Simple Summary
Gorlin syndrome (GS) is a genetic disorder characterized by multiple basal cell carcinomas (BCCs) due to mutations in the hedgehog signaling pathway. Patients with GS may need dozens or even hundreds of surgical procedures in their lifetime, which can leave them severely scarred, deformed, and disfigured. In 16 patients with GS, we examined the effectiveness, safety, and length of response to oral hedgehog inhibitors. According to our retrospective study, sonidegib inhibited the growth of both newly diagnosed and pre-existing basal cell carcinomas more successfully and safely than vismodegib.
Abstract
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome (GS), is a genetic disorder characterized by the development of multiple cutaneous BCCs due to mutations in the hedgehog signaling pathway. The use of hedgehog pathway inhibitors—vismodegib and sonidegib—has emerged as a promising therapeutic strategy for managing BCCs in individuals with GS. In a retrospective study conducted between March 2012 and January 2024, a cohort of 16 Gorlin syndrome patients who received treatment with either sonidegib or vismodegib were analyzed. The primary objectives of the study were to evaluate the efficacy, safety profile, and duration of response to oral hedgehog inhibitors in this patient population. The study assessed various parameters, including the number of new BCCs that developed before and after treatment initiation, the duration and sustainability of treatment responses, as well as the incidence of adverse effects associated with hedgehog inhibitor therapy. The findings of the study revealed that sustained treatment with hedgehog inhibitors could effectively suppress the progression of both new and existing BCCs. Furthermore, the results indicated that sonidegib exhibited superior efficacy and safety compared to vismodegib in the treatment of BCCs in individuals with GS. Notably, adjustments to the administration schedule of sonidegib were found to improve tolerability without compromising therapeutic efficacy, potentially leading to prolonged durations of treatment response and disease control.
1. Introduction
Gorlin syndrome (GS), also known as Gorlin–Goltz syndrome, nevoid basal cell carcinoma syndrome, or basal cell nevus syndrome (BCNS), is an autosomal dominant familial cancer syndrome that is characterized by the early onset of multiple BCCs and/or mandibular odontogenic keratocysts (OKCs) [1]. Macrocephaly, frontal bossing, facial dysmorphism such as cleft lip/palate, and face milia are common characteristics in approximately 60% of patients. By the age of 20, over 90% of individuals develop ectopic calcification of the falx cerebri. Skeletal anomalies and palmar or plantar pits (asymmetrical, 2–3 mm in diameter, 1–3 mm in depth, and developing in the second decade) are described. Additional features include ocular anomalies, such as cataracts, colobomas, and microphthalmos, as well as lymphomesenteric cysts. A susceptibility to either benign or malignant tumors, such as fibrosarcoma, nephroblastoma, ovarian fibroma (often bilateral and calcified), meningioma, medulloblastoma, and cardiac papillary fibroelastoma, is reported [1].
Few investigations on GS prevalence have been conducted, as reviewed by Evans et al. [2]. The prevalence rate of 1:57,000 that is most frequently cited originates from research conducted in northwest England, UK, including four million people. There is a theoretical range of 1:30,827 to 1:164,000. GS appears to be more prevalent among Caucasians, despite the possibility of ascertainment bias associated with BCC development being less prevalent among other races. In line with expectations, autosomal dominant inheritance causes a similar incidence of Gorlin syndrome in both sexes.
About 20–30% of GS cases are caused by a de novo pathogenetic variation, whereas 70–80% of cases have a relevant family history.
Loss-of-function mutations in the tumor suppressor gene PTCH1 (9q22.1–q31), which codes for the sonic hedgehog ligand receptor, cause GS. The clinical presentation’s varying expressivity may be attributed to modifier genes (SUFU and PTCH2) and environmental exposure.
Several diagnostic criteria for GS have been proposed. It should be noted that no molecular testing is required to meet the diagnostic criteria. When a patient meets two major diagnostic criteria and one minor diagnostic criteria, or one major diagnostic criteria and three minor diagnostic criteria, a diagnosis of GS is established [2]. Kimonis et al. developed a similar series of diagnostic criteria [3]. To date, no study has been able to determine which set of diagnostic criteria offers the optimal balance between specificity and sensitivity.
