Aggressive Angiomyxoma of the Lower Female Genital Tract in Pregnancy: A Review of the MITO Rare Tumors Group
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
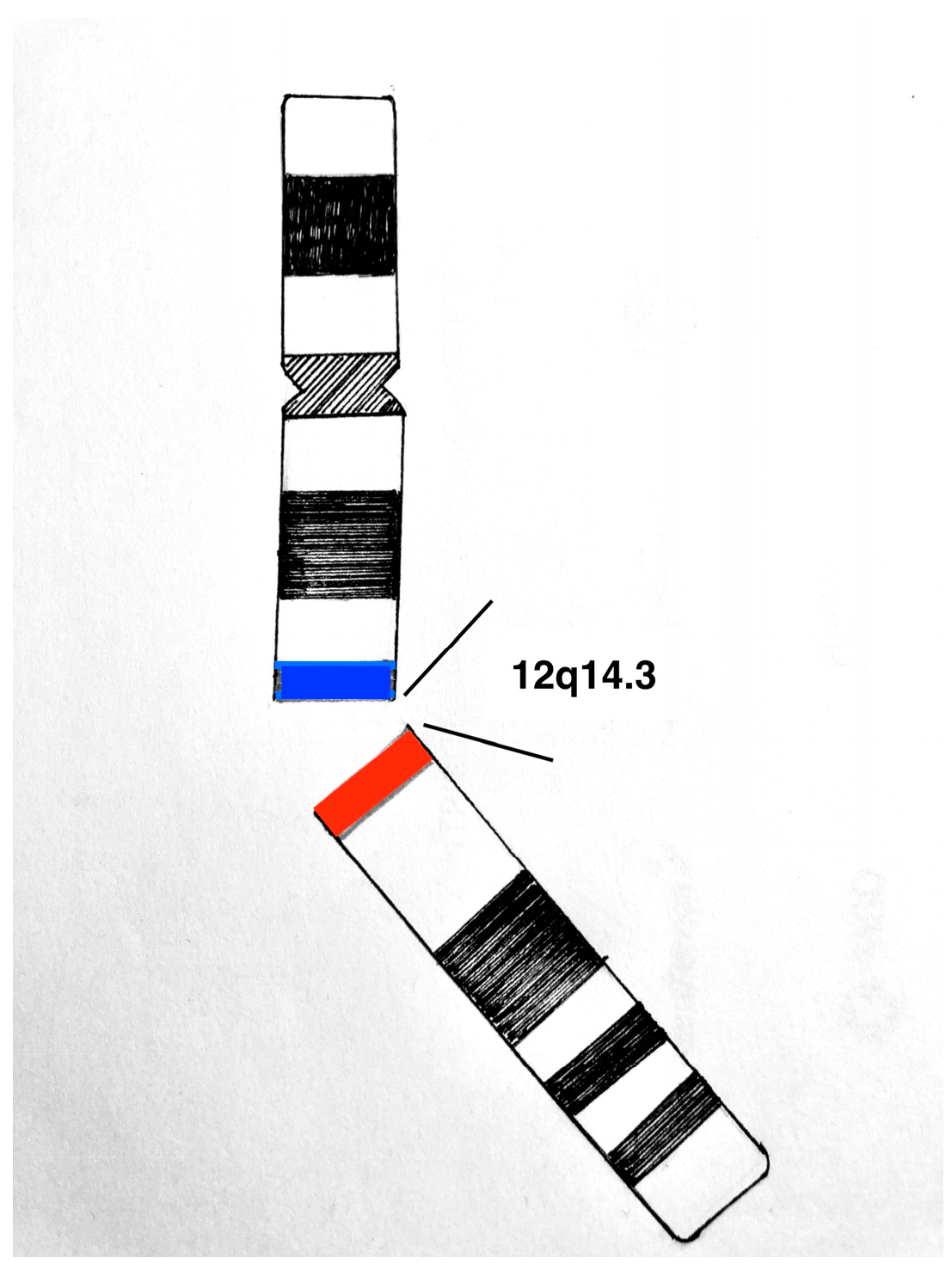
3.1. Etiopathogenesis
3.2. Clinical Features
3.3. Pathological Examination
3.4. Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ywasa, Y.; Fletcher, C.; Flucke, U. WHO Classification of Soft Tissue and Bone Tumours; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Magro, G.; Angelico, G.; Michal, M.; Broggi, G.; Zannoni, G.F.; Covello, R.; Marletta, S.; Salvatorelli, L.; Parenti, R. The Wide Morphological Spectrum of Deep (Aggressive) Angiomyxoma of the Vulvo-Vaginal Region: A Clinicopathologic Study of 36 Cases, including Recurrent Tumors. Diagnostics 2021, 11, 1360. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.J.; Laudadio, J. Aggressive angiomyxoma. Arch. Pathol. Lab. Med. 2012, 136, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Htwe, M.; Deppisch, L.M.; Saint-Julien, J.S. Hormone-dependent, aggressive angiomyxoma of the vulva. Obstet. Gynecol. 1995, 86, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Bagga, R.; Keepanasseril, A.; Suri, V.; Nijhawan, R. Aggressive angiomyxoma of the vulva in pregnancy: A case report and review of management options. Medscape Gen. Med. 2007, 9, 16. [Google Scholar]
- Aye, C.; Jefferis, H.; Chung, D.Y.; Manek, S.; Kehoe, S. A case of multi-modal managed vulval aggressive angiomyxoma diagnosed before conception and monitored during pregnancy. Gynecol. Oncol. 2009, 115, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Agrawal, D.; Sehgal, S.; Ghosh, S.; Kumar, A.; Singh, S. Aggressive angiomyxoma in pregnancy. Rare Tumors 2014, 6, 5362. [Google Scholar] [CrossRef]
- Sampaio, J.; Sarmento-Goncalves, I.; Ramada, D.; Amaro, T.; Tiago-Silva, P. Aggressive Angiomyxoma in Pregnancy: A Rare Condition, a Common Misdiagnosis. Case Rep. Obstet. Gynecol. 2016, 2016, 8539704. [Google Scholar] [CrossRef]
- Pisacane, A.; Cascardi, E.; Berrino, E.; Polidori, A.; Sarotto, I.; Casorzo, L.; Panero, M.; Boccaccio, C.; Verginelli, F.; Benvenuti, S.; et al. Real-world histopathological approach to malignancy of undefined primary origin (MUO) to diagnose cancers of unknown primary (CUPs). Virchows Arch. 2022, 482, 463–475. [Google Scholar] [CrossRef]
- Dellino, M.; Carriero, C.; Silvestris, E.; Capursi, T.; Paradiso, A.; Cormio, G. Primary Vaginal Carcinoma Arising on Cystocele Mimicking Vulvar Cancer. J. Obstet. Gynaecol. Can. 2020, 42, 1543–1545. [Google Scholar] [CrossRef]
- Dellino, M.; Gargano, G.; Tinelli, R.; Carriero, C.; Minoia, C.; Tetania, S.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Casamassima, P.; et al. A strengthening the reporting of observational studies in epidemiology (STROBE): Are HE4 and CA 125 suitable to detect a Paget disease of the vulva? Medicine 2021, 100, e24485. [Google Scholar] [CrossRef]
