Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario
Abstract
Simple Summary
Abstract
1. Introduction
2. Immune Checkpoints Role and Rationale for ICIs Use
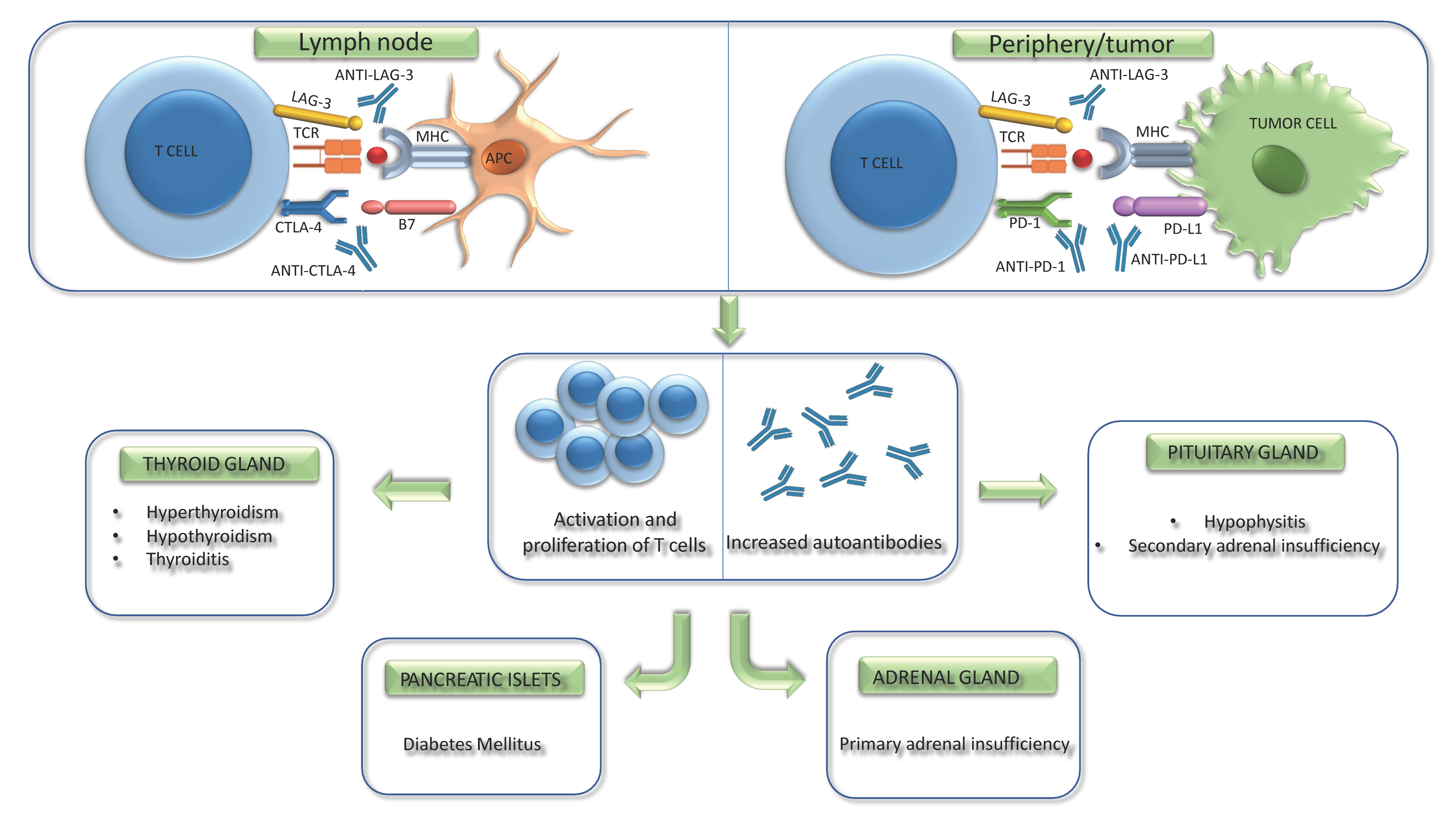
3. Physiopathology of ICI-Related Adverse Effects
4. Endocrine Adverse Effects
4.1. Thyroid Disorders
4.1.1. Epidemiology and Clinical Presentation
4.1.2. Diagnosis and Treatment
4.2. Pituitary Disorders
4.2.1. Epidemiology and Clinical Presentation
4.2.2. Diagnosis and Treatment
4.3. Adrenal Disorders
4.3.1. Epidemiology and Clinical Presentation
4.3.2. Diagnosis and Treatment
5. Metabolic Adverse Effects
5.1. Diabetes
5.1.1. Epidemiology and Clinical Presentation
5.1.2. Diagnosis and Treatment
6. Potential Biomarkers of irAEs Development and irAEs Impact on Clinical Outcomes
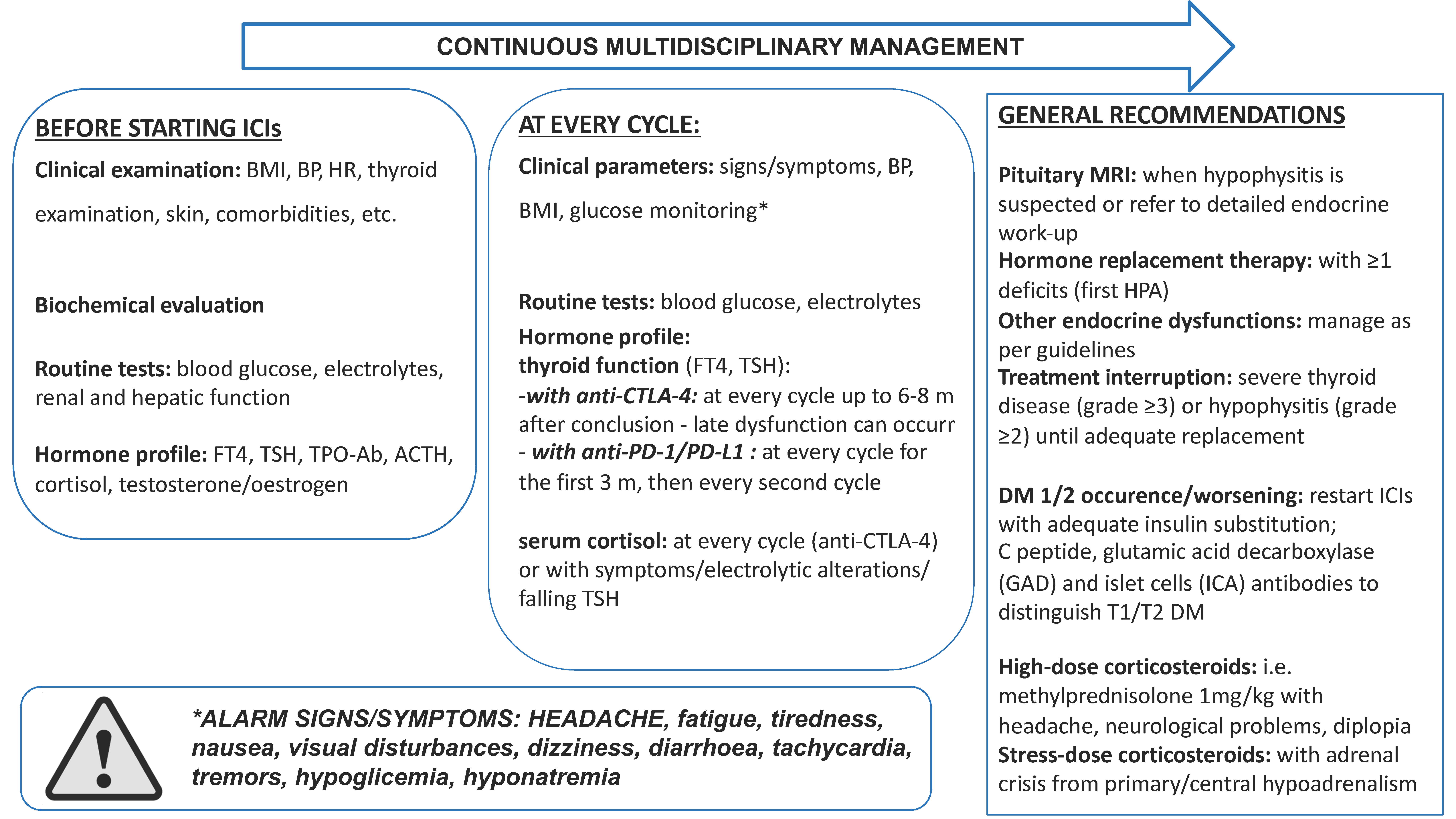
7. Multidisciplinary Management of irAEs in Real-Life
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stewart, T.J.; Smyth, M.J. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011, 30, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Del Rivero, J.; Cordes, L.M.; Klubo-Gwiezdzinska, J.; Madan, R.A.; Nieman, L.K.; Gulley, J.L. Endocrine-Related Adverse Events Related to Immune Checkpoint Inhibitors: Proposed Algorithms for Management. Oncologist 2020, 25, 290–300. [Google Scholar] [CrossRef]
- Inno, A.; Metro, G.; Bironzo, P.; Grimaldi, A.M.; Grego, E.; Di Nunno, V.; Picasso, V.; Massari, F.; Gori, S. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori J. 2017, 103, 405–421. [Google Scholar] [CrossRef]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campenni, A.; Giuffrida, G.; Trimboli, P.; Giovanella, L.; Trimarchi, F.; Cannavo, S. Endocrine and metabolic adverse effects of immune checkpoint inhibitors: An overview (what endocrinologists should know). J. Endocrinol. Investig. 2019, 42, 745–756. [Google Scholar] [CrossRef]
- Fiala, O.; Sorejs, O.; Pesek, M.; Finek, J. Immunotherapy in the Treatment of Lung Cancer. Klin. Onkol. 2017, 30, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.G.; Hegmans, J.P. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013, 73, 2381–2388. [Google Scholar] [CrossRef]
- Salagianni, M.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. New insights into the role of NK cells in cancer immunotherapy. Oncoimmunology 2012, 1, 205–207. [Google Scholar] [CrossRef]
- Vermaelen, K.; Pauwels, R. Pulmonary dendritic cells. Am. J. Respir. Crit. Care Med. 2005, 172, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Sun, Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning cold tumors hot: From molecular mechanisms to clinical applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubata, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef]
- Wang, X.B.; Zheng, C.Y.; Giscombe, R.; Lefvert, A.K. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand. J. Immunol. 2001, 54, 453–458. [Google Scholar] [CrossRef]
- Massafra, M.; Passalacqua, M.I.; Gebbia, V.; Macri, P.; Lazzari, C.; Gregorc, V.; Buda, C.; Altavilla, G.; Santarpia, M. Immunotherapeutic Advances for NSCLC. Biologics 2021, 15, 399–417. [Google Scholar] [CrossRef]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef]
- Egen, J.G.; Kuhns, M.S.; Allison, J.P. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002, 3, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Jen, T.; Skulski, R. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J. Oncol. Pharm. Pract. 2017, 23, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Iwai, Y.; Tanaka, Y.; Okazaki, T.; Freeman, G.J.; Minato, N.; Honjo, T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol. Lett. 2002, 84, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Erfani, N.; Mehrabadi, S.M.; Ghayumi, M.A.; Haghshenas, M.R.; Mojtahedi, Z.; Ghaderi, A.; Amani, D. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC). Lung Cancer 2012, 77, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Erfani, N.; Khademi, B.; Haghshenas, M.R.; Mojtahedi, Z.; Khademi, B.; Ghaderi, A. Intracellular CTLA-4 and regulatory T cells in patients with laryngeal squamous cell carcinoma. Immunol. Investig. 2013, 42, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Stelmachowska-Banas, M.; Czajka-Oraniec, I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: An updated review. Endocr. Connect. 2020, 9, R207–R228. [Google Scholar] [CrossRef]
