Simple Summary
Myxofibrosarcomas (MFS) are malignant soft tissue tumors, frequently located in the extremities. Owing to the infiltrative growth pattern of MFS, neoadjuvant radiotherapy (nRT) is commonly used before surgery to improve local control. Nevertheless, high local recurrence rates are typical in MFS. Data on prognostic factors for poor clinical outcomes are lacking. This retrospective study investigates the prognostic relevance of magnetic resonance imaging (MRI) characteristics before and after nRT in 40 MFS patients. The presence of a vascular pedicle, defined as extra-tumoral vessels at the tumor periphery, was prognostic for both worse disease-free survival (DFS) and overall survival. Additionally, the presence of an infiltrative pattern, referred to as a tail sign, was prognostic for worse DFS. These MRI characteristics could support the identification of patients at risk for poor clinical outcomes after nRT.
Abstract
To improve local control, neoadjuvant radiotherapy (nRT) followed by surgery is the standard of care in myxofibrosarcoma (MFS) because of its infiltrative growth pattern. Nevertheless, local recurrence rates are high. Data on prognostic factors for poor clinical outcomes are lacking. This retrospective study thus investigates the prognostic relevance of magnetic resonance imaging (MRI) characteristics before and after nRT in 40 MFS patients, as well as their association with disease-free survival (DFS) and overall survival (OS). A vascular pedicle, defined as extra-tumoral vessels at the tumor periphery, was observed in 12 patients (30.0%) pre-nRT and remained present post-nRT in all cases. Patients with a vascular pedicle had worse DFS (HR 5.85; 95% CI 1.56–21.90; p = 0.009) and OS (HR 9.58; 95% CI 1.91–48.00; p = 0.006). An infiltrative growth pattern, referred to as a tail sign, was observed in 22 patients (55.0%) pre-nRT and in 19 patients (47.5%) post-nRT, and was associated with worse DFS post-nRT (HR 6.99; 95% CI 1.39–35.35; p = 0.019). The percentage of tumor necrosis estimated by MRI was increased post-nRT, but was not associated with survival outcomes. The presence of a tail sign or vascular pedicle on MRI could support the identification of patients at risk for poor clinical outcomes after nRT.
1. Introduction
Myxofibrosarcoma (MFS) is a histological subtype of soft tissue sarcoma (STS), histologically characterized by pleomorphism, myxoid stroma, and curvilinear vasculature [1]. This malignant lesion of mesenchymal origin represents approximately 5% of all sarcoma entities, with an age-standardized incidence rate of 0.19 per 100,000 persons-year in Europe [2]. MFS most commonly presents in the extremities or trunk of patients in the sixth to eighth decades of life, with a particular predilection for the lower limbs [1,3]. The mainstay of treatment for localized primary disease involves surgical resection, commonly applied in conjunction with neoadjuvant radiotherapy (nRT) to optimize local control. MFS is described as a locally aggressive tumor with a distinctive infiltrative pattern of growth, resulting in particularly high rates of local recurrence (LR), ranging from 20 to 60% [3,4,5,6,7,8,9,10,11,12]. Distant metastasis will eventually develop in 20–40% of patients, despite adequate treatment of the primary tumor [3,4,5,6,7,8,9,10,11,12]. Several prognostic factors for LR and overall survival (OS) have been described in MFS, including tumor size and patient age and sex [3,4,5,6,8,11,13]. A recently published large series from the Netherlands comprising 908 MFS patients reported a median OS of 155 months, which was significantly lower in patients who experienced LR (64.0 months) or metastatic disease (34.3 months) [3]. A comprehensive understanding of prognostic factors for patients at risk of poor clinical outcomes despite adequate initial treatment might support the optimization of primary treatment and follow-up schedules.
Recently, neoadjuvant radiotherapy has been replacing adjuvant radiotherapy as the preferred treatment modality, as it enables more accurate definition of the treatment field and thus limits damage to adjacent structures [14,15]. The role of nRT has been investigated in different histological subtypes of STS, but not in MFS, where positive surgical margins and high tumor necrosis have been identified as predictors for worse clinical outcome [16,17,18,19,20,21]. A high percentage of tumor necrosis, histologically assessed, after nRT is the most commonly used surrogate marker for treatment response; however, this has not been validated in STS and small studies present conflicting data [18,19,20,22].
Magnetic resonance imaging (MRI) is the standard of care in MFS diagnosis and restaging after nRT. Specific MRI features at diagnosis, in particular the presence of longitudinal spreading, also referred to as a tail sign, has been recognized as an MRI predictor prognostic for LR and worse OS [5,6,23,24]. The value of post-nRT MRI characteristics as prognostic factors for disease recurrence or OS remains ambiguous and should be explored in further detail. Being able to identify high-risk patients in an early stage might support the development of intensified primary treatment and follow-up strategies for these patients. We thus aim to evaluate the prognostic relevance of MRI characteristics in MFS patients who received nRT.
