PSMA PET for the Evaluation of Liver Metastases in Castration-Resistant Prostate Cancer Patients: A Multicenter Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
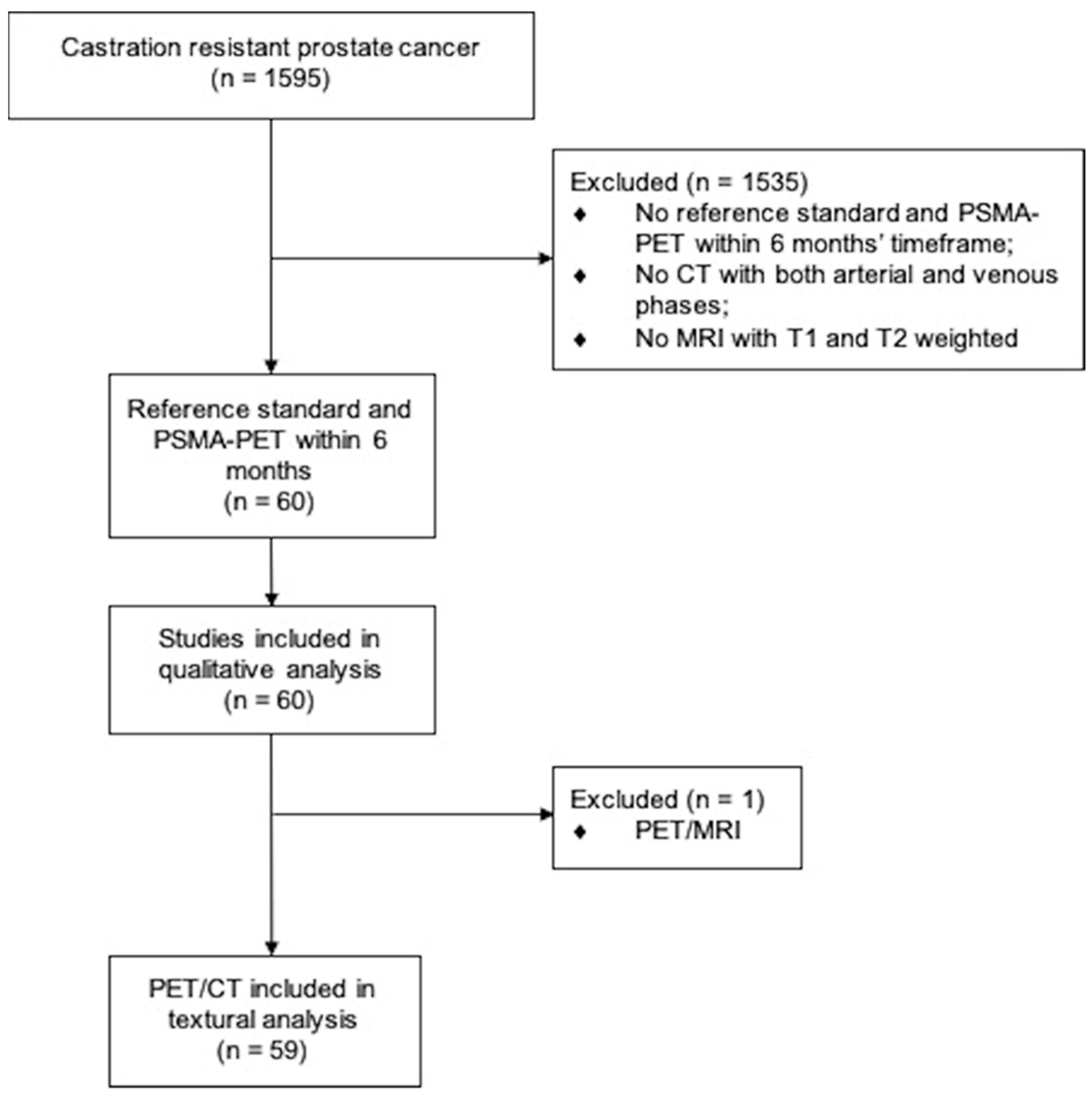
2.1. Study Design and Participants
2.2. PSMA-PET Imaging Procedures
2.3. Reference Standard Procedures
2.3.1. CT Imaging
2.3.2. MRI Imaging
2.3.3. Liver Biopsy
2.4. Image Interpretation
2.5. Radiomic Extraction
