Simple Summary
Febrile neutropenia is a common complication in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Its genesis is often attributed to infections; however, a specific cause frequently cannot be defined. We hypothesize that the composition of the intestinal flora may contribute to the genesis of the neutropenic fever. We analyzed the microbial composition of stool samples from pediatric patients from three European centers and assessed the relationship with the duration of the fever during neutropenia. We found that a more stable composition of the microbiota during the transplantation course is associated with a shorter duration of fever. Moreover, patients with a higher duration of fever presented higher levels of Collinsella, Megasphaera, Prevotella, Roseburia, Eggerthella and Akkermansia in the stool.
Abstract
Febrile neutropenia (FN) is a common complication in pediatric patients receiving allogeneic hematopoietic stem cell transplantation (HSCT). Frequently, a precise cause cannot be identified, and many factors can contribute to its genesis. Gut microbiota (GM) has been recently linked to many transplant-related complications, and may also play a role in the pathogenesis of FN. Here, we conducted a longitudinal study in pediatric patients receiving HSCT from three centers in Europe profiling their GM during the transplant course, particularly at FN onset. We found that a more stable GM configuration over time is associated with a shorter duration of fever. Moreover, patients with longer lasting fever exhibited higher pre-HSCT levels of Collinsella, Megasphaera, Prevotella and Roseburia and increased proportions of Eggerthella and Akkermansia at the engraftment. These results suggest a possible association of the GM with the genesis and course of FN. Data seem consistent with previous reports on the relationship of a so-called “healthy” GM and the reduction of transplant complications. To our knowledge, this is the first report in the pediatric HSCT setting. Future studies are warranted to define the underling biological mechanisms and possible clinical implications.
1. Introduction
Fever during neutropenia, (hereinafter termed as febrile neutropenia (FN)), is almost universally present in pediatric allogeneic hematopoietic stem cell transplantation (HSCT) recipients [1]. Bloodstream infections (BSI) represent the most frequent cause of fever, complicated by high mortality, particularly in cases related to Gram-negative bacteria [2,3]. However, only in half of the patients can an actual infectious etiology be identified [2]. Indeed, fever can also be attributed to other factors, including viral and fungal infections, graft-versus-host disease (GvHD) and cytokine release [4].
Growing evidence is pointing to a pivotal role of the microbial community inhabiting the gastrointestinal tract (i.e., the gut microbiota, GM) during anticancer therapies, and particularly during allogenic HSCT (allo-HCST) [5]. The disruption of the symbiosis between the host and the GM is well documented in pediatric patients receiving allo-HSCT [6,7], mainly due to many factors acting early in the neutropenic period, such as antibiotic administration, nutritional deficiencies, immunosuppression and chemotherapy-related toxicity [8,9,10,11,12]. In recent years, the relationship between GM and allo-HSCT outcomes has been extensively explored, with a particular focus on overall survival [13], relapse [14], GvHD [15], veno-occlusive disease [16] and BSI [6,17,18]. However, whether intestinal commensal bacteria could have a role in the genesis and course of FN has not yet been addressed. In transplanted adults, GM profiles have been observed to diverge between patients who developed FN and those who did not [19]. In particular, patients with FN showed enrichment in Mogibacterium, Bacteroides fragilis and Parabacteroides distasonis, while they showed a depletion in Prevotella, Ruminococcus, Dorea, Blautia and Collinsella. Recent evidence has also suggested that Akkermansia expansion in the gut of adult patients with acute leukemia could be a predictive signature for FN development [20]. However, evidence in the pediatric setting is still lacking to date. Improving HSCT outcomes could represent a crucial issue in improving outcomes of high-risk cancer patients since often it represents the best treatment option. Thus, the aim of this study was to characterize the GM dynamics in pediatric patients undergoing HSCT, with a focus on the neutropenic phase and the relationship between GM configurations and FN characteristics.