2. Materials and Methods
2.1. Study Objectives
The primary objective of this retrospective study was to evaluate the prognostic relevance of pre- and post-nRT MRI characteristics in MFS patients. The secondary objective of this study was to identify factors prognostic for DFS and OS. In addition, the effects of the WHO performance status, surgical margin status, the time interval between nRT and restaging MRI, and the time interval between nRT and surgery on DFS and OS were investigated.
2.2. Study Population
Data from patients diagnosed with MFS and treated with nRT in a tertiary sarcoma expertise center in the Netherlands between 2014 and 2022 were retrospectively collected. Histological diagnosis of MFS was confirmed by an experienced sarcoma pathologist (U.E.F.). Patients were included in this study if both pre- and post-nRT MRI data were available. This study was conducted according to the principles of the declaration of Helsinki. Written informed consent was provided by all participating subjects (NCT05373810).
2.3. Magnetic Resonance Imaging
Only patients with MRI examinations with the following minimum requirements were included in this study: all MRI studies were performed on a 1.5 Tesla scanner using a protocol including T1-weighted (T1w) and T2-weighted (T2w) sequences with fat saturation, and T1w images with fat saturation after intravenous administration of Gadolinium (Gd). All MRI studies, both pre- and post-nRT, were revised by an experienced musculoskeletal radiologist (J.W.J.d.R.). The musculoskeletal radiologist was blinded to the clinical and histopathological data.
Data on pre- and post-nRT MRI characteristics were systematically collected. Gd enhancement was evaluated on T1w sequences with fat saturation using the grading system proposed by Sambri et al. [5,6]. T1w, T2w, and Gd-enhanced studies were used to evaluate the parameters described below, including the presence of peritumoral edema or intratumoral signs of bleeding, vascular pedicle, necrosis percentage, tumor volume, and tumor size. Myxoid matrix content was recognized as the presence of high signal on fluid-sensitive sequences (T2w), slightly less than the signal of water [5,6]. A vascular pedicle was defined as abnormal tortuous feeding extra-tumoral vessels, whether or not coalescing as a clump, at one site along the lesion periphery. This peri-tumoral neo-angiogenesis can be identified as prominent flow voids (i.e., signal loss) [25,26]. The percentage of tumor necrosis was evaluated using T1w sequences with fat saturation after Gd contrast enhancement on a semi-quantitative base (<50.0% or ≥50.0%). Tumor size was measured as the largest diameter of the mass in any direction of the imaging plane. Tumor shape was classified as monolobular, lobular, or polylobulated. Tumor location was recorded as superficial (above the fascia) or deep (below the fascia). The number of anatomical compartments involved was registered, as was the tumor demarcation (unsharp, moderately sharp, sharp). The presence of an infiltrative growth pattern, referred to as a tail sign, was determined and differentiated from peritumoral edema by its enhancement on post-contrast images. A tail sign was deemed present if the tail was at least 10 mm in length and 2 mm in width [23,27].
2.4. Clinical and Histopathological Data
Clinical data were retrieved from a prospective clinical registry database and included patient age and BMI at diagnosis, sex, tumor site, WHO performance status, date and dose schedule of nRT, timing of MRI studies, the occurrence of LR, time interval between surgery and LR, treatment for LR, the occurrence of metastasis, time interval between surgery and metastasis, treatment for metastasis, and date of death or last follow-up. Histopathological data included tumor grading and classification of surgical margins according to the guidelines of the American Joint Committee on Cancer (R0: tumor-free margin, R1: microscopic positive margin, and R2: macroscopic positive margin) [28].
2.5. Statistical Analysis
Descriptive statistics were used to describe baseline patient characteristics. Mean or median values were described as applicable. Continuous variables were compared through t-test or Mann–Whitney U test. To compare categorical variables, McNemar-(Bowker) tests were used. Values were considered significant with a p-value ≤ 0.05. Univariate survival analyses were performed according to the Kaplan–Meier method. Multivariable Cox regression was performed using forward Wald selection. Variables that revealed a p-value ≤ 0.10 were included in the multivariable model. Statistical analyses were performed using IBM SPSS statistics 25 (version 3.6.2) and RStudio (version 1.1.463).
