Post-CDK 4/6 Inhibitor Therapy: Current Agents and Novel Targets
Abstract
Simple Summary
Abstract
1. Introduction
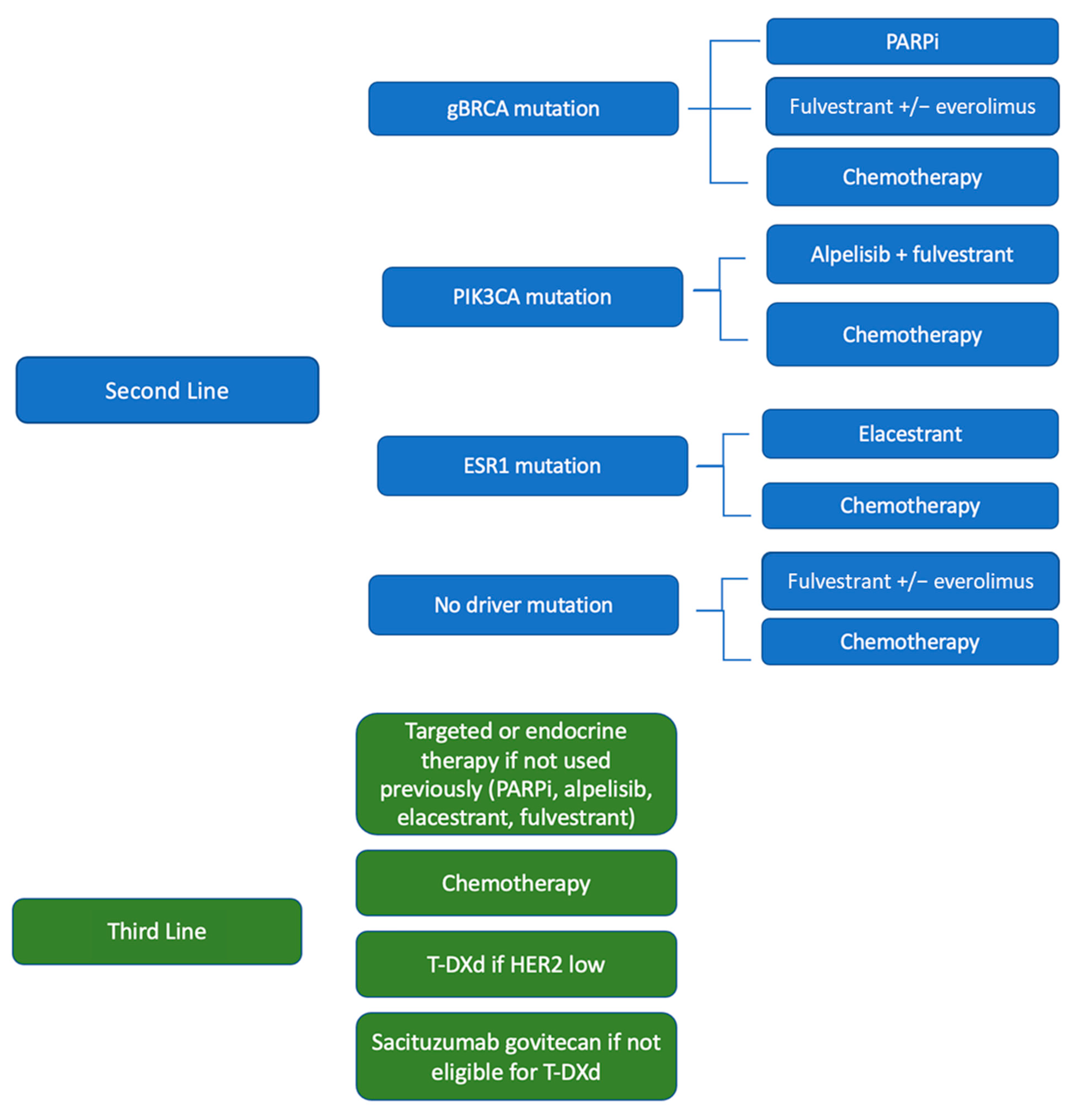
2. Second-Line Therapy Options
2.1. Poly (ADP-Ribose) Polymerase Inhibitors
2.2. Alpelisib
2.3. Fulvestrant
2.4. Exemestane and Everolimus
2.5. Trastuzumab Deruxtecan
2.6. Sacituzumab Govitecan
2.7. Continuing CDK4/6i
3. Targeting the Estrogen Receptor
3.1. Oral Selective Estrogen Receptor Degraders
3.2. Proteolysis-Targeting Chimeras (PROTAC)
3.3. Selective Estrogen Receptor Covalent Antagonists
3.4. Complete Estrogen Receptor Antagonists (CERANs)
3.5. Selective Estrogen Receptor Modulators (SERMs)
4. Overcoming Endocrine Resistance
4.1. Immunotherapy
4.2. Capivasertib and Fulvestrant
4.3. Samuraciclib plus Fulvestrant
4.4. Selective Androgen Receptor Modulators
4.5. Fibroblast Growth Factor Receptor (FGFR) Inhibitors
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 809–815. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2022. 2022. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on 6 February 2023).
