Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems
Abstract
Simple Summary
Abstract
1. A Brief Insight into Anticancer Peptides
2. Bioactive Molecules from Marine Organisms
3. Review Methodology
4. Marine Animals as Sources of Anticancer Peptides: In Vitro Evidence
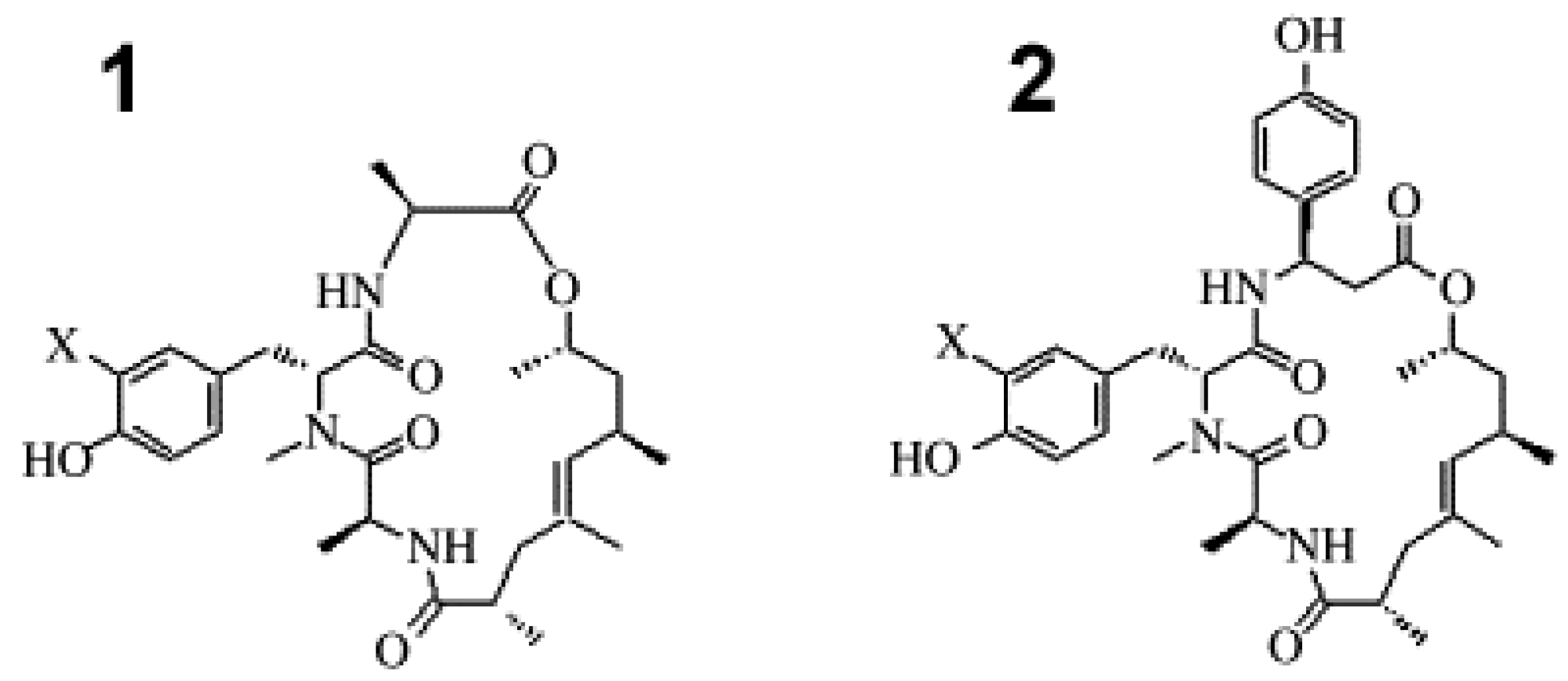
4.1. Porifera—Demospongiae
4.2. Cnidaria—Antozoa
4.3. Mollusca—Bivalvia
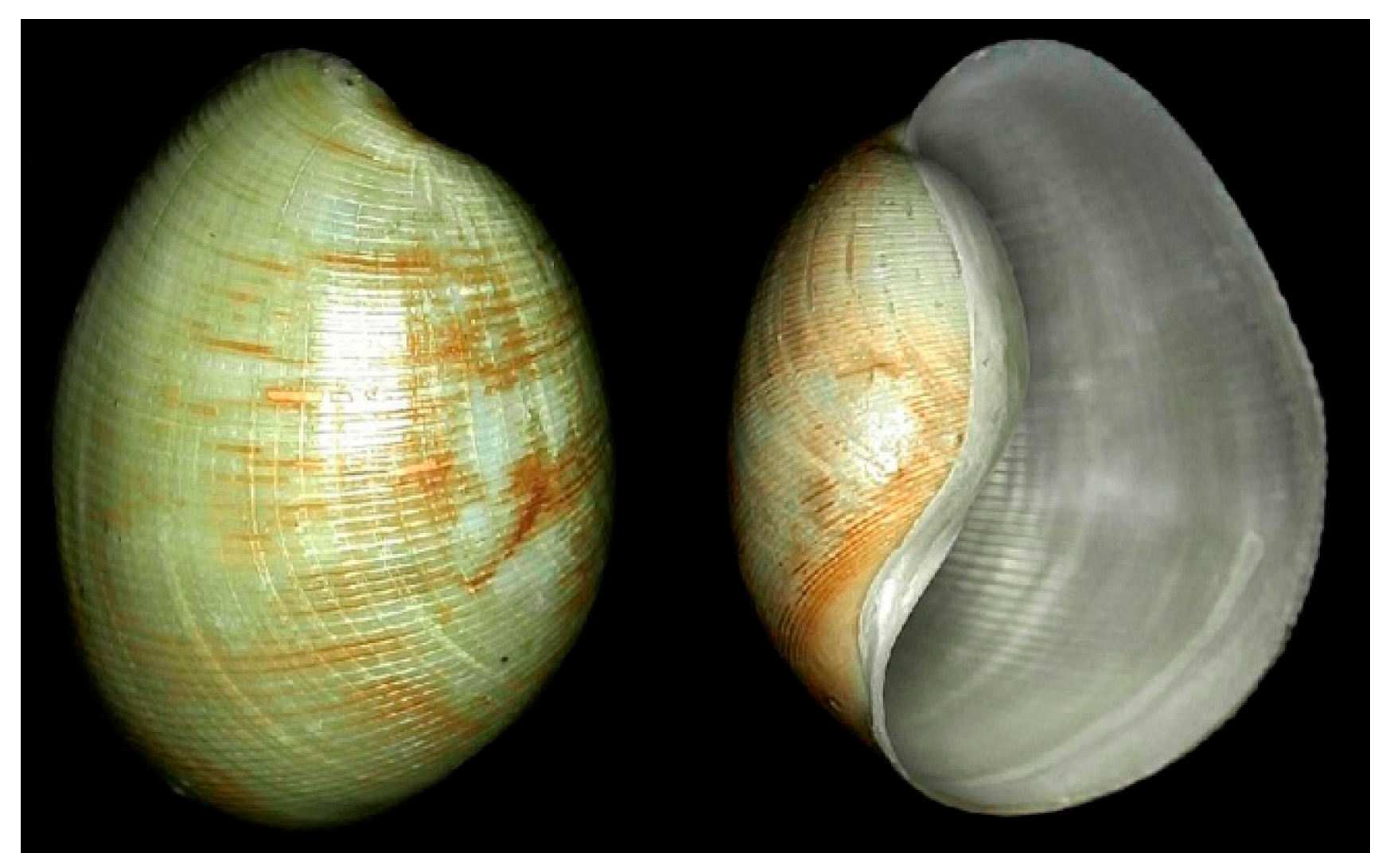
4.4. Mollusca—Gastropoda
4.5. Mollusca—Cephalopoda
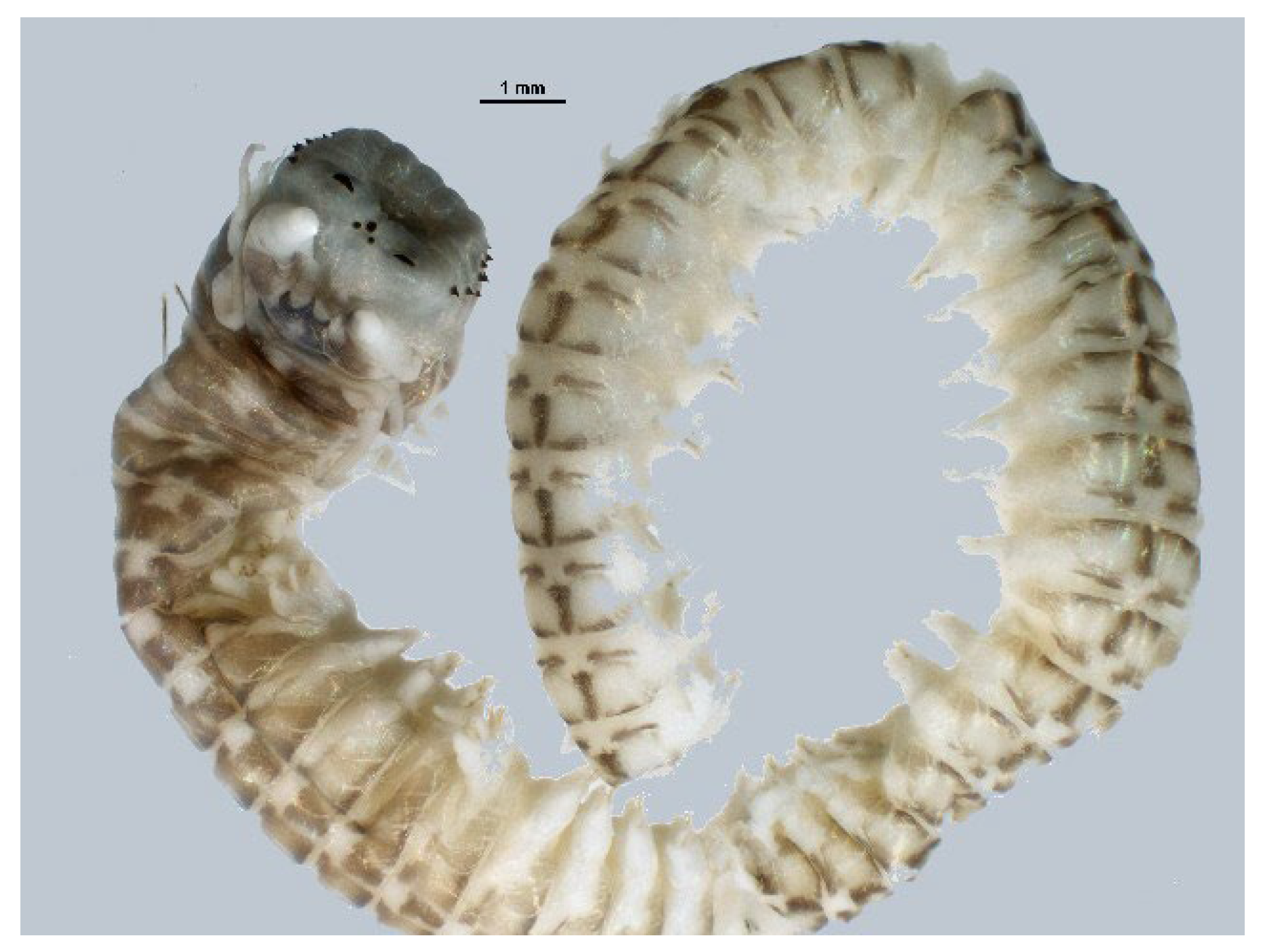
4.6. Anellida—Polychaeta
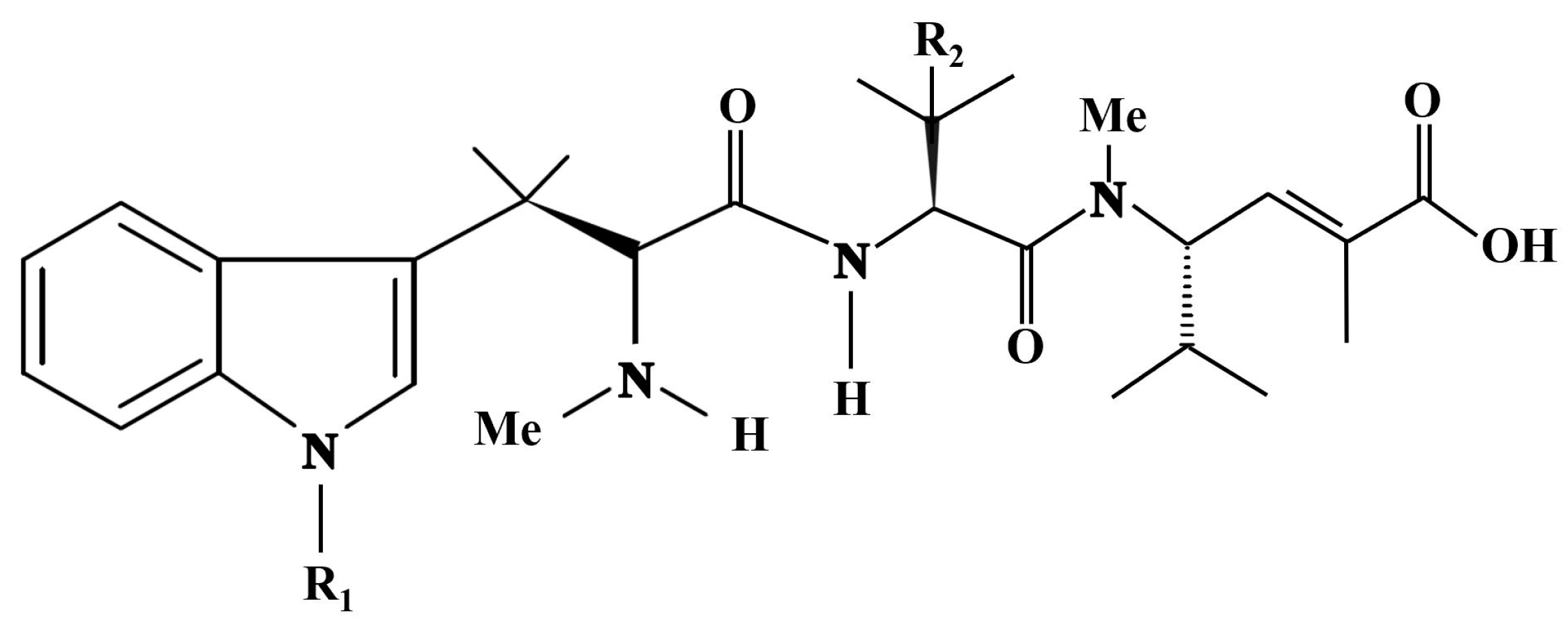
4.7. Arthropoda—Malacostraca
4.8. Echinodermata
4.9. Chordata—Ascidiacea
4.10. Chordata—Elasmobranchii
4.11. Chordata—Teleostei
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghaly, G.; Tallima, H.; Dabbish, E.; Badr ElDin, N.; Abd El-Rahman, M.K.; Ibrahim, M.A.A.; Shoeib, T. Anti-Cancer Peptides: Status and Future Prospects. Molecules 2023, 28, 1148. