Prognostic Value of the Lung Immune Prognostic Index on Recurrence after Radical Surgery for High-Risk Renal Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Surgery and Follow-Up Protocol
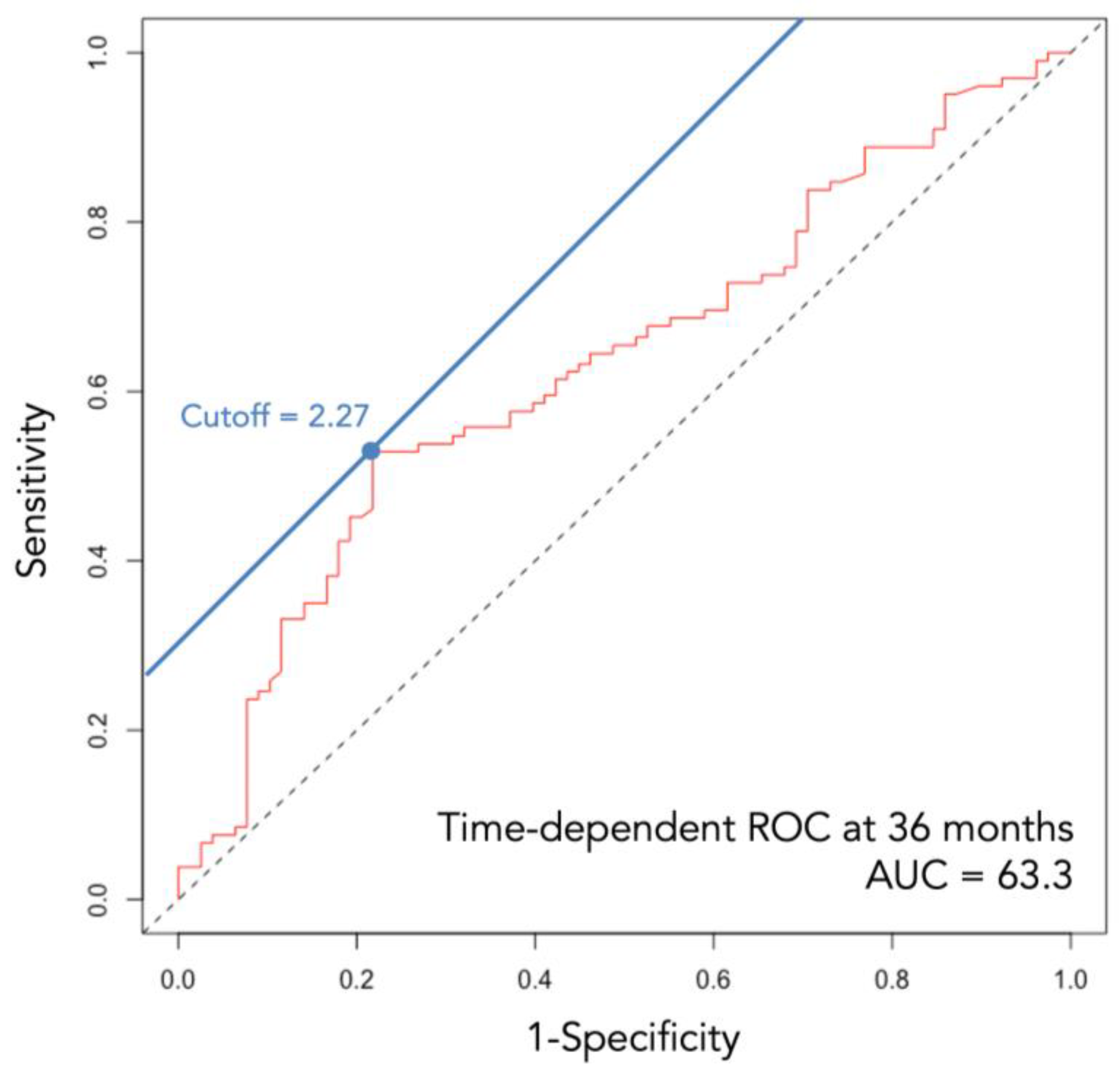
2.3. Study Design and Statistical Analyses
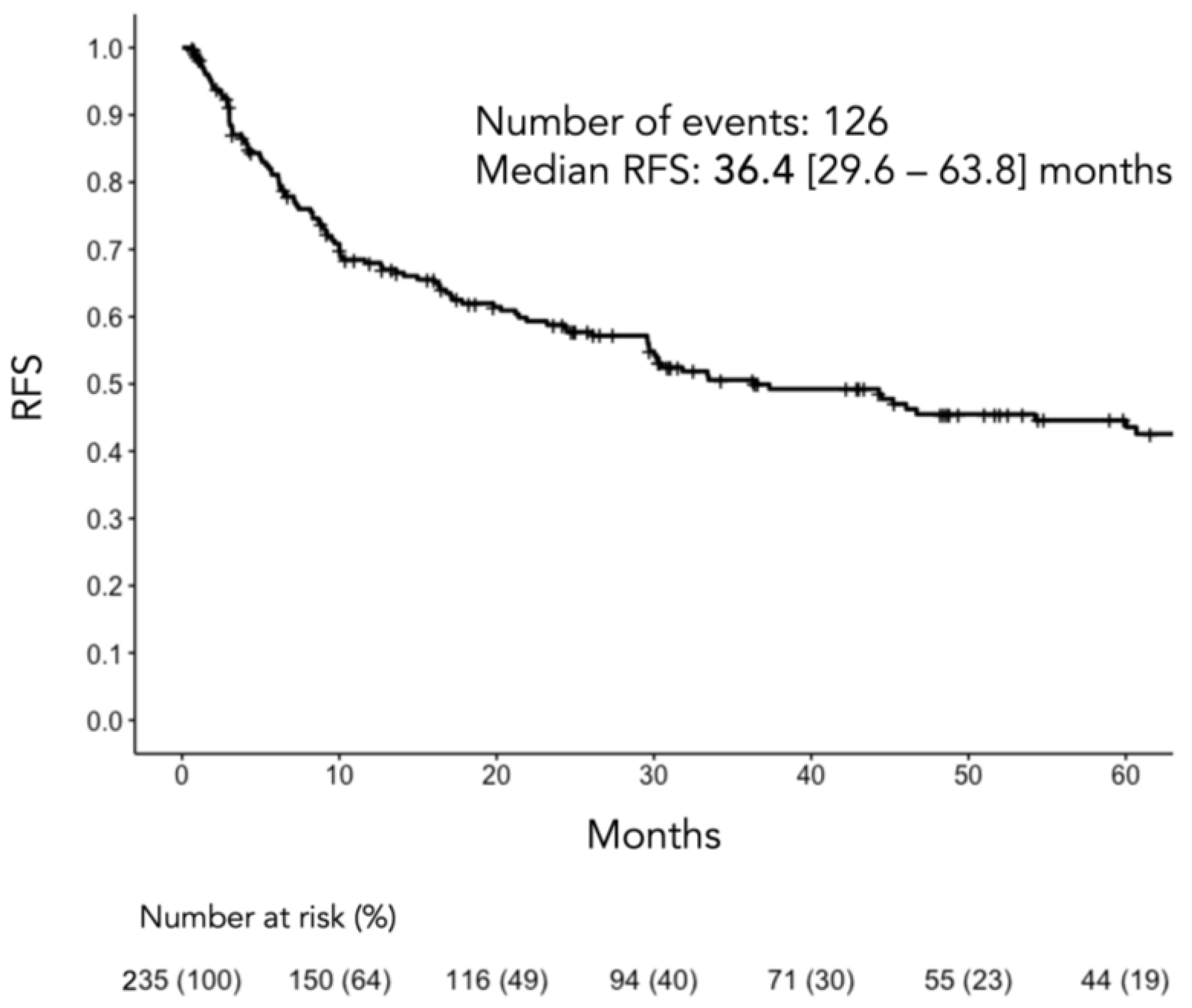
3. Results
3.1. Patient Characteristics
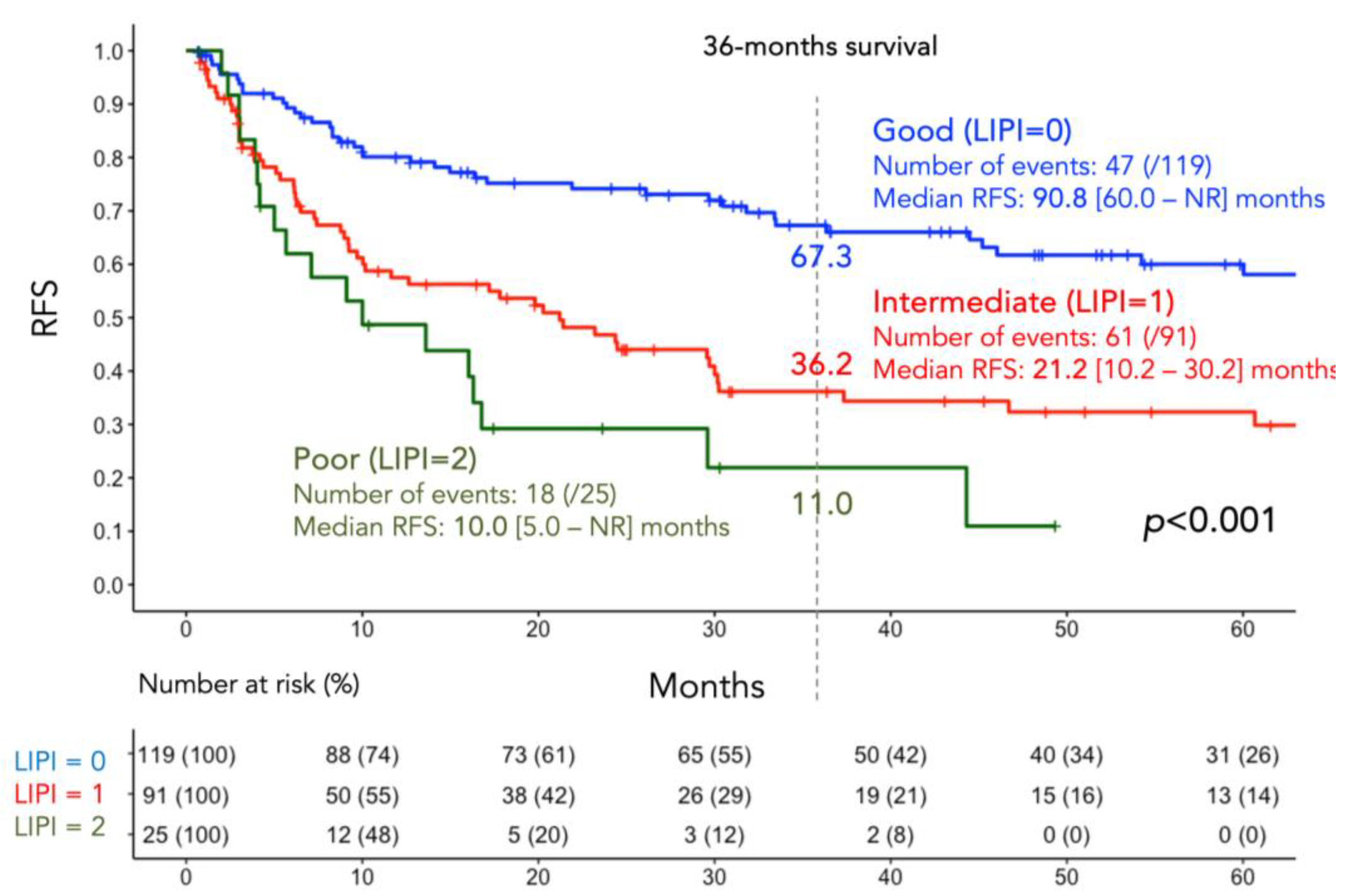
3.2. RFS According to LIPI Groups
3.3. Factors Associated with RFS
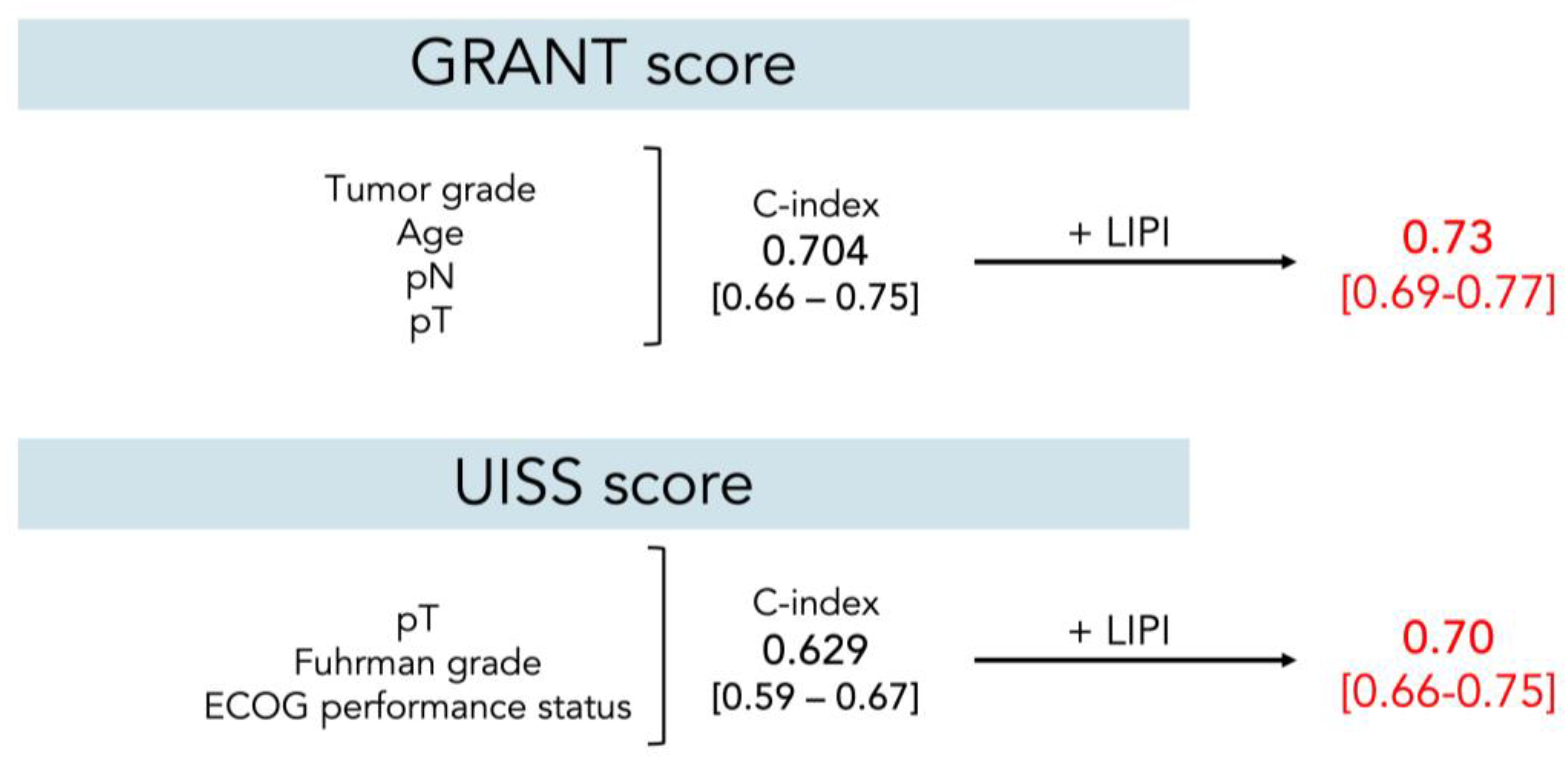
3.4. Additive Role of the LIPI to Preexisting Prediction Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dabestani, S.; Thorstenson, A.; Lindblad, P.; Harmenberg, U.; Ljungberg, B.; Lundstam, S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: A population-based study. World J. Urol. 2016, 34, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Karakiewicz, P.I.; Briganti, A.; Chun, F.K.; Trinh, Q.D.; Perrotte, P.; Ficarra, V.; Cindolo, L.; De la Taille, A.; Tostain, J.; Mulders, P.F.; et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J. Clin. Oncol. 2007, 25, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Leibovich, B.C.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Frank, I.; Kwon, E.D.; Weaver, A.L.; Parker, A.S.; Zincke, H. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Marconi, L.; Sun, M.; Beisland, C.; Klatte, T.; Ljungberg, B.; Stewart, G.D.; Dabestani, S.; Choueiri, T.K.; Bex, A. Prevalence, Disease-free, and Overall Survival of Contemporary Patients With Renal Cell Carcinoma Eligible for Adjuvant Checkpoint Inhibitor Trials. Clin. Genitourin. Cancer 2021, 19, e92–e99. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Gurney, H.; Chang, Y.H.; Lee, J.L.; et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Kondo, T.; Ishihara, H.; Yoshida, K.; Iizuka, J.; Tanabe, K.; Takagi, T. C-reactive protein kinetics to predict recurrence of high-risk renal cell carcinoma after radical surgery. Int. J. Clin. Oncol. 