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated Results from MONALEESA-2, a Phase III Trial of First-Line Ribociclib Plus Letrozole Versus Placebo Plus Letrozole in Hormone Receptor-Positive, HER2−Negative Advanced Breast Cancer. Ann. Oncol. 2019, 30, 1842. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 Final PFS: A Randomized Study of Abemaciclib as Initial Therapy for Advanced Breast Cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib Plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib Plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Goetz, M.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.; Campone, M.; Chen, S.; Manso Sanchez, L.; Paluch-Shimon, S.; et al. LBA15—MONARCH 3: Interim overall Survival (OS) Results of Abemaciclib Plus a Nonsteroidal Aromatase Inhibitor (NSAI) in Patients (Pts) with HR+, HER2− Advanced Breast Cancer (ABC). Ann. Oncol. 2022, 33, S1384. [Google Scholar] [CrossRef]
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Chavez Mac Gregor, M.; Bananis, E.; et al. Overall Survival (OS) with First-Line Palbociclib Plus Letrozole (PAL+LET) Versus Placebo Plus Letrozole (PBO+LET) in Women with Estrogen Receptor–positive/Human Epidermal Growth Factor Receptor 2–negative Advanced Breast Cancer (ER+/HER2− ABC): Analyses from PALOMA-2. JCO 2022, 40, LBA1003. [Google Scholar]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.S.; Kiedrowski, L.A. Olaparib in Hormone Receptor-Positive, HER2−Negative Metastatic Breast Cancer with a Somatic BRCA2 Mutation. Ther. Adv. Med. Oncol. 2021, 13, 17588359211006962. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD Final overall Survival and Tolerability Results: Olaparib Versus Chemotherapy Treatment of Physician’s Choice in Patients with a Germline BRCA Mutation and HER2−Negative Metastatic Breast Cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Ostinelli, A.; Waisberg, F.; Enrico, D.; Ponce, C.; Rivero, S.; Blanco, A.; Zarba, M.; Loza, M.; Fabiano, V.; et al. Cyclin-Dependent Kinase 4/6 Inhibitor Outcomes in Patients with Advanced Breast Cancer Carrying Germline Pathogenic Variants in DNA Repair-Related Genes. JCO Precis Oncol. 2022, 6, e2100140. [Google Scholar] [CrossRef] [PubMed]
- Safonov, A.; Bandlamudi, C.; de Lara, P.T.; Ferraro, E.; Derakhshan, F.; Will, M.; Donoghue, M.; Selenica, P.; Drago, J.; Rosen, E.; et al. Abstract GS4-08: Comprehensive Genomic Profiling of Patients with Breast Cancer Identifies Germline-Somatic Interactions Mediating Therapy Resistance. Cancer Res. 2022, 82, GS4-08. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Huang, B.; Li, X.; Yang, L.; Hu, X.; Jiang, Y.; Shao, Z.; Wang, Z. Efficacy and Mechanism of the Combination of PARP and CDK4/6 Inhibitors in the Treatment of Triple-Negative Breast Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 122. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib Plus Fulvestrant for PIK3CA-Mutated, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor-2–negative Advanced Breast Cancer: Final overall Survival Results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Turner, N.; Rugo, H.; Ciruelos, E.; Ruiz-Borrego, M.; Drullinsky, P.; Lerebours, F.; Prat, A.; Bachelot, T.; Chia, S.; Balbin, A.; et al. Abstract PD15-01: Impact of ESR1 Mutations on Endocrine Therapy (ET) Plus Alpelisib Benefit in Patients with Hormone Receptor-Positive (HR+), Human Epidermal Growth Factor Receptor 2-Negative (HER2−), PIK3CA -Mutated, Advanced Breast Cancer (ABC) Who Progressed on Or After Prior Cyclin-Dependent Kinase Inhibitor (CDK4/6i) Therapy in the BYLieve Trial. Cancer Res. 2022, 82, PD15-01. [Google Scholar]
- Rugo, H.S.; Neven, P.; Saffie, I.; Park, Y.H.; De Laurentiis, M.; Lerebours, F.; Ciruelos, E.M.; Turner, N.; Juric, D.; Gu, E.; et al. Abstract PD13-05: Alpelisib + Fulvestrant in Patients with PIK3CA-Mutated, HR+, HER− Advanced Breast Cancer (ABC) Who Received Chemotherapy Or Endocrine Therapy (ET) as Immediate Prior Treatment: BYLieve Cohort C Primary Results and Exploratory Biomarker Analyses. Cancer Res. 2021, 82, PD13-05. [Google Scholar]