3. Results
3.1. Patient Characteristics
The total cohort comprised 40 MFS patients. Patient characteristics are depicted in Table 1. The majority of primary MFS tumors were located in the lower extremities (77.5%). Most lesions were located below the fascia (70.0%) and were of high histologic grade (92.5%). The most frequently applied nRT dose was 50 Gray (Gy) in 25 fractions (25 × 2 Gy) (87.5%). Four patients (10.0%) received 45 Gy in 15 fractions (15 × 3 Gy) and one patient (2.5%) received 25 Gy in 5 fractions (5 × 5 Gy) based on the physicians’ choice. All patients with MFS involving the extremities who received nRT underwent limb-sparing resection. The median time intervals between nRT and restaging MRI or surgery were 32 (range 12–61) days and 51 (range 26–117) days, respectively. Negative surgical margins (R0) were reported in 90.0% of cases. Microscopic-positive surgical margins (R1) were described in 5.0% of patients and 5.0% of cases had macroscopic-positive surgical margins (R2).

Table 1.
Baseline patient and MRI characteristics. All MRI characteristics reflected in this table were described based on pre-nRT imaging.
3.2. Neoadjuvant Radiotherapy Induced Changes in MRI Characteristics
Pre-nRT MRI studies were predominantly performed at the primary referral center and, therefore, there was a large variance in MRI devices and pulse sequences. An overview of the observed pre- and post-nRT MRI characteristics is provided in Table 2. The median tumor diameter (81 mm pre-nRT versus (vs.) 92 mm post-nRT, p = 0.002) and median tumor volume (224 cm3 pre-nRT vs. 298 cm3 post-nRT, p = 0.008) were significantly increased post-nRT. The number of patients with a tumor necrosis percentage ≥50.0% significantly increased post-nRT (10.0% pre-nRT vs. 45.0% post-nRT, p = 0.046). Three patients showed no signs of tumor necrosis post-nRT. Furthermore, there was a significant increase in the number of cases that showed tumor bleeding (47.5% pre-nRT vs. 67.5% post-nRT, p = 0.021). Peritumoral edema was present in 97.5% of cases pre-nRT and in 100.0% of cases post-nRT (p = 1.000).

Table 2.
Pre- and post-neoadjuvant radiotherapy MRI characteristics.
A vascular pedicle, defined as abnormal tortuous feeding extra-tumoral vessels, whether or not coalescing as a clump, at one site along the lesion periphery (Figure 1), was observed in 12 patients pre-nRT (30.0%) and remained present post-nRT in all cases. No new cases with a vascular pedicle were identified on post-nRT MRI. When a vascular pedicle was present, 1 out of 12 patients had positive surgical margins, categorized as R1 or R2. In the absence of a vascular pedicle, 3 out of 28 patients had positive surgical margins (p = 0.824). An infiltrative pattern with extensions of ≥10 mm in length and ≥2 mm in width (Figure 1), also referred to as a tail sign, was present in 22 cases pre-nRT (55.0%) and in 19 cases (47.5%) post-nRT (p = 0.453). All lesions with a tail sign observed post-nRT were already observed pre-nRT. No new cases with a tail-sign-containing lesion were identified post-nRT. Furthermore, no significant changes were observed in tail sign length (p = 0.134) or tail sign width (p = 0.201) post-nRT. In the presence of a tail sign post-nRT, 4 out of 19 patients had positive surgical margins. In its absence, 0 out of 21 patients had positive surgical margins (p = 0.027).
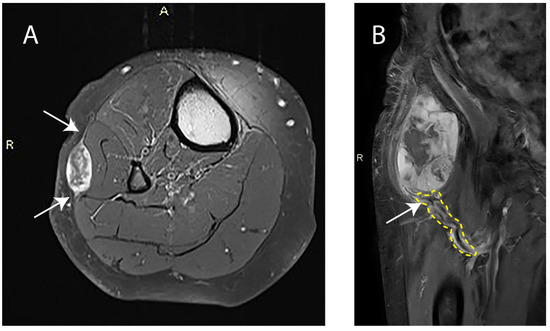
Figure 1.
(A) Axial-contrast-enhanced T1-weighted image with fat saturation of a right lower leg with a superficial myxofibrosarcoma. Arrows indicate the tail sign. A: Anterior orientation; R: Right side orientation. (B) Coronal-contrast-enhanced T1-weighted image of a right upper leg with a deep myxofibrosarcoma. The area outlined in yellow highlights the pathologic tumor-feeding vasculature and the arrow indicates the vascular pedicle. R: Right side orientation.
3.3. Prognostic Relevance of Post-nRT MRI Characteristics
The median follow-up after surgery was 44 (range 2–103) months. Throughout the follow-up period, LR occurred in four patients (10.0%) and nine patients (22.5%) developed distant metastases. Nine patients (22.5%) deceased, of whom seven passed away from disease-related causes. Figure 2 depicts the Kaplan–Meier estimates for DFS and OS for the entire patient cohort over time. Median DFS and OS were not reached during the follow-up period.