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Norouzi, P.; Mirmohammadi, M.; Houshdar Tehrani, M.H. Anticancer peptides mechanisms, simple and complex. Chem. Biol. Interact. 2022, 368, 110194. [Google Scholar] [CrossRef]
- Chinnadurai, R.K.; Khan, N.; Meghwanshi, G.K.; Ponne, S.; Althobiti, M.; Kumar, R. Current research status of anti-cancer peptides: Mechanism of action, production, and clinical applications. Biomed. Pharmacother. 2023, 164, 114996. [Google Scholar] [CrossRef]
- Rogers, A.D.; Appeltans, W.; Assis, J.; Balance, L.T.; Cury, P.; Duarte, C.; Favoretto, F.; Hynes, L.A.; Kumagai, J.A.; Lovelock, C.E.; et al. Discovering marine biodiversity in the 21st century. Adv. Mar. Biol. 2022, 93, 23–115. [Google Scholar]
- Beetul, K.; Gopeechund, A.; Kaullysing, D.; Mattan-Moorgawa, S.; Puchooa, D.; Bhagooli, R. Challenges and Opportunities in the Present Era of Marine Algal Applications. Algae—Organisms for Imminent Biotechnology. 29 June 2016. Available online: https://www.intechopen.com/chapters/50671 (accessed on 10 February 2023).
