Glioma Stem Cells in Pediatric High-Grade Gliomas: From Current Knowledge to Future Perspectives
Simple Summary
Abstract
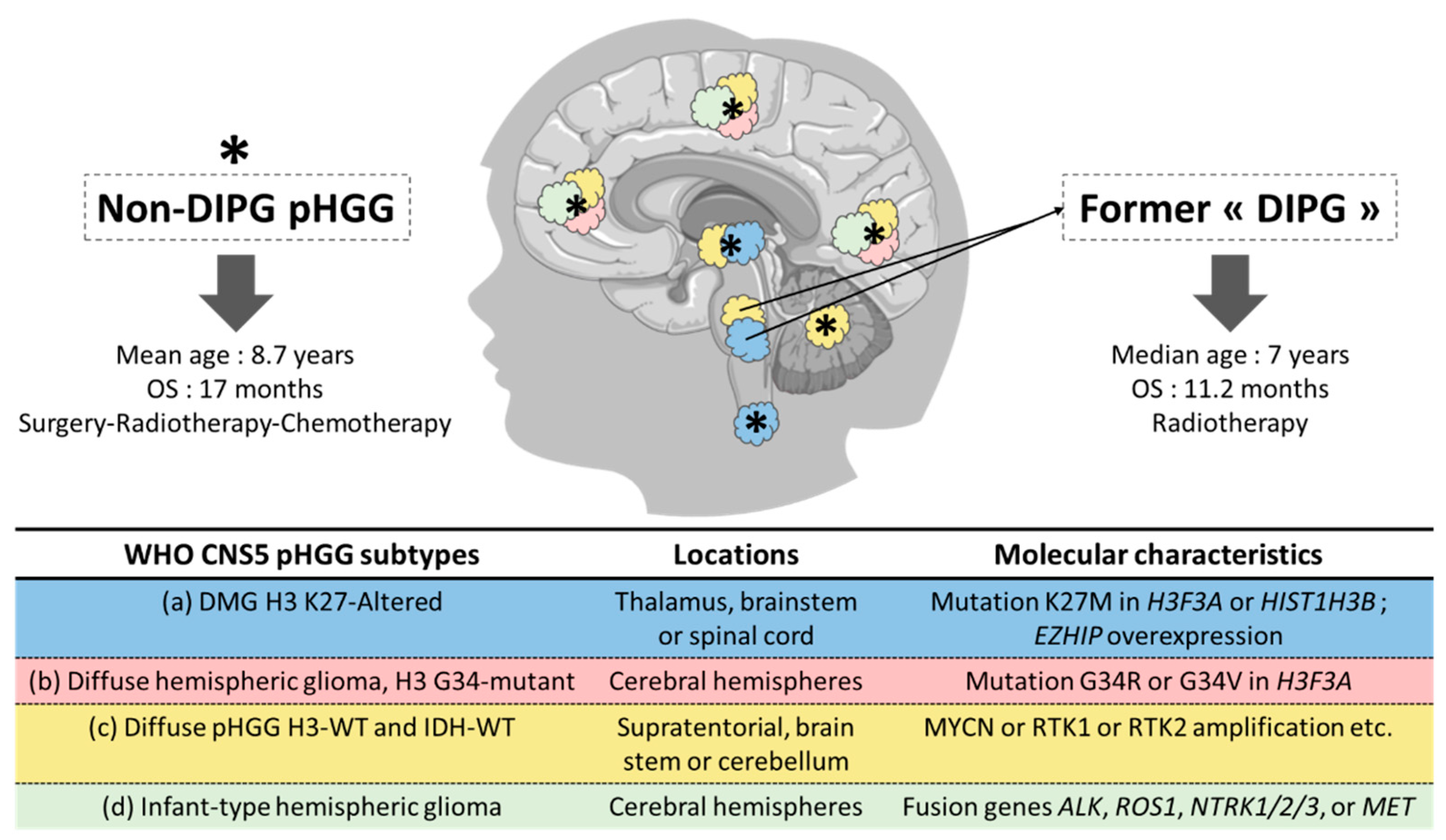
1. Pediatric High-Grade Gliomas: From Histologic to Histomolecular Classification
2. From Stem Cells to Glioma Stem Cells
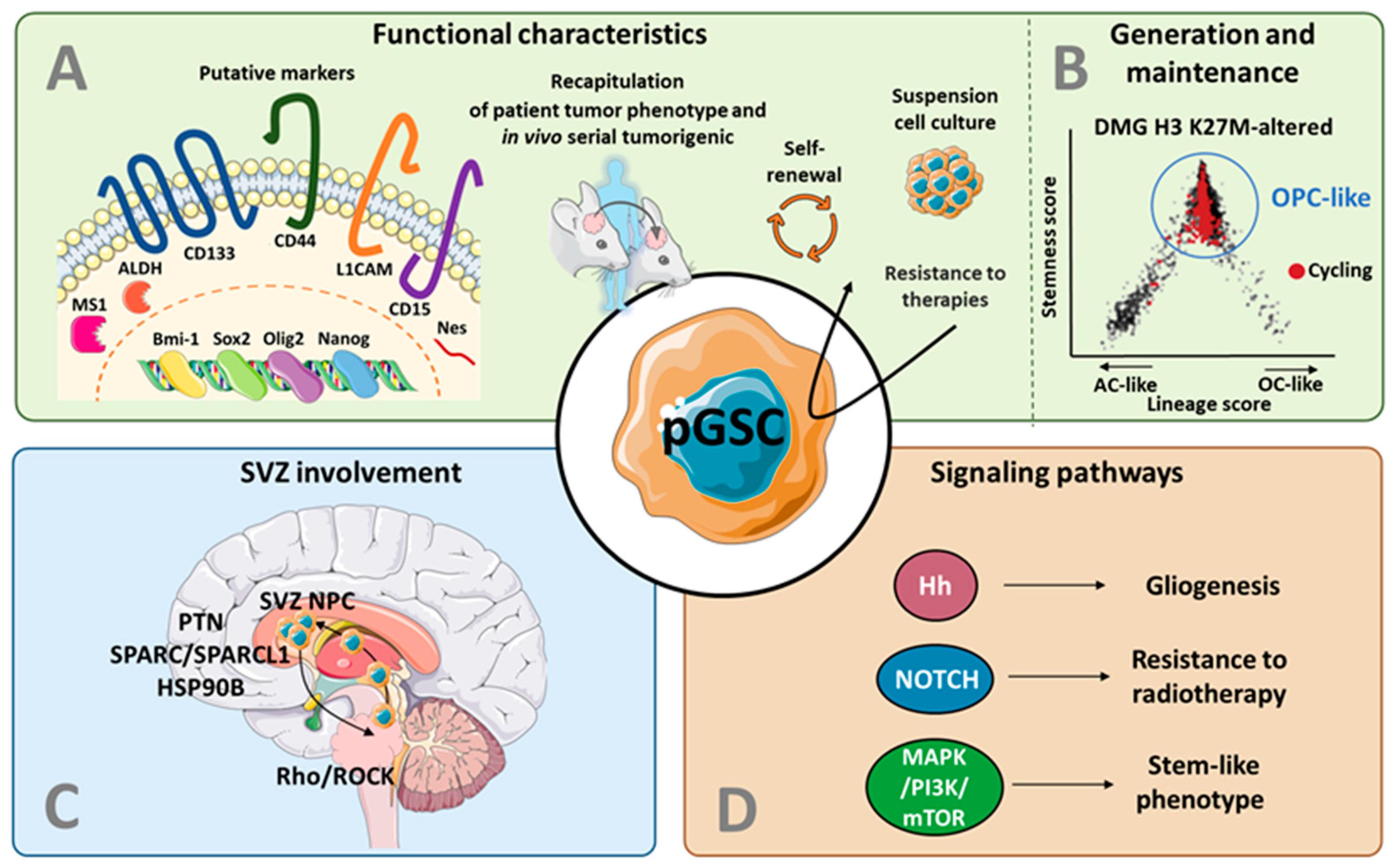
3. From Adult to Pediatric Glioma Stem Cells
3.1. Putative Markers for GSCs in pHGG
3.1.1. CD133
3.1.2. Bmi-1
3.1.3. ALDH
3.1.4. L1CAM
3.1.5. Mushashi-1
3.1.6. Nestin
3.1.7. Sox2
3.1.8. Olig2
3.1.9. Nanog
3.1.10. CD44
3.1.11. CD15
3.2. Recapitulation of Patient Tumor Features
3.3. Models for GSC Generation and Maintenance in pHGG
4. The Subventricular Zone as a Key Actor in pHGG:
4.1. The Cell of Origin in pHGG
4.2. The Potential Role of GSCs and SVZ in pHGG Recurrence
4.3. The Prognostic Impact of an SVZ Involvement in pHGG
5. From the Concept of Pediatric GSC to Targeted Therapies
5.1. Targeting of the GSC Signaling Pathways and Metabolism
5.2. Oncolytic Virotherapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, IV1–IV96. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro-Oncology 2015, 16 (Suppl. 10), x1–x35. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Sturm, D.; Witt, H.; Hovestadt, V.; Khuong-Quang, D.A.; Jones, D.T.W.; Konermann, C.; Pfaff, E.; Tönjes, M.; Sill, M.; Bender, S.; et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell 2012, 22, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.; Tang, Y.; Lindroth, A.M.; Hovestadt, V.; Jones, D.T.W.; Kool, M.; Zapatka, M.; Northcott, P.A.; Sturm, D.; Wang, W.; et al. Reduced H3K27me3 and DNA Hypomethylation Are Major Drivers of Gene Expression in K27M Mutant Pediatric High-Grade Gliomas. Cancer Cell 2013, 24, 660–672. [Google Scholar] [CrossRef]
- Lewis, P.W.; Müller, M.M.; Koletsky, M.S.; Cordero, F.; Lin, S.; Banaszynski, L.A.; Garcia, B.A.; Muir, T.W.; Becher, O.J.; Allis, C.D. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science 2013, 340, 857–861. [Google Scholar] [CrossRef]
- Chan, K.M.; Fang, D.; Gan, H.; Hashizume, R.; Yu, C.; Schroeder, M.; Gupta, N.; Mueller, S.; David James, C.; Jenkins, R.; et al. The Histone H3.3K27M Mutation in Pediatric Glioma Reprograms H3K27 Methylation and Gene Expression. Genes Dev. 2013, 27, 985. [Google Scholar] [CrossRef] [PubMed]
- Herz, H.M.; Morgan, M.; Gao, X.; Jackson, J.; Rickels, R.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Eissenberg, J.C.; Shilatifard, A. Histone H3 Lysine-to-Methionine Mutants as a Paradigm to Study Chromatin Signaling. Science 2014, 345, 1065–1070. [Google Scholar] [CrossRef]
- Lee, J.; Solomon, D.A.; Tihan, T. The Role of Histone Modifications and Telomere Alterations in the Pathogenesis of Diffuse Gliomas in Adults and Children. J. Neurooncol. 2017, 132, 1–11. [Google Scholar] [CrossRef]
- Castel, D.; Kergrohen, T.; Tauziède-Espariat, A.; Mackay, A.; Ghermaoui, S.; Lechapt, E.; Pfister, S.M.; Kramm, C.M.; Boddaert, N.; Blauwblomme, T.; et al. Histone H3 Wild-Type DIPG/DMG Overexpressing EZHIP Extend the Spectrum Diffuse Midline Gliomas with PRC2 Inhibition beyond H3-K27M Mutation. Acta Neuropathol. 2020, 139, 1109–1113. [Google Scholar] [CrossRef]
- Lowe, B.R.; Maxham, L.A.; Hamey, J.J.; Wilkins, M.R.; Partridge, J.F. Histone H3 Mutations: An Updated View of Their Role in Chromatin Deregulation and Cancer. Cancers 2019, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Schrimpf, D.; Ryzhova, M.; Sturm, D.; Chavez, L.; Hovestadt, V.; Sharma, T.; Habel, A.; Burford, A.; Jones, C.; et al. H3-/IDH-Wild Type Pediatric Glioblastoma Is Comprised of Molecularly and Prognostically Distinct Subtypes with Associated Oncogenic Drivers. Acta Neuropathol. 2017, 134, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Mackay, A.; Ismer, B.; Pickles, J.C.; Tatevossian, R.G.; Newman, S.; Bale, T.A.; Stoler, I.; Izquierdo, E.; Temelso, S.; et al. Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov. 2020, 10, 942–963. [Google Scholar] [CrossRef]
