Simple Summary
This study aimed to identify early predictive factors associated with the success of conversion surgery with R0 resection in patients with metastatic gastric cancer who underwent systemic chemotherapy. Patients with carcinoembryonic antigen > 5.0 ng/mL at the initial Response Evaluation Criteria in Solid Tumors evaluation showed less expectancy of undergoing conversion surgery with R0 resection.
Abstract
This retrospective study examined early the predictive factors for successful conversion surgery (CS) with R0 resection in patients with metastatic gastric cancer (MGC) who underwent systemic chemotherapy. This study included 204 patients diagnosed with metastatic gastric adenocarcinoma, who received chemotherapy between 2009 and 2019. Of these patients, 31 (15%) underwent CS with R0 resection. The incidence of CS with R0 resection was not affected by the volume of metastatic lesions or the presence of peritoneal metastasis. The overall survival time of the CS with R0 resection group was significantly longer than that of the non-CS group (hazard ratio, 0.12; 95% confidence interval, 0.07–0.23; p < 0.0001), with a 5 year overall survival rate of 50.2%. Multivariate analysis of 150 patients, excluding those with disease progression until the initial Response Evaluation Criteria in Solid Tumors (RECIST) evaluation, showed that carcinoembryonic antigen > 5.0 ng/mL at the initial RECIST evaluation was an independent, significant, and unfavorable predictor of CS with R0 resection (odds ratio, 0.21; p = 0.0108), whereas systemic chemotherapy with trastuzumab for HER2-positive cancer was a favorable factor (odds ratio, 4.20; p = 0.0119). Monitoring serum carcinoembryonic antigen levels during chemotherapy may be a useful predictor of the CS implementation in patients with MGC.
1. Introduction
Systemic chemotherapy is the standard treatment to palliate symptoms and prolong survival in metastatic gastric cancer (MGC) [1]. Although the median survival time remains unsatisfactory, between 6 and 14 months, complete or near-complete response (CR or near-CR) of the metastatic lesions sometimes occurs. This renders the primary lesion ± the remains of the metastases resectable [2]. This surgery has recently been referred to as conversion surgery (CS), and several retrospective studies have shown its favorable outcomes [3,4,5,6,7,8,9,10,11]. Nevertheless, the clinical significance of CS remains unclear, owing to the lack of prospective clinical trials.
In general, CS should only be performed on patients with excellent responses to pharmacotherapy, with hopes for R0 resection, and who are physically and mentally prepared to undergo major surgery. However, owing to the diversity in the type and volume of metastatic lesions in more complex conditions and the frequent presence of lesions undetected by conventional imaging studies, it is challenging to design an appropriate clinical trial to explore CS for MGC. Thus, before conducting such a trial, an objective and simple index should be developed to select suitable candidates for CS. Furthermore, it is known that tumor response evaluated by computed tomography (CT) imaging and a marked decrease in serum tumor marker values during chemotherapy correlate with better prognosis in various malignancies, including MGC [12,13,14,15,16,17].
In this study, we aimed to retrospectively analyze patients diagnosed with MGC and who were treated with systemic chemotherapy at our institution in order to identify the early predictive factors associated with the success of CS with R0 resection.
2. Materials and Methods
2.1. Patients
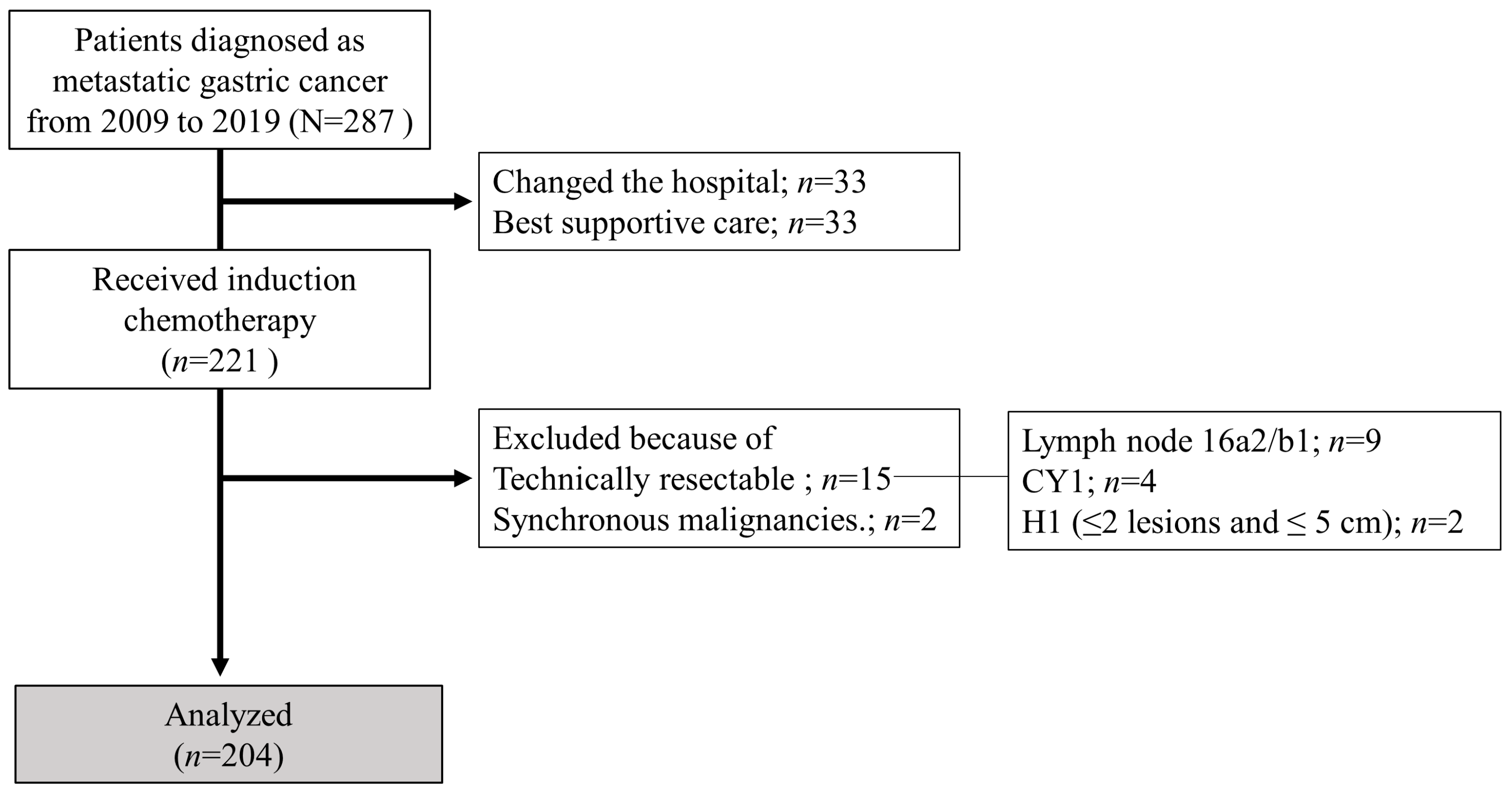
Consecutive patients diagnosed with metastatic gastric adenocarcinoma who received systemic chemotherapy at Nagoya University Hospital between January 2009 and December 2019 were included in this study. We classified them into two groups based on whether they underwent CS (CS and non-CS groups). In this study, patients with technically resectable metastases at the initial diagnosis, or synchronous malignancies, were excluded. Additionally, patients with esophagogastric junction cancer were also excluded. Using previous biological categories (Yoshida classification) [18], technically resectable metastasis was defined and classified as one of the following: (1) ≤2 metastatic nodules in the liver with ≤5 cm diameter (maximum), (2) positive cytology status (CY1), or (3) suspected para-aortic lymph node metastases in the region between the celiac axis and inferior mesenteric artery (no. 16a2 and/or 16b1). Tumor staging and histopathological grading were performed according to the 15th edition of the Japanese Classification of Gastric Carcinoma [19]. Distant metastases, other than technically resectable metastases as defined by the Yoshida classification, were defined as non-curable factors. Hematologic metastasis was defined as metastasis to the bone, liver, lung, and other organs, and lymphogenous metastasis was defined as distant lymph node metastasis. The flow diagram of the patients enrolled in this study is shown in Figure 1.
