Simple Summary
BRCA genetic testing is available for UK Jewish individuals through the National Health Service or private providers. This study evaluated how well UK organisations (UKO), UK Jewish community organisations (JCO), and genetic testing providers (GTP) provide information about BRCA online. Google was used to find relevant websites and assessed the first 100 links. We reviewed the sites for how accessible, comprehensive, detailed, accurate, and high-quality the information was, giving each site a score out of 5. From 6856 search results, we found 45 UKOs, 16 JCOs, and 18 GTPs that provided BRCA information. While most sites (84%) were easy to access, the information was often incomplete. Only 35% of sites covered more than half of the important BRCA topics. Most sites mentioned breast and ovarian cancer (82%), but fewer mentioned other BRCA-related cancers. Overall, the quality of information was low-to-moderate. This highlights a need for better online BRCA information.
Abstract
BRCA genetic testing is available for UK Jewish individuals but the provision of information online for BRCA is unknown. We aimed to evaluate online provision of BRCA information by UK organisations (UKO), UK Jewish community organisations (JCO), and genetic testing providers (GTP). Google searches for organisations offering BRCA information were performed using relevant sets of keywords. The first 100 website links were categorised into UKOs/JCOs/GTPs; additional JCOs were supplemented through community experts. Websites were reviewed using customised questionnaires for BRCA information. Information provision was assessed for five domains: accessibility, scope, depth, accuracy, and quality. These domains were combined to provide a composite score (maximum score = 5). Results were screened (n = 6856) and 45 UKOs, 16 JCOs, and 18 GTPs provided BRCA information. Accessibility was high (84%,66/79). Scope was lacking with 35% (28/79) addressing >50% items. Most (82%, 65/79) described BRCA-associated cancers: breast and/or ovarian cancer was mentioned by 78%(62/79), but only 34% (27/79) mentioned ≥1 pancreatic, prostate, melanoma. Few websites provided carrier frequencies in the general (24%,19/79) and Jewish populations (20%,16/79). Only 15% (12/79) had quality information with some/minimal shortcomings. Overall information provision was low-to-moderate: median scores UKO = 2.1 (IQR = 1), JCO = 1.6 (IQR = 0.9), and GTP = 2.3 (IQR = 1) (maximum-score = 5). There is a scarcity of high-quality BRCA information online. These findings have implications for UK Jewish BRCA programmes and those considering BRCA testing.
1. Introduction
BRCA1 and BRCA2 pathogenic variants (PVs) are associated with significantly increased lifetime risks of cancers of the breast, ovary, pancreas, prostate, and melanoma [1,2,3]. A number of effective risk management options are available for breast cancer (BC) and ovarian cancer (OC) prevention in BRCA PV carriers, including screening (e.g., mammography/MRI for BC), preventive medication (e.g., tamoxifen or anastrozole for BC), risk-reducing surgery (e.g., risk-reducing mastectomy (RRM) for BC or risk-reducing salpingo-oophorectomy (RRSO) for OC prevention) [4,5,6], and pre-implantation genetic diagnosis [7,8,9,10,11].
Public awareness, availability, and accessibility of BRCA genetic testing has increased over time. Traditionally, genetic testing provision has been restricted to individuals meeting strict clinical/family history criteria with a pre-test BRCA probability of ≥10% [12]. Genetic testing is available free of cost through the UK National Health Service (NHS) or via private testing providers at a charge. Implementation of mainstream genetic testing for cancer diagnosis and associated cascade testing is identifying more unaffected BRCA carriers. A London pilot UK NHS programme of BRCA testing for all women diagnosed with BC commenced in July 2023. Long-term trials have demonstrated feasibility, acceptability, high satisfaction, reduced anxiety, no detrimental impact on quality of life, and cost-effectiveness of population-based BRCA testing in the Jewish population [13,14,15,16]. Resultantly, NHS England Cancer Programme introduced clinical implementation of population-based BRCA1 and BRCA2 testing for all UK Jewish adults in January 2024 [17,18,19]. Consequently, there is an even stronger demand and need for BRCA information for individuals considering testing and to support identified PV carriers [20]. Although clinicians/health professionals (including clinical geneticists, genetic counsellors, medical/surgical oncologists, clinical nurses, and others) provide pre- and post-test counselling, many individuals require and seek further information and support from online resources. According to the UK Office of National Statistics, 60% of UK adults searched for health-related information online in 2020 [21]. This highlights the need for high-quality BRCA information online as the demand for testing increases and information is sought online.
There has been very limited research into the availability and quality of online information on BRCA. One study, not specific to BRCA information, of UK direct-to-consumer genetic testing services found very limited compliance with good practice principles developed by the UK Human Genetics Commission [22]. There has been no research into the quality and provision of BRCA specific information provided by community organisations, charities, cancer organisations, or testing providers.
Given the interest in, need for, and impact of online information on user behaviour and health-related decisions, it is crucial for healthcare organisations, advocacy/patient support/charity groups, and other stakeholders to offer accurate and easily accessible information about BRCA1/BRCA2. Several websites provide online information, support, and other services relating to BRCA; these include cancer organisations and community-based organisations with interests in health and community support. Organisations working with the UK Jewish community may also provide information about BRCA given the higher carrier frequency of BRCA PVs in the Jewish population (1:40 compared to ~1:200 for the general UK population). Furthermore, several companies offer private genetic testing, either via clinician or direct-to-consumer, with associated online resources and testing information. These may be promoted with search engine advertisements or optimisations and are likely to be read by interested individuals.
This study aimed to evaluate the online provision and quality of BRCA1/BRCA2 information, including that related to genetic testing and its implications, by UK organisations (UKO), UK Jewish community organisations (JCO), and (private) genetic testing providers (GTP).
2. Materials and Methods
2.1. Organisation Groups
UKOs were defined as UK-based organisations offering information regarding BRCA-associated cancers (breast, ovarian, pancreatic, prostate cancer, or melanoma), BRCA testing, or BRCA support. JCOs were defined as organisations working primarily in the UK Jewish community in areas of health, welfare, community education/awareness raising, signposting, or community leadership. GTPs were defined as private companies offering BRCA1 and BRCA2 testing to UK residents.