- Conte, M.; Fontana, E.; Nebbioso, A.; Altucci, L. Marine-Derived Secondary Metabolites as Promising Epigenetic Bio-Compounds for Anticancer Therapy. Mar. Drugs 2021, 19, 15. [Google Scholar] [CrossRef]
- Lazzara, V.; Arizza, V.; Luparello, C.; Mauro, M.; Vazzana, M. Bright Spots in the Darkness of Cancer: A Review of Starfishes-Derived Compounds and Their Anti-Tumor Action. Mar. Drugs 2019, 17, 617. [Google Scholar] [CrossRef]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Celi, M.; Arizza, V.; Vazzana, M. Cytotoxic Potential of the Coelomic Fluid Extracted from the Sea Cucumber Holothuria tubulosa against Triple-Negative MDA-MB231 Breast Cancer Cells. Biology 2019, 8, 76. [Google Scholar] [CrossRef]
- Mauro, M.; Lazzara, V.; Punginelli, D.; Arizza, V.; Vazzana, M. Antitumoral compounds from vertebrate sister group: A review of Mediterranean ascidians. Dev. Comp. Immunol. 2020, 108, 103669. [Google Scholar] [CrossRef]
- Luparello, C.; Mauro, M.; Lazzara, V.; Vazzana, M. Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates. Molecules 2020, 25, 2471. [Google Scholar] [CrossRef]
- Luparello, C.; Mauro, M.; Arizza, V.; Vazzana, M. Histone Deacetylase Inhibitors from Marine Invertebrates. Biology 2020, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Arizza, V.; Vazzana, M. Cell-Free Coelomic Fluid Extracts of the Sea Urchin Arbacia lixula Impair Mitochondrial Potential and Cell Cycle Distribution and Stimulate Reactive Oxygen Species Production and Autophagic Activity in Triple-Negative MDA-MB231 Breast Cancer Cells. J. Mar. Sci. Eng. 2020, 8, 261. [Google Scholar] [CrossRef]
- Luparello, C.; Branni, R.; Abruscato, G.; Lazzara, V.; Drahos, L.; Arizza, V.; Mauro, M.; Di Stefano, V.; Vazzana, M. Cytotoxic capability and the associated proteomic profile of cell-free coelomic fluid extracts from the edible sea cucumber Holothuria tubulosa on HepG2 liver cancer cells. EXCLI J. 2022, 21, 722–743. [Google Scholar] [PubMed]
- Luparello, C.; Branni, R.; Abruscato, G.; Lazzara, V.; Sugár, S.; Arizza, V.; Mauro, M.; Di Stefano, V.; Vazzana, M. Biological and Proteomic Characterization of the Anti-Cancer Potency of Aqueous Extracts from Cell-Free Coelomic Fluid of Arbacia lixula Sea Urchin in an In Vitro Model of Human Hepatocellular Carcinoma. J. Mar. Sci. Eng. 2022, 10, 1292. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Li, C.X.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Extraction and Characterization of Bioactive Fish By-Product Collagen as Promising for Potential Wound Healing Agent in Pharmaceutical Applications: Current Trend and Future Perspective. Int. J. Food Sci. 2022, 2022, 9437878. [Google Scholar] [CrossRef] [PubMed]
- Punginelli, D.; Catania, V.; Abruscato, G.; Luparello, C.; Vazzana, M.; Mauro, M.; Cunsolo, V.; Saletti, R.; Di Francesco, A.; Arizza, V.; et al. New Bioactive Peptides from the Mediterranean Seagrass Posidonia oceanica (L.) Delile and Their Impact on Antimicrobial Activity and Apoptosis of Human Cancer Cells. Int. J. Mol. Sci. 2023, 24, 5650. [Google Scholar] [CrossRef] [PubMed]
- Vicente, T.F.L.; Félix, C.; Félix, R.; Valentão, P.; Lemos, M.F.L. Seaweed as a Natural Source against Phytopathogenic Bacteria. Mar. Drugs 2023, 21, 23. [Google Scholar] [CrossRef]
- Abruscato, G.; Chiarelli, R.; Lazzara, V.; Punginelli, D.; Sugár, S.; Mauro, M.; Librizzi, M.; Di Stefano, V.; Arizza, V.; Vizzini, A.; et al. In Vitro Cytotoxic Effect of Aqueous Extracts from Leaves and Rhizomes of the Seagrass Posidonia oceanica (L.) Delile on HepG2 Liver Cancer Cells: Focus on Autophagy and Apoptosis. Biology 2023, 12, 616. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Cantalapiedra, J.; Zapata, C.; Franco, J.M.; Franco, D. Aquaculture and by-products: Challenges and opportunities in the use of alternative protein sources and bioactive compounds. Adv. Food Nutr. Res. 2020, 92, 127–185. [Google Scholar]
- Rebouças, J.S.A.; Oliveira, F.P.S.; Araujo, A.C.S.; Gouveia, H.L.; Latorres, J.M.; Martins, V.G.; Prentice Hernández, C.; Tesser, M.B. Shellfish industrial waste reuse. Crit. Rev. Biotechnol. 2023, 43, 50–66. [Google Scholar] [CrossRef]
- Van Soest, R.W.M. The Indonesian sponge fauna: A status report. Neth. J. Sea Res. 1989, 23, 223–230. [Google Scholar] [CrossRef]
- Hooper, J.N.A. Revision of Microcionidae (Porifera: Poecilosclerida: Demospongiae), with description of Australian species. Mem. Queens. Mus. 1996, 40, 1–626. [Google Scholar]
- Mokhlesi, A.; Stuhldreier, F.; Wex, K.W.; Berscheid, A.; Hartmann, R.; Rehberg, N.; Sureechatchaiyan, P.; Chaidir, C.; Kassack, M.U.; Kalscheuer, R.; et al. Cyclic Cystine-Bridged Peptides from the Marine Sponge Clathria basilana Induce Apoptosis in Tumor Cells and Depolarize the Bacterial Cytoplasmic Membrane. J. Nat. Prod. 2017, 80, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.S.; Sandes, J.; Guimaraes, C.R.; Muricy, G. Taxonomy of Geodia and Rhabdastrella from the Brazilian coast: A new species, new synonyms and redescription of Geodia tylastra (Demospongiae: Astrophorina: Geodiidae and Ancorinidae). Zootaxa 2021, 4995, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Prado, M.P.; Konno, K.; Naoki, H.; Freitas, J.C.; Machado-Santelli, G.M. Cytoskeleton alterations induced by Geodia corticostylifera depsipeptides in breast cancer cells. Peptides 2006, 27, 2047–2057. [Google Scholar] [CrossRef]
- Freitas, V.M.; Rangel, M.; Bisson, L.F.; Jaeger, R.G.; Machado-Santelli, G.M. The geodiamolide H, derived from Brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J. Cell. Physiol. 2008, 216, 583–594. [Google Scholar] [CrossRef]
- Rangel, M.; Ionta, M.; Pfister, S.C.; Sant’anna Ferreira, R.A.; Machado-Santelli, G.M. Marine sponge depsipeptide increases gap junction length in HTC cells transfected with Cx43-GFP. Cell Biol. Int. Rep. 2010, 2010, e00003. [Google Scholar] [CrossRef]
- Anderson, H.J.; Coleman, J.E.; Andersen, R.J.; Roberge, M. Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother. Pharmacol. 1997, 39, 223–226. [Google Scholar] [CrossRef]
- Bai, R.; Durso, N.A.; Sackett, D.L.; Hamel, E. Interactions of the sponge-derived antimitotic tripeptide hemiasterlin with tubulin: Comparison with dolastatin 10 and cryptophycin 1. Biochemistry 1999, 38, 14302–14310. [Google Scholar] [CrossRef]
- Kingston, D.G. Tubulin-interactive natural products as anticancer agents. J. Nat. Prod. 2009, 72, 507–515. [Google Scholar] [CrossRef]
