Low Expectancy of Conversion Surgery with R0 Resection in Patients with CEA > 5.0 ng/mL at the Initial RECIST Evaluation for Metastatic Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
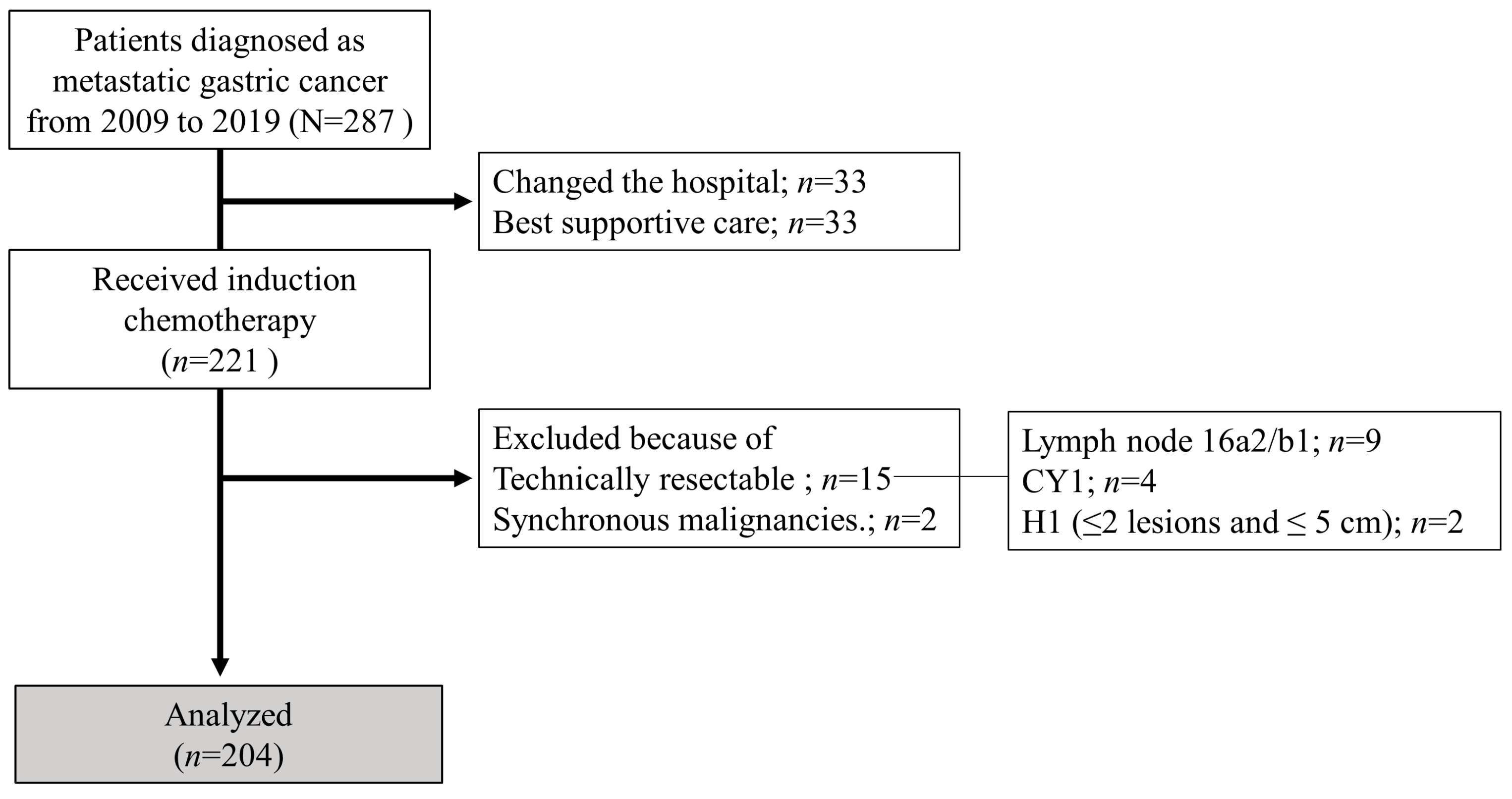
2.1. Patients
2.2. Chemotherapy, Surgery, and Postoperative Management
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. CS
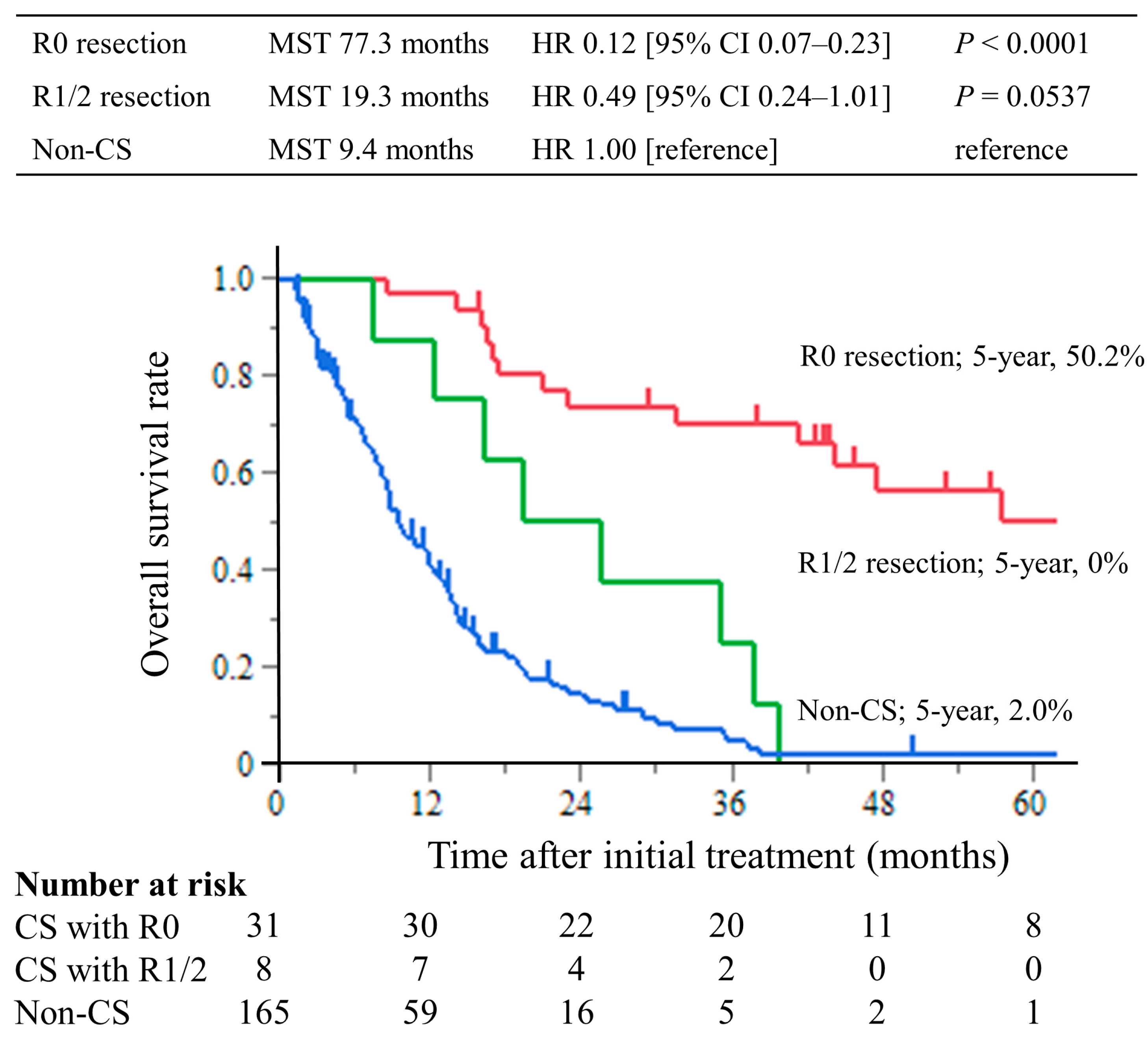
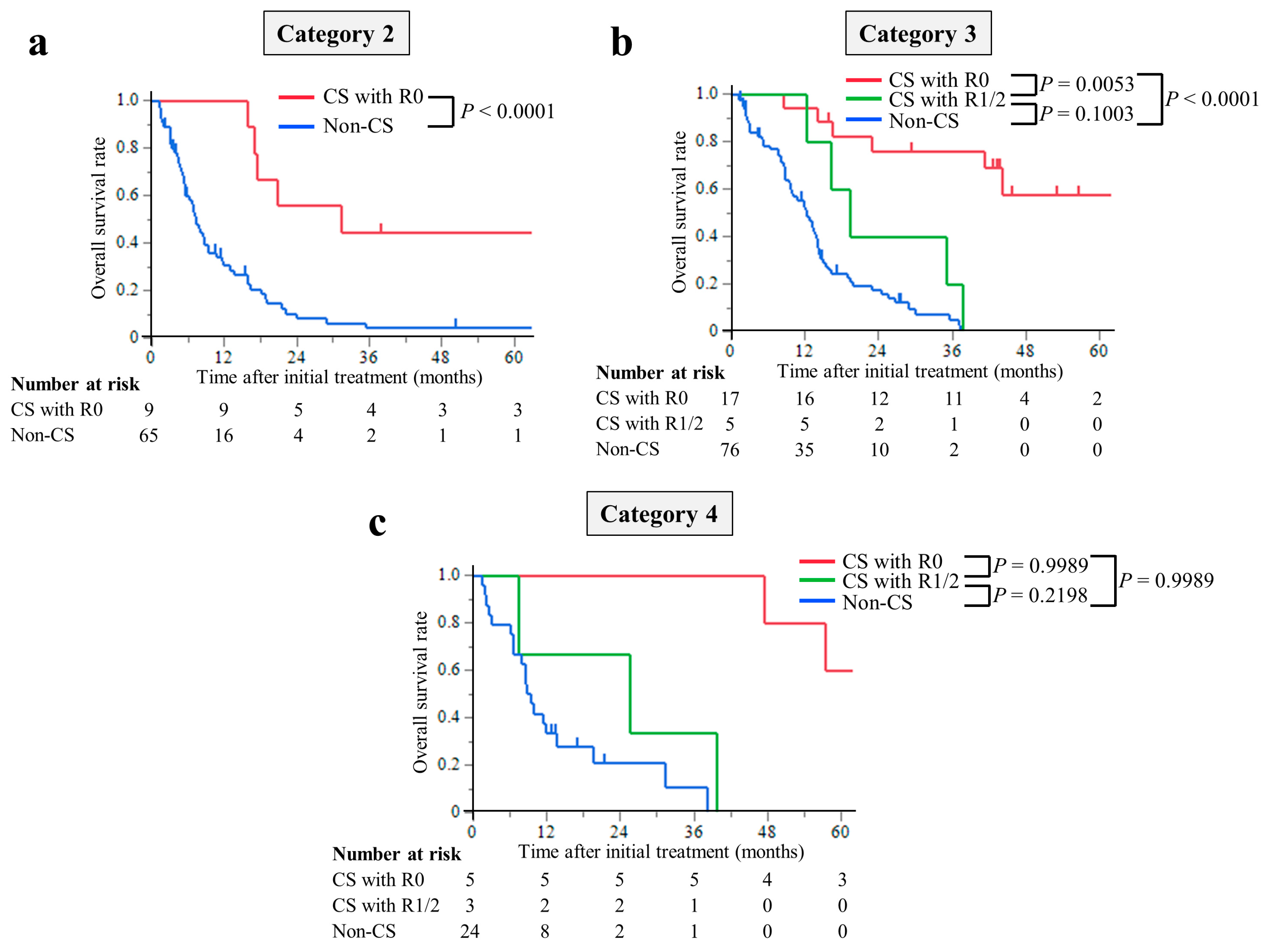
3.3. Survival Outcomes
3.4. Early Predictive Factors for CS with R0 Resection
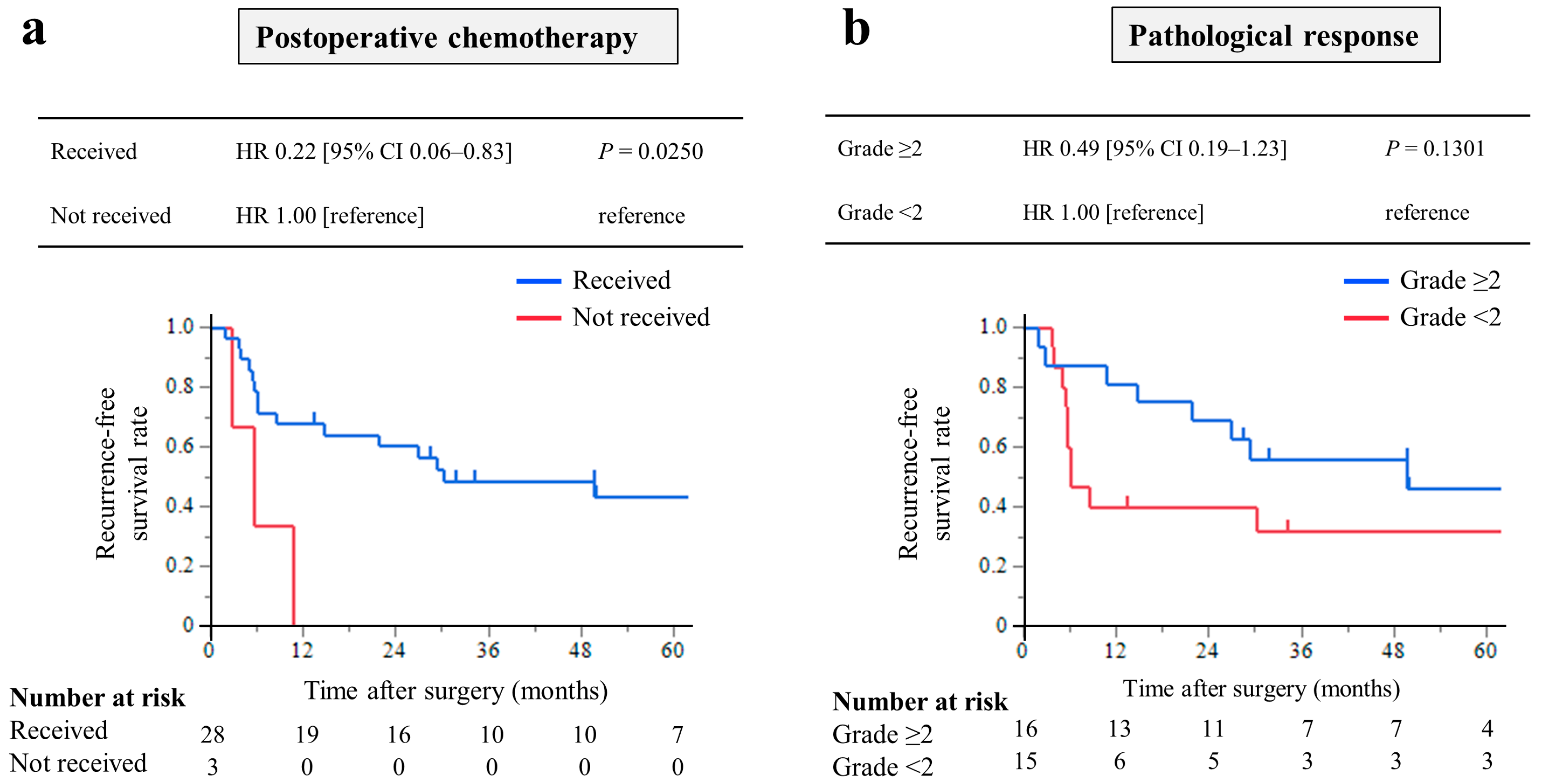
3.5. Relationship between Changes in CEA Levels and CS with R0 Resection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Muro, K.; Van Cutsem, E.; Narita, Y.; Pentheroudakis, G.; Baba, E.; Li, J.; Ryu, M.-H.; Wan Zamaniah, W.I.; Yong, W.-P.; Yeh, K.-H.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 19–33. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanabe, K.; Taomoto, J.; Yamamoto, H.; Tokumoto, N.; Yoshida, K.; Ohdan, H. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol. Lett. 2010, 1, 743–747. [Google Scholar] [CrossRef]
- Terashima, M. Conversion therapy for gastric cancer: Who can make conversion as successful as Goromaru? Gastric Cancer 2016, 19, 685–686. [Google Scholar] [CrossRef]
- Solaini, L.; Ministrini, S.; Bencivenga, M.; D’ignazio, A.; Marino, E.; Cipollari, C.; Molteni, B.; Mura, G.; Marrelli, D.; Graziosi, L.; et al. Conversion gastrectomy for stage IV unresectable gastric cancer: A GIRCG retrospective cohort