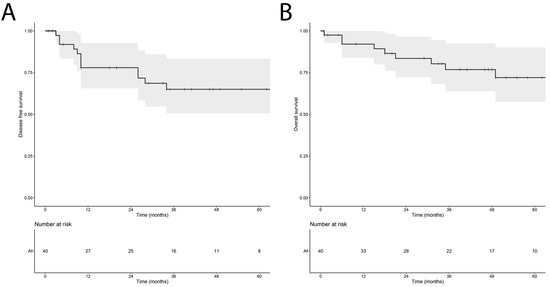
Figure 2.
Kaplan–Meier estimate reflecting the disease-free survival (A) and overall survival (B) of the cohort of 40 myxofibrosarcoma patients, including the 95% confidence interval.
An exploratory multivariable Cox regression analysis was performed using forward Wald selection on the MRI characteristics depicted in Table 2, with the inclusion of WHO performance status, surgical margin, tumor depth, myxoid type on MRI, and Gd enhancement. The presence of a vascular pedicle (hazard ratio (HR) 5.85; 95% confidence interval (CI) 1.56–21.90; p = 0.009) and a tail sign (HR 6.99; 95% CI 1.39–35.35; p = 0.019) post-nRT were associated with worse DFS. The presence of a vascular pedicle post-nRT was the sole factor associated with worse OS (HR 9.58; 95% CI 1.91–48.00; p = 0.006). Figure 3 depicts the Kaplan–Meier estimates for DFS and OS in relation to the presence of a vascular pedicle or tail sign post-nRT. None of the other remaining patient or MRI characteristics post-nRT demonstrated a significant association with DFS or OS.
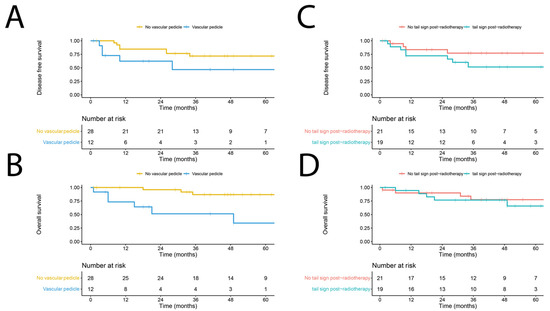
Figure 3.
Kaplan–Meier estimate reflecting the disease-free survival (DFS) (A) and overall survival (OS) (B) of myxofibrosarcoma in the presence or absence of a vascular pedicle on MRI, and DFS (C) and OS (D) of patients in the presence or absence of a tail sign on post-neoadjuvant radiotherapy MRI.
4. Discussion
In this large retrospective MFS series investigating the prognostic relevance of MRI characteristics in patients who received nRT, the presence of a vascular pedicle or tail sign on post-nRT MRI was prognostic for worse survival outcomes. The presence of a vascular pedicle on post-nRT MRI was prognostic for both worse DFS and OS, whereas the post-nRT presence of a tail-sign-containing lesion was exclusively prognostic for worse DFS. These MRI characteristics could serve as prognostic biomarkers to support the non-invasive identification of patients at risk of worse clinical outcomes in an early stage.
We identified a vascular pedicle in 30.0% of cases post-nRT, of whom 41.7% eventually developed LR (n = 1, 8.3%) or distant metastasis (n = 4, 33.3%). In the absence of a vascular pedicle, only 25.0% of cases developed LR (n = 2, 7.1%), distant metastasis (n = 4, 14.3%), or both (n = 1, 3.6%). We found no relationship between the presence of a vascular pedicle and surgical margin status. Furthermore, we observed no changes in the presence of a vascular pedicle between pre- and post-nRT MRI. This suggests that the presence of a vascular pedicle on pre-nRT MRI has similar prognostic value to its presence on post-nRT MRI. While the presence of a vascular pedicle has been reported in different histological subtypes of STS, this feature has not yet been described in MFS. Ledoux et al. identified abnormal peritumor vascularization in 17% of STS patients using MRI in a cohort of 157 STS cases, including 24 MFS patients. The authors found that peritumoral flow voids might be associated with a higher risk of metastatic relapse and poorer OS. They hypothesize that abnormal vascularization might favor the occurrence of hematogenous metastasis through the formation of endovascular thrombi of tumor cells [26,29]. In the current study, we report that patients with a vascular pedicle, defined as either a real clump of peri-tumoral vessels or as abnormal feeding peri-tumoral vascularization, are at increased risk of developing recurrent disease after nRT. As most patients with disease recurrence had metastatic disease, we suggest that the vascular pedicle could be involved in the hematogenic spread of cancer cells. Future studies using dynamic-contrast-enhanced (DCE) MRI may quantify tumor-related perfusion and further characterize peritumoral microstructures. Further research is needed to investigate the optimal surgical strategy regarding a vascular pedicle. One might hypothesize that, when a surgeon is informed about the presence of a vascular pedicle, this should also be resected to further prevent hematogenic spread of cancer cells. The same holds for considerations regarding adjuvant systemic therapy.
