A Comprehensive Review of Genistein’s Effects in Preclinical Models of Cervical Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Cervical Cancer
1.2. Genistein
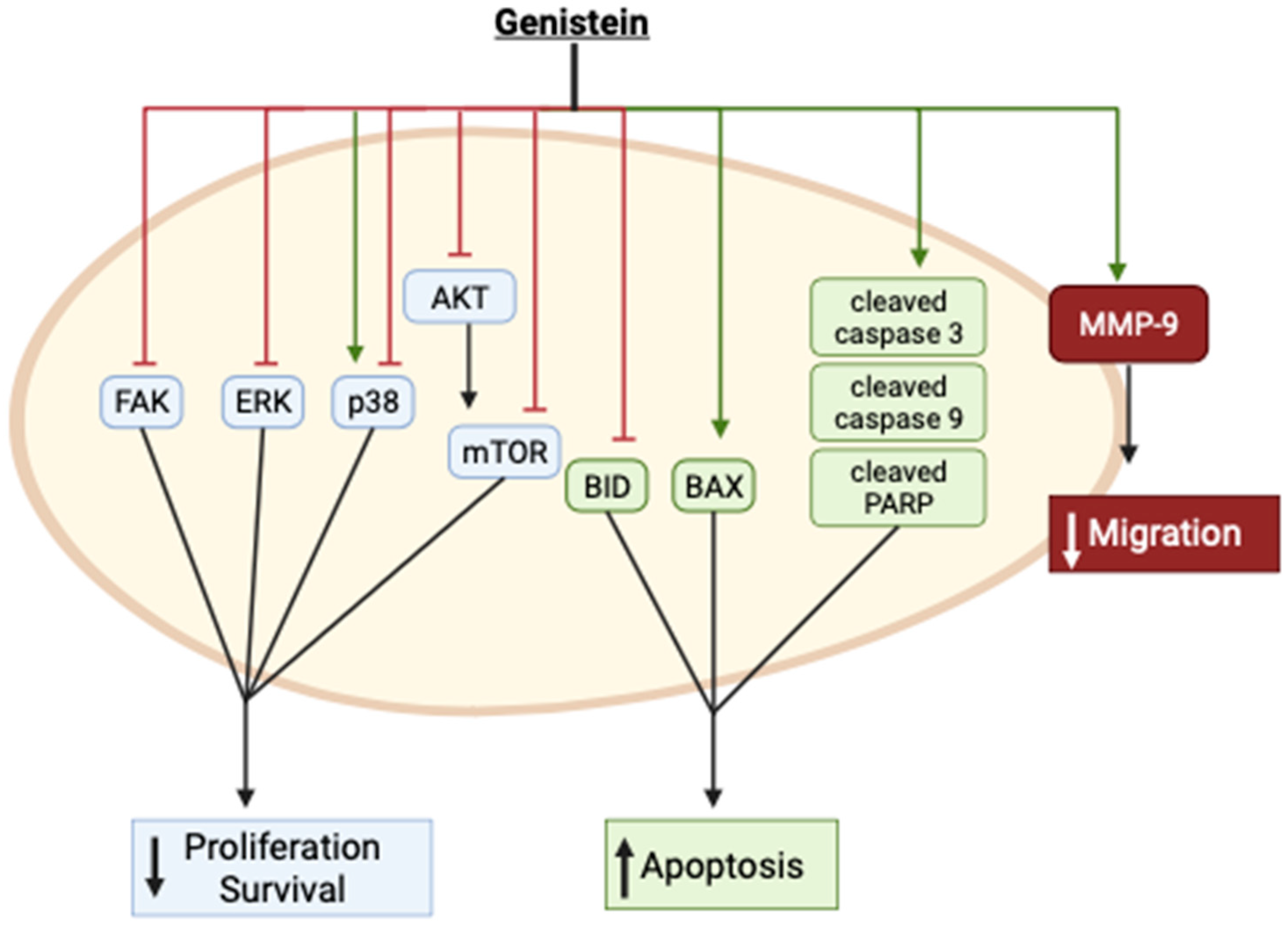
2. Genistein against Cervical Cancer
2.1. Genistein against Cervical Cancer: In Vitro Studies
| Cell Line | Genistein Concentration/Duration | Effect | Reference |
|---|---|---|---|
| HeLa ME-180 | IC50 35 and 60 µM | ↑ Cell cycle arrest S phase (HeLa) ↑ Cell cycle arrest G2/M phase (ME-180) ↓ Invasion ↑ Apoptosis | [30] |
| ME-180 CaSki | 10–40 µM 6 days (colony formation) 48 h | ↓ Colony formation ↑ Cytochrome c | [31] |
| HeLa | 25–150 µM 48 h | ↓ Cell survival ↑ Cell cycle arrest ↑ PARP cleavage ↓ Cyclin B1 ↓ cdc2 ↓ P-Tyr cdc2 | [32] |
| HeLa CaSki | 5–80 µM 24–48 h | ↓ Cell growth ↓ Cell viability ↓ p-Akt ↓ p-ERK ↑ p-p38 ↑ JNK | [33] |
| HeLa CaSki C33A | 5–60 µM 24–48 h | ↓ Cell viability ↑ Apoptosis ↓ Procaspase-3, -8, -9 ↑ Cleaved PARP ↓ Bid ↑ Bax | [34] |
| SiHa | 80 µM 48–72 h | ↓ Cell viability ↑ Apoptosis ↓ RARβ2 methylation | [35] |
| HeLa | 75, 100, and 150 µM 24 and 48 h | ↓ Cell viability ↑ Apoptosis G2/M Cell cycle arrest ↓ Migration ↓ MMP-9 mRNA expression ↑ TIMP-1 mRNA expression | [36] |
| HeLa | 25 µM 24 h | ↓ Cell viability ↓ NF-kB p65B ↓ p-mTOR ↓ p-p70S6K1 ↓ p-4E-BP1 ↓ p-Akt | [37] |
| HeLa | 0–100 µM 48 h | ↓ Cell viability ↑ Apoptosis ↑ Cleaved caspase-3 ↑ Cleaved PARP ↑ GRP78 ↑ CHOP | [38] |
| HeLa | 5 µM 24–72 h | ↓ Cell viability ↑ Cleaved caspase -9 ↑ Cleaved caspase -3 ↑ DNA fragmentation | [39] |
| HeLa | 0–150 µM 24–48 h | ↓ Proliferation ↓ Migration ↓ Invasion ↓ p-FAK ↓ p-paxillin ↓ p-p38 ↓ p-p42/44 ↓ β-catenin ↓ Vimentin ↓ p-paxillin mRNA levels ↓ p-p38 mRNA levels ↓ Snail mRNA levels ↓ twist mRNA levels | [40] |