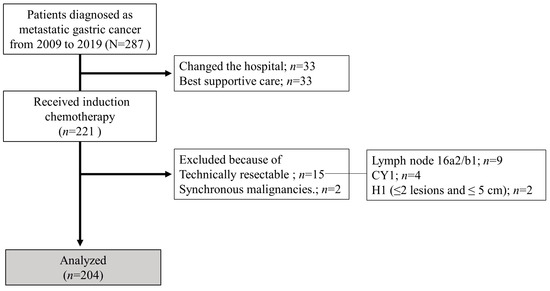
Figure 1.
Flowchart of patient enrolment.
2.2. Chemotherapy, Surgery, and Postoperative Management
Chemotherapy was administered according to the latest Japanese gastric cancer guidelines or clinical trial regimens at that time. Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [20]. Serum levels of carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) were measured in all patients before and during chemotherapy (every 6 ± 2 weeks). The cutoff values for CEA and CA19-9 were set at the upper limit of the normal value for each tumor marker at our institution: CEA, 5.0 ng/mL and CA19-9, 37 U/mL. CS was defined as curative-intent gastrectomy with ≥D1 lymph node dissection and was considered when R0 resection was possible. This study did not have a set protocol for CS implementation; however, it was undertaken in the following circumstances: (1) for peritoneal dissemination, if the lavage cytology showed negative results, and a representative peritoneal nodule was confirmed to be pathologically negative at the staging laparoscopy, with no evidence of other distant metastases; (2) for liver metastasis, when a CR or a reduction to 1 or 2 nodules with a maximum diameter of <5 cm was ensured; and (3) for lymph nodes or other distant sites, when a CR was confirmed in the distant metastasis. Lesions in which CR was achieved did not necessarily require surgical removal.
The Clavien–Dindo classification was employed to comprehensively evaluate postoperative complications [21,22], such as the clinically relevant grade ≥ II complications. The pathological response of the primary tumor after preoperative chemotherapy was assessed according to the 5th edition of the Japanese Classification of Gastric Carcinoma [19]. The regimen and duration of postoperative chemotherapy were at the physician’s discretion, considering the surgical and pathological findings, as well as the patient’s condition.
2.3. Statistical Analysis
The correlation between each group was analyzed using the chi-squared test or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables, where appropriate. Survival rates were estimated using the Kaplan–Meier method, and the overall differences between survival curves were compared using the Cox proportional hazards model. Overall survival (OS) was calculated as the time between the start of chemotherapy and death/last follow-up date. Recurrence-free survival (RFS) was defined as the time between surgery and the first evidence of disease recurrence or progression and was calculated for patients who underwent R0 resection. Multivariable logistic analysis was performed to identify important factors for CS with R0 resection. JMP version 15 (SAS Institute Inc., Cary, NC, USA) was used for all of the analyses. Statistical significance was set at p < 0.05.
3. Results
3.1. Patients’ Characteristics
In this study, 15 patients with technically resectable metastases were excluded (2 cases of ≤2 metastatic nodules in the liver with ≤5 cm maximum diameter, 9 cases of positive cytology, and 9 cases of para-aortic lymph node metastases confined to 16a2 and/or 16b1). A total of 204 patients were included in this analysis, of whom 39 underwent CS (conversion rate, 19%). Table 1 summarizes the patient characteristics of the two groups. Patients in the CS group had significantly better ECOG-PS scores, were female, and were younger than those in the non-CS group. There were no significant differences between the two groups in terms of histological type, distant metastasis site, or number of non-curable factors. However, the proportion of patients who received systemic chemotherapy combined with intraperitoneal administration and trastuzumab was significantly higher in the CS group than that in the non-CS group.

Table 1.
Patients’ characteristics of the study population.
3.2. CS
Table 2 summarizes the operative variables in the CS group. R0 resection was achieved in 31 patients (conversion surgery with R0 resection rate, 15%). At the time of CS, CR, partial response (PR), stable disease (SD), and non-CR/non-PD with chemotherapy were established in 1 (2.6%), 26 (67%),4 (10%), and 8 (20%) patients, respectively. The percentage of CS cases with R0 resection was 12% for Yoshida classification category 2, 17% for category 3, and 16% for category 4, with no significant variation between the categories (p = 0.6425). The surgical procedures for combined resection included the following: three partial hepatectomies, one lateral segmentectomy, and two distal pancreatectomies. Postoperative complications occurred in 8 (21%) patients. Postoperative chemotherapy was administered to 35 (90%) patients. The pathological response assessment of primary tumors showed that 10 (26%) patients had a grade 2a, 2 (5%) patients had a grade 2b, and 5 (13%) patients had a grade 3 response.

