Simple Summary
Penile squamous cell carcinoma (PSCC) is a rare and aggressive cancer. About half of all PSCC cases are thought to be related to human papillomavirus (HPV) or have increased expression of p16 by immunohistochemistry (IHC), a surrogate marker for HPV infection. HPV/p16 positivity is generally a marker of better outcomes in more common squamous cell carcinomas, but the studies evaluating its prognostic value in PSCC are conflicting. In this systematic review, we aim to summarize the existing literature on the impact of these biomarkers on PSCC prognosis and determine their potential value in identifying patients with more favorable biology.
Abstract
PSCC is a rare cancer, with approximately half of all cases related to HPV. While HPV and p16 IHC testing have proven their prognostic value for oropharyngeal cancer, this is not yet established for PSCC. The current level of evidence exploring the relation between PSCC and HPV is moderate, so we conducted a systematic review following PRISMA guidelines to evaluate the prognostic role of HPV and p16 IHC in PSCC clinical outcomes. We searched the PubMed, Embase, and Cochrane databases and identified 34 relevant studies that met our inclusion criteria. Of these, 33 were retrospective cohort studies, and one was a cross-sectional study. Nine studies reported that HPV-positive and p16-positive PSCC had better overall survival (OS) and disease-free survival (DFS). This study highlights the need for a meta-analysis to determine the role of routine HPV status or p16 staining testing as part of the initial diagnosis and staging of PSCC patients worldwide.
1. Introduction
Penile squamous cell carcinoma (PSCC) is a rare genitourinary cancer with fewer than 2500 newly diagnosed cases and 500 deaths annually reported in the United States [1]. Incidence and mortality are even higher in developing countries [2]. Although surgery can be curative in localized cases, lymph node involvement in locally advanced and advanced PSCC confers a significantly worse prognosis and typically warrants a multimodal therapeutic approach [3,4,5,6,7].
The development of PSCC is frequently linked to multiple risk factors, such as inadequate hygiene, absence of circumcision, tobacco smoking, specific genetic conditions, and the presence of HPV, which can be found in 30–50% of cases [8,9,10,11,12].
Two markers, namely p16 overexpression and HPV status, are associated with HPV in PSCC and play a crucial role in determining prognostic significance. Generally, PSCC cases that are positive for HPV and p16 exhibit a more favorable prognosis compared to those that are HPV-negative. Consequently, determining the HPV and p16 status can aid in estimating prognosis and making informed treatment decisions [8].
While p16-positive status through immunohistochemistry (IHC) is considered a robust marker of HPV infection in oropharyngeal squamous cell carcinoma, according to the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines, its value in PSCC is less clear [13,14]. Studies on the prognostic potential and predictive value of HPV and p16 in PSCC have yielded contradictory results [8,15]. Currently, testing HPV status in the initial diagnostic workup of PSCC carries a level 2a evidence grade in the updated ASCO–European Association of Urology (EAU) guidelines, which emphasize the need for more data to support the recommendation of universally assessing HPV status in all PSCC patients [16].
A previous systematic review and meta-analysis of twenty studies concluded that men with HPV-positive or p16-positive PSCC had significantly better disease-specific survival (DSS) [8]. However, the relationship between these biomarkers and overall survival (OS) or disease-free survival (DFS) was inconsistent among individual studies, and no statistically significant associations were found [8]. To complement this prior effort, we conducted an updated systematic review with a larger sample size and more widespread biomarker testing to explore the prognostic significance of HPV and p16 status on PSCC outcomes [8].
2. Materials and Methods
2.1. Search Strategy
We performed a systematic literature search in the electronic databases PubMed, Embase, and the Cochrane Library, covering articles published between March 1992 and December 2022. The database search was filtered to include articles written in English. The search terms used were “Prognosis” OR “Prevalence” AND “HPV” OR “Human Papillomavirus” OR “Human Papillomaviruses” OR “Papillomaviridae” OR “human papillomavirus” OR “Cyclin-Dependent Kinase Inhibitor p16” OR “p16INK4” OR “Cyclin-Dependent Kinase Inhibitor-2A” OR “p16INK4A Protein” OR “CDKN2A Protein” OR “CDKN2 Protein” AND “Penile Neo-plasms” OR “Penile Neoplasms” OR “Penis Neoplasms” OR “Penis Neoplasm” OR “Penile Neoplasm” OR “Penis Cancer” OR “Penile Cancer” OR “Penile carcinoma” OR “penis carcinoma” OR “penis tumor” OR “penile cancers”.