2.2. Search Strategy
One author (A.K.) performed online searches (www.google.co.uk, accessed on 27 February 2022) between February to May 2022, using Mozilla Firefox version-97.0.1 (64-bit), from a UK desktop PC, with pre-defined sets of keywords relating to BRCA information to identify websites (Supplementary File: A). To avoid bias from personalised search algorithms, as described previously [23], we used a non-university/NHS internet connection, a private browser window, and changed IP-address and deleted browser data/history before each search.
Forty searches were performed to identify UKOs and JCOs websites with BRCA information. For every search with different keywords, details, and website links of the first 100 search results (including advertisements) were recorded. GTP websites were identified through 40 keyword searches and supplemented by the UKO/JCO search results. Website links were recorded for the first 100 (UKO and JCO) and 50 GTP search results, as results thereafter only included duplicates. Additional JCO websites were identified through lists from the Jewish Leadership Council, Board of Directors, Jewish Charity Guide, and three Jewish community experts. Duplicates were removed.
2.3. Website Selection
We included organisations based on their mission/aims/remit, which on assessment should provide/support information relevant to BRCA. This included UKOs focused on BRCA-associated cancers, BRCA genetic testing, BRCA support, or cancer in general. We included JCOs with stated interests of Jewish community health, providing health-related emotional or wellbeing support and/or health education or focused on women’s health or cancer.
We included UKO or JCO websites from organisations with a national reach that provided BRCA information in one of the following formats: raising awareness or support for BRCA-associated cancers or BRCA testing, information provision on a dedicated webpage, mentioning BRCA on a webpage, or in published media (e.g., an article/blog/leaflet/news item/podcast/post), outreach talk/workshop, online forum, signposting, support group, or telephone helpline. All GTPs who stated they provided BRCA1 and BRCA2 genetic testing to UK individuals were included.
All websites were screened for eligibility by two authors (A.K., K.S.). UKO/GTP websites excluded were as follows: not UK-based/unavailable to UK residents; inaccessible (faulty link); NHS hospital websites with information relevant only to their own service(s); scientific publications; health organisations unrelated to genetics/cancer; organisations not related to BRCA-associated cancers; websites without information targeted towards laypeople; or news media websites. JCO websites excluded were as followed: not UK-based; solely fundraising/grant giving; interfaith/peace organisations or primarily religion-based; focusing on medical research/childhood cancers/politics/poverty/helping disadvantaged groups/servicemen/volunteering; non-BRCA patient groups; focusing on the holocaust; focusing on art/history/culture/architectural preservation; and schools (primary/nursery/secondary). Websites were reviewed as below.
2.4. Data Extraction and Website Content Evaluation
Selected websites, were reviewed, evaluated and data extracted by seven authors independently (T.A.G., K.S., A.K., M.S., S.O., N.D, A.G., Se.G., A.T.), and subsequently reviewed by three authors together (T.A.G., C.T.F., K.S.) to ensure consistency and resolve discrepancies, between September 2022 to March 2023, using a customised REDCap database.
We developed customised questionnaires (Supplementary File: B. Questionnaire: A1, A2, A3) to assess overall website BRCA information provision for JCO, UKO, and GTP organisations, respectively. For UKOs/JCOs these comprised seven questions (26 items) about organisational remit, relevant general BRCA information, BRCA genetic testing and cancer risk management options, further signposting, awareness raising/educational activities, carrier/family support services, genetic testing provision/access. The GTP questionnaire comprised eight questions (28 items) about organisational remit, genetic testing services offered, test information, testing service access (eligibility/referral/counselling/booking), service components and process, service costs, BRCA relevant clinical information, and other hereditary cancer information.
Websites were scored across five domains for BRCA information provided (Table 1). These domains were selected to broadly encompass what we felt someone looking for BRCA information would need to be able to access information, and how that information fitted with the expectation of information that is comprehensive, fully explained, and correct. Quality was added as this is an important domain and has been previously used in assessment of written information on websites [24,25,26]. These domains included (1) accessibility (2 items: search bar presence on website, number of (≤3) clicks needed to access BRCA information, each scored 0/1); (2) scope (5 questions [Q2–Q6], 23 items for UKO/JCO; 7 questions [Q2–Q8], 26 items for GTP); (3) depth (6 items, each scored 0 [brief/no information] or 1 [in-depth]); (4) accuracy (6 items, each scored 0 [inaccurate] or 1 [accurate]); and (5) quality of written information (assessed by the validated 16-item DISCERN-questionnaire [27,28], adapted for BRCA1/BRCA2 information provision (Supplementary File: C).

Table 1.
Website scoring. Per domain a score between 0–1 can be awarded, up to a maximum score of 5 for all domains combined.
Domains 1–4 were scored as , with a maximum score = 1 for each domain. For quality (domain 5), overall DISCERN score ranges from 1 to 5 (1 = serious shortcomings, 5 = minimal/no shortcomings) with the quality of information provided.
To facilitate overall comparisons between websites, domain scores were converted into summary scores. The DISCERN score was divided by five and then scores of all five domains were summed-up/aggregated and divided by five.
3. Results
Search results returned 6856 websites (Figure 1). Duplicates (n = 4422) and ineligible websites (n = 2257) were initially excluded. We reviewed 84 UKOs, 75 JCOs, and 18 GTPs in full, leading to 80 UKOs, 41 JCOs, and 18 GTPs as websites expected to have BRCA information based on their remit or organisation type (Figure 1). Of the UKOs, 73% (58/80) were organisations specific for one of the BRCA-associated cancers, women’s cancer, or women’s health (Supplementary Table S1). JCOs were classified as 37% (15/41) health/welfare-related, 32% (13/41) leadership, 17% (7/41) religious organisations, or 14% (6/41) educational (Supplementary Table S2). Only 45/80 (56%) UKOs and 16/41 (39%) JCOs had any information about BRCA1/BRCA2. Of these, 60% (27/45) of UKOs were focused on a specific BRCA-associated cancer or women’s health/cancer and 38% (6/16) of JCOs were health/welfare-related.