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Lesion Detection
3.2.1. Reference Standard
3.2.2. PSMA-PET
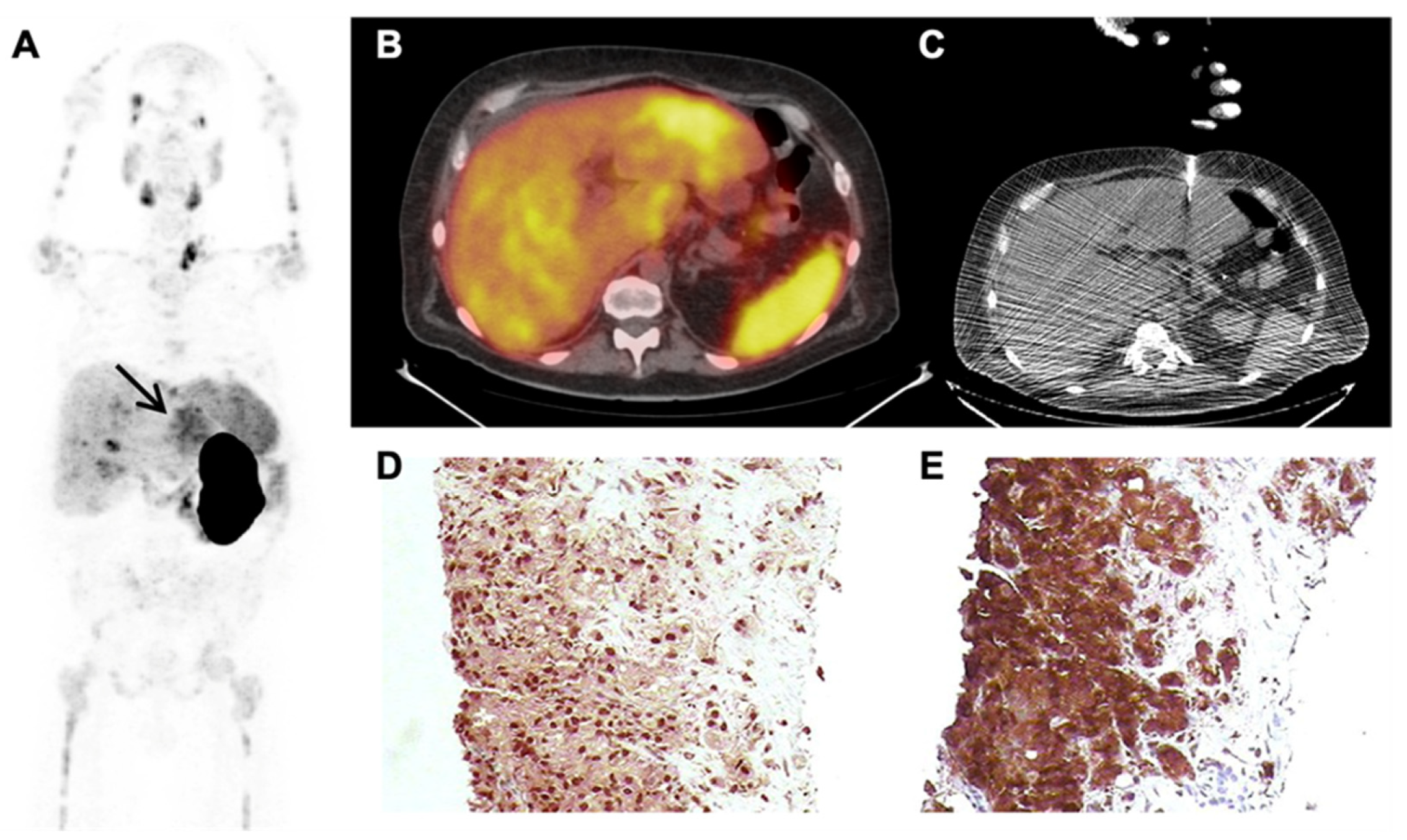
3.2.3. False Positive PSMA-PET Findings
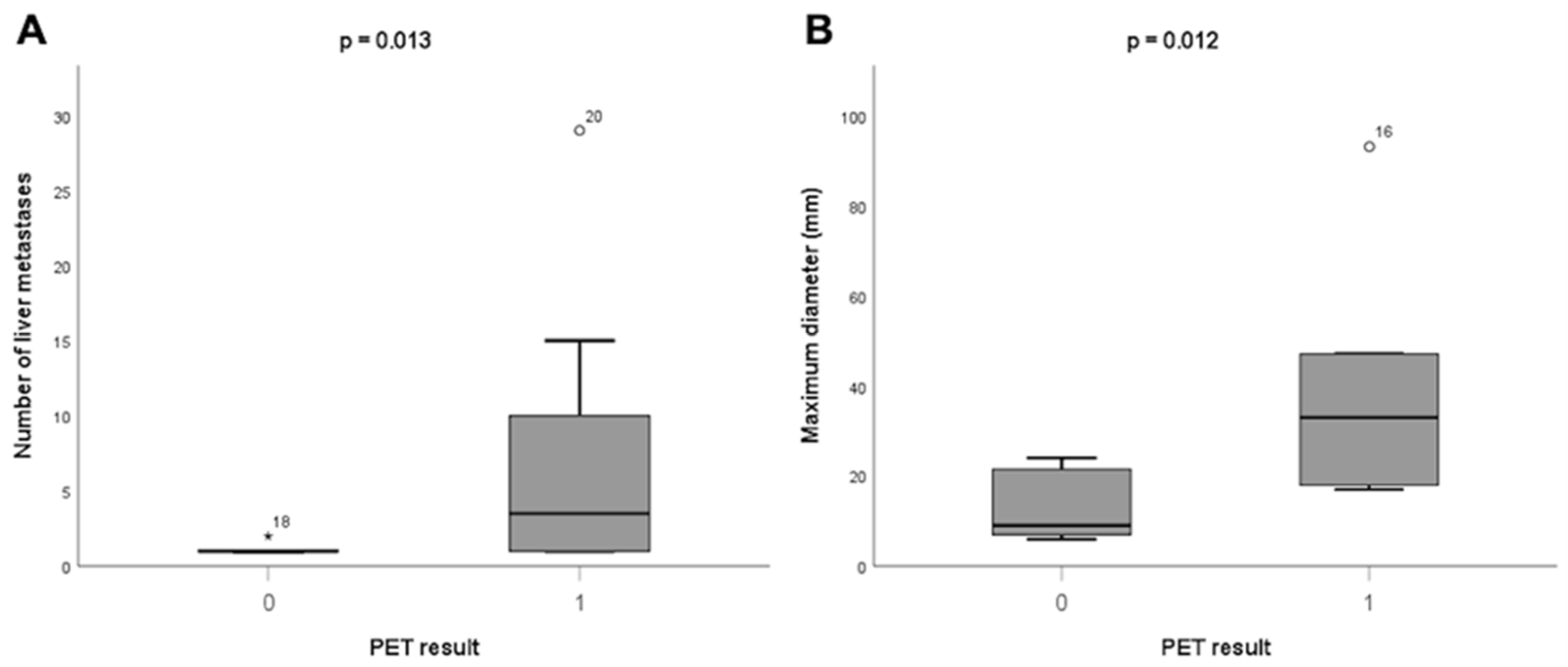
3.2.4. Factors Associated with Liver Metastases
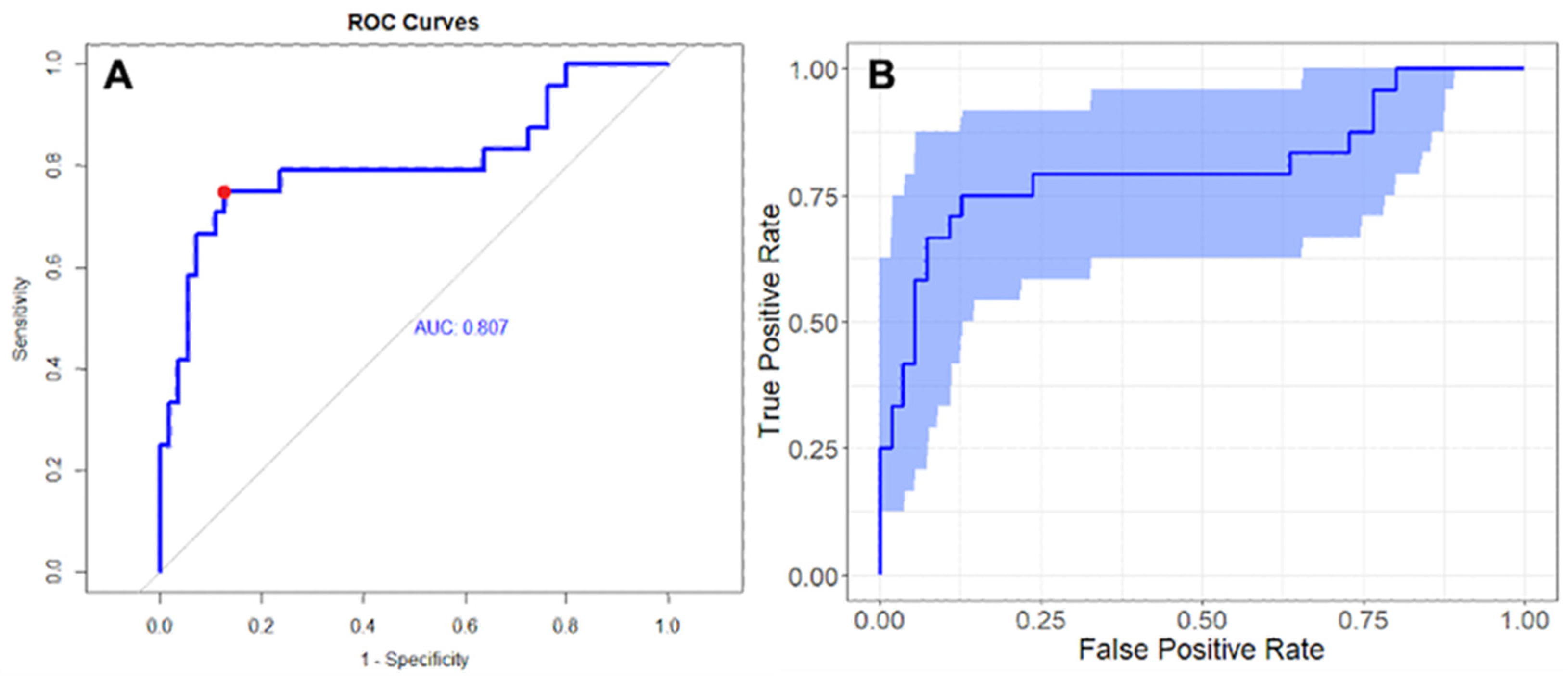
3.3. Radiomic Analysis
4. Discussion
4.1. [68Ga]Ga-PSMA-11 PET in CRPC Patients with Liver Metastases
4.2. Diagnostic Performance of PSMA-PET in the Detection of Liver Metastases Compared to Conventional Imaging/Liver Biopsy
4.3. Potential Use of Radiamics
4.4. Strengths of the Study
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Pouessel, D.; Gallet, B.; Bibeau, F.; Avancès, C.; Iborra, F.; Sénesse, P.; Culine, S. Liver metastases in prostate carcinoma: Clinical characteristics and outcome. BJU Int. 2007, 99, 807–811. [Google Scholar] [CrossRef]
- Vinjamoori, A.H.; Jagannathan, J.P.; Shinagare, A.B.; Taplin, M.E.; Oh, W.K.; Van den Abbeele, A.D.; Ramaiya, N.H. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am. J. Roentgenol. 2012, 199, 367–372. [Google Scholar] [CrossRef]
- FDA Approves First PSMA-Targeted PET Drug. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 11N. Available online: https://pubmed.ncbi.nlm.nih.gov/33468545/ (accessed on 15 November 2022).