- La Regina, G.; Coluccia, A.; Naccarato, V.; Silvestri, R. Towards modern anticancer agents that interact with tubulin. Eur. J. Pharm. Sci. 2019, 131, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Fitt, W.K.; Pardy, R.L.; Luttker, M.M. Photosynthesis, respiration, and contribution to community productivity of the symbiotic sea anemone Anthopleura elegantissima. J. Exp. Mar. Biol. Ecol. 1982, 61, 213–232. [Google Scholar] [CrossRef]
- Williams, R.B. Acrorhagi catch tentacles and sweeper tentacles: A synopsis of ‘aggression’ of actiniarian and scleractinian Cnidaria. Hydrobiologia 1991, 216, 539–545. [Google Scholar] [CrossRef]
- Wu, J.; Wu, X.; Lian, K.; Lin, B.; Guo, L.; Ding, Z. Overexpression of potassium channel ether à go-go in human osteosarcoma. Neoplasma 2012, 59, 207–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menéndez, S.T.; Villaronga, M.A.; Rodrigo, J.P.; Alvarez-Teijeiro, S.; García-Carracedo, D.; Urdinguio, R.G.; Fraga, M.F.; Pardo, L.A.; Viloria, C.G.; Suárez, C.; et al. Frequent aberrant expression of the human ether à go-go (hEAG1) potassium channel in head and neck cancer: Pathobiological mechanisms and clinical implications. J. Mol. Med. 2012, 90, 1173–1184. [Google Scholar] [CrossRef]
- Moreels, L.; Peigneur, S.; Galan, D.T.; De Pauw, E.; Béress, L.; Waelkens, E.; Pardo, L.A.; Quinton, L.; Tytgat, J. APETx4, a Novel Sea Anemone Toxin and a Modulator of the Cancer-Relevant Potassium Channel KV10.1. Mar. Drugs 2017, 15, 287. [Google Scholar] [CrossRef]
- Hernández-Meza, J.M.; Mares-Sámano, S.; Garduño-Juárez, R. Insights into the Molecular Inhibition of the Oncogenic Channel KV10.1 by Globular Toxins. J. Chem. Inf. Model. 2021, 61, 2328–2340. [Google Scholar] [CrossRef]
- Den Hartog, J.C.; Vennam, J. Some Actiniaria (Cnidaria: Anthozoa) from the west coast of India. Zool. Meded. 1993, 67, 601–637. [Google Scholar]
- Li, X.; Tang, Y.; Yu, F.; Sun, Y.; Huang, F.; Chen, Y.; Yang, Z.; Ding, G. Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Mar. Drugs 2018, 16, 325. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Ding, G.-F.; Huang, F.-F.; Yang, Z.-S.; Yu, F.-M.; Tang, Y.-P.; Jia, Y.-L.; Zheng, Y.-Y.; Chen, R. Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells. Mar. Drugs 2018, 16, 125. [Google Scholar] [CrossRef]
- Desrita, I.; Susetya, E.; Suriani, M.; Rahman, A. Biology and growth of Asiatic Hard Clam (Meretrix meretrix) population in Tanjung Balai, North Sumatera. IOP Conf. Ser. Earth Environ. Sci. 2019, 260, 012108. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Cheng, L.; Wei, J.; Wu, N.; Zheng, L.; Lin, X. A novel polypeptide from Meretrix meretrix Linnaeus inhibits the growth of human lung adenocarcinoma. Exp. Biol. Med. 2012, 237, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, J.; Wu, N.; Liu, M.; Wang, C.; Zhang, Y.; Wang, F.; Liu, H.; Lin, X. Mere15, a novel polypeptide from Meretrix meretrix, inhibits adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMPs. Pharm. Biol. 2013, 51, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Guo, M.; Ma, L.; Farooqi, A.A.; Wang, L.; Qiao, G.; Liu, M.; Zuo, L.; Ye, H.; Lin, X.; et al. Mere15, a novel polypeptide from Meretrix meretrix, inhibits proliferation and metastasis of human non-small cell lung cancer cells through regulating the PI3K/Akt/mTOR signaling pathway. Neoplasma 2021, 68, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xu, Q.; Wang, C.; Lin, X.; Zhang, Q.; Wu, N. A tropomyosin-like Meretrix meretrix Linnaeus polypeptide inhibits the proliferation and metastasis of glioma cells via microtubule polymerization and FAK/Akt/MMPs signaling. Int. J. Biol. Macromol. 2020, 145, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Kim, Y.-S.; Hwang, J.-W.; Lee, J.S.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum. Process Biochem. 2013, 48, 1086–1090. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Wang, H.; Liu, Y.; Chen, Y. Studies on mechanism of action of anti-cancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Zhu, J.; Huang, W.; Luo, Y.; Shi, H.; Yu, D.; Chen, L.; Song, L.; Yu, R. A Novel Peptide Derived from Arca inflata Induces Apoptosis in Colorectal Cancer Cells through Mitochondria and the p38 MAPK Pathway. Mar. Drugs 2022, 20, 110. [Google Scholar] [CrossRef]
- Strafella, P.; Ferrari, A.; Fabi, G.; Salvalaggio, V.; Punzo, E.; Cuicchi, C.; Santelli, A.; Cariani, A.; Tinti, F.; Tassetti, A.N.; et al. Anadara Kagoshimensis (Mollusca: Bivalvia: Arcidae) in Adriatic Sea: Morphological Analysis, Molecular Taxonomy, Spatial Distribution, and Prediction. Medit. Mar. Sci. 2018, 18, 443–453. [Google Scholar] [CrossRef]
- Hu, X.; Song, L.; Huang, L.; Zheng, Q.; Yu, R. Antitumor effect of a polypeptide fraction from Arca subcrenata in vitro and in vivo. Mar. Drugs 2012, 10, 2782–2794. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, W.; Luo, Y.; Ou, X.; Song, L.; Zhang, S.; He, T.; Guo, Z.; Zhu, J.; Shi, H.; et al. Arca subcrenata Polypeptides Inhibit Human Colorectal Cancer HT-29 Cells Growth via Suppression of IGF-1R/Akt/mTOR Signaling and ATP Production. Nutr. Cancer 2020, 72, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, X.; Song, L.; Zhu, J.; Yu, R. The inhibitory effect of a novel polypeptide fraction from Arca subcrenata on cancer-related inflammation in human cervical cancer HeLa cells. Sci. World J. 2014, 2014, 768938. [Google Scholar]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, T.; Ding, G.-F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Switzer-Dunlap, M.; Hadfield, M.G. Observations on development, larval growth and metamorphosis of four species of aplysiidae (gastropoda: Opisthobranchia) in laboratory culture. J. Exp. Mar. Biol. Ecol. 1977, 29, 245–261. [Google Scholar] [CrossRef]
- Maki, A.; Diwakaran, H.; Redman, B.; Al-Asfar, S.; Pettit, G.R.; Mohammad, R.M.; Al-Katib, A. The bcl-2 and p53 oncoproteins can be modulated by bryostatin 1 and dolastatins in human diffuse large cell lymphoma. Anticancer Drugs 1995, 6, 392–397. [Google Scholar] [CrossRef]
- Maki, A.; Mohammad, R.; Raza, S.; Saleh, M.; Govindaraju, K.D.; Pettit, G.R.; Al-Katib, A. Effect of dolastatin 10 on human non-Hodgkin’s lymphoma cell lines. Anticancer Drugs 1996, 7, 344–350. [Google Scholar] [CrossRef]
- Turner, T.; Jackson, W.H.; Pettit, G.R.; Wells, A.; Kraft, A.S. Treatment of human prostate cancer cells with dolastatin 10, a peptide isolated from a marine shell-less mollusc. Prostate 1998, 34, 175–181. [Google Scholar] [CrossRef]
- Ali, M.A.; Rosati, R.; Pettit, G.R.; Kalemkerian, G.P. Dolastatin 15 induces apoptosis and BCL-2 phosphorylation in small cell lung cancer cell lines. Anticancer Res. 1998, 18, 1021–1026. [Google Scholar]