- Bardia, A.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.; Sohn, J.H.; Vuylsteke, P.; Harnden, K.K.; Khong, H.; et al. Elacestrant, an Oral Selective Estrogen Receptor Degrader (SERD), Vs Investigator’s Choice of Endocrine Monotherapy for ER+/HER2− Advanced/Metastatic Breast Cancer (mBC) Following Progression on Prior Endocrine and CDK4/6 Inhibitor Therapy: Results of EMERALD Phase 3 Trial. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 7–10 December 2021; 2021, p. GS2-02. [Google Scholar]
- Lindeman, G.J.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.M.; McCune, S.L.; Armstrong, A.; Shannon, C.M.; Bertelli, G.; et al. Results from VERONICA: A Randomized, Phase II Study of Second-/Third-Line Venetoclax (VEN) + Fulvestrant (F) Versus F Alone in Estrogen Receptor (ER)-Positive, HER2−Negative, Locally Advanced, Or Metastatic Breast Cancer (LA/MBC). JCO 2021, 39, 1004. [Google Scholar] [CrossRef]
- Dhakal, A.; Antony Thomas, R.; Levine, E.G.; Brufsky, A.; Takabe, K.; Hanna, M.G.; Attwood, K.; Miller, A.; Khoury, T.; Early, A.P.; et al. Outcome of Everolimus-Based Therapy in Hormone-Receptor-Positive Metastatic Breast Cancer Patients After Progression on Palbociclib. Breast Cancer. 2020, 14, 1178223420944864. [Google Scholar]
- Lupichuk, S.M.; Recaldin, B.; Nixon, N.; Mututino, A.; Joy, A.A. Abstract P4-13-06: Real-World Experience using Exemestane and Everolimus in Patients with Hormone Receptor Positive/HER2 Negative Breast Cancer with and without Prior CDK4/6 Inhibitor Exposure. Cancer Res. 2019, 79, 4. [Google Scholar] [CrossRef]
- Cook, M.M.; Al Rabadi, L.; Kaempf, A.J.; Saraceni, M.M.; Savin, M.A.; Mitri, Z.I. Everolimus Plus Exemestane Treatment in Patients with Metastatic Hormone Receptor-Positive Breast Cancer Previously Treated with CDK4/6 Inhibitor Therapy. Oncologist 2021, 26, 101–106. [Google Scholar] [CrossRef]
- Nakayama, T.; Fujisawa, F. Therapy Options After CDK4/6 Inhibitors for HR+, HER2− Postmenopausal Metastatic/Recurrent Breast Cancer in Japan: A Role for Mammalian Target of Rapamycin Inhibitors? Future Oncol. 2020, 16, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Diamond, J.R.; Vahdat, L.T.; Tolaney, S.M.; Juric, D.; O’Shaughnessy, J.; Moroose, R.L.; Mayer, I.A.; Abramson, V.G.; Goldenberg, D.M.; et al. Sacituzumab Govitecan in Previously Treated Hormone Receptor-Positive/HER2−Negative Metastatic Breast Cancer: Final Results from a Phase I/II, Single-Arm, Basket Trial. Ann. Oncol. 2020, 31, 1709–1718. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.M.; Dalenc, F.; Gomez Pardo, P.; et al. LBA76—Overall Survival (OS) Results from the Phase III TROPiCS-02 Study of Sacituzumab Govitecan (SG) Vs Treatment of Physician’s Choice (TPC) in Patients (Pts) with HR+/HER2− Metastatic Breast Cancer (mBC). Ann. Oncol. 2022, 33, S808–S869. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.; Dalenc, F.; Gómez Pardo, P.; et al. Primary Results from TROPiCS-02: A Randomized Phase 3 Study of Sacituzumab Govitecan (SG) Versus Treatment of Physician’s Choice (TPC) in Patients (Pts) with Hormone Receptor–positive/HER2−Negative (HR+/HER2−) Advanced Breast Cancer. JCO 2022, 40, LBA1001. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2−Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; Raptis, G.; Baer, L.N.; Young Oh, S.; et al. A Randomized, Phase II Trial of Fulvestrant Or Exemestane with Or without Ribociclib After Progression on Anti-Estrogen Therapy Plus Cyclin-Dependent Kinase 4/6 Inhibition (CDK 4/6i) in Patients (Pts) with Unresectable Or Hormone Receptor–positive (HR+), HER2−Negative Metastatic Breast Cancer (MBC): MAINTAIN Trial. JCO 2022, 40, LBA1004. [Google Scholar]
- Mayer, E.L.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.; Miller, K.D.; Jolly, T.; et al. PACE: Palbociclib After CDK and Endocrine Therapy A Randomized Phase II Study of Fulvestrant +/− Palbociclib After Progression on CDK4/6 Inhibitor for HR+/HER2− Metastatic Breast Cancer. J. Clin. Oncol. 2022, 36, TPS1104. [Google Scholar] [CrossRef]
- Lv, M.; Mao, Y.; Ma, T.; Wang, Y.; Liu, X.; Song, Y.; Wang, H. Real-World Efficacy of Fulvestrant Monotherapy as the First Treatment Or Maintenance Treatment in Patients with Metastatic Breast Cancer. Breast Care 2021, 16, 368–375. [Google Scholar] [CrossRef]
- Skinner, K.E.; Olufade, T.; Walker, M.S.; Schwartzberg, L.S. Real-World Effectiveness of Fulvestrant Monotherapy as First Endocrine Treatment in Patients with Metastatic Breast Cancer. Breast J. 2020, 26, 112–119. [Google Scholar] [CrossRef]