The presence of a tail sign on MRI is the most recognized feature prognostic for worse DFS in MFS. In this study, we report the presence of a tail sign in 55.0% of cases pre-nRT and in 47.5% of cases post-nRT. This finding is in line with previous studies describing the presence of tail-like lesions in MFS. Lefkowitz et al. reported the presence of a tail sign in 64.0–77.0% of cases on MRI in a cohort of 44 MFS patients, which was associated with worse DFS, but not OS. We found a relationship between the presence of a tail sign and positive surgical margins. This finding could be consistent with the worse DFS observed in the presence of a tail sign. The presence of a tail sign has also been reported as an independent adverse prognostic factor for local control and metastasis-free survival after surgery in a cohort of 89 STS [30]. The histological effect of nRT on tail-like lesions was investigated in a cohort of 18 STS cases, comprising 8 MFS patients and 10 cases of undifferentiated pleomorphic sarcoma. Viable tumor cells remained present after treatment with nRT in 8/18 cases, of whom three patients developed locally recurrent disease [31]. We found no significant changes after nRT in the presence, length, or width of the tail sign. The complete disappearance or shrinkage of tail-like lesions after neoadjuvant treatment has been reported in 33.3% of cases in a cohort of 36 STS patients, including 13 MFS cases, but did not impact the oncological outcomes [32].
Tumor necrosis after nRT is the most studied prognostic factor in sarcoma. We found no correlation between the tumor necrosis percentage estimated by MRI and survival outcomes. Quantification of the tumor necrosis percentage by means of MRI is challenging owing to tissue heterogeneity, inter-observer variability, and a wide variety of pulse sequences. Diffusion-weighted imaging (DWI) MRI is not yet implemented in standard care, while it has the best capacity to quantify tumor necrosis. In the case of histopathological assessment, the estimation of the tumor necrosis percentage is subject to the selection of microscopic fields and sampling heterogeneity. Furthermore, within the European Organisation for Research and Treatment of Cancer (EORTC) scoring system for necrosis, cutoff percentages have been arbitrarily chosen [33]. Not surprisingly, data on the tumor necrosis percentage in STS in relation to clinical outcomes are contradictory [18,20]. The data obtained in this study support the conclusion that, in contrast to bone sarcomas, tumor necrosis has no clear prognostic value in MFS [19,34].
The current integration of nRT into the standard of care of MFS is hindered by the increased incidence of wound healing complications compared with adjuvant radiotherapy. Because of the lack of literature on the nRT-to-surgery time interval and its prognostic value in STS, we explored the influence of the time interval between the end of nRT and surgery on DFS and OS in MFS, and could not identify a clear cut-off. Currently, the EORTC recommends that imaging should not be performed earlier than 4 weeks after nRT [35]. Collier et al. reported, in a large retrospective database study investigating the nRT-surgery interval in STS, that a delay in surgery of up to 120 days after nRT was not associated with worse survival [36]. An interval of six weeks between the end of nRT and surgery was associated with fewer wound complications in another study [37]. The time interval between the end of nRT and surgery in STS warrants further investigation to minimize wound complications after nRT without affecting survival outcomes.
The next step after our retrospective study should be to perform a prospective trial using multiparametric MRI in a cohort of MFS patients to assess tumor cellularity and vascularization after nRT. Multiparametric MRI should be performed at multiple time points between the end of nRT and planned surgery using the recommendations of the EORTC Soft Tissue and Bone Sarcoma Imaging Group [35,38,39]. In the past, several adjuvant chemotherapy trials in different histological subtypes of STS failed to improve the survival of heterogeneous cohorts of STS patients. This has been attributed to the lack of selection of real high-risk patients with chemotherapy-sensitive STS histiotypes [40]. The use of MRI characteristics as prognostic biomarkers to select high-risk patients and to individualize further follow-up or treatment should be investigated in further detail.
5. Conclusions
The presence of a tail sign after nRT is prognostic for worse DFS and the presence of a vascular pedicle is prognostic for both worse DFS and OS, both pre- and post-nRT. These MRI characteristics could serve as biomarkers to support the identification of MFS patients at risk for dismal clinical outcomes in an early stage.
Author Contributions
Conceptualization: I.M.E.D., J.W.J.d.R. and S.G.v.R.; methodology: S.G.v.R., I.M.E.D., J.W.J.d.R., T.C.P.W. and M.J.L.N.; formal analysis: S.G.v.R., M.J.L.N. and T.C.P.W.; investigation: all; resources: S.G.v.R., M.J.L.N., T.C.P.W., I.M.E.D. and J.W.J.d.R.; data curation: S.G.v.R., M.J.L.N., T.C.P.W. and J.W.J.d.R.; writing—original draft preparation: S.G.v.R., M.J.L.N., T.C.P.W., I.M.E.D. and J.W.J.d.R.; writing—review and editing: all; visualization: S.G.v.R. and M.J.L.N.; supervision: I.M.E.D. and J.W.J.d.R.; project administration: I.M.E.D. and J.W.J.d.R.; funding acquisition: I.M.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Radboud University Medical Center. The protocol is registered under the identification number 108026.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study (NCT05373810).
