Simple Summary
Alzheimer disease (AD) and cancer have been reported to be inversely correlated in epidemiological studies. However, the mechanism behind it is not clear. The aim of our retrospective study was to assess the 11 risk factors, including pain, for subsequent AD death in patients with cancer. We examined a SEER Research Plus population of 25,512 cases and 127,560 controls. We found that pain was related to lower AD risk in all subgroups except for digestive cancer. In addition, age, sex, race, number of in situ/malignant tumors, number of benign/borderline tumors, cancer site, cancer-directed surgery, radiation, chemotherapy and survival years were independent factors of AD risk in cancer patients. The risk factors varied by cancer site and race. This study demonstrated pain as a novel protective factor of AD and suggests the uniqueness of the digestive system in interacting with the central nervous system, which provide new perspectives for future studies.
Abstract
Objective: Alzheimer disease (AD) and cancer have been reported to be inversely correlated in incidence, but the mechanism remains elusive. Methods: A case-control study was conducted, based on the SEER (Surveillance, Epidemiology, and End Results) Research Plus data, to evaluate 12 factors in patients with cancer. Results: Severe pain was related to reduced AD risk, while older age at cancer diagnosis, female, longer survival years after tumor diagnosis, more benign/borderline tumors, less cancer-directed surgery, and more chemotherapy were associated with higher AD risk. In addition, patients of different races or with different cancer sites were associated with different risks of getting AD. Cases had a higher prevalence of severe pain than controls in all race and cancer site subgroups, except for in digestive cancer, where the result was the opposite. Conclusions: This study indicated pain as a novel protective factor for AD in patients with cancer. The mechanism behind it may provide new perspective on AD pathogenesis and AD-cancer association, which we discussed in our own hypothesis of the mechanism of pain action. In addition, digestive cancer pain had an opposite impact on AD risk from other cancer pains, which suggests the uniqueness of digestive system in interacting with the central nervous system.
1. Introduction
Alzheimer disease (AD) and cancer are among the leading causes of human death around the world []. Both are age-related diseases possibly with some common molecular pathways in their pathogenesis []. However, there is still a lack of satisfactory understanding of their mechanisms, which to some extent impedes the development of effective treatments for each disease []. Several prior epidemiological studies have demonstrated a relationship between AD and cancer: cancer survivors had a lower risk of AD than cancer-free people [,,,,], cancer risk was lower among AD patients than those without AD [,,,], and survivors of certain types of cancer, such as prostate cancer, were found to have a slightly elevated AD risk []. However, the biological mechanism behind the association remains widely debated. Risk factors that have been studied previously include age, race, sex, comorbidities [], and cancer types. Existing main biological theories include the Warburg effect theory, the two-hit hypothesis theory, the unfolded protein response theory, chronic inflammation, age-related metabolic deregulation, epigenetic causes, family history, and exogenous infection [,,]. However, there have been no studies on pain and AD risk in cancer patients. Four previous studies reported the positive association between non-cancer pain and subsequent AD, but most of them attributed it to the mediation of mood or sleep disorders caused by pain [,,,]. A new perspective on pain and other risk factors in the etiopathology of cancer and AD may provide new chances for effective therapeutics.
The objective of this study was to examine pain and other risk factors for AD in cancer patients, both in the total sample and in subgroups. In this article, we calculated the prevalence, single factor difference, and odds ratio (OR) in an univariable and multivariable logistic regression model for established and novel risk factors, including pain. Then we analyzed them stratified by race and cancer site, which delineated the unique risk patterns for different race and cancer site groups, especially for digestive cancer. We also examined the predictive accuracy, sensitivity, and specificity of the logistic regression models by constructing receiver operating characteristic (ROC) curves.
2. Materials and Methods
In this case-control study, we used the Surveillance, Epidemiology, and End Results (SEER) Research Plus Data in 8 registries from 1975 to 2019 to obtain study samples. The SEER program, which is supported by the National Cancer Institute (NCI), collects cancer data from various state registries that cover approximately 35% of the US population. Since the SEER data is deidentified, this study is exempt from full board review by the institutional review boards at the participating research institutions. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
2.1. Study Populations
We included patients diagnosed at the age of over 20 with all primary cancers other than neural, head, face, or neck cancer between 1975 and 2019. We defined cancers using the International Classification of Diseases for Oncology, Version 3 (ICD-O-3)/WHO 2008. Only cases that were confirmed microscopically at diagnosis and by autopsy or death certificate at death were selected. We exclude those having brain or perineural invasion. The cause of death (COD) in the case group was Alzheimer disease defined by ICD-9 and 10. In the control group, the COD was defined besides its own or other cancer/neoplasm, AD, cerebrovascular disease (CVD), and all kinds of social events (e.g., accidents, suicide, homicide). We initially identified 29,040 cases and 440,355 controls, including 62 types of subdivided cancers. Then, we excluded those with invalid race, cancer-direct surgery, radiation data, those with age at death ≤65, and those with malignant behavior code but survival year <1. We selected control samples through random digit dialing to make case and control group 1:5 matched. The final sample had 25,512 cases and 127,560 controls. Figure 1 shows the flowchart of this study.
