Simple Summary
This research aims to investigate the effectiveness of combining Atezolizumab and Bevacizumab as a potential treatment for advanced liver cancer (hepatocellular carcinoma or HCC). The authors reviewed multiple studies and clinical trials to assess the impact of this combination therapy on patient survival and disease progression. While the results show promising benefits in terms of increased overall survival for HCC patients, the treatment also carries significant side effects. Additionally, there is a lack of consensus on specific biomarkers to predict treatment outcomes. This study highlights the need for personalized treatment approaches and further research to optimize the management of this deadly disease.
Abstract
Liver cancer, particularly hepatocellular carcinoma, is a global concern. This study focuses on the evaluation of Atezolizumab and Bevacizumab combination therapy as a promising alternative in the treatment of advanced hepatocellular carcinoma. The objectives of this systematic review include evaluating the efficacy of Atezolizumab and Bevacizumab combination therapy compared to conventional therapies with Sorafenib and other conventional therapies, analyzing the associated adverse effects, and exploring prognostic factors in the setting of advanced hepatocellular carcinoma. A systematic literature review was carried out using the PubMed and Web of Science databases. Fifteen related articles were included and evaluated according to their level of evidence and recommendation. Results: The combination therapy of Atezolizumab and Bevacizumab, along with Sorafenib, showed positive results in the treatment of patients with advanced hepatocellular carcinoma. Significant adverse effects were identified, such as gastrointestinal bleeding, arterial hypertension, and proteinuria, which require careful attention. In addition, prognostic factors, such as transforming growth factor beta (TGF-β), alpha-fetoprotein (AFP), and vascular invasion, were highlighted as key indicators of hepatocellular carcinoma progression. Conclusions: The combination of Atezolizumab and Bevacizumab is shown to be effective in the treatment of advanced hepatocellular carcinoma, although it is essential to take into consideration the associated adverse effects. The prognostic factors identified may provide valuable information for the clinical management of this disease. This study provides a comprehensive overview of a promising emerging therapy for liver cancer.
1. Introduction
Hepatocellular carcinoma (HCC) is the most prevalent primary neoplasm affecting the liver, emerging as a preeminent cause of mortality, particularly in patients with liver cirrhosis. The incidence of this cancer varies according to geographic location and risk factors, with a higher frequency in males [1,2]. Globally, HCC ranks sixth in terms of prevalence among neoplasms and is the third-leading cause of death from oncologic diseases. The age of HCC onset and survival rates are subject to regional variations. In nations such as Taiwan and Japan, where it tends to be diagnosed later in life, there is longer survival due to early detection. In contrast, in areas such as Korea, China, North America, and Europe, most cases are identified in intermediate or advanced stages of the disease [3].
In Spain, in 2019, a total of 6499 cases of liver cancer were reported [2,4,5], being more prevalent in men. Risk factors include liver inflammation, which can lead to cirrhosis, as well as the presence of diabetes, obesity, dyslipidemia, and excessive alcohol consumption. HCC is associated with viral infections, such as hepatitis B virus (HBV) and hepatitis C virus (HCV), in addition to alcoholic cirrhosis and nonalcoholic fatty liver disease (NAFLD), with diabetes and obesity acting as contributing factors [3,6]. Additional risk factors include male gender, advanced age (greater than 65 years), the presence of cirrhosis, chronic alcohol abuse, genotype 3, diabetes, metabolic syndrome, low albumin/platelet levels, and elevated alpha-fetoprotein (AFP) levels. In contrast, protective factors include HBV vaccination, antiviral therapy in the setting of chronic hepatitis, abstention from coffee and alcohol consumption in chronic liver conditions, and the adoption of a healthy lifestyle. In the context of chronic liver conditions and the risk of HCC, adopting a healthy lifestyle involves practices such as maintaining a balanced diet, engaging in regular physical activity, abstaining from excessive alcohol consumption, and avoiding harmful habits like smoking. These lifestyle choices are considered protective factors that may contribute to reducing the likelihood of developing liver cancer, complementing medical interventions and antiviral therapies for chronic liver diseases [5,7,8].