Table 2.
Surgical, pathological, and postoperative details of patients who underwent conversion surgery.
3.3. Survival Outcomes
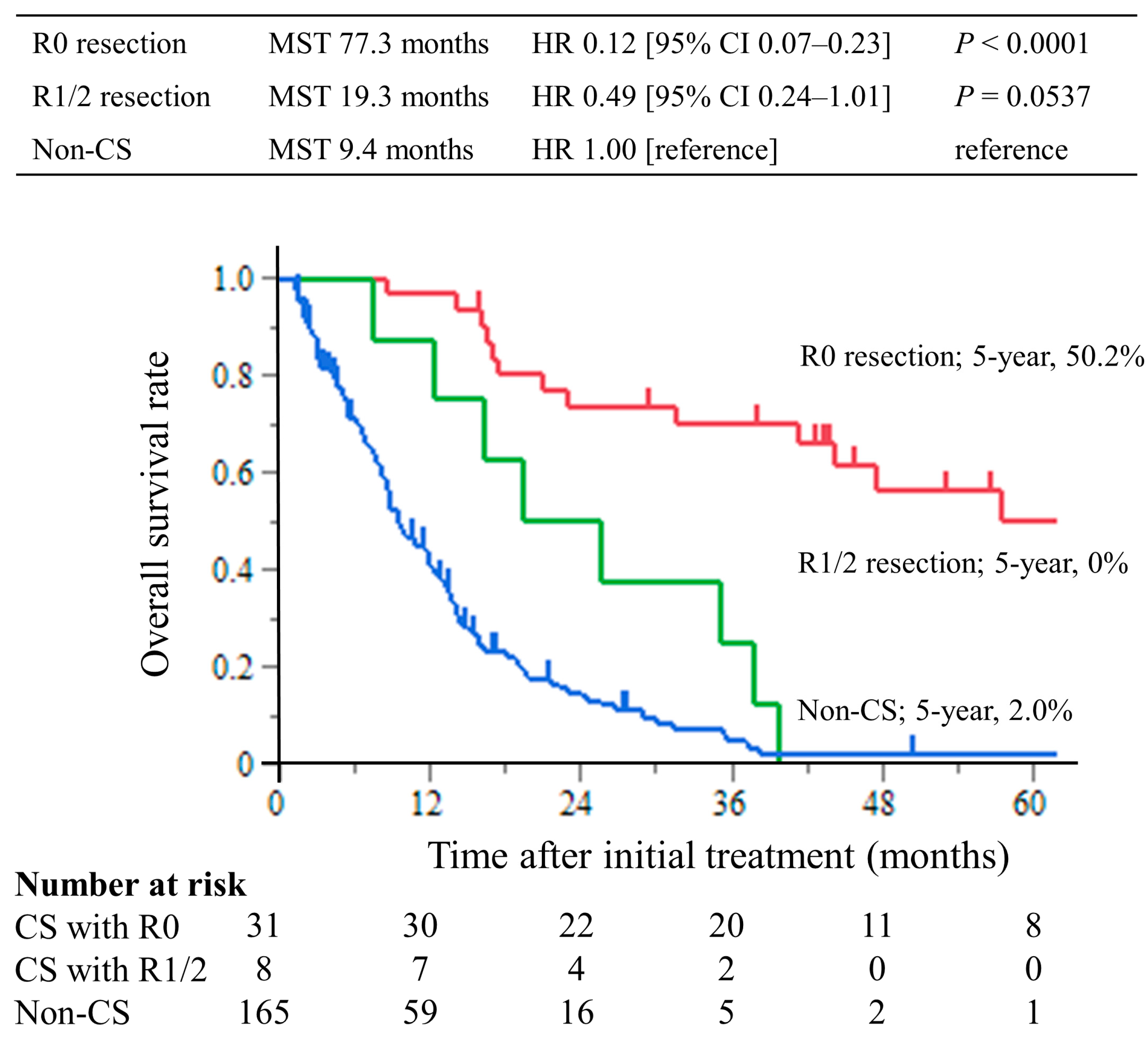
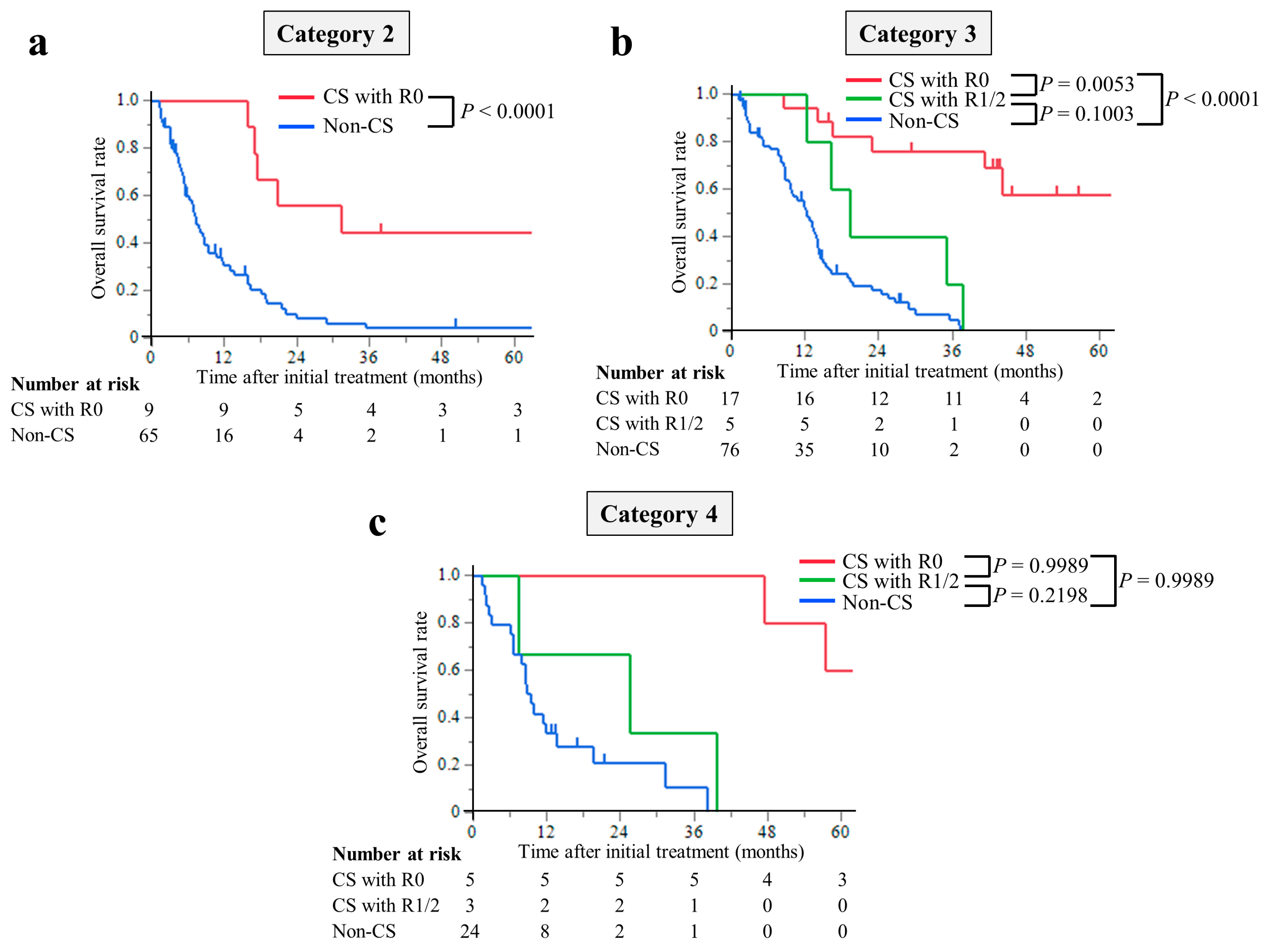
The median follow-up period was 8.7 months (interquartile range (IQR), 4.3–14.4 months) in the non-CS group and 39.8 months (IQR, 17.3–56.6 months) in the CS group. The median survival times (MST) were 77.3 months in the CS with R0 resection group, 19.3 months in the R1/2 resection group, and 9.4 months in the non-CS group. The hazard ratios for OS in the R0 resection group were 0.12 (95% CI, 0.07–0.23; p < 0.0001) compared with the non-CS group, while in the R1/2 resection group, it was 0.49 (95% CI, 0.24–1.01; p = 0.0537). The hazard ratios for OS compared with the R1/2 group were 0.25 (95% CI, 0.11–0.63; p = 0.0029) in the R0 resection group (Figure 2). This trend was the same for all of the Yoshida classification categories (Figure 3).
