Simple Summary
In patients with hepatitis C virus-related liver disease, direct-acting antivirals (DAAs) suppress the development of hepatocellular carcinoma (HCC). However, it is unclear whether their use after curative HCC treatment suppresses its recurrence in patients with hepatitis C virus-related liver disease. We retrospectively evaluated the inhibitory effect of DAAs on HCC recurrence using propensity score matching. Both the first and second HCC recurrence rates in the DAA-treated group were lower than those in the non-DAA-treated group, suggesting that the inhibitory effect of DAA therapy on HCC recurrence is sustained.
Abstract
It remains unclear whether hepatocellular carcinoma (HCC) recurrence in hepatitis C virus (HCV)-infected patients can be suppressed by the elimination of the virus using direct-acting antivirals (DAAs) after radical HCC treatment. We evaluated the sustained inhibitory effect of DAAs on HCC recurrence after curative treatment. This multicenter retrospective study included 190 HCV-positive patients after radical treatment for early-stage HCC. Patients were classified into the DAA treatment group (n = 70) and the non-DAA treatment group (n = 120) after HCC treatment. After propensity score matching (PSM), 112 patients were assessed for first and second recurrences using the Kaplan–Meier method and analyzed using a log-rank test. The first recurrence rates at 1 and 3 years were 3.6% and 42.1% in the DAA treatment group and 21.7% and 61.9% in the non-DAA treatment group, respectively (p = 0.0026). Among 85 patients who received radical treatment, the second recurrence rate at 3 years was 2.2% in the DAA treatment group and 33.9% in the non-DAA treatment group (p = 0.0128). In HCV-positive patients with early-stage HCC, the first and second recurrences were suppressed by DAA therapy after radical treatment, suggesting that the inhibitory effect of DAA therapy on HCC recurrence was sustained.
1. Introduction
Recent studies report approximately 95% efficacy of direct-acting antiviral (DAA) therapy in the elimination of the virus in hepatitis C virus (HCV)-related chronic liver disease (CLD) [1,2], as well as improvement of liver fibrosis [3,4]. Furthermore, the incidence of HCC has been reported to decrease in patients in whom the virus had been eliminated by DAA therapy [5,6,7]. However, it remains unclear whether DAA therapy after radical treatment of HCC inhibits its recurrence. Many studies have reported that DAA treatment increases the survival rate but does not change the recurrence rate of HCC [8,9,10]. In contrast, DAA therapy after HCC treatment has been reported to cause rapid HCC recurrence [11]. Few reports have shown a decrease in recurrence rates [12,13,14]. Even if HCC is detected early and subjected to radical treatment, new cancerous nodules may later develop at other sites; this is known as multicentric recurrence [15]. Among HCC-causing background liver diseases, such as hepatitis B virus- or HCV-related CLD, alcoholic liver disease, non-alcoholic steatohepatitis, and autoimmune liver diseases, the highest incidence of HCC can be found in those with HCV-related CLD [16,17]. In addition, the incidence rate of multicentricity is highest in patients with HCV-related CLD, as compared to patients with hepatitis B virus-related CLD or non-B/non-C liver disease [18,19]. Suppression of multicentric recurrence is important for the prognosis and prolongation of survival in patients with HCV-positive HCC. In this study, using retrospective multicenter data, the rates of first and second recurrences were compared in patients who had achieved sustained virological response (SVR) upon receiving DAA treatment and in patients who had not been treated with DAA after radical treatment of HCC. Based on these findings, our study examined (1) whether DAA therapy early after radical treatment of HCC had an inhibitory effect on recurrence and (2) whether the inhibitory effect on HCC was sustained.
2. Materials and Methods
2.1. Patient Enrollment and Study Design
Patients with HCC within Barcelona Clinic Liver Cancer (BCLC) stage A [20], who were treated radically with hepatectomy or radiofrequency ablation between January 2010 and December 2017 at Kurume University Hospital (Kurume, Japan), and from affiliated institutions, namely Kurume University Medical Center (Kurume, Japan), Kurume Central Hospital (Kurume, Japan), Yame General Hospital (Yame, Japan), Saga Central Hospital (Saga, Japan), Kumamoto Central Hospital (Kumamoto, Japan), Chikugo City Hospital (Chikugo, Japan), Yanagawa Hospital (Yanagawa, Japan), and Yasumoto Hospital (Kurume, Japan), were retrospectively included in this study. The final observation was conducted in October 2020. The exclusion criteria were as follows: death within 1 year after radical treatment of HCC and achievement of SVR upon receiving interferon (IFN) and DAA therapy before HCC treatment or after treatment of HCC recurrence. Patients who did not wish to be treated with DAA because of high age, financial problems, inactivity of hepatitis, or concerns about adverse effects were included in the untreated group. All patients of the non-DAA-treated group were confirmed positive for HCV-RNA.