Primary prevention focuses on the reduction of HCC in low-income countries through measures to prevent hepatitis B virus (HBV) transmission, the sterilization of surgical instruments, and the quality control of blood products. Secondary prevention focuses on early detection through ultrasound scans performed every 6 months in patients considered to be at high risk, including those with cirrhosis and chronic hepatitis B. Early diagnosis is of critical importance and is based on both radiological screening techniques, such as abdominal ultrasound, and serological screening, including measurement of AFP. The combination of both strategies proves to be more effective [4,5].
The diagnosis is based on specific radiological features evaluated by computed tomography (CT) and magnetic resonance imaging (MRI) [9]. In patients with cirrhosis, the diagnosis is considered without resorting to a liver biopsy. For nodules less than 1 cm in diameter, follow-up every 3–4 months is recommended. For nodules 1–3 cm, the diagnosis can be established without pathological confirmation in patients with cirrhosis or chronic hepatitis B. However, for other cases, follow-up every 3–4 months and further pathological confirmation are recommended [5,10,11,12,13].
The therapeutic approach to HCC encompasses various modalities, including surgical resection, ablation, radiotherapy, immunotherapy, liver transplantation, chemotherapy, and targeted therapy. However, recurrence is a common event, particularly after resection or ablation. Liver resection is characterized by its high cure rate, although recurrence persists as a relevant complication. As for liver transplantation, it represents a definitive option if there are no metastases present. Local ablation, through radiofrequency, is effective, especially in the treatment of small tumors.
In the context of systemic treatment, Sorafenib and Lenvatinib are agents used in the first line of therapy, while Regorafenib and other agents are reserved for the second line. The choice of treatment is based on patient- and tumor-specific characteristics [5,14,15,16]. Transforming growth factor beta (TGF-β) plays a critical role in biological processes and the progression of HCC. Alterations in it signaling pathway can drive tumor progression. Mutations in SMAD and TGF-β receptor genes have been identified in several types of cancer, supporting their suppressive role. Their influence on immune responses varies depending on the context. In summary, HCC manifests as a complex disease that involves multiple risk factors and shows remarkable geographic variations in its incidence and survival [17,18,19,20].
The combination of Atezolizumab and Bevacizumab represents a therapeutic approach for advanced hepatocellular carcinoma (HCC), operating through distinct mechanisms: Atezolizumab serves as an immune checkpoint inhibitor, targeting proteins known as immune checkpoints, which regulate the immune response. Atezolizumab exerts its action by antagonizing the PD-1 receptor expressed on T cells and the PD-L1 protein found on tumor cells. This antagonism results in the disruption of an inhibitory interaction, enabling T cells to mount a more robust assault against tumor cells. Conversely, Bevacizumab is a monoclonal antibody that selectively targets the vascular endothelial growth factor (VEGF), a pivotal protein orchestrating angiogenesis—the formation of new blood vessels. By obstructing VEGF, Bevacizumab effectively curtails the growth of blood vessels that nourish tumors. The confluence of these two distinct mechanisms engenders a synergistic therapeutic effect that surpasses the efficacy of each drug in isolation. More specifically, Atezolizumab enhances the capability of the immune system’s T cells to identify and eliminate tumor cells. Bevacizumab, on the other hand, disrupts the tumor cells’ supply of vital nutrients and oxygen, impeding their capacity for growth [17,18,19,20,21,22,23] (Figure 1).
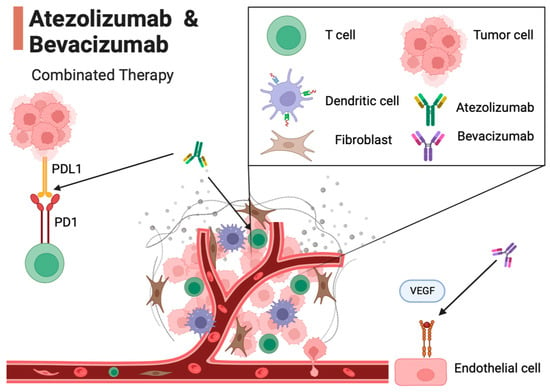
Figure 1.