The Consensus Statement from the First International Colloquium on Basal Cell Nevus Syndrome (BCNS) states that the following are diagnostic standards for GS [4].
Major criteria include BCC before the age of 20 or a significantly elevated number of BCCs compared to skin type and previous sun exposure; OKCs of the jaw before the age of 20; palmar or plantar pitting; lamellar calcification of the falx cerebri; medulloblastoma, usually desmoplastic; and first-degree family members with BCNS.
Minor criteria include rib abnormalities; macrocephaly; cleft lip and palate; ovarian or cardiac fibroma; lymphomesenteric cysts; ocular abnormalities (for example, strabismus, hypertelorism, congenital cataracts, glaucoma, or coloboma); and other skeletal malformations (for example, vertebral anomalies, kyphoscoliosis, short fourth metacarpals, or postaxial polydactyly).
A heterozygous germline PTCH1 or SUFU pathogenic or potentially pathogenic mutation may be found by molecular genetic analysis. Genetic confirmation validates a diagnosis if clinical signs are not totally evident. Mutations in PTCH2 have occasionally been reported in patients with NBCCS [5].
Patients with GS may need dozens or even hundreds of surgical procedures in their lifetime, which can leave them severely scarred, deformed, and disfigured [6].
Recent studies have identified pathogenic mutations in the hedgehog pathway, especially in “patched” (PTCH1 and PTCH2) genes, in Gorlin syndrome [7]. Vismodegib and sonidegib, known as “hedgehog inhibitors” (HHIs), are inhibitors of downstream signaling that target “smoothened” (SMO). These oral medications have demonstrated notable clinical efficacy in treating locally advanced or metastatic sporadic basal cell carcinomas [8,9]. This is because somatic (non-inherited) PTCH mutations are typically expressed in sporadic BCCs [10].
HHI drugs are associated with class-specific adverse effects (AEs), including dysgeusia (44–58%), fatigue (32–39%), hair loss (49–66%), muscular spasms (54–71% for sonidegib–vismodegib), and weight loss (44–56%). A post hoc analysis of the duration and severity of treatment-emergent adverse events (TEAEs) in patients receiving the two HHIs revealed that patients receiving sonidegib (200 mg) had a lower incidence of dysgeusia, alopecia, and muscle spasms than those receiving vismodegib (150 mg) [11]. Regarding treatment, there is a dearth of clinical evidence to support immunotherapy (particularly cemiplimab) in patients with GS, and there are few publications regarding the long-term efficacy and safety of HHIs in patients with this condition. Nevertheless, the most recent European consensus-based interdisciplinary guideline for the diagnosis and management of basal cell carcinoma recognizes multiple BCCs in patients with this syndrome as locally advanced BCCs, and treatment with Hedgehog inhibitors is advised for Gorlin patients who are not responsive to surgery or radiation therapy [12]. Based on these assumptions, we conducted a case study on 16 Gorlin patients (with clinical and genetic diagnoses) to detect differences between the two drugs in terms of efficacy, safety, and handling. It is imperative to enhance treatment compliance and minimize drug exposure in patients diagnosed with Gorlin syndrome, as they necessitate long-term therapy to prevent the onset of new BCCs.
2. Materials and Methods
2.1. Study Population
Our retrospective investigation included individuals with GS who received oral HHIs (vismodegib or sonidegib) between March 2012 and January 2024, identified by a search through our NMSC patient databases. The BCNS Consensus Statement defined diagnostic criteria for Gorlin syndrome. Patients taking vismodegib or sonidegib for other reasons, such as spontaneous basal cell carcinomas, were excluded from the study.