| HeLa CaSki | 120–180 µM 24 h | ↓ Cell Viability | [41] |
| HeLa | 50 µM 48 h | ↑ Cell cycle arrest G2/M phase ↑ Nuclear condensation ↑ Nuclear fragmentation ↑ NO levels | [42] |
| HeLa | 0.01–1 µM 48–72 h | ↑ Cell proliferation ↑ S-phase arrest ↓ Apoptosis ↑ p-Akt ↑ Erα ↑ Nuclear NF-kB | [44] |
2.2. Genistein Analogues and Nanoparticles against Cervical Cancer: In Vitro Studies
| Cell Line | Nanoparticle/Conjugate | Effect | Reference |
|---|---|---|---|
| HeLa | 1 & 10 µM 3 days | ↓ Proliferation ↑ Inhibitory rate | [47] |
| HeLa | Folic acid chitosan conjugate | ↓ Cell viability ↑ Apoptosis | [48] |
2.3. Genistein in Combination with Other Compounds
| Cell Line | Genistein/Combination | Effect | Reference |
|---|---|---|---|
| HeLa | Genistein 5 µM 24–72 h Resveratrol 5 µM | ↓ Cell viability ↑ Cleaved caspase-9 ↑ Cleaved caspase-3 ↓ Mitochondrial membrane potential ↑ DNA fragmentation ↓ HDM2 gene | [39] |
| HeLa CaSki | Genistein 80 µM 24 h Cisplatin 6 µM | ↓ Cell viability ↓ p-ERK1/2 ↑ p53 ↑ Cleaved casp-3 ↓ Bcl2 | [41] |
2.4. Genistein in Combination with Radiotherapy
| Cell Line | Genistein/Combination | Effect | Reference |
|---|---|---|---|
| ME-180 CaSki | Genistein 10–40 µM 200, 500, or 800 cGy radiation | ↑ Cytochrome c induction ↓ Mcl-1 protein expression ↓ Akt & p-Akt protein | [31] |
| HeLa | Genistein 20–100 µM 400 cGy radiation | ↓ Cell viability ↑ Apoptosis ↓ Survivin mRNA and protein | [49] |
2.5. Genistein against Cervical Cancer: In Vivo Animal Studies
| Model | Genistein Concentration/Duration | Effect | Reference |
|---|---|---|---|
| TC-1 (cervical cancer cells) Injected subcutaneously in the right flank of mice | 20 mg/kg Oral gavage Daily | ↓ Tumor volume ↑ Lymphocyte proliferation ↑ cytotoxicity in spleen ↑ IFN-y concentration | [50] |