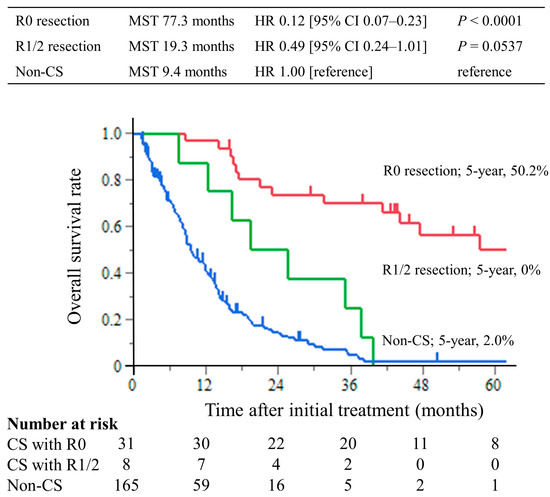
Figure 2.
Kaplan–Meier curves of overall survival. The overall survival time of the CS with R0 resection group was significantly longer than that of the non-CS group, with a 5 year overall survival rate of 50.2%.
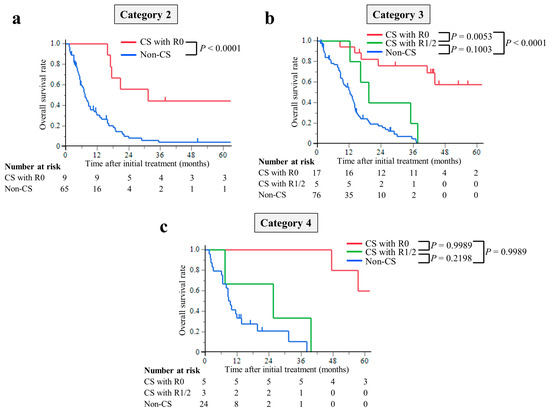
Figure 3.
Kaplan–Meier curves for overall survival of patients stratified using the Yoshida classification: (a) Category 2, (b) Category 3, and (c) Category 4. The overall survival time of the CS with R0 resection group was significantly longer than that of the non-CS group in any Yoshida classification category.
Fifteen patients with technically resectable metastases underwent induction chemotherapy. Of these, 14 patients subsequently underwent radical surgery, with 2 achieving an R1 resection and 12 achieving a R0 resection. The 5 year OS rate for R0 resection was 62.5%.