study. Gastric Cancer 2019, 22, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yasufuku, I.; Terashima, M.; Rha, S.Y.; Bae, J.M.; Li, G.; Katai, H.; Watanabe, M.; Seto, Y.; Noh, S.H.; et al. International retrospective cohort study of conversion therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann. Gastroenterol. Surg. 2022, 6, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Morgagni, P.; Solaini, L.; Framarini, M.; Vittimberga, G.; Gardini, A.; Tringali, D.; Valgiusti, M.; Monti, M.; Ercolani, G. Conversion surgery for gastric cancer: A cohort study from a western center. Int. J. Surg. 2018, 53, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kunisaki, C.; Tanaka, Y.; Sato, K.; Miyamoto, H.; Yukawa, N.; Kosaka, T.; Akiyama, H.; Endo, I.; Misumi, T. Curative-intent surgery for Stage IV advanced gastric cancer: Who can undergo surgery and what are the prognostic factors for long-term survival? Ann. Surg. Oncol. 2019, 26, 4452–4463. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yoshida, K.; Tanahashi, T.; Takahashi, T.; Matsuhashi, N.; Tanaka, Y.; Tanabe, K.; Ohdan, H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer 2018, 21, 315–323. [Google Scholar] [CrossRef]
- Ohnuma, H.; Sato, Y.; Onoyama, N.; Hamaguchi, K.; Hayasaka, N.; Sato, M.; Murase, K.; Takada, K.; Miyanishi, K.; Murakami, T.; et al. Survival benefit of conversion surgery after intensive chemotherapy for unresectable metastatic gastric cancer: A propensity score-matching analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Ishiguro, T.; Ogata, K.; Suzuki, O.; Kumagai, Y.; Ishibashi, K.; Ishida, H.; Kuwano, H.; Mochiki, E. Prognostic role of conversion surgery for unresectable gastric cancer. Ann. Surg. Oncol. 