3. Discussion
3.1. General Overview
3.2. Uncertainties and Variations
3.3. Bioavailability
3.4. Organoids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 4 December 2023).
- Caird, H.; Simkin, J.; Smith, L.; Van Niekerk, D.; Ogilvie, G. The Path to Eliminating Cervical Cancer in Canada: Past, Present and Future Directions. Curr. Oncol. 2022, 29, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Takekida, S.; Fujiwara, K.; Nagao, S.; Yamaguchi, S.; Yoshida, N.; Kitada, F.; Kigawa, J.; Terakawa, N.; Nishimura, R. Phase II Study of Combination Chemotherapy with Docetaxel and Carboplatin for Locally Advanced or Recurrent Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2010, 20, 1563–1568. [Google Scholar]
- Coronel, J.; Cetina, L.; Candelaria, M.; González-Fierro, A.; Arias, D.; Cantu, D.; Dueñas-González, A. Weekly Topotecan as Second- or Third-Line Treatment in Patients with Recurrent or Metastatic Cervical Cancer. Med. Oncol. 2009, 26, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Marcus, S.; Garg, S.; Kansal, A. Redefining Role of 5-Fluorouracil and Exploring the Impact of Taxanes and Cisplatin in Locally Advanced and Recurrent Carcinoma Cervix in Concurrent Setting with Radiotherapy: A Literature Review. Cureus 2020, 12, e11645. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Yao, G.-M.; Li, Y.-M.; Lu, J.-H.; Lin, C.-J.; Wang, X.; Kong, C.-H. 5-Fluorouracil Derivatives from the Sponge Phakellia fusca. J. Nat. Prod. 2003, 66, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- van Oosterom, A.T.; Schrijvers, D. Corrected to Schrijvers, D. Docetaxel (Taxotere), a Review of Preclinical and Clinical Experience. Part II: Clinical Experience. Anticancer. Drugs 1995, 6, 356–368. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and Insights into Drug Resistance in Cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Pan, S.-T.; Li, Z.-L.; He, Z.-X.; Qiu, J.-X.; Zhou, S.-F. Molecular Mechanisms for Tumour Resistance to Chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Zhang, W.; Shen, Z.; Hu, X.; Zhu, X. Molecular Mechanisms of Cisplatin Resistance in Cervical Cancer. Drug Des. Dev. Ther. 2016, 10, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Osei Appiah, E.; Amertil, N.P.; Oti-Boadi Ezekiel, E.; Lavoe, H.; Siedu, D.J. Impact of Cervical Cancer on the Sexual and Physical Health of Women Diagnosed with Cervical Cancer in Ghana: A Qualitative Phenomenological Study. Womens Health 2021, 17, 17455065211066075. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Ahsan, F. Badruddeen an Overview on Genistein and Its Various Formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and Pharmacokinetics of Genistein: Mechanistic Studies on Its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Gupta, D.S.; Abjani, N.K.; Kaur, G.; Mohan, C.D.; Kaur, J.; Aggarwal, D.; Rani, I.; Ramniwas, S.; Abdulabbas, H.S.; et al. Genistein: A Promising Modulator of Apoptosis and Survival Signaling in Cancer. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 2893–2910. [Google Scholar] [CrossRef] [PubMed]
- Rahman Mazumder, M.A.; Hongsprabhas, P. Genistein as Antioxidant and Antibrowning Agents in in Vivo and in Vitro: A Review. Biomed. Pharmacother. Biomedecine Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial Activity of the Soy Isoflavone Genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef]
- LeCher, J.C.; Diep, N.; Krug, P.W.; Hilliard, J.K. Genistein Has Antiviral Activity against Herpes B Virus and Acts Synergistically with Antiviral Treatments to Reduce Effective Dose. Viruses 2019, 11, 499. [Google Scholar] [CrossRef]