Seventy patients started receiving DAA within 1 year after HCC treatment and achieved SVR and were classified into the DAA-treated group, and 120 cases were classified into the non-DAA-treated group. The DAA-treated and non-DAA-treated groups, following propensity score matching (PSM) to reduce confounding, were compared on the basis of recurrence rates and re-recurrence rates (Supplementary Figure S1), and factors involved in recurrence and re-recurrence were analyzed.
The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Kurume University (approval number: 14178). Informed consent was obtained from the patients through a specific from on the website. Patients who denied consent were excluded.
2.2. Diagnosis, Treatment, and Assessment of Treatment Effect in HCC and Follow-Up Schedule
HCC was diagnosed in accordance with standard guideline [21]. Whether HCC was within BCLC stage A was determined using liver tumor biopsy and/or at least two of the following imaging modalities: dynamic computed tomography, magnetic resonance imaging, and contrast-enhanced ultrasonography. Radical treatment of HCC was done by hepatectomy, or radiofrequency ablation conducted percutaneously, laparoscopically, or by laparotomy. The treatment effect was determined 1 to 3 months after treatment by conducting dynamic computed tomography or magnetic resonance imaging and using the modified response evaluation criteria in solid tumors guideline [22]. Post-HCC treatment follow-up consisted of abdominal ultrasonography and laboratory tests, including tumor markers, every 2–4 months and dynamic computed tomography or magnetic resonance imaging every 6 months.
2.3. Treatment Using DAA
DAA treatment was initiated within 1 year of HCC therapy. Dynamic computed tomography or magnetic resonance imaging was performed to confirm the absence of HCC recurrence within 3 months before the initiation of DAA treatment. DAA treatment was administered for 8–24 weeks using medication regimens selected according to the genotype: daclatasvir and asunaprevir, sofosbuvir and ribavirin, ledipasvir/sofosbuvir, and lecaprevir/pibrentasvir. SVR was defined as the absence of detectable HCV RNA at 24 weeks after the end of treatment.
2.4. Collection of Clinical and Laboratory Data
The following data were collected: age, sex, hepatitis B surface antigen, alcohol consumption history, presence or absence of diabetes mellitus, clinical data (including history of prior interferon (IFN) therapy), platelet count, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), tumor diameter, and the number of tumors at diagnosis of HCC. Child–Pugh (CP) class, FIB-4 index, and modified albumin–bilirubin (mALBI) score [23,24] were calculated from these data.
2.5. PSM Analysis
To reduce confounders, a PSM analysis was used. Propensity scores were estimated using logistic regression models in which the following were used as covariates: age; sex; presence of hepatitis B surface antigen; alcohol consumption history; presence or absence of diabetes; history of IFN therapy; platelet count; albumin; AST, ALT, AFP, and DCP levels at the time of HCC diagnosis; tumor diameter; number of tumors; CP class; FIB-4 index; and modified ALBI score. The cut-off values for age; platelet count; and AST, ALT, and albumin levels were determined based on receiver operating characteristics, and reference values were used as cut-off values for AFP and DCP levels. Fifty-six pairs of patients were selected using the 1:1 nearest neighbor matching algorithm with an optimal caliper of 0.2, without replacement.
2.6. Statistical Analysis
The number of cases or the median value (range) is mentioned in the data. All statistical analyses were performed using JMP Pro version 15 (SAS Institute Inc., Cary, NC, USA). To compare factors between the two groups before and after PSM, the chi-square test was used for categorical variables and the Mann–Whitney U test (non-normal distribution data) and Student’s t-test (normal distribution data) were used for continuous variables. Statistical significance was set at p < 0.05. The recurrence and second recurrence rates were determined using the Kaplan–Meier method, and the differences between the two groups were analyzed using the log-rank test. The factors contributing to recurrence and second recurrence were examined using the Cox proportional hazards model.