2015, 22, 3618–3624. [Google Scholar] [CrossRef]
- Williams, J.L.; Kadera, B.E.; Nguyen, A.H.; Muthusamy, V.R.; Wainberg, Z.A.; Hines, O.J.; Reber, H.A.; Donahue, T.R. CA19-9 normalization during preoperative treatment predicts longer survival for patients with locally progressed pancreatic cancer. J. Gastrointest. Surg. 2016, 20, 1331–1342. [Google Scholar] [CrossRef]
- Reni, M.; Zanon, S.; Balzano, G.; Nobile, S.; Pircher, C.; Chiaravalli, M.; Passoni, P.; Arcidiacono, P.; Nicoletti, R.; Crippa, S.; et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann. Oncol. 2017, 28, 2786–2792. [Google Scholar] [CrossRef]
- Michelakos, T.; Pergolini, I.; Castillo, C.F.-D.; Honselmann, K.C.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef]
- Ogura, A.; Uehara, K.; Aiba, T.; Sando, M.; Tanaka, A.; Ohara, N.; Murata, Y.; Sato, Y.; Hattori, N.; Nakayama, G.; et al. Indications for neoadjuvant treatment based on risk factors for poor prognosis before and after neoadjuvant chemotherapy alone in patients with locally advanced rectal cancer. Eur. J. Surg. Oncol. 2021, 47, 1005–1011. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamada, D.; Asukai, K.; Wada, H.; Hasegawa, S.; Hara, H.; Shinno, N.; Ushigome, H.; Haraguchi, N.; Sugimura, K.; et al. Clinical implications of the serum CA19-9 level in “biological borderline resectability” and “biological downstaging” in the setting of preoperative chemoradiation therapy for pancreatic cancer. Pancreatology 2020, 20, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, Y.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Prognostic significance of perioperative tumor marker levels in stage II/III gastric cancer. World J. Gastrointest. Oncol. 2019, 11, 17–27. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 15th ed.; Kanehara Publisher: Tokyo, Japan, 2017. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Fujitani, K.; Yang, H.-K.; Mizusawa, J.; Kim, Y.-W.; Terashima, M.; Han, S.-U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Yamada, Y.; Boku, N.; Mizusawa, J.; Iwasa, S.; Kadowaki, S.; Nakayama, N.; Azuma, M.; Sakamoto, T.; Shitara, K.; Tamura, T.; et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): An open-label, phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Delay in initiation of postoperative adjuvant chemotherapy with S-1 monotherapy and prognosis for gastric cancer patients: Analysis of a multi-institutional dataset. Gastric Cancer 2019, 22, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; Tanaka, C.; et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. 2019, 8, 5194–5201. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Chen, L.-T.; Ryu, M.-H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]

| Non-CS Group n = 165 | CS Group n = 39 | p Value | |
|---|---|---|---|
| Sex | 0.0257 | ||