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vanni, S.; De Vita, A.; Gurrieri, L.; Fausti, V.; Miserocchi, G.; Spadazzi, C.; Liverani, C.; Cocchi, C.; Calabrese, C.; Bongiovanni, A.; et al. Myxofibrosarcoma landscape: Diagnostic pitfalls, clinical management and future perspectives. Ther. Adv. Med. Oncol. 2022, 14, 17588359221093973. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, B.; Penel, N.; Coindre, J.M.; Ray-Coquard, I.; Ligier, K.; Delafosse, P.; Bouvier, A.M.; Plouvier, S.; Gallet, J.; Lacourt, A.; et al. Incidence and time trends of sarcoma (2000–2013): Results from the French network of cancer registries (FRANCIM). BMC Cancer 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, C.A.; Bongers, S.L.; Versleijen-Jonkers, Y.M.; Ho, V.K.; Braam, P.M.; Flucke, U.E.; de Wilt, J.H.; Desar, I.M. Overall Survival of Patients with Myxofibrosarcomas: An Epidemiological Study. Cancers 2022, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Look Hong, N.J.; Hornicek, F.J.; Raskin, K.A.; Yoon, S.S.; Szymonifka, J.; Yeap, B.; Chen, Y.L.; DeLaney, T.F.; Nielsen, G.P.; Mullen, J.T. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann. Surg. Oncol. 2013, 20, 80–86. [Google Scholar] [CrossRef]
- Spinnato, P.; Clinca, R.; Vara, G.; Cesari, M.; Ponti, F.; Facchini, G.; Longhi, A.; Donati, D.M.; Bianchi, G.; Sambri, A. MRI Features as Prognostic Factors in Myxofibrosarcoma: Proposal of MRI Grading System. Acad. Radiol. 2021, 28, 1524–1529. [Google Scholar] [CrossRef]
- Sambri, A.; Spinnato, P.; Bazzocchi, A.; Tuzzato, G.M.; Donati, D.; Bianchi, G. Does pre-operative MRI predict the risk of local recurrence in primary myxofibrosarcoma of the extremities? Asia Pac. J. Clin. Oncol. 2019, 15, e181–e186. [Google Scholar] [CrossRef]
- Odei, B.; Rwigema, J.C.; Eilber, F.R.; Eilber, F.C.; Selch, M.; Singh, A.; Chmielowski, B.; Nelson, S.D.; Wang, P.C.; Steinberg, M.; et al. Predictors of Local Recurrence in Patients with Myxofibrosarcoma. Am. J. Clin. Oncol. 2018, 41, 827–831. [Google Scholar] [CrossRef]
- Mühlhofer, H.M.L.; Lenze, U.; Gersing, A.; Lallinger, V.; Burgkart, R.; Obermeier, A.; VON Eisenhart-Rothe, R.; Knebel, C. Prognostic Factors and Outcomes for Patients with Myxofibrosarcoma: A 13-Year Retrospective Evaluation. Anticancer Res. 2019, 39, 2985–2992. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Miceli, R.; Grosso, F.; Fiore, M.; Puma, E.; Pennacchioli, E.; Barisella, M.; Sangalli, C.; Mariani, L.; Casali, P.G.; et al. Myxofibrosarcoma: Prognostic Factors and Survival in a Series of Patients Treated at a Single Institution. Ann. Surg. Oncol. 2011, 18, 720–725. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Leung, D.H.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the Prognostic Significance of Microscopic Margins in 2,084 Localized Primary Adult Soft Tissue Sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lal, P.; Qin, J.; Brennan, M.F.; Antonescu, C.R. Low-Grade Myxofibrosarcoma: A Clinicopathologic Analysis of 49 Cases Treated at a Single Institution with Simultaneous Assessment of the Efficacy of 3-Tier and 4-Tier Grading Systems. Hum. Pathol. 2004, 35, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Teurneau, H.; Engellau, J.; Ghanei, I.; Vult von Steyern, F.; Styring, E. High Recurrence Rate of Myxofibrosarcoma: The Effect of Radiotherapy Is Not Clear. Sarcoma 2019, 2019, 8517371. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Bianchi, G.; Righi, A.; Ferrari, C.; Donati, D. Surgical Margins Do Not Affect Prognosis in High Grade Myxofibrosarcoma. Eur. J. Surg. Oncol. 2016, 42, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.A.; Beltrami, G.; Scoccianti, G.; Frenos, F.; Capanna, R. Combining limb-sparing surgery with radiation therapy in high-grade soft tissue sarcoma of extremities—Is it effective? Eur. J. Surg. Oncol. 