3. Results
3.1. Background Factors
Table 1 shows the clinical characteristics of the DAA-treated and untreated groups. No significant differences were found in terms of sex, alcohol consumption, presence or absence of diabetes, hepatitis B surface antigen positivity, tumor diameter, CP class indicating liver cirrhosis or liver fibrosis, FIB-4 index, and platelet count. Age was higher (p = 0.0002) and ALT levels (p = 0.0114) were lower in the non-DAA-treated group, and significantly more patients had a history of IFN therapy in the DAA-treated group (p = 0.0156). The serum AFP levels showed no significant difference. Serum DCP levels were significantly higher in the non-DAA-treated group (p = 0.0156); however, the median levels were below the reference values in both groups. There was no significant difference in the observation period from the time of HCC treatment between the two groups.

Table 1.
Patient characteristics before propensity score matching.
As indicated in Table 2, the comparison of 112 cases after PSM showed no significant differences in DCP and ALT levels which had shown a significant difference before PSM. The non-DAA-treated group had significantly more patients who were older and/or untreated with IFN. No significant differences were found in the duration of the observation.

Table 2.
Patient characteristics after propensity score matching.
3.2. Comparison of Recurrence Rates in the DAA-Treated Group and Non-DAA-Treated Group after PSM
The overall recurrence rates at 1, 2, and 3 years were 12.6%, 35.2%, and 51.6% (13.7/100 person-years at risk), respectively (Figure 1a). In the DAA-treated group, recurrence was found in 29 cases with a median observation period of 57.8 months, whereas in the non-DAA-treated group, recurrence was found in 38 cases with a median period of 43.3 months. The 1-year, 2-year, 3-year, 4-year, and 5-year recurrence rates were 3.6%, 27.2%, 42.1%, 46.2%, and 49.2% (11.5/100 person-years at risk), respectively, in the DAA-treated group, which were significantly lower than the 21.7%, 42.9%, 61.9%, 75.5%, and 75.5% (16.1/100 person-years at risk) in the non-DAA-treated group (p = 0.0026; Figure 1b). Recurrence was observed in 67 of 112 patients, and among these, radical treatment was performed in 40 patients. In the 85 cases including both recurrence-free cases and cases in which curative treatment was performed after recurrence, second recurrence was found in 11 cases with a median observation period of 57.8 months in the DAA-treated group, whereas recurrence was found in 16 cases with the median period of 46.7 months in the non-DAA-treated group. The rates of overall second recurrences at 1, 2, and 3 years were 3.5%, 12.2%, and 16.5% (7.3/100 person-years at risk), namely 0%, 2.2%, and 2.2% (5.4/100 person-years at risk) for the 45 cases in the DAA-treated group and 7.5%, 23.7%, and 33.9% (9.5/100 person-years at risk) for the 40 cases in the non-DAA-treated group, respectively, showing that the rates of second recurrences were significantly lower in the DAA-treated group (p = 0.0128; Figure 2a,b).
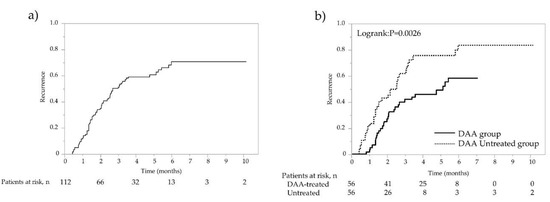
Figure 1.
Recurrence rate after propensity score matching (PSM) analysis. (a) Overall recurrence rate of patients after curative treatment for HCC. (b) Recurrence rates of patients with and without DAA treatment following HCC curative treatment. Solid line represents DAA therapy group; dotted line represents non-DAA-treated group.
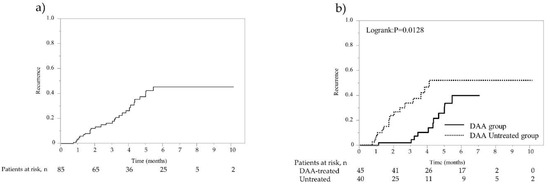
Figure 2.
Second recurrence rate after propensity score matching (PSM) analysis. (a) Overall second recurrence rate of 85 patients with curative treatment for initial and first recurrent HCC. (b) Second recurrence rates of 85 patients with curative treatment for initial and first recurrent HCC. Solid line represents DAA therapy group; dotted line represents non-DAA-treated group.