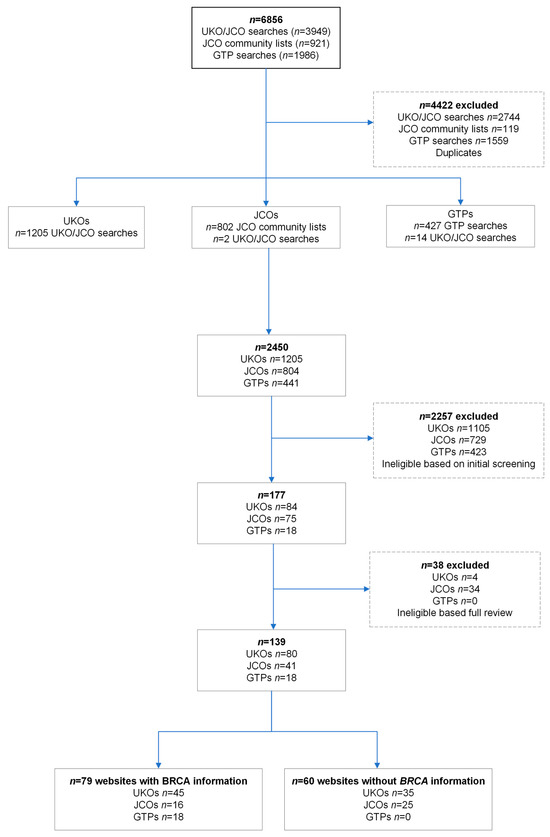
Figure 1.
Flowchart of inclusion of UK organisations (UKOs), UK Jewish community organisations (JCOs), and genetic testing providers (GTPs).
3.1. Information Provision: UKOs and JCOs
Of the websites, 61% (37/61) (28/45 UKOs, 9/16 JCOs) signposted to further BRCA resources. Table 2 lists the formats of information found on UKO and JCO websites, with mentioning of BRCA1, BRCA2, or BRCA in published media and signposting being the commonest.
Of UKOs, 10/45 (22%) had a dedicated BRCA webpage and 14/45 (31%) mentioned BRCA1, BRCA2, or BRCA on a webpage. Thirty-one UKOs mentioned BRCA1, BRCA2, or BRCA in published media and 10/31 had this as their only format of information. Six (13%) UKOs provided ≥4 formats and 13/45 (29%) provided only one format of information.
Of the JCOs, 8/16 (50%) provided information in one format, 7/16 (44%) in two formats, and only 1/16 (6%) in three different formats (Table 2). No JCOs provided an online forum or telephone helpline, only 1/16 (6%) had a support group, and only 13% (2/16) had a dedicated BRCA webpage.


Table 2.
BRCA information provided by JCOs and UKOs.
Table 2.
BRCA information provided by JCOs and UKOs.
| Organisation (Total Number) | ||
|---|---|---|
| BRCA Information Format | JCO (n = 16) | UKO (n = 45) |
| Dedicated webpage | 2 (13%) | 10 (22%) |
| Mentioned on webpage | 2 (13%) | 14 (31%) |
| Mention in published media | 8 (50%) | 31 (69%) |
| Outreach talk or workshop | 3 (19%) | 4 (9%) |
| Online forum | 0 | 4 (9%) |
| Signposting | 10 (63%) | 28 (62%) |
| Support group | 1 (6%) | 8 (18%) |
| Telephone helpline | 0 | 6 (13%) |
Percentages add up to more than 100% as organisations may provide more than one activity.
3.2. Genetic Testing Providers (GTPs)
Eighteen GTPs offering private BRCA genetic testing in the UK were included. GTPs varied widely based on type of testing provided, who could order the test, cost, pre- or post-test counselling, eligibility, and time to return results (Table 3). Clear information for these key factors was lacking across multiple websites, where it was unclear what the test included (56%, 10/18), which test to choose (28%, 5/18), and/or how to order the test (44%, 8/18). Regarding counselling, 33% (6/18) provided pre- and post-test counselling, 11% (2/18) post-test counselling only, 6% (1/18) pre-test counselling only, and 50% (9/18) lacked clarity. The test cost included counselling for 33% (6/18), did not for 28% (5/18), and lacked clarity in 39% (7/18).

Table 3.
GTP characteristics.
3.3. Domain 1: Accessibility
BRCA1/BRCA2 information was accessible within 3 clicks on 84% (66/79) of websites (37/45 UKOs, 14/16 JCOs, 15/18 GTPs; Table 4). Most (85%) websites (38/45 UKOs, 15/16 JCOs, 14/18 GTPs) had a search bar available. However, in 13% (10/79) of websites (6/45 UKOs, 3/16 JCOs, 1/18 GTPs), the BRCA1/BRCA2 information within the website was not accessible through the search bar.
3.4. Domain 2: Scope
Overall, the scope of information available on websites was limited (Table 4). Of those with information, only 30% (18/61) of UKOs and JCOs addressed >50% (5/9) items about general BRCA information (Supplementary File: B, Questions 2a–i on Questionnaires A1 and A2). Of the GTPs, 56% (10/18) addressed >50% (4/7) items about BRCA relevant clinical information (Supplementary File: B, Questions 7a–g on Questionnaire A3).
Only 37% (29/79) of websites (31%, 14/45 UKOs; 19%, 3/16 JCOs; 67%, 12/18 GTPs) provided information covering ≥50% items (see methods) across the scope domain (Table 4). One JCO did not have information about any of the items.


Table 4.
Summary statistics for items assessed within each of the five domains for UKOs, JCOs, and GTPs.
Table 4.
Summary statistics for items assessed within each of the five domains for UKOs, JCOs, and GTPs.