- Ovacik, M.; Lin, K. Tutorial on Monoclonal Antibody Pharmacokinetics and Its Considerations in Early Development. Clin. Transl. Sci. 2018, 11, 540–552. [Google Scholar] [CrossRef]
- Geraud, A.; Gougis, P.; Vozy, A.; Anquetil, C.; Allenbach, Y.; Romano, E.; Funck-Brentano, E.; Moslehi, J.J.; Johnson, D.B.; Salem, J.E. Clinical Pharmacology and Interplay of Immune Checkpoint Agents: A Yin-Yang Balance. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 85–112. [Google Scholar] [CrossRef]
- Dirks, N.L.; Meibohm, B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharm. 2010, 49, 633–659. [Google Scholar] [CrossRef]
- Romano, E.; Kusio-Kobialka, M.; Foukas, P.G.; Baumgaertner, P.; Meyer, C.; Ballabeni, P.; Michielin, O.; Weide, B.; Romero, P.; Speiser, D.E. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Peppas, N.A. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 2006, 11, 905–910. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Melanoma drug wins US approval. Nature 2011, 471, 561. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.P.; Tang, X.Y.; Xiong, Y.L.; Zheng, K.F.; Liu, Y.J.; Shi, X.G.; Lv, Y.; Jiang, T.; Ma, N.; Zhao, J.B. Immune Checkpoint LAG3 and Its Ligand FGL1 in Cancer. Front. Immunol. 2021, 12, 785091. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Goppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Sifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, T.K.; Hassel, J.C.; Berking, C.; Aberle, J.; Bachmann, O.; Grunwald, V.; Kahler, K.C.; Loquai, C.; Reinmuth, N.; Steins, M.; et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat. Rev. 2016, 45, 7–18. [Google Scholar] [CrossRef]
- Joshi, M.N.; Whitelaw, B.C.; Palomar, M.T.; Wu, Y.; Carroll, P.V. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: Clinical review. Clin. Endocrinol. 2016, 85, 331–339. [Google Scholar] [CrossRef]
- Weber, J.S.; Dummer, R.; de Pril, V.; Lebbe, C.; Hodi, F.S.; Investigators, M.D.X. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013, 119, 1675–1682. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Guaraldi, F.; La Selva, R.; Sama, M.T.; D’Angelo, V.; Gori, D.; Fava, P.; Fierro, M.T.; Savoia, P.; Arvat, E. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: A long-term prospective study from a referral institution. J. Endocrinol. Investig. 2018, 41, 549–556. [Google Scholar] [CrossRef]
- Chalan, P.; Di Dalmazi, G.; Pani, F.; De Remigis, A.; Corsello, A.; Caturegli, P. Thyroid dysfunctions secondary to cancer immunotherapy. J. Endocrinol. Investig. 2018, 41, 625–638. [Google Scholar] [CrossRef]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential Immune-Related Adverse Events Associated with Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef]
- O’Malley, G.; Lee, H.J.; Parekh, S.; Galsky, M.D.; Smith, C.B.; Friedlander, P.; Yanagisawa, R.T.; Gallagher, E.J. Rapid Evolution of Thyroid Dysfunction in Patients Treated with Nivolumab. Endocr. Pract. 2017, 23, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.; Salari, F.; Kashat, L.; Walfish, P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J. Clin. Endocrinol. Metab. 2015, 100, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, I.; Sakane, Y.; Fukuda, Y.; Fujii, T.; Taura, D.; Hirata, M.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid 2017, 27, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Paderi, A.; Giorgione, R.; Giommoni, E.; Mela, M.M.; Rossi, V.; Doni, L.; Minervini, A.; Carini, M.; Pillozzi, S.; Antonuzzo, L. Association between Immune Related Adverse Events and Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Cancers 2021, 13, 860. [Google Scholar] [CrossRef] [PubMed]
