Simple Summary
There are limited treatment options beyond chemotherapy for patients with hormone receptor positive metastatic breast cancer after progression on first line therapy with CDK4/6 inhibitors and endocrine therapy. Recently, encouraging evidence has emerged from multiple drugs in this space with the potential to delay chemotherapy and improve outcomes. The most promising agents include the AKT inhibitor capivasertib, the oral selective estrogen receptor degrader (SERD) elacestrant, and PARP inhibitors for patients harboring pathogenic germline BRCA1/2 mutations. Additionally, a subset of patients may also potentially be candidates for continuation of CDK4/6 inhibitors beyond progression. In this review, we highlight clinical data supporting the use of these agents and critically analyze the available evidence for their use. We also provide an algorithm to guide clinicians in their daily practice for patients with progression following first line CDK4/6 inhibitors and endocrine therapy.
Abstract
The rise of cyclin-dependent kinase (CDK)4/6 inhibitors has rapidly reshaped treatment algorithms for hormone receptor (HR)-positive metastatic breast cancer, with endocrine treatment (ET) plus a CDK4/6-inhibitor currently representing the standard of care in the first line setting. However, treatment selection for those patients experiencing progression while on ET + CDK4/6-inhibitors remains challenging due to the suboptimal activity or significant toxicities of the currently available options. There is also a paucity of data regarding the efficacy of older regimens, such as everolimus + exemestane, post-CDK4/6 inhibition. In this setting of high unmet need, several clinical trials of novel drugs have recently reported encouraging results: the addition of the AKT-inhibitor capivasertib to fulvestrant demonstrated a significant improvement in progression-free survival (PFS); the oral selective estrogen receptor degrader (SERD) elacestrant prolonged PFS compared to traditional ET in a phase 3 trial, particularly among patients with detectable ESR1 mutations; finally, PARP inhibitors are available treatment options for patients with pathogenic BRCA1/2 germline mutations. Overall, a plethora of novel endocrine and biologic treatment options are finally filling the gap between first-line ET and later line chemotherapy. In this review article, we recapitulate the activity of these novel treatment options and their potential role in future treatment algorithms.
1. Introduction
Breast cancer is the most commonly diagnosed cancer in women worldwide and represents the second most common cause of cancer related death in women in the United States, with approximately 43,600 deaths reported in 2021 [1,2]. Despite significant advancements in cancer treatments, patients with metastatic breast cancer (MBC) remain incurable, with a median overall survival (OS) of approximately five years with CDK4/6 inhibitor based therapy [2,3]. About 70% of all breast cancers express the estrogen receptor (ER), the progesterone receptor (PR), or both [4]. These tumors are often sensitive to hormonal manipulation with various drugs including selective estrogen receptor modulators (SERMs) like tamoxifen, aromatase inhibitors (AI) like letrozole, anastrozole and exemestane and selective estrogen receptor degraders (SERDs) like fulvestrant.
Since the first report of efficacy of the cyclin dependent kinase 4/6 (CDK4/6) inhibitor palbociclib in hormone receptor (HR)-positive advanced breast cancer (ABC) [5], multiple large randomized phase three trials have established the efficacy of CDK4/6 inhibitors (palbociclib, ribociclib and abemaciclib) in the first-line setting in both pre-menopausal and post-menopausal women in combination with ET [6,7,8,9,10,11]. OS benefit has been demonstrated for ribociclib plus letrozole/fulvestrant for postmenopausal women, with a median OS of greater than five years in patients receiving the CDK4/6 inhibitor [10,11]. The OS data from the second interim analysis of abemaciclb from the MONARCH-3 trial showed numerical improvement in the experimental arm (67.1 months vs. 54.2 months, hazard ratio 0.754, 0.57–0.97, p = 0.0301) but did not meet the pre-specified boundary for statistical significance at this timepoint; the final OS analysis is anticipated later in 2023 [12] Ribociclib in combination with endocrine therapy (ET) has also demonstrated an improvement in OS in premenopausal women and is considered the standard of care in first line setting [9]. Although the relative benefit in progression free survival (PFS) is similar in all first line studies of different CDK4/6 inhibitors with ET, recent survival data from PALOMA-2 trial demonstrated no significant OS benefit with palbociclib [13]. Whether this is related to differential efficacy of different CDK4/6 inhibitors or due to differences in patient population or loss of many patients for survival follow-up remains an open question. Given these findings, ribociclib recently received a category 1 recommendation for first line treatment for women with HR-positive MBC in the NCCN guidelines, whereas palbociclib and abemaciclib still have a category 2A recommendation [14] For patients who have endocrine sensitive disease (de-novo metastatic disease or progression > 12 months after completing adjuvant endocrine therapy), either AI or fulvestrant as ET partner is reasonable given similar efficacy in the PARSIFAL study [15]; however, AI is preferred in the clinic due to oral administration and more data for fulvestrant post progression on AI. For patients with endocrine resistant disease, fulvestrant is the preferred ET partner with a CDK4/6 inhibitor [11,16,17,18].
Eventually, most patients develop resistance to CDK4/6 inhibitors and require a change in therapy. PFS on first line CDK4/6 inhibitor ranges from 2–3 years; however, the median OS of about five years suggests limited efficacy of subsequent anti-cancer treatments. For instance, several recent trials have shown that fulvestrant achieves a median PFS of 2–3 months after progression to CDK4/6 inhibitors, warranting the development of better treatment strategies to extend the endocrine treatment window before moving to cytotoxic chemotherapy [19,20,21] Importantly, in the past few years, the mechanisms of resistance to CDK4/6 inhibitors and endocrine therapy are starting to be unraveled, allowing for an expansion in the pipeline of effective agents in this setting [22,23]. Multiple randomized phase 2 and 3 trials have recently reported positive results, leading to significant changes in treatment algorithms for HR-positive MBC.
In this article, we will review the current practice patterns beyond first line therapy in HR-positive ABC, common resistance mechanisms to CDK4/6 inhibitors and ET, describe the rationale and data for continuation of CDK4/6 inhibitors beyond progression and also delve into the novel endocrine and biological treatment options which may bridge the gap between first line CDK4/6 inhibitors + ET and subsequent chemotherapy for which data have been published or presented in the last few years. Finally, we will discuss how these treatments could be incorporated into future treatment algorithms.
2. Current Practice Standards after Progression on 1st Line CDK4/6 Inhibitor and ET
Data on therapies after progression on 1st line CDK4/6 inhibitor from major randomized trials show that single agent ET was the most commonly pursued strategy (50–60%) followed by chemotherapy (30–35%), mTOR inhibition with everolimus and exemestane (10–15%) and continuation of a CDK4/6 inhibitor (<10%) [24]. Real world studies suggest higher use of chemotherapy in the second line setting compared to a different endocrine strategy, possibly reflecting a fear among oncologists of loss of endocrine sensitivity [25,26,27,28]. Short progression free survival (PFS) under three months with ET monotherapy control arms including fulvestrant in recent randomized and single arm trials provides further credibility to this hypothesis and suggests an urgent need for alternative therapeutic approaches in this patient population [19,20,21].
3. Mechanisms of Resistance to CDK4/6 Inhibitors and Endocrine Therapy
Multiple mechanisms of resistance to CDK4/6 inhibitors have been described, including increased activity of the CDK4/6 checkpoint kinase, bypassing the checkpoint through activation of CCNE1/CDK2 leading to downstream phosphorylation of retinoblastoma (RB) protein or acquired RB1 loss of function mutations [29,30]. Several retrospective studies and post-hoc analysis of randomized trials have demonstrated that 5–10% of patients develop acquired RB1 mutation as a mechanism of resistance to CDK4/6 inhibitors, whereas these are a less common cause of intrinsic drug resistance (0–5%) [31,32,33,34,35]. Other mechanisms including c-MET mutations [36], aberrant cyclin E1 signaling [37], CDK6 amplification [38], loss of FAT1 [39] and activation of tyrosine kinase receptor signaling including the PI3K/AKT/mTOR pathway [40]. Resistance to ET (primarily AI) is often mediated by mutations in the alpha subunit of the ER (ESR1 driver mutations) in an endocrine dependent manner or due to constitutive activation of the PI3K/AKT/mTOR pathway in an endocrine independent manner [31,41,42,43]. About 30–40% of patients develop an ESR1 mutation while on treatment with a CDK4/6 inhibitor plus AI, reflecting endocrine resistance; these patients may still retain sensitivity to CDK4/6 inhibition, providing rationale for maintaining CDK4/6 inhibitor beyond progression and targeting ESR1, through a switch in endocrine therapy to a SERD with activity against this mutation [31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Moreover, various drugs targeting the upstream pathways [(PI3 kinase (alpelisib), AKT (capivasertib) and mTOR (everolimus)] have shown clinical benefit in randomized controlled trials and will be discussed below.
