.; Roth, C.; Needleman, L.; Rosato, E.; Hann, H.-W. Vagaries of the Host Response in the Development of Hepatitis B-related Hepatocellular Carcinoma: A Case Series. Curr. Cancer Ther. Rev. 2020, 16, 253–258. [Google Scholar] [CrossRef]
- Noverati, N.; Nguyen, A.; Chalikonda, D.; Halegoua-DeMarzio, D.; Hann, H.W. The Role of Host in the Spectrum of Outcomes in Family Clusters of Hepatitis Infection: From Asymptomatic to Hepatocellular Carcinoma. Case Rep. Gastroenterol. 2023, 17, 104–110. [Google Scholar] [CrossRef]
- Akcay, I.M.; Katrinli, S.; Ozdil, K.; Doganay, G.D.; Doganay, L. Host genetic factors affecting hepatitis B infection outcomes: Insights from genome-wide association studies. World J. Gastroenterol. 2018, 24, 3347–3360. [Google Scholar] [CrossRef]
- Russ, T.C.; Kivimäki, M.; Morling, J.R.; Starr, J.M.; Stamatakis, E.; Batty, G.D. Association between Psychological Distress and Liver Disease Mortality: A Meta-analysis of Individual Study Participants. Gastroenterology 2015, 148, 958–966.e4. [Google Scholar] [CrossRef]
- Joung, J.Y.; Cho, J.H.; Kim, Y.H.; Choi, S.H.; Son, C.G. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019, 9, e01235. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, H.; Li, X.; Zhao, Y. Psychological stress exerts effects on pathogenesis of hepatitis B via type-1/type-2 cytokines shift toward type-2 cytokine response. PLoS ONE 2014, 9, e105530. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009. [Google Scholar] [CrossRef]
- Han, Y.F.; Zhao, J.; Ma, L.Y.; Yin, J.H.; Chang, W.J.; Zhang, H.W.; Cao, G.W. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 4258–4270. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Loomba, R.; Berg, T.; Aguilar Schall, R.E.; Yee, L.J.; Dinh, P.V.; Flaherty, J.F.; Martins, E.B.; Therneau, T.M.; Jacobson, I.; et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015, 121, 3631–3638. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Chan, H.L.; Hansen, B.E.; Janssen, H.L.; Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J. Hepatol. 2015, 62, 956–967. [Google Scholar] [CrossRef]
- Singal, A.K.; Salameh, H.; Kuo, Y.F.; Fontana, R.J. Meta-analysis: The impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment. Pharmacol. Ther. 2013, 38, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Chan, H.L.Y.; Ahn, S.H.; Seto, W.K.; Ning, Q.; Agarwal, K.; Janssen, H.L.A.; Pan, C.Q.; Chuang, W.L.; Izumi, N.; et al. Tenofovir alafenamide and tenofovir disoproxil fumarate reduce incidence of hepatocellular carcinoma in patients with chronic hepatitis B. JHEP Rep. 2023, 5, 100847. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Manolakopoulos, S.; Touloumi, G.; Nikolopoulou, G.; Raptopoulou-Gigi, M.; Gogos, C.; Vafiadis-Zouboulis, I.; Karamanolis, D.; Chouta, A.; Ilias, A.; et al. Hepatocellular carcinoma risk in HBeAg-negative chronic hepatitis B patients with or without cirrhosis treated with entecavir: HepNet.Greece cohort. J. Viral Hepat. 2015, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sinn, D.H.; Kim, K.; Kim, H.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Lamivudine versus Entecavir for Newly Diagnosed Hepatitis B Virus-Related Hepatocellular Carcinoma. Gut Liver 2016, 10, 939–947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, S.C.; Lee, C.M.; Hu, T.H.; Wang, J.H.; Lu, S.N.; Hung, C.H.; Changchien, C.S.; Chen, C.H. Virological response to entecavir reduces the risk of liver disease progression in nucleos(t)ide analogue-experienced HBV-infected patients with prior resistant mutants. J. Antimicrob. Chemother. 2013, 68, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Ahn, S.J.; Park, S.Y.; Song, G.W.; Cheong, J.Y.; Cho, S.W. 652 virological response to entecavir is associated with low probability of developing hepatocellular carcinoma in chronic Hepatitis B patients with cirrhosis. J. Hepatol. 2013, 58, S265. [Google Scholar] [CrossRef]
