Simple Summary
Crossing biological barriers is often required for drug delivery. Cell-penetrating peptides (CPPs) are short strands of amino acids that have been widely used as a delivery vehicle to overcome barriers for various applications. This review aims to emphasize the role of CPPs as permeation enhancers for targeted drug delivery applications. We also discuss the prospect of clinical translation of CPP-functionalized drug delivery systems in oncology. This may help facilitate the development of new types of CPPs for preventing or treating cancer.
Abstract
Cell-Penetrating Peptides (CPPs) are short peptides consisting of <30 amino acids. Their ability to translocate through the cell membrane while carrying large cargo biomolecules has been the topic of pre-clinical and clinical trials. The ability to deliver cargo complexes through membranes yields potential for therapeutics and diagnostics for diseases such as cancer. Upon cellular entry, some CPPs have the ability to target specific organelles. CPP-based intracellular targeting strategies hold tremendous potential as they can improve efficacy and reduce toxicities and side effects. Further, recent clinical trials show a significant potential for future CPP-based cancer treatment. In this review, we summarize recent advances in CPPs based on systematic searches in PubMed, Embase, Web of Science, and Scopus databases until 30 September 2022. We highlight targeted delivery and explore the potential uses for CPPs as diagnostics, drug delivery, and intrinsic anti-cancer agents.
1. Introduction
The selective semi-permeability of the cell membrane protects the cell interior from harsh materials from outside of the cell while also allowing vital nutrients and materials to pass in and out. This same mechanism presents a real challenge when trying to deliver selective molecules into the cell. Current attempts to deliver therapeutics and diagnostic agents into cells are arduous and prone to restrictions and errors [1,2]. Despite extensive research efforts on drug delivery, it generally yields low cell specificity and leads to significant toxicity [1,2]. The ideal goal is to deliver the cargo molecules to the desired cancerous cells and avoid the healthy cells. Cell-penetrating peptides (CPPs) or protein transduction domains (PTDs) are relatively short and, in many cases, cationic amino acids that possess the titular ability to penetrate the cellular membrane [2]. Some CPPs can selectively interact with target cells with high accuracy and efficiency, and operate even at low concentrations. Several modes of CPPs internalization have been discussed [3,4,5,6,7]. Moreover, one of the most attractive aspects of CPPs is their ability to covalently link to macromolecular cargos, such as DNA, RNA, and proteins and deliver them into the cell. Thus, larger biomolecules that would normally be restricted from entering the cell, because of the selective impermeability of the cell membrane, can be translocated into the cell while being escorted by CPPs. Early indicators suggest that some of CPPs have a higher uptake efficacy and delivery efficiency while remaining less cytotoxic than other similar treatments, such as nanoparticles or virus vectors [8].
Since their discovery in 1988, the high potential and variety of CPPs have led to scores of different peptides being cataloged, all with different specificity and cargo-carrying capabilities. In recent decades, upwards of 1850 CPPs have been cataloged within CPPsite 2.0 [9], each with a unique peptide sequence, target cell specificity, and covalent cargo binding capabilities [10]. For example, the Trans-activator of Transcription (TAT) protein identified from the HIV1 virus allows for penetration of the cellular membranes and allows for direct interaction with the cell nucleus [11]. The chimeric approaches have been made to combine known CPPs such as TAT, Transportan, or pVEC with novel peptides [12,13,14,15,16,17,18,19]. All this is to highlight that the discovery and further research into the domain of CPPs yield countless possibilities on how to attack cancer by targeting specific intracellular molecules or organelles, whether it be by disrupting oncogenic pathways as a preventative measure, or by introducing therapeutic or diagnostic agents to cancer cells. In this review, we highlighted and summarized the recent updates of CPPs-based targeted delivery along with the therapeutic and diagnostic potential.
2. Types of CPPs
CPPs achieve a wide range of uses and cargo capabilities, in part, because there is a vast array of peptides to choose from, offering case-by-case specifications for CPP-based therapeutics. There are several ways to classify and categorize CPPs and in this review, we describe the different types of CPPs based on their physical characteristics, specifically charge.