- van Zanten, S.E.M.V.; Jansen, M.H.; Sanchez Aliaga, E.; van Vuurden, D.G.; Vandertop, W.P.; Kaspers, G.J. A Twenty-Year Review of Diagnosing and Treating Children with Diffuse Intrinsic Pontine Glioma in The Netherlands. Expert Rev. Anticancer Ther. 2015, 15, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Odia, Y.; Allen, J.E.; Tarapore, R.; Khatib, Z.; Niazi, T.N.; Daghistani, D.; Schalop, L.; Chi, A.S.; Oster, W.; et al. First Clinical Experience with DRD2/3 Antagonist ONC201 in H3 K27M–Mutant Pediatric Diffuse Intrinsic Pontine Glioma: A Case Report. J. Neurosurg. Pediatrics 2019, 23, 719–725. [Google Scholar] [CrossRef]
- Aziz-Bose, R.; Monje, M. Diffuse Intrinsic Pontine Glioma: Molecular Landscape and Emerging Therapeutic Targets. Curr. Opin. Oncol. 2019, 31, 522–530. [Google Scholar] [CrossRef]
- Cooney, T.; Lane, A.; Bartels, U.; Bouffet, E.; Goldman, S.; Leary, S.E.S.; Foreman, N.K.; Packer, R.J.; Broniscer, A.; Minturn, J.E.; et al. Contemporary Survival Endpoints: An International Diffuse Intrinsic Pontine Glioma Registry Study. Neuro-Oncology 2017, 19, 1279–1280. [Google Scholar] [CrossRef]
- da Costa, M.D.S.; Camargo, N.C.; Dastoli, P.A.; Nicácio, J.M.; Benevides Silva, F.A.; Sucharski Figueiredo, M.L.; Chen, M.J.; Cappellano, A.M.; da Silva, N.S.; Cavalheiro, S. High-Grade Gliomas in Children and Adolescents: Is There a Role for Reoperation? J. Neurosurg. Pediatr. 2020, 27, 160–169. [Google Scholar] [CrossRef]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.J.; Gupta, N.; Hawkins, C.; et al. Pediatric High-Grade Glioma: Biologically and Clinically in Need of New Thinking. Neuro-Oncology 2017, 19, 153–161. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Doulatov, S.; Notta, F.; Laurenti, E.; Dick, J.E. Hematopoiesis: A Human Perspective. Cell Stem Cell 2012, 10, 120–136. [Google Scholar] [CrossRef]
- Clevers, H. The Intestinal Crypt, a Prototype Stem Cell Compartment. Cell 2013, 154, 274. [Google Scholar] [CrossRef]
- Temple, S. The Development of Neural Stem Cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Furth, J.; Kahn, M.C. The Transmission of Leukemia of Mice with a Single Cell. Am. J. Cancer 1937, 31, 276–282. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A Cell Initiating Human Acute Myeloid Leukaemia after Transplantation into SCID Mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human Acute Myeloid Leukemia Is Organized as a Hierarchy That Originates from a Primitive Hematopoietic Cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Clevers, H. The Cancer Stem Cell: Premises, Promises and Challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; de Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and Characterization of Tumorigenic, Stem-like Neural Precursors from Human Glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Curtin, J.; Xiong, Y.; Liu, G.; Waschsmann-Hogiu, S.; Farkas, D.L.; Black, K.L.; Yu, J.S. Isolation of Cancer Stem Cells from Adult Glioblastoma Multiforme. Oncogene 2004, 23, 9392–9400. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; de Maria, R. Identification and Expansion of Human Colon-Cancer-Initiating Cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Rahmathulla, G.; Toms, S.A.; Weil, R.J. The Molecular Biology of Brain Metastasis. J. Oncol. 2012, 2012, 723541. [Google Scholar] [CrossRef]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective Identification of Tumorigenic Prostate Cancer Stem Cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic Characterization of Human Colorectal Cancer Stem Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A Human Colon Cancer Cell Capable of Initiating Tumour Growth in Immunodeficient Mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of Pancreatic Cancer Stem Cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef]
- Ignatova, T.N.; Kukekov, V.G.; Laywell, E.D.; Suslov, O.N.; Vrionis, F.D.; Steindler, D.A. Human Cortical Glial Tumors Contain Neural Stem-like Cells Expressing Astroglial and Neuronal Markers in vitro. Glia 2002, 39, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous Stem Cells Can Arise from Pediatric Brain Tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; de Sousa e Melo, F.; Richel, D.J.; Medema, J.P. The Developing Cancer Stem-Cell Model: Clinical Challenges and Opportunities. Lancet Oncol. 2012, 13, e83–e89. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma Stem Cells: Lessons from the Tumor Hierarchy in a Lethal Cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Ahmad, Z.; Jasnos, L.; Gil, V.; Howell, L.; Hallsworth, A.; Petrie, K.; Sawado, T.; Chesler, L. Molecular and In Vivo Characterization of Cancer-Propagating Cells Derived from MYCN-Dependent Medulloblastoma. PLoS ONE 2015, 10, e0119834. [Google Scholar] [CrossRef]