- Johnston, S.R.; Kilburn, L.S.; Ellis, P.; Dodwell, D.; Cameron, D.; Hayward, L.; Im, Y.H.; Braybrooke, J.P.; Brunt, A.M.; Cheung, K.L.; et al. Fulvestrant Plus Anastrozole Or Placebo Versus Exemestane Alone After Progression on Non-Steroidal Aromatase Inhibitors in Postmenopausal Patients with Hormone-Receptor-Positive Locally Advanced Or Metastatic Breast Cancer (SoFEA): A Composite, Multicentre, Phase 3 Randomised Trial. Lancet Oncol. 2013, 14, 989–998. [Google Scholar]
- Chia, S.; Gradishar, W.; Mauriac, L.; Bines, J.; Amant, F.; Federico, M.; Fein, L.; Romieu, G.; Buzdar, A.; Robertson, J.F.; et al. Double-Blind, Randomized Placebo Controlled Trial of Fulvestrant Compared with Exemestane After Prior Nonsteroidal Aromatase Inhibitor Therapy in Postmenopausal Women with Hormone Receptor-Positive, Advanced Breast Cancer: Results from EFECT. J. Clin. Oncol. 2008, 26, 1664–1670. [Google Scholar] [CrossRef]
- Bidard, F.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results from the Randomized Phase III EMERALD Trial. JCO 2022, 40, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Zaiss, M.; Harper-Wynne, C.; Ferreira, M.; Dubey, S.; Chan, S.; Makris, A.; Nemsadze, G.; Brunt, A.M.; Kuemmel, S.; et al. Fulvestrant Plus Vistusertib Vs Fulvestrant Plus Everolimus Vs Fulvestrant Alone for Women with Hormone Receptor–Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1556–1564. [Google Scholar] [CrossRef]
- Bidard, F.C.; Hardy-Bessard, A.C.; Dalenc, F.; Bachelot, T.; Pierga, J.Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.S.; Ferrero, J.M.; et al. Switch to Fulvestrant and Palbociclib Versus no Switch in Advanced Breast Cancer with Rising ESR1 Mutation during Aromatase Inhibitor and Palbociclib Therapy (PADA-1): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Schott, A.F.; Ma, C.; Hamilton, E.P.; Nanda, R.; Zahrah, G.; Hunter, N.; Tan, A.R.; Telli, M.L.; Mesias, J.A.; et al. ARV-471, a PROTAC® Estrogen Receptor (ER) Degrader in Advanced ER-Positive/Human Epidermal Growth Factor Receptor 2 (HER2)-Negative Breast Cancer: Phase 2 Expansion (VERITAC) of a Phase 1/2 Study. In Proceedings of the SABCS 2022, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Wander, S.A.; Han, H.S.; Zangardi, M.L.; Niemierko, A.; Mariotti, V.; Kim, L.S.L.; Xi, J.; Pandey, A.; Dunne, S.; Nasrazadani, A.; et al. Clinical Outcomes with Abemaciclib After Prior CDK4/6 Inhibitor Progression in Breast Cancer: A Multicenter Experience. J. Natl. Compr. Cancer Netw. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Boni, V.; Sohn, J.; Villanueva-Vásquez, R.; Bardia, A.; Schmid, P.; Lim, E.; Patel, J.M.; Perez-Fidalgo, J.; Loi, S.; et al. Safety and Activity of Single-Agent Giredestrant (GDC-9545) from a Phase Ia/B Study in Patients (Pts) with Estrogen Receptor-Positive (ER+), HER2−Negative Locally Advanced/Metastatic Breast Cancer (LA/mBC). JCO 2021, 39, 1017. [Google Scholar] [CrossRef]
- Bardia, A.; Kaklamani, V.; Wilks, S.; Weise, A.; Richards, D.; Harb, W.; Osborne, C.; Wesolowski, R.; Karuturi, M.; Conkling, P.; et al. Phase I Study of Elacestrant (RAD1901), a Novel Selective Estrogen Receptor Degrader, in ER-Positive, HER2−Negative Advanced Breast Cancer. JCO 2021, 39, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.; Oliveira, M.; Gil, E.M.C.; Patel, M.R.; Bermejo de las Heras, B.; Ruiz-Borrego, M.; García-Corbacho, J.; Armstrong, A.; Banerji, U.; Twelves, C.; et al. Abstract PS11-05: Updated Data from SERENA-1: A Phase 1 Dose Escalation and Expansion Study of the Next Generation Oral SERD AZD9833 as a Monotherapy and in Combination with Palbociclib, in Women with ER-Positive, HER2−Negative Advanced Breast Cancer. Cancer Res. 2021, 81, PS11-05. [Google Scholar] [CrossRef]
- Aftimos, P.; Neven, P.; Pegram, M.; van Oordt, C.W.M.V.D.H.; Dees, E.C.; Schröder, C.; Jager, A.; Bulat, I.; Chap, L.; Maglakelidze, M.; et al. Abstract PS12-04: Rintodestrant (G1T48), an Oral Selective Estrogen Receptor Degrader in ER+/HER2− Locally Advanced Or Metastatic Breast Cancer: Updated Phase 1 Results and Dose Selection. Cancer Res. 2021, 81, PS12-04. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Jeselsohn, R.; Lim, E.; Hamilton, E.P.; Yonemori, K.; Beck, J.T.; Kaufman, P.A.; Sammons, S.; Bhave, M.A.; Saura, C.; et al. A Phase 1a/B Trial of Imlunestrant (LY3484356), an Oral Selective Estrogen Receptor Degrader (SERD) in ER-Positive (ER+) Advanced Breast Cancer (aBC) and Endometrial Endometrioid Cancer (EEC): Monotherapy Results from EMBER. JCO 2022, 40, 1021. [Google Scholar] [CrossRef]