2.1. Cationic CPPs
At physiological pH, cationic CPPs yield a net positive charge and show great affinity at being able to penetrate the cell and circumvent the need to interact with the cell through receptors [10]. The property of certain molecules’ intrinsic ability to penetrate the cell membrane better than others was noticed as early as 1965 when cationic polymers such as polylysine and polyarginine, induced significantly higher cellular uptake of albumin by cultured cancer cells [2]. Since then, numerous comparisons have been made differentiating the uptake potential of positively charged, short peptide chains against their long or net-neutral counterparts. Specifically, it was shown that short homopolymers of arginine (R) had higher uptake compared to other amino acids including polylysine chains [20,21,22,23]. Attempts to utilize and mimic this ability have been undertaken, achieving excellent results. Different types of peptides present certain advantages or disadvantages over others and each utilizes differing pathways for cellular penetration. Arginine is one of the few cationic natural amino acids (pKa > 12) and possesses the ability to interact with negatively charged integrated proteins and induce translocation into the cell, carrying with it the specified cargo [24]. The uptake efficacy increases with arginine length, but beyond 8 to 10 arginine residues, while translocation can occur, but it may result in damage to the membrane, possibly inhibiting future CPP applications [24]. For instance, pVEC (LLIILRRRIRKQAHAHSK) [25] has multiple positively charged lysine and arginine amino groups, as well as the HIV-1-derived TAT protein (RKKRRQRRR) which also possesses these same characteristic arginine residues [26] (Table 1).

Table 1.
Common CPPs from various sources and their applications.
2.2. Amphipathic CPPs
Amphipathic CPPs contain a combination of polar and nonpolar amino acid residues. The nonpolar residues such as alanine (A), leucine (L), isoleucine (I), glycine (G), and valine (V) can interact with the nonpolar lipid head groups and insert the polar region into the membrane [1]. Among cataloged CPPs, those with amphipathic properties are the most common. Peptides such as ELP (VPGXG)n where X is valine (V), alanine (A), or glycine (G) [70,71]; this peptide works as an Elastin-like protein (ELP) and is proposed to use as a hyperthermic approach against cancer [72,73]. Most naturally and synthetically occurring CPPs utilize the differences in polarity to infiltrate the cell through the membrane.
CPPs mimicking the properties of naturally occurring CPPs to create more efficient and specific synthetic proteins have been designed. These CPPs are also combined with either natural or synthetic peptides, which can guide the CPP, along with its desired cargo, to the cell where it is needed. For instance, a chimeric combination between CPP pVEC (LLIILRRRIRKQAHAHSK-NH2) and a peptide designed to target glioma was successfully used to introduce fluorescent indicators into the cell [74]. The glioma homing peptide or gHo (NHQQQNPHQPPM-NH2) was combined with FAM (5-carboxyfluorescein) a fluorescent tag, which was identified to be able to translocate the tag cargo into the glioma [75]. Examples of CPPs that take advantage of this ability are Pep1 (KETWWETWWTEWSQPKKKRKV), which is a combination of a nonpolar amino group, and the NLS SV40 (Nuclear locating sequence) [39]. These types of combinations between the penetrating tail and the locating sequence are the basis of all CPP target specificity.
2.3. Anionic CPP, p28, A Fragment of Azurin
Another distinct category of the amphipathic CPPs is the negatively charged anionic CPPs. These peptide chains target and enter the cells differently than their cationic counterparts. A CPP, p28, has been found to have an ability of cancer preferential entry [59,60,61,62,63,64,76,77,78]. p28 is an anionic peptide made up of 28 amino acid residues (LSTAADMQGWTDGMASGLDKDYLKPDD) [65,66,79,80,81,82]. Specifically, the residues between Leu50-Asp77 within a protein known as azurin that are secreted by an opportunistic pathogen, Pseudomonas aeruginosa [67,68,83]. Azurin has been extensively studied as an electron transfer protein but has found itself to be the subject of many studies in the last few years due to its intrinsic ability to track down and enter cancer cells. The p28 region, a fragment of azurin, forms an alpha helix and interacts with beta microdomains called lipid rafts, which are generally overexpressed in cancer cells, along the cellular membrane and enter the cell.
3. Intracellular-Targeted Delivery by CPPs
Particularly powerful characteristics of CPPs are the ability to not only penetrate the cellular membrane but also home in on certain organelles to increase the precision of target site specificity. Targeting specific organelles within the cell when it comes to fighting cancer is important due to the fact that targeted delivery to the specific intracellular targets can result in enhanced therapeutic efficacy and reduced toxicity [84,85,86,87,88]. Combining a specific organelle-locator sequence to the CPP, along with the therapeutic cargo, results in even greater control over the delivery of anti-cancer drugs. In this section, we summarize the different types of organelle-targeting strategies, as well as their current and potential future, uses in cancer therapeutics (Figure 1).
