Simple Summary
There is no clear preference between S-1 and capecitabine in combination with platinum agent as first-line therapy for patients with HER2-negative unresectable advanced or recurrent gastric cancer (GC) with measurable disease. Although a distinguishing use of S-1 versus capecitabine based on histological classification has been studied, the present integrated analysis, using 162 individual participant data of GC patients with measurable disease, is the first to show that S-1 plus cisplatin (SP) achieves deeper tumor shrinkage than capecitabine plus cisplatin (XP), leading a longer overall survival although no differences in response rate or progression-free survival in differentiated GC. On the other hand, in undifferentiated GC, SP consistently showed better clinical results than XP. These findings thus have implications for the choice of oral fluoropyrimidine in the era of first line therapy in combination with oxaliplatin and immune checkpoint inhibitor.
Abstract
It has been suggested that the therapeutic efficacy of S-1 + cisplatin (SP) and capecitabine + cisplatin (XP) may differ depending on the histology of the tumor, but no clear evidence exists. Individual participant data were obtained from three randomized phase II trials in which such patients received either SP (S-1 [40–60 mg twice daily for 21 days] plus cisplatin [60 mg/m2 on day 8], every 5 weeks) or XP (capecitabine [1000 mg/m2 twice daily for 14 days] plus cisplatin [80 mg/m2 on day 1], every 3 weeks). A total of 162 patients were included, with 79 patients in the SP arm and 83 patients in the XP arm. Although there was also no difference between arms in ORR according to histological classification, differentiated tumors showed a significantly better OS (but not PFS) for SP versus XP that was associated with a deeper tumor shrinkage. Undifferentiated tumors showed a consistently better OS, and PFS for SP versus XP, likely because cases without tumor shrinkage tended to be fewer for SP. Our data thus showed that SP was superior to XP in this setting, but there were qualitative differences in therapeutic efficacy dependent on tumor histology.
1. Introduction
Gastric cancer remains one of the most common and fatal cancers worldwide, with GLOBOCAN data for 2020 showing it to be the fifth most frequent and fourth most deadly cancer, with an estimated 769,000 deaths [1]. Individuals with newly diagnosed gastric cancer often present with unresectable or metastatic disease. For these patients, as well as the small number of cases of recurrence after radical surgery, cytotoxic chemotherapy is administered to alleviate symptoms, improve quality of life, and prolong survival. Most advanced gastric tumors are negative for human epidermal growth factor receptor 2 (HER2), with the standard first-line chemotherapy for these cases being a two-drug combination of platinum and a fluoropyrimidine, the latter of which is usually oral S-1 or capecitabine in Japan.
S-1 has three components in a molar ratio of 1:0.4:1: tegafur, a prodrug of 5-fluorouracil (5-FU); gimeracil, which selectively inhibits the metabolic degradation of 5-FU; and potassium oteracil, which limits mucosal damage [2]. In Japan, the combination of S-1 and cisplatin (SP) has been regarded as a standard of care for advanced gastric cancer on the basis of the results of the SPIRITS study, in which 5-week cycles of SP showed an overall survival (OS) benefit versus S-1 alone [3]. Capecitabine is a prodrug of 5-FU that is hydrolyzed by carboxylesterase in the liver to 5′-deoxy-5-fluorocytidine (5′-DFCR), which is then converted to 5′-deoxy-5-fluorouridine (5′-DFUR) by cytidine deaminase in the liver and tumor tissue, and 5′-DFUR is finally converted to 5-FU by thymidine phosphorylase in tumor tissue [4]. Capecitabine plus cisplatin (XP) in 3-week cycles has been administered as a standard of care for advanced gastric cancer in several global trials [5,6,7] because of its noninferiority to 5-FU plus cisplatin [8]. Both SP and XP are listed in the Japanese guidelines as first-line treatments for advanced gastric cancer [9]. Given the difference in design and concept for S-1 and capecitabine, however, direct comparison of these two oral fluoropyrimidine drugs in combination with cisplatin is desirable to discriminate the use of SP versus XP.
As far as we are aware, three randomized phase II trials have prospectively compared SP and XP for HER2-negative unresectable advanced or recurrent gastric cancer in Japan: the HERBIS-2 [10] and HERBIS-4A [11] trials performed by the Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG) and the XParTS II trial [12] conducted by the Epidemiological and Clinical Research Information Network (ECRIN). However, these trials reported different results. In the pooled analysis of HERBIS-2 and HERBIS-4A, SP showed a longer progression-free survival (median PFS: 6.4 vs. 5.1 months, with a hazard ratio [HR] of 0.666, p = 0.062), OS (median of 14.8 vs. 10.6 months, with an HR of 0.695, p = 0.099), and time to treatment failure (median TTF: 4.6 vs. 3.6 months, with an HR of 0.668, p = 0.045) compared with XP, whereas there was no difference in overall response rate (ORR: 54.5% vs. 51.1%, {respectively, p = 1.000) [10]. On the other hand, the XParTS II study found that the ORR was significantly lower for SP than for XP (42.4% vs. 69.4%, p = 0.0237), with a comparable outcome of SP versus XP with regard to PFS (5.6 vs. 5.1 months, respectively, with an HR of 1.126, p = 0.5626) and OS (13.5 vs. 12.6 months, with an HR of 0.942, p = 0.7769) [12]. Exploratory analysis based on histological classification did not detect any difference in efficacy between treatments in any of these trials.