- Kalemkerian, G.P.; Ou, X.; Adil, M.R.; Rosati, R.; Khoulani, M.M.; Madan, S.K.; Pettit, G.R. Activity of dolastatin 10 against small-cell lung cancer in vitro and in vivo: Induction of apoptosis and bcl-2 modification. Cancer Chemother. Pharmacol. 1999, 43, 507–515. [Google Scholar] [CrossRef]
- Sato, M.; Sagawa, M.; Nakazato, T.; Ikeda, Y.; Kizaki, M. A natural peptide, dolastatin 15, induces G2/M cell cycle arrest and apoptosis of human multiple myeloma cells. Int. J. Oncol. 2007, 30, 1453–1459. [Google Scholar] [CrossRef]
- Lopus, M. Mechanism of mitotic arrest induced by dolastatin 15 involves loss of tension across kinetochore pairs. Mol. Cell. Biochem. 2013, 382, 93–102. [Google Scholar] [CrossRef]
- Malaquias, M.A.E. Systematics, phylogeny, and natural history of Bullacta exarata (Philippi, 1849): An endemic cephalaspidean gastropod from the China Sea. J. Nat. Hist. 2010, 44, 2015–2029. [Google Scholar] [CrossRef]
- Ma, J.; Huang, F.; Lin, H.; Wang, X. Isolation and purification of a peptide from Bullacta exarata and its impaction of apoptosis on prostate cancer cell. Mar. Drugs 2013, 11, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Natsukari, Y.; Tashiro, M. Neritic squid resources and cuttlefish resources in Japan. Mar. Behav. Physiol. 1991, 18, 149–226. [Google Scholar] [CrossRef]
- Huang, F.; Jing, Y.; Ding, G.; Yang, Z. Isolation and purification of novel peptides derived from Sepia ink: Effects on apoptosis of prostate cancer cell PC-3. Mol. Med. Rep. 2017, 16, 4222–4228. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Jiang, X.-M.; Zheng, Z.-M.; Jin, C.-H. Biological characeristics of Perinereis aibuhitensis Grube and status of its utilization. Mod. Fish. Inf. 2002, 17, 33–34. [Google Scholar]
- Jiang, S.; Jia, Y.; Tang, Y.; Zheng, D.; Han, X.; Yu, F.; Chen, Y.; Huang, F.; Yang, Z.; Ding, G. Anti-Proliferation Activity of a Decapeptide from Perinereies aibuhitensis toward Human Lung Cancer H1299 Cells. Mar. Drugs 2019, 17, 122. [Google Scholar] [CrossRef]
- Liao, I.C.; Chien, Y.H. The Pacific white shrimp, Litopenaeus vannamei, in Asia: The world’s most widely cultured alien crustacean. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology, Impacts; Invading Nature—Springer Series in Invasion Ecology; Galil, B., Clark, P., Carlton, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 6, pp. 489–519. [Google Scholar]
- Liu, S.; Aweya, J.J.; Zheng, L.; Wang, F.; Zheng, Z.; Zhong, M.; Lun, J.; Zhang, Y. A Litopenaeus vannamei Hemocyanin-Derived Antimicrobial Peptide (Peptide B11) Attenuates Cancer Cells’ Proliferation. Molecules 2018, 23, 3202. [Google Scholar] [CrossRef]
- Hu, H.; Guo, L.; Overholser, J.; Wang, X. Mitochondrial VDAC1: A Potential Therapeutic Target of Inflammation-Related Diseases and Clinical Opportunities. Cells 2022, 11, 3174. [Google Scholar] [CrossRef]
- Liu, S.; Aweya, J.J.; Zheng, L.; Zheng, Z.; Huang, H.; Wang, F.; Yao, D.; Ou, T.; Zhang, Y. LvHemB1, a novel cationic antimicrobial peptide derived from the hemocyanin of Litopenaeus vannamei, induces cancer cell death by targeting mitochondrial voltage-dependent anion channel 1. Cell Biol. Toxicol. 2022, 38, 87–110. [Google Scholar] [CrossRef]
- Khafage, A.R.; Taha, S.M.; Attallah, M.A. Presence of tiger shrimp Penaeus monodon Fabricius, 1798 (Penaeidae) in the Egyptian commercial shrimp catch, Alexandria, Egypt. Egypt. J. Aquat. Res. 2019, 45, 183–187. [Google Scholar] [CrossRef]
- Lin, M.C.; Lin, S.B.; Chen, J.C.; Hui, C.F.; Chen, J.Y. Shrimp anti-lipopolysaccharide factor peptide enhances the antitumor activity of cisplatin in vitro and inhibits HeLa cells growth in nude mice. Peptides 2010, 31, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Le Vay, L.; Ut, V.N.; Walton, M. Population ecology of the mud crab Scylla paramamosain (Estampador) in an estuarine mangrove system; a mark-recapture study. Mar. Biol. 2007, 151, 1127–1135. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, F.; Chen, H.-Y.; Peng, H.; Hao, H.; Wang, K.-J. A Novel Antimicrobial Peptide Scyreprocin from Mud Crab Scylla paramamosain Showing Potent Antifungal and Anti-biofilm Activity. Front. Microbiol. 2020, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, H.-Y.; Hao, H.; Wang, K.-J. The Anticancer Activity Conferred by the Mud Crab Antimicrobial Peptide Scyreprocin through Apoptosis and Membrane Disruption. Int. J. Mol. Sci. 2022, 23, 5500. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, F.; Hu, M.; Wang, Y. Research Development on Horseshoe Crab: A 30-Year Bibliometric Analysis. Front. Mar. Sci. 2020, 7, 41. [Google Scholar] [CrossRef]
- Ouyang, G.L.; Li, Q.F.; Peng, X.X.; Liu, Q.R.; Hong, S.G. Effects of tachyplesin on proliferation and differentiation of human hepatocellular carcinoma SMMC-7721 cells. World J. Gastroenterol. 2002, 8, 1053–1058. [Google Scholar] [CrossRef]
- Li, Q.F.; Ou-Yang, G.L.; Peng, X.X.; Hong, S.G. Effects of tachyplesin on the regulation of cell cycle in human hepatocarcinoma SMMC-7721 cells. World J. Gastroenterol. 2003, 9, 454–458. [Google Scholar] [CrossRef]
- Shi, S.L.; Wang, Y.Y.; Liang, Y.; Li, Q.F. Effects of tachyplesin and n-sodium butyrate on proliferation and gene expression of human gastric adenocarcinoma cell line BGC-823. World J. Gastroenterol. 2006, 12, 1694–1698. [Google Scholar] [CrossRef]
- Ding, H.; Jin, G.; Zhang, L.; Dai, J.; Dang, J.; Han, Y. Effects of tachyplesin I on human U251 glioma stem cells. Mol. Med. Rep. 2015, 11, 2953–2958. [Google Scholar] [CrossRef]
- Li, X.; Dai, J.; Tang, Y.; Li, L.; Jin, G. Quantitative Proteomic Profiling of Tachyplesin I Targets in U251 Gliomaspheres. Mar. Drugs 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, X.; Zhang, J.; Chen, J.; Wang, Y.; Wei, T.; Ma, J.; Li, Y.; Mo, T.; He, Z.; et al. Tachyplesin induces apoptosis in non-small cell lung cancer cells and enhances the chemosensitivity of A549/DDP cells to cisplatin by activating Fas and necroptosis pathway. Chem. Biol. Drug Des. 2021, 97, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Keesing, J.K.; Liu, D. A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev. Fish. Sci. Aquac. 2016, 24, 326–341. [Google Scholar] [CrossRef]
- Wei, W.; Fan, X.M.; Jia, S.H.; Zhang, X.P.; Zhang, Z.; Zhang, X.J.; Zhang, J.X.; Zhang, Y.W. Sea Cucumber Intestinal Peptide Induces the Apoptosis of MCF-7 Cells by Inhibiting PI3K/AKT Pathway. Front. Nutr. 2021, 8, 763692. [Google Scholar] [CrossRef] [PubMed]
- Shenkar, N.; Gittenberger, A.; Lambert, G.; Rius, M.; Moreira da Rocha, R.; Swalla, B.J.; Turon, X. Ascidiacea World Database. Ciona savignyi Herdman, 1882. World Register of Marine Species. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=250292 (accessed on 17 February 2023).