- Huang, G.H.; Xu, Q.F.; Cui, Y.H.; Li, N.; Bian, X.W.; Lv, S.Q. Medulloblastoma Stem Cells: Promising Targets in Medulloblastoma Therapy. Cancer Sci. 2016, 107, 583–589. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Poppiti, R.J. Medulloblastoma Cancer Stem Cells: Molecular Signatures and Therapeutic Targets. J. Clin. Pathol. 2020, 73, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Meco, D.; Servidei, T.; Lamorte, G.; Binda, E.; Arena, V.; Riccardi, R. Ependymoma Stem Cells Are Highly Sensitive to Temozolomide in Vitro and in Orthotopic Models. Neuro-Oncology 2014, 16, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Poppleton, H.; Fuller, C.; Su, X.; Liu, Y.; Jensen, P.; Magdaleno, S.; Dalton, J.; Calabrese, C.; Board, J.; et al. Radial Glia Cells Are Candidate Stem Cells of Ependymoma. Cancer Cell 2005, 8, 323–335. [Google Scholar] [CrossRef]
- Milde, T.; Kleber, S.; Korshunov, A.; Witt, H.; Hielscher, T.; Koch, P.; Kopp, H.G.; Jugold, M.; Deubzer, H.E.; Oehme, I.; et al. A Novel Human High-Risk Ependymoma Stem Cell Model Reveals the Differentiation-Inducing Potential of the Histone Deacetylase Inhibitor Vorinostat. Acta Neuropathol. 2011, 122, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Servidei, T.; Lucchetti, D.; Navarra, P.; Sgambato, A.; Riccardi, R.; Ruggiero, A. Cell-of-Origin and Genetic, Epigenetic, and Microenvironmental Factors Contribute to the Intra-Tumoral Heterogeneity of Pediatric Intracranial Ependymoma. Cancers 2021, 13, 6100. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.G.; Tirosh, I.; Hovestadt, V.; Shaw, M.L.; Escalante, L.E.; Mathewson, N.D.; Neftel, C.; Frank, N.; Pelton, K.; Hebert, C.M.; et al. Developmental and Oncogenic Programs in H3K27M Gliomas Dissected by Single-Cell RNA-Seq. Science 2018, 360, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer Stem Cells in Glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The Role of CD133 in Cancer: A Concise Review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct Isolation of Human Central Nervous System Stem Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef]
- Sun, Y.; Kong, W.; Falk, A.; Hu, J.; Zhou, L.; Pollard, S.; Smith, A. CD133 (Prominin) Negative Human Neural Stem Cells Are Clonogenic and Tripotent. PLoS ONE 2009, 4, e5498. [Google Scholar] [CrossRef]
- Shu, Q.; Wong, K.K.; Su, J.M.; Adesina, A.M.; Yu, L.T.; Tsang, Y.T.M.; Antalffy, B.C.; Baxter, P.; Perlaky, L.; Yang, J.; et al. Direct Orthotopic Transplantation of Fresh Surgical Specimen Preserves CD133+ Tumor Cells in Clinically Relevant Mouse Models of Medulloblastoma and Glioma. Stem Cells 2008, 26, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.A.; Lin, Q.; Mao, H.; Kogiso, M.; Zhao, X.; Liu, Z.; Huang, Y.; Voicu, H.; Gurusiddappa, S.; Su, J.M.; et al. Silencing BMI1 Eliminates Tumor Formation of Pediatric Glioma CD133+ Cells Not by Affecting Known Targets but by Down-Regulating a Novel Set of Core Genes. Acta Neuropathol. Commun. 2014, 2, 160. [Google Scholar] [CrossRef] [PubMed]
- Thirant, C.; Bessette, B.; Varlet, P.; Puget, S.; Cadusseau, J.; Tavares, S.d.R.; Studler, J.M.; Silvestre, D.C.; Susini, A.; Villa, C.; et al. Clinical Relevance of Tumor Cells with Stem-Like Properties in Pediatric Brain Tumors. PLoS ONE 2011, 6, e16375. [Google Scholar] [CrossRef]
- Kumar, S.S.; Sengupta, S.; Lee, K.; Hura, N.; Fuller, C.; DeWire, M.; Stevenson, C.B.; Fouladi, M.; Drissi, R. BMI-1 Is a Potential Therapeutic Target in Diffuse Intrinsic Pontine Glioma. Oncotarget 2017, 8, 62962–62975. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, R.K.; Ferris, S.F.; Apfelbaum, A.; Espinoza, C.; Mehta, R.K.; Monchamp, K.; Sirihorachai, V.R.; Bedi, K.; Ljungman, M.; Galban, S. Transcriptomic Analysis of Diffuse Intrinsic Pontine Glioma (DIPG) Identifies a Targetable ALDH-Positive Subset of Highly Tumorigenic Cancer Stem-like Cells. Mol. Cancer Res. 2021, 19, 223–239. [Google Scholar] [CrossRef]