It has therefore remained unknown whether S-1 or capecitabine is more suitable as a first-line treatment for this setting. Even though the two drugs are intrinsically different, the appropriate use of each remains unclear. One reason for this ambiguity is the dearth of significant differences in the clinical trials as a result of their limited sample sizes. The integration of individual participant data (IPD) would allow analysis of a larger number of patients. Given that the regimens administered in each study are identical, and that the eligibility and exclusion criteria as well as the enrollment periods (2012–2014, 2011–2016, and 2011–2013 for HERBIS-2, HERBIS-4A, and XParTS II, respectively) of the studies largely overlap, we have now performed an integrated analysis of these three trials. In this analysis, we here reveal qualitative differences in therapeutic efficacy between SP and XP for HER2-negative unresectable advanced or recurrent gastric cancer with a measurable lesion that are dependent on histology.
2. Patients and Methods
2.1. Study Design and Treatment
The analysis was designed in 2020. Both OGSG and ECRIN gave their approval according to a formal protocol. Each of the selected randomized phase II trials compared SP versus XP with the same dosing, and the treatment methods were identical. In all three trials, patients were randomly assigned to receive either SP (S-1 at 40–60 mg twice daily for 21 days plus cisplatin at 60 mg/m2 on day 8) every 5 weeks or XP (capecitabine at 1000 mg/m2 twice daily for 14 days plus cisplatin at 80 mg/m2 on day 1) every 3 weeks. Each treatment was continued until disease progression or the development of intolerable toxicity. Inclusion and exclusion criteria for patient enrollment (see below) were described previously [10,11,12].
We first verified the integrity of IPD from the HERBIS-2, HERBIS-4A, and XParTS II trials. All clinical data were extracted and held centrally at the data center of OGSG for HERBIS-2 and HERBIS-4A and at that of ECRIN for XParTS II. This analysis was conducted according to the Declaration of Helsinki. All patients in the three trials provided written consent after being informed about the purpose and investigational nature of the respective studies. The institutional review boards or ethics committees of all participating centers reviewed and approved the protocol for the present analysis.
2.2. Patients
Patients with histologically confirmed HER2-negative unresectable advanced or recurrent gastric cancer were eligible for all three trials. HER2 positivity was defined as 3+ staining by immunohistochemistry or as HER2 gene amplification (HER2:CEP17 signal ratio of ≥2.0) as detected by in situ hybridization. For HERBIS-4A, patients were limited to those who were naïve to systemic chemotherapy [11], whereas those with disease recurrence during or within 6 months after the completion of adjuvant therapy with S-1 were eligible for HERBIS-2 [10]. For XParTS II, eligibility stipulated no previous chemotherapy or radiotherapy, with the exception of adjuvant chemotherapy if completed >6 months before enrollment [12]. Other eligibility criteria were as follows: age of ≥20 years (and ≤74 years for XParTS II); estimated life expectancy of ≥12 weeks; written informed consent; an Eastern Cooperative Oncology Group performance status of ≤2; and adequate organ function as reflected by a white blood cell count of ≥3000/mm3 for HERBIS-2 and -4A, neutrophil count of ≥1500/mm3, platelet count of ≥100,000/mm3, hemoglobin level of ≥8.0 g/dL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of ≤100 IU/L (≤150 IU/L in cases with metastasis to the liver) for HERBIS-2 and -4A or of ≤2.5× the upper limit of normal (ULN) at each institution (≤5× in cases with metastasis to the liver) for XParTS II, total bilirubin level of ≤1.50 mg/dL for HERBIS-2 and -4A or of ≤1.5× the ULN at each institution for XParTS II, serum creatinine level of ≤1.20 mg/dL for HERBIS-2 and -4A, and creatinine clearance of ≥60 mL/min (as estimated by the Cockcroft-Gault equation). Exclusion criteria included additional malignancies and serious comorbidities.
2.3. Endpoints and Assessments
The primary objective of the present study was to integrate the prospectively collected IPD from the HERBIS-2, HERBIS-4A, and XParTS II trials in order to compare SP with XP and to determine the optimal first-line chemotherapy for patients with HER2-negative unresectable advanced or recurrent gastric cancer. We examined differences in response between SP and XP, focusing on tumor histology and the form of the response such as the distribution of tumor shrinkage and changes in tumor burden, and how they might affect survival.