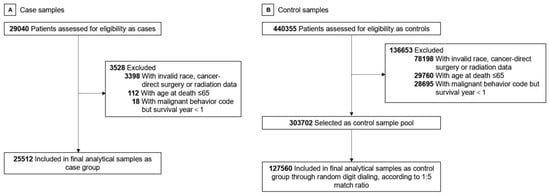
Figure 1.
Study Flowchart. (A) Case sample selection. (B) Control sample selection.
2.2. Outcome, Exposure, and Risk Factors
We chose the candidate variables based on previous literature and our biological inference. The corresponding data for case and control groups were then collected from SEER’ s electronic system. After calculation and categorization, we identified 11 potential risk factors, including age, sex, race (White, Asian/Pacific Islander, Black, American Indian/Alaska Native), pain rating (I, II), total number of in situ/malignant tumors (number 1), total number of benign/borderline tumors (number 2), cancer site, radiation therapy (Yes, No), cancer-directed surgery (Yes, No), chemotherapy (Yes, No/Unknown), and survival years after primary tumor diagnosis. The pain rating I was defined as in situ behavior, while pain rating II was defined as malignant behavior with survival years >1. The initial 62 cancer sites were further divided into 11 types according to the organ system, including bone and joint, breast, digestive, endocrine, hematological, Kaposi sarcoma, mesothelioma, miscellaneous, respiratory, skin and soft tissue, and urogenital cancers. The main outcome is whether or not death is due to AD.
2.3. Statistical Analysis
Descriptive statistics were used to describe the baseline characteristics of our study samples, both in total and after stratification by pain rating, race, and cancer site. Unadjusted case-control association analyses of the 11 potential risk factors were assessed using independent t-tests for continuous variables (age, number 1, number 2, and survival years) and a Χ2 test for categorical variables (sex, race, pain rating, cancer site, radiation, cancer-directed surgery, and chemotherapy). Risk factors that were p < 0.05 were selected for subsequent regression analysis. Multivariable statistical analyses of 2-level risk factors, including sex, pain rating, radiation therapy, cancer-directed surgery, and chemotherapy were performed using multivariable logistic regression. Multivariable statistical analyses for multi-level risk factors race and cancer site were assessed also using multivariable logistic regression, but with dummy variables, in which White race and urogenital cancer were set as the reference categories. In these analyses, univariable logistic regression models for risk of AD were first constructed. Then the multivariable logistic models were constructed, with forward stepwise selection to retain only those factors that were p < 0.05. Unadjusted odds ratio (OR) and adjusted OR were calculated to account for the risk level.
Then, the abovementioned analyses were repeated to explore case-control associations stratified by race and cancer site. Only subgroups with a population greater than 800 in both case and control groups were included. They were: White, Asian/Pacific Islander, Black, breast cancer, digestive cancer, skin and soft tissue cancer, and urogenital cancer groups. Missing data were not calculated. Statistical analyses were performed using SPSS version 26.0 (SPSS, Inc., Chicago, IL, USA) software. All statistical tests were two-sided, and statistical significance was set at p < 0.05.
3. Results
3.1. Overall Sample Characteristics
There were 25,512 case patients and 127,560 control patients. The average age of the case group was older than that of the control group (mean (SD) age, 71.92 (9.76) vs. 71.72 (9.80) years; p < 0.001) (Table 1). The case group had a significantly larger proportion of female, White, and American Indian/Alaska Native compared with the control group (16,670 (65.3%) vs. 65,911 (51.7%); 23,355 (91.5%) vs. 106,957 (83.8%), 49 (0.2%) vs. 126 (0.1%)) (age, sex: p < 0.001) and a smaller proportion of male, Asian/Pacific Islander, and Black (Figures S1 and S2). A substantially lower rate of case individuals had pain rating II compared to the controls (23,394 (91.7%) vs. 119,391 (93.6%), p < 0.001). Case patients had a significantly smaller number of in situ/malignant tumors (1.17 (0.45) vs. 1.20 (0.50), p < 0.001), a significantly larger number of benign/borderline tumors (0.40 × 10−2 (0.07) vs. 0.18 × 10−2 (0.04), p < 0.001), and substantially longer survival years after first cancer diagnosis (15.62 (8.86) vs. 11.71 (8.16) years, p < 0.001). Cancer sites were significantly different between case and control groups (p < 0.001; Figure S3). Case group had a significantly higher rate of cancer-directed surgery or chemotherapy compared to control group. No difference was detected in radiation therapy between the 2 groups.

Table 1.
Baseline characteristics of cases and controls.