- Chen, R.; Nishimura, M.C.; Bumbaca, S.M.; Kharbanda, S.; Forrest, W.F.; Kasman, I.M.; Greve, J.M.; Soriano, R.H.; Gilmour, L.L.; Rivers, C.S.; et al. A Hierarchy of Self-Renewing Tumor-Initiating Cell Types in Glioblastoma. Cancer Cell 2010, 17, 362–375. [Google Scholar] [CrossRef]
- Wang, J.; Sakariassen, P.; Tsinkalovsky, O.; Immervoll, H.; Bøe, S.O.; Svendsen, A.; Prestegarden, L.; Røsland, G.; Thorsen, F.; Stuhr, L.; et al. CD133 Negative Glioma Cells Form Tumors in Nude Rats and Give Rise to CD133 Positive Cells. Int. J. Cancer 2008, 122, 761–768. [Google Scholar] [CrossRef]
- Beier, D.; Hau, P.; Proescholdt, M.; Lohmeier, A.; Wischhusen, J.; Oefner, P.J.; Aigner, L.; Brawanski, A.; Bogdahn, U.; Beier, C.P. CD133(+) and CD133(−) Glioblastoma-Derived Cancer Stem Cells Show Differential Growth Characteristics and Molecular Profiles. Cancer Res. 2007, 67, 4010–4015. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Banerjee Mustafi, S.; Street, M.; Dey, A.; Dwivedi, S.K.D. Bmi-1: At the Crossroads of Physiological and Pathological Biology. Genes Dis. 2015, 2, 225–239. [Google Scholar] [CrossRef]
- Ballester, L.Y.; Wang, Z.; Shandilya, S.; Miettinen, M.; Burger, P.C.; Eberhart, C.G.; Rodriguez, F.J.; Raabe, E.; Nazarian, J.; Warren, K.; et al. Morphologic Characteristics and Immunohistochemical Profile of Diffuse Intrinsic Pontine Gliomas. Am. J. Surg. Pathol. 2013, 37, 1357–1364. [Google Scholar] [CrossRef]
- Kreso, A.; van Galen, P.; Pedley, N.M.; Lima-Fernandes, E.; Frelin, C.; Davis, T.; Cao, L.; Baiazitov, R.; Du, W.; Sydorenko, N.; et al. Self-Renewal as a Therapeutic Target in Human Colorectal Cancer. Nat. Med. 2014, 20, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 3904645. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in Human Cancer. Int. J. Cancer 2016, 138, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; Li, Z.; Sathornsumetee, S.; Wang, H.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. Targeting Cancer Stem Cells through L1CAM Suppresses Glioma Growth. Cancer Res. 2008, 68, 6043. [Google Scholar] [CrossRef]
- Glazer, R.I.; Wang, X.Y.; Yuan, H.; Yin, Y. Musashi1: A Stem Cell Marker No Longer in Search of a Function. Cell Cycle 2008, 7, 2635–2639. [Google Scholar] [CrossRef]
- Pötschke, R.; Haase, J.; Glaß, M.; Simmermacher, S.; Misiak, C.; Penalva, L.O.F.; Kühnöl, C.D.; Hüttelmaier, S. MSI1 Promotes the Expression of the GBM Stem Cell Marker CD44 by Impairing MiRNA-Dependent Degradation. Cancers 2020, 12, 3654. [Google Scholar] [CrossRef]
- Pötschke, R.; Gielen, G.; Pietsch, T.; Kramm, C.; Klusmann, J.H.; Hüttelmaier, S.; Kühnöl, C.D. Musashi1 Enhances Chemotherapy Resistance of Pediatric Glioblastoma Cells in vitro. Pediatric Res. 2020, 87, 669–676. [Google Scholar] [CrossRef]
- Bernal, A.; Arranz, L. Nestin-Expressing Progenitor Cells: Function, Identity and Therapeutic Implications. Cell. Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef]
- Qin, E.Y.; Cooper, D.D.; Abbott, K.L.; Vogel, H.; Jackson, P.K.; Correspondence, M.M. Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma. Cell 2017, 170, 845–849.e19. [Google Scholar] [CrossRef]
- Xu, C.; Liu, X.; Geng, Y.; Bai, Q.; Pan, C.; Sun, Y.; Chen, X.; Yu, H.; Wu, Y.; Zhang, P.; et al. Patient-Derived DIPG Cells Preserve Stem-like Characteristics and Generate Orthotopic Tumors. Oncotarget 2017, 8, 76644–76655. [Google Scholar] [CrossRef]
- Mamun, M.A.; Mannoor, K.; Cao, J.; Qadri, F.; Song, X. SOX2 in Cancer Stemness: Tumor Malignancy and Therapeutic Potentials. J. Mol. Cell Biol. 2020, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.; Hallou, C.; Dewari, P.; Pollard, S.; Pathania, M.; Bulstrode, H.; Rowitch, D. Characterising a SOX2+ OLIG2+ Glioma Stem Cell Using Crispr-Engineered Fluorescent Reporters in Primary Patient-Derived GBM and DIPG Cells. Neuro-Oncology 2019, 21, iv9. [Google Scholar] [CrossRef]
- Otero, J.J.; Rowitch, D.; Vandenberg, S. OLIG2 Is Differentially Expressed in Pediatric Astrocytic and in Ependymal Neoplasms. J. Neuro-Oncol. 2011, 104, 423–438. [Google Scholar] [CrossRef]