2022, 27, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Sacdalan, D.B.; Lucero, J.A.; Sacdalan, D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta-analysis. Onco Targets Ther. 2018, 11, 955–965. [Google Scholar] [CrossRef]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015, 54, 961–970. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Meyers, D.E.; Stukalin, I.; Vallerand, I.A.; Lewinson, R.T.; Suo, A.; Dean, M.; North, S.; Pabani, A.; Cheng, T.; Heng, D.Y.C.; et al. The Lung Immune Prognostic Index Discriminates Survival Outcomes in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors. Cancers 2019, 11, 1713. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.L.; Yang, X.Y.; Dong, Z.R.; Chen, Z.Q.; Hong, J.G.; Li, T. The Prediction Potential of the Pretreatment Lung Immune Prognostic Index for the Therapeutic Outcomes of Immune Checkpoint Inhibitors in Patients With Solid Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 691002. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Miki, J.; Fukuokaya, W.; Yanagisawa, T.; Kimura, S.; Tsuzuki, S.; Kimura, T.; Egawa, S. The prognostic value of the preoperative lung immune prognostic index in patients with urothelial bladder cancer undergoing radical cystectomy. Int. J. Clin. Oncol. 2022, 27, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Carril-Ajuria, L.; Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Hammers, H.J.; Plimack, E.R.; Donskov, F.; Rini, B.I.; Jiang, R.; Lee, C.-W.; et al. Prognostic value of the lung immune prognostic index in patients with untreated advanced renal cell carcinoma (aRCC) receiving nivolumab plus ipilimumab (N+I) or sunitinib (SUN) in the CheckMate 214 trial. J. Clin. Oncol. 2022, 40, 4538. [Google Scholar] [CrossRef]