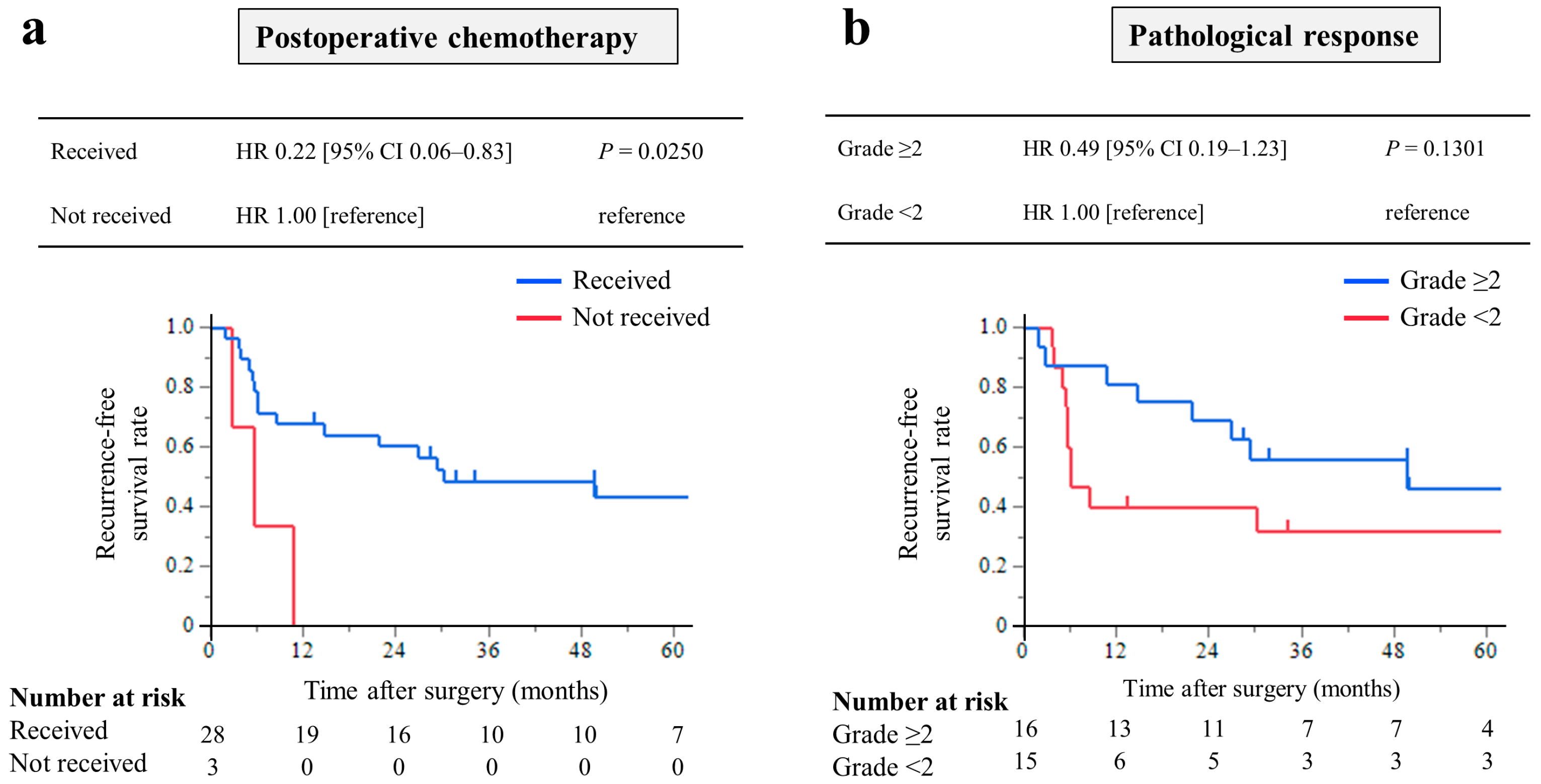
The RFS was analyzed in 31 patients who underwent CS with R0 resection. The median RFS was 29.4 months (IQR, 5.7–49.9 months), and the 3-year RFS rate was 43.8%. Among these, patients who received postoperative chemotherapy had significantly longer RFS times than those who did not (HR, 0.22; 95% CI, 0.06–0.83; p = 0.0250, Figure 4a). Patients with pathological response grade ≥ 2 had a longer RFS times, but the difference was not significant (HR, 0.49; 95% CI, 0.19–1.23; p = 0.1301, Figure 4b).
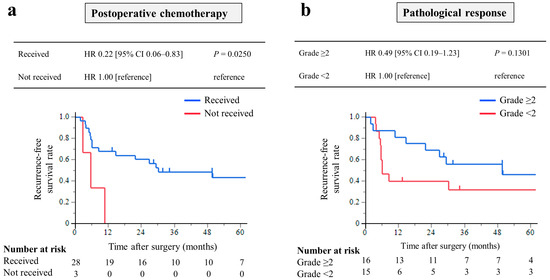
Figure 4.
Kaplan–Meier curves of recurrence-free survival of 31 patients who underwent conversion surgery with R0 resection: Analysis of the prognostic effect of (a) patients who received postoperative chemotherapy had significantly longer RFS times than those who did not, and (b) patients with pathological response grade ≥ 2 had a longer RFS rate, but the difference was not significant.
3.4. Early Predictive Factors for CS with R0 Resection
We analyzed the factors that influenced the early prediction of CS until the initial RECIST evaluation. This analysis was conducted on a subset of 150 patients, excluding those with progressive disease or who had discontinued chemotherapy until the initial RECIST evaluation. Patient and tumor characteristics, initial RECIST evaluation, and serum tumor markers at the initial RECIST evaluation were considered to be variables for the early prediction of CS with R0 resection (Table 3). Univariate analysis revealed that the following predictive factors were significant when implementing CS with R0 resection: CEA > 5.0 ng/mL at the initial RECIST evaluation and chemotherapy combined with trastuzumab administration. Multivariate analysis revealed that CEA > 5.0 ng/mL at the initial RECIST evaluation was an independent and unfavorable factor when implementing CS with R0 resection (odds ratio (OR), 0.21; 95% CI, 0.07–0.70; p = 0.0108), whereas chemotherapy with trastuzumab administration was an independent and favorable factor (OR, 4.20; 95% CI, 1.37–12.9; p = 0.0119).

Table 3.
Predictive factors for conversion surgery with R0 resection; univariate and multivariate analyses.