2016, 42, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.M.; Hasibuzzaman, M.M.; Rodman, S.N.; Goetz, J.E.; Mapuskar, K.A.; Petronek, M.S.; Steinbach, E.J.; Miller, B.J.; Pulliam, C.F.; Coleman, M.C.; et al. Neoadjuvant Radiotherapy-Related Wound Morbidity in Soft Tissue Sarcoma: Perspectives for Radioprotective Agents. Cancers 2020, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, A.A.; Bateni, S.B.; Monjazeb, A.M.; Darrow, M.A.; Thorpe, S.W.; Kirane, A.R.; Bold, R.J.; Canter, R.J. Neoadjuvant Radiotherapy Is Associated with R0 Resection and Improved Survival for Patients with Extremity Soft Tissue Sarcoma Undergoing Surgery: A National Cancer Database Analysis. Ann. Surg. Oncol. 2017, 24, 3252–3263. [Google Scholar] [CrossRef]
- Gannon, N.P.; King, D.M.; Ethun, C.G.; Charlson, J.; Tran, T.B.; Poultsides, G.; Grignol, V.; Howard, J.H.; Tseng, J.; Roggin, K.K.; et al. The role of radiation therapy and margin width in localized soft-tissue sarcoma: Analysis from the US Sarcoma Collaborative. J. Surg. Oncol. 2019, 120, 325–331. [Google Scholar] [CrossRef]
- Gannon, N.P.; Stemm, M.H.; King, D.M.; Bedi, M. Pathologic necrosis following neoadjuvant radiotherapy or chemoradiotherapy is prognostic of poor survival in soft tissue sarcoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1321–1330. [Google Scholar] [CrossRef]
- Lozano-Calderón, S.A.; Albergo, J.I.; Groot, O.Q.; Merchan, N.A.; El Abiad, J.M.; Salinas, V.; Gomez Mier, L.C.; Montoya, C.S.; Ferrone, M.L.; Ready, J.E.; et al. Complete tumor necrosis after neoadjuvant chemotherapy defines good responders in patients with Ewing sarcoma. Cancer 2023, 129, 60–70. [Google Scholar] [CrossRef]
- Salah, S.; Lewin, J.; Amir, E.; Abdul Razak, A. Tumor necrosis and clinical outcomes following neoadjuvant therapy in soft tissue sarcoma: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Bonvalot, S.; Wunder, J.; Gronchi, A.; Broto, J.M.; Turcotte, R.; Rastrelli, M.; Papai, Z.; Radaelli, S.; Lindner, L.H.; Shumelinsky, F.; et al. Complete pathological response to neoadjuvant treatment is associated with better survival outcomes in patients with soft tissue sarcoma: Results of a retrospective multicenter study. Eur. J. Surg. Oncol. 2021, 47, 2166–2172. [Google Scholar] [CrossRef]
- Reijers, S.J.M.; Gennaro, N.; Bruining, A.; van Boven, H.; Snaebjornsson, P.; Bekers, E.M.; van Coevorden, F.; Scholten, A.N.; Schrage, Y.; van der Graaf, W.T.A.; et al. Correlation of radiological and histopathological response after neoadjuvant radiotherapy in soft tissue sarcoma. Acta Oncol. 2023, 62, 25–32. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hong, S.H.; Kang, Y.; Choi, J.Y.; Moon, K.C.; Kim, H.S.; Han, I.; Yi, M.; Kang, H.S. MR imaging of myxofibrosarcoma and undifferentiated sarcoma with emphasis on tail sign; diagnostic and prognostic value. Eur. Radiol. 2014, 24, 1749–1757. [Google Scholar] [CrossRef]
- Kikuta, K.; Kubota, D.; Yoshida, A.; Morioka, H.; Toyama, Y.; Chuuman, H.; Kawai, A. An analysis of factors related to the tail-like pattern of myxofibrosarcoma seen on MRI. Skeletal Radiol. 2015, 44, 55–62. [Google Scholar] [CrossRef]
- Swami, V.G.; Demicco, E.G.; Naraghi, A.; White, L.M. Soft tissue solitary fibrous tumors of the musculoskeletal system: Spectrum of MRI appearances and characteristic imaging features. Skeletal Radiol. 2022, 51, 807–817. [Google Scholar] [CrossRef]
- Ledoux, P.; Kind, M.; Le Loarer, F.; Stoeckle, E.; Italiano, A.; Tirode, F.; Buy, X.; Crombé, A. Abnormal vascularization of soft-tissue sarcomas on conventional MRI: Diagnostic and prognostic values. Eur. J. Radiol. 2019, 117, 112–119. [Google Scholar] [CrossRef]