- Silva, C.A.C.; Zoppi, S.; Reni, A.; Piccinno, G.; Lahmar, I.; Cerbone, L.; Carril-Ajuria, L.; Colomba, E.; Flippot, R.; Sow, C.; et al. The Lung Immune Prognostic Index (LIPI) stratifies prognostic groups and correlates with gut microbiota (GM) in patients with advanced renal cell carcinoma (RCC). J. Clin. Oncol. 2023, 41, 719. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–663. [Google Scholar] [CrossRef]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef]
- Blute, M.L.; Inman, B.A.; Gelpi, F.J. Venal Caval Thrombectomy. In Hinman’s Atlas of Urologic Surgery, 4th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Gregg, J.; Scarpato, K. Surgical Approaches for Open Renal Surgery, Including Open Radical Nephrectomy. In Hinman’s Atlas of Urologic Surgery, 4th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Orden, M.; Landman, J. Laparoscopic Nephrectomy. In Hinman’s Atlas of Urologic Surgery, 4th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Buti, S.; Puligandla, M.; Bersanelli, M.; DiPaola, R.S.; Manola, J.; Taguchi, S.; Haas, N.B. Validation of a new prognostic model to easily predict outcome in renal cell carcinoma: The GRANT score applied to the ASSURE trial population. Ann. Oncol. 2017, 28, 2747–2753. [Google Scholar] [CrossRef]
- Zisman, A.; Pantuck, A.J.; Dorey, F.; Said, J.W.; Shvarts, O.; Quintana, D.; Gitlitz, B.J.; deKernion, J.B.; Figlin, R.A.; Belldegrun, A.S. Improved prognostication of renal cell carcinoma using an integrated staging system. J. Clin. Oncol. 2001, 19, 1649–1657. [Google Scholar] [CrossRef]
- Motzer, R.J.; McDermott, D.F.; Escudier, B.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Barthelemy, P.; Plimack, E.R.; Porta, C.; George, S.; et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022, 128, 2085–2097. [Google Scholar] [CrossRef]