- Kosty, J.; Lu, F.; Kupp, R.; Mehta, S.; Lu, Q.R. Harnessing OLIG2 Function in Tumorigenicity and Plasticity to Target Malignant Gliomas. Cell Cycle 2017, 16, 1654–1660. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Silva, J.C.R. Switching on Pluripotency: A Perspective on the Biological Requirement of Nanog. Philos. Trans. R. Soc. B: Biol. Sci. 2011, 366, 2222–2229. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, M.; Yan, G.; Ma, Q.; Yan, Z.; Wang, L.; Yang, K.; Guo, D. Nanog Promotes Stem-like Traits of Glioblastoma Cells. Front. Biosci.-Landmark 2021, 26, 552–565. [Google Scholar] [CrossRef]
- Anido, J.; Sáez-Borderías, A.; Gonzàlez-Juncà, A.; Rodón, L.; Folch, G.; Carmona, M.A.; Prieto-Sánchez, R.M.; Barba, I.; Martínez-Sáez, E.; Prudkin, L.; et al. TGF-β Receptor Inhibitors Target the CD44(High)/Id1(High) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell 2010, 18, 655–668. [Google Scholar] [CrossRef]
- Hartheimer, J.S.; Park, S.; Rao, S.S.; Kim, Y. Targeting Hyaluronan Interactions for Glioblastoma Stem Cell Therapy. Cancer Microenviron. 2019, 12, 47–56. [Google Scholar] [CrossRef]
- Wang, H.H.; Liao, C.C.; Chow, N.H.; Huang, L.L.H.; Chuang, J.I.; Wei, K.C.; Shin, J.W. Whether CD44 Is an Applicable Marker for Glioma Stem Cells. Am. J. Transl. Res. 2017, 9, 4785. [Google Scholar]
- Mooney, K.L.; Choy, W.; Sidhu, S.; Pelargos, P.; Bui, T.T.; Voth, B.; Barnette, N.; Yang, I. The Role of CD44 in Glioblastoma Multiforme. J. Clin. Neurosci. 2016, 34, 1–5. [Google Scholar] [CrossRef]
- Georgescu, M.M.; Islam, M.Z.; Li, Y.; Circu, M.L.; Traylor, J.; Notarianni, C.M.; Kline, C.N.; Burns, D.K. Global Activation of Oncogenic Pathways Underlies Therapy Resistance in Diffuse Midline Glioma. Acta Neuropathol. Commun. 2020, 8, 111. [Google Scholar] [CrossRef]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent Antitumor Efficacy of Anti-GD2 CAR T Cells in H3-K27M+ Diffuse Midline Gliomas Letter. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef]
- Hoeman, C.M.; Cordero, F.J.; Hu, G.; Misuraca, K.; Romero, M.M.; Cardona, H.J.; Nazarian, J.; Hashizume, R.; McLendon, R.; Yu, P.; et al. ACVR1 R206H Cooperates with H3.1K27M in Promoting Diffuse Intrinsic Pontine Glioma Pathogenesis. Nat. Commun. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Behrooz, A.B.; Abuhamad, A.Y.; Syahir, A. Understanding Glioblastoma Biomarkers: Knocking a Mountain with a Hammer. Cells 2020, 9, 1236. [Google Scholar] [CrossRef]
- Donovan, L.K.; Potter, N.E.; Warr, T.; Pilkington, G.J. A Prominin-1-Rich Pediatric Glioblastoma: Biologic Behavior Is Determined by Oxygen Tension-Modulated CD133 Expression but Not Accompanied by Underlying Molecular Profiles. Transl. Oncol. 2012, 5, 141–154. [Google Scholar] [CrossRef]
- De, P.C.; Hamer, W.; van Tilborg, A.; Eijk, P.P.; Sminia, P.; Troost, D.; van Noorden, C.; Ylstra, B.; Leenstra, S. The Genomic Profile of Human Malignant Glioma Is Altered Early in Primary Cell Culture and Preserved in Spheroids. Oncogene 2008, 27, 2091–2096. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor Stem Cells Derived from Glioblastomas Cultured in BFGF and EGF More Closely Mirror the Phenotype and Genotype of Primary Tumors than Do Serum-Cultured Cell Lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef]
- Wenger, A.; Larsson, S.; Danielsson, A.; Elbæk, K.J.; Kettunen, P.; Tisell, M.; Sabel, M.; Lannering, B.; Nordborg, C.; Schepke, E.; et al. Stem Cell Cultures Derived from Pediatric Brain Tumors Accurately Model the Originating Tumors. Oncotarget 2017, 8, 18626–18639. [Google Scholar] [CrossRef]
- Larsson, S.; Wenger, A.; Dósa, S.; Sabel, M.; Kling, T.; Carén, H. Cell Line-Based Xenograft Mouse Model of Paediatric Glioma Stem Cells Mirrors the Clinical Course of the Patient. Carcinogenesis 2018, 39, 1304–1309. [Google Scholar] [CrossRef]
- He, C.; Xu, K.; Zhu, X.; Dunphy, P.S.; Gudenas, B.; Lin, W.; Twarog, N.; Hover, L.D.; Kwon, C.H.; Kasper, L.H.; et al. Patient-Derived Models Recapitulate Heterogeneity of Molecular Signatures and Drug Response in Pediatric High-Grade Glioma. Nat. Commun. 2021, 12, 4089. [Google Scholar] [CrossRef]