- Karhapaa, H.; Makela, S.; Lauren, H.; Jaakkola, M.; Schalin-Jantti, C.; Hernberg, M. Immune checkpoint inhibitors, endocrine adverse events, and outcomes of melanoma. Endocr. Connect. 2022, 11, e210562. [Google Scholar] [CrossRef]
- Cheung, Y.M.; Wang, W.; McGregor, B.; Hamnvik, O.R. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2022, 71, 1795–1812. [Google Scholar] [CrossRef]
- Kotwal, A.; Ryder, M. Survival benefit of endocrine dysfunction following immune checkpoint inhibitors for nonthyroidal cancers. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 517–524. [Google Scholar] [CrossRef]
- Lima Ferreira, J.; Costa, C.; Marques, B.; Castro, S.; Victor, M.; Oliveira, J.; Santos, A.P.; Sampaio, I.L.; Duarte, H.; Marques, A.P.; et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 299–309. [Google Scholar] [CrossRef]
- Luongo, C.; Morra, R.; Gambale, C.; Porcelli, T.; Sessa, F.; Matano, E.; Damiano, V.; Klain, M.; Schlumberger, M.; Salvatore, D. Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J. Endocrinol. Investig. 2021, 44, 1927–1933. [Google Scholar] [CrossRef]
- Muir, C.A.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e3704–e3713. [Google Scholar] [CrossRef]
- Basak, E.A.; van der Meer, J.W.M.; Hurkmans, D.P.; Schreurs, M.W.J.; Oomen-de Hoop, E.; van der Veldt, A.A.M.; Bins, S.; Joosse, A.; Koolen, S.L.W.; Debets, R.; et al. Overt Thyroid Dysfunction and Anti-Thyroid Antibodies Predict Response to Anti-PD-1 Immunotherapy in Cancer Patients. Thyroid 2020, 30, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, I.; Yasoda, A.; Matsumoto, S.; Sakamori, Y.; Kim, Y.H.; Nomura, M.; Otsuka, A.; Yamasaki, T.; Saito, R.; Kitamura, M.; et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE 2019, 14, e0216954. [Google Scholar] [CrossRef] [PubMed]
- Rubino, R.; Marini, A.; Roviello, G.; Presotto, E.M.; Desideri, I.; Ciardetti, I.; Brugia, M.; Pimpinelli, N.; Antonuzzo, L.; Mini, E.; et al. Endocrine-related adverse events in a large series of cancer patients treated with anti-PD1 therapy. Endocrine 2021, 74, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, G.; Magazzu, G.; Campenni, A.; Lucanto, M.C.; Trimarchi, F.; Cannavo, S.; Ruggeri, R.M. Cystic Fibrosis as a Cause of Malabsorption and Increased Requirement of Levothyroxine. Thyroid 2020, 30, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Vaidya, A.; Becker, C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol. 2011, 164, 303–307. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Giuffrida, G.; Campenni, A. Autoimmune endocrine diseases. Minerva Endocrinol. 2018, 43, 305–322. [Google Scholar] [CrossRef]
- Omata, Y.; Satake, M.; Maeda, R.; Saito, A.; Shimazaki, K.; Yamauchi, K.; Uzuka, Y.; Tanabe, S.; Sarashina, T.; Mikami, T. Reduction of the infectivity of Toxoplasma gondii and Eimeria stiedai sporozoites by treatment with bovine lactoferricin. J. Vet. Med. Sci. 2001, 63, 187–190. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Shields, C.L.; Orloff, M.; Sato, T.; Shields, J.A. CHECKPOINT INHIBITOR IMMUNE THERAPY: Systemic Indications and Ophthalmic Side Effects. Retina 2018, 38, 1063–1078. [Google Scholar] [CrossRef]
- Sagiv, O.; Kandl, T.J.; Thakar, S.D.; Thuro, B.A.; Busaidy, N.L.; Cabanillas, M.; Jimenez, C.; Dadu, R.; Graham, P.H.; Debnam, J.M.; et al. Extraocular Muscle Enlargement and Thyroid Eye Disease-like Orbital Inflammation Associated with Immune Checkpoint Inhibitor Therapy in Cancer Patients. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 50–52. [Google Scholar] [CrossRef]