- Allaf, M.; Kim, S.E.; Harshman, L.C.; McDermott, D.F.; Master, V.A.; Signoretti, S.; Cole, S.; Moon, H.; Adra, N.; Singer, E.A.; et al. LBA67 Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. Ann. Oncol. 2022, 33, S1432–S1433. [Google Scholar] [CrossRef]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; Abu-Ghanem, Y.; et al. The 2022 Updated European Association of Urology Guidelines on the Use of Adjuvant Immune Checkpoint Inhibitor Therapy for Renal Cell Carcinoma. Eur. Urol. 2023, 83, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Lyon, T.D.; Thompson, R.H.; Shah, P.H.; Lohse, C.M.; Boorjian, S.A.; Costello, B.A.; Cheville, J.C.; Leibovich, B.C. Complete Surgical Metastasectomy of Renal Cell Carcinoma in the Post-Cytokine Era. J. Urol. 2020, 203, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Takagi, T.; Kondo, T.; Fukuda, H.; Tachibana, H.; Yoshida, K.; Iizuka, J.; Kobayashi, H.; Ishida, H.; Tanabe, K. Prognostic impact of metastasectomy in renal cell carcinoma in the postcytokine therapy era. Urol. Oncol. 2021, 39, 77.e17–77.e25. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Fukuda, H.; Ishihara, H.; Yoshida, K.; Kondo, T.; Kobayashi, H.; Iizuka, J.; Okumi, M.; Ishida, H.; Omae, K.; et al. Predictive factors for recurrence after complete metastasectomy in patients with metastatic renal cell carcinoma in the targeted therapy era. Urol. Oncol. 2020, 38, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef]
- Van Wilpe, S.; Koornstra, R.; Den Brok, M.; De Groot, J.W.; Blank, C.; De Vries, J.; Gerritsen, W.; Mehra, N. Lactate dehydrogenase: A marker of diminished antitumor immunity. Oncoimmunology 2020, 9, 1731942. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Zhu, D.; JiRiGaLa; Yu, D.; Wang, C.; WuYunBiLiGe; Amin; ZhiHong; Yu, H.; et al. Prognostic role of pretreatment lactate dehydrogenase in patients with metastatic renal cell carcinoma: A systematic review and meta-analysis. Int. J. Surg. 2020, 79, 66–73. [Google Scholar] [CrossRef]