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.S.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Small, E.J.; Smith, M.R.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef]
- Farolfi, A.; Hirmas, N.; Gafita, A.; Weber, M.; Barbato, F.; Wetter, A.; Mei, R.; Pianori, D.; Hadaschik, B.; Herrmann, K.; et al. PSMA-PET identifies PCWG3 target populations with superior accuracy and reproducibility when compared to conventional imaging: A multicenter retrospective study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 675–678. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Nehmeh, S.A.; Erdi, Y.E. Respiratory motion in positron emission tomography/computed tomography: A review. Semin. Nucl. Med. 2008, 38, 167–176. [Google Scholar] [CrossRef]
- Damjanovic, J.; Janssen, J.C.; Furth, C.; Diederichs, G.; Walter, T.; Amthauer, H.; Makowski, M.R. 68 Ga-PSMA-PET/CT for the evaluation of pulmonary metastases and opacities in patients with prostate cancer. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2018, 18, 20. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Cerci, J.J.; Pereira Neto, C.C.; Krauzer, C.; Sakamoto, D.G.; Vitola, J.V. The impact of coaxial core biopsy guided by FDG PET/CT in oncological patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.M.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Pond, G.R.; Sonpavde, G.; De Wit, R.; Eisenberger, M.A.; Tannock, I.F.; Armstrong, A.J. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur. Urol. 2014, 65, 3–6. [Google Scholar] [CrossRef]
- Gandaglia, G.; Karakiewicz, P.I.; Briganti, A.; Passoni, N.M.; Schiffmann, J.; Trudeau, V.; Graefen, M.; Montorsi, F.; Sun, M. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur. Urol. 2015, 68, 325–334. [Google Scholar] [CrossRef]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men with Castration-Resistant Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
- Pepe, P.; Pennisi, M. Should 68Ga-PSMA PET/CT Replace CT and Bone Scan in Clinical Staging of High-risk Prostate Cancer? Anticancer Res. 2022, 42, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Pepe, L.; Cosentino, S.; Ippolito, M.; Pennisi, M.; Fraggetta, F. Detection Rate of 68Ga-PSMA PET/CT vs. mpMRI Targeted Biopsy for Clinically Significant Prostate Cancer. Anticancer Res. 2022, 42, 3011–3015. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Calais, J.; Allen-Auerbach, M.; Bluemel, C.; Eberhardt, N.; Emmett, L.; Gupta, P.; Hartenbach, M.; Hope, T.A.; Okamoto, S.; et al. 68Ga-PSMA-11 PET/CT Interobserver Agreement for Prostate Cancer Assessments: An International Multicenter Prospective Study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, J.; Janssen, J.C.; Prasad, V.; Diederichs, G.; Walter, T.; Brenner, W.; Makowski, M.R. 68Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2019, 19, 37. [Google Scholar] [CrossRef]
- Derlin, T.; Werner, R.A.; Lafos, M.; Henkenberens, C.; von Klot, C.A.J.; Sommerlath Sohns, J.M.; Ross, T.L.; Bengel, F.M. Neuroendocrine Differentiation and Response to PSMA-Targeted Radioligand Therapy in Advanced Metastatic Castration-Resistant Prostate Cancer: A Single-Center Retrospective Study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Okada, Y.; Minei, S.; Takimoto, Y.; Nemoto, N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur. Urol. 2004, 45, 586–592; discussion 592. [Google Scholar] [CrossRef]
- Usmani, S.; Ahmed, N.; Marafi, F.; Rasheed, R.; Amanguno, H.G.; Al Kandari, F. Molecular Imaging in Neuroendocrine Differentiation of Prostate Cancer: 68Ga-PSMA Versus 68Ga-DOTA NOC PET-CT. Clin. Nucl. Med. 2017, 42, 410–413. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Stephens, M.; Bhatt, M.; Thomas, P.A. Prostate-Specific Membrane Antigen PET/CT Findings for Hepatic Hemangioma. Clin. Nucl. Med. 2016, 41, 968–969. [Google Scholar] [CrossRef]
- Kesler, M.; Levine, C.; Hershkovitz, D.; Mishani, E.; Menachem, Y.; Lerman, H.; Zohar, Y.; Shibolet, O.; Even-Sapir, E. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: A prospective pilot study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 185–191. [Google Scholar] [CrossRef]