- Lefkowitz, R.A.; Landa, J.; Hwang, S.; Zabor, E.C.; Moskowitz, C.S.; Agaram, N.P.; Panicek, D.M. Myxofibrosarcoma: Prevalence and diagnostic value of the "tail sign" on magnetic resonance imaging. Skeletal Radiol. 2013, 42, 809–818. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Setsu, N.; Yoshida, A.; Takahashi, F.; Chuman, H.; Kushima, R. Histological analysis suggests an invasion-independent metastatic mechanism in alveolar soft part sarcoma. Hum. Pathol. 2014, 45, 137–142. [Google Scholar] [CrossRef]
- Iwata, S.; Yonemoto, T.; Araki, A.; Ikebe, D.; Kamoda, H.; Hagiwara, Y.; Ishii, T. Impact of infiltrative growth on the outcome of patients with undifferentiated pleomorphic sarcoma and myxofibrosarcoma. J. Surg. Oncol. 2014, 110, 707–711. [Google Scholar] [CrossRef]
- Imanishi, J.; Slavin, J.; Pianta, M.; Jackett, L.; Ngan, S.Y.; Tanaka, T.; Charoenlap, C.; DI Bella, C.; Choong, P.F. Tail of Superficial Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma After Preoperative Radiotherapy. Anticancer Res. 2016, 36, 2339–2344. [Google Scholar] [PubMed]
- Aiba, H.; Ikuta, K.; Asanuma, K.; Kawanami, K.; Tsukushi, S.; Matsumine, A.; Ishimura, D.; Nagano, A.; Shido, Y.; Kozawa, E.; et al. Effect of neoadjuvant therapies on soft tissue sarcomas with tail-like lesions: A multicenter retrospective study. Cancers 2021, 13, 3901. [Google Scholar] [CrossRef]
- Wardelmann, E.; Haas, R.L.; Bovée, J.V.; Terrier, P.; Lazar, A.; Messiou, C.; LePechoux, C.; Hartmann, W.; Collin, F.; Fisher, C.; et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur. J. Cancer 2016, 53, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Tsoi, K.; Parry, M.C.; Stevenson, J.D.; Fujiwara, T.; Sumathi, V.; Jeys, L.M. Impact of chemotherapy-induced necrosis on event-free and overall survival after preoperative MAP chemotherapy in patients with primary high-grade localized osteosarcoma. Bone Joint J. 2020, 102-B, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Bonvalot, S.; Gronchi, A.; Vanel, D.; Meyer, M.; Robinson, P.; Morosi, C.; Bloem, J.L.; Terrier, P.H.; Lazar, A.; et al. Evaluation of response after pre-operative radiotherapy in soft tissue sarcomas; the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) and Imaging Group recommendations for radiological examination and reporting with an emphasis on magnetic resonance imaging. Eur. J. Cancer 2016, 56, 37–44. [Google Scholar]
- Collier, C.D.; Kim, C.Y.; Liu, R.W.; Getty, P.J. The Interval Between Preoperative Radiation and Surgery Is Not Associated with Overall Survival for Soft-tissue Sarcomas: An Analysis of the National Cancer Database. Clin. Orthop. Relat. Res. 2021, 479, 506–517. [Google Scholar] [CrossRef]
- Collier, C.D.; Su, C.A.; Reich, M.S.; Tu, L.A.; Getty, P.J. Six-Week Interval Between Preoperative Radiation and Surgery Is Associated With Fewer Major Wound Complications in Soft Tissue Sarcoma. Am. J. Clin. Oncol. 2020, 43, 491–495. [Google Scholar] [CrossRef]
- Soldatos, T.; Ahlawat, S.; Montgomery, E.; Chalian, M.; Jacobs, M.A.; Fayad, L.M. Multiparametric Assessment of Treatment Response in High-Grade Soft-Tissue Sarcomas with Anatomic and Functional MR Imaging Sequences. Radiology 2016, 278, 831–840. [Google Scholar] [CrossRef]
- Winfield, J.M.; Miah, A.B.; Strauss, D.; Thway, K.; Collins, D.J.; deSouza, N.M.; Leach, M.O.; Morgan, V.A.; Giles, S.L.; Moskovic, E.; et al. Utility of Multi-Parametric Quantitative Magnetic Resonance Imaging for Characterization and Radiotherapy Response Assessment in Soft-Tissue Sarcomas and Correlation With Histopathology. Front. Oncol. 2019, 9, 280. [Google Scholar] [CrossRef]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; Van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.; et al. EORTC Soft Tissue and Bone Sarcoma Group and the NCIC Clinical Trials Group Sarcoma Disease Site Committee. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).