The results were similar to those of the comparison of recurrence rates in the DAA-treated and non-DAA-treated groups before PSM (Supplementary Figure S2).
3.3. Factors Contributing to Recurrence-Free and Second-Recurrence-Free Survival after PSM
Univariate analysis of factors involved in recurrence-free survival in 112 cases after PSM showed that the following were significant: treatment with DAAs (p = 0.0032), presence of a single tumor (p = 0.0030), and AFP levels of 10 ng/mL or lower (p = 0.0304). Multivariate analysis showed that treatment with DAAs (p = 0.0034) and the presence of a single tumor (p = 0.0344) were significant factors (Table 3). A significant factor involved in the second-recurrence-free survival in 85 cases was only DAAs (p = 0.0394) in multivariate analysis (Table 4).

Table 3.
Univariate and multivariate analyses of HCC recurrence-free survival after PSM.

Table 4.
Univariate and multivariate analyses of 2nd HCC recurrence-free survival after PSM.
3.4. Factors Contributing to Recurrence-Free and Second-Recurrence-Free Survival in the Daa-Treated Group after PSM
For the 56 patients in the DAA-treated group, no significant factors contributing to recurrence-free survival were found in either univariate or multivariate analysis. The presence of a single tumor tended to contribute to recurrence in multivariate analysis (p = 0.0731). In contrast, the only significant factor contributing to second recurrence in both univariate (p = 0.0051) and multivariate analyses (p = 0.0016)) was the presence of a single tumor (Table 5, Supplementary Figure S3).

Table 5.
Univariate and multivariate analyses of 1st and 2nd HCC recurrence-free survival in DAA-treated group after PSM.
4. Discussion
The purpose of this study was to examine the effect of DAA therapy on tumor recurrence after radical treatment of BCLC stage A HCC. Our findings showed that DAA therapy after radical treatment of HCC suppressed both recurrence and second recurrence. The suppressive effect on recurrence was particularly prominent in patients with a single tumor.
With the former IFN therapy, HCC development was suppressed when SVR was achieved [25,26]. Furthermore, after radical treatment of HCC, tumor recurrence is suppressed by achieving SVR with IFN or long-term administration of low doses of IFN without SVR [27,28,29,30]. In contrast, although a number of studies have reported that achievement of SVR upon receiving DAA therapy inhibited the development of HCC [5,6,7], it remains unclear whether using DAA after HCC treatment suppresses recurrence. Reig et al. previously reported that, in some cases, the use of DAAs after HCC treatment was found to cause early recurrence [11]. Singal et al. refuted the aforementioned by stating in a retrospective study of 793 HCC patients that a comparison between the DAA-treated group and the untreated group showed no difference in recurrence rates after HCC treatment [31]. Kinoshita et al. reported no significant difference in the HCC recurrence rate between IFN and DAA [32]. In a prospective study, Cabibbo et al. reported no significant difference in recurrence rates between DAA-treated patients and untreated controls [8]). Several studies have reported that recurrence rates were reduced by the use of DAAs after treatment for HCC [12,13,14,33,34]. However, the short median observation period has been a major issue in reports published to date. Other reasons are that the studies included cases in which DAA therapy was carried out after treatment of a recurrent HCC rather than after treatment of initial HCC, the cases examined in those studies included patients who had received treatment other than radical treatment, and the duration of the period from the radical treatment of HCC until the administration of DAA therapy was unclear.
Periodic observation after radical treatment for HCC was performed using almost the same method in all patients. The median observation period after HCC treatment was 56 months, which was longer than that in previous reports. In addition, confounding factors were reduced using PSM analysis. The results showed that the recurrence rate was reduced by DAA therapy after radical treatment of HCC. It was suggested that the decrease in HCC recurrence rate was associated with suppression of inflammation and fibrosis by DAA therapy, similar to the reduced HCC incidence after IFN and DAA therapy [35,36].
We further confirmed that DAA therapy prevented second recurrences. Although suppression of first recurrences by long-term low-dose IFN treatment without achievement of SVR after radical treatment of HCC is unclear, suppression of second and third recurrences has been reported [28,37,38]. No previous report has stated that DAA therapy after radical cure of HCC suppressed the first and second recurrences, and the results described above suggested that similar to IFN therapy, the suppressive effect of DAA treatment on HCC was persistent. The findings of our present study further clarify the need for DAA treatment after the radical cure of HCC. In the DAA-treated group, the presence of the first cancer as a single tumor is a significant factor contributing to recurrence-free and secondary-recurrence-free survival rates. The results may be meaningful in determining the intervals between and duration of surveillance.