- Campredon, P.; Imbert, P.; Mouly, C.; Grunenwald, S.; Mazieres, J.; Caron, P. Severe Inflammatory Ophthalmopathy in a Euthyroid Patient during Nivolumab Treatment. Eur. Thyroid. J. 2018, 7, 84–87. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra245. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Piticchio, T.; Le Moli, R.; Malaguarnera, R.; Campenni, A.; Cannavo, S.; Ruggeri, R.M. Recent insights into the pathogenesis of autoimmune hypophysitis. Expert. Rev. Clin. Immunol. 2021, 17, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.T.; Sullivan, R.; Lawrence, D.; Tritos, N.A.; Fadden, R.; Klibanski, A.; Nachtigall, L. Ipilimumab-induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J. Clin. Endocrinol. Metab. 2014, 99, 4078–4085. [Google Scholar] [CrossRef]
- Araujo, P.B.; Coelho, M.C.; Arruda, M.; Gadelha, M.R.; Neto, L.V. Ipilimumab-induced hypophysitis: Review of the literature. J. Endocrinol. Investig. 2015, 38, 1159–1166. [Google Scholar] [CrossRef]
- Marlier, J.; Cocquyt, V.; Brochez, L.; Van Belle, S.; Kruse, V. Ipilimumab, not just another anti-cancer therapy: Hypophysitis as side effect illustrated by four case-reports. Endocrine 2014, 47, 878–883. [Google Scholar] [CrossRef]
- Dillard, T.; Yedinak, C.G.; Alumkal, J.; Fleseriu, M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: Serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 2010, 13, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwama, S.; Sugiyama, D.; Yasuda, Y.; Okuji, T.; Ito, M.; Ito, S.; Sugiyama, M.; Onoue, T.; Takagi, H.; et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002493. [Google Scholar] [CrossRef] [PubMed]
- Kanie, K.; Iguchi, G.; Bando, H.; Urai, S.; Shichi, H.; Fujita, Y.; Matsumoto, R.; Suda, K.; Yamamoto, M.; Fukuoka, H.; et al. Mechanistic insights into immune checkpoint inhibitor-related hypophysitis: A form of paraneoplastic syndrome. Cancer Immunol. Immunother. 2021, 70, 3669–3677. [Google Scholar] [CrossRef]
- Wallace, J.; Krupa, M.; Brennan, J.; Mihlon, F. Ipilimumab cystic hypophysitis mimicking metastatic melanoma. Radiol. Case Rep. 2018, 13, 740–742. [Google Scholar] [CrossRef]
- Giuffrida, G.; Ferrau, F.; Alessi, Y.; Cannavo, S. Shrinkage of a pituitary metastasis of melanoma induced by pembrolizumab: A case report. J. Med. Case Rep. 2021, 15, 555. [Google Scholar] [CrossRef]
- Min, L.; Ibrahim, N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013, 1, e15. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.A.; Krajewski, K.M.; Jagannathan, J.P.; Braschi-Amirfarzan, M.; Tirumani, S.H.; Shinagare, A.B.; Ramaiya, N.H. A New Look at Toxicity in the Era of Precision Oncology: Imaging Findings, Their Relationship With Tumor Response, and Effect on Metastasectomy. AJR Am. J. Roentgenol. 2016, 207, 4–14. [Google Scholar] [CrossRef]
- Pociot, F.; Lernmark, A. Genetic risk factors for type 1 diabetes. Lancet 2016, 387, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Brorsson, C.A.; Nielsen, L.B.; Andersen, M.L.; Kaur, S.; Bergholdt, R.; Hansen, L.; Mortensen, H.B.; Pociot, F.; Storling, J.; Hvidoere Study Group on Childhood Diabetes. Genetic Risk Score Modelling for Disease Progression in New-Onset Type 1 Diabetes Patients: Increased Genetic Load of Islet-Expressed and Cytokine-Regulated Candidate Genes Predicts Poorer Glycemic Control. J. Diabetes Res. 2016, 2016, 9570424. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef]