- Cheng, L.; Wang, C.; Liu, H.; Wang, F.; Zheng, L.; Zhao, J.; Chu, E.; Lin, X. A novel polypeptide extracted from Ciona savignyi induces apoptosis through a mitochondrial-mediated pathway in human colorectal carcinoma cells. Clin. Color. Cancer 2012, 11, 207–214. [Google Scholar] [CrossRef]
- Liu, G.; Liu, M.; Wei, J.; Huang, H.; Zhang, Y.; Zhao, J.; Xiao, L.; Wu, N.; Zheng, L.; Lin, X. CS5931, a novel polypeptide in Ciona savignyi, represses angiogenesis via inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs). Mar. Drugs 2014, 12, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Rigby, C.L.; Dulvy, N.K.; Derrick, D.; Dyldin, Y.V.; Herman, K.; Ishihara, H.; Jeong, C.H.; Semba, Y.; Tanaka, S.; Volvenko, I.V.; et al. Okamejei kenojei. The IUCN Red List of Threatened Species. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-1.RLTS.T161645A124520681.en (accessed on 5 November 2023).
- Pan, X.; Zhao, Y.-Q.; Hu, F.-Y.; Chi, C.-F.; Wang, B. Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sadovy, Y. Gonad development during sexual differentiation in hatchery-produced orangespotted grouper (Epinephelus coioides) and humpback grouper (Cromileptes altivelis) (Pisces: Serranidae, Epinephelinae). Aquaculture 2009, 287, 191–202. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Lee, M.F.; Chen, C.Y.; Chang, C.F. Development of gonadal tissue and aromatase function in the protogynous, orange-spotted grouper Epinephelus coioides. Zool. Stud. 2011, 50, 693–704. [Google Scholar]
- Lin, W.J.; Chien, Y.L.; Pan, C.Y.; Lin, T.L.; Chen, J.Y.; Chiu, S.J.; Hui, C.F. Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides 2009, 30, 283–290. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, W.J.; Wu, J.L.; Her, G.M.; Hui, C.F. Epinecidin-1 peptide induces apoptosis which enhances antitumor effects in human leukemia U937 cells. Peptides 2009, 30, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Su, B.C.; Wu, T.H.; Hsu, C.H.; Chen, J.Y. Distribution of positively charged amino acid residues in antimicrobial peptide epinecidin-1 is crucial for in vitro glioblastoma cytotoxicity and its underlying mechanisms. Chem. Biol. Interact. 2020, 315, 108904. [Google Scholar] [CrossRef] [PubMed]
- Su, B.C.; Li, C.C.; Horng, J.L.; Chen, J.Y. Calcium-Dependent Calpain Activation-Mediated Mitochondrial Dysfunction and Oxidative Stress Are Required for Cytotoxicity of Epinecidin-1 in Human Synovial Sarcoma SW982 Cells. Int. J. Mol. Sci. 2020, 21, 2109. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, F.S.B. Descriptions of major farmed aquatic animal species. In Aquaculture Pathophysiology Volume I. Finfish Diseases; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.-M., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 1, pp. 1–44. [Google Scholar]
- Ting, C.H.; Liu, Y.-C.; Lyu, P.-C.; Chen, J.-Y. Nile Tilapia Derived Antimicrobial Peptide TP4 Exerts Antineoplastic Activity Through Microtubule Disruption. Mar. Drugs 2018, 16, 462. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J.; Silphaduang, U.; Park, N.G.; Seo, J.K.; Stephenson, J.; Kozlowicz, S. Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 152, 299–305. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Bryan, L.; Silphadaung, U.; Noga, E.; Wade, D.; Rollins-Smith, L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 2004, 323, 268–275. [Google Scholar] [CrossRef]