- Shi, J.; Wang, K.; Xiong, Z.; Yuan, C.; Wang, C.; Cao, Q.; Yu, H.; Meng, X.; Xie, K.; Cheng, Z.; et al. Impact of inflammation and immunotherapy in renal cell carcinoma. Oncol. Lett. 2020, 20, 272. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Motzer, R.J.; Bacik, J.; Murphy, B.A.; Russo, P.; Mazumdar, M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J. Clin. Oncol. 2002, 20, 289–296. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Antonini Cappellini, G.C.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandala, M.; et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2018, 29, 524. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, Y.; Nemoto, Y.; Ishihara, H.; Tachibana, H.; Fukuda, H.; Shinmura, H.; Hashimoto, Y.; Yoshida, K.; Iizuka, J.; et al. Association between lung immune prognostic index and survival of patients with metastatic urothelial carcinoma treated with pembrolizumab. Int. J. Clin. Oncol. 2023, 28, 913–921. [Google Scholar] [CrossRef]
- Blum, K.A.; Gupta, S.; Tickoo, S.K.; Chan, T.A.; Russo, P.; Motzer, R.J.; Karam, J.A.; Hakimi, A.A. Sarcomatoid renal cell carcinoma: Biology, natural history and management. Nat. Rev. Urol. 2020, 17, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Majdoub, M.; Yanagisawa, T.; Quhal, F.; Laukhtina, E.; von Deimling, M.; Kawada, T.; Rajwa, P.; Bianchi, A.; Pallauf, M.; Mostafaei, H.; et al. Role of clinicopathological variables in predicting recurrence and survival outcomes after surgery for non-metastatic renal cell carcinoma: Systematic review and meta-analysis. Int. J. Cancer 2023, 154, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, G.B.; Dragomir, A.; Wood, L.A.; Kollmannsberger, C.; Basappa, N.S.; Kapoor, A.; Soulières, D.; Finelli, A.; Heng, D.Y.C.; Castonguay, V.; et