JC conducted the initial search, and subsequently, KP and AM independently verified the abstracts and selected manuscripts. Disagreements were resolved through collaborative discussions to achieve consensus. A fourth author, RS, addressed any unresolved conflicts. Supplementary information was reviewed using reference lists to ensure comprehensiveness. The study adhered to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Figure 1) [17].
Figure 1.
PRISMA diagram.
2.2. Study Selection
Only peer-reviewed studies in English or with available translations were eligible for inclusion in the analysis. In cases where a study population was reported in multiple papers, only data from the most up-to-date paper and providing the most complete information were utilized. Case reports, abstracts, letters to the editor, and review articles were excluded from the analysis.
2.3. Quality Assessment
Two reviewers independently evaluated the quality of the chosen papers using a modified Newcastle–Ottawa Scale (NOS). The scale comprised 8 items divided into three domains: subject selection criteria (0–4 points), comparability of subjects (0–2 points), and outcomes (0–3 points). The maximum achievable score was 9 points, with a score of more than 5 points indicating high quality. Supplemental Table S1 provides a summary of the assessment of the NOS scores with a median total score of 7 (IQR 7–8.25).
2.4. Data Extraction
A total of 34 articles were identified and selected for data extraction. Data tables were created to systematically capture relevant information from the texts, tables, and figures of each included study. These tables included details such as the first author, publication year, country, year of sample collection, follow-up time, type of tissue testing method, p16 testing technique, the definition of p16 positivity (overexpression), number of evaluators for p16 staining, sample size, the number of HPV-positive and HPV-negative PSCC cases, the number of p16-positive PSCC cases, and various survival endpoints. The examined survival outcomes encompassed overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS).
2.5. Mean Age Adjustment
During our research, we encountered studies that presented the median age of patients, along with either the interquartile range (IQR) or range, rather than providing the mean and standard deviation. To approximate the mean age, we adopted the methodology introduced by Wan et al. [18] in 2014, which provides formulas to estimate the sample mean and standard deviation based on factors such as the sample size, median, and either the IQR or range [18]. By employing these formulas, we successfully estimated the mean age of patients in the aforementioned studies and calculated the overall mean for our sample size.
3. Results
3.1. Study Characteristics
In this systematic review, we conducted an analysis of the data derived from a total of 34 studies encompassing 3994 patients diagnosed with PSCC. These studies spanned the period from 1970 to 2022. The estimated mean age of the patients was determined to be 62.3 years. Among the studies included in our analysis, Brazil and the United States both had the highest number of studies, with each country contributing eight studies (refer to Figure 2 for details). Fourteen studies reported on both HPV and p16 IHC status [4,13,19,20,21,22,23,24,25,26,27,28,29,30], fifteen studies [15,31,32,33,34,35,36,37,38,39,40,41,42,43,44] focused solely on HPV, and five studies [45,46,47,48,49] reported only on p16 status. For a summary of HPV and p16 positivity across the 34 included studies, please refer to Table 1 and Supplemental Table S2.
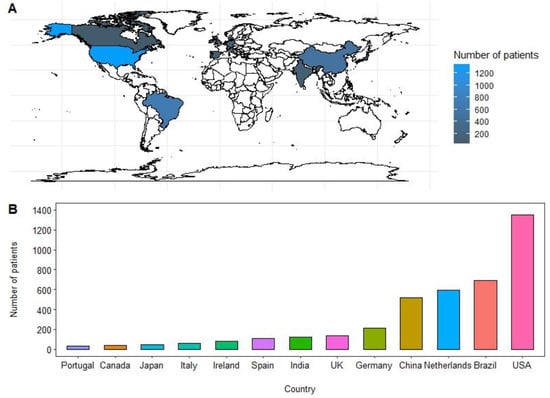
Figure 2.
(A,B) Original geographical distribution and bar graph representation of number of patients included in the study from 1970–2022.

Table 1.
Prevalence of HPV and p16 positivity across the included studies.
3.2. HPV Detection Methods
Among the studies included in our analysis, a total of twenty-nine studies reported HPV status. Out of these, eighteen studies employed the polymerase chain reaction (PCR) method for HPV detection [13,15,19,20,22,23,25,26,27,28,31,32,33,35,36,37,38,39], six studies utilized in situ hybridization (ISH) [4,21,24,29,34,40], and four studies did not specify the method used for HPV detection [41,42,43,44]. Additionally, one study employed a Quantus fluorometer for the detection of HPV nucleic acid [30]. For further details, please refer to Supplemental Table S2.