- Dellino, M.; Cicogna, S.; Falcone, F.; Mitidieri, M.; Mazzeo, R.; Pignata, S.; Mangili, G.; Cormio, G. “Intestinal-Type” Vulvar Adenocarcinoma: A Review of the MITO Rare Tumors Group. Cancers 2022, 14, 5171. [Google Scholar] [CrossRef]
- Steeper, T.A.; Rosai, J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am. J. Surg. Pathol. 1983, 7, 463–475. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Fishman, A.; Otey, L.; Poindexter, A., 3rd; Shannon, R.; Girtanner, R.; Kaplan, A.L. Aggressive angiomyxoma of the pelvis and perineum. A case report. J. Reprod. Med. 1995, 40, 665–669. [Google Scholar]
- Wolf, C.A.; Kurzeja, R.; Fietze, E.; Buscher, U. Aggressive angiomyxoma of the female perineum in pregnancy. Acta Obstet. Gynecol. Scand. 2003, 82, 484–485. [Google Scholar] [CrossRef]
- Ribaldone, R.; Piantanida, P.; Surico, D.; Boldorini, R.; Colombo, N.; Surico, N. Aggressive angiomyxoma of the vulva. Gynecol. Oncol. 2004, 95, 724–728. [Google Scholar] [CrossRef]
- Han-Geurts, I.J.; van Geel, A.N.; van Doorn, L.; Bakker, M.d.; Eggermont, A.M.; Verhoef, C. Aggressive angiomyxoma: Multimodality treatments can avoid mutilating surgery. Eur. J. Surg. Oncol. 2006, 32, 1217–1221. [Google Scholar] [CrossRef]
- Haldar, K.; Martinek, I.E.; Kehoe, S. Aggressive angiomyxoma: A case series and literature review. Eur. J. Surg. Oncol. 2010, 36, 335–339. [Google Scholar] [CrossRef]
- Sinha, V.; Dave, K.S.; Bhansali, R.P.; Arora, R.S. Aggressive angiomyxoma of vulva which grew with pregnancy and attained a huge size rarely seen in literature. J. Obstet. Gynaecol. India 2014, 64, 90–91. [Google Scholar] [CrossRef]
- Ashraf, T.; Haroon, S. Aggressive angiomyxoma in pregnancy. J. Coll. Physicians Surg. Pak. 2014, 24, 24–26. [Google Scholar]
- Zangmo, R.; Kumar, S.; Singh, N.; Meena, J. Aggressive Angiomyxoma of Vulva in Pregnancy: A Case Report. J. Obstet. Gynaecol. India 2016, 66, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Orfanelli, T.; Kim, C.S.; Vitez, S.F.; Van Gurp, J.; Misra, N. A Case Report of Aggressive Angiomyxoma in Pregnancy: Do Hormones Play a Role? Case Rep. Obstet. Gynecol. 2016, 2016, 6810368. [Google Scholar] [CrossRef] [PubMed]
- Malukani, K.; Varma, A.V.; Choudhary, D.; Dosi, S. Aggressive angiomyxoma in pregnancy: A rare and commonly misdiagnosed entity. J. Lab. Physicians 2018, 10, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, P.; Xu, R.; Wang, L.; Shi, Y. Aggressive angiomyxoma in pregnancy: A case report and literature review. J. Int. Med. Res. 2020, 48, 300060520936414. [Google Scholar] [CrossRef]
- Espejo-Reina, M.P.; Prieto-Moreno, M.; De-Miguel-Blanc, M.; Perez-Martinez, D.M.; Jimenez-Lopez, J.S.; Monis-Rodriguez, S. Genital Prolapse in Pregnant Woman as a Presentation of Aggressive Angiomyxoma: Case Report and Literature Review. Medicina 2022, 58, 107. [Google Scholar] [CrossRef]