| Number of Websites with: | UKO | JCO | GTP | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 45 | % | n = 16 | % | n = 18 | % | n = 79 | % | ||
| Accessibility | ≤3 mouse clicks | 37 | 82.2 | 14 | 87.5 | 15 | 83.3 | 66 | 83.5 |
| A search bar present | 38 | 84.4 | 15 | 93.8 | 14 | 77.8 | 67 | 84.8 | |
| Accessibility score of 1 | 31 | 68.9 | 13 | 81.3 | 12 | 66.7 | 56 | 71 | |
| Scope | >50% total items addressed | 14 | 31.1 | 3 | 18.8 | 12 | 66.7 | 29 | 36.7 |
| >50% total BRCA information | 13 | 28.9 | 5 | 31.3 | 10 | 55.6 | 28 | 35.4 | |
| Scope score of 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Depth | BRCA-associated cancers (BC and OC, and/or ProC, PanC, or melanoma) | 23 | 51.1 | 4 | 25.0 | 10 | 55.6 | 37 | 46.8 |
| All BRCA carrier risk management options | 6 | 13.3 | 3 | 18.8 | 5 | 27.8 | 14 | 17.7 | |
| Difference in carrier frequency in AJ vs non-AJ populations | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 1 | 1.3 | |
| Explanation of BRCA inheritance | 8 | 17.8 | 3 | 18.8 | 9 | 50.0 | 20 | 25.3 | |
| Increased cancer risks for BRCA carriers for each gene and/or multiple cancers | 16 | 35.6 | 1 | 6.3 | 6 | 33.3 | 23 | 29.1 | |
| Signposting to >2 resources | 11 | 24.4 | 4 | 25.0 | 3 | 16.7 | 18 | 22.8 | |
| Depth score of 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Accuracy | Accurate BRCA-associated cancers | 38 | 84.4 | 8 | 50.0 | 16 | 88.9 | 62 | 78.5 |
| Accurate BRCA carrier risk management options | 18 | 40.0 | 3 | 18.8 | 7 | 38.9 | 28 | 35.4 | |
| Accurate carrier frequency in AJ population | 5 | 11.1 | 5 | 31.3 | 5 | 27.8 | 15 | 19.0 | |
| Accurate carrier frequency in the general population | 0 | 0.0 | 3 | 18.8 | 2 | 11.1 | 5 | 6.3 | |
| Accurate increased risks for BRCA: carriers female BC and OC | 13 | 28.9 | 2 | 12.5 | 3 | 16.7 | 18 | 22.8 | |
| Accurate increased risks for BRCA: carriers male BC, ProC, PanC, & melanoma | 7 | 15.6 | 2 | 12.5 | 1 | 5.6 | 10 | 12.7 | |
| Accuracy score of 1 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 1 | 1.3 | |
| Quality | DISCERN overall score of 5 | 2 | 4.4 | 0 | 0.0 | 0 | 0.0 | 2 | 2.5 |
| Quality score of 1 | 2 | 4.4 | 0 | 0.0 | 0 | 0.0 | 2 | 2.5 | |
| Overall score >3.5 out of 5 | 5 | 11.1 | 2 | 12.5 | 2 | 11.1 | 9 | 11.4 | |
Score of 1 means that all criteria of domain are fulfilled. See Table 1 for more information, AJ: Ashkenazi Jewish; BC: breast cancer; OC: ovarian cancer; PanC: pancreatic cancer; ProC: prostate cancer.
3.5. Domain 3: Depth
BRCA1/BRCA2 association with BC and/or OC was mentioned in 78% (62/79) of websites (84%, 38/45 UKOs; 50%, 8/16 JCOs; 89%, 16/18 GTPs). Only 34% (27/79) of websites (51%, 23/45 UKOs; 25%, 4/16 JCOs; 56%, 10/18 GTPs) mentioned one or more of the additional associated cancers: pancreatic, prostate, and/or melanoma. BRCA1/BRCA2/BRCA carrier cancer risks were specified by 47% (37/79) of websites (58%, 26/45 UKOs; 19%, 3/16 JCOs; 44%, 8/18 GTPs). Regarding management options for BRCA1/BRCA2 carriers, only 18% (14/79) of websites (13%, 6/45 UKOs; 19%, 3/16 JCOs; 28%, 5/18 GTPs) mentioned medical prevention, risk-reducing surgery, and screening, while 25% (20/79) mentioned only one or two of these options, and the remaining 57% lacked any information.
Inheritance pattern for BRCA1/BRCA2 PVs was not explained by 75% (59/79; 82% (37/45) of UKOs; 81% (13/16) of JCOs; 50% (9/18) of GTPs. The rest either mentioned a 50% chance or autosomal dominant pattern and inheritance from either biological parent.
Signposting to further BRCA information was offered by 54% (43/79) of websites, but only 23% (18/79; 24%, 11/45 UKOs; 25%, 4/16 JCOs; 17%, 3/18 GTPs) signposted to >2 resources.
Overall, 76% (60/79; 80%, 36/45 UKOs; 81%, 13/16 JCOs; 61%, 11/18 GTPs) of organisations scored poorly (<0.5/1) in the depth domain.
3.6. Domain 4: Accuracy
The majority–82% (65/79)—of websites (87%, 39/45 UKOs; 50%, 8/16 JCOs; 89%, 16/18 GTPs) described BRCA-associated cancers. However, only four (5%) websites (UKOs = 3, JCO = 1) provided accurate risk estimates for all BRCA-associated cancers (breast, ovarian, prostate, and pancreatic cancer) by gene and sex. Overall, 42% (33/79; 49%, 22/45 UKOs; 19%, 3/16 JCOs; 44%, 8/18 GTPs) provided information about BRCA1 and/or BRCA2-associated female BC and OC risks. Additionally, of these, 41% (9/22) of UKOs, 33% (1/3) of JCOs, and 63% (5/8) of GTPs provided inaccurate information (Table 1, Supplementary Table S3), with overestimation of BC risks. Only 29% (22/79) of websites (38%, 17/45 UKOs; 13%, 2/16 JCOs; 17%, 3/18 GTPs) provided information about BRCA1/BRCA2-associated male BC, prostate, pancreatic cancers, and/or melanoma risks. Of these, risk estimates were inaccurate in 59% (10/17) of UKOs, 0% (0/2) of JCOs, and 67% (2/3) of GTPs, with overestimation of male BC risks, inaccurate description of prostate cancer risks or increased sarcoma/gastric cancer risks. Melanoma risk estimates of 3–5% and 2–6% for BRCA2 were provided by two websites. Over half (53%, 42/79) of websites (42%, 19/45 UKOs; 81%, 13/16 JCOs; 56%, 10/18 GTPs) did not have information about BRCA-associated cancer risks.
Only 24% (19/79) of websites reported BRCA carrier frequency in the general population (1:200–1:300) [30,31], of which 26% (5/19; 0%, 0/6 UKOs; 60%, 3/5 JCOs; 25%, 2/8 GTPs) were accurate. Jewish population BRCA carrier frequency (1:40) [32] was provided by 20% (16/79) of websites, of which 94% (15/16; 100%, 5/5 UKOs; 100%, 5/5 JCOs; 83%, 5/6 GTPs) were accurate.
Although 63% (50/79) lacked information, almost all (97%, 28/29) websites with information provided accurate information on management options for BRCA1/BRCA2 PV carriers. One JCO mentioned OC screening as a management option, but this was not available/recommended under UK NHS care at that time [33,34,35].