- Kim, J.H.; Sinn, D.H.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 2017, 66, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Dargan, A.; Wong, S.Y.; Coben, R.; Conn, M.; Dimarino, A.J.; Hann, H.W. Persistent risk for hepatocellular carcinoma after more than a decade of successful hepatitis B virus suppression. Minerva Gastroenterol. Dietol. 2017, 63, 74–76. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Manolakopoulos, S.; Touloumi, G.; Vourli, G.; Raptopoulou-Gigi, M.; Vafiadis-Zoumbouli, I.; Vasiliadis, T.; Mimidis, K.; Gogos, C.; Ketikoglou, I.; et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: Results of the nationwide HEPNET. Greece cohort study. Gut 2011, 60, 1109–1116. [Google Scholar] [CrossRef]
- Shinn, B.J.; Martin, A.; Coben, R.M.; Conn, M.I.; Prieto, J.; Kroop, H.; DiMarino, A.J.; Hann, H.W. Persistent risk for new, subsequent new and recurrent hepatocellular carcinoma despite successful anti-hepatitis B virus therapy and tumor ablation: The need for hepatitis B virus cure. World J. Hepatol. 2019, 11, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannakos, J.; Papatheodoridis, G. Hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. World J. Gastroenterol. 2013, 19, 8822–8830. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Hann, H.W.; Coben, R.; Conn, M.; DiMarino, A.J. Update Treatment for HBV Infection and Persistent Risk for Hepatocellular Carcinoma: Prospect for an HBV Cure. Diseases 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Park, Y.N.; Park, J.Y.; Chang, H.Y.; Lee, J.M.; Shin, J.E.; Han, K.H.; Park, C.; Moon, Y.M.; Chon, C.Y. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J. Hepatol. 2005, 42, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Gounder, P.P.; Bulkow, L.R.; Snowball, M.; Negus, S.; Spradling, P.R.; Simons, B.C.; McMahon, B.J. Nested case-control study: Hepatocellular carcinoma risk after hepatitis B surface antigen seroclearance. Aliment. Pharmacol. Ther. 2016, 43, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Ho, M.C.; Lin, Y.Y.; Tzeng, S.T.; Chen, Y.J.; Pai, H.Y.; Wang, Y.C.; Chen, C.L.; Lee, Y.H.; Chen, D.S.; et al. Cell-Free Virus-Host Chimera DNA From Hepatitis B Virus Integration Sites as a Circulating Biomarker of Hepatocellular Cancer. Hepatology 2020, 72, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Development of hepatocellular carcinoma in treated and untreated patients with chronic hepatitis B virus infection. Clin. Mol. Hepatol. 2023, 29, 605–622. [Google Scholar] [CrossRef]
- Coffin, C.S.; Mulrooney-Cousins, P.M.; Peters, M.G.; van Marle, G.; Roberts, J.P.; Michalak, T.I.; Terrault, N.A. Molecular characterization of intrahepatic and extrahepatic hepatitis B virus (HBV) reservoirs in patients on suppressive antiviral therapy. J. Viral Hepat. 2011, 18, 415–423. [Google Scholar] [CrossRef]
- Mak, L.Y.; Wong, D.K.; Pollicino, T.; Raimondo, G.; Hollinger, F.B.; Yuen, M.F. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J. Hepatol. 2020, 73, 952–964. [Google Scholar] [CrossRef]