4. Continuation of CDK4/6 Inhibitors beyond Progression
4.1. CDK4/6 Inhibitors plus ET
Potential benefit for continuation of a CDK4/6 inhibitor beyond initial progression was initially demonstrated in retrospective studies; however, most of these studies were small single institutional studies with heterogenous patient populations with heavily pre-treated patients, thus not allowing for any firm conclusions [45,46,47,48]. A larger multicentric study from six academic centers in the United States of abemaciclib post progression on palbociclib showed a median PFS of 5.6 months and median OS of 17.2 months [32].
Prospective data on this strategy are derived from the randomized phase II MAINTAIN trial [21], which enrolled 120 patients with HR-positive MBC. In this study, patients that progressed on prior CDK4/6 inhibitor plus ET were randomized to ribociclib plus switch of ET vs. placebo plus switch ET. Notably, most patients received an AI as initial ET, 83% of the patients received palbociclib as initial CDK4/6 inhibitor and more than two thirds had previously received a CDK4/6 inhibitor for >12 months. A small number of patients had received chemotherapy for MBC (around 10%). The study was powered for PFS as the primary end point which was met with an approximately 2.5 months improvement in PFS in the ribociclib plus ET arm compared to the placebo arm (median 5.29 months vs. 2.76 months, hazard ratio 0.57 (0.39–0.95), p = 0.006). The objective response rate (ORR) with ribociclib was 20% compared to 11% with ET alone. Data on OS and safety are not yet available [21]. In an exploratory analysis, there was no benefit in patients who had an ESR1 mutation at study entry with equally poor outcomes in both arms (median PFS 3 months). However, this analysis is limited by small numbers of patients with an ESR1 mutation (n = 33) and higher number of patients with CCND1 (24%) and FGFR1 (9%) alterations among the ESR1 mutant cohort, which might have limited the benefit of CDK4/6 inhibitor continuation in this subgroup [21].
The second prospective trial in this space for which results have been recently reported is the PACE phase two randomized study. Patients in this study were randomized to palbociclib plus fulvestrant vs. placebo plus fulvestrant, with the comparison among these two arms being the primary end point. A third arm tested triple therapy with palbociclib, fulvestrant and avelumab. The study enrolled a similar patient population to the MAINTAIN trial, with the majority of patients having received prior palbociclib as the CDK4/6 inhibitor (>90%) with duration of exposure of >12 months (76%) [49]. More than half of patients had visceral disease (60%), and only a minority had received prior chemotherapy for MBC (16%). Contrary to the findings from MAINTAIN, there was no benefit for continuing palbociclib beyond progression in terms of either PFS (median PFS 4.6 months vs. 4.8 months, hazard ratio 1.11 (0.74–1.66) or OS (median OS 24.6 months vs. 27.5 months, hazard ratio 1.02 (95% CI 0.67–1.56)). Again, contrary to MAINTAIN data, there was a trend towards PFS benefit in patients who had an ESR1 mutation with combination but no benefit in those who were ESR1 wild type. However, these analyses were exploratory.
Given the conflicting results of two prospective studies and the phase two nature of these trials, the benefits of continuing CDK4/6 inhibitor beyond progression remain controversial. It is plausible that switch to a different CDK4/6 inhibitor as tested in the MAINTAIN trial may be worthwhile in some patients rather than continuing the same agent, as was done in PACE. There are known biological and pharmacological differences among CDK4/6 inhibitors which might contribute to different mechanisms of resistance and differential efficacy in this setting [50]. Of note, the NATALEE phase 3 trial of adjuvant ribociclib was recently announced to meet its primary endpoint of invasive disease free survival, whereas Palbociclib failed to improve outcomes in two trials conducted in a similar setting (PALLAS and PENELOPE-B). Moreover, none of the maintenance studies tested a switch to abemaciclib, which has the highest single agent activity of all CDK4/6 inhibitors in a heavily pre-treated MBC population [51], and can also be modestly improved by adding tamoxifen to abemaciclib as seen in the nextMONARCH trial [52]. In the single arm Phase II ELAINE II study, abemaciclib and the novel selective estrogen receptor modulator lasofoxifene were trialed post-CDK4/6 inhibitors in 29 patients harboring ESR1 mutations. The median PFS was 13.9 months with an ORR of 33.3% (ASCO 2022) [53].
Ongoing randomized trials are likely to provide further evidence into this critical question. The postMONARCH phase 3 trial will evaluate the role of adding abemaciclib to fulvestrant in patients that have experienced progression to CDK4/6 inhibition. The EMBER 3 phase 3 trial will evaluate the novel SERD imlunestrant, alone or with abemaciclib, compared to investigator choice of endocrine treatment. The PALMIRA study will look at palbociclib rechallenge (similar to PACE) but in a population that has previously documented clinical benefit to palbociclib; this study has completed accrual and results are pending [54]. A similar single arm phase II study ongoing in Italy is currently recruiting [55]. ELAINE 3 will evaluate lasofoxifine/abemaciclib versus fulvestrant/abemaciclib in patients with prior progression on ribociclib or Palbociclib. A phase 1/2 study looking at dual CDK2 and CDK4/6 inhibitor after progression on CDK4/6 inhibitors in multiple tumor types is also ongoing and addresses a key resistance mechanism to these agents [56] (Table 1).

Table 1.
Ongoing/completed 2nd line and beyond clinical trials.
4.2. CDK4/6 Inhibitors plus Other Targeted Agents including Immunotherapy
Pre-clinical evidence suggests an interaction between checkpoint inhibitors and CDK4/6 inhibitors which might be mediated through programmed death-ligand 1 (PD-L1) degradation by SPOP or direct stimulation of PD-L1 expressing T cells by CDK 4/6 inhibitors [68,69], thus providing rationale for testing combination strategies using these drugs. One arm of the PACE study mentioned above tested the PD-L1 inhibitor avelumab in combination with palbociclib and fulvestrant in patients previous treated with palbociclib [49]. Superiority of this triplet over fulvestrant alone was a secondary end point of the study. Interestingly, both PFS and OS with triplet regimen were numerically longer in the PACE trial than either fulvestrant or combination of palbociclib plus fulvestrant, although the differences were not statistically significant. No major toxicity signals were identified, different from previous studies that have found concerning toxicities when CDK4/6 inhibitors and immune checkpoint inhibitors have been combined [70,71,72]. Overall, this was a small trial, underpowered to detect this difference and this observation is hypothesis generating and warrants evaluation in future trials. The approach is current being tested with atezolizumab in different combinations with other targeted agents [57] (Table 1).
Synergistic activity for CDK4/6 inhibitors with PI3K or mTOR inhibitors has also been observed and is currently being tested in clinical trials [73]. One of the first studies testing this concept using a combination of palbociclib with everolimus and exemestane was limited by severe toxicity (including high grade mucositis and neutropenia) and limited efficacy [74]. Similar toxicity concerns were observed in another study looking at ribociclib in combination with everolimus and exemestane [75]. This is consistent with the poor tolerability often observed with everolimus in this setting [76]. Other combinations including those effecting upstream signaling [58] or having dual mTOR/PI3K inhibitor activity [59] are being explored. Clinical trials are also testing newer generation FGFR inhibitors like erdafitinib [60] (Table 1). Data on these targeted approaches are awaited with interest and are likely to influence management of patients who have previously progressed on CDK4/6 inhibitors.
5. Fulvestrant and Oral SERDs
The benefit of fulvestrant as second line ET was established in the pre CDK4/6 inhibitor era. In the FALCON study, PFS was superior with fulvestrant compared to AI in ET naïve MBC patients [77]. In the phase III EFFECT trial, efficacy of fulvestrant was similar to exemestane in patients who had progressed on a aromatase inhibitor [78]. However, in this study, fulvestrant was administered at 250 mg rather than the current standard of 500 mg. The superior efficacy of 500 mg dose over 250 mg was in the phase III CONFIRM study, which demonstrated a marginal improvement in both PFS and OS with the 500 mg dose [79]. Although fulvestrant has been frequently used as an ET partner with CDK4/6 inhibitors in phase III trials in both first- and second-line setting, the efficacy of single agent fulvestrant post progression on CDK4/6 inhibitor remains limited. Recent studies using fulvestrant as control arm in this setting have shown median PFS ranging from 1.9–4.7 months [20,21,80].
To improve upon the activity of fulvestrant, which is limited by poor bioavailability and weak permeation, and provide an easier route of administration, multiple oral SERDs have been tested in clinical trials with mixed results (Table 2). For instance, the pivotal randomized trials of amcenestrant and giredestrant failed to demonstrate a meaningful improvement in PFS, despite encouraging early-phase trial results, leading to discontinuation of their future development and further clinical trials by their respective pharmaceutical companies [81,82,83]. On the other hand, encouraging results for select patients were reported with elacestrant, which was tested in the phase 3 EMERALD study [19]. This was a phase III trial, that compared elacestrant to standard of care (SoC) ET (fulvestrant or an aromatase inhibitor) in 477 patients who had progressed on prior treatment with a CDK 4/6 inhibitor. Most patients in the SOC arm received fulvestrant (70%). More than 40% of patients in both arms had received two prior endocrine therapies and 20% had received chemotherapy for MBC. The study had coprimary end points of PFS in all patients and PFS in patients with an ESR1 mutations. Overall, there was a modest improvement in median PFS with elacesterant (2.8 vs. 1.9 months, hazard ratio 0.70, 0.55–0.88, p = 0.0018) with a significant proportion of patients in both arms progressing in the first six months suggesting endocrine resistance for nearly 50% patients in this setting. Six month and 12-month PFS rates were also improved with elacestrant (34.3% and 22% in elacestrant vs. 20.4% and 9.4% respective in SOC). Nausea was the most common side effect, with 35% experiencing nausea with elacestrant. Treatment discontinuations were observed in 3.4% of the patients with elacestrant compared to 0.9% in SOC arm.