Mechanism of action of Atezolizumab and Bevacizumab in the tumor microenvironment. In this figure, key interactions in the tumor microenvironment are illustrated. T cells are activated by Atezolizumab, an immune checkpoint inhibitor. Atezolizumab inhibits the interaction between the PD-1 receptor on T cells and the PD-L1 on tumor cells, enabling T cells to target tumor cells. Endothelial cells are influenced by Bevacizumab, a monoclonal antibody that targets VEGF. Bevacizumab hinders the growth of new blood vessels (angiogenesis) by blocking VEGF, preventing tumor cells from receiving the nutrients and oxygen required for growth. This combination of mechanisms of action demonstrates a synergistic effect in the battle against cancer, potentially extending the survival of patients with advanced HCC.
The main objective of this systematic review will therefore be to evaluate the influence of the use of Atezolizumab in combination with Bevacizumab compared to Sorafenib treatment in patients with hepatocellular carcinoma, as well as to analyze its adverse effects.
2. Search Methodology
This systematic review was conducted in accordance with the criteria set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [24,25]. The protocol has not been registered. The literature search was performed in PubMed, Cochrane, and Web of Science. The search strategy was carried out by combining the following MeSH terms using Boolean operators: “Transforming Growth Factor β”, “liver cancer”, “liver neoplasms”, “hepatocellular cancer”, hepatocellular carcinoma”, “Sorafenib”, “Atezolizumab”, and “Bevacizumab”. The search equation was ((((((((TGF β) OR (Transforming Growth Factor β)) AND (liver cancer)) OR (liver neoplasms)) OR (hepatocellular cancer)) OR (hepatocellular carcinoma)) AND (Sorafenib)) AND (Atezolizumab)) AND (Bevacizumab).
The initial search resulted in a total of 1101 articles. Human studies published in the last 5 years in full text in English evaluating the combination of Atezolizumab and Bevacizumab in the treatment of HCC and its adverse effects were included. The flow diagram in Figure 2 describes the screening and selection process. All studies were based on randomized controlled trials, and the quality of these articles was high, as assessed by the Joanna Briggs Institute (JBI) checklist for randomized clinical trials [26]. The bibliographic search continued with narrowed results after establishing the selection criteria for the articles of interest. Inclusion criteria were studies conducted with human subjects, published in the last 5 years in the English language, with full-text availability and with high scientific evidence. Studies conducted exclusively with animals or studies on TGF-β based solely on cancer at the general level, with no direct relation to the combination of Atezolizumab + Bevacizumab in the treatment of HCC, were considered as exclusion criteria [17].
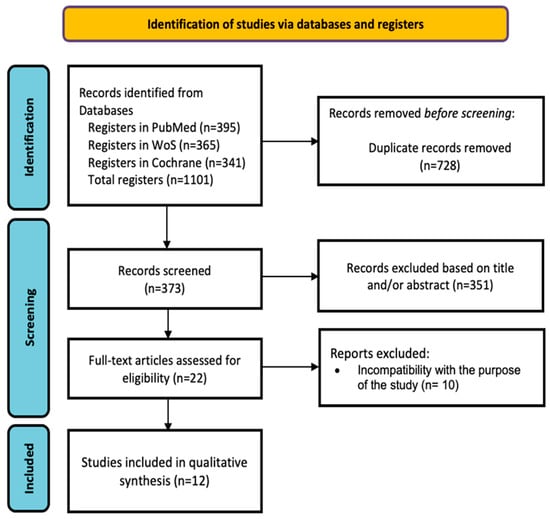
Figure 2.
PRISMA flowchart of the study selection process.
