The Impact of PSMA PET/CT on Modern Prostate Cancer Management and Decision Making—The Urological Perspective
Abstract
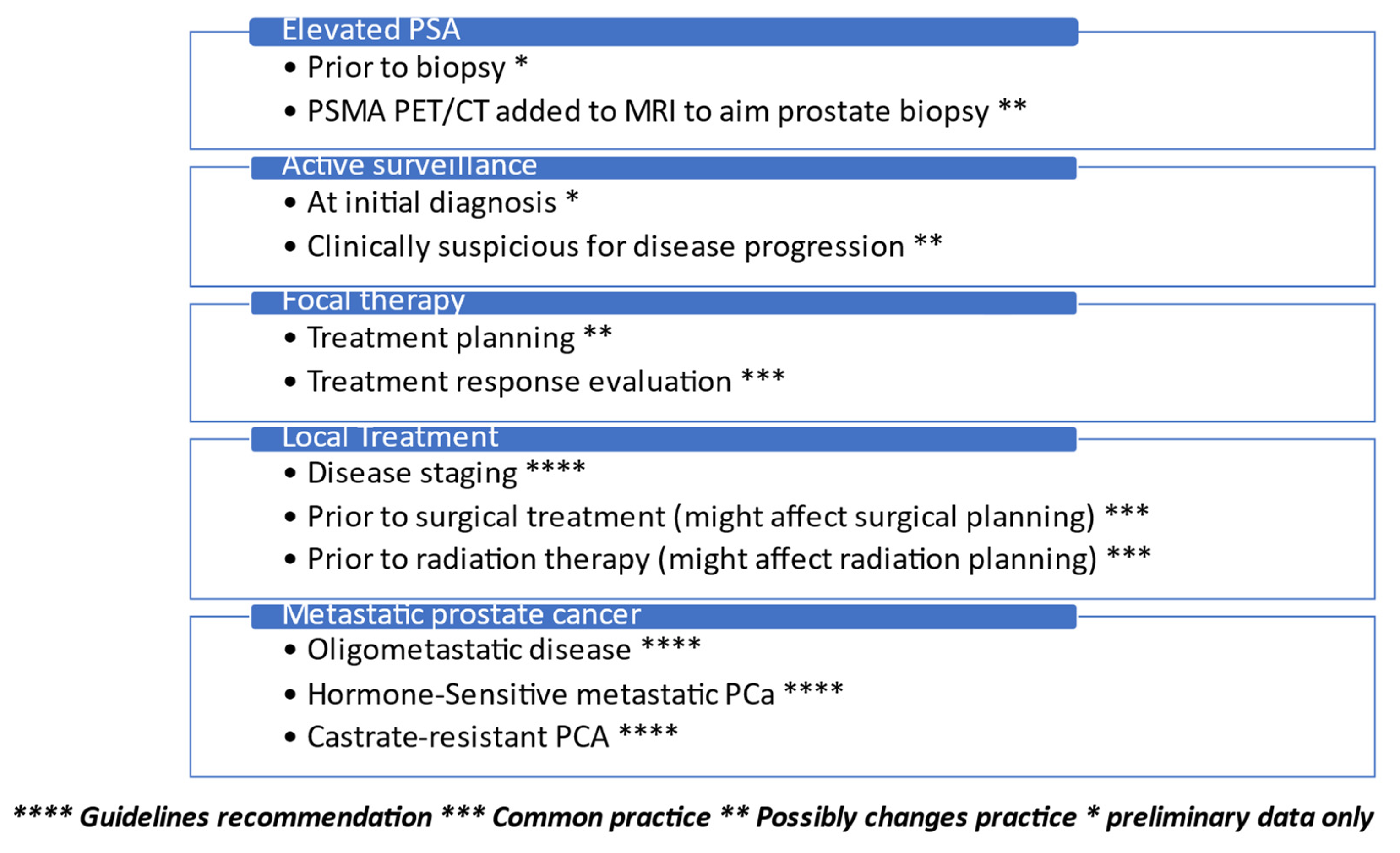
Simple Summary
Abstract
1. Introduction
2. PSMA PET/CT in Metastatic Prostate Cancer
3. PSMA PET/CT in the Detection of Prostate Cancer Bone Metastasis
4. PSMA PET/CT in Organ-Confined and Locally Advanced Prostate Cancer Treatment
5. PSMA PET/CT Use in Active Surveillance and Focal Treatment for Prostate Cancer
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Awenat, S.; Piccardo, A.; Carvoeiras, P.; Signore, G.; Giovanella, L.; Prior, J.O.; Treglia, G. Diagnostic Role of 18F-PSMA-1007 PET/CT in Prostate Cancer Staging: A Systematic Review. Diagnostics 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Ghosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Friedman, N.C.; Hines, E., Jr. The Will Rodgers Phenomenon and PSMA PET/CT. J. Nucl. Med. 2022, 63, 966. [Google Scholar] [CrossRef]
- Kase, A.M.; Tan, W.; Copland, J.A., 3rd; Cai, H.; Parent, E.E.; Madan, R.A. The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer. Cancers 2022, 14, 1361. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Jiao, J.; Kang, F.; Zhang, J.; Quan, Z.; Wen, W.; Zhao, X.; Ma, S.; Wu, P.; Yang, F.; Guo, W.; et al. Establishment and prospective validation of an SUVmax cutoff value to discriminate clinically significant prostate cancer from benign prostate diseases in patients with suspected prostate cancer by 68Ga-PSMA PET/CT: A real-world study. Theranostics 2021, 11, 8396–8411. [Google Scholar] [CrossRef]
- van der Sar, E.C.A.; Kühr, A.J.S.; Ebbers, S.C.; Henderson, A.M.; de Keizer, B.; Lam, M.G.E.H.; Braat, A.J.A.T. Baseline Imaging Derived Predictive Factors of Response Following [177Lu]Lu-PSMA-617 Therapy in Salvage Metastatic Castration-Resistant Prostate Cancer: A Lesion- and Patient-Based Analysis. Biomedicines 2022, 10, 1575. [Google Scholar] [CrossRef]
- Shagera, Q.A.; Artigas, C.; Karfis, I.; Critchi, G.; Chanza, N.M.; Sideris, S.; Peltier, A.; Paesmans, M.; Gil, T.; Flamen, P. 68Ga-PSMA PET/CT for Response Assessment and Outcome Prediction in Metastatic Prostate Cancer Patients Treated with Taxane-Based Chemotherapy. J. Nucl. Med. 2022, 63, 1191–1198. [Google Scholar] [CrossRef]
- Anton, A.; Hasan, O.K.; Ballok, Z.; Bowden, P.; Costello, A.J.; Harewood, L.; Corcoran, N.M.; Dundee, P.; Peters, J.S.; Lawrentschuk, N.; et al. Use of prostate-specific membrane antigen positron-emission tomography/CT in response assessment following upfront chemohormonal therapy in metastatic prostate cancer. BJU Int. 2020, 126, 433–435. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Kumar, A.S.R.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef]