3.3. p16 Detection Methods
Nineteen studies included in our analysis examined samples to evaluate the p16+ status using immunohistochemical (IHC) staining. Two different methodologies were employed for grading p16 on IHC. Six studies utilized a quantitative measurement approach, where the cut-off for considering a sample as p16-positive ranged from >10% to >75% of cells exhibiting positive staining [4,20,24,26,29,48]. Conversely, twelve studies employed subjective criteria to define p16 positivity, using descriptions such as strong and diffuse staining, vital staining of proliferative cells, continuous and complete cytoplasmic staining, intense confluent staining, or focal scattering of staining in the cytoplasm and nucleus of cells [13,19,21,22,23,25,28,30,42,45,47,49]. Bethune et al. [46] adopted a mixed methodology, considering a qualitative cut-off of >30% staining with nuclear and cytoplasmic staining, moderate sample staining with at least 30% of cells staining, or strong cytoplasmic and nuclear staining as positive for p16 [46]. One study did not specify the criteria used to determine p16 positivity [27]. For further details, please refer to Supplemental Table S2.
3.4. HPV + and p16+ Impact on Overall Survival
None of the included studies demonstrated a significant association between HPV positivity and improved overall survival (OS). However, p16 status was found to be associated with improved OS in three independent analyses [4,29,46]. Notably, Chahoud et al. [4] investigated both biomarkers in the same population and discovered a positive correlation between p16-positive cases and superior OS, while no such association was observed for HPV+ cases [4]. These discrepancies could potentially be attributed to the variations in defining p16 positivity during IHC analysis, indicating the presence of heterogeneity among the studies. For further details on the impact HPV and p16 positivity had on overall survival, please refer to Table 2.

Table 2.
HPV+ and p16+ on overall survival (OS).
3.5. HPV+ and p16+ Impact on Disease-Free Survival
The presence of HPV positivity was associated with improved disease-free survival (DFS) and was statistically significant in four of the studies [13,26,28,39]. Similarly, p16 positivity was also associated with improved DFS and was statistically significant in two of the studies [42,47]. For further details on the impact HPV and p16 positivity had on disease-free survival, please refer to Table 3.

Table 3.
HPV+ and p16 + on disease-free survival (DFS).
3.6. HPV+ and p16+ Impact on Disease-Specific Survival
In three of the studies, HPV positivity was associated with improved disease-specific survival (DSS) [28,32,37]. Conversely, p16 positivity was linked with improved DSS in four of the studies [4,24,28,45]. Notably, the study conducted by Chu et al. [28] demonstrated statistically significant associations for both biomarkers within the same population [28]. For further details on the impact HPV and p16 positivity had on disease-specific survival, please refer to Table 4.

Table 4.
HPV+ and p16+ on disease-specific survival (DSS).
4. Discussion
This systematic review presents an analysis of 34 studies, aiming to provide an up-to-date overview of the prognostic significance of HPV status and p16 expression in PSCC. The inclusion of recent studies has significantly enhanced previous efforts on this topic, notably by substantially increasing the sample size and incorporating a more diverse patient population from various global sites.
The risk factors for PSCC can vary across countries and regions. While some risk factors, like HPV, are consistent globally, others may be more prevalent in specific populations. For example, Brazil has been identified as having one of the highest incidence rates of PSCC worldwide, possibly due to a high prevalence of HPV infection, lower rates of circumcision, and socioeconomic factors such as limited access to healthcare and education about safe sex practices [11]. On the other hand, westernized countries like Portugal may have a lower incidence of PSCC due to public health campaigns and educational initiatives that raise awareness about the disease and promote preventive measures such as good hygiene and regular medical check-ups. These efforts may facilitate timely diagnosis and treatment, potentially reducing the occurrence of PSCC [50].
Certain high-risk HPV genotypes, particularly HPV-16 and HPV-18, are strongly associated with p16 overexpression in infected cells. HPV-16—being the most oncogenic and prevalent high-risk genotype—is strongly linked to the development of various cancers, including cervical, anal, vulvar, vaginal, certain head and neck cancers, and penile cancers [51,52]. In contrast, low-risk HPV genotypes like HPV-6 and HPV-11 are commonly associated with benign lesions and typically not associated with p16 overexpression [53,54].
It is crucial to note that combining multiple prevention strategies, including HPV vaccination, screening, and safe sexual practices, can have the greatest impact on reducing HPV-related diseases. However, challenges such as vaccine hesitancy, lack of awareness, and limited access to vaccination programs can hinder vaccine uptake. Sociocultural factors and stigma surrounding discussions about sexual health and genital cancers may also create barriers to open dialogue, education, and prevention efforts [55,56,57].