Tumor responses were analyzed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 in patients with at least one measurable lesion at baseline, and they were classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The ORR was defined as the proportion of patients with a CR or PR, and the disease control rate (DCR) as the proportion of patients with a CR, PR, or SD. Deepness of response (DpR) was defined as the maximum tumor shrinkage (%) observed throughout treatment.
Tumor histology was based on the Japanese classification of gastric carcinoma [9], with differentiated-type tumors being defined as papillary or tubular adenocarcinoma and undifferentiated-type tumors as poorly differentiated adenocarcinoma, signet ring cell carcinoma, or mucinous adenocarcinoma. Other histology types were designated as “other”.
2.4. Statistical Analysis
OS was defined as time from randomization to death, PFS as time from randomization to radiographic progression or death. Time to response (TTR) was defined as the interval between the start of therapy and the first confirmation of a response, and duration of response (DOR) as the time from documentation of a response to that of disease progression. Survival curves were constructed as time-to-event plots with the Kaplan–Meier method. Time-to-event curves were compared with the log-rank test, and HRs were estimated with Cox regression models. The confidence coefficient for confidence intervals (CIs) of median OS, PFS, DOR, TTR, and DpR values as well as of HRs was set to 95% (p < 0.05). The ORR and DCR were compared between arms with Fisher’s exact test. All statistical analysis was performed with the use of R version 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC, USA). A p value of <0.05 was considered statistically significant.
3. Results
3.1. Patients
A total of 211 patients was included in the initial trial database, with 49 patients being subsequently excluded from the study, 8 of 17 patients in HERBIS-2 and 41 of 110 patients in XParTS II because of the lack of a measurable lesion. All of the HERBIS-4A patients (n = 84) were included. IPD from a total of 162 patients were thus included in the current integrated analysis, with 79 patients in the SP arm and 83 patients in the XP arm (Supplementary Figure S1). Baseline characteristics were well balanced between the study arms, with potential imbalances including fewer female patients (14.5% vs. 24.1%), gastric body tumors (30.1% vs. 41.8%), and differentiated tumor types (38.6% vs. 49.4%) in the XP arm compared with the SP arm (Table 1).

Table 1.
Baseline characteristics of patients in the SP and XP arms for the integrated analysis.
3.2. Survival
In the current integrated analysis, SP showed a better survival outcome over XP. Median PFS was thus more favorable for SP compared with XP at 5.9 months (95% CI, 4.5–7.4 months) versus 5.1 months (95% CI, 4.1–5.8 months), with an HR of 0.717 (95% CI, 0.512–1.005) and p value of 0.052 (Figure 1a). Median OS in the SP arm (14.2 months, 95% CI of 12.4–16.7 months) was significantly longer than that in the XP arm (11.0 months, 95% CI of 8.5–14.2 months), with an HR of 0.704 (95% CI, 0.500–0.989) and p value of 0.048 (Figure 1b).
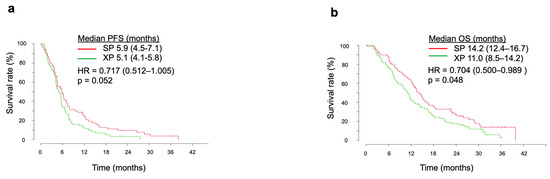
Figure 1.
Kaplan–Meier analysis of PFS (a), and OS (b) in the SP and XP arms in the integrated analysis. The p values were determined with the log-rank test.
ORR, Distribution of Tumor Shrinkage, and Changes in Tumor Burden
The ORR was similar between the SP and XP arms (48.1% vs. 50.6%, respectively, p = 0.755), whereas the DCR tended to be higher in the SP arm (83.5% vs. 72.3%, p = 0.137) (Supplementary Table S1). We also determined the DOR and TTR for patients who achieved a CR or PR (SP, n = 38; XP, n = 42). The median DOR for SP (7.0 months, 95% CI of 5.0–10.7 months) was double that for XP (3.5 months, 95% CI of 2.8–5.4 months), with this difference being statistically significant (p = 0.011) (Supplementary Figure S2a). On the other hand, the median TTR was similar between the SP and XP arms (2.1 months [95% CI, 1.8–2.3 months] vs. 1.8 months [95% CI, 1.6–2.2 months], respectively; HR of 0.741 [95% CI, 0.469–1.170]; p = 0.192) (Supplementary Figure S2b).
The distribution of tumor shrinkage for the SP and XP arms was visualized with waterfall plots (Figure 2a,b). Median DpR was 41.0% (95% CI, 15.4–64.0%) for the SP arm and 37.5% (95% CI, 8.47–53.5%) for the XP arm. Changes in tumor burden (assessed as the sum of the longest lesion dimensions) over time were visualized with spider plots (except for four and five cases of SP and XP arm, respectively, for which data were uncertain). Tumor reduction appeared to be more durable in patients who received SP (n = 75) (Figure 2c) than in those who received XP (n = 78) (Figure 2d), consistent with the significant differences in DOR between the two arms.