- Furusawa, C.; Kaneko, K. Chaotic Expression Dynamics Implies Pluripotency: When Theory and Experiment Meet. Biol. Direct 2009, 4, 17. [Google Scholar] [CrossRef]
- Lan, X.; Jörg, D.J.; Cavalli, F.M.G.; Richards, L.M.; Nguyen, L.V.; Vanner, R.J.; Guilhamon, P.; Lee, L.; Kushida, M.M.; Pellacani, D.; et al. Fate Mapping of Human Glioblastoma Reveals an Invariant Stem Cell Hierarchy. Nature 2017, 549, 227–232. [Google Scholar] [CrossRef]
- Tirosh, I.; Venteicher, A.S.; Hebert, C.; Escalante, L.E.; Patel, A.P.; Yizhak, K.; Fisher, J.M.; Rodman, C.; Mount, C.; Filbin, M.G.; et al. Single-Cell RNA-Seq Supports a Developmental Hierarchy in Human Oligodendroglioma. Nature 2016, 539, 309–313. [Google Scholar] [CrossRef]
- Suvà, M.L.; Tirosh, I. The Glioma Stem Cell Model in the Era of Single-Cell Genomics. Cancer Cell 2020, 37, 630–636. [Google Scholar] [CrossRef]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem Cell-Associated Heterogeneity in Glioblastoma Results from Intrinsic Tumor Plasticity Shaped by the Microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Hoffman, M.; Gillmor, A.H.; Kunz, D.J.; Johnston, M.J.; Nikolic, A.; Narta, K.; Zarrei, M.; King, J.; Ellestad, K.; Dang, N.H.; et al. Intratumoral Genetic and Functional Heterogeneity in Pediatric Glioblastoma. Cancer Res. 2019, 79, 2111–2123. [Google Scholar] [CrossRef]
- Franjic, D.; Skarica, M.; Ma, S.; Arellano, J.I.; Tebbenkamp, A.T.N.; Choi, J.; Xu, C.; Li, Q.; Morozov, Y.M.; Andrijevic, D.; et al. Transcriptomic Taxonomy and Neurogenic Trajectories of Adult Human, Macaque, and Pig Hippocampal and Entorhinal Cells. Neuron 2022, 110, 452–469.e14. [Google Scholar] [CrossRef]
- Goffart, N.; Lombard, A.; Lallemand, F.; Kroonen, J.; Nassen, J.; di Valentin, E.; Berendsen, S.; Dedobbeleer, M.; Willems, E.; Robe, P.; et al. CXCL12 Mediates Glioblastoma Resistance to Radiotherapy in the Subventricular Zone. Neuro-Oncology 2017, 19, 66–77. [Google Scholar] [CrossRef]
- Piccirillo, S.G.M.; Spiteri, I.; Sottoriva, A.; Touloumis, A.; Ber, S.; Price, S.J.; Heywood, R.; Francis, N.J.; Howarth, K.D.; Collins, V.P.; et al. Contributions to Drug Resistance in Glioblastoma Derived from Malignant Cells in the Sub-Ependymal Zone. Cancer Res. 2015, 75, 194–202. [Google Scholar] [CrossRef]
- Baker, S.J.; Ellison, D.W.; Gutmann, D.H. Pediatric Gliomas as Neurodevelopmental Disorders. Glia 2016, 64, 879. [Google Scholar] [CrossRef]
- Alcantara Llaguno, S.; Chen, J.; Kwon, C.H.; Jackson, E.L.; Li, Y.; Burns, D.K.; Alvarez-Buylla, A.; Parada, L.F. Malignant Astrocytomas Originate from Neural Stem/Progenitor Cells in a Somatic Tumor Suppressor Mouse Model. Cancer Cell 2009, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.-Y.; Kim, W.K.; Lee, J.-K.; Park, J.; et al. Human Glioblastoma Arises from Subventricular Zone Cells with Low-Level Driver Mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, D.; Chen, Y.J.; Xie, X.; Shi, Y.; Tabar, V.; Brennan, C.W.; Bale, T.A.; Jayewickreme, C.D.; Laks, D.R.; et al. Cell Lineage-Based Stratification for Glioblastoma. Cancer Cell 2020, 38, 366–379.e8. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Mitra, S.S.; Freret, M.E.; Raveh, T.B.; Kim, J.; Masek, M.; Attema, J.L.; Li, G.; Haddix, T.; Edwards, M.S.B.; et al. Hedgehog-Responsive Candidate Cell of Origin for Diffuse Intrinsic Pontine Glioma. Proc. Natl. Acad. Sci. USA 2011, 108, 4453–4458. [Google Scholar] [CrossRef]
- Chen, C.C.L.; Deshmukh, S.; Jessa, S.; Hadjadj, D.; Lisi, V.; Andrade, A.F.; Faury, D.; Jawhar, W.; Dali, R.; Suzuki, H.; et al. Histone H3.3G34-Mutant Interneuron Progenitors Co-Opt PDGFRA for Gliomagenesis. Cell 2020, 183, 1617–1633.e22. [Google Scholar] [CrossRef]
- Lombard, A.; Digregorio, M.; Delcamp, C.; Rogister, B.; Piette, C.; Coppieters, N. The Subventricular Zone, a Hideout for Adult and Pediatric High-Grade Glioma Stem Cells. Front. Oncol. 2021, 10, 3197. [Google Scholar] [CrossRef]
- Caretti, V.; Bugiani, M.; Freret, M.; Schellen, P.; Jansen, M.; van Vuurden, D.; Kaspers, G.; Fisher, P.G.; Hulleman, E.; Wesseling, P.; et al. Subventricular Spread of Diffuse Intrinsic Pontine Glioma. Acta Neuropathol. 2014, 128, 605–607. [Google Scholar] [CrossRef]