- Yoneda, S.; Imagawa, A.; Hosokawa, Y.; Baden, M.Y.; Kimura, T.; Uno, S.; Fukui, K.; Goto, K.; Uemura, M.; Eguchi, H.; et al. T-Lymphocyte Infiltration to Islets in the Pancreas of a Patient Who Developed Type 1 Diabetes After Administration of Immune Checkpoint Inhibitors. Diabetes Care 2019, 42, e116–e118. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Salem, J.E.; Johnson, D.B.; Lebrun-Vignes, B.; Stamatouli, A.; Thomas, J.W.; Herold, K.C.; Moslehi, J.; Powers, A.C. Increased Reporting of Immune Checkpoint Inhibitor-Associated Diabetes. Diabetes Care 2018, 41, e150–e151. [Google Scholar] [CrossRef] [PubMed]
- Tsang, V.H.M.; McGrath, R.T.; Clifton-Bligh, R.J.; Scolyer, R.A.; Jakrot, V.; Guminski, A.D.; Long, G.V.; Menzies, A.M. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct From Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 5499–5506. [Google Scholar] [CrossRef]
- de Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: A case report and systematic review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Quandt, Z.; Young, A.; Anderson, M. Immune checkpoint inhibitor diabetes mellitus: A novel form of autoimmune diabetes. Clin. Exp. Immunol. 2020, 200, 131–140. [Google Scholar] [CrossRef]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.J.; Rhinehart, A.S.; Shaefer, C.F., Jr.; Neuman, A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann. Intern. Med. 2016, 164, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Leiter, A.; Gnjatic, S.; Fowkes, M.; Kim-Schulze, S.; Laface, I.; Galsky, M.D.; Gallagher, E.J. A Common Pituitary Autoantibody in Two Patients with Immune Checkpoint Inhibitor-Mediated Hypophysitis: Zcchc8. AACE Clin. Case Rep. 2020, 6, e151–e160. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Hong, A.R.; Kim, H.K.; Kang, H.C. Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors. Endocrinol. Metab. 2021, 36, 413–423. [Google Scholar] [CrossRef]
- Brilli, L.; Danielli, R.; Campanile, M.; Secchi, C.; Ciuoli, C.; Calabro, L.; Pilli, T.; Cartocci, A.; Pacini, F.; Di Giacomo, A.M.; et al. Baseline serum TSH levels predict the absence of thyroid dysfunction in cancer patients treated with immunotherapy. J. Endocrinol. Investig. 2021, 44, 1719–1726. [Google Scholar] [CrossRef]
- Osawa, T.; Inoue, S.; Umeda, M.; Hasegawa, T.; Makino, T.; Hori, A.; Tanaka, K.; Yasuda, M.; Mizui, T.; Sawa, T.; et al. Predictors of Nivolumab-Induced Skin Reactions. Cancer Chemother. 2018, 45, 1533–1535. [Google Scholar]
- Nakamura, Y.; Tanaka, R.; Maruyama, H.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujimoto, M.; Fujisawa, Y. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn. J. Clin. Oncol. 2019, 49, 431–437. [Google Scholar] [CrossRef]
- Keenan, T.E.; Burke, K.P.; Van Allen, E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019, 25, 389–402. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Wang, J.; Nadeem, Z.; Patel, N.; Postow, M.A.; Shoushtari, A.N.; Plitas, G.; Balachandran, V.P.; et al. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat. Genet. 2021, 53, 11–15. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef] [PubMed]
- Hsiehchen, D.; Naqash, A.R.; Espinoza, M.; Von Itzstein, M.S.; Cortellini, A.; Ricciuti, B.; Owen, D.H.; Laharwal, M.; Toi, Y.; Burke, M.; et al. Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology 2022, 11, 2017162. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martinez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Spakowicz, D.; Hoyd, R.; Muniak, M.; Husain, M.; Bassett, J.S.; Wang, L.; Tinoco, G.; Patel, S.H.; Burkart, J.; Miah, A.; et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: Causal modeling, timing, and classes of concomitant medications. BMC Cancer 2020, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Longo, D.L. The Surprisingly Positive Association Between Obesity and Cancer Immunotherapy Efficacy. JAMA 2019, 321, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Rogado, J.; Sanchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Levi, A.; Arranz, R.; Lorenzo, A.; Gullon, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Toi, Y.; Sugawara, S.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Tsurumi, K.; Suzuki, K.; Shimizu, H.; Sugisaka, J.; et al. Association of Immune-Related Adverse Events with Clinical Benefit in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab. Oncologist 2018, 23, 1358–1365. [Google Scholar] [CrossRef]