3.5. Relationship between Changes in CEA Levels and CS with R0 Resection
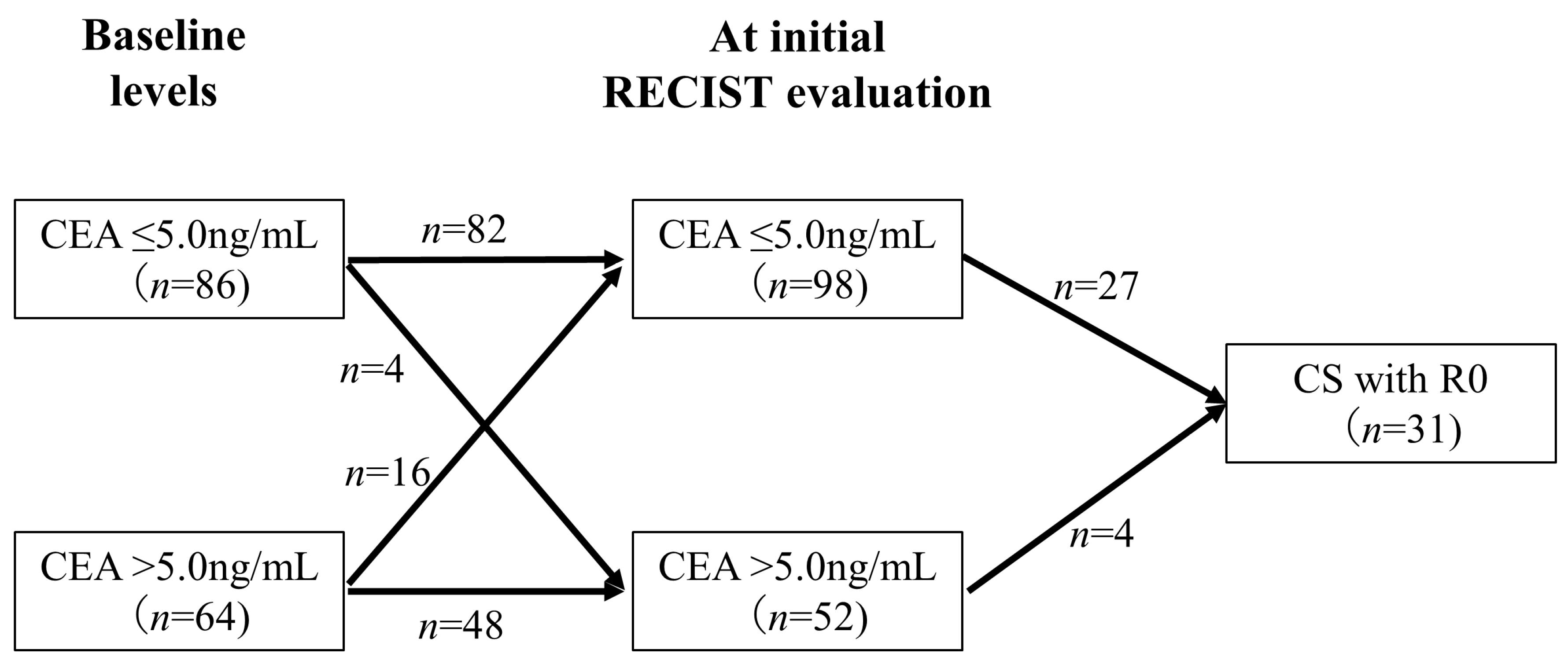
Figure 5 shows the association between changes in CEA levels from baseline to the initial RECIST evaluation and CS with R0 resection. Among the 64 patients with CEA levels > 5.0 ng/mL at baseline, 16 (25.0%) exhibited a decrease in CEA levels to ≤5.0 ng/mL at the initial RECIST evaluation. In contrast, among the 86 patients with CEA levels ≤ 5.0 ng/mL at baseline, 4 (4.7%) had an increase in CEA levels to >5.0 ng/mL at the initial RECIST evaluation, and none of these patients achieved CS with R0 resection. Of the patients with CEA levels ≤ 5.0 ng/mL at the initial RECIST evaluation (n = 98), 27 (28%) achieved CS with R0 resection. In contrast, only 4 (7.7%) patients with CEA levels > 5.0 ng/mL at the initial RECIST evaluation (n = 52) achieved CS with R0 resection, which was significantly less frequent (p = 0.0043).
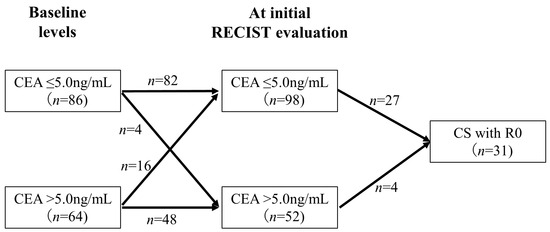
Figure 5.
Relationship between changes in CEA levels and CS with R0 resection. This figure shows the association between changes in CEA levels from baseline to the initial RECIST evaluation and CS. Only 7.7% of the patients with CEA levels ≥ 5.0 ng/mL at the initial RECIST evaluation achieved CS with R0 resection, which was significantly less frequent.
4. Discussion
We retrospectively analyzed patients diagnosed with MGC who were treated with systemic chemotherapy at our institution in order to identify the early predictive factors associated with the success of CS with R0 resection. The incidence of CS with R0 resection was 15%, and the patients who underwent the procedure had a better prognosis. However, patients with CEA > 5.0 ng/mL at the initial RECIST evaluation had a low indication for surgery.
The reported incidence of CS varies from 17.7% to 34.9% in large-scale retrospective studies [8,9,10,11]. This is slightly higher than our findings, potentially because patients with technically resectable metastases (Yoshida classification category 1) were excluded from our study. Chemotherapy for such patients could be regarded as induction chemotherapy, and we believe that our eligibility criteria were reasonable. Additionally, there may have been patients in our series who missed the timing for the implementation of CS because of our cautious approach toward the procedure. The incidence of CS observed in prospective clinical trials of chemotherapy for advanced/metastatic cancer is much lower, at 4.2% and 6%, respectively [23,24]. This may be because our series included several HER2-positive patients treated with trastuzumab-containing regimens and those with peritoneal disease who were treated with intraperitoneal chemotherapy. Moreover, all investigators who participated in the previous clinical trials were keen on conducting CS. We are currently conducting a multi-institutional prospective observational study, using the same inclusion and exclusion criteria, to evaluate the clinical significance and status of CS (UMIN000042244).
We recently reported that postoperative complications, massive intraoperative blood loss, and delayed postoperative adjuvant chemotherapy adversely affect the prognosis of patients with gastric cancer [25,26,27]. Thus, our CS strategy avoided extended surgery (e.g., extended lymph node dissection, pancreaticoduodenectomy, and Appleby’s procedure). We also made efforts in this series to continue chemotherapy after surgery. Our subgroup analysis showed that patients who received postoperative chemotherapy had better prognoses than those who did not.