3.2. Pain and AD Risk
In multivariable analysis, pain rating II was found to be associated with a lower AD risk in cancer patients (aOR = 0.849; 95% CI, 0.805–0.896; p < 0.001) (Table 2). The adjusted ORs for age and female sex were 1.055 (95% CI, 1.053–1.057; p < 0.001) and 1.315 (95% CI, 1.269–1.362; p < 0.001). Compared to White, the adjusted Ors for Asian/Pacific Islander, Black, and American Indian/Alaska Native are 0.408 (95% CI, 0.384–0.434; p < 0.001), 0.670 (95% CI, 0.623–0.721; p < 0.001), and 2.179 (95% CI, 1.545–3.075; p < 0.001). AD risk in cancer patients was correlated with fewer in situ/malignant tumors (aOR = 0.764, 95% CI, 0.740–0.788; p < 0.001) and more benign/borderline tumors (aOR = 1.987, 95% CI, 1.573–2.509; p < 0.001). Compared with the reference urogenital cancer, AD risks in skin and soft tissue (aOR = 1.190; 95% CI, 1.114–1.272; p < 0.001) and breast cancer (aOR = 1.090; 95% CI, 1.047–1.136; p < 0.001) were higher, whereas AD risks in digestive (aOR = 0.784; 95% CI, 0.753–0.816; p < 0.001), hematological (aOR = 0.683; 95% CI, 0.597–0.781; p < 0.001), respiratory cancer (aOR = 0.533; 95% CI, 0.486–0.586; p < 0.001) were lower. The difference in AD risks between urogenital and bone and joint (aOR = 0.829; 95% CI, 0.416–1.652; p = 0.59), endocrine (aOR = 1.040; 95% CI, 0.897–1.204; p = 0.61), mesothelioma (aOR = 0.769; 95% CI, 0.170–3.474; p = 0.73), or miscellaneous cancer (aOR = 2.133; 95% CI, 0.484–9.402; p < 0.32) were not significant. The adjusted OR for cancer-directed surgery, chemotherapy, and survival years were 0.902 (95% CI, 0.857–0.951; p < 0.001), 1.450 (95% CI, 1.370–1.534; p < 0.001), and 1.096 (95% CI, 1.093–1.098; p < 0.001). Radiation therapy had no significant relationship with AD risk in total cancer patients. Figure 2A shows the area under the receiver operating characteristic curve (AUC) for AD risk in overall cancer patients was 0.700 (95% CI, 0.697–0.703). The Youden’s index’s best cutoff value was 0.295. The cut-off value with the highest Youden Index was 0.295, with sensitivity of 71.2% and specificity of 58.4%.

Table 2.
Analyses of risk factors for AD in cancer patients.
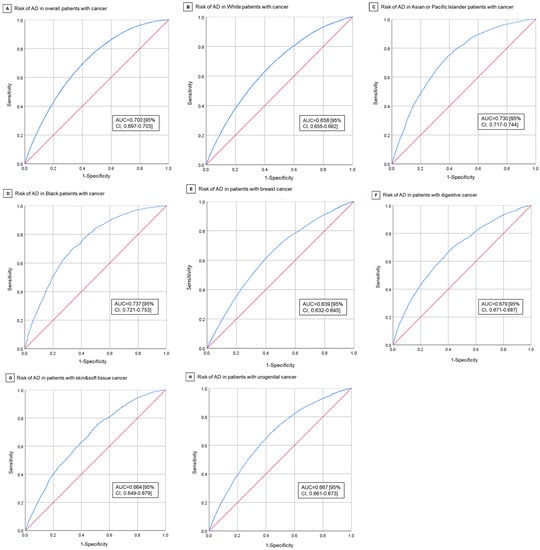
Figure 2.
Receive-Operating Characteristics Curves for Risk of AD in Cancer Patients. (A) Risk of AD in overall cancer patients. (B) Risk of AD in White cancer patients. (C) Risk of AD in Asian/Pacific Islander cancer patients. (D) Risk of AD in Black cancer patients. (E) Risk of AD in breast cancer patients. (F) Risk of AD in digestive system cancer patients. (G) Risk of AD in skin and soft tissue cancer patients. (H) Risk of AD in urogenital cancer patients.
3.3. Patterns of Pain Rating
We further explored the different characteristics between the pain rating I and rating II groups (Table 3). In the case group, there were 2118 (8.3%) pain rating I patients and 23,394 (91.7%) pain rating II patients, while in the control group, there were 8169 (6.4%) pain rating I patients and 119,391 (93.6%) pain rating II patients. In both the case and control groups, there was a significant difference in the distribution of age, sex, number of in situ/malignant tumors, cancer site, radiation therapy, cancer-directed surgery, chemosurgery, and survival years between pain rating I and II patients. In both groups, pain rating II patients tended to be older, male, fewer in situ/malignant tumors, received more radiation therapy, less cancer-directed surgery, more chemotherapy, and shorter survival years compared to pain rating I patients.

Table 3.
Sample Characteristics Stratified by Pain Rating a.