2.2. Study Methods
Records were collected in a spreadsheet format (Microsoft Excel version 16.77.1; Microsoft, Redmond, WA, USA) and comprised demographics, comorbid illnesses, and the type and the length of HHI treatment. The early response to HHIs was described, along with the TEAEs and drug response/resistance patterns. The number of BCCs before and during HHI therapy was determined using pathological examinations and dermatologist records. The time to progression was estimated from the beginning of HHI treatment up to the date of the histological documented recurrence. Each patient received a randomly generated patient number, and all patient information was de-identified prior to analysis.
2.3. Treatment Regimens
For many decades, individuals with GS were managed surgically, with several basal cell carcinoma excisions. Nowadays, if the number of BCCs is too large for appropriate surgical management, or if the patient is considered challenging to treat or unsuitable for radiation or surgical treatment after an evaluation by a multidisciplinary team, oral HHIs are prescribed.
2.4. Response Assessment
The effectiveness of HHI treatment was measured through a comparison of the number of BCCs after 4 months of HHI treatment with the number of lesions before therapy. A complete response was referred to as 100% clearance of all cutaneous BCCs. A partial response was described as a reduction in the size and number of existing BCCs, without any new lesions occurring. Progression was defined as the recurrent growth of BCCs or the formation of new BCCs. Time to progression (TTP) was determined by the occurrence of biopsy-proven new lesions while taking an HHI. Relapses were classified as local or generalized based on the size and location(s) of new or recurring BCCs. Localized resistance has been defined as the development of 1–3 new superficial resectable BCCs while being treated with HHI. Generalized resistance was defined as the occurrence of >3 new BCCs. Progression-free survival was defined as the period of time between the start of HHI and the first BCC recurrence.
2.5. Statistical Analysis
Statistical analyses were carried out using IBM SPSS statistics, version 29.0.1.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were reported as medians and interquartile ranges (IQRs), according to their distribution, and were compared using non-parametric tests. Discrete variables were described as numbers and percentages and compared through Fisher’s exact test. The Kaplan–Meyer method was used to describe the progression-free survival (PFS) of the two treatments, vismodegib and sonidegib. Follow-up of PFS was presented as the mean ± standard error and median value if present. A p-value < 0.05 was considered statistically significant.
3. Results
We collected data from a total of 16 patients with a median age of 55 (interquartile range (IQR): 49.5–70.75). The median age of diagnosis expressed in years was 38 (25–57), while the median age of onset of symptoms was 16 (14–28). Our sample was almost equally divided by sex (eight males and eight females). All patients (100%) had a genetic mutation in PTCH1/PTCH2 or SUFU genes. Nine out of sixteen patients (56%) had an affected family member, and four out of these nine patients (44%) had two affected family members. All patients developed BCCs with a median age at diagnosis of the first BCC of 27 (17–35); in fact, only 5/16 (31.3%) developed the first BCC before the age of 20. As many as 15/16 (93.8%) had numerous BCCs not justifiable by photoexposure or phenotype. Pits were present in 10/16 (62.5%), with a median age at diagnosis of 30 (14–47.5). Keratocysts were present in 14/16 (87.5%), with a median age at diagnosis corresponding to 17 (14.50–25.75). Falx cerebri calcification was present in 6/16 patients (37.55%). Only 3/16 (18.75%) patients developed a medulloblastoma. Table 1 shows the patients’ major and minor diagnostic criteria and comorbidities.

Table 1.
Patient characteristics.
3.1. Comparison of Vismodegib and Sonidegib
In our samples, 10/16 patients received only sonidegib, 3/16 patients received vismodegib first and were later switched to sonidegib due to progression, and 3/16 patients received only vismodegib. After four months of HHI treatment, about 61.5% of the sonidegib patients achieved clinical remission, while only 16.7% of the vismodegib patients achieved it (Figure 1A). A partial response was obtained in 66.6% of patients with vismodegib and in 23.1% of those treated with sonidegib. After four months of treatment, 16.7% of patients on vismodegib and 15.4% of patients on sonidegib experienced a progression of the disease (Figure 1A).