Table 2.
Positive studies of oral SERDs (including PROTACs).
Previous studies have suggested that about 30–40% patients develop an ESR1 mutation as a mechanism of resistance to ET under pressure from AI’s, a mechanism that can be potentially overcome by SERDs [31,86,87]. An ESR1 mutation was seen in 47.8% of patients in EMERALD. Retrospective analysis from SoFEA study and data from plasmaMATCH study suggest some activity of fulvestrant in patients with an ESR1 mutation [88,89]. Similarly, ESR1 mutation predicted for higher benefit in the EMERLALD study with a median PFS of 3.8 months with elacestrant and 6- and 12-month PFS rates of 41% and 27%. Of note, there was no benefit observed in ESR1 wild type and no OS benefit was observed overall. Data on the role of ESR1 mutation and length of prior CDK4/6 inhibitor treatment were recently presented, showing a 6 months PFS benefit (median 8.6 vs. 1.91 months, hazard ratio 0.41, 0.26–0.63) in patients with a ESR1 mutation who had remained on a prior CDK4/6 inhibitor for ≥12 months indicating that this might be the subgroup deriving most benefit from elacestrant [90]. On the basis of these data, the United States Food and Drug Administration (US FDA) approved elacestrant for postmenopausal patients with ER-positive, HER2-negative, ESR1-mutated ABC and disease progression after at least one line of ET [91]. Approval was also provided for Guardant360 CDx assay as a companion diagnostic to identify ESR1 mutation to select patients for this agent.
Camizestrant is another oral SERD which has shown efficacy in this setting. The phase 2 SERENA-2 trial was meant to compare each of the 75 mg and 150 mg doses of camizestrant with fulvestrant with a primary end point of PFS [84]. The trial enrolled a less heavily pre-treated population compared to EMERALD with only 50% patients having received prior CDK4/6 inhibitors and no patient received more than one line of ET for MBC prior to enrolment. More importantly, prior exposure to fulvestrant or oral SERD was not allowed, a condition which was not mandatory in EMERALD study. Both doses of camizestrant produced a statistically significant improvement in PFS with median PFS for 75 mg being 7.2 months (3.7–10.9, hazard ratio 0.58, 0.41–0.81) and 150 mg being 7.7 months (5.5–12.9, hazard ratio 0.67, 0.48–0.92) compared to fulvestrant [median PFS 3.7 months [2,3,4,5,6]]. The response rates were modest (15% at 75 mg, 20% at 150 mg and 11% with fulvestrant). As with elacestrant, the benefits seem to be limited to patients with an ESR1 mutation. Currently, SERENA-6 study is comparing a switch to camizestrant 75 mg once daily vs. continuation on an AI in combination with CDK4/6 inhibitor in patients who have a detectable ESR1 mutation on ctDNA during first line treatment with an AI and CDK4/6 inhibitor [61] (Table 3). A similar strategy yielded promising results in the PADA-1 phase 3 trial when an AI was switched to fulvestrant on detection of an ESR1 mutation and may represent a biomarker based strategy to change treatment on development of resistance before clinical progression [92].

Table 3.
Positive studies of PI3K/AKT/mTOR inhibitors.
Preliminary data have also been presented for imlunestrant [96], and the ongoing phase 3 EMBER-3 study will further clarify its role in the treatment of patients with HR-positive MBC [62].
6. Proteolysis Targeting Chimeras (PROTACs)
PROTACs are a relatively new class of small molecules which possess the ability to target proteins for degradation via the ubiquitin-proteasome inhibition [97]. Unlike fulvestrant (which requires high affinity binding to ER for its action, thus requiring higher dose and more frequent administration and making it more prone to resistance due to point mutations in ER) the PROTAC ARV-471 (vepdegestrant) binds to E3 ubiquitin ligase and ER to trigger ER degradation via the proteasome pathway [98]. In breast xenograft models, ARV-471 yielded higher ER degradation and tumor growth inhibition than fulvestrant which led to its testing in clinical trials [99]. The results of the phase 2 dose expansion of ARV-471 (VERITAC) were recently presented [85] where a total of 71 patients with heavily pre-treated (median 3 lines) HR-positive MBC were treated at two dose levels of ARV-741 (200 mg and 500 mg QD). The overall clinical benefit rate (CBR), which was the primary end point, was 38% and was similar in both dose levels. Most of the responses were of stable disease with only two partial responses noted and with a median PFS of 3.7 months. A higher CBR (51.2%) was noted in patients with an ESR1 mutation, but numbers were small. A phase 3 study comparing ARV-471 to fulvestrant in patients who have previously progressed on a CDK4/6 inhibitor is currently ongoing and will help to define the role of this new class of compounds in the management of HR-positive MBC [63].
7. Targeting the PI3K/AKT/mTOR Pathway
7.1. PI3K Inhibitors
Mutation in PIK3CA (which encodes for isoform 110 alpha of PI3K) lead to constitutive activation of PI3K and are seen in 30–50% of HR-positive MBC [100,101]. Most of these mutations are seen in exon 9 and exon 20, are considered early events in breast cancer pathogenesis [100,101,102] and can be detected either in plasma or tissue with good concordance [103,104]. Pan-PI3K inhibitors like buparlisib and taselesib were initially tested in MBC, but their development was halted due to significant toxicity concerns [105,106].
Alpelisib, an α-selective PI3K inhibitor, was the first PI3Kα inhibitor to demonstrate an improvement in PFS in patients with HR-positive HER2− MBC with activating PIK3CA mutations. The SOLAR-1 trial included postmenopausal women who were resistant to endocrine therapy, with disease progression on or after prior aromatase inhibitor. Only around 6% of patients previously received a CDK 4/6 inhibitor. Patients were randomized to receive alpelisib or placebo plus fulvestrant. The primary endpoint was PFS in the PIK3CA-mutated cohort. With a median follow-up of 20 months, the median PFS for the PIK3CA-mutated cohort was almost double with the addition of alpelisib, 11.0 vs. 5.7 months (hazard ratio 0.65 [95% CI 0.50–0.85]; [93]. The experimental arm was, however, burdened by a high rate of side effects, with key grade 3 and 4 toxicities being hyperglycemia in 36.6%, rash in 9.9% and diarrhea in 6.7% of the patients receiving alpelisib. In the final OS analysis, OS did not cross the pre-specified boundary (p ≤ 0.0161) for the PIK3CA-mutated cohort, although median OS was numerically prolonged by 7.9 months for patients in the alpelisib plus fulvestrant arm [94]. These interpretations are limited somewhat due to higher treatment discontinuations in the alpelesib and fulvestrant arm due to higher toxicity of the combination, leading to the risk of informative censoring. Subsequently reported patient reported outcomes showed a numerical worsening in quality of life with alpelesib compared to placebo, with deterioration in multiple symptom subscales possibly related to toxicity [107]. The data for alpelesib plus fulvestrant post CDK4/6 inhibitor progression are derived from a phase 2 open label BYLieve study [108]. Updated data from both cohort A (progression post CDK4/6 inhibitor and AI) and cohort B (progression post CDK4/6 inhibitor and fulvestrant) were presented at ASCO 2022, which showed a median PFS of 8.2 months (5.6–9.5) in cohort A and 5.6 months (3.7–7.1) in cohort B [109]. About 26% patients had >grade 3 side effects including hyperglycemia, rash and diarrhea [108]. Other α-selective mutant-degrading PI3K inhibitors (such as inavolisib) are currently being studied in this setting and data on efficacy and especially safety are awaited with great interest [64] (Table 1). Additionally, mutant-selective PI3K inhibitors like LOXO-783 and RLY-2608 are currently under clinical development and may allow to retain the activity of this class of drugs but reduce off-target toxicities [110,111].
7.2. AKT Inhibitors
Protein kinase B (AKT) is a key element of the PI3K/AKT/mTOR signaling pathway. AKT inhibitors in combination with ET showed preliminary clinically meaningful activity in early trials conducted in HR-positive HER2-negative MBC. However, the identification of biomarkers of response and resistance to AKT inhibition is crucial and still represents an unmet need [112,113].