- Oliveira, M.; Pominchuk, D.; Nowecki, Z.; Hamilton, E.; Kulyaba, Y.; Andabekov, T.; Hotko, Y.; Melkadze, T.; Nemsadze, G.; Neven, P.; et al. Camizestrant, a Next-Generation Oral SERD vs. Fulvestrant in Post-Menopausal Women with Advanced ER-Positive HER2−Negative Breast Cancer: Results of the Randomized, Multi-Dose Phase 2 SERENA-2 Trial. In Proceedings of the SABCS 2022, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Bardia, A.; Chandarlapaty, S.; Linden, H.M.; Ulaner, G.A.; Gosselin, A.; Cartot-Cotton, S.; Cohen, P.; Doroumian, S.; Paux, G.; Celanovic, M.; et al. AMEERA-1 Phase 1/2 Study of Amcenestrant, SAR439859, in Postmenopausal Women with ER-Positive/HER2−Negative Advanced Breast Cancer. Nat. Commun. 2022, 13, 4116–4118. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Oliveira, M.; Banerji, U.; Hernando, C.; Garcia-Corbacho, J.; Armstrong, A.; Ciruelos, E.; Patel, M.R.; Incorvati, J.; Twelves, C.; et al. A Phase I Dose Escalation and Expansion Study of the Next Generation Oral SERD AZD9833 in Women with ER-Positive, HER2−Negative Advanced Breast Cancer. JCO 2020, 38, 1024. [Google Scholar] [CrossRef]
- Oliveira, M.; Hamilton, E.P.; Incorvati, J.; Bermejo de la Heras, B.; Calvo, E.; García-Corbacho, J.; Ruiz-Borrego, M.; Vaklavas, C.; Turner, N.C.; Ciruelos, E.M.; et al. Serena-1: Updated Analyses from a Phase 1 Study (Parts C/D) of the Next-Generation Oral SERD Camizestrant (AZD9833) in Combination with Palbociclib, in Women with ER-Positive, HER2−Negative Advanced Breast Cancer. JCO 2022, 40, 1032. [Google Scholar] [CrossRef]
- Bardia, A.; Bidard, F.C.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. EMERALD Phase 3 Trial of Elacestrant Versus Standard of Care Endocrine Therapy in Patients with ER+/HER2− Metastatic Breast Cancer: Updated Results by Duration of Prior CDK4/6i in Metastatic Setting. In Proceedings of the SABC 2022, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Reed, J. Press Release: Sanofi Provides Update on Amcenestrant Clinical Development Program; Sanofi: Paris, France, 2022. [Google Scholar]
- Martin Jimenez, M.; Lim, E.; Chavez Mac Gregor, M.; Bardia, A.; Wu, J.; Zhang, Q.; Nowecki, Z.; Cruz, F.; Safin, R.; Kim, S.; et al. Giredestrant (GDC-9545) Vs Physician Choice of Endocrine Monotherapy (PCET) in Patients (Pts) with ER+, HER2– Locally Advanced/Metastatic Breast Cancer (LA/mBC): Primary Analysis of the Phase II, Randomised, Open-Label acelERA BC Study. Ann. Oncol. 2022, 33, S633–S634. [Google Scholar] [CrossRef]
- Snyder, L.B.; Flanagan, J.J.; Qian, Y.; Gough, S.M.; Andreoli, M.; Bookbinder, M.; Cadelina, G.; Bradley, J.; Rousseau, E.; Chandler, J. The Discovery of ARV-471, an Orally Bioavailable Estrogen Receptor Degrading PROTAC for the Treatment of Patients with Breast Cancer. In Proceedings of the 112th Annual Meeting of the American Association for Cancer Research, Online, 10–15 April, 17–21 May 2021. [Google Scholar]
- Turner, N.C.; Oliveira, M.; Howell, S.; Dalenc, F.; Cortes, J.; Gomez, H.; Hu, X.; Jhaveri, K.; Loibl, S.; Morales Murillo, S.; et al. Capivasertib and Fulvestrant for Patients with Aromatase Inhibitor-Resistant Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results from the Phase III CAPItello-291 Trial. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 9 December 2021. [Google Scholar]
- Hamilton, E.P.; Wang, J.S.; Pluard, T.J.; Johnston, S.R.D.; Morikawa, A.; Dees, E.C.; Jones, R.H.; Haley, B.B.; Armstrong, A.C.; Cohen, A.L.; et al. Phase I/II Study of H3B-6545, a Novel Selective Estrogen Receptor Covalent Antagonist (SERCA), in Estrogen Receptor Positive (ER+), Human Epidermal Growth Factor Receptor 2 Negative (HER2−) Advanced Breast Cancer. JCO 2021, 39, 1018. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Wang, J.S.; Pluard, T.; Morikawa, A.; Dees, E.C.; Jones, R.H.; Haley, B.; Armstrong, A.; Cohen, A.L.; Munster, P.; et al. Abstract P1-17-10: H3B-6545, a Novel Selective Estrogen Receptor Covalent Antagonist (SERCA), in Estrogen Receptor Positive (ER+), Human Epidermal Growth Factor Receptor 2 Negative (HER2−) Advanced Breast Cancer—A Phase II Study. Cancer Res. 2022, 82, P1-10. [Google Scholar] [CrossRef]