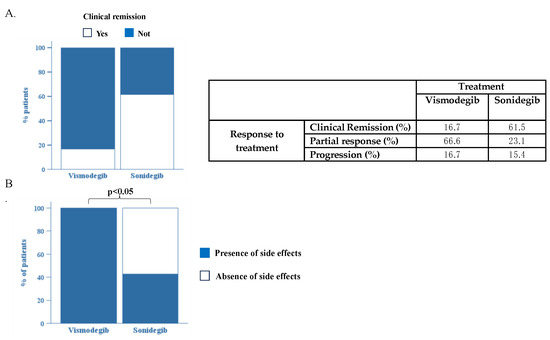
Figure 1.
(A). Comparison of efficacy of treatment with vismodegib and sonidegib. (B). Comparison of patients who developed side effects during treatment between groups of patients treated with vismodegib and sonidegib. p < 0.05 via Fisher’s exact test.
Although the number of our samples was limited, the data we obtained show a clear superiority of sonidegib over vismodegib in terms of effectiveness, but large, confirmatory prospective clinical trials are needed.
Interestingly, our analysis highlights the superiority of sonidegib over vismodegib in term of safety. In fact, all (100%) patients treated with vismodegib developed at least one side effect compared to only 57.9% of the patients on sonidegib (p < 0.05) (Figure 1B).
The median of follow-up for patients treated with vismodegib (n = 6) was 20.5 months (5.0–57.75), and, of those, three out of six stopped treatment and three out of six switched to sonidegib. The median of follow-up for patients treated with sonidegib was 21 months (11.75–25), and all patients are still under treatment. It is interesting to note that in the sonidegib group, the median of PFS was not reached; thus, during all the time of surveillance of these patients, more than 50% were free from progression (Figure 2B). On the contrary, it should be recorded that in the vismodegib group (Figure 2A), the Kaplan–Meyer curve showed a median PFS of 53.0 months.
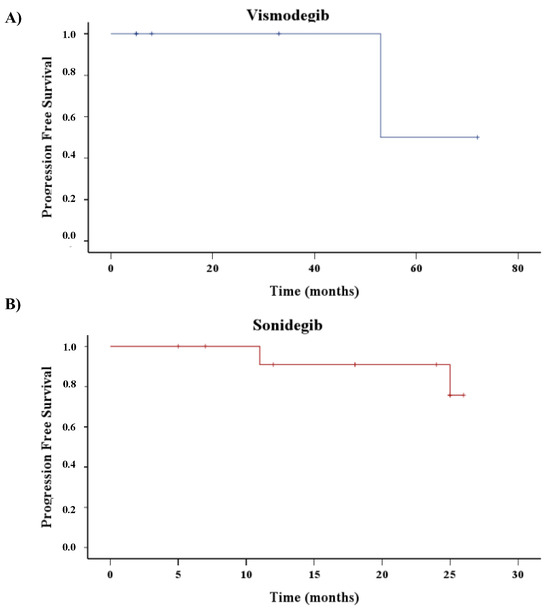
Figure 2.
Time to first new BCC recurrence from the beginning of HHI treatment. Hash marks represent censored patients. Kaplan-Meyer curves of patients treated with Vismodegib (A) or Sonidegib (B).
3.2. Association between Response to Sonidegib and Clinicopathological Features
We then evaluated whether or not any clinical features present at baseline could be associated with the therapeutic response to sonidegib (Supplementary Table S1). According to our analysis, patients who experienced clinical remission following 4 months of sonidegib treatment had BCC counts < 100 (p < 0.05), fewer than 4 BCC counts annually (p < 0.05), and fewer than 2 disease recurrences in high-risk locations (p < 0.05).
Photo S1 illustrates clinical and histological remission of laBCC after three months of Sonidegib.