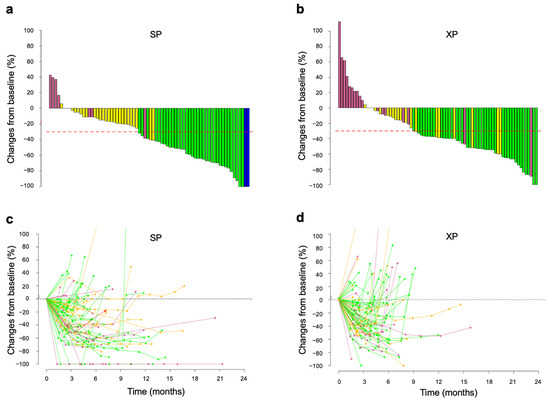
Figure 2.
Changes in tumor size during treatment with SP or XP. (a,b) Best change from baseline in the sum of the longest target lesion diameters for each patient receiving SP (a) or XP (b). Best responses are color-coded: blue, CR; green, PR; yellow, SD; pink, PD. Red dashed lines indicate tumor shrinkage of 30%. (c,d) Time course of the percentage change in the sum of the longest target lesion diameters from baseline in each patient receiving SP (c) or XP (d).
3.3. Differences in Treatment Effects between SP and XP According to Histology
We further investigated whether the treatment effects of SP and XP differed according to tumor histology. This analysis was performed for 77 patients in the SP arm (differentiated, n = 39; undifferentiated, n = 38) and 78 patients in the XP arm (differentiated, n = 32; undifferentiated, n = 46), excluding other histological types (2 and 5 cases in the SP and XP arms, respectively). We first examined differences in survival according to histology type. As seen in the overall analysis, there was no significant difference in median PFS between SP and XP arms for differentiated-type tumors (5.9 months [95% CI, 4.4–10.2 months] vs. 5.6 months [95% CI, 3.9–8.3 months], respectively; HR of 0.791 [95% CI, 0.479–1.307]; p = 0.358), whereas there was a trend for median PFS to be longer in the SP arm for undifferentiated-type tumors (5.9 months [95% CI, 4.4–10.2 months] vs. 4.4 months [95% CI, 4.1–5.8 months]; HR of 0.655 [95% CI, 0.404–1.061]; p = 0.086) (Figure 3a). A significant difference in median OS was apparent between the SP and XP arms for both tumor types, although the HR was lower for differentiated-type tumors (13.5 months [95% CI, 11.2–24.2 months] vs. 10.3 months [95% CI, 8.0–14.7 months]; HR of 0.506 [95% CI, 0.298–0.859]; p = 0.011) than for undifferentiated-type tumors (14.2 months [95% CI, 11.4–22.6 months] vs. 11.4 months [95% CI, 7.1–16.3 months]; HR of 0.790 [95% CI, 0.493–1.268]; p = 0.0011) (Figure 3b). The difference in OS between SP and XP arms was thus greater than that in PFS for differentiated tumors, whereas differences in PFS between the two arms appeared to consistently reflect that in OS for undifferentiated tumors.
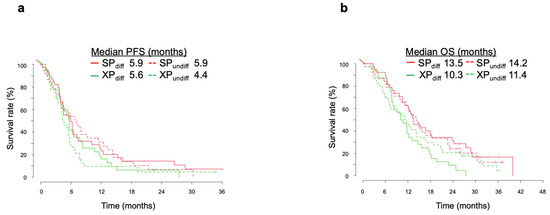
Figure 3.
Kaplan–Meier analysis of PFS (a), and OS (b) according to differentiated (diff, solid lines) or undifferentiated (undiff, dotted lines) tumor types in the SP and XP arms of the integrated analysis.