- Patel, M.; Alemany, C.; Mitri, Z.; Makower, D.; Borges, V.; Sparano, J.; Le, T.; Klein, P.; Lawrence, J.; Kushner, P.; et al. Abstract P1-17-12: Preliminary Data from a Phase I/II, Multicenter, Dose Escalation Study of OP-1250, an Oral CERAN/SERD, in Subjects with Advanced and/Or Metastatic Estrogen Receptor (ER)-Positive, HER2−Negative Breast Cancer. Cancer Res. 2022, 82, P1-12. [Google Scholar] [CrossRef]
- Coombes, C.; Howell, S.; Krebs Matthew, G.; Lord, S.; Kenny, L.; Bahl, A.; Clack, G.; Ainscow, E.; Dickinson, P.A.; Fostea, R.; et al. Study of Samuraciclib (CT7001), a First-in-Class, Oral, Selective Inhibitor of CDK7, in Combination with Fulvestrant in Patients with Advanced Hormone Receptor-Positive, HER2−Negative Breast Cancer. In Proceedings of the 2021 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 9 December 2021. [Google Scholar]
- Palmieri, C.; Linden, H.M.; Birrell, S.; Lim, E.; Schwartzberg, L.S.; Rugo, H.S.; Cobb, P.W.; Jain, K.; Vogel, C.L.; O’Shaughnessy, J.; et al. Efficacy of Enobosarm, a Selective Androgen Receptor (AR) Targeting Agent, Correlates with the Degree of AR Positivity in Advanced AR+/Estrogen Receptor (ER)+ Breast Cancer in an International Phase 2 Clinical Study. JCO 2021, 39, 1020. [Google Scholar] [CrossRef]
- Palmieri, C.; Linden, H.; Birrell, S.; Lim, E.; Schwartzberg, L.S.; Rugo, H.S.; Cobb, P.; Jain, K.; Vogel, C.; O’Shaughnessy, J.A.; et al. Abstract PD8-10: Efficacy and Safety of Enobosarm, a Selective Androgen Receptor Modulator, to Target AR in Women with Advanced ER+/AR+ Breast Cancer—Final Results from an International Phase 2 Randomized Study. Cancer Res. 2021, 81, PD8-10. [Google Scholar] [CrossRef]
- Chae, Y.K.; Hong, F.; Vaklavas, C.; Cheng, H.H.; Hammerman, P.; Mitchell, E.P.; Zwiebel, J.A.; Ivy, S.P.; Gray, R.J.; Li, S.; et al. Phase II Study of AZD4547 in Patients with Tumors Harboring Aberrations in the FGFR Pathway: Results from the NCI-MATCH Trial (EAY131) Subprotocol W. J. Clin. Oncol. 2020, 38, 2407–2417. [Google Scholar] [CrossRef]
- Korpal, M.; Furman, C.; Puyang, X.; Zhang, Z.; Wu, Z.; Banka, D.; Das, S.; Destenaves, B.; Gao, L.; Hamilton, E.; et al. Abstract PS12-23: Development of H3B-6545, a First-in-Class Oral Selective ER Covalent Antagonist (SERCA), for the Treatment of ERaWT and ERaMUT Breast Cancer. Cancer Res. 2021, 81, PS12-23. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Pluard, T.J.; Wang, J.S.; Hamilton, E.P.; Juric, D.; Scholz, C.R.; Hnitecki, E.; Gao, L.; Cantagallo, L.; Korpal, M.; et al. Phase 1b Study of H3B-6545 in Combination with Palbociclib in Women with Metastatic Estrogen Receptor–positive (ER+), Human Epidermal Growth Factor Receptor 2 (HER2)-Negative Breast Cancer. JCO 2021, 39, e13025. [Google Scholar] [CrossRef]
- Hodges-Gallagher, L.; Richard Sun, R.; Myles, D.; Harmon, C.; Kushner, P. OP-1250 is a Complete Estrogen Receptor Antagonist (CERAN) that Lacks Agonist Activity on Cell Signaling and Proliferation in Breast Cancer Cells. In Proceedings of the 32nd EORTC-NCI-AACR Symposium, Barcelona, Spain, 24–25 October 2020. [Google Scholar]
- Lainé, M.; Fanning, S.W.; Chang, Y.F.; Green, B.; Greene, M.E.; Komm, B.; Kurleto, J.D.; Phung, L.; Greene, G.L. Lasofoxifene as a Potential Treatment for Therapy-Resistant ER-Positive Metastatic Breast Cancer. Breast Cancer Res. 2021, 23, 54. [Google Scholar] [CrossRef]
- Goetz, M.; Plourde, P.; Stover, D.; Bagegni, N.; Vidal, G.; Brufsky, A.; Rugo, H.; Portman, D.J.; Gal-Yam, E. LBA20—Open-Label, Randomized Study of Lasofoxifene (LAS) Vs Fulvestrant (Fulv) for Women with Locally Advanced/Metastatic ER+/HER2− Breast Cancer (mBC), an Estrogen Receptor 1 (ESR1) Mutation, and Disease Progression on Aromatase (AI) and Cyclin-Dependent Kinase 4/6 (CDK4/6i) Inhibitors. Ann. Oncol. 2022, 33, S1387–S1388. [Google Scholar]
- Cummings, S.R.; Ensrud, K.; Delmas, P.D.; LaCroix, A.Z.; Vukicevic, S.; Reid, D.M.; Goldstein, S.; Sriram, U.; Lee, A.; Thompson, J.; et al. Lasofoxifene in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2010, 362, 686–696. [Google Scholar] [CrossRef]