4. Discussion
The maintenance of somatic stem and pluripotent cells as well as the development of different organ systems are crucial functions of the three human “hedgehog” (HH) peptide family members: Sonic, Desert, and Indian Hedgehog [13]. These soluble proteins have varying tissue-regulated patterns of expression, which account for their apparent functional variations. The binding of all three HH isoforms to the 12-pass transmembrane receptor “patched” (PTCH) allows them to function. Two PTCH homologs are currently known in mammals: Patched1 (PTCH1) and Patched2 (PTCH2). Each isoform has a tissue-specific expression, regulates different cellular development patterns by binding all three HH peptides with equal affinity, and suppresses SMO activity. Mutations within PTCH genes have been identified in Gorlin syndrome and sporadic basal cell carcinomas. Medulloblastomas are typically related to “suppressor of fused homologue” (SUFU) gene modifications [1].
BCC carcinogenesis is ligand-independent because the HH pathway is constitutively activated by changes in its components, involving gain-of-function (GOF) mutations in the SMO gene and loss-of-function (LOF) mutations in the PTCH1 or SUFU genes [1].
Approximately 90% of individuals with sporadic BCC have a detectable monoallelic LOF mutation in PTCH1, 30% have biallelic inactivation, and 10% have GOF mutations in SMO. The majority of sporadic BCCs have increased expressions of the proteins PTCH1 and GLI [14].
In patients with GS, a monoallelic PTCH1 mutation on chromosome 9 is frequently identified. It is noteworthy that 27% of individuals with clinically diagnosed Gorlin do not exhibit any detectable mutation in either PTCH1 or SUFU, suggesting the possibility of further undiscovered causative mutations [1].
Cyclopamine was the first hedgehog inhibitor to be developed. This drug suppresses downstream hedgehog signaling by binding to SMO [15]. Nevertheless, cyclopamine causes quite serious birth defects. Safer synthetic equivalents of cyclopamine have been developed and approved: sonidegib (LDE225) and vismodegib (GDC-0449).
These new oral HHIs have demonstrated effectiveness and safety in the management of laBCC. When HHIs bind selectively to SMO, the hedgehog pathway is deactivated. As reported by Dummer et al. [16], the ORRs of HHIs for laBCC were recorded as 47.6% for vismodegib over a 21-month follow-up period and 60.6% for sonidegib during an 18-month follow-up period. At the 30-month follow-up, the centrally evaluated mDOR for sonidegib was 26.1 months, and at the 21-month follow-up, it was 9.5 months for vismodegib. At the 30-month follow-up, the centrally evaluated mPFS for sonidegib was 22.1 months, whereas at the 21-month follow-up, it was 9.5 months for vismodegib. A review of published data from both pivotal trials (ERIVANCE and BOLT) showed that sonidegib exhibited around a 10% lower incidence of most adverse events (AEs) compared to vismodegib. In general, TEAEs associated with sonidegib were slightly less common and milder than those associated with vismodegib. Except for fatigue, the time to onset of AEs for individuals treated with sonidegib suggested that AEs may occur slightly later in relation to vismodegib.
There are just a few case reports and three published case studies including individuals with Gorlin syndrome who received HHI treatment in the literature [17,18,19,20,21,22,23,24,25,26,27]. All these studies showed a decrease in the quantity and size of BCCs, along with a rapid onset of response. The effectiveness and tolerance of long-term therapy have not been extensively investigated.
In another real-world experience with sonidegib, Nazzaro et al. documented 11 patients, 4 of whom were diagnosed with GS [27]. Of the patients, seven (63.6%) had adverse events (AEs); however, only three had their medication stopped because of toxicity. Four patients (50%) had complete remission (CR) confirmed by biopsy, while three patients (37.5%) had a partial response (PR). In total, 12.5% of the patients had a stable illness (SD). Sonidegib treatment was able to manage the disease in all four of the GS patients. Numerous novel HHI agents have been investigated as a result of vismodegib’s and sonidegib’s activity in BCCs [28,29]. These include topical medications like patigedib and medications that target mutations that cause drug resistance (taladegib, TAK441, and LEQ506). Cemiplimab, a PD-1-directed monoclonal antibody, has also demonstrated notable therapeutic effectiveness against sporadic BCCs [30]. The relevance of these newer medications in Gorlin disease therapy is still being investigated.