- Medeiros, F.; Erickson-Johnson, M.R.; Keeney, G.L.; Clayton, A.C.; Nascimento, A.G.; Wang, X.; Oliveira, A.M. Frequency and characterization of HMGA2 and HMGA1 rearrangements in mesenchymal tumors of the lower genital tract. Genes Chromosom. Cancer 2007, 46, 981–990. [Google Scholar] [CrossRef]
- Lee, M.Y.; da Silva, B.; Ramirez, D.C.; Maki, R.G. Novel HMGA2-YAP1 fusion gene in aggressive angiomyxoma. BMJ Case Rep. 2019, 12, e227475. [Google Scholar] [CrossRef]
- Bartuma, H.; Hallor, K.H.; Panagopoulos, I.; Collin, A.; Rydholm, A.; Gustafson, P.; Bauer, H.C.; Brosjo, O.; Domanski, H.A.; Mandahl, N.; et al. Assessment of the clinical and molecular impact of different cytogenetic subgroups in a series of 272 lipomas with abnormal karyotype. Genes Chromosom. Cancer 2007, 46, 594–606. [Google Scholar] [CrossRef]
- Mine, N.; Kurose, K.; Nagai, H.; Doi, D.; Ota, Y.; Yoneyama, K.; Konishi, H.; Araki, T.; Emi, M. Gene fusion involving HMGIC is a frequent aberration in uterine leiomyomas. J. Hum. Genet. 2001, 46, 408–412. [Google Scholar] [CrossRef]
- Micci, F.; Panagopoulos, I.; Bjerkehagen, B.; Heim, S. Deregulation of HMGA2 in an aggressive angiomyxoma with t(11;12)(q23;q15). Virchows Arch. 2006, 448, 838–842. [Google Scholar] [CrossRef]
- Rabban, J.T.; Dal Cin, P.; Oliva, E. HMGA2 rearrangement in a case of vulvar aggressive angiomyxoma. Int. J. Gynecol. Pathol. 2006, 25, 403–407. [Google Scholar] [CrossRef]
- McCluggage, W.G. Recent developments in vulvovaginal pathology. Histopathology 2009, 54, 156–173. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Mentzel, T.; Dei Tos, A.P. The contribution of Juan Rosai to the pathology of soft tissue tumors. Pathologica 2021, 113, 396–409. [Google Scholar] [CrossRef]
- Angelico, G.; Marletta, S.; Broggi, G.; Vigneri, P.; Vecchio, G.M.; Salvatorelli, L.; Magro, G. Practical Approach to the Diagnosis of the Vulvo-Vaginal Stromal Tumors: An Overview. Diagnostics 2022, 12, 357. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Patterson, A.; Maxwell, P. Aggressive angiomyxoma of pelvic parts exhibits oestrogen and progesterone receptor positivity. J. Clin. Pathol. 2000, 53, 603–605. [Google Scholar] [CrossRef]
- Harkness, R.; McCluggage, W.G. HMGA2 Is a Useful Marker of Vulvovaginal Aggressive Angiomyxoma But May Be Positive in Other Mesenchymal Lesions at This Site. Int. J. Gynecol. Pathol. 2021, 40, 185–189. [Google Scholar] [CrossRef]
- Fuca, G.; Hindi, N.; Ray-Coquard, I.; Colia, V.; Dei Tos, A.P.; Martin-Broto, J.; Brahmi, M.; Collini, P.; Lorusso, D.; Raspagliesi, F.; et al. Treatment Outcomes and Sensitivity to Hormone Therapy of Aggressive Angiomyxoma: A Multicenter, International, Retrospective Study. Oncologist 2019, 24, e536–e541. [Google Scholar] [CrossRef]
- Dellino, M.; Cascardi, E.; Lagana, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect. Agent Cancer 2022, 17, 53. [Google Scholar] [CrossRef]