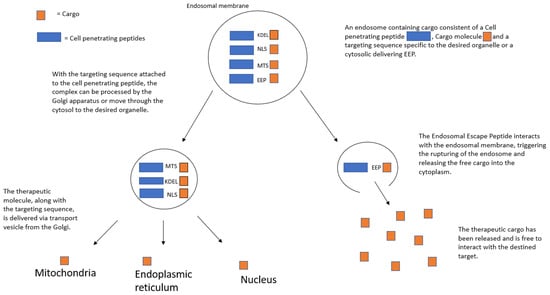
Figure 1.
CPP-guided organelle targeting.
3.1. Nuclear Localization Signal (NLS)
The nucleus is the membrane-bound organelle that houses the cell’s complete genetic makeup in the form of DNA. Given that many genetic diseases originate from an error(s) in the genetic code, efforts are underway to target the nucleus more precisley. While the aforementioned HIV1 derivative, TAT, possesses an intrinsic ability to target the nucleus [89], better uptake rates can be achieved by combining the CPP with a nuclear-locating sequence. Interaction with the nucleus is achieved through the nuclear pore complex (NPC), which normally prohibits large molecules from entering the nucleus. Using an NLS, which can assist the cargo molecule, induces active transport through the nuclear pore, where the administration of the desired drug can then be achieved. For example, an NLS derived from the Simian virus antigen (SV40) can be covalently bound to both a CPP such as TAT and a DNA-repairing cargo to attack the disease at its genetic source [89]. Another study showed that a conjugation between a viral gene vector, adenovirus, and CPP-PEG resulted in an uptake increase into the nucleus by nearly 80 times when compared to the adenovirus alone [90] The combination of specified nuclear targeting sequences with their respective CPP shows great potential in the ability to not only penetrate the normally impermeable nuclear membrane, but also efficiently administer drugs or gene editing molecules to tackle the disease at the source.
3.2. Mitochondrial Targeting
The mitochondria are an integral part of the survival and overall health of the cell biome, as it is in charge of producing cellular energy in the form of ATP as well as being involved in cellular signaling pathways [91,92]. For these reasons, the mitochondria are an attractive target for therapeutics as disorders within the mitochondria can result in neurodegenerative or cancerous disease down the line. Mitochondrial-specific delivery is achieved by employing a mitochondrion-targeting sequence (MTS) with CPPs to deliver therapeutics or diagnostic dyes into the mitochondrial matrix. When the MTS is bound to the carboxy terminus of CPPs, the whole complex is processed by the mitochondrial membrane and is delivered into the matrix [93,94,95,96]. A common MTS is a positively charged lipid, triphenyl-phosphonium, or TPP, which uses its charge to interact with the anionic mitochondrial membrane, allowing for entry into the organelle [94]. Another example of a specific MTS combination (MLRAALSTARRGPRLSRLL) was bound to H3R8, a CPP, and was successful in delivering 5-FAM dye into the mitochondria. The dye itself is normally impenetrable to the mitochondrial membrane and is delivered better than when compared to the MTS dye complex alone [89].
3.3. Endoplasmic Reticulum Targeting
The folding of the endoplasmic reticulum (ER) is important to maximize the organelle’s surface area and volume to carry out the main ER functions which are protein folding and biosynthesizing lipids [89]. Since these functions are critical to biochemical pathways within the cell, disorders in the ER can lead to a myriad of diseases, including cancer. Proteins formed by ribosomes reach the ER either by the ribosome being embedded in the organelle or by being trafficked there by utilizing receptors on the outer ER membrane [97,98]. The receptors (KDEL-R) bind with a short locator sequence called KDEL (Lys-Asp-Glu-Leu) to allow synthesized proteins into the cell [99]. By using KDEL, ER-targeting CPPs have been created, and gold nanoparticles (AuNP) bound to the KDEL sequence showed a targeted localization at the ER [99].