The 2022 World Health Organization (WHO) classification of penile cancer, as suggested by Menon et al., recommends the utilization of p16 screening whenever possible to differentiate between HPV-associated and HPV-independent PSCC [58]. This updated recommendation is expected to result in increased p16 testing and the accumulation of additional studies, which can contribute to the statistical power of future meta-analyses.
In our review, we conducted a multivariate analysis and found that p16 positivity was associated with improved OS and DSS in a subset of published studies. Specifically, in 3 out of 11 (27%) studies, p16 positivity was associated with improved OS, and in 4 out of 7 (57%) studies, p16 positivity was associated with improved DSS. In comparison, HPV positivity was not significantly associated with improved OS in any of the 10 studies analyzed (0%), and it was associated with improved DSS in only 3 out of 10 (30%) studies. Most of the included studies in our review demonstrated good methodological quality, with a majority achieving a Newcastle–Ottawa Scale (NOS) score higher than 7 and a median total score of 7 (IQR 7–8.25). However, it is important to acknowledge the limitations of our systematic review. We did not include unpublished studies or studies published in languages other than English, which may introduce publication bias and limit the comprehensiveness of the evidence base. Furthermore, another limitation in our study arises from the observed variability in the definitions of p16 positivity among the twenty included studies that assessed p16 status. Out of these studies, six [4,20,24,26,29,48] employed a quantitative measurement approach, while twelve [13,19,21,22,23,25,28,30,42,45,47,49] relied on descriptive criteria. Additionally, Bethune et al. [46] and Chahoud et al. [4] utilized both methodologies, reporting both quantitative and descriptive elements to define p16 positivity. The existence of such diverse definitions for p16 positivity presents a challenge in drawing definitive conclusions and synthesizing the findings across studies.
The inclusion of fourteen additional studies, since the publication of the meta-analysis by Sand et al. [8], provides significant additions to the current body of evidence regarding the prognostic significance of HPV and p16 biomarkers in PSCC. Although the systematic review has not yielded conclusive results regarding the overall data on HPV and P16 biomarkers, it has provided new evidence of the possible correlation of p16 using IHC with better prognosis in PSCC and highlighted some discrepancy between p16 and HPV status in PSCC.
The current guidelines from EAU–ASCO recommend conducting p16 testing through IHC in all individuals diagnosed with PSCC [16]. This underscores the importance of assessing p16 expression as a valuable diagnostic tool in PSCC management. Looking ahead, we anticipate that an upcoming meta-analysis will provide additional clarity and a deeper understanding of this subject in the future, further advancing our knowledge of the prognostic significance of HPV and p16 biomarkers in PSCC.
5. Conclusions
Our systematic review highlights the importance of conducting an updated comprehensive meta-analysis to define the potential role of these biomarkers in PSCC. We anticipate that this will address the discordance between p16 and HPV and uncover a potentially stronger prognostic value for p16 positivity on the outcomes of PSCC patients.
Supplementary Materials
The following information can be downloaded at https://www.mdpi.com/article/10.3390/cancers15143713/s1. Supplemental Table S1 provides a summary of the assessment of the NOS scores with a median total score of 7 (IQR 7–8.25). Supplemental Table S2 is demographic data and method of HPV and or P16 Detection of the 34 studies included in our retrospective analysis.