3. Results
As shown in Figure 2, 22 articles were selected for full-text review, of which 12 studies met the inclusion criteria. Table 1 shows the main characteristics of each study, and Table 2 shows the quality assessment of the studies.

Table 1.
Main characteristics of each study analyzed.

Table 2.
Studies appraised using the Joanna Briggs Institute critical appraisal checklist for randomized controlled trials.
This systematic analysis summarizes key findings from various studies evaluating the efficacy and safety of the combination of Atezolizumab and Bevacizumab in the treatment of advanced HCC. The studies reveal that this combination demonstrates a positive response in patients with advanced HCC, with higher overall survival and progression-free survival rates compared to the previous standard, Sorafenib [27,28,36]. Table 3 presents the overall survival (OS) data reported in the analyzed studies of treatment for advanced hepatocellular carcinoma. The data indicate that treatment with Atezolizumab and Bevacizumab provides a significant increase in survival compared to other treatments such as Atezolizumab alone, Sorafenib, Nivolimab, Lenvatinib, Linifanib, or Sunitinib.

Table 3.
Hazard ratio for the overall survival of the analyzed studies.
Furthermore, the importance of considering prognostic factors, such as PD-L1 expression and biomarkers like VEGF receptor 2 [34,35], for more precise patient selection is emphasized. While the efficacy of Atezolizumab and Bevacizumab is highlighted, the urgency to develop more robust biomarkers for further personalized HCC treatment is underscored [29,30,31,32]. The studies also address treatment sequencing, highlighting that this combination might be especially promising in patients with virally induced HCC [35,36,37]. Despite its benefits, the side effects, although manageable, suggest the need for close monitoring during therapy. Overall, these studies emphasize the need for a personalized approach in managing HCC [28,30,35], considering the diversity of patient subgroups and the lack of robust biomarkers in therapeutic decision-making [28,37,38]. The combination of Atezolizumab and Bevacizumab emerges as a promising option, but careful management of its adverse effects and the precise identification of suitable patients are essential to maximize the benefits and minimize the risks [37].
4. Discussion
In the present systematic review, we address key aspects related to hepatocellular carcinoma, a liver cancer of great relevance in the field of oncology. HCC manifests as the predominant type of primary hepatic neoplasm and is frequently associated with high mortality rates, especially in patients with a history of liver cirrhosis. Currently, HCC therapeutics include a variety of first- and second-line drugs.
This systematic review focuses on comprehensively investigating the results of representative studies evaluating the combination therapy of the drugs Atezolizumab and Bevacizumab as an alternative to conventional systemic treatment in patients with HCC in the adult population. In addition, the adverse effects associated with this therapy are addressed, and possible predictive biomarkers of disease progression are explored.
In the phase III IMBrave150 study, led by Cheng et al. [34], the combination therapy of Atezolizumab and Bevacizumab was compared to the conventional treatment, Sorafenib, in terms of overall survival and progression-free survival. A survival benefit was seen in patients treated with the combination of Atezolizumab and Bevacizumab, supporting the efficacy of this therapeutic approach. These results were corroborated in the work of Zhang et al. [21] regarding overall survival. In addition, Fulgenzi et al. [36] noted a significantly higher rate of a radiologically measurable response in patients treated with this combination therapy.
Consistent findings were observed in the work of Sonbol et al. [31] in comparing Sorafenib with the combination of Atezolizumab and Bevacizumab and in a comparative study by Lee et al. [29] in comparing Atezolizumab and Bevacizumab with Atezolizumab monotherapy. Finn et al. [27] conducted a global open-label phase III trial that also supported the superiority of combination therapy in terms of overall survival at 12 months, particularly in previously untreated patients. However, Pinter et al. [23] warned about the contraindication of this combination therapy in patients who had previously received an organ transplant.