- Wong, D.K.; Cheng, S.C.Y.; Mak, L.L.; To, E.W.; Lo, R.C.; Cheung, T.T.; Seto, W.K.; Fung, J.; Man, K.; Lai, C.L.; et al. Among Patients with Undetectable Hepatitis B Surface Antigen and Hepatocellular Carcinoma, a High Proportion Has Integration of HBV DNA into Hepatocyte DNA and No Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 449–456. [Google Scholar] [CrossRef]
- Paterlini, P.; Driss, F.; Nalpas, B.; Pisi, E.; Franco, D.; Berthelot, P.; Brechot, C. Persistence of hepatitis B and hepatitis C viral genomes in primary liver cancers from HBsAg-negative patients: A study of a low-endemic area. Hepatology 1993, 17, 20–29. [Google Scholar] [CrossRef]
- Pollicino, T.; Squadrito, G.; Cerenzia, G.; Cacciola, I.; Raffa, G.; Craxi, A.; Farinati, F.; Missale, G.; Smedile, A.; Tiribelli, C.; et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004, 126, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Michalak, T.I.; Pardoe, I.U.; Coffin, C.S.; Churchill, N.D.; Freake, D.S.; Smith, P.; Trelegan, C.L. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology 1999, 29, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.S.; Gill, U.S.; Litwin, S.; Zhou, Y.; Peri, S.; Pop, O.; Hong, M.L.; Naik, S.; Quaglia, A.; Bertoletti, A.; et al. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology 2016, 151, 986–998.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lim, Y.S.; Han, S.; Choi, J.; Shim, J.H.; Kim, K.M.; Lee, H.C.; Lee, Y.S. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut 2018, 67, 945–952. [Google Scholar] [CrossRef]
- Zheng, B.; Yang, Y.; Han, Q.; Yin, C.; Pan, Z.; Zhang, J. STAT3 directly regulates NKp46 transcription in NK cells of HBeAg-negative CHB patients. J. Leukoc. Biol. 2019, 106, 987–996. [Google Scholar] [CrossRef]
- Faure-Dupuy, S.; Delphin, M.; Aillot, L.; Dimier, L.; Lebosse, F.; Fresquet, J.; Parent, R.; Matter, M.S.; Rivoire, M.; Bendriss-Vermare, N.; et al. Hepatitis B virus-induced modulation of liver macrophage function promotes hepatocyte infection. J. Hepatol. 2019, 71, 1086–1098. [Google Scholar] [CrossRef]
- Zhou, K.; Terrault, N. Immune tolerant HBV and HCC: Time to revise our tolerance levels for therapy? AME Med. J. 2018, 3, 27. [Google Scholar] [CrossRef]
- Howell, J.; Chan, H.L.Y.; Feld, J.J.; Hellard, M.E.; Thompson, A.J. Closing the Stable Door After the Horse Has Bolted: Should We Be Treating People With Immune-Tolerant Chronic Hepatitis B to Prevent Hepatocellular Carcinoma? Gastroenterology 2020, 158, 2028–2032. [Google Scholar] [CrossRef]
- Chan, H.L.; Chan, C.K.; Hui, A.J.; Chan, S.; Poordad, F.; Chang, T.T.; Mathurin, P.; Flaherty, J.F.; Lin, L.; Corsa, A.; et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 2014, 146, 1240–1248. [Google Scholar] [CrossRef]
- Jonas, M.M.; Block, J.M.; Haber, B.A.; Karpen, S.J.; London, W.T.; Murray, K.F.; Narkewicz, M.R.; Rosenthal, P.; Schwarz, K.B.; McMahon, B.J.; et al. Treatment of children with chronic hepatitis B virus infection in the United States: Patient selection and therapeutic options. Hepatology 2010, 52, 2192–2205. [Google Scholar] [CrossRef]
- Boortalary, T.; Shinn, B.; Coben, R.M.; Conn, M.I.; Prieto, J.; Kroop, H.; Dimarino, A.; Hann, H. Are We Close to Achieving a HBV Cure? Risk for Hepatocellular Carcinoma Persists Despite Long-term HBV Suppression: An Update on Our Experience. Arch. Gastroenterol. Res. 2020, 1, 105–110. [Google Scholar]
- Boortalary, T.; Shinn, B.; Halegoua-DeMarzio, D.; Hann, H.W. Achieving a Cure: The Next Frontier in Hepatitis B Treatment. In Liver Cancer [Internet]; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2021; Chapter 6; Available online: https://www.ncbi.nlm.nih.gov/books/NBK569795/ (accessed on 26 December 2023).