The main limitation of our retrospective study is the small sample size for a rare disorder. Furthermore, the number of BCCs identified before therapy varied significantly across individuals. A bias in lead time might have arisen from this. Additionally, there was a chance of ascertainment bias when determining the quantity of BCCs before and after therapy. It is possible that tumors were excised or eliminated without a histological examination, as is common with non-melanoma skin cancer. Further, data were sometimes unavailable for more than 5–7 years due to the extended duration during which individuals were susceptible to developing BCC. Furthermore, the patients in our study switched doctors often, making it challenging to collect all previous biopsy reports.
5. Conclusions
Our retrospective investigation assessed the efficacy and safety of HHIs in treating 16 individuals with clinically and genetically confirmed GS. After four months of HHI treatment, about 61.5% of sonidegib patients and just 16.7% of vismodegib patients achieved clinical remission. A partial response was obtained in 66.6% of patients treated with vismodegib and in 23.1% of those treated with sonidegib. According to our analysis, patients who experienced clinical remission following 4 months of sonidegib treatment had BCC counts < 100, fewer than 4 BCC counts annually, and fewer than 2 disease recurrences in high-risk locations. Interestingly, our analysis highlights the superiority of sonidegib over vismodegib in term of safety. In fact, all (100%) patients treated with vismodegib developed at least one side effect compared to only 57.9% of patients on sonidegib (p < 0.05). Studies of the pharmacokinetic profiles point out that sonidegib seems to be more lipophilic than vismodegib, with a volume of distribution of >9.000 L, indicating extensive distribution in tissues, while vismodegib has a volume of distribution of 16–27 L, suggesting that it is largely confined to the plasma. In theory, this evidence indicates that sonidegib is more distributed in the skin compared with vismodegib, which may potentially explain the differences in efficacy and toxicity observed in our case series [31].
Since the use of long-term HHI medication in patients with GS appears to be extremely effective, future research with longer follow-ups should investigate HHIs’ effectiveness and safety in these patients. Our case series proved the superiority in terms of effectiveness and safety of sonidegib over vismodegib in the treatment of BCCs in Gorlin syndrome. These data, together with the option of dose interruptions, supportive medications to better manage the adverse events, and on-label dose reduction for sonidegib to the “every other day” schedule [32,33], make this molecule a candidate for the long-term management of these chronic patients and one to be investigated with large, controlled trials. Since drug resistance may be caused by mutations that confer resistance to sonidegib or vismodegib [21,34], the development of new HHIs may also be significant. Resection of possibly resistant BCCs is advised in patients with a small number of progressing lesions, as it may extend the duration of the benefit of HHI treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16122166/s1. Supplementary Table S1: Baseline clinicopathological features of patients treated with sonidegib predicting clinical remission. Photo S1: (A) Clinical and histological presentation of laBCC before sonidegib treatment. (B) Clinical and histological remission after three months of sonidegib.
Author Contributions
Conceptualization, G.M. and A.V.M.; methodology, G.M., L.V. and E.P.; validation, N.D. and G.N.; formal analysis, G.M. and L.V.; investigation, G.M. and G.N.; data curation, G.M.; writing—original draft preparation, G.M. and L.V.; writing—review and editing, G.M., V.B. and P.B.; visualization, P.B.; supervision, E.P. and A.V.M.; project administration, E.P. and A.V.M.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SUNPHARMA 9741.
Institutional Review Board Statement
This study was performed based on the guidelines established by the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects (accessed on 4 June 2024)).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The de-identified data underlying this case series will be shared upon reasonable request to the corresponding authors.