3.4. Association of the Longer OS in the SP Arm with Deeper Tumor Shrinkage for Differentiated Tumors
We further investigated whether the survival differences between SP and XP arms dependent on tumor histology were due to differences in tumor response rate or shrinkage, the latter analysis being performed for 73 patients in the SP arm (differentiated, n = 37; undifferentiated, n = 36) and 76 patients in the XP arm (differentiated, n = 31; undifferentiated, n = 45), excluding for four and five cases of SP and XP arm, respectively, for which data were uncertain. The ORR and DCR tended to be higher for differentiated tumors (ORR, 53.8% for SP and 56.3% for XP, p = >0.999; DCR, 87.2% for SP and 78.1% for XP, p = 0.354) than for undifferentiated tumors (ORR, 44.7% for SP and 45.7% for XP, p = >0.999; DCR, 81.6% for SP and 69.6% for XP, p = 0.311), with no differences between the treatment arms. However, deeper tumor shrinkage was apparent in the SP arm than in the XP arm for differentiated tumor types (Figure 4a,b), as shown by the fact that cases that achieved a >60% reduction were significantly more common in the SP arm than in the XP arm (29.7% [11/37] vs. 6.4% [2/31]; p = 0.038, Fisher’s exact test), suggesting that this deeper shrinkage may have contributed to the longer OS for the SP arm. In contrast, DpR appeared similar between SP and XP arms for undifferentiated tumor types (Figure 4c,d), but patients whose tumors increased in size from baseline tended to be less frequent in the SP arm than in the XP arm (2.8% [1/36] vs. 13.3% [6/45]; p = 0.123, Fisher’s exact test). Spider plots for each treatment arm according to histological type (Figure 4e–h) showed that a transient response in the XP arm was apparent for both tumor types, but was more pronounced for undifferentiated tumors, reflecting the shorter PFS in the XP arm than in the SP arm for this subgroup. Overall, these findings thus suggested that the therapeutic benefit of SP over XP as first-line treatment varies greatly depending on histological type.
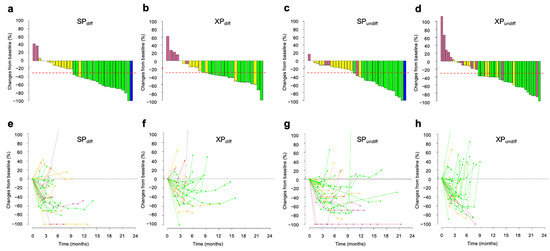
Figure 4.
Changes in tumor size according to tumor histology during treatment with SP or XP. (a–d) Best change from baseline in the sum of the longest target lesion diameters for differentiated tumors (diff, a,b) or undifferentiated tumors (undiff, c,d) in each patient treated with SP (a,c) or XP (b,d). Best responses are color-coded: blue, CR; green, PR; yellow, SD; pink, PD. Red dashed lines indicate tumor shrinkage of 30%. (e–h) Time course of the percentage change in the sum of the longest target lesion diameters from baseline for differentiated tumors (diff, e,f) or undifferentiated tumors (undiff, g,h) in each patient treated with SP (e,g) or XP (f,h).
Finally, we performed subgroup analysis for PFS and OS according to tumor histology (differentiated type vs. undifferentiated type) and tumor shrinkage (tumor size reduction of ≥30% from baseline vs. <30%) (Figure 5). Although both PFS and OS tended to be better for SP than for XP in most subgroups, a significant benefit of SP versus XP was apparent only for OS in patients with differentiated tumors and a tumor size reduction of ≥30% (23.7 months [95% CI, 13.2–not available] vs. 11.7 months [95% CI, 7.8–19.6 months]; HR of 0.339 [95% CI, 0.163–0.705]; interaction p = 0.003; Supplementary Figure S4), suggesting that deeper tumor shrinkage induced by SP compared with XP contributed to prolongation of OS for patients with differentiated tumors.
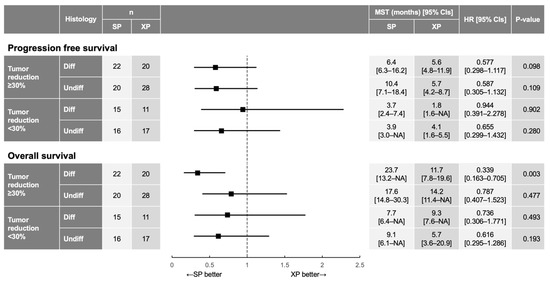
Figure 5.
HR and 95% CI for PFS and OS in patient subgroups defined by the extent of tumor reduction and tumor histology. The p values were determined with the log-rank test. MST, median survival time; NA, not available.
4. Discussion
There have been various comparisons between S-1–based and capecitabine-based regimens for first-line treatment of advanced gastric cancer, but none of the individual trials [10,11,12] or combined abstract-based analyses [13,14,15,16,17,18] has shown a significant survival difference. The present analysis showed that SP significantly outperformed XP in terms of OS, with a trend toward a better PFS also for the SP arm. As far as we are aware, this is the first evidence of a significant OS benefit for SP over XP. This significant finding may have emerged as a result both of the increased number of patients in our combined analysis of the three original trials and of the fact that the analysis was limited to patients with measurable disease. In addition, it is important to note that the present analysis was based on IPD rather than abstract based, with the latter approach evaluating only median values. On the other hand, there was no significant difference in the ORR between SP and XP. Given that the ORR corresponds to the percentage of patients whose tumors shrink by ≥30%, which is commonly adopted as an indicator of treatment efficacy, it serves as a “temporary measure” during treatment and may not reveal efficacy over the entire treatment period. We found that the pattern of tumor shrinkage differed between the SP and XP arms, with spider plots clarifying that this difference likely accounts for the significant difference in DOR between the two arms. A high DCR and durability of tumor shrinkage are advantageous for gastric cancer patients, given that worsening tumor control is directly related to loss of quality of life as a result of the inability to eat.