- Garrido, D.; Block, P.; Lin, S.; Halegoua-DeMarzio, D.; Hann, H.W. Survival Disparity Between Antiviral-Treated and Antiviral-Naïve Patients Who Develop Their First HBV-Associated Hepatocellular Carcinoma. Arch. Gastroenterol. Res. 2021, 2, 86–94. [Google Scholar]
- Chowdhury, S.; Garrido, D.; Halegoua-DeMarzio, D.; Roth, C.; Hann, H.W. Poor Prognosis in HBV-associated Hepatocellular Carcinoma After Successful Viral Suppression: A Case Series Highlighting a Need for a Cure. J. Immunol. Sci. 2023, 7, 1–8. [Google Scholar] [CrossRef]
- Li, N.; Lai, E.C.; Shi, J.; Guo, W.X.; Xue, J.; Huang, B.; Lau, W.Y.; Wu, M.C.; Cheng, S.Q. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann. Surg. Oncol. 2010, 17, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Zhang, A.; Lian, J.; Wang, J.; Chang, T.T.; Lin, Y.J.; Song, W.; Su, Y.H. Recurrent HBV Integration Targets as Potential Drivers in Hepatocellular Carcinoma. Cells 2021, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152–4163. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lv, Z.; Wang, M.; Zhang, D.; Liu, D.; Zhu, F. HBV Enhances Sorafenib Resistance in Hepatocellular Carcinoma by Reducing Ferroptosis via SRSF2-Mediated Abnormal PCLAF Splicing. Int. J. Mol. Sci. 2023, 24, 3263. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Lv, Z.; Xue, X.; Xing, Z.Y.; Zhu, F. Canonical WNT Signaling Activated by WNT7B Contributes to L-HBs-Mediated Sorafenib Resistance in Hepatocellular Carcinoma by Inhibiting Mitophagy. Cancers 2022, 14, 5781. [Google Scholar] [CrossRef]
- Zhang, S.; Li, N.; Sheng, Y.; Chen, W.; Ma, Q.; Yu, X.; Lian, J.; Zeng, J.; Yang, Y.; Yan, J. Hepatitis B virus induces sorafenib resistance in liver cancer via upregulation of cIAP2 expression. Infect. Agent. Cancer 2021, 16, 20. [Google Scholar] [CrossRef]