- Rugo, H.S.; Delord, J.; Im, S.; Ott, P.A.; Piha-Paul, S.A.; Bedard, P.L.; Sachdev, J.; Le Tourneau, C.; van Brummelen, E.; Varga, A.; et al. Abstract S5-07: Preliminary Efficacy and Safety of Pembrolizumab (MK-3475) in Patients with PD-L1–positive, Estrogen Receptor-Positive (ER+)/HER2−Negative Advanced Breast Cancer Enrolled in KEYNOTE-028. Cancer Res. 2016, 76, 5. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an Anti-PD-L1 Antibody, in Patients with Locally Advanced Or Metastatic Breast Cancer: A Phase 1b JAVELIN Solid Tumor Study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Liu, M.C.; Yau, C.; Asare, S.; Hylton, N.; Veer, L.V.; Perlmutter, J.; Wallace, A.M.; Chien, A.J.; Forero-Torres, A.; et al. Pembrolizumab Plus Standard Neoadjuvant Therapy for High-Risk Breast Cancer (BC): Results from I-SPY 2. JCO 2017, 35, 506. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, J.S.; Yost, S.E.; Frankel, P.H.; Ruel, C.; Egelston, C.A.; Guo, W.; Padam, S.; Tang, A.; Martinez, N.; et al. Phase I/II Trial of Palbociclib, Pembrolizumab and Letrozole in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Eur. J. Cancer 2021, 154, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Prat, A.; Salgado, R.F.; Reinisch, M.; Saura, C.; Ruiz Borrego, M.; Nikolinakos, P.; Filian, J.; Ades, F.; Huang, N.; et al. 92MO Neoadjuvant Nivolumab (NIVO) + Palbociclib (PALBO) + Anastrozole (ANA) for Estrogen Receptor-Positive (ER+)/Human Epidermal Growth Factor Receptor 2-Negative (HER2−) Primary Breast Cancer (BC): CheckMate 7A8. Ann. Oncol. 2022, 33, S165–S166. [Google Scholar] [CrossRef]
- Howell, S.J.; Casbard, A.; Carucci, M.; Ingarfield, K.; Butler, R.; Morgan, S.; Meissner, M.; Bale, C.; Bezecny, P.; Moon, S.; et al. Fulvestrant Plus Capivasertib Versus Placebo After Relapse Or Progression on an Aromatase Inhibitor in Metastatic, Oestrogen Receptor-Positive, HER2−Negative Breast Cancer (FAKTION): Overall Survival, Updated Progression-Free Survival, and Expanded Biomarker Analysis from a Randomised, Phase 2 Trial. Lancet Oncol. 2022, 23, 851–864. [Google Scholar]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The Androgen Receptor is a Tumor Suppressor in Estrogen Receptor-Positive Breast Cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef]
- Freelander, A.; Laven-Law, G.; Eshraghi, L.; Chia, K.M.; Pickering, M.; Yong, A.; Wilkinson, A.; Alexandrou, S.; Caldon, C.E.; Hickey, T.E.; et al. Abstract PD2-02: Combination CDK4/6 Inhibition and AR Agonism Suppresses the Growth of CDK4/6 Inhibitor Resistant Breast Cancers. Cancer Res. 2022, 82, PD2-02. [Google Scholar] [CrossRef]
- Mayer, I.A.; Haley, B.B.; Abramson, V.G.; Brufsky, A.; Rexer, B.; Stringer-Reasor, E.; Jhaveri, K.L.; Sanders, M.; Ericsson-Gonzalez, P.I.; Ye, F.; et al. Abstract PD1-03: A Phase Ib Trial of Fulvestrant + CDK4/6 Inhibitor (CDK4/6i) Palbociclib + Pan-FGFR Tyrosine Kinase Inhibitor (TKI) Erdafitinib in FGFR-Amplified/ER+/HER2−Negative Metastatic Breast Cancer (MBC). Cancer Res. 2021, 81, PD1-03. [Google Scholar] [CrossRef]
| Drug | Trial | n | Prior Lines of Therapy | Prior CDK4/6i | PFS | Response Rate | Reference | |
|---|---|---|---|---|---|---|---|---|
| Alpelisib fulvestrant | Prospective Phase II, BYLieve trial, Cohort A | 121 |
| 99% | 7.3 months | ORR 17% CBR 55% | [21] | |
| Prospective Phase II, BYLieve trial, Cohort C | 112 |
| 84% | 5.6 months |
ORR 28% CBR 43% | [22] | ||
| Fulvestrant | Prospective, Phase III EMERALD trial | 165 |
| 100% | 1.9 months | 6-month PFS rate 23% 12-month PFS rate 10% | [23] | |
| Prospective, Phase II, VERONICA trial | 51 |
| 100% | 1.94 months | CBR 13.7% | [24] | ||
| Exemestane and everolimus | Retrospective | 41 |
| 100% | 4.2 months | ORR 17.1% CBR 17.1% | [25] | |
| Retrospective | 20 |
| 100% | 5.8 months | NA | [26] | ||
| Retrospective | 17 |
| 100% | 5.7 months | 6-month PFS 18% | [27] | ||
| Retrospective | 12 |
| 100% | 11.7 months | NA | [28] | ||
| Sacituzumab govitecan | Prospective, Phase I/II | 32 |
| 100% | 3.8 months | ORR 8% CBR 12% | [29] | |
| Prospective, Phase III TROPiCs-02 | 272 |
| 98% | 5.5 months | ORR at 21% Improvement PFS compared to control: 1.5 months Improvement in OS compared to control: 3.2 months | [30,31] | ||
| Trastuzumab deruxtecan | Prospective, Phase III DESTINY-Breast04 * | 331 |
| 70.4% | 10.1 months | CBR: 71.2%, ORR 52.6% Improvement PFS compared to control: 4.7 months Improvement in OS compared to control: 6.4 months | [32] | |
| Continuing CDK4/6i | Prospective, Phase II MAINTAIN | 120 |