2. Materials and Methods
2.1. Study Population and Stool Sampling
This prospective, multi-center study enrolled pediatric patients undergoing allo-HSCT in three pediatric centers in Europe (Bologna, Italy; Verona, Italy; Wroclaw, Poland) between January 2019 and December 2020. Inclusion criteria were age ≤ 18 years and informed consent provided. Exclusion criteria included previous fecal microbiota transplantation. Enrolled patients could be candidates for HSCT for any underlying condition, with any HSCT characteristics. The sampling protocol was approved by the Ethics Committee of the CE-AVEC of Emilia-Romagna, Italy (ref. number 19/2013/U/Tess). The study was conducted in accordance with the Declaration of Helsinki. Stool samples were collected at baseline (before transplant; hereafter referred to as PRE); at the onset of neutropenia (day −2/+2; P2); at the onset of fever (day +4/+5; P5); at engraftment (absolute neutrophil count (ANC) ≥ 500 × 106/L for at least 3 days; TAKE) and after engraftment (day +20/+30; P20). Stool samples were collected in sterile tubes and stored at −80 °C and shipped in dry ice to the Department of Pharmacy and Biotechnology (FABIT), University of Bologna, Italy where the analysis was performed. Neutropenia was defined as ANC < 500 × 106/L and fever was defined as a body temperature ≥ 38 °C proven by two different determinations. Episodes of BSI were defined as positive cultures from a blood sample from both the central venous catheter and peripheral veins.
2.2. Microbial DNA Extraction and 16S rRNA Gene Library Preparation
Microbial DNA was extracted from 0.25 g of fecal sample using the repeated bead-beating protocol [21], with only a few modifications [22]. In short, stool samples were resuspended in 1 mL of lysis buffer in presence of four 3-mm glass beads and 0.5 g of 0.1-mm zirconia beads (BioSpec Products, Bartlesville, OK, USA), and bead-beaten in a FastPrep homogenizer (MP Biomedicals, Irvine, CA, USA) at 5.5 movements/s for 1 min three times. After 15-min incubation at 95 °C, supernatants were separated by centrifugation at 13,000 rpm for 5 min and incubated first with 260 µL of 10 M ammonium acetate and then with one volume of isopropanol for 30 min. The nucleic acid pellets were washed with 70% ethanol and resuspended in 100 µL of TE buffer. RNA was removed by 15-min incubation at 37 °C with 2 µL of DNase-free RNase (10 mg/mL). For the subsequent DNA purification steps, the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) was used. DNA concentration and quality were assessed using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
The V3-V4 hypervariable regions of the 16S rRNA gene were amplified using the 341F and 785R primes with Illumina adapter overhang sequences, as previously described [22]. PCR products were purified using a magnetic bead-based system (Agencourt AMPure XP; Beckman Coulter, Brea, CA, USA), followed by sample indexing through a limited-cycle PCR with Nextera technology. Indexed libraries were purified with another clean-up step, as described above, and then pooled at the equimolar concentration of 4 nM. Sequencing was performed by loading the denatured and 5 pM diluted library into the Illumina MiSeq platform, using the 2 × 250 bp paired-end protocol, per the manufacturer’s instructions (Illumina, San Diego, CA, USA).
2.3. Bioinformatic and Statistical Analysis
Raw sequences were processed using a pipeline combining PANDAseq [23] and QIIME 2 [24]. After length and quality filtering, sequences were binned into amplicon sequence variants (ASVs) using the DADA2 pipeline [25]. The taxonomic assignment was performed using the VSEARCH algorithm [26] against the Greengenes database (May 2013 release). Sequence reads were deposited in the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA; BioProject ID PRJNA780429).