The combination of capivasertib (an AKT inhibitor) and fulvestrant was explored in a randomized, placebo-controlled phase II trial (FAKTION) in postmenopausal women with HR-positive HER2-negative MBC progressing after or on an aromatase inhibitor. The primary endpoint was PFS. In the overall population, the addition of the AKT inhibitor to endocrine therapy provided a statistically significant 5.5-month gain in median PFS, 10.3 months in the capivasertib arm vs. 4.8 months in the control arm (hazard ratio 0.58 (95% CI 0.39–0.84); p = 0.004). Median OS in the experimental vs. placebo arms was 29.3 vs. 23.4 months (hazard ratio 0.66 (95% CI 0.45–0.97); p = 0.035) [114]. The benefits in this study were restricted to patients who had a mutation in the PIK3CA, AKT1 or PTEN; however, the analysis was exploratory due to limited number of patients in subgroups. The most common grade 3–4 adverse events were hypertension (32%), diarrhea (14%), and rash (20%) [115].
The results of the confirmatory phase III trial (CAPItello-291) of capivasertib and fulvestrant in HR-positive HER2- MBC patients after progression to an aromatase inhibitor-based therapy were recently presented [95] (Table 3). The study included pre/perimenopausal or postmenopausal ABC patients that progressed after aromatase inhibitor treatment, with or without a CDK4/6 inhibitor. The primary endpoint was PFS in the overall patient population and in the population of patients whose tumors had qualifying alterations in the AKT pathway (PIK3CA, AKT1 or PTEN genes). Of the 708 patients randomized, 41 percent had genetic alterations in the AKT pathway (31% being PIK3CA, 4.7% AKT and 5.2% PTEN) and 69 percent previously received a CDK4/6 inhibitor. The median PFS for the capivasertib/fulvestrant arm was 7.2 months compared with 3.6 months for placebo/fulvestrant (hazard ratio 0.60 (95% CI 0.51–0.61)). For patients in the AKT-altered population, the median PFS was 7.3 months for the capivasertib/fulvestrant group compared with 3.2 months for the placebo/fulvestrant group (hazard ratio 0.50 (95% CI 0.38–0.65)). In an exploratory analysis, the benefits were similar in patients with no mutation (including unknown status) in the AKT pathway (median PFS 7.2 months vs. 3.7 months, hazard ratio 0.70, 0.56–0.88). However, it should be noted that 16% of patients had an unknown mutation status and the hazard ratio for PFS excluding those patients was 0.79 (0.61–1.02). Further data from ctDNA based profiling is awaited to better understand the benefits in the unaltered group. The OS data remain immature but also showed a trend towards improvement in the overall population and AKT pathway mutated population. Major adverse events included diarrhea (72.4%, 9.3% grade 3–4) and rash (38%, grade 3–4 11.6%) with 9.3% patients discontinuing capivasertib due to adverse events [95]. Ongoing trials like FINER are testing other AKT inhibitors like ipatasertib post CDK4/6 inhibitor progression [65] and are expected to provide confirmatory evidence of efficacy of AKT inhibitors in this population (Table 1). Ongoing trials like CAPITELLO-292 are also looking at the efficacy of a combination of capivasertib, palbociclib and fulvestrant as first line treatment for HR + MBC [116].
7.3. mTOR Inhibitors
The mTOR signaling pathway regulates cell proliferation, autophagy, apoptosis, and is involved in malignant transformation. Better understanding of the complex regulatory mechanisms of the mTOR signaling pathway have been important in the development of mTOR inhibitors for treatment of cancer and in identifying predictors of response or resistance [117,118].
Everolimus has been tested with various endocrine partners. In the Pre0102 trial, addition of everolimus to fulvestrant improved median PFS (10.1 months to 5.3 months, hazard ratio 0.61 (0.40–0.92). Toxicities observed in the study included oral mucositis (53% vs. 12%), fatigue (42% vs. 22%), rash (38% vs. 5%), diarrhea (23% vs. 8%), hyperglycemia (19% vs. 5) and pneumonitis (17% vs. 0%) among others [119]. In the BOLERO-2 trial, the addition of everolimus to exemestane in postmenopausal women with HR-positive HER2-negative MBC who had progressed on aromatase inhibitor, improvement in median PFS with the combination was demonstrated (median PFS 10.6 months vs. 4.1 months according to central assessment, hazard ratio 0.36, 0.27–0.47) [76] (Table 3). However, there was a higher rate of discontinuation of everolimus due to adverse events, 29% compared to 5% in the control arm, leading to informative censoring that likely influenced these results [120]. Furthermore, an update of the trial reported no improvement in OS with a median of 31.0 months in the combination group compared to 26.6 months in the group receiving Exemestane and placebo (p = 0.14) [121]. Everolimus was also evaluated in combination with exemestane compared to exemestane or capecitabine alone in the BOLERO-6 trial. Consistent with the BOLERO-2 study, it showed evidence of superiority in median PFS for everolimus plus exemestane vs. everolimus alone but not vs. capecitabine alone [122]. Moreover, serious adverse events were more common with everolimus exemestane (36%) compared to capecitabine (29%), again raising questions about toxicity of this potential chemotherapy free approach. An ongoing phase 3 study (eVERA) is currently evaluating the combination of oral SERD giredestrant and everolimus compared to everolimus and exemestane in patients who have previously progressed on a CDK4/6 inhibitor (Table 1). However, given the previous negative data for single agent giredestrant and safety concerns with everolimus, it is critical to be able to identify which patients would benefit from these drugs [66].
8. PARP Inhibitors
Two randomized phase III studies were conducted to evaluate the efficacy of PARP inhibitors (olaparib and talazoparib) in germline BRCA mutated MBC patients in the pre CDK 4/6 inhibitor era [123,124,125,126]. About half of the patients in both these trials had HR-positive disease, and all patients had received at least one prior chemotherapy regimen. The trials effectively excluded patients resistant to platinum, and platinum was not allowed as one of the investigator choice chemotherapy regimens. Overall, both trials demonstrated an improvement in ORR and PFS (median improvement 3 months) with the PARP inhibitor but failed to demonstrate an improvement in OS [124,125]. How prior CDK4/6 inhibitor treatment would modify these treatment effects remains unknown. However, for selected patients with germline BRCA mutation, PARP inhibitors remain a reasonable alternative to chemotherapy beyond CDK4/6 inhibitors in the endocrine refractory setting [127].
9. Role of Genomic Testing to Select Treatment after CDK4/6-Inhibitors
The approval of elacestrant post progression on CDK4/6 inhibitors for patients with a ESR1 mutation has added another layer of complexity to genomic testing and its role for patients with HR-positive MBC. Previously, alpelisib was approved for patients with a PIK3CA mutation, which could be detected on either tissue or blood with good concordance and at any time point in the disease course since it is a founder mutation [100,101,102,103,104]. However, ESR1 mutation usually develops as a resistance mechanism to an AI and prevalence of ESR1 mutation at the time of primary diagnosis is low (usually <5%, <1% in AI naïve MBC) [44,124,128]. Moreover, the prevalence of ESR1 mutations increases over time in patients with MBC and a recent liquid biopsy assay to detect this alteration is required rather than relying on a remote test [129,130].
Therefore, for the optimal selection of treatment after CDK4/6-inhibitors, next-generation sequencing with tissue or preferentially liquid biopsy is recommended, to understand the most updated ESR1 (and PIK3CA) status of the disease, which is key for treatment decisions.
It is worth noticing that testing by either method does add cost and complexity and the clinical implications of using one method over the other remain to be defined. Moreover, deeper knowledge regarding the complexities of ESR1 mutations is warranted. The recent plasmaMATCH study, for instance, emphasized the importance of ESR1 variant allele frequency (VAF) when no responses to fulvestrant were seen when VAF was <50% [89]. The correlation of VAF and outcomes with oral SERDs thus needs further study.
10. How to Approach a Patient with Hormone Receptor Positive MBC Who Has Progressed on First Line CDK4/6 Inhibitor and ET in 2023?
Several aspects need to be considered to select the most adequate treatment after progression on first line CDK4/6 inhibitor plus ET (Figure 1). Among these, key factors are represented by prior duration of exposure to CDK4/6 inhibitors, patient preference, comorbidities, recent somatic mutation status including ESR1, PIK3CA and germline BRCA1/2 results. Although elacestrant has been approved in all patients with an ESR1 mutation, the majority of the benefit seems to be derived in patients who are endocrine sensitive with a prolonged exposure to prior CDK 4/6 inhibitors. Therefore, the adoption of second line elacestrant seems best suited for patients with ESR1-mutant disease that experienced prolonged benefit (at least 12 months) from prior CDK4/6-inhibitors.
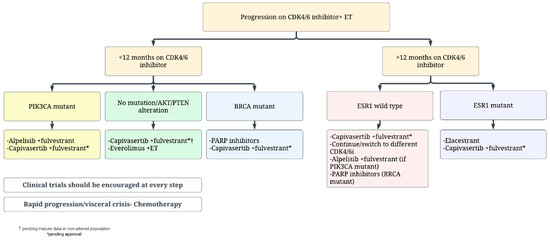
Figure 1.
Proposed second line treatment algorithm for patients with hormone receptor positive MBC.