- Okada, N.; Kawazoe, H.; Takechi, K.; Matsudate, Y.; Utsunomiya, R.; Zamami, Y.; Goda, M.; Imanishi, M.; Chuma, M.; Hidaka, N.; et al. Association Between Immune-Related Adverse Events and Clinical Efficacy in Patients with Melanoma Treated With Nivolumab: A Multicenter Retrospective Study. Clin. Ther. 2019, 41, 59–67. [Google Scholar] [CrossRef]
- Grangeon, M.; Tomasini, P.; Chaleat, S.; Jeanson, A.; Souquet-Bressand, M.; Khobta, N.; Bermudez, J.; Trigui, Y.; Greillier, L.; Blanchon, M.; et al. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non-small-cell Lung Cancer. Clin. Lung Cancer 2019, 20, 201–207. [Google Scholar] [CrossRef]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018, 115, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Schlaak, M.; Hassel, J.C.; Loquai, C.; Stoffels, I.; Gutzmer, R.; Patzold, S.; Mohr, P.; Keller, U.; Starz, H.; et al. Effectiveness and tolerability of ipilimumab: Experiences from 198 patients included in a named-patient program in various daily-practice settings and multiple institutions. J. Immunother. 2014, 37, 374–381. [Google Scholar] [CrossRef]
- Downey, S.G.; Klapper, J.A.; Smith, F.O.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Kammula, U.S.; Hughes, M.S.; Allen, T.E.; Levy, C.L.; et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 2007, 13, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; O’Day, S.; Urba, W.; Powderly, J.; Nichol, G.; Yellin, M.; Snively, J.; Hersh, E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5950–5956. [Google Scholar] [CrossRef]
- Ribas, A.; Camacho, L.H.; Lopez-Berestein, G.; Pavlov, D.; Bulanhagui, C.A.; Millham, R.; Comin-Anduix, B.; Reuben, J.M.; Seja, E.; Parker, C.A.; et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 2005, 23, 8968–8977. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef]
- Al Ashi, S.I.; Thapa, B.; Flores, M.; Ahmed, R.; Rahim, S.E.G.; Amir, M.; Alomari, M.; Chadalavada, P.; Morrison, S.L.; Bena, J.F.; et al. Endocrine Toxicity and Outcomes in Patients With Metastatic Malignancies Treated with Immune Checkpoint Inhibitors. J. Endocr. Soc. 2021, 5, bvab100. [Google Scholar] [CrossRef]
- von Itzstein, M.S.; Gonugunta, A.S.; Wang, Y.; Sheffield, T.; Lu, R.; Ali, S.; Fattah, F.J.; Xie, D.; Cai, J.; Xie, Y.; et al. Divergent prognostic effects of pre-existing and treatment-emergent thyroid dysfunction in patients treated with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2022, 71, 2169–2181. [Google Scholar] [CrossRef]
- Inaba, H.; Ariyasu, H.; Iwakura, H.; Kurimoto, C.; Takeshima, K.; Morita, S.; Furuta, H.; Hotomi, M.; Akamizu, T. Distinct clinical features and prognosis between persistent and temporary thyroid dysfunctions by immune-checkpoint inhibitors. Endocr. J. 2021, 68, 231–241. [Google Scholar] [CrossRef]
- Muir, C.A.; Tsang, V.H.M.; Menzies, A.M.; Clifton-Bligh, R.J. Immune Related Adverse Events of the Thyroid—A Narrative Review. Front. Endocrinol. 2022, 13, 886930. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
| Target | Agent | Indication | Year of Approval |
|---|---|---|---|
| PD-1 | Pembrolizumab | Unresectable or metastatic melanoma (as single agent or in combination with ipilimumab) | 2015 |
| Adjuvant treatment of melanoma (with lymph node(s) involvement) after complete resection | 2021 | ||
| Advanced Non-Small Cell Lung Cancer (NSCLC) | 2016 | ||
| Recurrent or metastatic or Head and Neck Squamous Cell Carcinoma (HNSCC) | 2016 | ||
| Advanced urothelial carcinoma | 2017 | ||