3.4. Lysosomal Targeting
The lysosomal function consists of cellular waste degradation, and apoptosis, as well as playing a key role in intracellular signaling pathways [100,101]. Their involvement with apoptosis makes the lysosome an attractive target for cancer treatments. One way of granting lysosomal targeting is to use Lysosome Sorting Peptides (LSPs), usually short motifs consistent with tyrosine [102]. For example, a short LSP known as YXXO (Y being tyrosine, X being any amino acid residue, and O being any large hydrophobic group such as phenylalanine or isoleucine, among others.) has been shown to greatly increase uptake efficacy into the lysosome by interacting with the adapter protein complexes that make up the lysosome [103]. The use of these LSP-CPP conjugations warrants more research and trials but these studies suggested the possibility of controlled apoptotic treatments utilizing the lysosome intrinsic abilities [102].
3.5. Cytoplasmic Targeting through Endosomal Escape Peptides
The ability to influence over the cytoplasm can prove to be a useful tool. While organelle targeting can be achieved by manipulating the natural intracellular transport mechanisms, such as the Golgi complex or chaperone proteins, cytoplasmic targeting requires a different approach. Once CPP induces endocytosis to gain entry into the cell, the large impermeable cargo molecule is generally trapped within the endosome [104]. Endosomal escape efficiency has remained a barrier and the rate-limiting step for delivering intracellular cytosolic therapeutic proteins [104,105]. The ability of these large biomolecules to breach the endosomal membrane to reach their target is a critical step in intracellular therapeutics and has caused the search for and use of Endosomal Escape Peptides (EEPs) to assist the delivery.
EEPs are a class of conjugate peptides that can be used to disrupt the endosomal lining from within, thus triggering the release of the cargo molecule into the cytoplasm [104]. Molecules such as chloroquine have been bound to cargo molecules as a way of flooding the endosome with water, thus rupturing the endosomal lining [106]. Attempts to utilize an EEP-TAT-PTD consistent with indole and aromatic rings induce endosomal escape through their highly hydrophobic properties interacting with the endosomal membrane while having a less cytotoxic effect [105]. Likewise, domains such as E5TAT, HA2 (isolated from influenza), and ZF5.3 (a derivative of an avian pancreatic domain) have been bound to luciferase and tested to find their relative escape efficacy when compared to the luminescent cargo itself [104]. Conducting a reliable test to determine the relative concentrations of the delivered cargo is difficult, due to the dilution of the luminescent cargo within the cytoplasm. With this considered, it is proposed that the inclusion of EEPs can increase cytosolic delivery by a rate of 7 to 30 times greater than when compared to the cargo alone [104]. While this means that a large quantity of the specified cargo molecules will remain trapped within the endosome, this is an important step in finding a more efficient and reliable method of cytosolic targeting.
Attempts have been made to further increase the rate at which cargos are released from the endosome into the cytoplasm. While the use of EEPs greatly increases the rate at which the CPP complex is released compared to the CPP alone, it still falls short of optimal release rates. A possible solution to this issue is the use of multivalent CPPs (MCCPs), which essentially introduce a higher concentration of the CPP by adding multiple copies of the peptide to increase the interaction of the endosome [6,107]. The results showed that the uptake and delivery rates of MCCPs are comparable to that of regular CPPs but include the additional benefit of achieving endosomal escape more easily [6]. Another attempt at increasing endosomal escape rates is the use of pH-dependent membrane-active peptides (PMAPs). PMAPs work in a similar way to the HA2 virus in that they breach the endosomal membrane by increasing the pH within the lumen [108]. One peptide that can achieve that result is named GALA (WEAALAEALAEALAEHLAEALAEALEALAA) [109]. In general, these peptides contain large hydrophobic groups such as leucine or alanine along with groups such as aspartate or glutamate. The interaction between these amino acid residues releases protons into the lumen, acidifying the endosome and triggering endosomal escape [109].
4. Potential Diagnostics and Therapeutics
It has been suggested that there is great potential for the use of CPPs in clinical trials to treat cancer. The manipulability of CPPs provides new opportunities to precisely control the cell biome through transmembrane translocation, and the beginning looks promising for the future of CPP treatments. The ability to covalently link self-assembly CPPs and carry large, normally impermeable cargo molecules makes CPPs great tools with new abilities to deliver therapeutic medicines or imaging molecules into normally difficult-to-penetrate environments [2,110]. Traditionally, cancer cells presented a challenge simply getting into the cell, let alone manipulating it in any way because of some altered membrane expression. This especially applies to brain cancers such as glioblastomas, because while they are within the already impermeable cancer cell membrane, they are also protected by the blood-brain barrier (BBB), making CPPs such as novel p28 treatments so attractive because of their inherent permeability through the BBB and the cell membrane.