| Male | 108 (65%) | 18 (46%) | |
| Female | 57 (35%) | 21 (54%) | |
| Age, years, median (IQR) | 66 (59, 73) | 61 (49, 68) | 0.0027 |
| ECOG-PS | 0.0073 | ||
| 0 | 96 (58%) | 32 (79%) | |
| 1 | 51 (31%) | 7 (21%) | |
| 2 | 18 (11%) | 0 (0%) | |
| ALP, median (IQR) | 188 (173, 228) | 231 (183, 261) | 0.0536 |
| Primary site | 0.8904 | ||
| U | 48 (29%) | 10 (26%) | |
| M | 66 (40%) | 17 (43%) | |
| L | 51 (31%) | 12 (31%) | |
| Macroscopic type | 0.1177 | ||
| Type 1 | 10 (6%) | 1 (3%) | |
| Type 2 | 17 (10%) | 8 (21%) | |
| Type 3 | 77 (47%) | 12 (31%) | |
| Type 4 | 61 (37%) | 18 (46%) | |
| Histologic type | 0.2694 | ||
| Differentiated | 53 (32%) | 9 (23%) | |
| Undifferentiated | 112 (68%) | 30 (77%) | |
| Baseline CEA, ng/mL, median (IQR) | 3.7 (1.6, 16.8) | 2.3 (1.5, 5.6) | 0.4986 |
| Baseline CA19-9, IU/mL, median (IQR) | 28 (8, 135) | 16 (6, 33) | 0.3070 |
| cT stage | 0.8172 | ||
| T2 | 2 (1%) | 0 (0%) | |
| T3 | 15 (9%) | 5 (13%) | |
| T4a | 131 (79%) | 30 (77%) | |
| T4b | 17 (10%) | 4 (10%) | |
| cN stage | 0.0804 | ||
| N (−) | 30 (18%) | 12 (31%) | |
| N (+) | 135 (82%) | 27 (69%) | |
| Yoshida classification | 0.1545 | ||
| 2 | 65 (39%) | 9 (23%) | |
| 3 | 76 (46%) | 22 (56%) | |
| 4 | 24 (15%) | 8 (21%) | |
| Distant metastatic site | |||
| Hematogenous | 51 (31%) | 8 (21%) | 0.1978 |
| Lymphogenous | 46 (28%) | 13 (33%) | 0.4992 |
| Peritoneal dissemination | 100 (61%) | 30 (77%) | 0.0566 |
| Non-curable factors | 0.3487 | ||
| 1 | 123 (75%) | 26 (67%) | |
| 2 | 34 (21%) | 9 (23%) | |