- Dellino, M.; Cascardi, E.; Vinciguerra, M.; Lamanna, B.; Malvasi, A.; Scacco, S.; Acquaviva, S.; Pinto, V.; Di Vagno, G.; Cormio, G.; et al. Nutrition as Personalized Medicine against SARS-CoV-2 Infections: Clinical and Oncological Options with a Specific Female Groups Overview. Int. J. Mol. Sci. 2022, 23, 9136. [Google Scholar] [CrossRef]
- Cascardi, E.; Cazzato, G.; Daniele, A.; Silvestris, E.; Cormio, G.; Di Vagno, G.; Malvasi, A.; Loizzi, V.; Scacco, S.; Pinto, V. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology 2022, 11, 1114. [Google Scholar] [CrossRef]
- Chen, L.; Schink, J.C.; Panares, B.N.; Barbuto, D.; Lagasse, L.D. Resection of a giant aggressive angiomyxoma in the Philippines. Gynecol. Oncol. 1998, 70, 435–439. [Google Scholar] [CrossRef] [PubMed]

| Reference | Age (Years) | GA at Diagnosis (Weeks) | Location | Tumor Size (cm) | IHC | Treatment | Delivery (GA Weeks) | Recurrence | PFS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| [15] | 37 | NA | Right vulva | 3 → 40 | NA | Pre-pregnancy LE | NA | Yes | NA |
| [4] | 41 | 18 | Left labium minus | 6 × 6 × 4 | PR+++, ER+, S100− | LE at 18 weeks | VB (40) | NA | NA |
| [16] | 32 | 32 | Vulva posterior commissura | 3 × 4 | ER+, PR− | LE 36 weeks | VB | No (9 months follow-up) | NA |
| [17] | 36 | Present before and during the pregnancy, grew very rapidly after the birth. | Right major labium, pelvic-perineal region (between the obturator and levator muscles of the anus) | 6.5 ≥ 15 | Vimentin++ SM actin++ desmin++ ER++ PR++ S100− CD34− | LE postpartum + transperineal surgery (ischio-rectal fossa toilette + external sphincterotomy and reconstruction) (R0) | CS for breech presentation (40) | NA | NA |
| [18] | 31 | NA (already present) | paravaginal/pararectal mass | NA | NA | Posterior exenteration (R1) and RT 60 Gy | NA | No recurrence after surgery (first suspected during 1st pregnancy) during second pregnancy 4 years later | 96 |
| [18] | 34 | 30 | Left and right labia majora | NA | NA | left labial surgery (30 weeks GA; several weeks later, right labial mass surgery was performed); vulvectomy (R1) | NA | No | 24 |
| [18] | 27 | Early pregnancy | Pelvis-perineum, in front of the bladder | NA | NA | Total exenteration (R1) | NA | No | 48 |
| [5] | 25 | 12 | Right labia majus | 2 → 4 | Desmin+ ER+ PR− | LE 16 weeks | VB (40) | No | 9 |
| [6] | 22 | Before pregnancy, recurrence at I trimester | Right vulva and pelvis | 8 (first diagnosis, before pregnancy) 3.1 × 1.9 × 2.2 (I trimester) → 5.1 × 4.6 × 3.5 (32 weeks), halved in size postpartum | NA | prenatal surgery (R1) + GnRH analogous + surgery + GnRH monitored in pregnancy | CS (38) (for mass) | Yes (in pregnancy) | NA |
| [19] | 22 | Before pregnancy, recurred three times (last one during pregnancy) | Right vulva and vagina extending into right ichiorectal-fossa and levator ani | NA | NA | 3 LWE → successful pregnancy after third surgery (R1) + GnRH analogue | NA | Yes (before and during pregnancy) | 5 |