- Mittlmeier, L.M.; Brendel, M.; Beyer, L.; Albert, N.L.; Todica, A.; Zacherl, M.J.; Wenter, V.; Herlemann, A.; Kretschmer, A.; Ledderose, S.T.; et al. Feasibility of Different Tumor Delineation Approaches for 18F-PSMA-1007 PET/CT Imaging in Prostate Cancer Patients. Front. Oncol. 2021, 11, 663631. [Google Scholar] [CrossRef]
- Kim, M.; Seifert, R.; Fragemann, J.; Kersting, D.; Murray, J.; Jonske, F.; Pomykala, K.L.; Egger, J.; Fendler, W.P.; Herrmann, K.; et al. Evaluation of thresholding methods for the quantification of [68Ga]Ga-PSMA-11 PET molecular tumor volume and their effect on survival prediction in patients with advanced prostate cancer undergoing [177Lu]Lu-PSMA-617 radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2196–2209. [Google Scholar] [CrossRef]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Zhu, Y.; Shi, Y.; Xu, L.; Huang, G.; Liu, J. The added value of 18F-FDG PET/CT compared to 68Ga-PSMA PET/CT in patients with castration-resistant prostate cancer. J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Bagni, O. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev. Anticancer Ther. 2020, 20, 823–829. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Mdlophane, A.; Maes, A.; Mokoala, K.; et al. mCRPC Patients Receiving 225Ac-PSMA-617 Therapy in the Post–Androgen Deprivation Therapy Setting: Response to Treatment and Survival Analysis. J. Nucl. Med. 2022, 63, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Pianou, N.K.; Stavrou, P.Z.; Vlontzou, E.; Rondogianni, P.; Exarhos, D.N.; Datseris, I.E. More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT. Hell J. Nucl Med. 2019, 22, 6–9. [Google Scholar]
- Marcus, C.; Butler, P.; Bagrodia, A.; Cole, S.; Subramaniam, R.M. Fluorine-18-Labeled Fluciclovine PET/CT in Primary and Biochemical Recurrent Prostate Cancer Management. Am. J. Roentgenol. 2020, 215, 267–276. [Google Scholar] [CrossRef]
- Filippi, L.; Bagni, O.; Schillaci, O. Digital PET/CT with 18F-FACBC in early castration-resistant prostate cancer: Our preliminary results. Expert Rev. Med. Devices 2022, 19, 591–598. [Google Scholar] [CrossRef]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.-C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical Validation of PSMA Expression Measured by 68Ga-PSMA PET/CT in Primary Prostate Cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef]
- Hofman, M.S.; Murphy, D.G.; Williams, S.G.; Nzenza, T.; Herschtal, A.; Lourenco, R.D.A.; Bailey, D.L.; Budd, R.; Hicks, R.J.; Francis, R.J.; et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): Clinical trial protocol. BJU Int. 2018, 122, 783–793. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, S.; Zhang, C.; Fu, Y.; Lv, X.; Zhang, Q.; Deng, Y.; Zhang, C.; Luo, R.; Zhao, X.; et al. Comparison of 68Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J. Transl. Med. 2017, 15, 230. [Google Scholar] [CrossRef]