Despite the difference in drug design, it has remained uncertain how to discriminate the use of S-1 from that of capecitabine. We have now shown that these two drugs differ qualitatively in terms of efficacy in a manner dependent on tumor histology. Of interest, no difference in the ORR was apparent between SP and XP for subgroups based on tumor histology. However, for undifferentiated tumors, the percentage of patients whose tumors did not shrink tended to be greater for XP than for SP. This difference may directly reflect the observed differences in PFS, TTF, and OS. On the other hand, for differentiated tumors, SP resulted in deeper tumor shrinkage compared with XP, as shown by the observation that the proportion of patients who achieved a tumor reduction of >60% was significantly greater for SP. The cutoff value for DpR of 60% seemed reasonable given that cutoff values of 50% or 62.4% were previously adopted as predictors of longer survival for first-line chemotherapy in Japanese patients with HER2-positive gastric cancer, for whom the median DpR was 56.8% [19], and in patients with colorectal cancer [20], respectively. Our subgroup analysis indicated that deeper tumor shrinkage induced by SP compared with XP contributed to OS prolongation in patients with differentiated tumors. Association of no difference in ORR and PFS with a large difference in OS, as observed for differentiated tumors in the present study, can occur when DpR is large, as has been seen in previous studies [21,22] and given that an increased DpR is more associated with prolongation of postprogression survival and therefore OS than with the ORR or PFS [21,22]. Together, our data have thus revealed that qualitative differences between the effects of SP and XP were highlighted by tumor histological classification.
Our study has several limitations. Although it was based on IPD from prospective studies, it was conducted in an ad hoc setting and should be interpreted with caution. In addition, our analysis was limited to patients with measurable lesions, and the results may therefore not be applicable to all patients. Furthermore, the platinum agent of the treatment regimens was cisplatin, and so it is not clear whether our results will also be applicable to regimens based on other platinum agents. In recent years, oxaliplatin has become more commonly administered than cisplatin as the platinum agent in combination with fluoropyrimidines for gastric cancer. Indeed, S-1 plus oxaliplatin (SOX) and capecitabine plus oxaliplatin (CapeOX) are currently recommended regimens in the treatment guidelines. A previous randomized phase II study showed no significant difference between SOX and CapeOX in efficacy such as OS, PFS, and ORR [23], but they lacked the information regarding DpR or histology type as an assessment of efficacy. Currently, nivolumab, an immune checkpoint inhibitor, in combination with oxaliplatin and fluoropyrimidines is a standard therapy for HER2-negative unresectable advanced or recurrent gastric cancer on the basis of recent phase III studies [24,25], raising the issue of whether SOX or CapeOX should be used as backbone chemotherapy. Of interest, in the phase II part of the latter (ATTRACTION-4) study [26], although the ORR was similar for the SOX-nivolumab arm and the CapeOX-nivolumab arm (66.7% vs. 70.6%, respectively), the waterfall plot is suggestive of a deeper tumor shrinkage in the SOX arm than in the CapeOX arm, consistent with our results. Further studies focusing on histology are needed to clarify the difference in efficacy of SOX versus CapeOX in combination with nivolumab, given that a recent study suggested an association between a high tumor burden and immunosuppressive phenotypes [27].
5. Conclusions
In conclusion, our data have shown that SP is superior to XP for patients with HER2-negative unresectable advanced or recurrent gastric cancer, but that there are qualitative differences between the effects of SP and XP that depend on the histological type of the tumor. For undifferentiated tumors, SP has fewer treatment failures than does XP, as is reflected in a better PFS, and OS. For differentiated tumors, SP achieves deeper tumor shrinkage compared with XP, which contributes to a longer OS, but not PFS. Further study is warranted to determine whether these differences for S-1 versus capecitabine are reproduced in combination with oxaliplatin and immune checkpoint inhibitors, the new standard of care for HER2-negative unresectable advanced or recurrent gastric cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14225673/s1, Figure S1: Patient flow for the integrated analysis of the SP and XP arms; Figure S2: Kaplan–Meier analysis of DOR (a) and TTR (b) for the SP and XP arms in the integrated analysis; Figure S3: Kaplan–Meier analysis of PFS (a and b) and OS (c and d) for patients with a tumor reduction of ≥30% (a and c) or <30% (b and d) from baseline according to differentiated (diff, solid lines) or undifferentiated (undiff, dotted lines) tumor types in the SP and XP arms of the integrated analysis. Table S1: Overall response for SP and XP in the integrated analysis.