| ≥3 | 8 (5%) | 4 (19%) | |
| Types of chemotherapy | 0.0443 | ||
| S-1 | 13 (8%) | 0 (0%) | |
| S-1 + paclitaxel | 12 (7%) | 6 (14%) | |
| S-1 + oxaliplatin | 36 (21%) | 6 (16%) | |
| S-1 + cisplatin | 66 (40%) | 14 (35%) | |
| S-1 + docetaxel + cisplatin | 2 (1%) | 1 (2%) | |
| Capecitabine + oxaliplatin | 3 (2%) | 1 (5%) | |
| Capecitabine + cisplatin | 18 (11%) | 8 (21%) | |
| Capecitabine + docetaxel + cisplatin | 5 (3%) | 3 (7%) | |
| Others | 10 (6%) | 0 (0%) | |
| Combined with trastuzumab administration | 19 (12%) | 10 (26%) | 0.0231 |
| Combined with intraperitoneal administration | 30 (18%) | 18 (46%) | 0.0002 |
| n = 39 | |
|---|---|
| Duration of chemotherapy, months, median (IQR) | 7.4 (5.1, 10.3) |
| RECIST response at conversion surgery | |
| CR/PR/SD/non-CR/non-PD | 1/26/4/8 |
| Type of gastrectomy | |
| Total gastrectomy/Non-Total gastrectomy | 25/14 |
| Surgical approach | |
| Open/Laparoscopy | 38/1 |
| Lymph node dissection | |
| D1/D1+/D2/D2+ | 3/2/31/3 |
| Combined resection of other organs | |
| None/Colon/Liver/Ovary/Pancreas/Spleen | 26/3/4/5/2/3 |
| (Partial hepatectomy, 3; Lateral segmentectomy, 1; Distal pancreatectomy, 2) | |
| Operation time, min | |
| Mean ± SD a | 276 ± 75 |
| Intraoperative blood loss, mL | |
| Mean ± SD a | 789 ± 684 |
| Tumor size, cm | |
| Mean ± SD a | 78 ± 43 |
| ypT stage | |
| 0/1/2/3/4a/4b | 5/3/5/10/14/2 |
| ypN stage | |
| 0/1/2/3a/3b | 13/7/4/5/10 |
| pStage | |
| pCR/IA/IB/IIA/IIA/IIIA/IIIB/IIIC/IV | 5/3/4/2/2/6/2/2/13 |
| Residual cancer | |