- Kroenke, M.; Mirzoyan, L.; Horn, T.; Peeken, J.C.; Wurzer, A.; Wester, H.-J.; Makowski, M.; Weber, W.A.; Eiber, M.; Rauscher, I. Matched-Pair Comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in Patients with Primary and Biochemical Recurrence of Prostate Cancer: Frequency of Non–Tumor-Related Uptake and Tumor Positivity. J. Nucl. Med. 2021, 62, 1082–1088. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 686–693. [Google Scholar] [CrossRef]
- Stabile, A.; Pellegrino, A.; Mazzone, E.; Cannoletta, D.; de Angelis, M.; Barletta, F.; Scuderi, S.; Cucchiara, V.; Gandaglia, G.; Raggi, D.; et al. Can Negative Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-analysis with Backup Histology as Reference Standard. Eur. Urol. Oncol. 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Franklin, A.; Yaxley, W.J.; Raveenthiran, S.; Coughlin, G.; Gianduzzo, T.; Kua, B.; McEwan, L.; Wong, D.; Delahunt, B.; Egevad, L.; et al. Histological comparison between predictive value of preoperative 3-T multiparametric MRI and 68 Ga-PSMA PET/CT scan for pathological outcomes at radical prostatectomy and pelvic lymph node dissection for prostate cancer. BJU Int. 2020, 127, 71–79. [Google Scholar] [CrossRef]
- Aydin, A.M.; Haberal, B.; Artykov, M.; Bilen, C.Y.; Yazici, S. Clinicopathological predictors of positive 68Ga-PSMA-11 PET/CT in PSA-only recurrence of localized prostate cancer following definitive therapy. Ann. Nucl. Med. 2019, 33, 326–332. [Google Scholar] [CrossRef]
- Luiting, H.B.; van Leeuwen, P.J.; Busstra, M.B.; Brabander, T.; van der Poel, H.G.; Donswijk, M.L.; Vis, A.N.; Emmett, L.; Stricker, P.D.; Roobol, M.J. Use of gallium-68 prostate-specific membrane antigen positron-emission tomography for detecting lymph node metastases in primary and recurrent prostate cancer and location of recurrence after radical prostatectomy: An overview of the current literature. BJU Int. 2019, 125, 206–214. [Google Scholar] [CrossRef]
- Boreta, L.; Gadzinski, A.J.; Wu, S.Y.; Xu, M.; Greene, K.; Quanstrom, K.; Nguyen, H.G.; Carroll, P.R.; Hope, T.A.; Feng, F.Y. Location of Recurrence by Gallium-68 PSMA-11 PET Scan in Prostate Cancer Patients Eligible for Salvage Radiotherapy. Urology 2019, 129, 165–171. [Google Scholar] [CrossRef]
- Metz, R.; Rauscher, A.; Vaugier, L.; Supiot, S.; Drouet, F.; Campion, L.; Rousseau, C. Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy. Cancers 2023, 15, 1898. [Google Scholar] [CrossRef]