- Gao, Y.; You, M.; Fu, J.; Tian, M.; Zhong, X.; Du, C.; Hong, Z.; Zhu, Z.; Liu, J.; Markowitz, G.J.; et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J. Hepatol. 2022, 76, 148–159. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, X.; Li, N.; Du, C.; Yang, B.; Qin, W.; Fu, J.; Markowitz, G.J.; Wang, H.; Ma, J.; et al. CCL22 signaling contributes to sorafenib resistance in hepatitis B virus-associated hepatocellular carcinoma. Pharmacol. Res. 2020, 157, 104800. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, J.; He, C.; Xu, K.; Liu, J.; Sun, J.; Wu, G.; Tan, C.; Zeng, Y.; Wang, J.; et al. Restoration of miR-193b sensitizes Hepatitis B virus-associated hepatocellular carcinoma to sorafenib. Cancer Lett. 2014, 352, 245–252. [Google Scholar] [CrossRef]
- He, H.; Zhou, J.; Cheng, F.; Li, H.; Quan, Y. MiR-3677-3p promotes development and sorafenib resistance of hepatitis B-related hepatocellular carcinoma by inhibiting FOXM1 ubiquitination. Hum. Cell 2023, 36, 1773–1789. [Google Scholar] [CrossRef]
- Witt-Kehati, D.; Fridkin, A.; Alaluf, M.B.; Zemel, R.; Shlomai, A. Inhibition of pMAPK14 Overcomes Resistance to Sorafenib in Hepatoma Cells with Hepatitis B Virus. Transl. Oncol. 2018, 11, 511–517. [Google Scholar] [CrossRef]
- Levrero, M.; Testoni, B.; Zoulim, F. HBV cure: Why, how, when? Curr. Opin. Virol. 2016, 18, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yardeni, D.; Chang, K.M.; Ghany, M.G. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology 2023, 164, 42–60.e6. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Lok, A.S.; Zoulim, F. New Perspectives on Development of Curative Strategies for Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2023, 21, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Pan, C.Q.; Han, S.H.; Trinh, H.N.; Fessel, W.J.; Rodell, T.; Massetto, B.; Lin, L.; Gaggar, A.; Subramanian, G.M.; et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016, 65, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Michler, T.; Kosinska, A.D.; Festag, J.; Bunse, T.; Su, J.; Ringelhan, M.; Imhof, H.; Grimm, D.; Steiger, K.; Mogler, C.; et al. Knockdown of Virus Antigen Expression Increases Therapeutic Vaccine Efficacy in High-Titer Hepatitis B Virus Carrier Mice. Gastroenterology 2020, 158, 1762–1775.e9. [Google Scholar] [CrossRef] [PubMed]
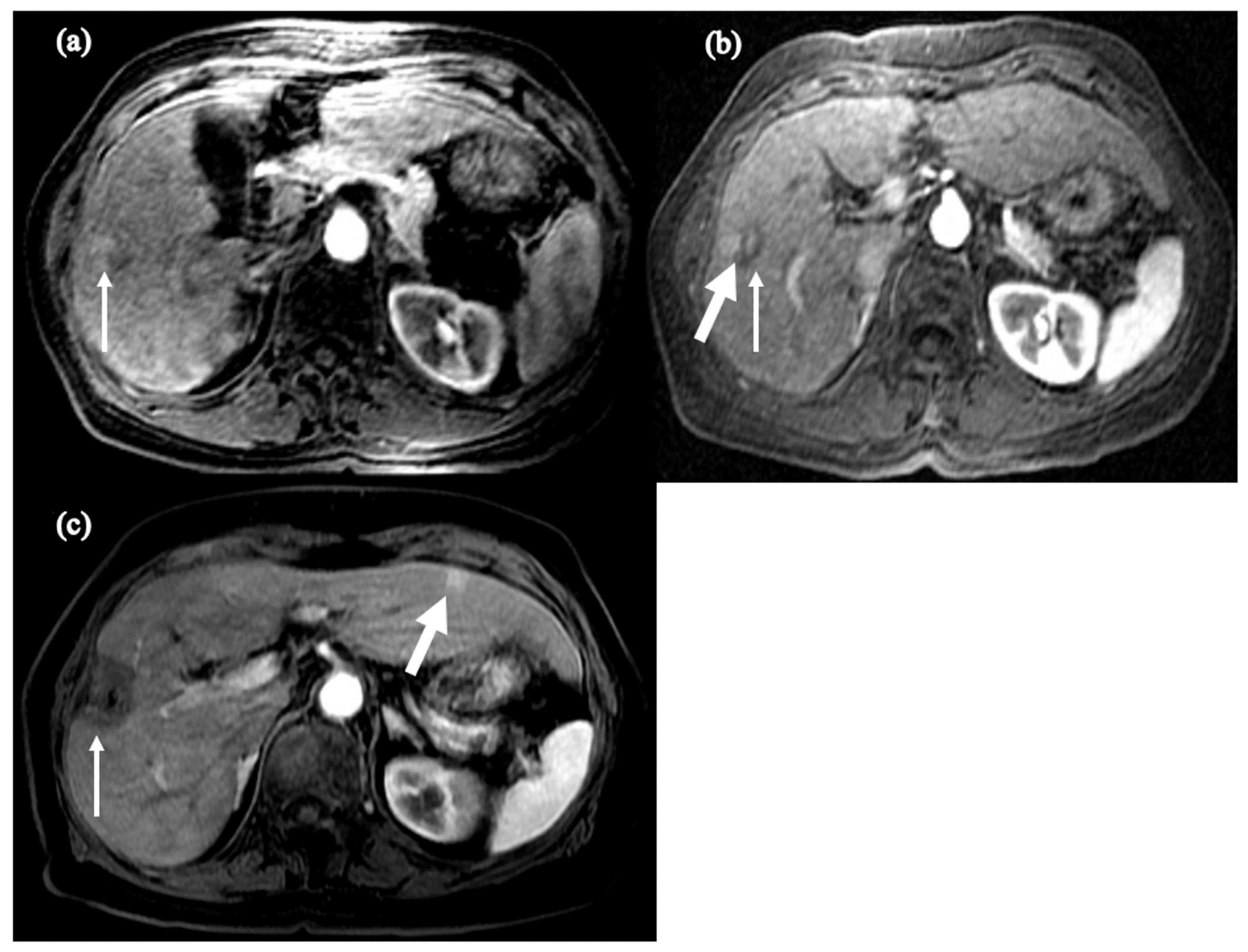
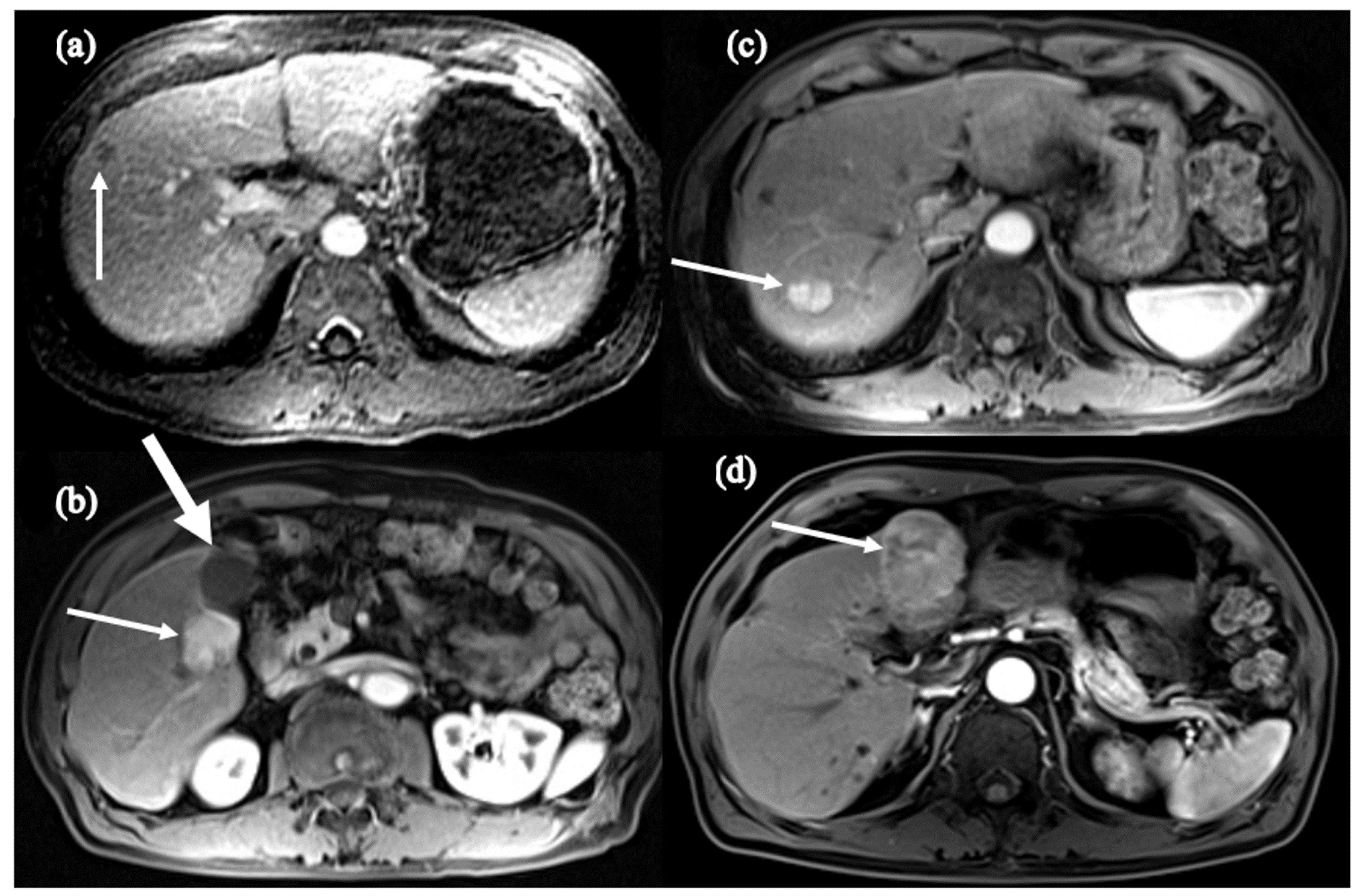
| Pt | Date StartTx | Change in Child Class on Tx | Date HCC Dx | Yrs on Anti-HBV Tx at HCC Dx | Yrs with HBV DNA (-) * | Age (Yr) at HCC Dx | Tumor Size at Dx | HBVDNA at HCC Dx | Anti-HBV Tx | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | April 1998 | B → A | July 2007 | 9 | 3 | 53 | 1.1 Junction | UD | LAM + TDF | Alive |
| 2 | January 1998 | B → A | March 2008 | 10 | 8 | 68 | 2.8 Rt | UD | LAM + TDF | Dead |
| 3 | May 1998 | A →A | February 2008 | 10 | 7 | 76 | 1.8 Lt | UD | LAM + TDF | Alive |
| 4 | July 2001 | B → B | September 2010 | 9 | 4 | 54 | 2.8 Rt | UD | LAM + TDF | Dead |
| 5 | August 2004 | B → B | November 2010 | 16 | 4 | 53 | 3.9 Rt | UD | LAM + TDF | Alive |
| 6 | July 2001 | B → B | January 2011 | 10 | 5 | 55 | 2.8 Rt | UD | LAM + TDF | Dead |
| 7 | February 2004 | A → A | June 2013 | 9 | 8 | 57 | 2.5 Lt med | UD | TDF | Dead |
| 8 | February 1996 | A → A | July 2013 | 17 | 10 | 73 | 1.6 Rt | UD | TDF | Dead |
| 9 | August 1997 | A → A | June 2014 | 17 | 6 | 54 | 2.2 Lt lat | UD | ETV | Alive |
| 10 | March 2004 | B → B | June 2013 | 9 | 7 | 57 | 2.5 Lt | UD | TDF | Dead |