Alcohol history, liver fibrosis, and diabetes have been reported as factors contributing to hepatic carcinogenesis after SVR [39]. However, in the present study, alcohol history, diabetes, platelet count, and ALBI grade (an indicator of liver fibrosis) did not contribute to recurrence or second recurrence in patients with HCC after SVR. This may be related to the shorter follow-up period after SVR in the present study compared to that of the previous one examining the risk of HCC after DAA treatment.
In the present study, the observation period was three years longer in the non-DAA-treated group than in the DAA-treated group. Although the same observation period is desirable, we added the 3-year period of 2010–2012 to the observation period of the untreated group to increase the number of patients for statistical robustness to minimize potential lead time bias. It seemed valid to prolong the observation period for the following reasons: during these three years, the quality of both the diagnostic modalities, including MRI and contrast-enhanced ultrasonography, and the radical treatment, including radiofrequency ablation therapy, were considered unchanged. Furthermore, there were no significant differences in patient backgrounds between the 70 patients in 2010–2012 and the 120 patients in 2013–2017.
This study had several limitations. First, it was a retrospective study with a small sample size. A prospective randomized study or cohort study of treatment would be desirable, but given the reported improvement in hepatic reserve and prognosis by DAA treatment, it would be difficult to accumulate untreated cases in the future. The second limitation is the inadequate investigation of risk factors for liver cancer other than HCV. In addition to diabetes and alcohol drinking, which were examined in this study, obesity and diabetes medications such as insulin should be considered.
5. Conclusions
In conclusion, first and second recurrences were suppressed by DAA therapy after radical treatment for HCC in patients with HCV-related HCC within BCLC stage A, suggesting that the inhibitory effect of DAA therapy on HCC recurrence was sustained.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14092295/s1, Figure S1: Study design; Figure S2: First recurrence rate after propensity score matching (PSM) analysis.; Figure S3: Recurrence and second recurrence rates with and without single tumor following HCC.
Author Contributions
R.K. (Ryoko Kuromatsu) participated in study conception and design, data acquisition and interpretation, analysis, and drafting of the manuscript. T.S., S.S., H.I., T.N., T.A.-H., R.K. (Reiichiro Kuwahara), Y.Z., H.T., and M.S. participated in data acquisition. S.O., Y.N., N.K., and M.N. participated in data acquisition, analysis, and interpretation of data. T.I., H.K., and T.T. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Kurume University School of Medicine (protocol code No. 14178).
Informed Consent Statement
Informed consent was obtained in the form of opt-out on the web-site “Review Status by Medical Ethics Committee of Kurume University, Available online: http://www.med.kurume-u.ac.jp/med/joint/rinri/sinsajokyo2014.html (accessed on 28 February 2021)”. Those who rejected were excluded.
Data Availability Statement
Data that support the findings of this study are available from the author, R.K. (Ryoko Kuromatsu), on reasonable request.