- Cytawa, W.; Kircher, S.; Kübler, H.; Werner, R.A.; Weber, S.; Hartrampf, P.; Bandurski, T.; Lass, P.; Połom, W.; Matuszewski, M.; et al. Diverse PSMA expression in primary prostate cancer: Reason for negative [68Ga]Ga-PSMA PET/CT scans? Immunohistochemical validation in 40 surgical specimens. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3938–3949. [Google Scholar] [CrossRef]
- Zamboglou, C.; Schiller, F.; Fechter, T.; Wieser, G.; Jilg, C.A.; Chirindel, A.; Salman, N.; Drendel, V.; Werner, M.; Mix, M.; et al. 68Ga-HBED-CC-PSMA PET/CT Versus Histopathology In Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics 2016, 6, 1619–1628. [Google Scholar] [CrossRef]
- Schollhammer, R.; Robert, G.; Asselineau, J.; Yacoub, M.; Vimont, D.; Balamoutoff, N.; Bladou, F.; Bénard, A.; Hindié, E.; de Clermont-Gallerande, H.H.; et al. Comparison of 68Ga-PSMA-617 PET/CT and68Ga-RM2 PET/CT in Patients with Localized Prostate Cancer Who Are Candidates for Radical Prostatectomy: A Prospective, Single-Arm, Single-Center, Phase II Study. J. Nucl. Med. 2022, 64, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.M.; Papa, N.; Buteau, J.; Ho, B.; Liu, V.; Roberts, M.; Thompson, J.; Moon, D.; Sheehan-Dare, G.; Alghazo, O.; et al. The PRIMARY Score: Using Intraprostatic 68Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J. Nucl. Med. 2022, 63, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Bernstine, H.; Groshar, D.; Ber, Y.; Nezrit, O.; Segal, N.; Yakimov, M.; Baniel, J.; Domachevsky, L. Diagnostic Performance of 68Ga Prostate-specific Membrane Antigen PET/MRI Compared with Multiparametric MRI for Detecting Clinically Significant Prostate Cancer. Radiology 2021, 301, 379–386. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Miederer, M.; Wieler, H.J.; Ruf, C.; Jakobs, F.M.; Schreckenberger, M. Diagnostic performance of 68Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with 18FEC PET/CT. Oncotarget 2017, 8, 111073–111083. [Google Scholar] [CrossRef]
- Chaloupka, M.; Apfelbeck, M.; Pyrgidis, N.; Marcon, J.; Weinhold, P.; Stief, C.G. Radical Prostatectomy without Prior Biopsy in Patients with High Suspicion of Prostate Cancer Based on Multiparametric Magnetic Resonance Imaging and Prostate-Specific Mem-brane Antigen Positron Emission Tomography: A Prospective Cohort Study. Cancers 2023, 15, 1266. [Google Scholar] [CrossRef]
- Zhang, H.; Rao, M.; Zhao, H.; Ren, J.; Hao, L.; Zhong, M.; Chen, Y.; Yang, X.; Feng, Y.; Yuan, G. Imageological/Structural Study regarding the Improved Pharmacokinetics by 68Ga-Labeled PEGylated PSMA Multimer in Prostate Cancer. Pharmaceuticals 2023, 16, 589. [Google Scholar] [CrossRef]
- Spohn, S.K.B.; Farolfi, A.; Schandeler, S.; Vogel, M.M.E.; Ruf, J.; Mix, M.; Kirste, S.; Ceci, F.; Fanti, S.; Lanzafame, H.; et al. The maximum standardized uptake value in patients with recurrent or persistent prostate cancer after radical prostatectomy and PSMA-PET-guided salvage radiotherapy—A multicenter retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 218–227. [Google Scholar] [CrossRef]