Alpha diversity was computed using several metrics, including the number of observed ASVs and the Shannon index. Beta diversity was estimated by weighted and unweighted UniFrac distances, which were used to build Principal Coordinates Analysis (PCoA) plots. All statistical analyses were performed on R and RStudio software. PCoA plots were constructed with the “Made4” [27] and “Vegan” (http://www.cran.r-project.org/package=vegan/packages, accessed on 26 February 2021) packages and data separation was tested by a permutation test with pseudo-F ratio (function “Adonis” in “Vegan”). To assess the significance of differences in GM alpha diversity and composition between groups, the Kruskal–Wallis test followed by post-hoc Wilcoxon tests (paired or unpaired as needed) was used. To reconstruct the trajectory over time (i.e., from the PRE to the P20 timepoint) of the major genera of GM in relation to the duration of FN, Circos plots were generated using the “Circos Table Viewer” software from Krzywinski et al. [28]. When appropriate, P values were corrected for multiple comparisons using the Benjamini–Hochberg method. A false discovery rate (FDR) ≤ 0.05 was considered as statistically significant.
Qualitative clinical variables were compared using Fisher’s exact or Pearson’s chi-square test, while means were compared using the Mann–Whitney U test. Cumulative incidences of BSI and GvHD were calculated using the Kalbfleisch and Prentice method and compared using the Gray test.
3. Results
3.1. Study Cohort Description
Thirty-seven patients were enrolled in the study. Clinical and transplant characteristics are summarized in Table 1.

Table 1.
Clinical and transplant characteristics of enrolled patients. Data are shown for the whole cohort and for each of the three pediatric centers. BM: Bone marrow; MAC: Myeloablative conditioning; MMUD: Mismatched unrelated donor; MSD: Matched sibling donor; MUD: Matched unrelated donor; PBSC: Peripheral blood stem cells; RIC: Reduced intensity conditioning.
Patients were stratified according to the median value of days of fever into two groups: less than or equal to three days (n = 16) and more than three days (n = 21). Clinical characteristics were comparable between the two groups (Table 2).

Table 2.
Comparison of clinical confounders between patients with longer and shorter duration of neutropenic fever. BM: Bone marrow; EN: Enteral nutrition; MAC: Myeloablative conditioning; MMUD: Mismatched unrelated donor; MSD: Matched sibling donor; MUD: Matched unrelated donor; PBSC: Peripheral blood stem cells; PN: Parenteral nutrition; RIC: Reduced intensity conditioning. Qualitative clinical variables were compared using Fisher’s exact or Pearson’s chi-square test.
FN was treated according to local protocols, using broad-spectrum antibiotics as the first line. Details of infections for enrolled patients are reported in Supplementary Table S1. A detailed summary of the administered antibiotics is provided in Supplementary Table S2. No differences were found in the total number of days of antibiotic administration between patients with longer or shorter duration of fever (median days, 20.5 vs. 17.0, p = 0.1).
3.2. Gut Microbiota Dynamics in Pediatric Allo-HSCT Patients in Relation to the Duration of Febrile Neutropenia
A total of 161 fecal samples were collected prior to HSCT and at various timepoints after FN development, up to 30 days later, to reconstruct the GM trajectory (Figure 1).
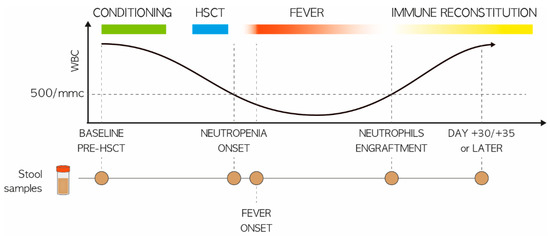
Figure 1.
Study design. Schematic representation of fecal sampling for pediatric patients undergoing HSCT, in relation to the development of FN. Circles indicate the sampling timepoints, i.e., at baseline (before transplant; PRE), at the onset of neutropenia (day −2/+2; P2), at the onset of fever (day +4/+5; P5), at engraftment (TAKE) and after engraftment (day +20/+30; P20). Patients were stratified based on the median of total fever days into two groups: (i) less than or equal to three days (n = 16) and (ii) more than three days (n = 21).