Acknowledgments
We appreciate the patients, families, clinic staff, and referring physicians who made this case series possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lo Muzio, L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J. Rare Dis. 2008, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Farndon, P.A. Nevoid Basal Cell Carcinoma Syndrome; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; GeneReviews: Seattle, WA, USA, 1993. [Google Scholar]
- Kimonis, V.E.; Goldstein, A.V.M.; Pastakia, B.; Yang, M.L.; Kase, R.; DiGiovanna, J.J.; Bale, A.E.; Bale, S.J. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 1997, 69, 299–308. [Google Scholar] [CrossRef]
- Bree, A.F.; Shah, M.R.; BCNS Colloquium Group. Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS). Am. J. Med. Genet. Part A 2011, 155A, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Ohashi, H.; Suzuki, M.; Hatsuse, H.; Shiohama, T.; Uchikawa, H.; Miyashita, T. Frameshift mutation in the PTCH2 gene can cause nevoid basal cell carcinoma syndrome. Fam. Cancer 2013, 12, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Padwa, B.L.; Granter, S.R. Nevoid Basal Cell Carcinoma Syndrome (Gorlin Syndrome). Head Neck Pathol. 2016, 10, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Onodera, S.; Nakamura, Y.; Azuma, T. Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research. Int. J. Mol. Sci. 2020, 21, 7559. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Lear, J.T.; Migden, M.R.; Lewis, K.D.; Chang, A.L.S.; Guminski, A.; Gutzmer, R.; Dirix, L.; Combemale, P.; Stratigos, A.; Plummer, R.; et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Gambini, D.; Passoni, E.; Nazzaro, G.; Beltramini, G.; Tomasello, G.; Ghidini, M.; Kuhn, E.; Garrone, O. Basal Cell Carcinoma and Hedgehog Pathway Inhibitors: Focus on Immune Response. Front. Med. 2022, 9, 893063. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Dreno, B.; Ascierto, P.A.; Dummer, R.; Basset-Seguin, N.; Fife, K.; Ernst, S.; Licitra, L.; Neves, R.I.; Peris, K.; et al. Characterization and Management of Hedgehog Pathway Inhibitor-Related Adverse Events in Patients with Advanced Basal Cell Carcinoma. Oncologist 2016, 21, 1218–1229. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Kaufmann, R.; Arenberger, P.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Brochez, L.; Del Marmol, V.; Dummer, R.; et al. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma-update 2023. Eur. J. Cancer 2023, 192, 113254. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.N.; Phi, L.T.H.; Jun, N.; Wijaya, Y.T.; Lee, S.; Kwon, H.Y. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells 2018, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Jia, M.; Luo, S.Y.; Li, F.Z.; Fang, S. Expression of Hedgehog Signaling Pathway Proteins in Basal Cell Carcinoma:Clinicopathologic Study. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2353–2361. [Google Scholar] [CrossRef]
- Cucchi, D.; Occhione, M.A.; Gulino, A.; De Smaele, E. Hedgehog signaling pathway and its targets for treatment in basal cell carcinoma. J. Exp. Pharmacol. 2012, 4, 173–185. [Google Scholar] [PubMed]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef]
- Russo, F.; Tognetti, L.; Santi, F.; Lazzeri, L.; Rubegni, P.; Taddeucci, P. Long-term efficacy of a Vismodegib regime including a 1-week drug holiday every month in two patients with Gorlin Goltz syndrome. Dermatol. Ther. 2022, 35, e15293. [Google Scholar] [CrossRef]
- Piccerillo, A.; Di Stefani, A.; Costantini, A.; Peris, K. Sonidegib after vismodegib discontinuation in a patient with Gorlin-Goltz syndrome and multiple basal cell carcinomas. Dermatol. Ther. 2021, 34, e15095. [Google Scholar] [CrossRef]