In the presence of a PIK3CA mutation, the use of fulvestrant plus alpelisib can be discussed with patients. The previously mentioned issues with toxicities of the regimen may turn this choice less preferrable in the presence of active alternatives. Among these, capivasertib may soon achieve regulatory approval based on the data of CAPITELLO-291 study [95]. If approved, it might be reasonable to use second line capivasertib plus fulvestrant, particularly for patients with PIK3CA, AKT or PTEN alterations, who seemed to derive a major benefit in the trial; the role of this combination in patients without mutations will require elucidation in future updates of the CAPITELLO-291 study. Fulvestrant plus everolimus also represents an available option in this setting, although with little data after progression to CDK4/6 inhibitors, and with non-negligible toxicities.
For patients with a germline BRCA mutation, it is reasonable to discuss a PARP inhibitor (either olaparib or talazoparib) as second or third line as a non-chemotherapy option after discussion of risks, benefits and limitations of no OS benefit yet demonstrated [124,125].
The continuation of CDK4/6 inhibitor beyond progression remains controversial given the conflicting data discussed above [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,68,69,70,71,72,73,74,75,76,77,78,79,80]. This approach is not standard clinical practice and the mentioned research studies are likely to provide more information about the efficacy of this approach and on patient selection. The signal for addition of immunotherapy in such patients from PACE trial is intriguing and clinical trials considering this approach and addition of other targeted agents to CDK4/6 inhibitors plus ET should be considered in these patients (Table 1).
Lastly, for patients with rapid clinical progression with impending visceral crisis, single agent chemotherapy remains the preferred approach with the choice of agent dictated by patient preference and side effect profile. These patients are also likely to benefit from antibody drug conjugates like trastuzumab deruxtecan or sacituzumab govitecan, and enrollment on clinical trials testing these strategies should be encouraged (Table 1).
11. Conclusions
The treatment of patients who experience disease progression on CDK4/6 inhibitors and ET remains challenging. Multiple novel biological and endocrine therapies have shown promise in this setting. A subset of patients, defined by the presence of an ESR1 mutation and a prolonged benefit from prior CDK4/6 inhibition, derive benefit from elacestrant, which is now an approved treatment option in the US. Adding the AKT inhibitor capivasertib to fulvestrant achieved an encouraging benefit in PFS in patients with progression while on CDK4/6 inhibitors and may soon become an available treatment option. Continuation of CDK4/6 inhibitors beyond progression remains controversial and more data is required before this approach can be considered standard. Despite the recent advances in treatment, outcomes for these patients remain suboptimal and enrolment into ongoing clinical trials is strongly encouraged at every step. Progression on CDK4/6 inhibitor therapy is a major change, demarcating nearly half of the survival time in a patient’s life facing HR-positive, HER2-negative MBC. It is an excellent time for the clinician to take a step back, reassess tumor biology and initiate discussions about the patients’ goals and preferences. Patient shared decision making is always encouraged.
Author Contributions
Conceptualization, A.M. and P.T.; methodology, A.M., C.M.V., F.T. and P.T.; investigation, A.M., C.M.V., F.T. and P.T.; resources, S.S., I.S., S.M.T., P.T.; data curation, A.M., C.M.V., F.T.; writing—original draft preparation, A.M., C.M.V., F.T.; writing—review and editing, All Authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
S.S. has served as an advisor/consultant for Foundation Medicine, AstraZeneca, Daichii Sankyo, Eli Lilly, Pfizer, Sermonix, and Novartis. S.M.T. has served as an advisor/consultant to Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, Odonate Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Gilead, Mersana, Certara, Chugai Pharma, Ellipses Pharma, Infinity, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics; and has received institutional research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, NanoString Technologies, Cyclacel, Nektar, Gilead, Odonate Therapeutics, Sanofi, Seattle Genetics. P.T. served as advisor/consultant for AstraZeneca, Daiichi Sankyo, Gilead and Eli Lilly. Other authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer—Statistics [Internet]. Cancer.Net. 2012. Available online: https://www.cancer.net/cancer-types/breast-cancer/statistics (accessed on 22 January 2023).
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martín, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Jatoi, I.; Chen, B.E.; Anderson, W.F.; Rosenberg, P.S. Breast Cancer Mortality Trends in the United States According to Estrogen Receptor Status and Age at Diagnosis. J. Clin. Oncol. 2007, 25, 1683–1690. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Abemaciclib Plus Aromatase Inhibitor in Advanced Breast Cancer [Internet]. Daily Reporter. Available online: https://dailyreporter.esmo.org/esmo-congress-2022/news/monarch-3-trend-for-improved-overall-survival-with-abemaciclib-plus-aromatase-inhibitor-in-advanced-breast-cancer (accessed on 18 February 2023).
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Chavez Mac Gregor, M.; Bananis, E.; et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022, 40, LBA1003. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil-Gil, M.; Ruíz-Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Fulvestrant-Palbociclib vs Letrozole-Palbociclib as Initial Therapy for Endocrine-Sensitive, Hormone Receptor-Positive, ERBB2-Negative Advanced Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.-A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Fernando, T.M.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.; McCune, S.; Armstrong, A.; Shannon, C.; et al. VERONICA: Randomized Phase II Study of Fulvestrant and Venetoclax in ER-Positive Metastatic Breast Cancer Post-CDK4/6 Inhibitors—Efficacy, Safety, and Biomarker Results. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 3256–3267. [Google Scholar] [CrossRef]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; Raptis, G.; Baer, L.N.; Young Oh, S.; et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J. Clin. Oncol. 2022, 40, LBA1004. [Google Scholar] [CrossRef]
- Cogliati, V.; Capici, S.; Pepe, F.F.; di Mauro, P.; Riva, F.; Cicchiello, F.; Maggioni, C.; Cordani, N.; Cerrito, M.G.; Cazzaniga, M.E. How to Treat HR+/HER2- Metastatic Breast Cancer Patients after CDK4/6 Inhibitors: An Unfinished Story. Life 2022, 12, 378. [Google Scholar] [CrossRef]
- McCartney, A.; Migliaccio, I.; Bonechi, M.; Biagioni, C.; Romagnoli, D.; De Luca, F.; Galardi, F.; Risi, E.; De Santo, I.; Benelli, M.; et al. Mechanisms of Resistance to CDK4/6 Inhibitors: Potential Implications and Biomarkers for Clinical Practice. Front. Oncol. 2019, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Munzone, E.; Pagan, E.; Bagnardi, V.; Montagna, E.; Cancello, G.; Dellapasqua, S.; Iorfida, M.; Mazza, M.; Colleoni, M. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2− metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open 2021, 6, 100332. [Google Scholar] [CrossRef]
- Princic, N.; Aizer, A.; Tang, D.H.; Smith, D.M.; Johnson, W.; Bardia, A. Predictors of systemic therapy sequences following a CDK 4/6 inhibitor-based regimen in post-menopausal women with hormone receptor positive, HEGFR-2 negative metastatic breast cancer. Curr. Med. Res. Opin. 2019, 35, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Biagioni, C.; McCartney, A.; Migliaccio, I.; Curigliano, G.; Sanna, G.; Moretti, E.; Minisini, A.M.; Cinieri, S.; Tondini, C.; et al. Clinical outcomes after palbociclib with or without endocrine therapy in postmenopausal women with hormone receptor positive and HER2-negative metastatic breast cancer enrolled in the TREnd trial. Breast Cancer Res. BCR 2019, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Puglisi, S.; Calabrese, A.; Pia, A.; Perotti, P.; Berruti, A.; Reimondo, G.; Terzolo, M. Unwanted Hormonal and Metabolic Effects of Postoperative Adjuvant Mitotane Treatment for Adrenocortical Cancer. Cancers 2020, 12, 2615. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Gong, C.; Zheng, Y.; Ouyang, Q.; Xie, N.; Qu, Q.; Ge, R.; Wang, B. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211022890. [Google Scholar] [CrossRef]