- Ting, C.H.; Chen, Y.C.; Wu, C.J.; Chen, J.Y. Targeting FOSB with a cationic antimicrobial peptide, TP4, for treatment of triple-negative breast cancer. Oncotarget 2016, 7, 40329–40347. [Google Scholar] [CrossRef]
- Ting, C.-H.; Chen, J.-Y. Nile Tilapia Derived TP4 Shows Broad Cytotoxicity toward to Non-Small-Cell Lung Cancer Cells. Mar. Drugs 2018, 16, 506. [Google Scholar] [CrossRef]
- Ting, C.-H.; Lee, K.-Y.; Wu, S.-M.; Feng, P.-H.; Chan, Y.-F.; Chen, Y.-C.; Chen, J.-Y. FOSB–PCDHB13 Axis Disrupts the Microtubule Network in Non-Small Cell Lung Cancer. Cancers 2019, 11, 107. [Google Scholar] [CrossRef]
- Su, B.-C.; Pan, C.-Y.; Chen, J.-Y. Antimicrobial Peptide TP4 Induces ROS-Mediated Necrosis by Triggering Mitochondrial Dysfunction in Wild-Type and Mutant p53 Glioblastoma Cells. Cancers 2019, 11, 171. [Google Scholar] [CrossRef]
- Su, B.-C.; Liu, Y.-C.; Ting, C.-H.; Lyu, P.-C.; Chen, J.-Y. Antimicrobial Peptide TP4 Targets Mitochondrial Adenine Nucleotide Translocator 2. Mar. Drugs 2020, 18, 417. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-C.; Hung, G.-Y.; Tu, Y.-C.; Yeh, W.-C.; Lin, M.-C.; Chen, J.-Y. Marine Antimicrobial Peptide TP4 Exerts Anticancer Effects on Human Synovial Sarcoma Cells via Calcium Overload, Reactive Oxygen Species Production and Mitochondrial Hyperpolarization. Mar. Drugs 2021, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Huang, T.C.; Muthusamy, S.; Lee, J.F.; Duann, Y.F.; Lin, C.H. Piscidin-1, an antimicrobial peptide from fish (hybrid striped bass Morone saxatilis x M. chrysops), induces apoptotic and necrotic activity in HT1080 cells. Zoolog. Sci. 2012, 29, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Pan, C.Y.; Chen, N.F.; Yang, S.N.; Hsieh, S.; Wen, Z.H.; Chen, W.F.; Wang, J.W.; Lu, W.H.; Kuo, H.M. Piscidin-1 Induces Apoptosis via Mitochondrial Reactive Oxygen Species-Regulated Mitochondrial Dysfunction in Human Osteosarcoma Cells. Sci. Rep. 2020, 10, 5045. [Google Scholar] [CrossRef] [PubMed]
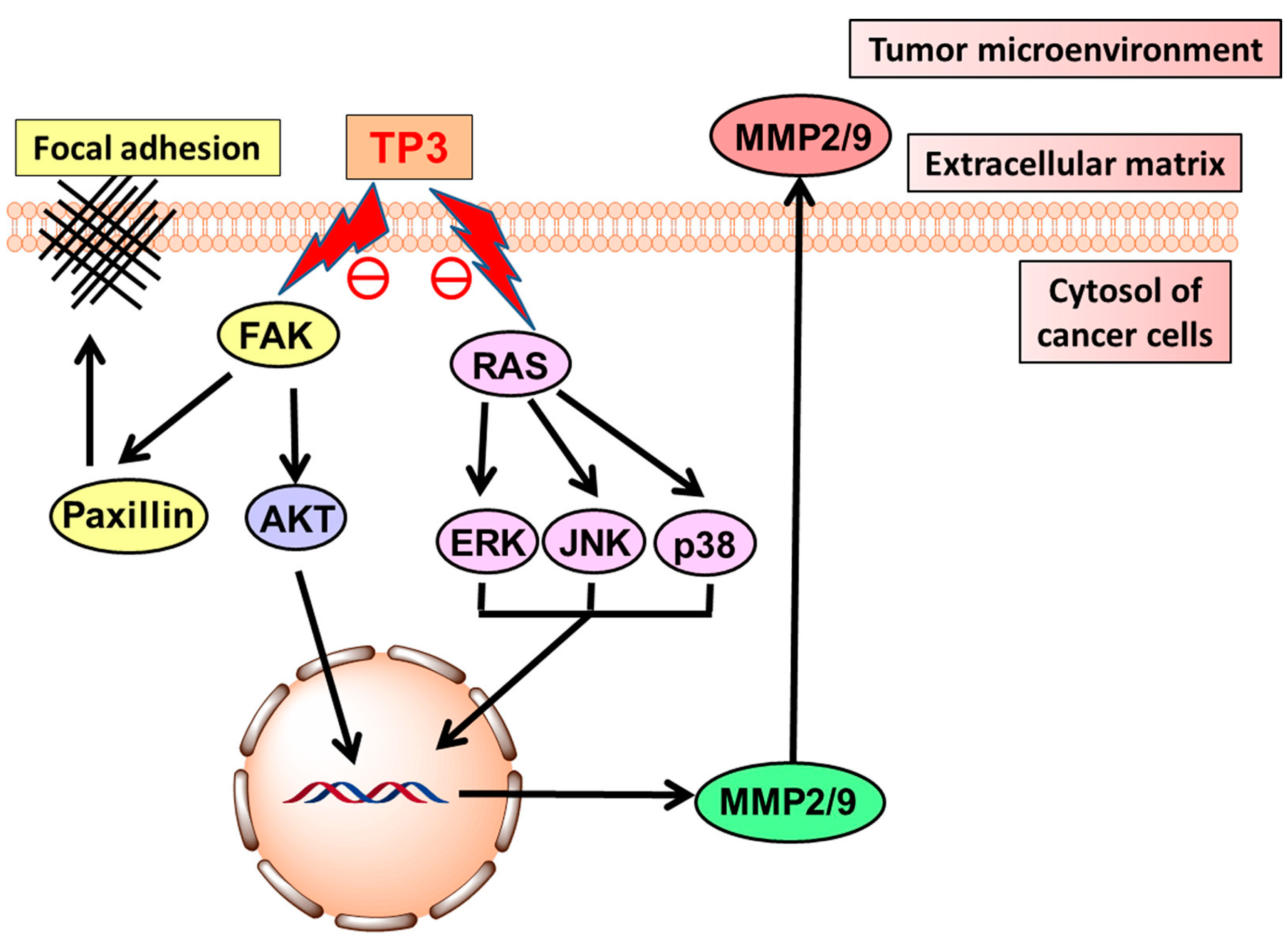
- Chen, Y.F.; Shih, P.C.; Kuo, H.M.; Yang, S.N.; Lin, Y.Y.; Chen, W.F.; Tzou, S.J.; Liu, H.T.; Chen, N.F. TP3, an antimicrobial peptide, inhibits infiltration and motility of glioblastoma cells via modulating the tumor microenvironment. Cancer Med. 2020, 9, 3918–3931. [Google Scholar] [CrossRef]
- Yuan, C.H.; Ma, Y.L.; Shih, P.C.; Chen, C.T.; Cheng, S.Y.; Pan, C.Y.; Jean, Y.H.; Chu, Y.M.; Lin, S.C.; Lai, Y.C.; et al. The antimicrobial peptide tilapia piscidin 3 induces mitochondria-modulated intrinsic apoptosis of osteosarcoma cells. Biochem. Pharmacol. 2020, 178, 114064. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Thuesen, P.A.; Thomson, F.E. A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev. Fish Biol. Fish. 2012, 22, 533–554. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, W.J.; Lin, T.L. A fish antimicrobial peptide, tilapia hepcidin TH2-3, shows potent antitumor activity against human fibrosarcoma cells. Peptides 2009, 30, 1636–1642. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.; Chen, J.Y. Pardaxin-induced apoptosis enhances antitumor activity in HeLa cells. Peptides 2011, 32, 1110–1116. [Google Scholar] [CrossRef]