| Bladder carcinoma non-muscle invasive (high-risk) | 2020 | ||
| Advanced MSI-H/dMMR cancers | 2017 | ||
| Advanced MSI-H/dMMR colorectal cancer | 2020 | ||
| Advanced gastric, esophageal or gastroesophageal junction cancer | 2017 | ||
| Advanced cervical cancer | 2018 | ||
| Advanced Hepatocellular Carcinoma (HCC) | 2018 | ||
| Advanced Merkel cell carcinoma | 2018 | ||
| Advanced renal cell carcinoma (with axitinib) | 2019 | ||
| Adjuvant treatment for Renal Cell Carcinoma (RCC) | 2021 | ||
| Advanced MSI-H/dMMR endometrial carcinoma (with lenvatinib) | 2019 | ||
| Advanced TMB-H cancers (≥10 mut/Mb) | 2020 | ||
| Advanced cutaneous Squamous Cell Carcinoma (cSCC) | 2020 | ||
| Advanced Triple-Negative Breast Cancer (TNBC) or high-risk early-stage TNBC | 2020 | ||
| Relapsed or refractory classical Hodgkin lymphoma | 2017 | ||
| Relapsed or refractory primary mediastinal large B-cell lymphoma | 2018 | ||
| Nivolumab | Advanced melanoma (as single agent or in combination with ipilimumab) | 2014 | |
| Advanced NSCLC | 2015 | ||
| Metastatic RCC after prior therapy | 2015 | ||
| Relapsed or refractory classical Hodgkin lymphoma | 2016 | ||
| Advanced or recurrent HNSCC after prior platinum-based therapy | 2016 | ||
| Locally advanced or metastatic urothelial carcinoma after prior platinum-based chemotherapy | 2017 | ||
| Advanced MSI-H/dMMR colorectal cancer after prior chemotherapy (as sigle agent or in combination with ipilimumab) | 2017 | ||
| Advanced HCC after prior treatment with sorafenib (as single agent or in combination with ipilimumab) | 2017 | ||
| Adjuvant treatment of melanoma [with lymph node(s) involvement] or metastatic disease | 2017 | ||
| Metastatic or recurrent esophageal squamous cell carcinoma after prior chemotherapy | 2020 | ||
| Advanced RCC (with cabozantinib) | 2021 | ||
| Adjuvant treatment of urothelial carcinoma (high risk of recurrence) | 2021 | ||
| Advanced malignant pleural mesothelioma (with ipilimumab) | 2020 | ||
| Early-stage NSCLC before surgery | 2022 | ||
| Unresectable advanced or metastatic esophageal squamous cell carcinoma in combination with chemotherapy or ipilimumab | 2022 | ||
| Neoadjuvant treatment of resectable NSCLC in combination with platinum-doublet chemotherapy | 2022 | ||
| Advanced NSCLC (PD-L1 ≥ 1%) or in combination with two cycles of platinum-based chemotherapy | 2020 | ||
| Cemiplimab | Metastatic or locally advanced cutaneous SCC | 2018 | |
| Locally advanced Basal Cell Carcinoma (BCC) | 2021 | ||
| Metastatic or locally advanced NSCLC (TPS ≥ 50%) | 2018 | ||
| Dostarlimab | Recurrent or advanced endometrial cancer with mismatch repair deficient (dMMR), after prior platinum-based therapy | 2021 | |
| PD-L1 | Atezolizumab | Metastatic or locally advanced or urothelial carcinoma, after prior platinum-based chemotherapy | 2016 |
| Advanced NSCLC after prior target therapy or platinum-based chemotherapy | 2016 | ||
| Metastatic or locally advanced or urothelial carcinoma not eligible for platinum- based chemotherapy (expressing PD-L1) | 2017 | ||
| Advanced NSCLC (squamous) without EGFR or ALK alterations (with carboplatin, paclitaxel and bevacizumab) | 2018 | ||
| SCLC (extensive disease) (with carboplatin and etoposide) | 2019 | ||
| Locally advanced or metastatic TNBC (PD-L1 ≥ 1%) (with nab-paclitaxel) | 2019 | ||
| Advanced NSCLC without EGFR or ALK alterations (with nab-paclitaxel/carboplatin) | 2019 | ||
| Advanced NSCLC with PD-L1 ≥ 50% and without EGFR or ALK alterations | 2020 | ||
| Metastatic HCC (with bevacizumab) | 2020 | ||
| Advanced melanoma BRAF V600 mutation–positive (with cobimetinib and vemurafenib) | 2020 | ||
| Adjuvant treatment for stage II-IIIA NSCLC (PD-L1 > 1%) | 2021 | ||
| Durvalumab | Unresectable NSCLC (stage III) after concurrent radiotherapy and platinum-based chemotherapy with nonprogressive disease | 2018 | |