- Valenzuela-Onate, C.A.; Magdaleno-Tapial, J.; Garcia-Legaz Martinez, M.; Perez-Pastor, G.; Sanchez Carazo, J.L. Drug holiday approach for Vismodegib treatment in patients with nevoid basal cell carcinoma syndrome: Three cases from real clinical practice. Dermatol. Ther. 2020, 33, e13540. [Google Scholar] [CrossRef] [PubMed]
- Kesireddy, M.; Mendiola, V.L.; Jana, B.; Patel, S. Long-term Response to Vismodegib in a Patient with Gorlin-Goltz Syndrome: A Case Report and Review of Pathological Mechanisms Involved. Cureus 2019, 11, e5383. [Google Scholar] [CrossRef]
- Hsu, S.W.; Lin, C.Y.; Wang, C.W.; Chung, W.H.; Yang, C.H.; Chang, Y.Y. Novel Patched 1 Mutations in Patients with Gorlin-Goltz Syndrome Strategic Treated by Smoothened Inhibitor. Ann. Dermatol. 2018, 30, 597–601. [Google Scholar] [CrossRef]
- Puig, S.; Serra-Guillen, C.; Perez-Pastor, G.; Martinez-Domenech, A.; Fernandez-de-Misa Cabrera, R. Experience with sonidegib in patients with advanced basal cell carcinoma: Case reports. Drugs Context 2022, 11, 2022-3-8. [Google Scholar] [CrossRef] [PubMed]
- Lear, J.T.; Hauschild, A.; Stockfleth, E.; Squittieri, N.; Basset-Seguin, N.; Dummer, R. Efficacy and Safety of Sonidegib in Adult Patients with Nevoid Basal Cell Carcinoma Syndrome (Gorlin Syndrome): Results from a Phase 2, Double-Blind, Randomized Trial. Clin. Cosmet. Investig. Dermatol. 2020, 13, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, O.K.; Yin, V.; Chou, E.; Ball, S.; Kies, M.; William, W.N.; Migden, M.; Thuro, B.A.; Esmaeli, B. Hedgehog Pathway Inhibition for Locally Advanced Periocular Basal Cell Carcinoma and Basal Cell Nevus Syndrome. Am. J. Ophthalmol. 2015, 160, 220–227.e2. [Google Scholar] [CrossRef] [PubMed]
- Verkouteren, B.J.A.; Wakkee, M.; Reyners, A.K.L.; Nelemans, P.; Aarts, M.J.B.; Racz, E.; Terra, J.B.; Devriese, L.A.; Alers, R.J.; Kapiteijn, E.; et al. Eight years of experience with vismodegib for advanced and multiple basal cell carcinoma patients in the Netherlands: A retrospective cohort study. Br. J. Cancer 2021, 124, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Wescott, R.; Samlowski, W. Sustained Suppression of Gorlin Syndrome-Associated Basal Cell Carcinomas with Vismodegib or Sonidegib: A Case Series. Curr. Oncol. 2023, 30, 9156–9167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nazzaro, G.; Benzecry, V.; Mattioli, M.A.; Denaro, N.; Beltramini, G.A.; Marzano, A.V.; Passoni, E. Sonidegib in Locally Advanced Basal Cell Carcinoma: A Monocentric Retrospective Experience and a Review of Published Real-Life Data. Cancers 2023, 15, 3621. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Dubey, S.K.; Singhvi, G. The Hedgehog pathway and its inhibitors: Emerging therapeutic approaches for basal cell carcinoma. Drug Discov. Today 2022, 27, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Scalvenzi, M. New Emerging Treatment Options for Advanced Basal Cell Carcinoma and Squamous Cell Carcinoma. Adv. Ther. 2022, 39, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef]
- Dreno, B.; Kunstfeld, R.; Hauschild, A.; Fosko, S.; Zloty, D.; Labeille, B.; Grob, J.J.; Puig, S.; Gilberg, F.; Bergstrom, D.; et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): A randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017, 18, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Ascierto, P.A.; Basset-Seguin, N.; Dreno, B.; Dummer, R.; Hauschild, A.; Mohr, P.; Kaufmann, R.; Pellacani, G.; Puig, S.; et al. Long-term strategies for management of advanced basal cell carcinoma with hedgehog inhibitors. Crit. Rev. Oncol. Hematol. 2023, 189, 104066. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, R.; Shao, W.; Sellers, W.R. Oncogene addiction: Pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015, 16, 280–296. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).