Author Contributions
H.K.: Conceptualization, Methodology, Project administration, Resources, Funding acquisition, Visualization, Writing—original draft, Writing—Reviewing and Editing. K.N.: Conceptualization, Methodology, Project administration, Resources, Visualization, Writing—Reviewing and Editing. T.S. (Toshio Shimokawa): Data curation, Formal analysis, Investigation, Software, Validation, Visualization. K.F.: Resources, Writing—Reviewing and Editing. S.T.: Resources, Writing—Reviewing and Editing. S.E.: Resources, Writing—Reviewing and Editing. M.K.: Resources, Writing—Reviewing and Editing. J.K.: Resources, Writing—Reviewing and Editing. Y.K.: Resources, Writing—Reviewing and Editing. A.T.: Resources, Writing—Reviewing and Editing. T.Y.: Resources, Writing—Reviewing and Editing. J.S.: Supervision, Writing—Reviewing and Editing. T.S. (Taroh Satoh): Supervision, Resources, Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Osaka Clinical Study Supporting Organization and in part by Mr. Futomitsu Toyokawa.
Institutional Review Board Statement
This analysis was conducted according to the Declaration of Helsinki. All patients in the three trials provided written consent after being informed about the purpose and investigational nature of the respective studies. The institutional review boards or ethics committees of all participating centers reviewed and approved the protocol for the present analysis.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, H.K., upon reasonable request.
Acknowledgments
We thank all the patients, investigators, and medical staff at the participating institutions who contributed to this study.
Conflicts of Interest
H.K. has received consulting fees from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., and Taiho Pharmaceutical Co. Ltd.; honoraria from Bristol-Myers Squibb Co. Ltd., Bayer Yakuhin Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Merck Biopharma Co. Ltd., Takeda Pharmaceutical Co. Ltd., Yakult Pharmaceutical Industry, Teijin Pharma Ltd., and Taiho Pharmaceutical Co. Ltd.; lecture fees from Glaxo Smith Kline K.K. and Otsuka Pharmaceutical Co. Ltd.; and research funding from Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Kobayashi Pharmaceutical Co. Ltd., and Eisai Co. Ltd. K.N. has received honoraria for lectures from Bristol-Myers Squibb Co. Ltd., Daiichi-Sankyo Co. Ltd., EA Pharma Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. S.E. has received lecture fees from Taiho Pharmaceutical Co., Ltd. and Ono Pharmaceutical Co., Ltd. Y. K. has received research funding from Yakult Honsha and Taiho Pharmaceutical and lecture fees from Yakult Honsha, Taiho Pharmaceutical, and Nippon Kayaku. T. Satoh has received departmental research grants from Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., and Yakult Honsha Co. Ltd., and honoraria from Taiho Pharmaceutical Co. Ltd., and Chugai Pharmaceutical Co. Ltd. All remaining authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shirasaka, T.; Shimamato, Y.; Ohshimo, H.; Yamaguchi, M.; Kato, T.; Yonekura, K.; Fukushima, M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anti-Cancer Drugs 1996, 7, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Miwa, M.; Ura, M.; Nishida, M.; Sawada, N.; Ishikawa, T.; Mori, K.; Shimma, N.; Umeda, I.; Ishitsuka, H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer 1998, 34, 1274–1281. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in Combination with Chemotherapy as First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. J. Clin. Oncol. 2011, 29, 3968–3976. [Google Scholar] [CrossRef]
- Lordick, F.; Kang, Y.-K.; Chung, H.-C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Shin, D.-B.; Chen, J.; Xiong, J.; Wang, J.; Lichinitser, M.; Guan, Z.; Khasanov, R.; Zheng, L.; Philco-Salas, M.; et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann. Oncol. 2009, 20, 666–673. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Kawakami, H.; for the Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG); Fujitani, K.; Matsuyama, J.; Akamaru, Y.; Tamura, S.; Endo, S.; Kimura, Y.; Makari, Y. Comparison of S-1–cisplatin every 5 weeks with capecitabine-cisplatin every 3 weeks for HER2-negative gastric cancer (recurrent after S-1 adjuvant therapy or chemotherapy-naïve advanced): Pooled analysis of HERBIS-2 (OGSG 1103) and HERBIS-4A (OGSG 1105) trials. Int. J. Clin. Oncol. 2020, 25, 1635–1643. [Google Scholar] [CrossRef]