Next-generation sequencing of the 16S rRNA gene yielded 25,563,052 high-quality reads (mean ± SD, 158,777 ± 52,330) clustered into 3279 ASVs. At baseline, the GM profile did not stratify for any of the possible confounding factors, i.e., transplant center, antibiotic prophylaxis, GvHD development, underlying disease, previous chemotherapy, and previous antibiotic therapies (permutation test with pseudo-F ratio, p ≥ 0.08) (Supplementary Figure S1). The GM dynamics were then reconstructed separately into the two groups of patients with different duration of fever (i.e., more vs. less than or equal to three days). According to both weighted and unweighted UniFrac metrics (Figure 2A; Supplementary Figure S2), samples from patients with more than three days of fever deviated significantly from the baseline over time (p ≤ 0.05).
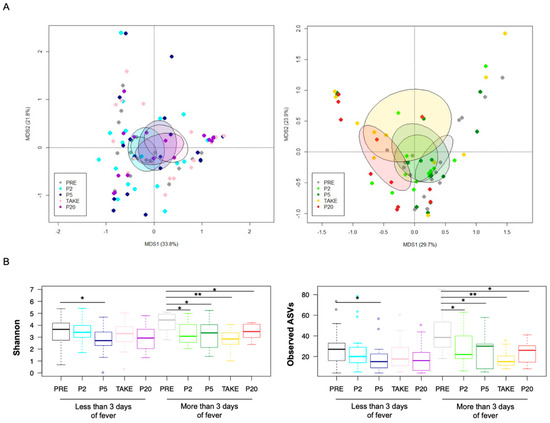
Figure 2.
Gut microbiota diversity in pediatric allo-HSCT patients with different duration of febrile neutropenia. (A) PCoA based on weighted UniFrac distances between the gut microbiota profiles of samples collected before transplant (PRE), at the onset of neutropenia (day −2/+2; P2), at the onset of fever (day +4/+5; P5), at engraftment (TAKE) and after engraftment (day +20/+30; P20), in pediatric allo-HSCT patients with less than or equal to (left) and more (right) than three days of fever. Ellipses include 95% confidence area based on the standard error of the weighted average of sample coordinates. Significant separation among groups was found only for patients with longer fever duration (permutation test with pseudo-F ratio, p = 0.03). See also Supplementary Figure S2; (B) Boxplots showing the dynamics of alpha diversity, estimated with the Shannon index (left) and the number of observed ASVs (right), in patients with less than or equal to vs. more than three days of fever. Wilcoxon test, * for p < 0.05, ** for p < 0.01.
In contrast, samples from patients with shorter fever duration overlapped regardless of timepoint (p ≥ 0.4), suggesting some temporal stability of the GM structure from before transplant up to 30 days after the onset of FN. This opposite trend was also corroborated by the alpha diversity analysis (Figure 2B). In fact, both the Shannon index and the number of observed ASVs showed a significant loss of diversity at all post-FN timepoints compared to baseline, in patients with a longer fever duration (Wilcoxon test, p < 0.05). On the other hand, patients with less than or equal to three days of fever were characterized by decreased diversity only at P2 compared to PRE (p < 0.05).
From the taxonomic standpoint (Supplementary Figure S3), as expected for pediatric allo-HSCT patients [7,15], the GM was overall dominated by the phylum Firmicutes (mean relative abundance across the dataset, 66.6%), and specifically by the Enterococcaceae, Ruminococcaceae, Lachnospiraceae, Streptococcaceae, Lactobacillaceae and Erysipelotrichaceae families. Bifidobacteriaceae and Coriobacteriaceae, both belonging to Actinobacteria (cumulative relative abundance, 12.6%), as well as Enterobacteriaceae and Bacteroidaceae (Proteobacteria and Bacteroidetes members, respectively; 11.8% and 5.9%) accounted for the remainder of the GM community. Consistent with the diversity data, when comparing the two groups of patients with different duration of fever, many compositional differences emerged (Figure 3).