3.4. Racial and Tumor Site Variation in AD Risk
Subgroup analyses stratified by race demonstrated the significant association of pain rating II (aOR = 0.862; 95% CI, 0.816–0.911), more in situ/malignant tumors (aOR = 0.751; 95% CI, 0.727–0.775), and cancer-directed surgery (aOR = 0.901; 95% CI, 0.853–0.952) with reduced AD risk in White cancer patients. On the contrary, female, more benign/borderline tumors, chemotherapy, and longer survival years after first cancer were associated with a higher risk of AD in White cancer patients. Compared with the reference urogenital cancer, skin and soft tissue (aOR = 1.205; 95% CI, 1.127–1.289) and breast cancer (aOR = 1.110; 95% CI, 1.064–1.157) were associated with an increased risk of AD in White, whereas in digestive (aOR = 0.848; 95% CI, 0.813–0.884; p < 0.001), hematological (aOR = 0.675; 95% CI, 0.588–0.775; p < 0.001) and respiratory cancers (aOR = 0.438; 95% CI, 0.397–0.483; p < 0.001) the association was inverse. Figure 2B shows the AUC for AD risk in White cancer patients is 0.658 (95% CI, 0.655–0.662), with the best cut-off value of 0.232 (sensitivity: 69.1% and specificity: 54.2%). In Asian/Pacific Islander cancer patients, AD risk was only statistically related to age (aOR = 1.092; 95% CI, 1.083–1.102), female gender (aOR = 1.701; 95% CI, 1.499–1.929), and survival years (aOR = 1.118; 95% CI, 1.107–1.129). The case group had a lower prevalence of pain rating II than control group, but the difference was not statistically significant. The AUC for AD risk in Asian/Pacific Islander cancer patients is 0.730 (95% CI, 0.717–0.744) (Figure 2C). In Black cancer patients, more in situ/malignant tumors (aOR = 0.641; 95% CI, 0.530–0.775) were associated with reduced AD risk, whereas age (aOR = 1.076; 95% CI, 1.066–1.086) and survival years (aOR = 1.121; 95% CI, 1.108–1.133) were associated with increased risk of AD. The case group had a lower proportion of pain rating II patients than the control group, but it was only significant in independent t test. The AUC for AD risk in Black cancer patients is 0.737 (95% CI, 0.721–0.753) (Figure 2D). Table S1 shows the distribution of risk factors in patients stratified by race. Table S2 shows the results of univariable and multivariable logistic regression.
Subgroup analyses stratified by cancer site demonstrated pain rating II as a protective factor for AD risk in breast (aOR = 0.772; 95% CI, 0.718–0.831) and skin and soft tissue cancers (aOR = 0.815; 95% CI, 0.710–0.934), but as a risk factor in digestive cancer (aOR = 1.130; 95% CI, 1.006–1.270) (Table S4). The case group with urogenital cancer had a significantly lower proportion of pain rating II patients (9358 [98.1%] vs. 49,995 [98.5%], p = 0.006), but it was not included in the regression model (Table S3). In all four types of cancers, Asian/Pacific Islander and Black had a lower risk of AD compared with the reference White group. The adjusted OR for Asian/Pacific Islander and black compared with White, respectively, were 0.394 (95% CI, 0.352–0.440) and 0.604 (95% CI, 0.531–0.686) in breast cancer, 0.461 (95% CI, 0.410–0.518) and 0.660 (95% CI, 0.568–0.767) in digestive cancer, 0.524 (95% CI, 0.331–0.829) and 0.359 (95% CI, 0.178–0.724) in skin and soft tissue cancer, and 0.382 (95% CI, 0.345–0.424) and 0.541 (95% CI, 0.483–0.607) in urogenital cancer. AD risk in all 4 cancers were also related to female, less in situ/malignant tumors, and longer survival years. The AUC for AD risk in the 4 cancers (breast, digestive, skin and soft tissue, urogenital) are 0.639 (95% CI, 0.632–0.645), 0.679 (95% CI, 0.671–0.687), 0.664 (95% CI, 0.649–0.679), and 0.667 (95% CI, 0.661–0.673) (Figure 2E–H).
4. Discussion
4.1. Pain as a Protective Factor for AD in Patients with Cancer
Despite progress in the study of AD, effective prevention or treatment for it is still lacking due to a lack of explicit understanding of its pathogenesis. In the last decade, there has been emerging research studying the AD-cancer association, aiming to find out the common and different mechanisms in these two intractable diseases []. Many epidemiological studies demonstrated the inverse relationship between AD and certain cancers, including a lower chance of developing cancer in AD patients and a lower chance of AD in cancer patients, whereas several other studies reported little or a positive association with certain cancers [,]. Main factors studied for AD risk in cancer patients include age, sex, cancer type, tumor stage, size, and grade, treatment, and comorbidities such as CVD []. In addition, there have been four studies reported about the positive relationship between AD and non-cancer chronic pain conditions (NCPCs) [,,,], and most of them attribute the cause to the patient’s depression/anxiety mood or sleep disorders. However, there is no literature regarding the association of pain with subsequent AD risk in cancer patients.