- Kawakami, H.; Takeno, A.; Endo, S.; Makari, Y.; Kawada, J.; Taniguchi, H.; Tamura, S.; Sugimoto, N.; Kimura, Y.; Tamura, T.; et al. Randomized, Open-Label Phase II Study Comparing Capecitabine-Cisplatin Every 3 Weeks with S-1-Cisplatin Every 5 Weeks in Chemotherapy-Naïve Patients with HER2-Negative Advanced Gastric Cancer: OGSG1105, HERBIS-4A Trial. Oncologist 2018, 23, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Tsuburaya, A.; Yoshikawa, T.; Kobayashi, M.; Kawada, J.; Fukushima, R.; Matsui, T.; Tanabe, K.; Yamaguchi, K.; Yoshino, S.; et al. A randomised phase II trial of capecitabine plus cisplatin versus S-1 plus cisplatin as a first-line treatment for advanced gastric cancer: Capecitabine plus cisplatin ascertainment versus S-1 plus cisplatin randomised PII trial (XParTS II). Eur. J. Cancer 2018, 101, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Qu, J.; Song, N.; Chen, Y.; Cheng, Y.; Zhang, S.; Qu, X.; Liu, Y. Clinical outcomes of capecitabine-based versus S-1-based regimens as first-line chemotherapy in patients with unresectable or metastatic gastric cancer: A propensity score matched single-center comparison. J. Gastrointest. Oncol. 2020, 11, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yan, P.; Hou, X.; Feng, J.; He, X.; Yang, K. The efficacy and safety of capecitabine-based versus S-1-based chemotherapy for metastatic or recurrent gastric cancer: A systematic review and meta-analysis of clinical randomized trials. Ann. Palliat. Med. 2020, 9, 883–894. [Google Scholar] [CrossRef] [PubMed]
- He, M.-M.; Wu, W.-J.; Wang, F.; Wang, Z.-Q.; Zhang, D.-S.; Luo, H.-Y.; Qiu, M.-Z.; Ren, C.; Zeng, Z.-L.; Xu, R.-H. S-1-Based Chemotherapy versus Capecitabine-Based Chemotherapy as First-Line Treatment for Advanced Gastric Carcinoma: A Meta-Analysis. PLoS ONE 2013, 8, e82798. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, J.; Rao, Y.; Yang, W. Should S-1 be better than capecitabine for patients with advanced gastric cancer in Asia? A systematic review and meta-analysis. OncoTargets Ther. 2018, 12, 269–277. [Google Scholar] [CrossRef]
- Ter Veer, E.; Ngai, L.L.; Van Valkenhoef, G.; Mohammad, N.H.; Anderegg, M.C.J.; Van Oijen, M.G.H.; Van Laarhoven, H.W.M. Capecitabine, 5-fluorouracil and S-1 based regimens for previously untreated advanced oesophagogastric cancer: A network meta-analysis. Sci. Rep. 2017, 7, 7142. [Google Scholar] [CrossRef]
- He, A.-B.; Peng, X.-L.; Song, J.; Zhang, J.-X.; Dong, W.-G.; Luo, R.-F.; Tang, Y. Efficacy of S-1vscapecitabine for the treatment of gastric cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 4358–4364. [Google Scholar] [CrossRef]
- Kadowaki, S.; Masuishi, T.; Eto, T.; Narita, Y.; Taniguchi, H.; Ura, T.; Ando, M.; Tajika, M.; Niwa, Y.; Yatabe, Y.; et al. Depth of response predicts the clinical outcome of advanced HER2-positive gastric cancer to trastuzumab-based first-line chemotherapy. Cancer Chemother. Pharmacol. 2017, 80, 807–813. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lonardi, S.; Masi, G.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Spadi, R.; Zaniboni, A.; et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: Results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann. Oncol. 2015, 26, 1188–1194. [Google Scholar] [CrossRef]
- Heinemann, V.; Stintzing, S.; Modest, D.P.; Giessen-Jung, C.; Michl, M.; Mansmann, U.R. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur. J. Cancer 2015, 51, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef]
- Kim, G.M.; Jeung, H.-C.; Rha, S.Y.; Kim, H.S.; Jung, I.; Nam, B.H.; Lee, K.H.; Chung, H.C. A randomized phase II trial of S-1-oxaliplatin versus capecitabine–oxaliplatin in advanced gastric cancer. Eur. J. Cancer 2012, 48, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 3, 27–40. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Chen, L.-T.; Ryu, M.-H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]
- Boku, N.; Ryu, M.-H.; Kato, K.; Chung, H.; Minashi, K.; Lee, K.-W.; Cho, H.; Kang, W.; Komatsu, Y.; Tsuda, M.; et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: Interim results of a randomized, phase II trial (ATTRACTION-4). Ann. Oncol. 2019, 30, 250–258. [Google Scholar] [CrossRef]
- Suzuki, S.; Haratani, K.; Hayashi, H.; Chiba, Y.; Tanizaki, J.; Kato, R.; Mitani, S.; Kawanaka, Y.; Kurosaki, T.; Hasegawa, Y.; et al. Association of tumour burden with the efficacy of programmed cell death-1/programmed cell death ligand-1 inhibitors for treatment-naïve advanced non-small-cell lung cancer. Eur. J. Cancer 2021, 161, 44–54. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).