Additionally, in studies involving the drug Lenvatinib, such as the analyses by Fulgenzi et al. [33] and Piñero et al. [28], superior survival was observed in patients treated with the Atezolizumab and Bevacizumab combination, followed by the Lenvatinib group. Han et al. [32] supported these results, although they noted a higher incidence of treatment interruptions due to adverse effects in combination therapy.
On the other hand, Rimini et al. [37] put forward a different perspective by suggesting a longer overall survival in patients with advanced HCC treated with Lenvatinib compared to the other two alternatives. These authors disagreed with previous studies, such as those mentioned above, which found no significant differences between patients treated with Atezolizumab and Bevacizumab and Sorafenib [34,38].
Concerning the adverse effects of Atezolizumab combined with Bevacizumab therapy for HCC, Cheng et al. [34] reported grade 3 adverse effects in 43% of patients, including intestinal bleeding and gastric ulcers. In this context, “grade 3” indicates the severity of adverse effects, with a higher grade signifying more significant complications. Specifically, a grade 3 adverse effect denotes substantial severity, as reported in 43% of patients, including instances of intestinal bleeding and gastric ulcers, according to Cheng et al. [34]. Finn et al. [27] also noted serious side effects in 38% of patients.
Han et al. [32] and Zhang et al. [21] reported the occurrence of adverse effects, although they did not provide specific details. Pinter et al. [35], in addition to gastrointestinal bleeding, reported cases of arterial hypertension and proteinuria, adverse effects that were also observed in the multicenter study by Lee et al. [29] and in the study of patients with HCC of viral etiology by Fulgenzi et al. [36].
As HCC is among the deadliest cancers worldwide, the identification of predictive biomarkers is of utmost importance. Pinter et al. [35] suggested TGF-β signaling as a biomarker, proposing that less-altered levels of this cytokine are associated with a better prognosis. However, these authors disagreed regarding PD-L1 expression and tumor burden as predictive factors. Zhang et al. [21] also mentioned TGF-β, correlating it with decreased survival, although they differed regarding PD-L1 expression, correlating it with accelerated HCC progression.
Cheng et al. [34] associated elevated VEGF levels with a greater benefit from therapy, a finding that was also proposed by Pinter et al. [35]. Piñero et al. [28] suggested the absence of extrahepatic pathology and viral etiology of hepatitis C as predictors of longer survival to treatment, whereas the presence of elevated AFP levels and vascular invasion at the macroscopic level were considered poor prognostic factors. On the other hand, Da Fonseca et al. [31] proposed age, cirrhosis, and portal hypertension as influential factors in the prognosis of HCC treatment.
In summary, this review addresses various aspects related to the treatment of HCC using the combination of the drugs Atezolizumab and Bevacizumab. It highlights the significant benefits in terms of survival and response rates observed in several studies, supporting the efficacy of this combination therapy in advanced HCC [37]. In addition, associated adverse effects and potential predictive biomarkers that may influence the prognosis and response to HCC treatment have been explored [38]. These findings offer valuable information for clinical decision-making in the management of this highly lethal disease.
Study Limitations
Despite efforts to comprehensively evaluate the effectiveness and adverse effects of the combination of the drugs Atezolizumab and Bevacizumab as an alternative to conventional systemic therapy in the treatment of HCC, it is essential to recognize certain limitations that affect the interpretation of the results and the generalizability of the conclusions.
First, we must point out that most of the studies included in this review were based on data from clinical trials and observational studies, which could introduce selection bias and potentially limit the representativeness of the HCC patient population. In addition, the variability in the design methodology of the selected studies, as well as differences in the patient populations, could influence the quality of the evidence and the ability to synthesize the results in a homogeneous manner.
Second, most of the included clinical trials and observational studies had short-term follow-ups in relation to the chronic and evolving nature of HCC. This limitation could influence the ability to assess long-term survival, duration of the treatment response, and the potential occurrence of late adverse effects fully and accurately. In addition, it is necessary to recognize that the assessment of adverse effects is based on the information available in the included studies, and the reporting of these events may be subject to reporting biases. Therefore, it is essential to consider the possibility of underestimation or overestimation of the frequency and intensity of adverse effects associated with Atezolizumab and Bevacizumab therapy [32].