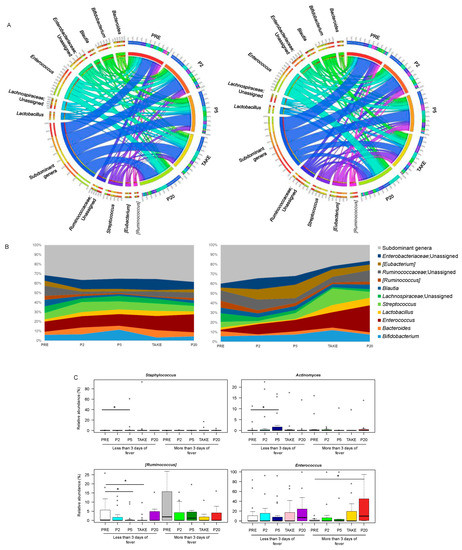
Figure 3.
Genus-level gut microbiota trajectory in pediatric allo-HSCT patients with different duration of febrile neutropenia. Circos (A) and area (B) plots showing the relative abundance over time of the major genera in the GM of patients with less than or equal to (left) vs. more (right) than three days of fever. Only taxa with mean relative abundance > 20% in at least five samples of the total dataset are shown. PRE, before transplant; P2, at the onset of neutropenia (day −2/+2); P5, at the onset of fever (day +4/+5); TAKE, at engraftment; P20, after engraftment (day +20/+30); (C) Boxplots showing the relative abundance distribution of genera significantly differentially represented over time in patients with different duration of fever. Wilcoxon test, * for p < 0.05.
In particular, the GM profile of patients with more than three days of fever showed a progressive thinning of subdominant genera, especially starting from TAKE, leaving room for the overabundance of potential pathobionts, such as Enterococcus. The proportions of the latter were in fact significantly higher at P20 than PRE in patients with more than three days of fever. On the other hand, the GM of patients with less than or equal to three days of fever was characterized by overall greater compositional stability over time, with only some early fluctuations in Actinomyces and Staphylococcus, which increased at P5 compared to PRE, and Ruminococcus whose relative abundance was lower at P5 and TAKE than PRE (p < 0.05). Finally, both patient groups showed a reduction over time in some health-associated, SCFA-producing GM members (i.e., Coprococcus, Dorea, Ruminococcus, Roseburia, Blautia and Faecalibacterium) (p < 0.05) (Supplementary Figure S4).
3.3. Early and Late Gut Microbiota Signatures of Febrile Neutropenia Duration in Pediatric Allo-HSCT Patients
To identify potential signatures of fever duration, between-group comparisons were made at the different timepoints. According to our findings, Collinsella, Megasphaera, Prevotella and Roseburia were more represented in PRE samples from patients with more than three days of fever (Wilcoxon test, p < 0.05) (Figure 4A).
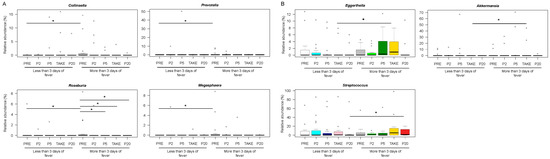
Figure 4.
Early and late gut microbiota signatures of febrile neutropenia duration. Boxplots showing the relative abundance distribution of genera significantly differentially represented between patients with less than or equal to vs. more than three days of fever, before transplant (A) and after the onset of fever (B). PRE, before transplant; P2, at the onset of neutropenia (day −2/+2); P5, at the onset of fever (day +4/+5); TAKE, at engraftment; P20, after engraftment (day +20/+30). Wilcoxon test, * for p < 0.05.
Patients with longer lasting fever were also distinguished by increased proportions of Eggerthella and Akkermansia at TAKE, as well as Streptococcus at P20 (p < 0.05) (Figure 4B) (Supplementary Figure S5).
3.4. Association between Febrile Neutropenia Duration and Development of Other Transplant Complications
Finally, we investigated whether fever duration was associated with other clinical outcomes, namely BSI and GvHD. No significant differences were found between patients with longer or shorter fever duration for BSI onset (4/16 vs. 5/21, p = 0.9), as well as for any grade GvHD (10/16 vs. 7/21, p = 0.1) or grade II-IV aGvHD (4/16 vs. 4/21, p = 0.7) (Table 2). We then assessed the impact of FN duration on length of hospitalization and found that patients with longer fever duration had longer hospitalization (median in days: 61.3 vs. 40.0, p = 0.001).