Acknowledgments
The authors would like to thank the members of the Kurume Liver Cancer Study Group of Japan, namely Chikugo City Hospital, Kumamoto Central Hospital, Kurume Central Hospital, Kurume University Hospital, Kurume University Medical Center, Saga Central Hospital, Yame General Hospital, and Yanagawa Hospital, for their valuable support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Charlton, M.; Everson, G.T.; Flamm, S.L.; Kumar, P.; Landis, C.; Brown, R.S., Jr.; Fried, M.W.; Terrault, N.A.; O’Leary, J.G.; Vargas, H.E.; et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015, 149, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itokawa, N.; Atsukawa, M.; Tsubota, A.; Ikegami, T.; Shimada, N.; Kato, K.; Abe, H.; Okubo, T.; Arai, T.; Iwashita, A.N.; et al. Efficacy of direct-acting antiviral treatment in patients with compensated liver cirrhosis: A multicenter study. Hepatol. Res. 2019, 49, 125–135. [Google Scholar] [CrossRef]
- Knop, V.; Mauss, S.; Goeser, T.; Geier, A.; Zimmermann, T.; Herzer, K.; Postel, N.; Friedrich-Rust, M.; Hofmann, W.P. Dynamics of liver stiffness by transient elastography in patients with chronic hepatitis C virus infection receiving direct-acting antiviral therapy-results from the German Hepatitis C-Registry. J. Viral Hepat. 2020, 27, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Backus, L.I.; Belperio, P.S.; Shahoumian, T.A.; Mole, L.A. Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology 2018, 68, 827–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, G.N.; Beste, L.A.; Green, P.K.; Singal, A.G.; Tapper, E.B.; Waljee, A.K. Increased risk for hepatocellular carcinoma persists up to 10 Years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 2019, 157, 1264–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, T.; Koga, H.; Nakano, M.; Hashimoto, S.; Yatsuhashi, H.; Higuchi, N.; Nakamuta, M.; Oeda, S.; Eguchi, Y.; Shakado, S.; et al. Direct-acting antiviral agents do not increase the incidence of hepatocellular carcinoma development: A prospective, multicenter study. Hepatol. Int. 2019, 13, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cabibbo, G.; Celsa, C.; Calvaruso, V.; Petta, S.; Cacciola, I.; Cannavo, M.R.; Madonia, S.; Rossi, M.; Magro, B.; Rini, F.; et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J. Hepatol. 2019, 71, 265–273. [Google Scholar] [CrossRef]
- Huang, A.C.; Mehta, N.; Dodge, J.L.; Yao, F.Y.; Terrault, N.A. Direct-acting antivivals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology 2018, 68, 449–461. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, Y.S.; Chang, C.W.; Chang, C.W.; Wang, T.E.; Wang, H.Y.; Chen, M.J. Impact of direct-acting antiviral therapy for hepatitis C-related hepatocellular carcinoma. PLoS ONE 2020, 15, e0233212. [Google Scholar] [CrossRef]
- Reig, M.; Marino, Z.; Perello, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Kawamura, Y.; Kobayashi, M.; Kominami, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Akuta, N.; Saitoh, S.; Suzuki, F.; et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig. Dis. Sci. 2017, 62, 2932–2942. [Google Scholar] [CrossRef]
- Virlogeux, V.; Pradat, P.; Hartig-Lavie, K.; Bailly, F.; Maynard, M.; Ouziel, G.; Poinsot, D.; Lebossé, F.; Ecochard, M.; Radenne, S.; et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017, 37, 1122–1127. [Google Scholar] [CrossRef]
- Ochi, H.; Hiraoka, A.; Hirooka, M.; Koizumi, Y.; Amano, M.; Azemoto, N.; Watanabe, T.; Yoshida, O.; Tokumoto, Y.; Mashiba, T.; et al. Direct-acting antivirals improve survival and recurrence rates after treatment of hepatocellular carcinoma within the Milan criteria. J. Gastroenterol. 2021, 56, 90–100. [Google Scholar] [CrossRef]
- Tsuda, H.; Oda, T.; Sakamoto, M.; Hirohashi, S. Different pattern of chromosomal allele loss in multiple hepatocellular carcinomas as evidence of their multifocal origin. Cancer Res. 1992, 52, 1504–1509. [Google Scholar] [PubMed]
- Tokushige KHashimoto, E.; Kodama, K. Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S4), 88–92. [Google Scholar] [CrossRef] [Green Version]
- Hino-Arinaga, T.; Ide, T.; Kuromatsu, R.; Miyajima, I.; Ogata, K.; Kuwahara, R.; Hisamochi, A.; Torimura, T.; Sata, M. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. J. Gastroenterol. 2012, 47, 569–576. [Google Scholar] [CrossRef]