- Mistry, A.M.; Mummareddy, N.; CreveCoeur, T.S.; Lillard, J.C.; Vaughn, B.N.; Gallant, J.-N.; Hale, A.T.; Griffin, N.; Wellons, J.C.; Limbrick, D.D.; et al. Association between Supratentorial Pediatric High-Grade Gliomas Involved with the Subventricular Zone and Decreased Survival: A Multi-Institutional Retrospective Study. J. Neurosurg. Pediatrics 2020, 26, 288–294. [Google Scholar] [CrossRef]
- Jalali, R.; Mallick, I.; Dutta, D.; Goswami, S.; Gupta, T.; Munshi, A.; Deshpande, D.; Sarin, R. Factors Influencing Neurocognitive Outcomes in Young Patients With Benign and Low-Grade Brain Tumors Treated With Stereotactic Conformal Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 974–979. [Google Scholar] [CrossRef]
- Khatua, S.; Dhall, G.; O’Neil, S.; Jubran, R.; Villablanca, J.G.; Marachelian, A.; Nastia, A.; Lavey, R.; Olch, A.J.; Gonzalez, I.; et al. Treatment of Primary CNS Germinomatous Germ Cell Tumors with Chemotherapy Prior to Reduced Dose Whole Ventricular and Local Boost Irradiation. Pediatric Blood Cancer 2010, 55, 42–46. [Google Scholar] [CrossRef]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The Role of the Hedgehog Signaling Pathway in Cancer: A Comprehensive Review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From Neural Development to Neurological Disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Pierfelice, T.J.; Schreck, K.C.; Eberhart, C.G.; Gaiano, N. Notch, Neural Stem Cells, and Brain Tumors. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 367–375. [Google Scholar] [CrossRef]
- Taylor, I.C.; Hütt-Cabezas, M.; Brandt, W.D.; Kambhampati, M.; Nazarian, J.; Chang, H.T.; Warren, K.E.; Eberhart, C.G.; Raabe, E.H. Disrupting NOTCH Slows Diffuse Intrinsic Pontine Glioma Growth, Enhances Radiation Sensitivity, and Shows Combinatorial Efficacy With Bromodomain Inhibition. J. Neuropathol. Exp. Neurol. 2015, 74, 778–790. [Google Scholar] [CrossRef]
- Madhunapantula, S.V.; Mosca, P.J.; Robertson, G.P. The Akt Signaling Pathway. Cancer Biol. Ther. 2011, 12, 1032–1049. [Google Scholar] [CrossRef]
- Faury, D.; Nantel, A.; Dunn, S.E.; Guiot, M.-C.; Haque, T.; Hauser, P.; Garami, M.; Bognár, L.; Hanzély, Z.; Liberski, P.P.; et al. Molecular Profiling Identifies Prognostic Subgroups of Pediatric Glioblastoma and Shows Increased YB-1 Expression in Tumors. J. Clin. Oncol. 2007, 25, 1196–1208. [Google Scholar] [CrossRef]
- Holland, E.C.; Celestino, J.; Dai, C.; Schaefer, L.; Sawaya, R.E.; Fuller, G.N. Combined Activation of Ras and Akt in Neural Progenitors Induces Glioblastoma Formation in Mice. Nat. Genet. 2000, 25, 55–57. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e5. [Google Scholar] [CrossRef]
- Wu, Y.L.; Maachani, U.B.; Schweitzer, M.; Singh, R.; Wang, M.; Chang, R.; Souweidane, M.M. Dual Inhibition of PI3K/AKT and MEK/ERK Pathways Induces Synergistic Antitumor Effects in Diffuse Intrinsic Pontine Glioma Cells. Transl. Oncol. 2017, 10, 221–228. [Google Scholar] [CrossRef]
- Duchatel, R.; Jackson, E.; Patabendige, A.; Cain, J.; Tsoli, M.; Monje, M.; Alvaro, F.; Ziegler, D.; Dun, M. Dipg-03. Targeting pi3k using the blood brain barrier penetrable inhibitor, gdc-0084, for the treatment of diffuse intrinsic pontine glioma (dipg). Neuro-Oncology 2019, 21, ii68. [Google Scholar] [CrossRef]
- Chang, R.; Tosi, U.; Voronina, J.; Adeuyan, O.; Wu, L.Y.; Schweitzer, M.E.; Pisapia, D.J.; Becher, O.J.; Souweidane, M.M.; Maachani, U.B. Combined Targeting of PI3K and MEK Effector Pathways via CED for DIPG Therapy. Neurooncol. Adv. 2019, 1, vdz004. [Google Scholar] [CrossRef] [PubMed]
- Gojo, J.; Pavelka, Z.; Zapletalova, D.; Schmook, M.T.; Mayr, L.; Madlener, S.; Kyr, M.; Vejmelkova, K.; Smrcka, M.; Czech, T.; et al. Personalized Treatment of H3K27M-Mutant Pediatric Diffuse Gliomas Provides Improved Therapeutic Opportunities. Front. Oncol. 2020, 9, 1436. [Google Scholar] [CrossRef]