| 97% | 5.29 months | 12-month PF rate 25% | [33] | |
| Prospective Phase II PACE | 220 |
| 99.5% | Fulvestrant: 4.8 months Fulvestrant and palbociclib: 4.6 monthsFulvestrant, palbociclib, avelumab: 8.1 months | CBR fulvestrant monotherapy: 29.1% CBR fulvestrant, palbociclib: 32.4% CBR fulvestrant, palbociclib, avelumab: 35.2% | [34] | ||
| Elacestrant (Radius) | Imlunestrant (Lilly) | Camizestrant (AstraZeneca) | Rintodestrant (G1 Therapeutics) | |
|---|---|---|---|---|
| Phase | III | I | II * | I |
| n | 237 | 141 | 73 | 67 |
| Total % Adverse Events | 92% | 92% | 90.4 | 70% |
| Adverse Events ≥ 10% (%) |
|
|
|
|
| Grade ≥ 3 AE (%) |
|
|
|
|
| References | [39] | [48] | [49] | [47] |
| Drugs Under Investigation | Monotherapy Trials | Prior CDK4/6i | CDK4/6i Combination Trials | Everolimus Combination Trials | Monotherapy Median PFS | Monotherapy Response Rate | References |
|---|---|---|---|---|---|---|---|
| Elacestrant (Radius) | Phase III EMERALD | 100% | Phase II (NCT04791384) CDK4/6i = abemaciclib | NA | Phase I—4.5 months Phase III EMERALD—2.8 months | Phase I CBR—42.6%, ORR 19.4% Phase III EMERALD – 12-month PFS rate 22.3% – PFS 2.8 months, improvement over fulvestrant 0.9 months | [39,50] |
| Giredestrant (Roche) | Phase II acelERA—trial suspended due to lack of PFS benefit at interim analysis | NA | Phase III persevERA (NCT04546009) CDK4/6i = palbociclib | Phase III evERA (NCT05306340) | Phase I—7.2 months Phase II acelERA—trial suspended due to lack of PFS benefit over physician’s choice of ET | Phase I CBR– 48% | [44] |
| Amcenestrant (Sanofi) | Phase II AMEERA-3—trial suspended due to lack of PFS benefit at interim analysis | NA | Phase III AMEERA-5—trial suspended due to lack of clinical benefit at interim analysis | NA | NA | Phase I CBR—28.3%, ORR 10.9% Phase II AMEERA-3—trial suspended due to lack of PFS benefit at interim analysis | [50] |
| Imlunestrant (Lilly) | Phase I EMBER (NCT04188548) | 92% | Phase 3 EMBER-3 (NCT04975308) CDK4/6i = abemaciclib | Phase I EMBER (NCT04188548) | Phase I EMBER, post-CDK4/6i—PFS 6.5 months | Phase I CBR 55%, ORR 8.0% | [48] |
| Camizestrant (AstraZeneca) | Phase II SERENA-2 | 50% | Phase III SERENA-4 (NCT04711252) CDK4/6i = palbociclib Phase III SERENA-6 (NCT04964934) CDK4/6i = palbociclib | NA | Phase II SERENA-2—7.2 months (75mg) in patients with prior CDK4/6i compared to 3.7 months on fulvestrant | Phase I CBR of 42.3%, ORR 16.3% Phase II SERENA-2 (75 mg) CBR 47%, ORR 16% | [49,51,52] |
| Ritodestrant (G1 Therapeutics) | Phase I | 69% | Phase II (NCT03455270) CDK4/6i = palbociclib | NA | NA | Phase I CBR 28% | [47] |
| Drug | ARV-471 | Capivasertib | H3B-6545 | OP-1250 | Samuraciclib plus Fulvestrant | Enobosarm | Erdafitinib |
|---|---|---|---|---|---|---|---|
| MOA | Proteolysis targeting chimeras (PROTAC) | AKT pathway inhibitor | Selective estrogen receptor covalent antagonist (SERCA) | Complete estrogen receptor antagonists (CERAN) | Selective inhibitor of CDK7 | Selective androgen receptor modulator | Fibroblast growth factor receptor (FGFR) Inhibitors |
| Phase | I/II | III | I/II | I | I/II | II | II |
| n | 71 | 355 | 94 | 27 | 31 | 50 | 48 |
| Prior CDK4/6i | 100% | 69% | 85% | 100% | 100% | NA | NA |
| Adverse Events (>10%) |
|
|
|
|
|
|
|
| PFS | 3.7 months | 7.2 months | 5.1 months | NA | TP53 WT: 32 weeks TPS mutant: 7.9 weeks | 5.6 months | 3.4 months |
| Response rate | CBR 38% | CBR 40.3% ORR 16.7% | CBR 21% ORR 9% | CBR 36% | AR ≥ 40% CBR = 80% AR < 40% CBR = 18% | 6-month PFS rate was 15% | |
| References | [42] | [57] | [58,59] | [60] | [61] | [62,63] | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashai, N.; Swain, S.M. Post-CDK 4/6 Inhibitor Therapy: Current Agents and Novel Targets. Cancers 2023, 15, 1855. https://doi.org/10.3390/cancers15061855
Ashai N, Swain SM. Post-CDK 4/6 Inhibitor Therapy: Current Agents and Novel Targets. Cancers. 2023; 15(6):1855. https://doi.org/10.3390/cancers15061855
Chicago/Turabian StyleAshai, Nadia, and Sandra M. Swain. 2023. "Post-CDK 4/6 Inhibitor Therapy: Current Agents and Novel Targets" Cancers 15, no. 6: 1855. https://doi.org/10.3390/cancers15061855
APA StyleAshai, N., & Swain, S. M. (2023). Post-CDK 4/6 Inhibitor Therapy: Current Agents and Novel Targets. Cancers, 15(6), 1855. https://doi.org/10.3390/cancers15061855