- Hirmas, N.; Leyh, C.; Sraieb, M.; Barbato, F.; Schaarschmidt, B.M.; Umutlu, L.; Nader, M.; Wedemeyer, H.; Ferdinandus, J.; Rischpler, C.; et al. [68Ga]Ga-PSMA-11 PET/CT improves tumor detection and impacts management in patients with hepatocellular carcinoma (HCC). J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 1235–1241. [Google Scholar] [CrossRef]
- Hoberück, S.; Driesnack, S.; Seppelt, D.; Michler, E.; Hölscher, T.; Kotzerke, J. Hepatic Vascular Malformation Mimics PSMA-Positive Prostate Cancer Metastasis. Clin. Nucl. Med. 2020, 45, e283–e284. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Koeber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Altazi, B.A.; Zhang, G.G.; Fernandez, D.C.; Montejo, M.E.; Hunt, D.; Werner, J.; Biagioli, M.C.; Moros, E.G. Reproducibility of F18-FDG PET radiomic features for different cervical tumor segmentation methods, gray-level discretization, and reconstruction algorithms. J. Appl. Clin. Med. Phys. 2017, 18, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Carles, M.; Torres-Espallardo, I.; Alberich-Bayarri, A.; Olivas, C.; Bello, P.; Nestle, U.; Martí-Bonmatí, L. Evaluation of PET texture features with heterogeneous phantoms: Complementarity and effect of motion and segmentation method. Phys. Med. Biol. 2017, 62, 652–668. [Google Scholar] [CrossRef]
| Mean ± SD | Median | IQR | |
|---|---|---|---|
| Age (years) | 70 (9) | 72 | 64–75 |
| PSA at time of PET scan (ng/mL) | 80.5 (179.9) | 6.3 | 1.6–45.3 |
| ∆ date PET—date reference standard (months) | 3 (3) | 1 | 0–5 |
| Frequency | % | ||
| ≥pT3a | 31/39 | 80 | |
| pN1 | 20/41 | 49 | |
| ISUP Grade Group ≥ 4 | 30/51 | 57 | |
| Previous therapies | |||
| Radical prostatectomy | 44/60 | 73 | |
| External beam radiation therapy | 4/60 | 7 | |
| Hormonal therapy | 60/60 | 100 | |
| Docetaxel | 12/60 | 20 | |
| Cabazitaxel | 6/60 | 10 | |
| Abiraterone/Enzalutamide/Apalutamide | 23/60 | 38 | |
| Radium-223 | 3/60 | 5 | |
| Palliative RT | 2/60 12/60 | 3 20 | |
| PSMA-RLT On-going hormonal therapy at the time of PET | 43/60 | 72 |
| Single Lesion (%) | Multiple Lesions (%) | Total (%) | Median SUVmax Lesion (IQR) | Median SUVmax Liver Parenchyma (IQR) | |
|---|---|---|---|---|---|
| Absent uptake | 1 (6) | - | 1 (6) | 4 (4–4) | 11 (11–11) |
| Increased uptake | 5 (29) | 9 (53) | 14 (82) | 20 (18–24) | 6 (4–8) |
| Heterogeneous uptake | - | 2 (12) | 2 (12) | 25 (12–22) | 6 (6–7) |
| Total | 6 (35) | 11 (65) | 17 (100) | 20 (17–24) | 6 (4–8) |
| Variable | Estimate | Standard Error | T Value | Pr(>|t|) | Odds Ratio | Sign 95% |
|---|---|---|---|---|---|---|
| (Intercept) | 2.53 | 0.63 | 3.99 | 0.00015 | 12.54 | [4.42; 35.56] |
| CT_Sphericity | −3.99 | 1.05 | −3.80 | 0.00029 | 0.018 | [0.003; 0.10] |
| PET_Idm | 1.67 | 0.57 | 2.93 | 0.00449 | 5.29 | [2.08; 13.48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattoni, S.; Farolfi, A.; Formaggio, F.; Bruno, G.; Caroli, P.; Cerci, J.J.; Eiber, M.; Fendler, W.P.; Golfieri, R.; Herrmann, K.; et al. PSMA PET for the Evaluation of Liver Metastases in Castration-Resistant Prostate Cancer Patients: A Multicenter Retrospective Study. Cancers 2022, 14, 5680. https://doi.org/10.3390/cancers14225680
Mattoni S, Farolfi A, Formaggio F, Bruno G, Caroli P, Cerci JJ, Eiber M, Fendler WP, Golfieri R, Herrmann K, et al. PSMA PET for the Evaluation of Liver Metastases in Castration-Resistant Prostate Cancer Patients: A Multicenter Retrospective Study. Cancers. 2022; 14(22):5680. https://doi.org/10.3390/cancers14225680
Chicago/Turabian StyleMattoni, Susanna, Andrea Farolfi, Fabio Formaggio, Gabriel Bruno, Paola Caroli, Juliano Julio Cerci, Matthias Eiber, Wolfgang Peter Fendler, Rita Golfieri, Ken Herrmann, and et al. 2022. "PSMA PET for the Evaluation of Liver Metastases in Castration-Resistant Prostate Cancer Patients: A Multicenter Retrospective Study" Cancers 14, no. 22: 5680. https://doi.org/10.3390/cancers14225680
APA StyleMattoni, S., Farolfi, A., Formaggio, F., Bruno, G., Caroli, P., Cerci, J. J., Eiber, M., Fendler, W. P., Golfieri, R., Herrmann, K., Matteucci, F., Mosconi, C., Paolani, G., Santoro, M., Strigari, L., Nanni, C., Castellucci, P., & Fanti, S. (2022). PSMA PET for the Evaluation of Liver Metastases in Castration-Resistant Prostate Cancer Patients: A Multicenter Retrospective Study. Cancers, 14(22), 5680. https://doi.org/10.3390/cancers14225680