Author Contributions
Conceptualization, K.P., J.C. (Jad Chahoud), C.A.P. and P.E.S.; methodology, K.P., A.M., F.I. and J.C. (Jad Chahoud); software, K.P. and A.M.; validation, A.M., F.I. and R.S.; formal analysis, K.P., A.M., S.A.E. and P.E.S.; investigation, A.M. and F.I.; resources, J.C. (Jad Chahoud); data curation, K.P., A.M., F.I., M.P., H.S., P.E.S. and J.C. (Jad Chahoud); writing—original draft preparation, K.P., A.M., F.I., R.S. and J.C. (Jad Chahoud); writing—review and editing, K.P., A.M., F.I., M.P., R.S., H.S., G.D.G., P.A.J., J.C. (Juskaran Chadha), N.Z., C.A.P., P.E.S. and J.C. (Jad Chahoud); supervision, J.C. (Jad Chahoud); project administration, K.P. and J.C. (Jad Chahoud). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
J.C. received consulting fees from Pfizer, Exelixis, Aveo. The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Tian, T.; Yao, K.; Chen, X.F.; Luo, G.; Gao, Y.; Lin, Y.F.; Wang, B.; Sun, Y.; Zheng, W.; et al. Global Pattern and Trends in Penile Cancer Incidence: Population-Based Study. JMIR Public Health Surveill. 2022, 8, e34874. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Chadha, J.; Chahoud, J. Penile Cancer: Updates in Systemic Therapy. Asian J. Urol. 2022, 9, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Chahoud, J.; Spiess, P.E. An Update on Treatment of Penile Cancer. Ther. Adv. Med. Oncol. 2022, 14, 17588359221127254. [Google Scholar] [CrossRef]
- Chahoud, J.; Pham, R.; Sonpavde, G. Innovative Systemic Therapies for Penile Cancer. Curr. Opin. Urol. 2022, 32, 8–16. [Google Scholar] [CrossRef]
- Chahoud, J.; Kohli, M.; Spiess, P.E. Management of Advanced Penile Cancer. Mayo Clin. Proc. 2021, 96, 720–732. [Google Scholar] [CrossRef]
- Chahoud, J.; Tamil, M.; Necchi, A. Second Line Salvage Systemic Therapy for Advanced Penile Cancer. Urol. Oncol. 2022, 40, 229–234. [Google Scholar] [CrossRef]
- Sand, F.L.; Rasmussen, C.L.; Frederiksen, M.H.; Andersen, K.K.; Kjaer, S.K. Prognostic Significance of HPV and P16 Status in Men Diagnosed with Penile Cancer: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1123–1132. [Google Scholar] [CrossRef]
- Chahoud, J.; Zacharias, N.M.; Pham, R.; Qiao, W.; Guo, M.; Lu, X.; Alaniz, A.; Segarra, L.; Martinez-Ferrer, M.; Gleber-Netto, F.O.; et al. Prognostic Significance of P16 and Its Relationship with Human Papillomavirus Status in Patients with Penile Squamous Cell Carcinoma: Results of 5 Years Follow-Up. Cancers 2022, 14, 6024. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Vieira, C.B.; Feitoza, L.; Pinho, J.; Teixeira-Júnior, A.; Lages, J.; Calixto, J.; Coelho, R.; Nogueira, L.; Cunha, I.; Soares, F.; et al. Profile of Patients with Penile Cancer in the Region with the Highest Worldwide Incidence. Sci. Rep. 2020, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Naghavi, A.O.; Johnstone, P.A.; Spiess, P.E.; Grass, G.D. Updates in the Use of Radiotherapy in the Management of Primary and Locally-Advanced Penile Cancer. Asian J. Urol. 2022, 9, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.A.; Pinho, J.D.; Teixeira Júnior, A.A.L.; Nogueira, L.R.; Silva, F.F.; Maulen, V.E.; Khayat, A.S.; Calixto, J.R.R.; Costa, H.A.; Ramalho, L.N.Z.; et al. P16INK4a Expression in Patients with Penile Cancer. PLoS ONE 2018, 13, e0205350. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Ismaila, N.; Ku, J.A.; Burtness, B.; Swiecicki, P.L.; Mell, L.; Beitler, J.J.; Gross, N.; Jones, C.U.; Kaufman, M.; et al. Role of Treatment Deintensification in the Management of P16+ Oropharyngeal Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, A.L.R.; Lopes, A.; Santiago, G.H.; Ribeiro, K.C.B.; Latorre, M.R.D.O.; Villa, L.L. Human Papillomavirus as a Prognostic Factor in Carcinoma of the Penis: Analysis of 82 Patients Treated with Amputation and Bilateral Lymphadenectomy. Cancer 2001, 91, 2315–2321. [Google Scholar] [CrossRef]