Finally, although a comprehensive effort has been made to identify and analyze the adverse effects associated with this combination therapy and to investigate the prognostic factors that influence the treatment of advanced HCC, the heterogeneity of the data and the lack of standardization in the presentation of the results could limit the ability to perform a robust quantitative analysis.
5. Conclusions
The study results suggest a high overall survival rate and superior benefits in patients with advanced HCC who are treated with the combination of Atezolizumab and Bevacizumab compared to conventional therapies. These findings support the efficacy of this combination therapy as a promising alternative in the treatment of this disease. Notwithstanding the successful results observed in terms of survival, it is essential to highlight that several authors reported the appearance of serious adverse effects in a significant percentage of the patients treated with Atezolizumab and Bevacizumab. These adverse effects included gastrointestinal bleeding, arterial hypertension, proteinuria, and gastric ulcers, which pose challenges in terms of the tolerability and safety of this combination therapy.
There is a lack of consensus and clarity regarding the predictive biomarkers of HCC. However, some authors have suggested that elevated levels of TGF-β, AFP, and vascular invasion are associated with an unfavorable prognosis for survival. On the other hand, it has been proposed that elevated VEGF levels, the absence of extrahepatic pathology, and the viral etiology of hepatitis C may predict longer survival in response to treatment. In addition, age, the presence of cirrhosis, and hypertension are mentioned as factors that may influence the prognosis of HCC treatment.
Despite the observed efficacy in terms of survival in patients with advanced HCC treated with Atezolizumab and Bevacizumab, the occurrence of serious adverse effects and the lack of definitive biomarkers must be considered. These results emphasize the need for an individualized approach in clinical decision-making and highlight the importance of future research to address the challenges and optimize the management of this disease.
Author Contributions
Conceptualization, I.V. and L.S.; methodology, M.P.-B., M.E.L.-G. and M.T.M.-L.; validation, I.V. and M.P.-B.; investigation, L.S.; writing—original draft preparation, I.V., L.S., M.P.-B., M.E.L.-G. and M.T.M.-L.; writing—review and editing, M.P.-B., M.E.L.-G. and M.T.M.-L.; supervision, I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Catholic University of Valencia San Vicente Mártir for their contribution and help in the payment of the Open Access publication fee.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Molinuevo, A.; Salmerón, D.; Marcos-Gragera, R.; Carulla, M.; Chirlaque, M.D.; Rodríguez Camblor, M.; Alemán, A.; Rojas, D.; Vizcaíno Batllés, A.; et al. Cancer survival in adults in Spain: A population-based study of the spanish network of cancer registries (REDECAN). Cancers 2022, 14, 2441. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M.; Burgos Peláez, R.; Rivera Irigoin, R. Guía Práctica ESPEN: Nutrición clínica en las enfermedades del hígado [ESPEN Practical Guideline: Clinical nutrition in liver disease]. Nutr. Hosp. 2022, 39, 434–472. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Heckley, G.A.; Jarl, J.; Asamoah, B.O.; G-Gerdtham, U. How the risk of liver cancer changes after alcohol cessation: A review and meta-analysis of the current 59 literature. BMC Cancer 2011, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Ávila, M.A.; Ayuso, C.; Mínguez, B.; Varela, M.; Bilbao, I.; Bilba, J.I.; Burrel, M.; Bustamante, J.; et al. Diagnosis and treatment of hepatocellular carcinoma. Update of the consensus document of the AEEH, AEC, SEOM, SERAM, SERVEI, and SETH. Med. Clin. 2021, 156, 463.e1–463.e30, (In Spanish with English abstract). [Google Scholar] [CrossRef]