- Andres, A.; Donovan, S.M.; Kuhlenschmidt, M.S. Soy Isoflavones and Virus Infections. J. Nutr. Biochem. 2009, 20, 563–569. [Google Scholar] [CrossRef]
- Kuryłowicz, A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. Int. J. Mol. Sci. 2021, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Liu, D. Anti-Diabetic Functions of Soy Isoflavone Genistein: Mechanisms Underlying Its Effects on Pancreatic β-Cell Function. Food Funct. 2013, 4, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y.; Xu, F.; Ding, H. Study on the Neuroprotective Effects of Genistein on Alzheimer’s Disease. Brain Behav. 2021, 11, e02100. [Google Scholar] [CrossRef]
- Li, R.; Robinson, M.; Ding, X.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Genistein: A Focus on Several Neurodegenerative Diseases. J. Food Biochem. 2022, 46, e14155. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, S.; Kumar, M.; Guarve, K.; Dhanawat, M.; Sharma, V. Therapeutic Potential of Genistein and Its Derivatives as a Target for Anticancer Agents. ChemistrySelect 2023, 8, e202204924. [Google Scholar] [CrossRef]
- Eumkeb, G.; Tanphonkrang, S.; Sirichaiwetchakoon, K.; Hengpratom, T.; Naknarong, W. The Synergy Effect of Daidzein and Genistein Isolated from Butea Superba Roxb. on the Reproductive System of Male Mice. Nat. Prod. Res. 2017, 31, 672–675. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, K.W.; Hsu, Y.T.; Chang, C.L.; Yang, Y.C. The Differential Inhibitory Effects of Genistein on the Growth of Cervical Cancer Cells in Vitro. Neoplasma 2001, 48, 227–233. [Google Scholar]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the Radiation Effect with Genistein in Cervical Cancer Cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Kalemi, T.G.; Zambouli, D.; Geromichalos, G.D.; Lambropoulos, A.F.; Kotsis, A.; Boutis, L.L.; Kortsaris, A.H. Synergistic Effects of Protein Tyrosine Kinase Inhibitor Genistein with Camptothecins against Three Cell Lines in Vitro. Cancer Lett. 2006, 233, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, S.-H.; Kim, Y.-B.; Jeon, Y.-T.; Lee, S.-C.; Song, Y.-S. Genistein Inhibits Cell Growth by Modulating Various Mitogen-Activated Protein Kinases and AKT in Cervical Cancer Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, S.-H.; Lee, S.-C.; Song, Y.-S. Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Apoptosis Induced by Genistein in Human Cervical Cancer Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Nikbakht, M.; Parashar, G.; Shrivastava, A.; Capalash, N.; Kaur, J. Reversal of Hypermethylation and Reactivation of the RARβ2 Gene by Natural Compounds in Cervical Cancer Cell Lines. Folia Biol. 2010, 56, 195–200. [Google Scholar]
- Hussain, A.; Harish, G.; Prabhu, S.A.; Mohsin, J.; Khan, M.A.; Rizvi, T.A.; Sharma, C. Inhibitory Effect of Genistein on the Invasive Potential of Human Cervical Cancer Cells via Modulation of Matrix Metalloproteinase-9 and Tissue Inhibitiors of Matrix Metalloproteinase-1 Expression. Cancer Epidemiol. 2012, 36, e387–e393. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Basak, N.; Caglayan, B.; Kilic, U.; Sahin, F.; Kucuk, O. Sensitization of Cervical Cancer Cells to Cisplatin by Genistein: The Role of NFκB and Akt/mTOR Signaling Pathways. J. Oncol. 2012, 2012, 461562. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Yang, Y.; Dai, W.-W.; Li, X.-M.; Ma, J.-Q.; Tang, L.-P. Genistein-Induced Apoptosis Is Mediated by Endoplasmic Reticulum Stress in Cervical Cancer Cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3292–3296. [Google Scholar]