- European Association of Urology. EAU-ASCO Guidelines on Penile Cancer. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-ASCO-Guidelines-on-Penile-Cancer-2023_2023-03-08-131333_piyo.pdf (accessed on 15 April 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Guerrero, D.; Guarch, R.; Ojer, A.; Casas, J.M.; Ropero, S.; Mancha, A.; Pesce, C.; Lloveras, B.; Garcia-Bragado, F.; Puras, A. Hypermethylation of the Thrombospondin-1 Gene Is Associated with Poor Prognosis in Penile Squamous Cell Carcinoma. BJU Int. 2008, 102, 747–755. [Google Scholar] [CrossRef]
- Ferrándiz-Pulido, C.; Masferrer, E.; De Torres, I.; Lloveras, B.; Hernandez-Losa, J.; Mojal, S.; Salvador, C.; Morote, J.; Ramon, Y.; Cajal, S.; et al. Identification and Genotyping of Human Papillomavirus in a Spanish Cohort of Penile Squamous Cell Carcinomas: Correlation with Pathologic Subtypes, P16INK4a Expression, and Prognosis. J. Am. Acad. Dermatol. 2013, 68, 73–82. [Google Scholar] [CrossRef]
- Bezerra, S.M.; Chaux, A.; Ball, M.W.; Faraj, S.F.; Munari, E.; Gonzalez-Roibon, N.; Sharma, R.; Bivalacqua, T.J.; Burnett, A.L.; Netto, G.J. Human Papillomavirus Infection and Immunohistochemical P16INK4a Expression as Predictors of Outcome in Penile Squamous Cell Carcinomas. Human. Pathol. 2015, 46, 532–540. [Google Scholar] [CrossRef]
- McDaniel, A.S.; Hovelson, D.H.; Cani, A.K.; Liu, C.J.; Zhai, Y.; Zhang, Y.; Weizer, A.Z.; Mehra, R.; Feng, F.Y.; Alva, A.S.; et al. Genomic Profiling of Penile Squamous Cell Carcinoma Reveals New Opportunities for Targeted Therapy. Cancer Res. 2015, 75, 5219–5227. [Google Scholar] [CrossRef] [PubMed]
- Steinestel, J.; Al Ghazal, A.; Arndt, A.; Schnoeller, T.J.; Schrader, A.J.; Moeller, P.; Steinestel, K. The Role of Histologic Subtype, P16INK4a Expression, and Presence of Human Papillomavirus DNA in Penile Squamous Cell Carcinoma. BMC Cancer 2015, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Zargar-Shoshtari, K.; Spiess, P.E.; Berglund, A.E.; Sharma, P.; Powsang, J.M.; Giuliano, A.; Magliocco, A.M.; Dhillon, J. Clinical Significance of P53 and P16ink4a Status in a Contemporary North American Penile Carcinoma Cohort. Clin. Genitourin. Cancer 2016, 14, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Afonso, L.A.; Carestiato, F.N.; Ornellas, A.A.; Ornellas, P.; Rocha, W.M.; Cordeiro, T.I.; Lisboa, D.C.; Alves, G.B.; Cavalcanti, S.M.B. Human Papillomavirus, Epstein-Barr Virus, and Methylation Status of P16ink4a in Penile Cancer. J. Med. Virol. 2017, 89, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Cunha, I.W.; Figliuolo, G.; Rondon, H.; de Souza, P.M.; Torres Silva, F.L.; Torres Silva, G.L.; de Souza Bastos, M.; de Castro, D.B.; Santana, M.F.; et al. Presence of HPV with Overexpression of P16INK4a Protein and EBV Infection in Penile Cancer—A Series of Cases from Brazil Amazon. PLoS ONE 2020, 15, e0232474. [Google Scholar] [CrossRef]
- Muresu, N.; Sotgiu, G.; Saderi, L.; Sechi, I.; Cossu, A.; Marras, V.; Meloni, M.; Martinelli, M.; Cocuzza, C.; Tanda, F.; et al. Italian Observational Study on HPV Infection, E6, and P16 Expression in Men with Penile Cancer. Virol. J. 2020, 17, 161. [Google Scholar] [CrossRef]
- Chu, C.; Chen, K.; Tan, X.; Lu, J.; Yang, Y.; Zhang, Y.J.; Yao, K.; Cao, Y. Prevalence of Human Papillomavirus and Implication on Survival in Chinese Penile Cancer. Virchows Arch. 2020, 477, 667–675. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Mishra, S.K.; Bhardwaj, N.; Sardana, R.; Jaiswal, S.; Pattnaik, N.; Pradhan, D.; Sharma, S.; Kaushal, S.; Baisakh, M.R.; et al. P53 and P16ink4a As Predictive and Prognostic Biomarkers for Nodal Metastasis and Survival in A Contemporary Cohort of Penile Squamous Cell Carcinoma. Clin. Genitourin. Cancer 2021, 19, 510–520. [Google Scholar] [CrossRef]