al. Real-world Assessment of Clinical Outcomes in Patients with Metastatic Renal Cell Carcinoma with or Without Sarcomatoid Features Treated with First-line Systemic Therapies. Eur. Urol. Oncol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Ornstein, M.C. Clinical Review on the Management of Metastatic Renal Cell Carcinoma. JCO Oncol. Pract. 2021, 18, 187–196. [Google Scholar] [CrossRef]
- Roussel, E.; Kinget, L.; Verbiest, A.; Debruyne, P.R.; Baldewijns, M.; Van Poppel, H.; Albersen, M.; Beuselinck, B. C-reactive protein and neutrophil-lymphocyte ratio are prognostic in metastatic clear-cell renal cell carcinoma patients treated with nivolumab. Urol. Oncol. 2021, 39, e217–e239. [Google Scholar] [CrossRef] [PubMed]
| Overall 235 | Good LIPI = 0 119 (50.6%) | Intermediate LIPI = 1 91 (38.7%) | Poor LIPI = 2 25 (10.6%) | p | |
|---|---|---|---|---|---|
| Age, years (median [IQR]) | 67.0 [61.0–73.0] | 67.0 [61.5–74.0] | 66.0 [61.5–72.0] | 69.0 [57.0–74.0] | 0.915 |
| Sex, male (%) | 166 (70.6) | 84 (70.6) | 65 (71.4) | 17 (68.0) | 0.946 |
| ECOG-PS (%) | 0.359 | ||||
| 0 | 196 (83.4) | 102 (85.7) | 72 (79.1) | 22 (88.0) | |
| ≥1 | 39 (16.6) | 17 (14.3) | 19 (20.9) | 3 (12.0) | |
| Symptoms at diagnosis | 104 (44.3) | 65 (54.6) | 31 (34.1) | 8 (32.0) | 0.011 |
| None | 95 (40.4) | 42 (35.3) | 43 (47.3) | 10 (40.0) | |
| Local | 36 (15.3) | 12 (10.1) | 17 (18.7) | 7 (28.0) | |
| Systemic | 104 (44.3) | 65 (54.6) | 31 (34.1) | 8 (32.0) | |
| Tumor size, mm (median [IQR]) | 77.0 [59.0–100.0] | 74.5 [57.0–90.0] | 85.0 [61.5–107.0] | 91.0 [65.0–113.0] | 0.014 |
| Pathological T stage (%) | <0.001 | ||||
| T1/T2 | 62 (26.4) | 42 (35.3) | 17 (18.7) | 3 (12.0) | |
| T3a | 101 (43.0) | 57 (47.9) | 35 (38.5) | 9 (36.0) | |
| T3b | 47 (20.0) | 11 (9.2) | 26 (28.6) | 10 (40.0) | |