Overall, 75% (59/79; 73%, 33/45 UKOs; 75%, 12/16 JCOs; 78%, 14/18 GTPs) of organisations scored <0.5 for accuracy.
3.7. Domain 5: Quality
Of BRCA information websites, 58% (46/79) had an overall DISCERN score = 1, indicating serious shortcomings in quality of information provision (42%, 9/45 UKOs; 75%, 12/16 JCOs; 83%, 15/18 GTPs), 11 (14%) scored = 2 (18%, 8/45 UKOs; 13%, 2/16 JCOs; 6%, 1/18 GTPs), 10 (13%) scored = 3, indicating moderate shortcomings (20%, 9/45 UKOs; 0%, 0/16 JCOs; 6%, 1/18 GTPs), and 10 (13%) scored = 4 (16%, 7/45 UKOs; 13%, 2/16 JCOs; 6%, 1/18 GTPs). Only two (2%) websites scored = 5, indicating minimal shortcomings (4%, 2/45 UKOs).
Overall, only 15% (12/79) of websites had quality information with some/minimal shortcomings.
3.8. Combined Scores across All Five Domains
Median scores for the five domains (accuracy, accessibility, scope, depth, and quality) across the three organisation types showed an overall low-to-moderate level of information provision (median = 1.9/5, IQR = 1.1; Table 5). Four websites (UKOs = 2; JCOs = 2) had overall scores < 1.

Table 5.
Combined scores across all five analysis domains.
Five (11%) UKOs had an overall score > 3.5/5. Two each had a dedicated BRCA webpage or genetic testing webpages with BRCA information and one provided an extensive patient information leaflet.
Two (13%) JCOs had an overall score > 4/5 and were the two highest-scoring JCOs across all five domains, providing high-quality BRCA information. These had a dedicated webpage with BRCA1/BRCA2 information, ≥2 formats of information, and one also provided a support group.
Two (11%) GTPs had an overall score > 3.5/5, and both offered pre- and post-test genetic counselling.
Overall, only 11% (9/79) of websites had combined scores > 3.5, indicating accessible, accurate, in-depth, quality information.
4. Discussion
4.1. Findings
We found that overall provision of BRCA1/BRCA2 information was lacking/limited across websites that had a health- or community-facing remit and those that provided BRCA information.
Within the organisation types that provided information, there was a noticeable difference in the quality and quantity/breadth of information provided, varying between one brief mention of BRCA1/BRCA2 and others that provide dedicated, easily accessible webpages with extensive information. Amongst GTPs, there was wide variation in testing information provided, including that relating to the nature of testing, costs, eligibility criteria, and the availability of and requirements for pre- and post-test counselling, with clear information regarding these issues lacking across multiple websites.
The overall accessibility of websites was high, but the information scope, depth, accuracy, and quality were poor for most, and only around half signposted to other information sources.
Very few websites provided complete information regarding all aspects of BRCA information (domain 2 [scope] and domain 3 [depth]). Inaccuracy of information was due to the information being out of date, but also not providing complete information regarding cancer risks and/or management. Only four (5%) provided accurate risk estimates for all BRCA-associated cancers by gene/sex and 53% lacked information about BRCA-associated cancer risks. Cancer risks provided should be up to date and be in line with risks provided in the UK Cancer Genetics Group guidelines [36]. Table 6 shows the risks that should be provided in public facing materials. Information on management options was lacking on 57% websites and only 18% covered both screening and medical/surgical prevention. Only 29% (22/79) of websites provided information about BRCA1/BRCA2-associated male breast, prostate, pancreatic cancers, and melanoma. Overall, 58% of websites had serious shortcomings and 27% had moderate shortcomings in the quality of information (DISCERN assessed) provided.

Table 6.
Cancer risks associated with BRCA1 and BRCA2 pathogenic variants to age 80.
Combined scores from all five domains (accessibility, scope, depth, accuracy, and quality) suggested a low-to-moderate level of information provision, with large variation across websites. Only 11% of websites had a high level of information, suggesting these websites would be beneficial for individuals to access at when looking for BRCA information.
4.2. Strengths and Weaknesses
To our knowledge, this is the first study evaluating websites on the provision of BRCA1/BRCA2 information, for individuals searching for health information online via a search engine. This is the first to evaluate organisational websites relating specifically to the Jewish community, as well as the general population. This is relevant, given the implementation of Jewish population-testing programmes in the UK and Israel. Validated tools to evaluate all relevant aspects of online information were unavailable, and so we developed a scoring system to facilitate a comprehensive and systematic review of website content across five domains. We considered the accessibility of information as equally important as accuracy, scope, depth, and quality for an interested individual, as this influences how likely information is to be viewed. We used the previously described ‘three click rule’ [37], which states that website information should be accessible within three clicks, based on the belief that users will stop searching if it takes them longer than this. However, some studies suggest that organisation, navigation, and structure of websites should also be taken into consideration [38,39]. We considered the number of clicks as simple and objective tool to assess this. We used a modified version of the validated DISCERN tool [27,28] to assess information quality. We attempted to minimise interpretation bias with DISCERN scoring by a senior reviewer (K.S.), training all reviewers, and review of any discordance/issues by two reviewers (T.A.G., K.S.).
We used the most common UK/worldwide search engine [40], and reviewed a large number of results, despite evidence that 91.5% of Google users stay on the first results page and only 4.8% click the second page [41] The number of relevant websites was too small to enable further statistical testing to quantitatively compare between websites. Although, we took appropriate steps to reduce biases in searches and website data extraction, we cannot guarantee results were free from bias, as search engine algorithms are not published. We restricted this analysis to websites relevant for a UK population. Further research will need to address information provision across a broader international population.
4.3. Interpretation
The finding that information provision varied across and within the three organisational groups is also consistent with findings in other studies on website information regarding cervical cancer, skin cancer, and BC screening [23,42,43]. If individuals have access to different information regarding a health-related topic, this can impact their decision-making process and choices they make. If information on a website is incomplete or poorly understood, it could cause concern and/or undermine this process [44].
Online information increases awareness and improves informed decision-making about genetic testing and/or risk management. Online provision of high-quality, trustworthy, relevant, and accessible information on BRCA is important given the large numbers of people who search for genetic testing information online. Around 44% of individuals having genetic testing for cancer susceptibility genes identified online searches as the primary source to look for additional information, outside of clinical pre- and post-test counselling, although it was difficult to identify trustworthy sources [45]. A one-size-fits-all approach to genetic testing may not be suitable, since individuals may need different amounts of information based on several different factors [46,47,48]. Hence, information provision must be tailored to individual circumstances, including desire for details.