- Vad, B.S.; Bertelsen, K.; Johansen, C.H.; Pedersen, J.M.; Skrydstrup, T.; Nielsen, N.C.; Otzen, D.E. Pardaxin permeabilizes vesicles more efficiently by pore formation than by disruption. Biophys. J. 2010, 98, 576–585. [Google Scholar] [CrossRef]
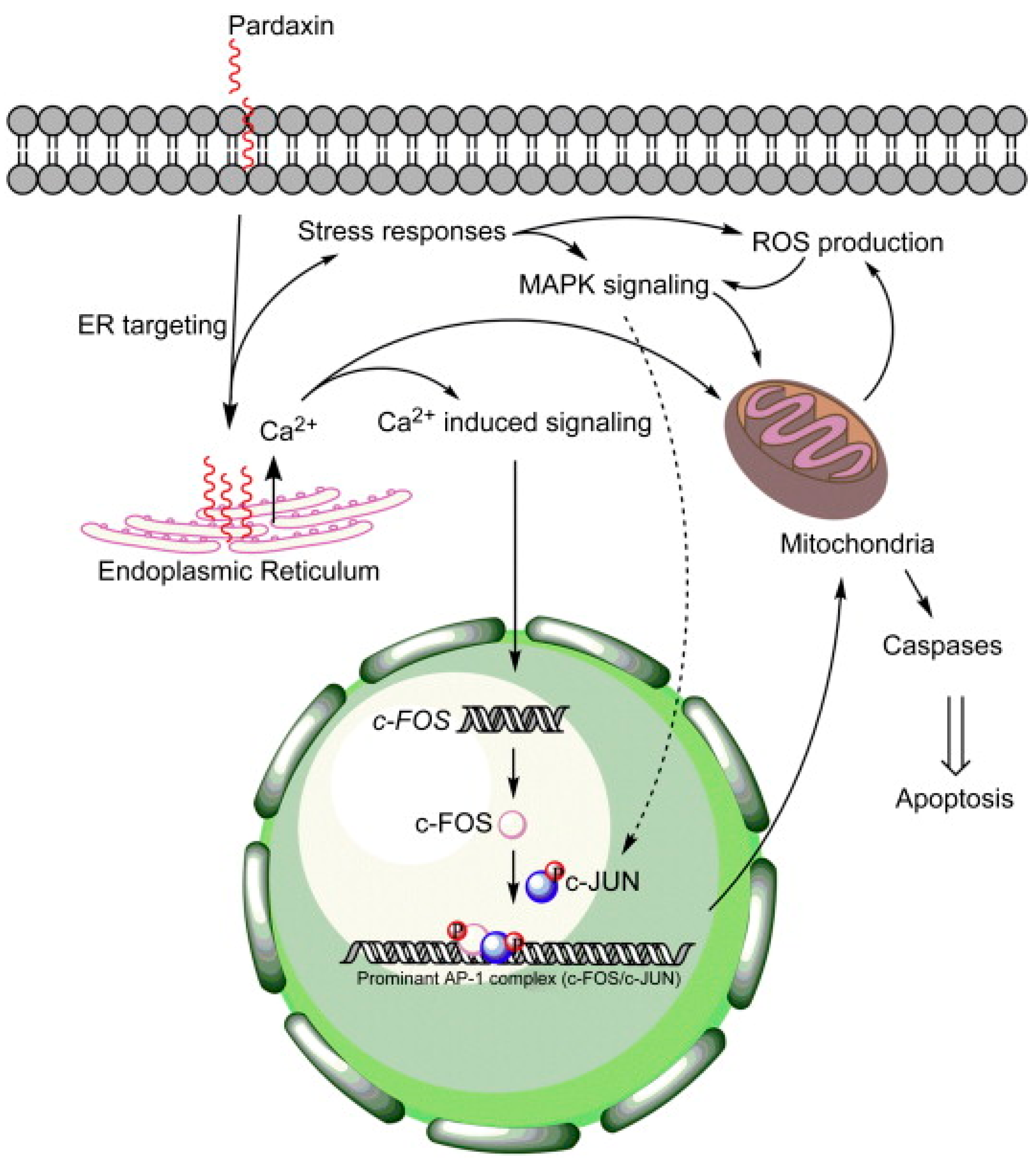
- Huang, T.C.; Chen, J.Y. Proteomic analysis reveals that pardaxin triggers apoptotic signaling pathways in human cervical carcinoma HeLa cells: Cross talk among the UPR, c-Jun and ROS. Carcinogenesis 2013, 34, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.H.; Huang, H.N.; Huang, T.C.; Wu, C.J.; Chen, J.Y. The mechanisms by which pardaxin, a natural cationic antimicrobial peptide, targets the endoplasmic reticulum and induces c-FOS. Biomaterials 2014, 35, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Shih, P.-C.; Feng, C.-W.; Wu, C.-C.; Tsui, K.-H.; Lin, Y.-H.; Kuo, H.-M.; Wen, Z.-H. Pardaxin Activates Excessive Mitophagy and Mitochondria-Mediated Apoptosis in Human Ovarian Cancer by Inducing Reactive Oxygen Species. Antioxidants 2021, 10, 1883. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Doucette, C.D.; Pinto, D.M.; Patrzykat, A.; Douglas, S.; Hoskin, D.W. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 2011, 13, R102. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Conrad, D.M.; Coombs, M.R.; Zemlak, T.; Doucette, C.D.; Liwski, R.S.; Hoskin, D.W. Pleurocidin-family cationic antimicrobial peptides mediate lysis of multiple myeloma cells and impair the growth of multiple myeloma xenografts. Leuk. Lymphoma 2013, 54, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
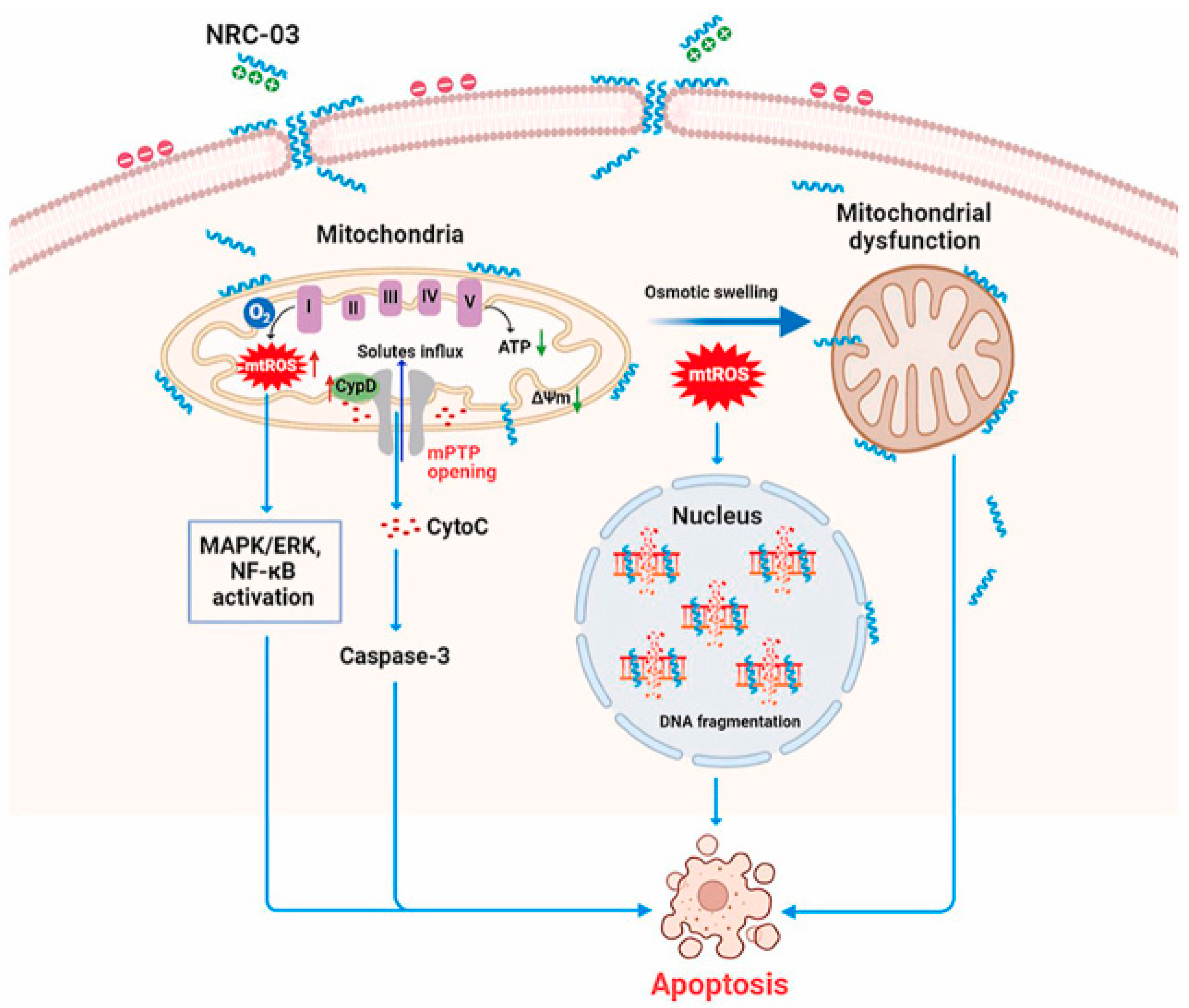
- Hou, D.; Hu, F.; Mao, Y.; Yan, L.; Zhang, Y.; Zheng, Z.; Wu, A.; Forouzanfar, T.; Pathak, J.L.; Wu, G. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022, 54, 102355. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Karami Fath, M.; Babakhaniyan, K.; Zokaei, M.; Yaghoubian, A.; Akbari, S.; Khorsandi, M.; Soofi, A.; Nabi-Afjadi, M.; Zalpoor, H.; Jalalifar, F.; et al. Anti-cancer peptide-based therapeutic strategies in solid tumors. Cell. Mol. Biol. Lett. 2022, 27, 33. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Eghtedari, M.; Jafari Porzani, S.; Nowruzi, B. Anticancer potential of natural peptides from terrestrial and marine environments: A review. Phytochem. Lett. 2021, 42, 87–103. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]



























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Librizzi, M.; Martino, C.; Mauro, M.; Abruscato, G.; Arizza, V.; Vazzana, M.; Luparello, C. Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems. Cancers 2024, 16, 36. https://doi.org/10.3390/cancers16010036
Librizzi M, Martino C, Mauro M, Abruscato G, Arizza V, Vazzana M, Luparello C. Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems. Cancers. 2024; 16(1):36. https://doi.org/10.3390/cancers16010036
Chicago/Turabian StyleLibrizzi, Mariangela, Chiara Martino, Manuela Mauro, Giulia Abruscato, Vincenzo Arizza, Mirella Vazzana, and Claudio Luparello. 2024. "Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems" Cancers 16, no. 1: 36. https://doi.org/10.3390/cancers16010036
APA StyleLibrizzi, M., Martino, C., Mauro, M., Abruscato, G., Arizza, V., Vazzana, M., & Luparello, C. (2024). Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems. Cancers, 16(1), 36. https://doi.org/10.3390/cancers16010036