In this study, we demonstrated that pain served as an independent protective factor for AD death. A meta-analysis revealed a high prevalence of pain in cancer patients, with 66.4% in advanced, metastatic, or terminal disease, 50.7% in all cancer stages, 39.3% after curative treatment, and 55.0% during anticancer treatment []. In addition, a meta-analysis found that progress in the pathophysiological mechanisms of pain and the wider use of antinociceptive therapies had not influenced the prevalence of pain in cancer patients []. In this study, we categorize pain into two ratings based on cancer behavior and survival years, representing both the degree and the duration of pain. The reason we take these two measurements of pain into account is that AD is a chronic disease, and the effect of any influencing factor in its pathogenesis, such as cancer pain, must have a certain degree and a long-lasting effect. Therefore, we categorize the cancer pain into rating I and II, which are defined as in situ cancer and malignant cancer with survival years >1 respectively. In both the total samples and all subgroups except for digestive cancer, cancer patients who died by AD had a lower prevalence of pain rating II compared to those who died by control causes. Among them, the group that had the most obvious relationship with cancer pain was breast cancer, with an OR of 0.772. A previous bioinformatic study recognized many shared transcription factors (TFs) and regulatory processes that were closely related to the adaptive immune response from dramatically different directions, which may play crucial roles in both AD and breast cancer pathogenesis []. We also found that digestive cancer patients had elevated AD risk by cancer pain, with an OR of 1.13.
Overall, our result regarding pain was opposite to the four previous studies that reported a positive relationship between pain and AD risk. The reasons might be as follows. First, in the four previous studies, the discrepancy in mood or sleep disorder degree between case and control groups may have been larger than in this study since they used individuals with no NCPCs as controls. Second, the sources of NCPCs in those studies included headache, neuropathic pain, and inflammatory pain (such as osteoarthritis), which may have confounders on AD risk apart from the pain itself, such as the effects of the primary disease on the central nervous system and on peripheral inflammation []. Although we didn’t subdivide pain character due to the limitations of the SEER database in our study, we excluded cancers appearing on the nervous system, head, face, or neck, as well as those with perineural invasion or metastasis to the brain. Therefore, the association of pain with AD risk in our study may be less confounded by other influencing factors on the central nervous system.
4.2. Hypothesis on Cancer-AD Association
It has been debated whether the association between AD and cancer is the consequence of their shared biological mechanisms or the pharmacological treatments given to patients []. Recently a meta-analysis proved that the inverse association between AD and cancer may be possibly attributable to shared inverse etiological mechanisms or survival bias, but was not likely due to competing risks bias, diagnostic bias, or inadequate control for potential confounding factors []. Since we set age, survival years, and cancer treatments including radiation, therapy, and cancer-directed surgery as independent risk factors in our study, we thus make the hypothesis that pain itself may have a direct influence on AD pathogenesis and that it intertwines with the common inverse etiological mechanisms that AD and cancer share. Nowadays, there are diverse proposed biological theories trying to explain the inverse correlation between AD and cancer, such as the Warburg effect theory, the two-hit hypothesis theory, the unfolded protein response theory, chronic inflammation, metabolic deregulation hypothesis, and epigenetic causes [], but none of them can completely explain the two diseases []. Therefore, there are also merging hypotheses which include a subset of molecules such as p53, Wnt, UPS, and PIN1 []. Based on our results and biological reference, we hypothesize that enduring pain in malignant cancer patients is related to inhibition of the systemic sympathetic nervous system, which can regulate whole-body metabolic homeostasis [], central nervous system (CNS) neural activity, and immune cell activity [], and thus have an impact on AD and cancer pathogenesis in two relatively opposite directions. This can explain why cancer patients who died by AD in our study had a lower proportion of individuals with the pain rating II. However, the specific molecular mechanisms involved need further experimental verification. Figure S4 describes our hypothesis of the mechanism of pain action in AD and cancer.
Our hypothesis can also explain why digestive cancer doesn’t fall within the range of pain’s protective influence. The digestive system has long been found to be tightly connected to the central nervous system in many ways, such as the gut-brain axis, gut microbiota, and intestinal sympathetic nervous system. Therefore, pain may have little influence on digestive cancer-AD association, due to the strong direct link between digestive cancer and the CNS. The elevated risk caused by pain in digestive cancer patients might be explained by the effect of the local intestinal sympathetic nervous system and gut microbiota, which can affect the brain microenvironment directly through the gut-brain axis, increase neuroinflammation in the brain, and thus increase the risk of getting AD. In both the total samples and the subgroups that had a difference in distribution among the two pain ratings, the difference was significant, except in Asian/Pacific Islander, where the difference existed but was not significant (case vs. control: 92.9% vs. 93.4%, p = 0.51). We can also explain it by our hypothesis for that the average level of systemic sympathetic activity might be relatively low in Asian/Pacific Islander, which needs further verification []. This makes cancer pain less influential in AD prevention in Asian/Pacific Islander compared with other races, though the pain association remained.