| T3c | 20 (8.5) | 8 (6.7) | 9 (9.9) | 3 (12.0) | |
| T4 | 5 (2.1) | 1 (0.8) | 4 (4.4) | 0 (0.0) | |
| pN stage (%) | |||||
| N0/Nx | 212 (90.2) | 109 (91.6) | 78 (85.7) | 25(100.0) | 0.080 |
| N1 | 23 (9.8) | 10 (8.4) | 13 (14.3) | 0 (0.0) | |
| Pathological histology (%) | 0.034 | ||||
| Clear cell | 202 (86.0) | 108 (90.8) | 76 (83.5) | 18 (72.0) | |
| Non-clear cell | 33 (14.0) | 11 (9.2) | 15 (16.5) | 7 (28.0) | |
| Tumor grade (%) | 0.041 | ||||
| 1 | 16 (6.8) | 10 (8.4) | 5 (5.5) | 1 (4.0) | |
| 2 | 97 (41.3) | 57 (47.9) | 32 (35.2) | 8 (32.0) | |
| 3 | 89 (37.9) | 44 (37.0) | 34 (37.4) | 11 (44.0) | |
| 4 | 33 (14.0) | 8 (6.7) | 20 (22.0) | 5 (20.0) | |
| Recurrence | 126 (53.6) | 47 (39.5) | 61 (67.0) | 18 (72.0) | <0.001 |
| Follow-up periods, months | 19.8 [5.9–48.3] | 31.8 [9.4–62.7] | 12.6 [3.8–30.5] | 9.1 [4.0–16.8] | <0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (continuous) | 1.00 | 0.99–1.02 | 0.837 | 1.00 | 0.98–1.02 | 0.836 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 1.01 | 0.69–1.49 | 0.953 | 0.86 | 0.56–1.33 | 0.503 |
| Presence of symptoms | ||||||
| None | Ref | |||||
| Local | 2.27 | 1.50–3.43 | 0.000 | 2.06 | 1.31–3.23 | 0.002 |
| Systemic | 4.34 | 2.65–7.11 | 0.000 | 2.19 | 1.26–3.82 | 0.006 |
| ECOG-PS | ||||||
| 0 | Ref | |||||
| ≥1 | 1.67 | 1.07–2.60 | 0.025 | 1.65 | 1.00–2.71 | 0.049 |
| Tumor size (continuous) | 1.01 | 1.00–1.01 | 0.001 | 1.01 | 1.00–1.01 | 0.062 |
| pT | ||||||
| T1/T2 | Ref | Ref | ||||
| T3a | 2.43 | 1.45–4.07 | 0.001 | 2.26 | 1.30–3.91 | 0.004 |
| T3b | 3.64 | 2.08–6.36 | 0.000 | 2.37 | 1.25–4.51 | 0.009 |
| T3c | 5.78 | 2.90–11.56 | 0.000 | 4.57 | 2.14–9.75 | <0.001 |
| T4 | 5.97 | 2.03–17.56 | 0.001 | 5.80 | 1.76–19.10 | 0.004 |
| pN | ||||||
| N0/Nx | Ref | |||||
| N1 | 2.28 | 1.37–3.82 | 0.002 | 4.05 | 2.09–7.84 | <0.001 |
| Tumor grade | ||||||
| 1 | Ref | Ref | ||||