- Asghar, U.S.; Kanani, R.; Roylance, R.; Mittnacht, S. Systematic Review of Molecular Biomarkers Predictive of Resistance to CDK4/6 Inhibition in Metastatic Breast Cancer. JCO Precis. Oncol. 2022, 6, e2100002. [Google Scholar] [CrossRef]
- Mavratzas, A.; Marmé, F. Treatment of Luminal Metastatic Breast Cancer beyond CDK4/6 Inhibition: Is There a Standard of Care in Clinical Practice? Breast Care 2021, 16, 115–128. [Google Scholar] [CrossRef]
- O’Leary, B.; Cutts, R.J.; Liu, Y.; Hrebien, S.; Huang, X.; Fenwick, K.; André, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018, 8, 1390–1403. [Google Scholar] [CrossRef]
- Wander, S.A.; Han, H.S.; Zangardi, M.L.; Niemierko, A.; Mariotti, V.; Kim, L.S.L.; Xi, J.; Pandey, A.; Dunne, S.; Nasrazadani, A.; et al. Clinical Outcomes with Abemaciclib after Prior CDK4/6 Inhibitor Progression in Breast Cancer: A Multicenter Experience. J. Natl. Compr. Cancer Netw. JNCCN 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Condorelli, R.; Spring, L.; O’Shaughnessy, J.; Lacroix, L.; Bailleux, C.; Scott, V.; Dubois, J.; Nagy, R.J.; Lanman, R.B.; Iafrate, A.J.; et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 640–645. [Google Scholar] [CrossRef]
- Finn, R.S.; Liu, Y.; Zhu, Z.; Martin, M.; Rugo, H.S.; Diéras, V.; Im, S.-A.; Gelmon, K.A.; Harbeck, N.; Lu, D.R.; et al. Biomarker Analyses of Response to Cyclin-Dependent Kinase 4/6 Inhibition and Endocrine Therapy in Women with Treatment-Naïve Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, T.-Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.-J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Stockwell, S.R.; Elbanna, M.; Ketteler, R.; Freeman, J.; Al-Lazikani, B.; Eccles, S.; De Haven Brandon, A.; Raynaud, F.; Hayes, A.; et al. Signalling involving MET and FAK supports cell division independent of the activity of the cell cycle-regulating CDK4/6 kinases. Oncogene 2019, 38, 5905–5920. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Liu, Y.; Zhu, Z.; Loi, S.; Colleoni, M.; Loibl, S.; DeMichele, A.; Harbeck, N.; André, F.; Bayar, M.A.; et al. Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1169–1178. [Google Scholar] [CrossRef]
- Finn, R.S.; Cristofanilli, M.; Ettl, J.; Gelmon, K.A.; Colleoni, M.; Giorgetti, C.; Gauthier, E.; Liu, Y.; Lu, D.R.; Zhang, Z.; et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: A joint analysis of PALOMA-2 and PALOMA-3 clinical trials. Breast Cancer Res. Treat. 2020, 184, 23–35. [Google Scholar] [CrossRef]
- Li, Z.; Razavi, P.; Li, Q.; Toy, W.; Liu, B.; Ping, C.; Hsieh, W.; Sanchez-Vega, F.; Brown, D.N.; Da Cruz Paula, A.F.; et al. Loss of the FAT1 Tumor Suppressor Promotes Resistance to CDK4/6 Inhibitors via the Hippo Pathway. Cancer Cell 2018, 34, 893–905.e8. [Google Scholar] [CrossRef]
- Wander, S.A.; Cohen, O.; Gong, X.; Johnson, G.N.; Buendia-Buendia, J.E.; Lloyd, M.R.; Kim, D.; Luo, F.; Mao, P.; Helvie, K.; et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor positive metastatic breast cancer. Cancer Discov. 2020, 10, 1174–1193. [Google Scholar] [CrossRef]
- Dustin, D.; Gu, G.; Fuqua, S.A. ESR1 Mutations in Breast Cancer. Cancer 2019, 125, 3714–3728. [Google Scholar] [CrossRef]
- Ahn, S.G.; Bae, S.J.; Kim, Y.; Ji, J.H.; Chu, C.; Kim, D.; Lee, J.; Cha, Y.J.; Lee, K.-A.; Jeong, J. Primary endocrine resistance of ER+ breast cancer with ESR1 mutations interrogated by droplet digital PCR. NPJ Breast Cancer 2022, 8, 58. [Google Scholar] [CrossRef]
- Vasan, N.; Toska, E.; Scaltriti, M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, x3–x11. [Google Scholar] [CrossRef]
- Brett, J.O.; Spring, L.M.; Bardia, A.; Wander, S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. BCR 2021, 23, 85. [Google Scholar] [CrossRef]
- dos Anjos, C.H.; Razavi, P.; Herbert, J.; Colon, J.; Gill, K.; Modi, S.; Bromberg, J.; Dang, C.T.; Liu, D.; Norton, L.; et al. A large retrospective analysis of CDK 4/6 inhibitor retreatment in ER+ metastatic breast cancer (MBC). J. Clin. Oncol. 2019, 37, 1053. [Google Scholar] [CrossRef]
- Mariotti, V.; Khong, H.T.; Soliman, H.H.; Costa, R.L.; Fisher, S.; Boulware, D.; Han, H.S. Efficacy of abemaciclib (abema) after palbociclib (palbo) in patients (pts) with metastatic breast cancer (MBC). J. Clin. Oncol. 2019, 37, e12521. [Google Scholar] [CrossRef]
- Eziokwu, A.S.; Varella, L.; Kruse, M.L.; Jia, X.; Moore, H.C.F.; Budd, G.T.; Abraham, J.; Montero, A.J. Real-world evidence evaluating continuation of CDK4/6 inhibitors beyond first progression in hormone receptor-positive (HR+) metastatic breast cancer. J. Clin. Oncol. 2019, 37, e12538. [Google Scholar] [CrossRef]
- Tamragouri, K.; Cobleigh, M.A.; Rao, R.D. Abemaciclib with or without fulvestrant for the treatment of hormone receptor-positive and HER2-negative metastatic breast cancer with disease progression following prior treatment with palbociclib. J. Clin. Oncol. 2019, 37, e12533. [Google Scholar] [CrossRef]
- Continuing CDK4/6 Inhibitor beyond Progression No Help in Metastatic Breast Cancer [Internet]. 2022. Available online: https://www.medpagetoday.com/meetingcoverage/sabcs/102159 (accessed on 26 January 2023).
- George, M.A.; Qureshi, S.; Omene, C.; Toppmeyer, D.L.; Ganesan, S. Clinical and Pharmacologic Differences of CDK4/6 Inhibitors in Breast Cancer. Front. Oncol. 2021, 11, 2471. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Cortes, J.; Ozyilkan, O.; Chen, S.-C.; Petrakova, K.; Manikhas, A.; Jerusalem, G.; Hegg, R.; Huober, J.; Chapman, S.C.; et al. nextMONARCH: Abemaciclib Monotherapy or Combined with Tamoxifen for Metastatic Breast Cancer. Clin. Breast Cancer 2021, 21, 181–190.e2. [Google Scholar] [CrossRef]
- Damodaran, S.; Plourde, P.V.; Moore, H.C.F.; Anderson, I.C.; Portman, D.J. Open-label, phase 2, multicenter study of lasofoxifene (LAS) combined with abemaciclib (Abema) for treating pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer and an ESR1 mutation after progression on prior therapies. J. Clin. Oncol. 2022, 40, 1022. [Google Scholar] [CrossRef]
- MedSIR. International, Multicenter, Randomized, Open-Label, Phase II to Evaluate the Efficacy and Safety of Continuation of Palbociclib+2nd Line Endocrine Therapy in HR+/HER2-ABC Patients Who Had Clinical Benefit during 1st Line Palbociclib. [Internet]. clinicaltrials.gov; Report No.: NCT03809988. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03809988 (accessed on 25 January 2023).
- Consorzio Oncotech. Palbociclib Plus Fulvestrant in Women with Hormone Receptor Positive and Human Epidermal Growth Factor Receptor Type 2 Negative Locally Advanced or Metastatic Breast Cancer Previously Treated with a CDK4/6 Inhibitor in Combination with Hormonal Therapy: A Multicenter, Phase II Trial [Internet]. clinicaltrials.gov; Report No.: NCT04318223. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04318223 (accessed on 25 January 2023).
- Pfizer. PHASE 1/2A Dose Escalation and Expansion Study Evaluating Safety, Tolerability, Pharmacokinetic, Pharmacodynamics and Anti-Tumor Activity of PF-06873600 as a Single Agent and in Combination with Endocrine Therapy [Internet]. clinicaltrials.gov; Report No.: NCT03519178. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03519178 (accessed on 25 January 2023).
- Hoffmann-La Roche. A Phase Ib/II, Open-Label, Multicenter, Randomized Umbrella Study Evaluating the Efficacy and Safety of Multiple Immunotherapy-Based Treatment Combinations in Patients with Hormone Receptor-Positive HER2-Negative Breast Cancer (MORPHEUS-HR+ Breast Cancer) [Internet]. clinicaltrials.gov; Report No.: NCT03280563. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03280563 (accessed on 25 January 2023).
- Boehringer Ingelheim. An Open Label, Phase Ib, Dose-Escalation Study Evaluating the Safety and Tolerability of Xentuzumab and Abemaciclib in Patients with Locally Advanced or Metastatic Solid Tumours and in Combination with Endocrine Therapy in Patients with Locally Advanced or Metastatic Hormone Receptor-Positive, HER2-, Breast Cancer, Followed by Expansion Cohorts. [Internet]. clinicaltrials.gov; Report No.: NCT03099174. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03099174 (accessed on 25 January 2023).
- Celcuity, Inc. Phase 1B Study to Assess the Safety, Tolerability, and Clinical Activity of Gedatolisib in Combination with Palbociclib and Either Letrozole or Fulvestrant in Women with Metastatic or Locally Advanced/Recurrent Breast Cancer (MBC) [Internet]. clinicaltrials.gov; Report No.: NCT02684032. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02684032 (accessed on 25 January 2023).
- Abramson, V. A Phase Ib Trial of Fulvestrant, Palbociclib (CDK4/6 Inhibitor) and Erdafitinib (JNJ-42756493, Pan-FGFR Tyrosine Kinase Inhibitor) in ER+/HER2-/FGFR-Amplified Metastatic Breast Cancer (MBC) [Internet]. clinicaltrials.gov; Report No.: NCT03238196. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03238196 (accessed on 25 January 2023).