Several studies have explored the effect of education and decision-making including uptake of genetic testing. Though few had equivocal results [44,49,50], multiple studies demonstrate information/education improves knowledge and satisfaction and reduces uncertainty and decisional conflict [14,51,52,53]. Some of these results may be impacted by the effect of in-person counselling, higher motivation, and/or pre-existing intention to test. However, this may not be representative or generalisable to those searching online, who may have a lower baseline level of information or intention to test. A US general population study found that repeated viewing of an online decision aid was associated with increased ease of decision, and increased likelihood to undergo testing [54]. Another US study of women undergoing BRCA genetic testing found 48% of participants decided to undergo genetic testing or not irrespective of clinician advice or attitude [46]. It has also been shown that higher BRCA-related knowledge increases the likelihood of accepting genetic testing [46,47]. Ensuring individuals have easy access to reliable BRCA information through not only clinicians but also websites can improve knowledge and decision-making regarding genetic testing.
The increasing awareness, along with expanding access and applicability of genomics and BRCA genetic testing to healthcare, is leading to many more individuals seeking trustworthy sources of information about testing tomake properly informed decisions. Hence, appropriate online BRCA1/BRCA2 information provision is increasingly important. This is exemplified by the new NHS England Jewish population BRCA genetic testing programme [17], expanding testing for all women with BC diagnosis in a new London based NHS pilot [55], and falling thresholds for clinical genetic testing [56].
A number of organisations, particularly JCOs, do not provide comprehensive information about BRCA1/BRCA2. However, a high-quality website should provide clear signposting to other websites or sources of information. It is important that UKOs, especially those for cancer in general and with a specific remit for BRCA-associated cancers, provide a high standard of information. GTPs providing BRCA1/BRCA2 genetic testing should provide comprehensive information regarding testing, especially those that offer direct-to-consumer tests. Our data highlight huge gaps in information provision that need addressing.
Although we evaluated online website information provision, this is not the only source of information people interested in obtaining BRCA information could use. Several studies show the use of social media for health-related information, including communication on BRCA1 and BRCA2 on several platforms, such as Facebook, Instagram, and Twitter [45,49,57]. Other examples of sources are telephone helplines and physical leaflets. We tried to identify these sources by capturing if this was provided by the organisations we investigated; however, an analysis of social media content was beyond the scope of this study and is a point for future research.
5. Conclusions
This is the first study to assess BRCA information provision available on websites of UK organisations (including Jewish-specific) and genetic testing providers. We find that overall provision of high-quality information is low, with large variation in quantity and quality across websites providing BRCA information. There is a need for improved accessible and high-quality online BRCA information provision, with signposting where appropriate to high-quality resources for organisations that are health- and/or community-related, and especially GTPs that offer BRCA1/BRCA2 genetic testing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16132324/s1, Supplementary File: A. Keywords to identify websites; B. Data extraction questionnaires; C. Modified DISCERN questionnaire; Supplementary Table S1: UK organisation types; Supplementary Table S2: Jewish community organisation types; Supplementary Table S3: Scores for organisations with BRCA-related activities across the five categories.
Author Contributions
Conception: K.S. and R.M.; planning: K.S., C.T.F., A.K., M.S. and R.M.; Conduct: K.S., A.K. and C.T.F.; Analysis: K.S., A.K., T.A.G., S.G.O., N.D., A.G., S.G. (Sevasti Glynou), A.T., S.G. (Subhasheenee Ganesan) and C.T.F.; Initial Draft: K.S., A.K., T.A.G., C.T.F., S.G.O. and R.M.; Manuscript Review, final writing and approval: K.S., A.K., T.A.G., S.G.O., N.D., A.G., S.G. (Sevasti Glynou), A.T., S.G. (Subhasheenee Ganesan), C.T.F., M.S., R.L., R.E., D.G.R.E., M.F., R.M,. A.K. and K.S. contributed equally to the work. C.T.F. and R.M. contributed equally to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by The Eve Appeal, Barts Charity, Rosetrees Trust, Jacobs family, Rosefield family, Dr Pauline Caller, Naftalin family and Breaking Down Barriers. Funders had no role in the conduct, analysis or writing of this work. D Gareth Evans is supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007) and Cancer Research UK ACED Alliance Early Detection Centre C19941/A27859.
Institutional Review Board Statement
This study was approved by the Queen Mary Research Ethics Committee on 18/01/2022 (QMERC20.593).
Informed Consent Statement
Not applicable.
Data Availability Statement
Additional data are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Michelle Janes, Jon Boyd, and Elaine Kerr for providing lists of Jewish community organisations.
Conflicts of Interest
RM declares research funding from the British Gynaecological Cancer Society, Barts & the London Charity, Yorkshire Cancer Research, NHS England outside this work, an honorarium for grant review from Israel National Institute for Health Policy Research and honorarium for advisory board membership from Astrazeneca/MSD/EGL/GSK. RM was supported by an NHS Innovation Accelerator (NIA) Fellowship for population testing. RM has received grant funding for the evaluation of the NHS Jewish BRCA Programme and the Small Business Research Initiative BRCA-DIRECT breast cancer testing programme. RM has been the Chair for the Trial Steering Committee for the BRCA-DIRECT trial. RM has received grant funding and is chief investigator for the PROTECT-C trial into population testing in the UK general population. RE declares honoraria from GU-ASCO, Janssen, University of Chicago, Dana Farber Cancer Institute USA as a speaker, and educational honorarium from Bayer and Ipsen. RE is a member of external expert committee to Astra Zeneca UK, a member of Active Surveillance Movember Committee, a member of the SAB of Our Future Health, and undertakes private practice in cancer genetic testing as a sole trader at The Royal Marsden NHS Foundation Trust and 90 Sloane Street SW1X 9PQ and 280 Kings Road SW3 4NX, London, UK. The other authors declare no conflicts of interest.