- Müller, T.; Demes, M.; Lehn, A.; Köllermann, J.; Vallo, S.; Wild, P.J.; Winkelmann, R. The Peri- and Intratumoral Immune Cell Infiltrate and PD-L1 Status in Invasive Squamous Cell Carcinomas of the Penis. Clin. Transl. Oncol. 2022, 24, 331–341. [Google Scholar] [CrossRef]
- Wiener, J.S.; Effert, P.J.; Humphrey, P.A.; Yu, L.; Liu, E.T.; Walther, P.J. Prevalence of Human Papillomavirus Types 16 and 18 in Squamous-Cell Carcinoma of the Penis: A Retrospective Analysis of Primary and Metastatic Lesions by Differential Polymerase Chain Reaction. Int. J. Cancer 1992, 50, 694–701. [Google Scholar] [CrossRef]
- Lont, A.P.; Kroon, B.K.; Horenblas, S.; Gallee, M.P.W.; Berkhof, J.; Meijer, C.J.L.M.; Snijders, P.J.F. Presence of High-Risk Human Papillomavirus DNA in Penile Carcinoma Predicts Favorable Outcome in Survival. Int. J. Cancer 2006, 119, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, M.A.; Campos, M.M.; Ornellas, A.A.; Chin, E.W.; Ornellas, M.H.; Andrada-Serpa, M.J. Human Papillomavirus and Penile Cancers in Rio de Janeiro, Brazil: HPV Typing and Clinical Features. Int. Braz. J. Urol. 2008, 34, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Perdonà, S.; Buonerba, C.; Sonpavde, G.; Gigantino, V.; Pannone, G.; Quarto, G.; Ferro, M.; Gaudioso, G.; Terracciano, D.; et al. Cytosolic Phosphorylated EGFR Is Predictive of Recurrence in Early Stage Penile Cancer Patients: A Retropective Study. J. Transl. Med. 2013, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.G.; Soares, F.A.; Burbano, R.R.; Silvestre, R.V.; Pinto, L.O.A.D. Human Papilloma Virus: Prevalence, Distribution and Predictive Value to Lymphatic Metastasis in Penile Carcinoma. Int. Braz. J. Urol. 2013, 39, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.Y.; Goodman, M.T.; Unger, E.R.; Steinau, M.; Powers, A.; Lynch, C.F.; Cozen, W.; Saber, M.S.; Peters, E.S.; Wilkinson, E.J.; et al. Human Papillomavirus Genotype Prevalence in Invasive Penile Cancers from a Registry-Based United States Population. Front. Oncol. 2014, 4, 9. [Google Scholar] [CrossRef]
- Djajadiningrat, R.S.; Jordanova, E.S.; Kroon, B.K.; Van Werkhoven, E.; De Jong, J.; Pronk, D.T.M.; Snijders, P.J.F.; Horenblas, S.; Heideman, D.A.M. Human Papillomavirus Prevalence in Invasive Penile Cancer and Association with Clinical Outcome. J. Urol. 2015, 193, 526–531. [Google Scholar] [CrossRef]
- de Araújo, L.A.; De Paula, A.A.P.; da Silva Cintra de Paula, H.; Porto Ramos, J.E.; de Oliveira, B.R.; De Carvalho, K.P.A.; Guimarães, R.A.; de Alencar, R.D.C.G.; Barroso Duarte, E.C.; Rabelo Santos, S.H.; et al. Human Papillomavirus (HPV) Genotype Distribution in Penile Carcinoma: Association with Clinic Pathological Factors. PLoS ONE 2018, 13, e0199557. [Google Scholar] [CrossRef]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Józwiak, K.; Heeren, A.M.; de Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front. Immunol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Takamoto, D.; Kawahara, T.; Kasuga, J.; Sasaki, T.; Yao, M.; Yumura, Y.; Uemura, H. The Analysis of Human Papillomavirus DNA in Penile Cancer Tissue by in Situ Hybridization. Oncol. Lett. 2018, 15, 8102–8106. [Google Scholar] [CrossRef]
- Wang, B.; Gu, W.; Wan, F.; Wei, Y.; Xiao, W.; Lu, X.; Zhang, G.; Zhou, J.; Wang, Q.; Ding, X.; et al. Prognosis of the 8th TNM Staging System for Penile Cancer and Refinement of Prognostication by Incorporating High Risk Human Papillomavirus Status. J. Urol. 2020, 203, 562–569. [Google Scholar] [CrossRef]
- Ashley, S.; Shanks, J.H.; Oliveira, P.; Lucky, M.; Parnham, A.; Lau, M.; Sangar, V. Human Papilloma Virus (HPV) Status May Impact Treatment Outcomes in Patients with Pre-Cancerous Penile Lesions (an EUROGEN Study). Int. J. Impot. Res. 2021, 33, 620–626. [Google Scholar] [CrossRef]