Our study suggests that changes in tumor marker levels could serve as an early indicator in patients with gastric cancer. Tumor shrinkage is an important indicator when considering whether CS is indicated in daily practice. However, relying solely on RECIST evaluation may not suffice, as conventional imaging studies may fail to detect lesions, such as those associated with peritoneal dissemination, which is often associated with gastric cancer. All cases of R1/2 resection presented with peritoneal dissemination at initial diagnosis. Furthermore, CS requires a CR or near-CR of the metastatic lesions. In this study, CEA > 5.0 ng/mL at the initial RECIST evaluation represented an independent and unfavorable factor for performing CS with R0 resection, whereas the RECIST assessment was not a significant predictor. We speculate that the elevated CEA level more sensitively reflects the presence of metastatic lesions than CT imaging assessments. If the CEA level decreases from baseline, tumor shrinkage may be expected. However, the presence of CEA > 5.0 ng/mL at the initial RECIST evaluation indicates that the cases with CEA > 5.0 ng/mL did not show the marked shrinkage necessary to implement CS, while those with CEA ≤ 5.0 ng/mL had tumor progression. Moreover, CEA trends offer an objective and less invasive method of assessment. However, the wide range of CEA levels (>5.0 ng/mL) may not apply to all cases; the optimal timing of CEA measurement for predicting later CS was unclear in this study. Moreover, the usefulness of other tumor markers, such as MRI and PET/CT, as predictors for implementing CS has not been evaluated. It is necessary to assess this correlation using studies with larger sample sizes. We plan to investigate the relationship between changes in tumor markers and the indication for CS in our ongoing prospective observational study.
Many practice-changing clinical trials have been reported in the literature, and more active agents have become increasingly available in clinical practice for the past decade. Accordingly, based on the results of the ToGA study [28], systemic chemotherapy combined with trastuzumab, the standard treatment for HER2-positive MGC, successfully predicted CS in our study. In combination with chemotherapy, nivolumab has recently become available for the treatment of HER2-negative MGC [29,30], which is expected to have a higher tumor response and may also be an important predictor of CS. However, these patients were not included in this study. Moreover, several patients received systemic chemotherapy combined with intraperitoneal administration in the clinical trial setting; this factor may have impacted a subset of patients with peritoneal disease. A future increase in biomarker-driven treatments may lead to more opportunities for performing CS with R0 resection. At that time, the monitoring of CEA levels during chemotherapy might become increasingly important as these become candidates for CS implementation.
Our study has several limitations. First, this was a single-center, nonrandomized, retrospective study. Second, CS was conducted at the physician’s discretion, and the criteria for implementing the procedure were not fixed. Third, as mentioned above, the availability of active agents in clinical practice has dramatically changed from that at the time of our study period. Fourth, we included patients who underwent clinical trials, and not all chemotherapy regimens were selected based on the Japanese gastric cancer guidelines. Notably, several patients received systemic chemotherapy combined with intraperitoneal administration in a clinical trial setting; this factor may have affected a subset of patients with peritoneal disease. Fifth, tumor markers do not always reflect the total tumor volume or the tumor response to chemotherapy. Sixth, complete metastasis resections were not performed, and the true achievement of R0 resection relies on clinical and imaging assessments. Seventh, although we performed staging laparoscopy before CS in patients with peritoneal dissemination, we still encountered a significant number of R1/2 resection cases. Finally, data regarding good responders to chemotherapy without CS were lacking, and the MST of CS with R1/2 had longer survival times than those in the non-CS group. However, it is important to note that R1/2 resection is considered incomplete and may not provide the same benefits as that of R0 resection in terms of long-term survival. Additionally, patients who undergo R1/2 resection may require further treatment, such as radiation or additional chemotherapy, which can affect overall survival.
5. Conclusions
In conclusion, our study highlights the significance of monitoring CEA levels during chemotherapy when predicting the likelihood of achieving CS with R0 resection. Patients with CEA > 5.0 ng/mL at the initial RECIST evaluation might be unfavorable candidates for CS. Monitoring CEA levels during chemotherapy may be a useful predictor of the CS implementation in patients with MGC. Although monitoring CEA trends is an objective and less invasive assessment method, it is essential to emphasize that making treatment decisions solely based on CEA levels is not advisable. Other critical factors, such as the patient’s overall health status, tumor location, and response to treatment, must be carefully considered in the decision-making process. Therefore, the circumstances of each patient should be carefully evaluated in order to determine the most appropriate treatment strategy.
Author Contributions
Conceptualization, K.N., C.T., M.K. and Y.K.; data curation, K.N., C.T., M.K., K.M., K.F., O.M., D.S., S.S., N.K. and S.F.; methodology, K.N., M.K. and Y.K.; software, C.T.; supervision, H.K., Y.A., T.E. and Y.K.; writing—original draft, K.N.; writing—review and editing, C.T., M.K., K.M., K.F., O.M., D.S., S.S., N.K., S.F., H.K., Y.A., T.E. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (No. 2020–0578).
Informed Consent Statement
Written informed consent for treatment was obtained from all patients. The clinical data were exclusively analyzed retrospectively without any intervention by the investigators; therefore, an opt-out recruitment strategy was followed according to Japanese government policy. Patients were excluded only when they specified that they were unwilling to participate.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Yasuhiro Kodera reports grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Chugai Pharma, grants from Takeda, grants and personal fees from MSD, grants and personal fees from Nihon Kayaku, grants, and personal fees from Yakult, grants, personal fees from Lilly Japan, grants and personal fees from Ono Pharmaceutical Co., Ltd., grants from Kaken Pharma, grants and personal fees from Covidien, grants from EA Pharma, grants and personal fees from Daiichi Sankyo Company, Ltd., grants from Otsuka, grants and personal fees from Tsumura, grants and personal fees from Johnson & Johnson, grants from Sanofi, grants from Abbot, grants from Bayer, grants and personal fees from Abbvie, grants from Pfizer, personal fees from Miyarisan, personal fees from Amgen, and personal fees from Olympus, outside the submitted work. Yuichi Ando reports grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Kyowa Kirin Co., Ltd., grants and personal fees from Nippon Kayaku Co., Ltd., grants and personal fees from Yakult Honsha Co., Ltd., personal fees from Eli Lilly Japan K.K., grants and personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Novartis Pharma K.K., personal fees from Bayer Holding Ltd., personal fees from Sawai Pharmaceutical Co., Ltd., grants and personal fees from Daiichi Sankyo Company, Ltd., grants and personal fees from Eisai Co., Ltd., personal fees from MSD K.K, personal fees from Astellas Pharma Inc., personal fees from Otsuka Holdings Co., Ltd., personal fees from Sanwa Kagaku Kenkyusho Co., Ltd., personal fees from Hisamitsu Pharmaceutical Co., Inc., personal fees from SymBio Pharceuticals, personal fees from Aptitude Health, grants from BeiGene, Ltd., and personal fees from Alfresa Pharma Corporation, outside the submitted work; The other authors declare that no financial or material support has been received for this study.