- AstraZeneca. A Phase III, Double-Blind, Randomised Study to Assess Switching to AZD9833 (a Next Generation, Oral SERD) + CDK4/6 Inhibitor (Palbociclib or Abemaciclib) vs Continuing Aromatase Inhibitor (Letrozole or Anastrozole)+ CDK4/6 Inhibitor in HR+/HER2-MBC Patients with Detectable ESR1Mutation without Disease Progression during 1L Treatment with Aromatase Inhibitor+ CDK4/6 Inhibitor–A ctDNA Guided Early Switch Study [Internet]. clinicaltrials.gov; Report No.: NCT04964934. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04964934 (accessed on 26 January 2023).
- Eli Lilly and Company. EMBER-3: A Phase 3, Randomized, Open-Label Study of Imlunestrant, Investigator’s Choice of Endocrine Therapy, and Imlunestrant Plus Abemaciclib in Patients with Estrogen Receptor Positive, HER2 Negative Locally Advanced or Metastatic Breast Cancer Previously Treated with Endocrine Therapy [Internet]. clinicaltrials.gov; Report No.: NCT04975308. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04975308 (accessed on 26 January 2023).
- Pfizer. A Phase 3, Randomized, Open-Label, Multicenter Trial of ARV-471 (PF-07850327) vs Fulvestrant in Participants with Estrogen Receptor-Positive, Her2-Negative Advanced Breast Cancer Whose Disease Progressed after Prior Endocrine Based Treatment for Advanced Disease (VERITAC-2) [Internet]. clinicaltrials.gov; Report No.: NCT05654623. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05654623 (accessed on 25 January 2023).
- Genentech, Inc. A Phase I, Open-Label, Dose-Escalation Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0077 as a Single Agent in Patients with Locally Advanced or Metastatic PIK3CA-Mutant Solid Tumors and in Combination with Endocrine and Targeted Therapies in Patients with Locally Advanced or Metastatic PIK3CA-Mutant Breast Cancer [Internet]. clinicaltrials.gov; Report No.: NCT03006172. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03006172 (accessed on 26 January 2023).
- Canadian Cancer Trials Group. A Double-Blind Placebo-Controlled Randomized Phase III Trial of Fulvestrant and Ipatasertib as Treatment for Advanced HER-2 Negative and Estrogen Receptor Positive (ER+) Breast Cancer Following Progression on First Line CDK 4/6 Inhibitor and Aromatase Inhibitor [Internet]. clinicaltrials.gov; Report No.: NCT04650581. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04650581 (accessed on 26 January 2023).
- Genentech, Inc. A Phase III, Randomized, Open-Label, Multicenter Study Evaluating the Efficacy and Safety of Giredestrant Plus Everolimus Compared with Exemestane Plus Everolimus in Patients with Estrogen Receptor-Positive, HER2-Negative, Locally Advanced or Metastatic Breast Cancer [Internet]. clinicaltrials.gov; Report No.: NCT05306340. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05306340 (accessed on 26 January 2023).
- AstraZeneca. A Phase 3, Randomized, Multi-Center, Open-Label Study of Trastuzumab Deruxtecan (T-DXd) versus Investigator’s Choice Chemotherapy in HER2-Low, Hormone Receptor Positive Breast Cancer Patients Whose Disease Has Progressed on Endocrine Therapy in the Metastatic Setting (DESTINY-Breast06) [Internet]. clinicaltrials.gov; Report No.: NCT04494425. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04494425 (accessed on 25 January 2023).
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.A.; Beckmann, R.P.; Dempsey, J.A.; Huber, L.; Forest, A.; Amaladas, N.; Li, Y.; Wang, Y.C.; Rasmussen, E.R.; Chin, D.; et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep. 2018, 22, 2978–2994. [Google Scholar] [CrossRef]
- Patnaik, A.; Yap, T.A.; Chung, H.C.; de Miguel, M.J.; Bang, Y.J.; Lin, C.C.; Su, W.C.; Italiano, A.; Chow, K.H.; Szpurka, A.M.; et al. Safety and Clinical Activity of a New Anti-PD-L1 Antibody as Monotherapy or Combined with Targeted Therapy in Advanced Solid Tumors: The PACT Phase Ia/Ib Trial. Clin. Cancer Res. 2021, 27, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.L.; Vansteenkiste, J.; Rodríguez, L.P.A.; Gregorc, V.; Mazieres, J.; Awad, M.; Jänne, P.A.; Chisamore, M.; Hossain, A.M.; Chen, Y.; et al. Abemaciclib in Combination with Pembrolizumab for Stage IV KRAS-Mutant or Squamous NSCLC: A Phase 1b Study. JTO Clin. Res Rep. 2021, 2, 100234. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lee, J.S.; Yost, S.E.; Frankel, P.H.; Ruel, C.; Egelston, C.A.; Guo, W.; Padam, S.; Tang, A.; Martinez, N.; et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur. J. Cancer 2021, 154, 11–20. [Google Scholar] [CrossRef]
- Korotchkina, L.G.; Leontieva, O.V.; Bukreeva, E.I.; Demidenko, Z.N.; Gudkov, A.V.; Blagosklonny, M.V. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging 2010, 2, 344–352. [Google Scholar] [CrossRef]
- Tolaney, S. A Phase 1b/2a Study of Palbociclib in Combination with Everolimus and Exemestane in Postmenopausal Women with Estrogen Receptor Positive and HER2 Negative Metastatic Breast Cancer [Internet]. clinicaltrials.gov; Report No.: NCT02871791. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02871791 (accessed on 25 January 2023).
- Bardia, A. Phase II Study of CDK 4/6 Inhibitor, LEE011 (Ribociclib), in Combination with Adjuvant Endocrine Therapy at Varying Duration for ER-Positive Breast Cancer (LEADER). [Internet]. clinicaltrials.gov; Report No.: NCT03285412. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03285412 (accessed on 14 July 2022).
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Chia, S.; Gradishar, W.; Mauriac, L.; Bines, J.; Amant, F.; Federico, M.; Fein, L.; Romieu, G.; Buzdar, A.; Robertson, J.F.R.; et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: Results from EFECT. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1664–1670. [Google Scholar] [CrossRef]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Final overall survival: Fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J. Natl. Cancer Inst. 2014, 106, djt337. [Google Scholar] [CrossRef]
- Palbociclib Plus Fulvestrant Fails to Improve PFS in ER+/HER2-Breast Cancer after Progression on a CDK4/6 Inhibitor [Internet]. OncLive. Available online: https://www.onclive.com/view/palbociclib-plus-fulvestrant-fails-to-improve-pfs-in-er-her2--breast-cancer-after-progression-on-a-cdk4-6-inhibitor (accessed on 28 January 2023).
- Garcia-Fructuoso, I.; Gomez-Bravo, R.; Schettini, F. Integrating new oral selective oestrogen receptor degraders in the breast cancer treatment. Curr. Opin. Oncol. 2022, 34, 635. [Google Scholar] [CrossRef] [PubMed]
- Sanofi Trial Failure Halts Work on Breast Cancer Treatment Amcenestrant | Reuters [Internet]. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/sanofi-ends-development-amcenestrant-breast-cancer-treatment-2022-08-17/ (accessed on 28 January 2023).
- Roche Breast Cancer Drug Fails in Phase 2 Following Similar Stumble by Rival Sanofi—MedCity News [Internet]. Available online: https://medcitynews.com/2022/04/roche-breast-cancer-drug-fails-in-phase-2-following-similar-stumble-by-rival-sanofi/ (accessed on 28 January 2023).
- SABCS 2022 [Internet]. Available online: https://www.eventscribe.net/2022/SABCS/fsPopup.asp?PresentationID=1156609&query=SERENA&Mode=presInfo (accessed on 28 January 2023).
- ARV-471 Monotherapy Demonstrates Clinical Benefit in ER+/HER2-Advanced Breast Cancer [Internet]. OncLive. Available online: https://www.onclive.com/view/arv-471-monotherapy-demonstrates-clinical-benefit-in-er-her2--advanced-breast-cancer (accessed on 26 January 2023).
- Clatot, F.; Perdrix, A.; Augusto, L.; Beaussire, L.; Delacour, J.; Calbrix, C.; Sefrioui, D.; Viailly, P.-J.; Bubenheim, M.; Moldovan, C.; et al. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget 2016, 7, 74448–74459. [Google Scholar] [CrossRef] [PubMed]
- De Santo, I.; McCartney, A.; Migliaccio, I.; Di Leo, A.; Malorni, L. The Emerging Role of ESR1 Mutations in Luminal Breast Cancer as a Prognostic and Predictive Biomarker of Response to Endocrine Therapy. Cancers 2019, 11, 1894. [Google Scholar] [CrossRef]
- Fribbens, C.; O’Leary, B.; Kilburn, L.; Hrebien, S.; Garcia-Murillas, I.; Beaney, M.; Cristofanilli, M.; Andre, F.; Loi, S.; Loibl, S.; et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2961–2968. [Google Scholar] [CrossRef]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- Nye, J. Duration of Prior CDK4/6 Inhibitor Treatment Tied to PFS with Elacestrant [Internet]. Cancer Therapy Advisor. 2022. Available online: https://www.cancertherapyadvisor.com/home/news/conference-coverage/san-antonio-breast-cancer-symposium-sabcs/sabcs-2022/cdk4-6-inhibitor-duration-pfs-elacestrant/ (accessed on 28 January 2023).