4.3. Other Risk Factors for AD in Patients with Cancer
Apart from pain, we also found that age, female sex, American Indian/Alaska Native ethnicity, more benign/borderline tumors, chemotherapy, and longer survival years were associated with a higher risk of AD in cancer patients, whereas more in situ/malignant tumors and cancer-directed surgery were linked to a reduced AD risk. In fact, the latter two factors are, to some extent, similar to pain because both of them can increase a patient’s physical pain. Age, sex, and race have long been discussed as risk factors for AD []. Older age, female sex, and Black were usually recognized as risk factors for AD []. There has been debate on the effect of chemotherapy on AD incidence. A study found chemotherapy was independently related to decreased AD risk []. Another study reported that chemotherapy used in colorectal cancer survivals was associated with reduced risk for AD and other neurocognitive disorders []. However, chemotherapy-induced cognitive impairment was also been reported in studies [].
4.4. Study Strengths
This study has strengths. To our knowledge, this is the first comprehensive investigational study exploring the influence of pain in the cancer-AD association. In addition, we proposed a new hypothesis about pain action and provided a new perspective on AD pathogenesis and the cancer-AD relationship. Another strength is the large size and proper setting of the study. More than 25,000 case and 120,000 control samples across the United States, with four main race categories, and 62 cancer types were included, which ensures the reliability of the study. In addition, we established strict inclusion and exclusion criteria to avoid potential confounding factors for the AD outcome, which ensures the study’ s rigorousness. We covered 11 widely-distributed risk factors including demographic characteristics, cancer features, and treatment information in our study, which allow us to explore cancer-AD relationship broadly. What’s more, we did subgroup analyses stratified by race and cancer site, which added depth and completeness to the study results. For the first time, we demonstrated the uniqueness of digestive cancer pain in AD pathogenesis compared with other cancers, which inferred the intricate interaction between the gastrointestinal system and the central nervous system through known or unknown mechanisms.
4.5. Study Limitations
This study also has limitations. First, it was based on a single database. Future multi-database and multicenter randomized controlled studies may be needed to verify the association between pain and AD. Second, the measurement of pain is semi-quantitative and indirect, which may lead to a loss of detailed evidence and residual confounding [,]. However, we think our grading of pain was enough to reflect the effect of pain degree and pain time on subsequent AD risk. Completely quantitative measurement of pain degree by detailed grading such as the 1–10 score system may be unnecessary. Additionally, the causes of pain in cancer patients may be extensive, such as physical pressure, neuropathic, treatment-induced, and comorbidity-induced pain. However, late-stage malignant cancer itself is the biggest source of pain, according to the literature [,]. Third, due to the limitation of variable items in the SEER database, other underlying or contributory causes of AD such as comorbidities [], socioeconomic conditions, neuroimaging changes [], genetic differences, histological nature of the cancer, and treatment for AD or pain were not included in the study, which may be potential confounders. Although they were not considered by us in our study and can be compromised by the large samples and exhaustive subgroup analyses, future studies based on a database that includes these variables may promote the research. In addition, the diagnosis sequence of cancer and AD is not known due to the lack of information at the baseline of our study. Although we did get a positive outcome showing an inverse association based on the limited data, the reverse association over time between AD and cancer can be explored in future studies.
We view our findings with caution. Although an association between cancer pain and subsequent AD risk was proved, causation cannot be inferred due to the nature of the case-control study. Further experimental study may be needed to address the exact causal relationship between pain and AD in cancer patients, as well as the molecular mechanisms behind it. Furthermore, we suggest that pain should be considered as a factor in studies regarding cancer-AD relationship in the future.
5. Conclusions
This study of pain and other risk factors for AD in cancer patients identified pain as a novel protective factor for AD in the total sample and all subgroups, except in digestive cancer, where it was a risk factor. We proposed a new hypothesis of pain action in AD and cancer pathogenesis. Our study provides a new perspective on the pathogenesis of AD and the mechanism behind the AD-cancer relationship. It also suggests the importance of recognizing pain as an influencing factor in future epidemiological studies of the cancer-AD association.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15010248/s1, Figure S1: Proportion of males and females in the case and control groups; Figure S2: Proportion of different races in the case and control groups; Figure S3: Proportion of different cancer sites in the case and control groups; Figure S4: Hypothesis of the mechanism of pain action in AD and cancer; Table S1: Baseline characteristics of cases and controls, stratified by race; Table S2: Risk factors for AD in patients with cancer, stratified by race; Table S3: Baseline characteristics of cases and controls, stratified by cancer site; Table S4: Risk factors for AD in patients with cancer, stratified by cancer site.