| [20] | 43 | tumour gradually increased for 9 years, suddenly grew during and after pregnancy | Left labium majus | Up to 55 (postpartum) | ER+ | LE 9 months postpartum | CS (NA) | No | 8 |
| [7] | 25 | 18 | Left labium majus | Up to 8 | NA | LE 18 weeks | VB (40) | No | 9 |
| [21] | 24 | 16 | Right labium majus | Up to 30 | ER+ PR+ desmin+ | LE 20 weeks (R0) | CS for failed labor induction (40) | No | 60 |
| [22] | 21 | 20 | Right labium majus | Up to 15 in pregnancy, up to 18 postpartum | NA | LE postpartum (6 weeks) (R0) | CS for vaginal mass (38) | No | NA |
| [8] | 25 | 9 | Vaginal fornix | 12 | Vimentin+ SMA+ ER+ PR+ s100−, EMA−, CD34− | LE 13 weeks | VB (40) | No, after surgery (suspected REL in pregnancy) | |
| [23] | 29 | 20 | Right labium majus | 2 → 7 | ER+ PR+ CD34+ | LE during CS (R1) | CS for vaginal mass (39) | No | 20 |
| [24] | 24 | 17 | Vaginal fornix (bleeding, pain, vaginal mass) | 11.4 × 11.3 × 9.95 | ER+ vimentin+ PR− S100− | LE during abortion | Induced abortion | NA | NA |
| [25] | 32 | 8 (2 months) | Right vulva | 3 → 5 (first pregnancy), up to 7 cm postpartum | Vimentin+++ ER++ PR++ desmin+ Ki-67 + (<1%) S100− | No treatment | VB (37) | Yes 40 months (during second pregnancy) | 8 |
| LE 22 postpartum | |||||||||
| 6 × 4 × 3 (second pregnancy) | spontaneuos regression postpartum | CS for fetal distress (39) | 8 months after the second pregnancy, postpartum | ||||||
| 5.3 × 5.1 × 4.2 | LE 11 months postpartum +GnRH analogous | ||||||||
| [26] | 36 | 20 | Prolapsed vaginal mass, vagina, vesico-vaginal space | 7 × 3 × 3.5 (enlarged in puerperium 7 × 7 × 7.5) | Vimentin+, Actin+/− CD34+/− S-100−, Desmin− ER+ PR+ EMA− Ki 67 < 1% | LE postpartum (R1) + GnRh analogues | CS for prolapsed mass (37) | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicogna, S.; Dellino, M.; Miano, S.T.; Magazzino, F.; Domenici, L.; Pignata, S.; Mangili, G.; Cormio, G. Aggressive Angiomyxoma of the Lower Female Genital Tract in Pregnancy: A Review of the MITO Rare Tumors Group. Cancers 2023, 15, 3403. https://doi.org/10.3390/cancers15133403
Cicogna S, Dellino M, Miano ST, Magazzino F, Domenici L, Pignata S, Mangili G, Cormio G. Aggressive Angiomyxoma of the Lower Female Genital Tract in Pregnancy: A Review of the MITO Rare Tumors Group. Cancers. 2023; 15(13):3403. https://doi.org/10.3390/cancers15133403
Chicago/Turabian StyleCicogna, Stefania, Miriam Dellino, Salvatora Tindara Miano, Francescapaola Magazzino, Lavinia Domenici, Sandro Pignata, Giorgia Mangili, and Gennaro Cormio. 2023. "Aggressive Angiomyxoma of the Lower Female Genital Tract in Pregnancy: A Review of the MITO Rare Tumors Group" Cancers 15, no. 13: 3403. https://doi.org/10.3390/cancers15133403
APA StyleCicogna, S., Dellino, M., Miano, S. T., Magazzino, F., Domenici, L., Pignata, S., Mangili, G., & Cormio, G. (2023). Aggressive Angiomyxoma of the Lower Female Genital Tract in Pregnancy: A Review of the MITO Rare Tumors Group. Cancers, 15(13), 3403. https://doi.org/10.3390/cancers15133403