- Research C for DE and FDA Approves Elacestrant for ER-Positive, HER2-Negative, ESR1-Mutated Advanced or Metastatic Breast Cancer. FDA [Internet]. 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer (accessed on 28 January 2023).
- Bidard, F.C.; Hardy-Bessard, A.C.; Dalenc, F.; Bachelot, T.; Pierga, J.Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.S.; Ferrero, J.M.; et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- SABCS 2022 [Internet]. Available online: https://www.eventscribe.net/2022/SABCS/fsPopup.asp?PresentationID=1156611&query=capivasertib&Mode=presInfo (accessed on 29 January 2023).
- Jhaveri, K.L.; Lim, E.; Hamilton, E.P.; Saura, C.; Meniawy, T.; Jeselsohn, R.; Beck, J.T.; Kaufman, P.A.; Sammons, S.; Banda, K.; et al. A first-in-human phase 1a/b trial of LY3484356, an oral selective estrogen receptor (ER) degrader (SERD) in ER+ advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Results from the EMBER study. J. Clin. Oncol. 2021, 39, 1050. [Google Scholar] [CrossRef]
- Xie, H.; Liu, J.; Alem Glison, D.M.; Fleming, J.B. The clinical advances of proteolysis targeting chimeras in oncology. Explor. Target. Anti-Tumor Ther. 2021, 2, 511–521. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.; Qian, Y.; Gough, S.; Andreoli, M.; Bookbinder, M.; Cadelina, G.; Bradley, J.; Rousseau, E.; Willard, R.; Pizzano, J.; et al. Abstract P5-04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Res. 2019, 79, P5-04-18. [Google Scholar] [CrossRef]
- Campbell, I.G.; Russell, S.E.; Choong, D.Y.H.; Montgomery, K.G.; Ciavarella, M.L.; Hooi, C.S.F.; Cristiano, B.E.; Pearson, R.B.; Phillips, W.A. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004, 64, 7678–7681. [Google Scholar] [CrossRef] [PubMed]
- Bachman, K.E.; Argani, P.; Samuels, Y.; Silliman, N.; Ptak, J.; Szabo, S.; Konishi, H.; Karakas, B.; Blair, B.G.; Lin, C.; et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol. Ther. 2004, 3, 772–775. [Google Scholar] [CrossRef]
- Miron, A.; Varadi, M.; Carrasco, D.; Li, H.; Luongo, L.; Kim, H.J.; Park, S.Y.; Cho, E.Y.; Lewis, G.; Kehoe, S.; et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2010, 70, 5674–5678. [Google Scholar] [CrossRef] [PubMed]
- Kodahl, A.R.; Ehmsen, S.; Pallisgaard, N.; Jylling, A.M.B.; Jensen, J.D.; Laenkholm, A.-V.; Knoop, A.S.; Ditzel, H.J. Correlation between circulating cell-free PIK3CA tumor DNA levels and treatment response in patients with PIK3CA-mutated metastatic breast cancer. Mol. Oncol. 2018, 12, 925–935. [Google Scholar] [CrossRef]
- Dumbrava, E.E.; Call, S.G.; Huang, H.J.; Stuckett, A.L.; Madwani, K.; Adat, A.; Hong, D.S.; Piha-Paul, S.A.; Subbiah, V.; Karp, D.D.; et al. PIK3CA mutations in plasma circulating tumor DNA predict survival and treatment outcomes in patients with advanced cancers. ESMO Open 2021, 6, 100230. [Google Scholar] [CrossRef]
- Criscitiello, C.; Viale, G.; Curigliano, G.; Goldhirsch, A. Profile of buparlisib and its potential in the treatment of breast cancer: Evidence to date. Breast Cancer 2018, 10, 23–29. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.M.; Rugo, H.S.; Mayer, I.A.; Levy, C.; Forget, F.; Delgado Mingorance, J.I.; Safra, T.; Masuda, N.; Park, Y.H.; Juric, D.; et al. Patient-Reported Outcomes in Patients with PIK3CA-Mutated Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer From SOLAR-1. J. Clin. Oncol. 2021, 39, 2005–2015. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Rugo, H.S.; Chia, S.K.L.; Lerebours, F.; Ruiz-Borrego, M.; Drullinsky, P.; Ciruelos, E.M.; Neven, P.; Park, Y.H.; Arce, C.H.; et al. Alpelisib (ALP) + endocrine therapy (ET) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–), PIK3CA-mutated (mut) advanced breast cancer (ABC): Baseline biomarker analysis and progression-free survival (PFS) by duration of prior cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) therapy in the BYLieve study. J. Clin. Oncol. 2022, 40, 1018. [Google Scholar] [CrossRef]
- PI3KΑ Inhibitor Molecule Overview | Loxo Oncology [Internet]. Available online: https://www.lillyloxooncologypipeline.com/molecule/pi3k-alpha-inhibitor (accessed on 18 February 2023).
- Relay Therapeutics, Inc. A First-in-Human Study of Mutant-Selective PI3Kα Inhibitor, RLY-2608, as a Single Agent in Advanced Solid Tumor Patients and in Combination with Fulvestrant in Patients with Advanced Breast Cancer [Internet]. clinicaltrials.gov; Report No.: NCT05216432. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05216432 (accessed on 17 February 2023).
- Smyth, L.M.; Tamura, K.; Oliveira, M.; Ciruelos, E.M.; Mayer, I.A.; Sablin, M.-P.; Biganzoli, L.; Ambrose, H.J.; Ashton, J.; Barnicle, A.; et al. Capivasertib, an AKT Kinase Inhibitor, as Monotherapy or in Combination with Fulvestrant in Patients with AKT1 E17K-Mutant, ER-Positive Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3947–3957. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Sanchez, C.; Gao, F.; Crowder, R.; Naughton, M.; Pluard, T.; Creekmore, A.; Guo, Z.; Hoog, J.; Lockhart, A.C.; et al. A Phase I Study of the AKT Inhibitor MK-2206 in Combination with Hormonal Therapy in Postmenopausal Women with Estrogen Receptor-Positive Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2650–2658. [Google Scholar] [CrossRef]
- Howell, S.J.; Casbard, A.; Carucci, M.; Ingarfield, K.; Butler, R.; Morgan, S.; Meissner, M.; Bale, C.; Bezecny, P.; Moon, S.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): Overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022, 23, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca. A Phase Ib/III Randomised Study of Capivasertib Plus Palbociclib and Fulvestrant Versus Placebo Plus Palbociclib and Fulvestrant in Hormone Receptor-Positive and Human Epidermal Growth Factor Receptor 2-Negative Locally Advanced, Unresectable or Metastatic Breast Cancer [Internet]. clinicaltrials.gov; Report No.: NCT04862663. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04862663 (accessed on 22 February 2023).
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; Amir, E.; Vera-Badillo, F.; Ocana, A.; Pond, G.R.; Tannock, I.F. Influence of censoring on conclusions of trials for women with metastatic breast cancer. Eur. J. Cancer 2015, 51, 721–724. [Google Scholar] [CrossRef]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; de Boer, R.H.; Hurvitz, S.; Yardley, D.A.; Kovalenko, E.; Ejlertsen, B.; Blau, S.; Özgüroğlu, M.; Landherr, L.; Ewertz, M.; et al. Everolimus Plus Exemestane vs Everolimus or Capecitabine Monotherapy for Estrogen Receptor–Positive, HER2-Negative Advanced Breast Cancer: The BOLERO-6 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Sammons, S.; Shastry, M.; Dent, S.; Anders, C.; Hamilton, E. Practical Treatment Strategies and Future Directions after Progression While Receiving CDK4/6 Inhibition and Endocrine Therapy in Advanced HR+/HER2− Breast Cancer. Clin. Breast Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Jeselsohn, R.; Yelensky, R.; Buchwalter, G.; Frampton, G.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; Ferrer-Lozano, J.; Perez-Fidalgo, J.A.; Cristofanilli, M.; Gómez, H.; et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1757–1767. [Google Scholar] [CrossRef]
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Tomiguchi, M.; Sueta, A.; Murakami, K.; Iwase, H. Clinical significance of plasma cell-free DNA mutations in PIK3CA, AKT1, and ESR1 gene according to treatment lines in ER-positive breast cancer. Mol. Cancer 2018, 17, 67. [Google Scholar] [CrossRef]
- Shibayama, T.; Low, S.-K.; Ono, M.; Kobayashi, T.; Kobayashi, K.; Fukada, I.; Ito, Y.; Ueno, T.; Ohno, S.; Nakamura, Y.; et al. Clinical significance of gene mutation in ctDNA analysis for hormone receptor-positive metastatic breast cancer. Breast Cancer Res. Treat. 2020, 180, 331–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).