- Dhandayuthapani, S.; Marimuthu, P.; Hörmann, V.; Kumi-Diaka, J.; Rathinavelu, A. Induction of Apoptosis in HeLa Cells via Caspase Activation by Resveratrol and Genistein. J. Med. Food 2013, 16, 139–146. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, S.; Ruan, X.; Liao, H.; Zhang, Y.; Sun, J.; Gao, J.; Deng, G. Genistein Inhibits Migration and Invasion of Cervical Cancer HeLa Cells by Regulating FAK-Paxillin and MAPK Signaling Pathways. Taiwan. J. Obstet. Gynecol. 2020, 59, 403–408. [Google Scholar] [CrossRef]
- Liu, H.; Lee, G.; Lee, J.I.; Ahn, T.-G.; Kim, S.A. Effects of Genistein on Anti-Tumor Activity of Cisplatin in Human Cervical Cancer Cell Lines. Obstet. Gynecol. Sci. 2019, 62, 322–328. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Khan, M.A.; Alalami, U.; Somvanshi, P.; Bhardwaj, T.; Pramodh, S.; Raina, R.; Shekfeh, Z.; Haque, S.; Hussain, A. Phytochemicals Induce Apoptosis by Modulation of Nitric Oxide Signaling Pathway in Cervical Cancer Cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11827–11844. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, J.; Li, M.; Wu, Q.; Cui, S. Genistein Inhibits Proliferation and Metastasis in Human Cervical Cancer Cells through the Focal Adhesion Kinase Signaling Pathway: A Network Pharmacology-Based In Vitro Study in HeLa Cells. Molecules 2023, 28, 1919. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Chen, S.-P.; Zheng, Q.-L.; Nie, S.-P.; Li, W.-J.; Hu, X.-J.; Xie, M.-Y. Genistein Promotes Proliferation of Human Cervical Cancer Cells Through Estrogen Receptor-Mediated PI3K/Akt-NF-κB Pathway. J. Cancer 2018, 9, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jangid, A.K.; Solanki, R.; Patel, S.; Pooja, D.; Kulhari, H. Genistein Encapsulated Inulin-Stearic Acid Bioconjugate Nanoparticles: Formulation Development, Characterization and Anticancer Activity. Int. J. Biol. Macromol. 2022, 206, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lin, Z.; Hou, J.; Liu, W.; Xu, J.; Guo, Y. Purification and Characterization of a Chicory Polysaccharide and Its Application in Stabilizing Genistein for Cancer Therapy. Int. J. Biol. Macromol. 2023, 242, 124635. [Google Scholar] [CrossRef]
- Xiong, P.; Wang, R.; Zhang, X.; Torre, E.D.; Leon, F.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.-H. Design, Synthesis, and Evaluation of Genistein Analogues as Anti-Cancer Agents. Anticancer Agents Med. Chem. 2015, 15, 1197–1203. [Google Scholar] [CrossRef]
- Cai, L.; Yu, R.; Hao, X.; Ding, X. Folate Receptor-Targeted Bioflavonoid Genistein-Loaded Chitosan Nanoparticles for Enhanced Anticancer Effect in Cervical Cancers. Nanoscale Res. Lett. 2017, 12, 509. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Pan, J.; Han, S.; Yin, X.; Wang, B.; Hu, G. Combined Treatment of Ionizing Radiation with Genistein on Cervical Cancer HeLa Cells. J. Pharmacol. Sci. 2006, 102, 129–135. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Razeghi, S.; Gorji, A.; Tabaraei, A.; Moradi, A.; Alizadeh, A.; Vakili, M.A. Genistein Induces a Protective Immunomodulatory Effect in a Mouse Model of Cervical Cancer. Iran. J. Immunol. IJI 2012, 9, 119–127. [Google Scholar]
- Murakami, T.; Murata, T.; Kawaguchi, K.; Kiyuna, T.; Igarashi, K.; Hwang, H.K.; Hiroshima, Y.; Hozumi, C.; Komatsu, S.; Kikuchi, T.; et al. Cervical Cancer Patient-Derived Orthotopic Xenograft (PDOX) Is Sensitive to Cisplatinum and Resistant to Nab-Paclitaxel. Anticancer Res. 2017, 37, 61–65. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Gao, S.; Xu, H.; Wu, B.; Kulkarni, K.; Singh, R.; Tang, L.; Hu, M. Simultaneous Determination of Genistein and Its Four Phase II Metabolites in Blood by a Sensitive and Robust UPLC-MS/MS Method: Application to an Oral Bioavailability Study of Genistein in Mice. J. Pharm. Biomed. Anal. 2010, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; van der Wildt, J.; Kinzel, J.; Attalla, H.; Wähälä, K.; Mäkelä, T.; Hase, T.; Fotsis, T. Lignan and Isoflavonoid Conjugates in Human Urine. J. Steroid Biochem. Mol. Biol. 1995, 52, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic Phenotype of Isoflavones Differ among Female Rats, Pigs, Monkeys, and Women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Shelnutt, S.R.; Cimino, C.O.; Wiggins, P.A.; Badger, T.M. Urinary Pharmacokinetics of the Glucuronide and Sulfate Conjugates of Genistein and Daidzein. Cancer Epidemiol. Biomark. Prev. 2000, 9, 413–419. [Google Scholar]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Virant, S.M.; Helferich, W.G. Soy Diets Containing Varying Amounts of Genistein Stimulate Growth of Estrogen-Dependent (MCF-7) Tumors in a Dose-Dependent Manner. Cancer Res. 2001, 61, 5045–5050. [Google Scholar] [PubMed]
- Nakamura, H.; Wang, Y.; Kurita, T.; Adomat, H.; Cunha, G.R.; Wang, Y. Genistein Increases Epidermal Growth Factor Receptor Signaling and Promotes Tumor Progression in Advanced Human Prostate Cancer. PLoS ONE 2011, 6, e20034. [Google Scholar] [CrossRef] [PubMed]
- Wietrzyk, J.; Mazurkiewicz, M.; Madej, J.; Dzimira, S.; Grynkiewicz, G.; Radzikowski, C.; Opolski, A. Genistein Alone or Combined with Cyclophosphamide May Stimulate 16/C Transplantable Mouse Mammary Cancer Growth. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004, 10, BR414–BR419. [Google Scholar]
- Lõhmussaar, K.; Oka, R.; Espejo Valle-Inclan, J.; Smits, M.H.H.; Wardak, H.; Korving, J.; Begthel, H.; Proost, N.; van de Ven, M.; Kranenburg, O.W.; et al. Patient-Derived Organoids Model Cervical Tissue Dynamics and Viral Oncogenesis in Cervical Cancer. Cell Stem Cell 2021, 28, 1380–1396.e6. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Organoid Systems to Recapitulate Cervical Cancer. Nat. Methods 2021, 18, 596. [Google Scholar] [CrossRef]
- Kutle, I.; Polten, R.; Hachenberg, J.; Klapdor, R.; Morgan, M.; Schambach, A. Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers 2023, 15, 2518. [Google Scholar] [CrossRef]
- Seol, H.S.; Oh, J.H.; Choi, E.; Kim, S.; Kim, H.; Nam, E.J. Preclinical Investigation of Patient-Derived Cervical Cancer Organoids for Precision Medicine. J. Gynecol. Oncol. 2022, 34, e35. [Google Scholar] [CrossRef] [PubMed]
- Di Fonte, R.; Strippoli, S.; Garofoli, M.; Cormio, G.; Serratì, S.; Loizzi, V.; Fasano, R.; Arezzo, F.; Volpicella, M.; Derakhshani, A.; et al. Cervical Cancer Benefits from Trabectedin Combination with the β-Blocker Propranolol: In Vitro and Ex Vivo Evaluations in Patient-Derived Organoids. Front. Cell Dev. Biol. 2023, 11, 1178316. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadile, M.; Kornel, A.; Sze, N.S.K.; Tsiani, E. A Comprehensive Review of Genistein’s Effects in Preclinical Models of Cervical Cancer. Cancers 2024, 16, 35. https://doi.org/10.3390/cancers16010035
Nadile M, Kornel A, Sze NSK, Tsiani E. A Comprehensive Review of Genistein’s Effects in Preclinical Models of Cervical Cancer. Cancers. 2024; 16(1):35. https://doi.org/10.3390/cancers16010035
Chicago/Turabian StyleNadile, Matteo, Amanda Kornel, Newman Siu Kwan Sze, and Evangelia Tsiani. 2024. "A Comprehensive Review of Genistein’s Effects in Preclinical Models of Cervical Cancer" Cancers 16, no. 1: 35. https://doi.org/10.3390/cancers16010035
APA StyleNadile, M., Kornel, A., Sze, N. S. K., & Tsiani, E. (2024). A Comprehensive Review of Genistein’s Effects in Preclinical Models of Cervical Cancer. Cancers, 16(1), 35. https://doi.org/10.3390/cancers16010035