Author Contributions
Conceptualization, validation, formal analysis, and investigation, S.X.; methodology and software, S.X. and X.Y.; resources, S.X. and G.C.; data curation, S.X.; writing—original draft preparation, S.X.; writing—review and editing, S.X., X.Y. and G.C.; visualization, S.X.; supervision, G.C.; project administration, G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key R & D Program of Zhejiang, grant number 2022C03133 and the Key Program of Zhejiang, grant number WKJ-ZJ-2004.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to that the SEER data is deidentified.
Informed Consent Statement
Patient consent was waived due to that the SEER data is deidentified.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s Disease and Cancer: When Two Monsters Cannot Be Together. Front. Neurosci. 2019, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.M.; Ahles, T.; Calderwood, S.; Carrillo, M.C.; Chen, H.; Chang, C.H.; Craft, S.; De Jager, P.; Driver, J.A.; Fillit, H.; et al. Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies: A convening of the Alzheimer’s Association & Alzheimer’s Drug Discovery Foundation. Alzheimers Dement. 2017, 13, 267–273. [Google Scholar] [CrossRef]
- Mezencev, R.; Chernoff, Y.O. Risk of Alzheimer’s Disease in Cancer Patients: Analysis of Mortality Data from the US SEER Population-Based Registries. Cancers 2020, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A.; Beiser, A.; Au, R.; Kreger, B.E.; Splansky, G.L.; Kurth, T.; Kiel, D.P.; Lu, K.P.; Seshadri, S.; Wolf, P.A. Inverse association between cancer and Alzheimer’s disease: Results from the Framingham Heart Study. BMJ 2012, 344, e1442. [Google Scholar] [CrossRef] [PubMed]
- Seddighi, S.; Houck, A.L.; Rowe, J.B.; Pharoah, P.D.P. Evidence of a Causal Association between Cancer and Alzheimer’s Disease: A Mendelian Randomization Analysis. Sci. Rep. 2019, 9, 13548. [Google Scholar] [CrossRef] [PubMed]
- Hanson, H.A.; Horn, K.P.; Rasmussen, K.M.; Hoffman, J.M.; Smith, K.R. Is Cancer Protective for Subsequent Alzheimer’s Disease Risk? Evidence From the Utah Population Database. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 1032–1043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Realmuto, S.; Cinturino, A.; Arnao, V.; Mazzola, M.A.; Cupidi, C.; Aridon, P.; Ragonese, P.; Savettieri, G.; D’Amelio, M. Tumor diagnosis preceding Alzheimer’s disease onset: Is there a link between cancer and Alzheimer’s disease? J. Alzheimers Dis. 2012, 31, 177–182. [Google Scholar] [CrossRef]
- Ren, R.J.; Huang, Q.; Xu, G.; Gu, K.; Dammer, E.B.; Wang, C.F.; Xie, X.Y.; Chen, W.; Shao, Z.Y.; Chen, S.D.; et al. Association between Alzheimer’s disease and risk of cancer: A retrospective cohort study in Shanghai, China. Alzheimers Dement. 2022, 18, 924–933. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, D.; Lee, J.H. Association between Alzheimer’s Disease and Cancer Risk in South Korea: An 11-year Nationwide Population-Based Study. Dement. Neurocogn. Disord. 2018, 17, 137–147. [Google Scholar] [CrossRef]
- Freedman, D.M.; Wu, J.; Chen, H.; Kuncl, R.W.; Enewold, L.R.; Engels, E.A.; Freedman, N.D.; Pfeiffer, R.M. Associations between cancer and Alzheimer’s disease in a U.S. Medicare population. Cancer Med. 2016, 5, 2965–2976. [Google Scholar] [CrossRef]
- Musicco, M.; Adorni, F.; Di Santo, S.; Prinelli, F.; Pettenati, C.; Caltagirone, C.; Palmer, K.; Russo, A. Inverse occurrence of cancer and Alzheimer disease: A population-based incidence study. Neurology 2013, 81, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Frain, L.; Swanson, D.; Cho, K.; Gagnon, D.; Lu, K.P.; Betensky, R.A.; Driver, J. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimers Dement. 2017, 13, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Song, L.; Schulz, P.E.; Xu, H.; Chan, W. Risk of Developing Alzheimer’s Disease and Related Dementias in Association with Cardiovascular Disease, Stroke, Hypertension, and Diabetes in a Large Cohort of Women with Breast Cancer and with up to 26 Years of Follow-Up. J. Alzheimers Dis. 2022, 87, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse Correlation between Alzheimer’s Disease and Cancer: Short Overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. microRNA diagnostic panel for Alzheimer’s disease and epigenetic trade-off between neurodegeneration and cancer. Ageing Res. Rev. 2019, 49, 125–143. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H. Association between widespread pain and dementia, Alzheimer’s disease and stroke: A cohort study from the Framingham Heart Study. Reg. Anesth. Pain Med. 2021, 46, 879–885. [Google Scholar] [CrossRef]