| R0/R1/R2 | 31/2/6 |
| Pathological response | |
| 0/1a/1b/2a/2b/3 | 2/14/6/10/2/5 |
| Postoperative complications b | |
| II/IIIa/IIIb/IVa/IVb/V | 3/4/1/0/0/0 |
| Postoperative chemotherapy | |
| Absent/Present | 4/35 |
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Sex | ||||||
| Male | 1 | |||||
| Female | 1.33 | 0.61–2.96 | 0.4705 | |||
| Age (years) | ||||||
| <70 | 1 | |||||
| ≥70 | 0.53 | 0.20–1.41 | 0.2030 | |||
| Yoshida classification | ||||||
| Category 2 | 1 | |||||
| Category 3 | 1.19 | 0.48–2.93 | 0.7079 | |||
| Category 4 | 1.20 | 0.35–4.11 | 0.7672 | |||
| Non-curable factors | ||||||
| 1 | 1 | |||||
| 2 | 1.07 | 0.41–2.80 | 0.8838 | |||
| ≥3 | 2.49 | 0.55–11.2 | 0.2368 | |||
| Histological type | ||||||
| Differentiated | 1 | |||||
| Undifferentiated | 1.10 | 0.46–2.63 | 0.8247 | |||
| Initial RECIST evaluation | ||||||
| SD, non-CR/non-PD | 1 | |||||
| PR | 1.70 | 0.71–4.06 | 0.2300 | |||
| CEA level at initial RECIST evaluation | ||||||
| ≤5.0 ng/mL | 1 | 1 | ||||
| >5.0 ng/mL | 0.22 | 0.07–0.67 | 0.0075 | 0.21 | 0.07–0.70 | 0.0108 |
| CA19-9 level at initial RECIST evaluation | ||||||
| ≤37 U/mL | 1 | |||||
| >37 U/mL | 0.59 | 0.26–1.37 | 0.2201 | |||
| Chemotherapy | ||||||
| S1/capecitabine + cisplatin/oxaliplatin | 1 | 1 | ||||
| Combined with intraperitoneal administration | 2.48 | 0.97–6.36 | 0.0592 | 1.91 | 0.68–4.78 | 0.2342 |