- Oikawa, T.; Ojima, H.; Yamasaki, S.; Takayama, T.; Hirohashi, S.; Sakamoto, M. Multistep and multicentric development of hepatocellular carcinoma: Histological analysis of 980 resected nodules. J. Hepatol. 2005, 42, 225–229. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Fujii, H.; Matsuda, M.; Kono, H. Multicentric occurrence of hepatocellular carcinoma: Diagnosis and clinical significance. J. Hepatobiliary Pancreat. Surg. 2001, 8, 435–440. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Makuuchi, M.; Kokudo, N.; Arii, S.; Futagawa, S.; Kaneko, S.; Kawasaki, S.; Matsuyama, Y.; Okazaki, M.; Okita, K.; Omata, M.; et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol. Res. 2008, 38, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Varidation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Ikeda, K.; Saitoh, S.; Arase, Y.; Chayama, K.; Suzuki, Y.; Kobayashi, M.; Tsubota, A.; Nakamura, I.; Murashima, N.; Kumada, H.; et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology 1999, 29, 1124–1130. [Google Scholar] [CrossRef]
- Bruno, S.; Stroffolini, T.; Colombo, M.; Bollani, S.; Benvegnu, L.; Mazzella, G.; Ascione, A.; Santantonio, T.; Piccinino, F.; Andreone, P.; et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: A retrospective study. Hepatology 2007, 45, 579–587. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Romito, R.; Chiavo, M.; Mariani, L.; Camerini, T.; Bhoori, S.; Capussotti, L.; Calise, F.; Pellicci, R.; Belli, G.; et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006, 44, 1543–1554. [Google Scholar] [CrossRef]
- Shiratori, Y.; Shiina, S.; Teratani, T.; Imamura, M.; Obi, S.; Sato, S.; Koike, Y.; Yoshida, H.; Omata, M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann. Intern. Med. 2003, 138, 299–306. [Google Scholar] [CrossRef]
- Shen, Y.C.; Hsu, C.; Chen, L.T.; Cheng, C.C.; Hu, F.C.; Cheng, A.L. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): A meta-regression approach. J. Hepatol. 2010, 52, 889–894. [Google Scholar] [CrossRef]
- Sakae, M.; Kubo, S.; Takemura, S.; Sakata, C.; Uenishi, T.; Kodai, S.; Shinkawa, H.; Urata, Y.; Ohata, K.; Kaneda, K.; et al. Effect of interferon therapy on first and second recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. Hepatol. Res. 2012, 42, 564–573. [Google Scholar] [CrossRef]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology 2019, 156, 1683–1692. [Google Scholar] [CrossRef]
- Kinoshita, M.N.; Minami, T.; Tateishi, R.; Wake, T.; Nakagomi, R.; Fujiwara, N.; Sato, M.; Uchino, K.; Enooku, K.; Nakagawa, H.; et al. Impact of direct-acting antivirals on early recurrence of HCV-related HCC: Comparison with interferon-based therapy. J. Hepatol. 2018, 70, 78–86. [Google Scholar] [CrossRef]
- ANRS Collaborative Study Group on Hepatocellular Carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J. Hepatol. 2016, 65, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.Y.; Choi, K.; Kramer, J.R.; Yu, X.; Cao, Y.; El-Serag, H.B.; Kanwal, F. Risk of hepatocellular cancer recurrence in hepatitis C virus+ patients treated with direct-acting antiviral agents. Dig. Dis. Sci. 2019, 64, 3328–3336. [Google Scholar] [CrossRef]
- Nagata, H.; Nakagawa, M.; Asahina, Y.; Sato, A.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Otani, S.; Kawai-Kitahata, F.; et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017, 67, 933–939. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Oze, T.; Takehara, T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon based antiviral therapy. Hepatol. Res. 2015, 45, 152–161. [Google Scholar] [CrossRef]
- Ikeda, K.; Arase, Y.; Saitoh, S.; Kobayashi, M.; Suzuki, Y.; Suzuki, F.; Tsubota, A.; Chayama, K.; Murashima, N.; Kumada, H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology 2000, 32, 228–232. [Google Scholar] [CrossRef]
- Kudo, M.; Sakaguchi, Y.; Chung, H.; Hatanaka, K.; Hagiwara, S.; Ishikawa, E.; Takahashi, S.; Kitai, S.; Inoue, T.; Minami, Y.; et al. Long-term interferon maintenance therapy improves survival in patients with HCV-related hepatocellular carcinoma after curative radiofrequency ablation. A matched case-control study. Oncology 2007, 72 (Suppl. S1), 132–138. [Google Scholar] [CrossRef]
- Váncsa, S.; Németh, D.; Hegyi, P.; Szakács, Z.; Farkas, Á.; Kiss, S.; Hegyi, P.J.; Kanjo, A.; Sarlós, P.; Erőss, B.; et al. Diabetes mellitus increases the risk of hepatocellular carcinoma after direct-acting antiviral therapy: Systemic review and meta-analysis. Front. Med. 2021, 8, 744512. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).