- Khalid, S.; Sambamoorthi, U.; Innes, K.E. Non-Cancer Chronic Pain Conditions and Risk for Incident Alzheimer’s Disease and Related Dementias in Community-Dwelling Older Adults: A Population-Based Retrospective Cohort Study of United States Medicare Beneficiaries, 2001–2013. Int. J. Environ. Res. Public Health 2020, 17, 5454. [Google Scholar] [CrossRef]
- Innes, K.E.; Sambamoorthi, U. The Association of Osteoarthritis and Related Pain Burden to Incident Alzheimer’s Disease and Related Dementias: A Retrospective Cohort Study of U.S. Medicare Beneficiaries. J. Alzheimers Dis. 2020, 75, 789–805. [Google Scholar] [CrossRef]
- Khalid, S.; Sambamoorthi, U.; Umer, A.; Lilly, C.L.; Gross, D.K.; Innes, K.E. Increased Odds of Incident Alzheimer’s Disease and Related Dementias in Presence of Common Non-Cancer Chronic Pain Conditions in Appalachian Older Adults. J. Aging Health 2022, 34, 158–172. [Google Scholar] [CrossRef]
- Ganguli, M. A reduced risk of Alzheimer’s disease in those who survive cancer. BMJ 2012, 344, e1662. [Google Scholar] [CrossRef] [PubMed]
- Ording, A.G.; Horváth-Puhó, E.; Veres, K.; Glymour, M.M.; Rørth, M.; Sørensen, H.T.; Henderson, V.W. Cancer and risk of Alzheimer’s disease: Small association in a nationwide cohort study. Alzheimers Dement. 2020, 16, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Bowles, E.J.A.; Walker, R.L.; Anderson, M.L.; Dublin, S.; Crane, P.K.; Larson, E.B. Risk of Alzheimer’s disease or dementia following a cancer diagnosis. PLoS ONE 2017, 12, e0179857. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Song, L.; Schulz, P.E.; Xu, H.; Chan, W. Associations Between Vascular Diseases and Alzheimer’s Disease or Related Dementias in a Large Cohort of Men and Women with Colorectal Cancer. J. Alzheimers Dis. 2022, 90, 211–231. [Google Scholar] [CrossRef] [PubMed]
- van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- van den Beuken-van Everdingen, M.H.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann. Oncol. 2007, 18, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Mou, X.; Deng, J.; Di, B.; Zhong, R.; Wang, S.; Yang, Y.; Zeng, W. Differences of immune disorders between Alzheimer’s disease and breast cancer based on transcriptional regulation. PLoS ONE 2017, 12, e0180337. [Google Scholar] [CrossRef]
- Cao, S.; Fisher, D.W.; Yu, T.; Dong, H. The link between chronic pain and Alzheimer’s disease. J. Neuroinflammation 2019, 16, 204. [Google Scholar] [CrossRef]
- Ospina-Romero, M.; Glymour, M.M.; Hayes-Larson, E.; Mayeda, E.R.; Graff, R.E.; Brenowitz, W.D.; Ackley, S.F.; Witte, J.S.; Kobayashi, L.C. Association Between Alzheimer Disease and Cancer with Evaluation of Study Biases: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2025515. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Martinez-Sanchez, N.; Sweeney, O.; Sidarta-Oliveira, D.; Caron, A.; Stanley, S.A.; Domingos, A.I. The sympathetic nervous system in the 21st century: Neuroimmune interactions in metabolic homeostasis and obesity. Neuron 2022, 110, 3597–3626. [Google Scholar] [CrossRef] [PubMed]
- Egerman, R.S.; Andersen, R.N.; Manejwala, F.M.; Sibai, B.M. Neuropeptide Y and nitrite levels in preeclamptic and normotensive gravid women. Am. J. Obstet. Gynecol. 1999, 181, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L. Major risk factors for Alzheimer’s disease: Age and genetics. Lancet Neurol. 2020, 19, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated with Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584–591. [Google Scholar] [CrossRef]
- Akushevich, I.; Yashkin, A.P.; Kravchenko, J.; Kertai, M.D. Chemotherapy and the Risk of Alzheimer’s Disease in Colorectal Cancer Survivors: Evidence From the Medicare System. JCO Oncol. Pract. 2021, 17, e1649–e1659. [Google Scholar] [CrossRef]
- Butterfield, D.A. The 2013 SFRBM discovery award: Selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic. Biol. Med. 2014, 74, 157–174. [Google Scholar] [CrossRef]
- Okereke, O.I.; Meadows, M.E. More Evidence of an Inverse Association Between Cancer and Alzheimer Disease. JAMA Netw. Open 2019, 2, e196167. [Google Scholar] [CrossRef]
- Nudelman, K.N.; Risacher, S.L.; West, J.D.; McDonald, B.C.; Gao, S.; Saykin, A.J. Association of cancer history with Alzheimer’s disease onset and structural brain changes. Front. Physiol. 2014, 5, 423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).