- Mbah, N.E.; Myers, A.L.; Chung, C.; Thompson, J.K.; Hong, H.S.; Sajjakulnukit, P.; Nwosu, Z.C.; Shan, M.; Sweha, S.R.; Maydan, D.D.; et al. Therapeutic Targeting of Differentiation State-Dependent Metabolic Vulnerabilities in DIPG. bioRxiv 2022, 15, 482555. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Collichio, F.A.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.; Spitler, L.; Puzanov, I.; Agarwala, S.; Milhem, M.; et al. Final Planned Overall Survival (OS) from OPTiM, a Randomized Phase III Trial of Talimogene Laherparepvec (T-VEC) versus GM-CSF for the Treatment of Unresected Stage IIIB/C/IV Melanoma (NCT00769704). J. ImmunoTherapy Cancer 2014, 2, P263. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A Phase 1 Trial of Oncolytic HSV-1, G207, given in Combination with Radiation for Recurrent GBM Demonstrates Safety and Radiographic Responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef]
- Friedman, G.K.; Langford, C.P.; Coleman, J.M.; Cassady, K.A.; Parker, J.N.; Markert, J.M.; Yancey Gillespie, G. Engineered Herpes Simplex Viruses Efficiently Infect and Kill CD133+ Human Glioma Xenograft Cells That Express CD111. J. Neuro-Oncol. 2009, 95, 199–209. [Google Scholar] [CrossRef]
- Wakimoto, H.; Kesari, S.; Farrell, C.J.; Curry, W.T.; Zaupa, C.; Aghi, M.; Kuroda, T.; Stemmer-Rachamimov, A.; Shah, K.; Liu, T.C.; et al. Human Glioblastoma-Derived Cancer Stem Cells: Establishment of Invasive Glioma Models and Treatment with Oncolytic Herpes Simplex Virus Vectors. Cancer Res. 2009, 69, 3472–3481. [Google Scholar] [CrossRef]
- Josupeit, R.; Bender, S.; Kern, S.; Leuchs, B.; Hielscher, T.; Herold-Mende, C.; Schlehofer, J.; Dinsart, C.; Witt, O.; Rommelaere, J.; et al. Pediatric and Adult High-Grade Glioma Stem Cell Culture Models Are Permissive to Lytic Infection with Parvovirus H-1. Viruses 2016, 8, 138. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Alfred Yung, W.K.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419. [Google Scholar] [CrossRef]
- Cobb, D.A.; de Rossi, J.; Liu, L.; An, E.; Lee, D.W. Targeting of the Alpha v Beta 3 Integrin Complex by CAR-T Cells Leads to Rapid Regression of Diffuse Intrinsic Pontine Glioma and Glioblastoma. J. ImmunoTherapy Cancer 2022, 10, e003816. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vélez, N.; Garcia-Moure, M.; Marigil, M.; González-Huarriz, M.; Puigdelloses, M.; Gallego Pérez-Larraya, J.; Zalacaín, M.; Marrodán, L.; Varela-Guruceaga, M.; Laspidea, V.; et al. The Oncolytic Virus Delta-24-RGD Elicits an Antitumor Effect in Pediatric Glioma and DIPG Mouse Models. Nat. Commun. 2019, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Q.; Zhou, X.; Zhao, H.; Wang, K.; Niu, H.; Wang, Y. Gospel of Malignant Glioma: Oncolytic Virus Therapy. Gene 2022, 818, 146217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, X.; Gao, L.; Wang, Y.; Guo, Y.; Xing, B.; Ma, W. Classification of Pediatric Gliomas Based on Immunological Profiling: Implications for Immunotherapy Strategies. Mol. Ther. Oncolytics 2021, 20, 34. [Google Scholar] [CrossRef]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Chastkofsky, M.I.; Pituch, K.C.; Katagi, H.; Zannikou, M.; Ilut, L.; Xiao, T.; Han, Y.; Sonabend, A.M.; Curiel, D.T.; Bonner, E.R.; et al. Mesenchymal Stem Cells Successfully Deliver Oncolytic Virotherapy to Diffuse Intrinsic Pontine Glioma. Clin. Cancer Res. 2021, 27, 1766–1777. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da-Veiga, M.-A.; Rogister, B.; Lombard, A.; Neirinckx, V.; Piette, C. Glioma Stem Cells in Pediatric High-Grade Gliomas: From Current Knowledge to Future Perspectives. Cancers 2022, 14, 2296. https://doi.org/10.3390/cancers14092296
Da-Veiga M-A, Rogister B, Lombard A, Neirinckx V, Piette C. Glioma Stem Cells in Pediatric High-Grade Gliomas: From Current Knowledge to Future Perspectives. Cancers. 2022; 14(9):2296. https://doi.org/10.3390/cancers14092296
Chicago/Turabian StyleDa-Veiga, Marc-Antoine, Bernard Rogister, Arnaud Lombard, Virginie Neirinckx, and Caroline Piette. 2022. "Glioma Stem Cells in Pediatric High-Grade Gliomas: From Current Knowledge to Future Perspectives" Cancers 14, no. 9: 2296. https://doi.org/10.3390/cancers14092296
APA StyleDa-Veiga, M.-A., Rogister, B., Lombard, A., Neirinckx, V., & Piette, C. (2022). Glioma Stem Cells in Pediatric High-Grade Gliomas: From Current Knowledge to Future Perspectives. Cancers, 14(9), 2296. https://doi.org/10.3390/cancers14092296