| SCLC (extensive disease) (with platinum-based chemotherapy and etoposide) | 2020 | ||
| Locally advanced or metastatic urothelial carcinoma | 2017 | ||
| Unresectable HCC (in combination with tremelimumab) | 2022 | ||
| Avelumab | Metastatic Merkel cell carcinoma | 2017 | |
| Locally advanced or metastatic urothelial carcinoma (progression after prior platinum-based chemotherapy or maintenance treatment) | 2017 | ||
| Advanced RCC (in combination with axitinib) | 2019 | ||
| CTLA-4 | Ipilimumab | Unresectable or metastatic melanoma | 2011 |
| Melanoma (stage III) high-risk after complete resection | 2015 | ||
| Tremelimumab | Unresectable HCC (in combination with durvalumab) | 2022 | |
| LAG 3 | Relatlimab | Unresectable or metastatic melanoma (in combination with nivolumab) | 2022 |
| Year | First Author | Ref. | Patients n. | M/F | ICI | irAEs n. (%) | Thyroid irAEs n. (%) | Hypothyroidism n. (%) | Thyrotoxicosis n. (%) | Previous Thyroid Disease n. (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | Guaraldi F | [48] | 52 | 22/30 | 52 (100%) ipilimumab 29 (55.8%) nivolumab for disease progression | - | 7 (13.4%) | 1 (1.9%, 4 euthyroid HT)) 7 (13.4%) | 3 (5.7%) (1 transient) | 3 (5.7%) |
| 2019 | Yamauchi I | [53] | 200 | 134/66 | 200 (100%) nivolumab | - | 67 (33.5%) 40 (20%) subclinical 27 (13.5%) overt | - - 11 (5.5%) post thyrotoxicosis | - - 17 (8.5%) | NA |
| 2021 | Paderi A | [54] | 43 | 35/8 | 33 (76.7%) nivolumab 10 (23.7%) nivolumab plus ipilimumab | 29 (67.4%) | 19 (44.2%) endocrine irAEs 15/19 (78.9%) thyroid irAEs | 15 (43.88%) | 8/19 early thyrotoxicosis | NA |
| 2022 | Karhapaa H | [55] | 140 | 75/65 | 21 (15%) ipilimumab, 46 (33%) nivolumab, 67 (48%) pembrolizumab, and 6 (4%) ipilimumab + nivolumab | - | 41 (29.2%) endocrine irAEs 36/41 (87.8%) thyroid irAEs | - 8 (22%) | - 14 (39%) | NA |
| 2021 | Ferreira JL | [58] | 161 | pembrolizumab, nivolumab, and ipilimumab | - | 29 (18%) | 8.7% primary 4.3% central 2.5% biphasic thyroiditis | 2.5% | NA | |
| 2021 | Luongo C | [59] | 96 | 66/30 | 67 (69.1%) nivolumab, 18 (18.5%) pembrolizumab, 9 (9.3%) ipilimumab | - | 36 (38%) | 11 (30.5%) | 25 (69.5%) transient | NA |
| 2021 | Muir CA | [60] | 1246 | 824/422 | 165 (13%) ipilimumab, 236 (19%) nivolumab, 448 (36%) pembrolizumab 285 (23%) ipilimumab + nivolumab, and 112 (9%) others | - | 518 (42%) | 100 (8%) | 388 (31%) | NA |
| 2020 | Basak EA | [61] | 168 | 103/65 | 118 (70%) nivolumab 50 (30%) pembrolizumab | - | 54 (32%) 34 (20%) subclinical 20 (12%) overt | - | - | 27 (16%) |
| 2021 | Rubino R | [63] | 251 | 119/62 | 154 (61.35%) nivolumab 97 (38.65%) pembrolizumab | 70 (27.89) endocrine irAEs 66/70 (94.28%) thyroid irAEs | 34 (51.52%) | 17 (22.72%) | 28 (73.68%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, C.C.; Giuffrida, G.; Cannavò, S.; Franchina, T.; Silvestris, N.; Ruggeri, R.M.; Santarpia, M. Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario. Cancers 2023, 15, 246. https://doi.org/10.3390/cancers15010246
Spagnolo CC, Giuffrida G, Cannavò S, Franchina T, Silvestris N, Ruggeri RM, Santarpia M. Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario. Cancers. 2023; 15(1):246. https://doi.org/10.3390/cancers15010246
Chicago/Turabian StyleSpagnolo, Calogera Claudia, Giuseppe Giuffrida, Salvatore Cannavò, Tindara Franchina, Nicola Silvestris, Rosaria Maddalena Ruggeri, and Mariacarmela Santarpia. 2023. "Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario" Cancers 15, no. 1: 246. https://doi.org/10.3390/cancers15010246
APA StyleSpagnolo, C. C., Giuffrida, G., Cannavò, S., Franchina, T., Silvestris, N., Ruggeri, R. M., & Santarpia, M. (2023). Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario. Cancers, 15(1), 246. https://doi.org/10.3390/cancers15010246