| 11 | July 2001 | A → A | June 2014 | 13 | 7 | 54 | 2.2 Lt | UD | TDF | Alive |
| 12 | May 1996 | A → A | October 2014 | 18 | 10 | 74 | 3.4 Rt | UD | LAM + TDF | Dead |
| 13 | February 2000 | A → A | October 2014 | 14 | 12 | 62 | 3.8 Rt | UD | ETV + TDF | Alive |
| 14 | February 2000 | A → A | April 2015 | 15 | 12 | 62 | 3.4 Rt | UD | TDF | Alive |
| 15 | February 2000 | B → A | May 2015 | 15 | 12 | 65 | 3.8 Rt | UD | TDF | Alive |
| 16 | December 1998 | A → A | August 2017 | 19 | 8 | 64 | 2.0 Rt | UD | LAM + TDF | Alive |
| 17 | February 2008 | A → A | June 2019 | 11 | 10 | 57 | 2.2 Rt | UD | ETV + TDF | Alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varghese, N.; Majeed, A.; Nyalakonda, S.; Boortalary, T.; Halegoua-DeMarzio, D.; Hann, H.-W. Review of Related Factors for Persistent Risk of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Cancers 2024, 16, 777. https://doi.org/10.3390/cancers16040777
Varghese N, Majeed A, Nyalakonda S, Boortalary T, Halegoua-DeMarzio D, Hann H-W. Review of Related Factors for Persistent Risk of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Cancers. 2024; 16(4):777. https://doi.org/10.3390/cancers16040777
Chicago/Turabian StyleVarghese, Nevin, Amry Majeed, Suraj Nyalakonda, Tina Boortalary, Dina Halegoua-DeMarzio, and Hie-Won Hann. 2024. "Review of Related Factors for Persistent Risk of Hepatitis B Virus-Associated Hepatocellular Carcinoma" Cancers 16, no. 4: 777. https://doi.org/10.3390/cancers16040777
APA StyleVarghese, N., Majeed, A., Nyalakonda, S., Boortalary, T., Halegoua-DeMarzio, D., & Hann, H.-W. (2024). Review of Related Factors for Persistent Risk of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Cancers, 16(4), 777. https://doi.org/10.3390/cancers16040777