References
- Chen, J.; Bae, E.; Zhang, L.; Hughes, K.; Parmigiani, G.; Braun, D.; Rebbeck, T.R. Penetrance of Breast and Ovarian Cancer in Women Who Carry a BRCA1/2 Mutation and Do Not Use Risk-Reducing Salpingo-Oophorectomy: An Updated Meta-Analysis. JNCI Cancer Spectr. 2020, 4, pkaa029. [Google Scholar] [CrossRef]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated With BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef]
- Nyberg, T.; Frost, D.; Barrowdale, D.; Evans, D.G.; Bancroft, E.; Adlard, J.; Ahmed, M.; Barwell, J.; Brady, A.F.; Brewer, C.; et al. Prostate Cancer Risks for Male BRCA1 and BRCA2 Mutation Carriers: A Prospective Cohort Study. Eur. Urol. 2020, 77, 24–35. [Google Scholar] [CrossRef]
- Gentile, D.; Losurdo, A.; Sagona, A.; Zuradelli, M.; Gatzemeier, W.; Barbieri, E.; Testori, A.; Errico, V.; Bianchi, P.; Biondi, E.; et al. Surgical management of BRCA-mutation carriers: A single institution experience. Eur. J. Surg. Oncol. 2022, 48, 1706–1712. [Google Scholar] [CrossRef]
- Slade, E.; Berg, L.; Dworzynski, K.; Manchanda, R.; Guideline, C. Ovarian cancer: Identifying and managing familial and genetic risk-summary of new NICE guidance. BMJ 2024, 385, q807. [Google Scholar] [CrossRef]
- Wei, X.; Sun, L.; Slade, E.; Fierheller, C.T.; Oxley, S.; Kalra, A.; Sia, J.; Sideris, M.; McCluggage, W.G.; Bromham, N.; et al. Cost-Effectiveness of Gene-Specific Prevention Strategies for Ovarian and Breast Cancer. JAMA Netw. Open 2024, 7, e2355324. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collee, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef] [PubMed]
- NICE. Familial Breast Cancer: Full Guideline; NICE: Manchester, UK, 2017. [Google Scholar]
- Nelson, H.D.; Fu, R.; Zakher, B.; Pappas, M.; McDonagh, M. Medication Use for the Risk Reduction of Primary Breast Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019, 322, 868–886. [Google Scholar] [CrossRef]
- Michaan, N.; Leshno, M.; Cohen, Y.; Safra, T.; Peleg-Hasson, S.; Laskov, I.; Grisaru, D. Preimplantation genetic testing for BRCA gene mutation carriers: A cost effectiveness analysis. Reprod. Biol. Endocrinol. 2021, 19, 153. [Google Scholar] [CrossRef]
- NHS England. Clinical Commissioning Policy: Genetic Testing for BRCA1 and BRCA2 Mutations; NHS England: London, UK, 2015. [Google Scholar]
- Manchanda, R.; Burnell, M.; Gaba, F.; Desai, R.; Wardle, J.; Gessler, S.; Side, L.; Sanderson, S.; Loggenberg, K.; Brady, A.F.; et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: Long-term outcomes. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Burnell, M.; Loggenberg, K.; Desai, R.; Wardle, J.; Sanderson, S.C.; Gessler, S.; Side, L.; Balogun, N.; Kumar, A.; et al. Cluster-randomised non-inferiority trial comparing DVD-assisted and traditional genetic counselling in systematic population testing for BRCA1/2 mutations. J. Med. Genet. 2016, 53, 472–480. [Google Scholar] [CrossRef]
- Manchanda, R.; Legood, R.; Burnell, M.; McGuire, A.; Raikou, M.; Loggenberg, K.; Wardle, J.; Sanderson, S.; Gessler, S.; Side, L.; et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J. Natl. Cancer Inst. 2015, 107, 380. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Sideris, M. Population-based genetic testing for cancer susceptibility genes: Quo vadis? BJOG Int. J. Obstet. Gynaecol. 2023, 130, 125–130. [Google Scholar] [CrossRef]
- NHS. Jewish Community’s NHS BRCA Screening Programme. Available online: https://nhsjewishbrcaprogramme.org.uk/ (accessed on 8 February 2024).
- Manchanda, R.; Loggenberg, K.; Sanderson, S.; Burnell, M.; Wardle, J.; Gessler, S.; Side, L.; Balogun, N.; Desai, R.; Kumar, A.; et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: A randomized controlled trial. J. Natl. Cancer Inst. 2015, 107, 379. [Google Scholar] [CrossRef]
- Venkatesan, P. BRCA testing launched for people of Jewish ancestry in England. Lancet Oncol. 2024, 25, 284. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Zhou, J.; Singh, P.; Wang, J.; Braun, D.; Hughes, K.S. Search Behavior Regarding Cancer Susceptibility Genes Using a Clinical Decision Support Tool for Gene-Specific Penetrance: Content Analysis. JMIR Cancer 2021, 7, e28527. [Google Scholar] [CrossRef]
- Eurostat. Digital Economy and Society Statistics—Households and Individuals; Eurostat: Luxembourg, 2022. [Google Scholar]
- Hall, J.A.; Gertz, R.; Amato, J.; Pagliari, C. Transparency of genetic testing services for ‘health, wellness and lifestyle’: Analysis of online prepurchase information for UK consumers. Eur. J. Hum. Genet. 2017, 25, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Eversbusch, C.; Gefeller, O.; Pfahlberg, A. Quality of Information for Skin Cancer Prevention: A Quantitative Evaluation of Internet Offerings. Healthcare 2021, 9, 229. [Google Scholar] [CrossRef]
- Pattenden, T.A.; Raleigh, R.A.; Pattenden, E.R.; Thangasamy, I.A. Quality and readability of online patient information on treatment for erectile dysfunction. BJUI Compass 2021, 2, 412–418. [Google Scholar] [CrossRef]
- Sansevere, M.E.; White, J.D. Quality Assessment of Online Complementary and Alternative Medicine Information Resources Relevant to Cancer. Integr. Cancer Ther. 2021, 20, 15347354211066081. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, F.; Shirreff, L.; Huang, L.N.; Jacobson, M.; Jarcevic, R.; Christakis, M.K. Quality and readability of online health information on menopausal hormone therapy in Canada: What are our patients reading? Menopause 2021, 29, 54–62. [Google Scholar] [CrossRef]
- Charnock, D.; Shepperd, S.; Needham, G.; Gann, R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J. Epidemiol. Community Health 1999, 53, 105–111. [Google Scholar] [CrossRef] [PubMed]
- The DISCERN Instrument. Available online: http://www.discern.org.uk/index.php (accessed on 16 May 2023).