- Neels, O.C.; Kopka, K.; Liolios, C.; Afshar-Oromieh, A. Radiolabeled PSMA Inhibitors. Cancers 2021, 13, 6255. [Google Scholar] [CrossRef]
- Schollhammer, R.; Quintyn Ranty, M.-L.Q.; de Clermont Gallerande, H.d.C.; Cavelier, F.; Valverde, I.E.; Vimont, D.; Hindié, E.; Morgat, C. Theranostics of Primary Prostate Cancer: Beyond PSMA and GRP-R. Cancers 2023, 15, 2345. [Google Scholar] [CrossRef]
- Francolini, G.; Ganovelli, M.; Di Cataldo, V.; Detti, B.; Caini, S.; Loi, M.; Simontacchi, G.; Desideri, I.; Greto, D.; Valzano, M.; et al. Early biochemical outcomes following PSMA guided approach for bIoCHEmical relapse after prostatectomy-PSICHE trial (NCT05022914): Preliminary results. Clin. Exp. Metastasis 2023, 40, 197–201. [Google Scholar] [CrossRef]
- Solomonidou, N.; Germanou, D.; Strouthos, I.; Karagiannis, E.; Farolfi, A.; Koerber, S.A.; Debus, J.; Peeken, J.C.; Vogel, M.E.; Vrachimis, A.; et al. PSMA-PET/CT-guided salvage radiotherapy in recurrent or persistent prostate cancer and PSA < 0.2 ng/ml. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2529–2536. [Google Scholar] [CrossRef]
- Koerber, S.A.; Sprute, K.; Kratochwil, C.; Winter, E.; Haefner, M.F.; Katayama, S.; Schlampp, I.; Herfarth, K.; Kopka, K.; Afshar-Oromieh, A.; et al. Clinical outcome of PSMA-guided radiotherapy for patients with oligorecurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 143–151. [Google Scholar] [CrossRef]
- Harsini, S.; Wilson, D.; Saprunoff, H.; Allan, H.; Gleave, M.; Goldenberg, L.; Chi, K.N.; Kim-Sing, C.; Tyldesley, S.; Bénard, F. Outcome of patients with biochemical recurrence of prostate cancer after PSMA PET/CT-directed radiotherapy or surgery without systemic therapy. Cancer Imaging 2023, 23, 27. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, A.; Amiel, G.E. The Impact of PSMA PET/CT on Modern Prostate Cancer Management and Decision Making—The Urological Perspective. Cancers 2023, 15, 3402. https://doi.org/10.3390/cancers15133402
Hoffman A, Amiel GE. The Impact of PSMA PET/CT on Modern Prostate Cancer Management and Decision Making—The Urological Perspective. Cancers. 2023; 15(13):3402. https://doi.org/10.3390/cancers15133402
Chicago/Turabian StyleHoffman, Azik, and Gilad E. Amiel. 2023. "The Impact of PSMA PET/CT on Modern Prostate Cancer Management and Decision Making—The Urological Perspective" Cancers 15, no. 13: 3402. https://doi.org/10.3390/cancers15133402
APA StyleHoffman, A., & Amiel, G. E. (2023). The Impact of PSMA PET/CT on Modern Prostate Cancer Management and Decision Making—The Urological Perspective. Cancers, 15(13), 3402. https://doi.org/10.3390/cancers15133402