- Chipollini, J.; Pollock, G.; Hsu, C.H.; Batai, K.; Recio-Boiles, A.; Lee, B.R. National Trends and Survival Outcomes of Penile Squamous Cell Carcinoma Based on Human Papillomavirus Status. Cancer Med. 2021, 10, 7466–7474. [Google Scholar] [CrossRef]
- Browne, E.; Foley, M.P.; O’Kelly, J.; Ríogh, A.N.A.; Shah, N.; Shilling, C.; Keane, J.P.; Daly, P.; Cullen, I.M. The Relationship of Human Papillomavirus Positivity with Tumor Characteristics in an Irish Penile Cancer Population. Can. Urol. Assoc. J. 2022, 16, 435–438. [Google Scholar] [CrossRef]
- Gunia, S.; Erbersdobler, A.; Hakenberg, O.W.; Koch, S.; May, M. P16 INK4a Is a Marker of Good Prognosis for Primary Invasive Penile Squamous Cell Carcinoma: A Multi-Institutional Study. J. Urol. 2012, 187, 899–907. [Google Scholar] [CrossRef]
- Bethune, G.; Campbell, J.; Rocker, A.; Bell, D.; Rendon, R.; Merrimen, J. Clinical and Pathologic Factors of Prognostic Significance in Penile Squamous Cell Carcinoma in a North American Population. Urology 2012, 79, 1092–1097. [Google Scholar] [CrossRef]
- Tang, D.H.; Clark, P.E.; Giannico, G.; Hameed, O.; Chang, S.S.; Gellert, L.L. Lack of P16ink4a over Expression in Penile Squamous Cell Carcinoma Is Associated with Recurrence after Lymph Node Dissection. J. Urol. 2015, 193, 519–525. [Google Scholar] [CrossRef]
- De Bacco, M.W.; Carvalhal, G.F.; MacGregor, B.; Marçal, J.M.B.; Wagner, M.B.; Sonpavde, G.P.; Fay, A.P. PD-L1 and P16 Expression in Penile Squamous Cell Carcinoma from an Endemic Region. Clin. Genitourin. Cancer 2020, 18, e254–e259. [Google Scholar] [CrossRef]
- Pereira-Lourenço, M.; Vieira e Brito, D.; Peralta, J.P.; Godinho, R.; Conceição, P.; Reis, M.; Rabaça, C.; Sismeiro, A.; Eliseu, M.; Castelo-Branco, N. Prognostic Value of P16INK4a Overexpression in Penile Cancer. Arch. Ital. Urol. Androl. 2020, 92, 11–16. [Google Scholar] [CrossRef]
- Douglawi, A.; Masterson, T.A. Penile Cancer Epidemiology and Risk Factors: A Contemporary Review. Curr. Opin. Urol. 2019, 29, 145–149. [Google Scholar] [CrossRef]
- Quinlan, J.D. Human Papillomavirus: Screening, Testing, and Prevention. Am. Fam. Physician 2021, 104, 152–159. [Google Scholar]
- Ramakrishnan, S.; Partricia, S.; Mathan, G. Overview of High-Risk HPV’s 16 and 18 Infected Cervical Cancer: Pathogenesis to Prevention. Biomed. Pharmacother. 2015, 70, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hoai, B.N.; Cao, T.N.; Luong Thi, L.A.; Nguyen, M.N.; Duong, H.-Q.; Than, V.T. Human Papillomavirus Prevalence and Genotype Distribution in Vietnamese Male Patients between 2016 and 2020. J. Med. Virol. 2022, 94, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Derbie, A.; Mekonnen, D.; Nibret, E.; Maier, M.; Woldeamanuel, Y.; Abebe, T. Human Papillomavirus Genotype Distribution in Ethiopia: An Updated Systematic Review. Virol. J. 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Mott, N.; Clark, S.J.; Harper, D.M.; Shuman, A.G.; Prince, M.E.P.; Dossett, L.A. HPV Vaccination among Young Adults in the US. JAMA 2021, 325, 1673–1674. [Google Scholar] [CrossRef]
- Thompson, E.L.; Garg, A.; Galvin, A.M.; Moore, J.D.; Kasting, M.L.; Wheldon, C.W. Correlates of HPV Vaccination Intentions among Adults Ages 27–45 Years Old in the U.S. J. Community Health 2021, 46, 893–902. [Google Scholar] [CrossRef]
- Walling, E.B.; Benzoni, N.; Dornfeld, J.; Bhandari, R.; Sisk, B.A.; Garbutt, J.; Colditz, G. Interventions to Improve HPV Vaccine Uptake: A Systematic Review. Pediatrics 2016, 138, e20153863. [Google Scholar] [CrossRef]
- Menon, S.; Moch, H.; Berney, D.M.; Cree, I.A.; Srigley, J.R.; Tsuzuki, T.; Compérat, E.; Hartmann, A.; Netto, G.; Rubin, M.A.; et al. WHO 2022 Classification of Penile and Scrotal Cancers: Updates and Evolution. Histopathology 2023, 82, 508–520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).