4. Discussion
To our knowledge, this is the first report in the pediatric HSCT setting that has investigated the relationship between the GM dynamics and FN. According to our data, an overall more stable GM configuration over time, in terms of both alpha and beta diversity, is associated with a shorter duration of fever. Interestingly, this was achieved for three different centers across Europe, suggesting that this association is robust to the so-called geographic effect [29]. These findings are in line with previous reports, which suggest that a more diverse GM is associated with the reduction of transplant complications [6,30,31]. Consistently, patients with long-lasting fever presented greater compositional instability of GM, with in particular an overabundance of Enterococcus, frequently known as a possible pathobiont [32]. Moreover, we found that patients with fever of longer duration exhibited higher pre-HSCT levels of Collinsella, Megasphaera, Prevotella and Roseburia, which could therefore represent possible predictive signatures of FN duration. This latter finding is partially in contrast with what was reported in adult patients undergoing HSCT, in whom a higher amount of Collinsella and Prevotella was associated with the absence of fever during the neutropenic period [19]. However, it should be remembered that in the pediatric subject, the composition (and functionality) of the GM is very different, which probably leads to the establishment of different ecological networks that affect the host health in a different way. Furthermore, Collinsella is often reported in the literature as a pathobiont and associated with impaired integrity of the intestinal barrier [33], which may support its role in the development of fever. The GM profile of patients with longer fever duration was also enriched in other potentially harmful components, such as Akkermansia, Eggerthella and Streptococcus. The former is a mucus degrader, previously associated with other pathological conditions, such as inflammatory bowel disease [34], colonic epithelial carcinogenesis [35] and multiple sclerosis [36]. Notably, the presence of Akkermansia has been related with the onset of FN in adults undergoing intensive chemotherapy, and suggested to contribute to oxidative stress as a possible mediator of FN [20]. On the other hand, Akkermansia has also been shown to have beneficial effects in other clinical settings, such as in patients with obesity [37] or in the response rate of cancer mice treated with anti-PD1 drugs [38]. Thus, its role should be better addressed in future studies.
While our data are insufficient to postulate any causal relationship between GM and FN duration in pediatric HSCT recipients, we can hypothesize that GM dysbiosis may be associated with a prolonged inflammatory state. An altered intestinal ecosystem, enriched in pathobionts and mucus degraders with an impoverished commensal community, can worsen the intestinal mucosa damage, allowing the translocation of microbial molecules and microbes [39], thus sustaining a systemic inflammatory process and leading to a longer duration of fever. Indirect markers to assess mucosal and endothelial damage, such as citrulline, lipopolysaccharides (LPS), regenerating islet-derived protein 3 alpha (REG-3-alpha) or urinary 3-indoxil-sulfate (3-IS), should be analyzed in the future alongside GM trajectories to further address this hypothesis [40].
On the other hand, the clinical impact of FN duration remains to be fully assessed. In fact, it must also be said that whether fever per se can be considered as a complication is still debated. In our cohort, we were unable to find a relationship between fever and BSI or GvHD, but we did find a difference in the length of hospitalization. Moreover, no differences in antibiotic exposure were found between the two groups. Probably, the number of enrolled patients was too small to draw firm conclusions, and this represents a limitation of this study. Another limitation is the enrolment of patients undergoing HSCT for any indications, thus representing a possible confounding factor. Lastly, assessing FN may be subject to a certain degree of bias, as it is known to be due to different causes. Therefore, confirmation from larger cohorts is highly awaited.
It has been reported that patients with FN experience non-specific symptoms that may impair general conditions and prolong hospitalization [41], as in our case, and thus targeting FN may present clinical benefits. While our study also included patients with non-oncological diseases, improving HSCT certainly has a clinical impact on cancer patients, representing a key treatment for high-risk patients. In fact, alongside the new drugs in development [42], HSCT represents the best therapeutic option also for cancer patients not responsive to conventional therapies.