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Wang, Q.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Grzymski, J.J.; Elhanan, G.; Morales Rosado, J.A.; Smith, E.; Schlauch, K.A.; Read, R.; Rowan, C.; Slotnick, N.; Dabe, S.; Metcalf, W.J.; et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat. Med. 2020, 26, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Roa, B.B.; Boyd, A.A.; Volcik, K.; Richards, C.S. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat. Genet. 1996, 14, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- UK National Screening Committee. Adult Screening Programme: Ovarian Cancer. Available online: https://view-health-screening-recommendations.service.gov.uk/ovarian-cancer/ (accessed on 15 July 2023).
- Kearns, B.C.J.; Whyte, S.; Calvert, N.; Preston, L. An Economic Evaluation of the Cost-Effectiveness of Screening for Ovarian Cancer Amongst Postmenopausal Women Who Are Not at High Risk of Ovarian Cancer; School of Health and Related Research (ScHARR), University of Sheffield: Sheffield, UK, 2016. [Google Scholar]
- UK Cancer Genetics Group. UKCGG One-Page Gene-Specific Management Guidelines. Available online: https://www.ukcgg.org/information-education/ukcgg-leaflets-and-guidelines/ (accessed on 9 April 2024).
- Zeldman, J. Taking Your Talent to the Web: A Guide for the Transitioning Designer; New Riders: Indianapolis, IN, USA, 2001. [Google Scholar]
- Laubheimer, P. The 3-Click Rule for Navigation Is False. Available online: https://www.nngroup.com/articles/3-click-rule/ (accessed on 16 May 2023).
- Porter, J. Testing the Three-Click Rule. 16 April 2003. Available online: https://articles.centercentre.com/three_click_rule/ (accessed on 17 May 2024).
- Statista. Market Share of Leading Search Engines in the United Kingdom. Available online: https://www.statista.com/statistics/280269/market-share-held-by-search-engines-in-the-united-kingdom/ (accessed on 12 June 2023).
- Chitika Insights. The Value of Google Result Positioning; Chitika, Inc.: Westborough, MA, USA, 2013. [Google Scholar]
- Jørgensen, K.J.; Gøtzsche, P.C. Presentation on websites of possible benefits and harms from screening for breast cancer: Cross sectional study. BMJ 2004, 328, 148. [Google Scholar] [CrossRef]
- Dawson, J.Q.; Davies, J.M.; Ingledew, P.A. Quality of Online Information Regarding Cervical Cancer. Cureus 2020, 12, e9511. [Google Scholar] [CrossRef]
- Lerman, C.; Biesecker, B.; Benkendorf, J.L.; Kerner, J.; Gomez-Caminero, A.; Hughes, C.; Reed, M.M. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J. Natl. Cancer Inst. 1997, 89, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.G.; Roberts, M.; Andersen, B.; Khoury, M.J. Communication About Hereditary Cancers on Social Media: A Content Analysis of Tweets About Hereditary Breast and Ovarian Cancer and Lynch Syndrome. J. Cancer Educ. 2020, 35, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Wolpe, P.R.; Jones, N.L.; Cho, M. How do women decide? Accepting or declining BRCA1/2 testing in a nationwide clinical sample in the United States. Community Genet. 2006, 9, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Burnell, M.; Gaba, F.; Sanderson, S.; Loggenberg, K.; Gessler, S.; Wardle, J.; Side, L.; Desai, R.; Brady, A.F.; et al. Attitude towards and factors affecting uptake of population-based BRCA testing in the Ashkenazi Jewish population: A cohort study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Sobocan, M.; Chandrasekaran, D.; Sideris, M.; Blyuss, O.; Fierheller, C.; Kalra, A.; Sia, J.; Miller, R.E.; Mills-Baldock, T.; Crusz, S.M.; et al. Patient decision aids in mainstreaming genetic testing for women with ovarian cancer: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2023, 131, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.L.; Jimenez-Marroquin, M.C.; Jadad, A.R. Seeking support on facebook: A content analysis of breast cancer groups. J. Med. Internet Res. 2011, 13, e16. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.C.J.; Bowen, D.; Lowry, D.; Durfy, S.; McTiernan, A.; Andersen, M.R. Genetic counseling for women with an intermediate family history of breast cancer. Am. J. Med. Genet. 2000, 90, 361–368. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Poll, A.; O’Connor, A.; Gershman, S.; Armel, S.; Finch, A.; Demsky, R.; Rosen, B.; Narod, S.A. Development and testing of a decision aid for breast cancer prevention for women with a BRCA1 or BRCA2 mutation. Clin. Genet. 2007, 72, 208–217. [Google Scholar] [CrossRef]
- Lieberman, S.; Lahad, A.; Tomer, A.; Cohen, C.; Levy-Lahad, E.; Raz, A. Population screening for BRCA1/BRCA2 mutations: Lessons from qualitative analysis of the screening experience. Genet. Med. 2017, 19, 628–634. [Google Scholar] [CrossRef]
- Stacey, D.; Legare, F.; Lewis, K.; Barry, M.J.; Bennett, C.L.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017, 4, CD001431. [Google Scholar] [CrossRef]
- Kaphingst, K.A.; McBride, C.M.; Wade, C.; Alford, S.H.; Brody, L.C.; Baxevanis, A.D. Consumers’ use of web-based information and their decisions about multiplex genetic susceptibility testing. J. Med. Internet Res. 2010, 12, e41. [Google Scholar] [CrossRef] [PubMed]
- Torr, B.; Jones, C.; Choi, S.; Allen, S.; Kavanaugh, G.; Hamill, M.; Garrett, A.; MacMahon, S.; Loong, L.; Reay, A.; et al. A digital pathway for genetic testing in UK NHS patients with cancer: BRCA-DIRECT randomised study internal pilot. J. Med. Genet. 2022, 59, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- NICE. Ovarian Cancer: Identifying and Managing Familial and Genetic Risk; Draft for consultation; NICE: Manchester, UK, 2023. [Google Scholar]
- Vicente, E.P.; De Faria, S.E.E.; Almeida, A.B.L.; Yamada, P.A.; Lucena, T.F.; Silva, T.M.; Bernuci, M.P. Cervical Cancer Prevention on Instagram: Content and Social Interaction Analysis of Brazilian Accounts. Asian Pac. J. Cancer Prev. 2022, 23, 3043–3049. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).