- Miyata, T.; Nagy, L.E. Programmed cell death in alcohol-associated liver disease. Clin. Mol. Hepatol. 2020, 26, 618–625. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Bianchi, L.; Rodríguez-Lope, C.; Reig, M.; García-Criado, M.A.; Rimola, J.; Solé, M.; Ayuso, C.; Bru, C.; et al. Lack of arterial hypervascularity at contrast-enhanced ultrasound should not define the priority for diagnostic work-up of nodules. J. Hepatol. 2015, 62, 150–155. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Regimbeau, J.M.; Colombat, M.; Mognol, P.; Durand, F.; Abdalla, E.; Degott, C.; Degos, F.; Farges, O.; Belghiti, J. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004, 10, S69–S70. [Google Scholar] [CrossRef]
- Bruix, J.; da Fonseca, L.G.; Reig, M. Insights into the success and failure of sys- temic therapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Saborowski, A. Current strategies for the treatment of intermediate and advanced hepatocelular carcinoma. Cancer Treat. Rev. 2020, 82, 101946. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.L.; Yau, T.; Furuse, J.; Won, P.J.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumabtreated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Gao, Y.; Shen, F. Identification of subtypes of hepatocellular carcinoma and screening of prognostic molecular diagnostic markers based on cell adhesion molecule related genes. Genet. Front. 2022, 13, 1042540. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ signalling in contex. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Metelli, A.; Salem, M.; Wallace, C.H.; Wu, B.X.; Li, A.; Li, X.; Li, Z. Immunoregulatory functions and the therapeutic implications of GARP-TGF-β in inflammation and cancer. J. Hematol. Oncol. 2018, 11, 24. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Yamazaki, K.; Masugi, Y.; Sakamoto, M. Molecular pathogenesis of hepatocellular carcinoma: Altering transforming growth factor-β signaling in hepatocarcinogenesis. Dig. Dis. 2011, 29, 284–288. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Li, H.Y.; Wang, Z.H.; Wu, J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188441. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Jain, R.K.; Duda, D.G. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: A Review. JAMA Oncol. 2021, 7, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Piñero, F.; Silva, M.; Iavarone, M. Sequencing of systemic treatment for hepatocellular carcinoma: Second line competitors. World J. Gastroenterol. 2020, 26, 1888–1900. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Riaz, I.B.; Naqvi, S.A.A.; Almquist, D.R.; Mina, S.; Almasri, J.; Shah, S.; Almader-Douglas, D.; Uson Junior, P.L.S.; Mahipal, A.; et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2020, 6, e204930. [Google Scholar] [CrossRef]
- Da Fonseca, L.G. Trial eligibility in advanced hepatocellular carcinoma: Does it support clinical practice in underrepresented subgroups? World J. Gastroenterol. 2021, 27, 3429–3439. [Google Scholar] [CrossRef]
- Han, Y.; Zhi, W.H.; Xu, F.; Zhang, C.B.; Huang, X.Q.; Luo, J.F. Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. World J. Gastroenterol. 2021, 27, 2415–2433. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, C.A.M.; Talbot, T.; Murray, S.M.; Silletta, M.; Vincenzi, B.; Cortellini, A.; Pinato, D.J. Immunotherapy in hepatocellular carcinoma. Curr. Treat. Options Oncol. 2021, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Scheiner, B.; Peck-Radosavljevic, M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups. Gut 2021, 70, 204–214. [Google Scholar] [CrossRef]
- Fulgenzi, C.A.M.; Cheon, J.; D’Alessio, A.; Nishida, N.; Ang, C.; Marron, T.U.; Wu, L.; Saeed, A.; Wietharn, B.; Cammarota, A.; et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: Results of the AB-real study. Eur. J. Cancer 2022, 175, 204–213. [Google Scholar] [CrossRef]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Fanizzi, A.; Massafra, R.; De Luca, R.; Brandi, G. Immune-based combinations versus Sorafenib as first-line treatment for advanced hepatocellular carcinoma: A Meta-Analysis. Curr. Oncol. 2023, 30, 749–757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).