5. Conclusions
In summary, we provided the first evidence of a correlation between FN duration and GM dynamics in pediatric patients undergoing HSCT. Future studies are needed to better define the biological mechanisms underpinning this relationship. Besides, studies on larger cohorts are needed to assess the clinical impact of FN duration, and whether novel approaches targeting the GM ecosystem [12,43] could be clinically relevant.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14081932/s1, Table S1: Detailed summary of infections in the two patient groups; Table S2: Detailed summary of administered antibiotics; Figure S1: Baseline gut microbiota diversity in pediatric allo-HSCT patients who developed febrile neutropenia; Figure S2: Beta diversity of the gut microbiota in pediatric allo-HSCT patients with different duration of febrile neutropenia; Figure S3: Gut microbiota trajectory in pediatric allo-HSCT patients with different duration of febrile neutropenia; Figure S4: Dynamics of typical health-associated taxa in pediatric allo-HSCT patients with different duration of febrile neutropenia; Figure S5: Boxplots showing the relative abundance distribution of Akkermansia in patients with different duration of febrile neutropenia (less vs. more than three days).
Author Contributions
R.M. was responsible for the study design, the interpretation of the results and critically reviewed the manuscript; F.D. and S.T. analyzed stool samples, performed the gut microbiome characterization and wrote the manuscript; D.Z. contributed to the study design, to the interpretation of the results and critically reviewed the manuscript; D.L. and E.M. collected the stool samples and wrote the manuscript; M.U., J.F., S.C., G.C., V.P., T.B., F.G., P.T. collected the stool samples and critically reviewed the manuscript; P.B. and A.P. contributed to the interpretation of the results and critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondazione Cassa di Risparmio in Bologna, grant number 2020CARISBO_MASETTI_R.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Comitato Etico Area Vasta Emilia Centro (CE- AVEC) (ref. number 19/2013/U/Tess) on 12 March 2013.
Informed Consent Statement
Written informed consent was obtained from each enrolled patient or parent/legal guardian.
Data Availability Statement
Sequence reads were deposited in the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA; BioProject ID PRJNA780429).
Acknowledgments
Authors would like to thank Maria Francesca Secchi, and all the nurses of the transplant units for their valuable contribution in sample collection.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Barton, C.D.; Waugh, L.K.; Nielsen, M.J.; Paulus, S. Febrile neutropenia in children treated for malignancy. J. Infect. 2015, 71, S27–S35. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.M.; Santolaya, M.; et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer International Publishing: Cham, Switzerland, 2019; ISBN 3030022781. [Google Scholar]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28711. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Zama, D.; Nastasi, C.; Consolandi, C.; Fiori, J.; Rampelli, S.; Turroni, S.; Centanni, M.; Severgnini, M.; Peano, C.; et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015, 50, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Zama, D.; Bossù, G.; Leardini, D.; Muratore, E.; Biagi, E.; Prete, A.; Pession, A.; Masetti, R. Insights into the role of intestinal microbiota in hematopoietic stem-cell transplantation. Ther. Adv. Hematol. 2020, 11, 2040620719896961. [Google Scholar] [CrossRef] [Green Version]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef]
- D’Amico, F.; Biagi, E.; Rampelli, S.; Fiori, J.; Zama, D.; Soverini, M.; Barone, M.; Leardini, D.; Muratore, E.; Prete, A.; et al. Enteral Nutrition in Pediatric Patients Undergoing Hematopoietic SCT Promotes the Recovery of Gut Microbiome Homeostasis. Nutrients 2019, 11, 2958. [Google Scholar] [CrossRef] [Green Version]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2017, 23, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Zama, D.; Gori, D.; Muratore, E.; Leardini, D.; Rallo, F.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A.; Masetti, R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2021, 27, 180.e1–180.e8. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal microbiota and Relapse after hematopoietic-Cell transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Zama, D.; Rampelli, S.; Turroni, S.; Brigidi, P.; Consolandi, C.; Severgnini, M.; Picotti, E.; Gasperini, P.; Merli, P.; et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med. Genomics 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Biagi, E.; Zama, D.; Muratore, E.; Amico, F.D.; Leardini, D.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. Early modifications of the gut microbiome in children with hepatic sinusoidal obstruction syndrome after hematopoietic stem cell transplantation. Sci. Rep. 2021, 11, 14307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Ward, D.V.; Severyn, C.J.; Arshad, M.; Heston, S.M.; Jenkins, K.; Martin, P.L.; McGill, L.; Stokhuyzen, A.; Bhattarai, S.K.; et al. Gut Colonization Preceding Mucosal Barrier Injury Bloodstream Infection in Pediatric Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2019, 25, 2274–2280. [Google Scholar] [CrossRef]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Murthy, H.S.; Gharaibeh, R.Z.; Al-Mansour, Z.; Kozlov, A.; Trikha, G.; Newsome, R.C.; Gauthier, J.; Farhadfar, N.; Wang, Y.; Kelly, D.L.; et al. Baseline Gut Microbiota Composition is Associated with Major Infections Early after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 2001–2010. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Nalluri, H.; Kaiser, T.; Ramamoorthy, S.; Holtan, S.G.; Khoruts, A.; Weisdorf, D.J.; et al. Altered microbiota-host metabolic cross talk preceding neutropenic fever in patients with acute leukemia. Blood Adv. 2021, 5, 3937–3950. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- D’Amico, F.; Perrone, A.M.; Rampelli, S.; Coluccelli, S.; Barone, M.; Ravegnini, G.; Fabbrini, M.; Brigidi, P.; De Iaco, P.; Turroni, S. Gut Microbiota Dynamics during Chemotherapy in Epithelial Ovarian Cancer Patients Are Related to Therapeutic Outcome. Cancers 2021, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Chase, J.; Pitman, T.A.; Shiffer, A.; Mercurio, W.; Dillon, M.R.; Caporaso, J.G. An Introduction to Applied Bioinformatics: A free, open, and interactive text. J. Open Source Educ. 2018, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Culhane, A.C.; Thioulouse, J.; Perrière, G.; Higgins, D.G. MADE4: An R package for multivariate analysis of gene expression data. Bioinformatics 2005, 21, 2789–2790. [Google Scholar] [CrossRef] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.X.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Cilieborg, M.S.; Lund, O.; Holmes, S.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome 2019, 7, 131. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Mordhorst, H.; Ifversen, M.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Microbiota long-term dynamics and prediction of acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Microbiome 2021, 9, 148. [Google Scholar] [CrossRef]
- Lebreton, F.; Manson, A.L.; Saavedra, J.T.; Straub, T.J.; Earl, A.M.; Gilmore, M.S. Tracing the Enterococci from Paleozoic Origins to the Hospital. Cell 2017, 169, 849–861.e13. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 2174. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; D’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Brigidi, P.; Pession, A. Microbiome-derived metabolites in allogeneic hematopoietic stem cell transplantation. Int. J. Mol. Sci. 2021, 22, 1197. [Google Scholar] [CrossRef]
- Van Der Velden, W.J.F.M.; Herbers, A.H.E.; Brüggemann, R.J.M.; Feuth, T.; Peter Donnelly, J.; Blijlevens, N.M.A. Citrulline and albumin as biomarkers for gastrointestinal mucositis in recipients of hematopoietic SCT. Bone Marrow Transplant. 2013, 48, 977–981. [Google Scholar] [CrossRef]
- Krell, D.; Jones, A.L. Impact of effective prevention and management of febrile neutropenia. Br. J. Cancer 2009, 101, S23–S26. [Google Scholar] [CrossRef]
- Lonetti, A.; Pession, A.; Masetti, R. Targeted Therapies for Pediatric AML: Gaps and Perspective. Front Pediatr. 2019, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Pession, A.; Zama, D.; Muratore, E.; Leardini, D.; Gori, D.; Guaraldi, F.; Prete, A.; Turroni, S.; Brigidi, P.; Masetti, R. Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J. Pers. Med. 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).