| Combined with Trastuzumab administration | 3.84 | 1.33–11.1 | 0.0130 | 4.20 | 1.37–12.9 | 0.0119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, K.; Tanaka, C.; Kanda, M.; Miyata, K.; Furukawa, K.; Maeda, O.; Shimizu, D.; Sugita, S.; Kakushima, N.; Furune, S.; et al. Low Expectancy of Conversion Surgery with R0 Resection in Patients with CEA > 5.0 ng/mL at the Initial RECIST Evaluation for Metastatic Gastric Cancer. Cancers 2023, 15, 5197. https://doi.org/10.3390/cancers15215197
Nakanishi K, Tanaka C, Kanda M, Miyata K, Furukawa K, Maeda O, Shimizu D, Sugita S, Kakushima N, Furune S, et al. Low Expectancy of Conversion Surgery with R0 Resection in Patients with CEA > 5.0 ng/mL at the Initial RECIST Evaluation for Metastatic Gastric Cancer. Cancers. 2023; 15(21):5197. https://doi.org/10.3390/cancers15215197
Chicago/Turabian StyleNakanishi, Koki, Chie Tanaka, Mitsuro Kanda, Kazushi Miyata, Kazuhiro Furukawa, Osamu Maeda, Dai Shimizu, Shizuki Sugita, Naomi Kakushima, Satoshi Furune, and et al. 2023. "Low Expectancy of Conversion Surgery with R0 Resection in Patients with CEA > 5.0 ng/mL at the Initial RECIST Evaluation for Metastatic Gastric Cancer" Cancers 15, no. 21: 5197. https://doi.org/10.3390/cancers15215197
APA StyleNakanishi, K., Tanaka, C., Kanda, M., Miyata, K., Furukawa, K., Maeda, O., Shimizu, D., Sugita, S., Kakushima, N., Furune, S., Kawashima, H., Ando, Y., Ebata, T., & Kodera, Y. (2023). Low Expectancy of Conversion Surgery with R0 Resection in Patients with CEA > 5.0 ng/mL at the Initial RECIST Evaluation for Metastatic Gastric Cancer. Cancers, 15(21), 5197. https://doi.org/10.3390/cancers15215197