| 2 | 1.66 | 0.66–4.19 | 0.286 | 1.35 | 0.51–3.54 | 0.549 |
| 3 | 3.28 | 1.31–8.26 | 0.011 | 2.43 | 0.92–6.44 | 0.074 |
| 4 | 6.48 | 2.45–17.16 | 0.000 | 2.94 | 1.05–8.25 | 0.040 |
| Histology | ||||||
| Clear cell | Ref | Ref | ||||
| Non-clear cell | 1.84 | 1.18–2.89 | 0.008 | 1.30 | 0.75–2.26 | 0.349 |
| LIPI | ||||||
| Good (0) | Ref | Ref | ||||
| Intermediate (1) | 2.32 | 1.58–3.41 | 0.000 | 1.59 | 1.04–2.44 | 0.033 |
| Poor (2) | 3.76 | 2.15–6.57 | 0.000 | 2.73 | 1.41–5.28 | 0.003 |
| dNLR | ||||||
| Continuous | 1.23 | 1.09–1.38 | 0.001 | NA | ||
| ≥2.28 (Ref: <2.28) | 2.14 | 1.50–3.06 | 0.000 | NA | ||
| LDH | ||||||
| Continuous | 1.74 | 1.39–2.19 | 0.000 | NA | ||
| ≥UNL (Ref: <UNL) | 2.13 | 1.45–3.14 | 0.000 | NA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiyama, Y.; Kondo, T.; Yoshida, K.; Iizuka, J.; Takagi, T. Prognostic Value of the Lung Immune Prognostic Index on Recurrence after Radical Surgery for High-Risk Renal Cell Carcinoma. Cancers 2024, 16, 776. https://doi.org/10.3390/cancers16040776
Ishiyama Y, Kondo T, Yoshida K, Iizuka J, Takagi T. Prognostic Value of the Lung Immune Prognostic Index on Recurrence after Radical Surgery for High-Risk Renal Cell Carcinoma. Cancers. 2024; 16(4):776. https://doi.org/10.3390/cancers16040776
Chicago/Turabian StyleIshiyama, Yudai, Tsunenori Kondo, Kazuhiko Yoshida, Junpei Iizuka, and Toshio Takagi. 2024. "Prognostic Value of the Lung Immune Prognostic Index on Recurrence after Radical Surgery for High-Risk Renal Cell Carcinoma" Cancers 16, no. 4: 776. https://doi.org/10.3390/cancers16040776
APA StyleIshiyama, Y., Kondo, T., Yoshida, K., Iizuka, J., & Takagi, T. (2024). Prognostic Value of the Lung Immune Prognostic Index on Recurrence after Radical Surgery for High-Risk Renal Cell Carcinoma. Cancers, 16(4), 776. https://doi.org/10.3390/cancers16040776