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Muro, K.; Van Cutsem, E.; Narita, Y.; Pentheroudakis, G.; Baba, E.; Li, J.; Ryu, M.-H.; Wan Zamaniah, W.I.; Yong, W.-P.; Yeh, K.-H.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 19–33. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanabe, K.; Taomoto, J.; Yamamoto, H.; Tokumoto, N.; Yoshida, K.; Ohdan, H. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol. Lett. 2010, 1, 743–747. [Google Scholar] [CrossRef]
- Terashima, M. Conversion therapy for gastric cancer: Who can make conversion as successful as Goromaru? Gastric Cancer 2016, 19, 685–686. [Google Scholar] [CrossRef]
- Solaini, L.; Ministrini, S.; Bencivenga, M.; D’ignazio, A.; Marino, E.; Cipollari, C.; Molteni, B.; Mura, G.; Marrelli, D.; Graziosi, L.; et al. Conversion gastrectomy for stage IV unresectable gastric cancer: A GIRCG retrospective cohort study. Gastric Cancer 2019, 22, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yasufuku, I.; Terashima, M.; Rha, S.Y.; Bae, J.M.; Li, G.; Katai, H.; Watanabe, M.; Seto, Y.; Noh, S.H.; et al. International retrospective cohort study of conversion therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann. Gastroenterol. Surg. 2022, 6, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Morgagni, P.; Solaini, L.; Framarini, M.; Vittimberga, G.; Gardini, A.; Tringali, D.; Valgiusti, M.; Monti, M.; Ercolani, G. Conversion surgery for gastric cancer: A cohort study from a western center. Int. J. Surg. 2018, 53, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kunisaki, C.; Tanaka, Y.; Sato, K.; Miyamoto, H.; Yukawa, N.; Kosaka, T.; Akiyama, H.; Endo, I.; Misumi, T. Curative-intent surgery for Stage IV advanced gastric cancer: Who can undergo surgery and what are the prognostic factors for long-term survival? Ann. Surg. Oncol. 2019, 26, 4452–4463. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yoshida, K.; Tanahashi, T.; Takahashi, T.; Matsuhashi, N.; Tanaka, Y.; Tanabe, K.; Ohdan, H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer 2018, 21, 315–323. [Google Scholar] [CrossRef]
- Ohnuma, H.; Sato, Y.; Onoyama, N.; Hamaguchi, K.; Hayasaka, N.; Sato, M.; Murase, K.; Takada, K.; Miyanishi, K.; Murakami, T.; et al. Survival benefit of conversion surgery after intensive chemotherapy for unresectable metastatic gastric cancer: A propensity score-matching analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Ishiguro, T.; Ogata, K.; Suzuki, O.; Kumagai, Y.; Ishibashi, K.; Ishida, H.; Kuwano, H.; Mochiki, E. Prognostic role of conversion surgery for unresectable gastric cancer. Ann. Surg. Oncol. 2015, 22, 3618–3624. [Google Scholar] [CrossRef]
- Williams, J.L.; Kadera, B.E.; Nguyen, A.H.; Muthusamy, V.R.; Wainberg, Z.A.; Hines, O.J.; Reber, H.A.; Donahue, T.R. CA19-9 normalization during preoperative treatment predicts longer survival for patients with locally progressed pancreatic cancer. J. Gastrointest. Surg. 2016, 20, 1331–1342. [Google Scholar] [CrossRef]
- Reni, M.; Zanon, S.; Balzano, G.; Nobile, S.; Pircher, C.; Chiaravalli, M.; Passoni, P.; Arcidiacono, P.; Nicoletti, R.; Crippa, S.; et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann. Oncol. 2017, 28, 2786–2792. [Google Scholar] [CrossRef]
- Michelakos, T.; Pergolini, I.; Castillo, C.F.-D.; Honselmann, K.C.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef]
- Ogura, A.; Uehara, K.; Aiba, T.; Sando, M.; Tanaka, A.; Ohara, N.; Murata, Y.; Sato, Y.; Hattori, N.; Nakayama, G.; et al. Indications for neoadjuvant treatment based on risk factors for poor prognosis before and after neoadjuvant chemotherapy alone in patients with locally advanced rectal cancer. Eur. J. Surg. Oncol. 2021, 47, 1005–1011. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamada, D.; Asukai, K.; Wada, H.; Hasegawa, S.; Hara, H.; Shinno, N.; Ushigome, H.; Haraguchi, N.; Sugimura, K.; et al. Clinical implications of the serum CA19-9 level in “biological borderline resectability” and “biological downstaging” in the setting of preoperative chemoradiation therapy for pancreatic cancer. Pancreatology 2020, 20, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, Y.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Prognostic significance of perioperative tumor marker levels in stage II/III gastric cancer. World J. Gastrointest. Oncol. 2019, 11, 17–27. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 15th ed.; Kanehara Publisher: Tokyo, Japan, 2017. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Fujitani, K.; Yang, H.-K.; Mizusawa, J.; Kim, Y.-W.; Terashima, M.; Han, S.-U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Yamada, Y.; Boku, N.; Mizusawa, J.; Iwasa, S.; Kadowaki, S.; Nakayama, N.; Azuma, M.; Sakamoto, T.; Shitara, K.; Tamura, T.; et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): An open-label, phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Delay in initiation of postoperative adjuvant chemotherapy with S-1 monotherapy and prognosis for gastric cancer patients: Analysis of a multi-institutional dataset. Gastric Cancer 2019, 22, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; Tanaka, C.; et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. 2019, 8, 5194–5201. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Chen, L.-T.; Ryu, M.-H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).