4.1. Imaging Tools and Diagnostics
As stated earlier, the covalent bonding of cargo molecules is the main attraction of CPPs and this has numerous possibilities when it comes to being able to manipulate the expression of cancer at a cellular level. The translational example of this potential is the ability to deliver bioluminescent molecules into cancer cells for diagnostic imaging and image-guided surgery. For instance, a difficulty, especially on the surgical front, is trying to precisely remove the tumor regions but not necessarily being able to fully differentiate between healthy and cancerous tissues without any image guidance [76,111]. This poses a challenge for surgeons aiming to both remove as much of the cancerous tissue as possible without affecting the surrounding normal tissues. For this purpose, ICG, a US FDA-approved nontoxic dye conjugated with a tumor-targeting CPP p28 (also known as ICG-p28) was created [59,60,76,77]. When viewed under near-infrared light wavelength, systemically administrated ICG-p28 provides a clear visualization of various types of tumors and identifies tumor margins that need to be removed surgically [59,60,76,77]. Removing the entire tumor region is critical for surgical cancer treatment, and the use of ICG-p28 aids in the visualization of hard to see deep tumor regions and can help prevent reproliferation [59,60,76,77]. The high tissue penetration, visibility, and differentiability of ICG-p28 mean that it is a prime candidate to be tested in clinical settings.
As cancer diagnostic tools, CPPs have also been proposed to deliver contrast agents for magnetic resonance imaging (MRI) [112,113,114,115], single photon emission computed tomography (SPECT) [116,117,118], positron emission tomography (PET) [119,120,121], and optical imaging [122,123]. CPPs have been chemically conjugated to contrast agents (e.g., metals, fluorescent, and radioactive materials) with the aim of more favorable pharmacokinetics/ biodistributions for cancer diagnosis [124]. In addition, to improve the tumor-specificity of CPPs, activatable CPPs have been proposed for more precise tumor visualization [10,125,126,127,128]. As CPPs are peptides that can be substrates for endogenous proteases. The activatable CPPs contain an amino acid sequence that can be targeted by matrix metalloproteases-2 (MMP-2) and MMP-9. Such metalloproteases are generally overexpressed in cancer cells [129,130]. When activated by MMP-2 and MMP-9, the targeted sequence within CPPs is cleaved, and their cellular uptake by tumor becomes greater [131]. There have been several attempts to design new activatable CPPs that can be activated by enzymatic activity, pH gradient, reactive oxygen species (ROS), and optical light. These approaches are summarized in a recent article [131].
Another promising area of development in CPPs to further increase their specificity is to use different combinations of transport domains such as nanoparticles. With further cell specificity, more detailed and reliable images of the tumor region can be possibly achieved. Dual targeting or dual-modality imaging binds nanoparticles with CPPs and they deliver the imaging agent jointly into the target cell [132]. Breast cancer cells treated with the dual imaging combination of CPP and NP yielded the highest concentration of DiR fluorescent dye when compared to CPP, NP, or DiR alone [133].
4.2. Anticancer Therapeutic Uses
Some CPPs have intrinsic anti-tumor properties along with cell-penetrating abilities. For example, p28, a fragment of Pseudomonas azurin protein, carries anticancer activity, along with the ability to enter tumor cells [61,63,64,65,66,76,77]. In preclinical testing, the anti-tumor efficacy of p28 was assessed on various types of human cancer cells such as brain, breast, prostate cancer, and melanoma. Upon cellular entry, p28 binds to a hydrophobic region within the DNA-binding domain of p53 and inhibits proteasomal degradation via an HDM2-independent pathway [64,65,66,134,135,136,137,138]. This results in an increase in the intracellular levels of p53 as well as its DNA-binding activity and elevates the cyclin-dependent kinase inhibitors, p21 and p27, thereby inducing cancer cell cycle arrest at G2/M and tumor growth inhibition [64,66].
The intrinsic anti-tumor properties of CPPs when paired with selected anticancer therapeutic cargos, also allow us to attack cancer at multiple levels. The specific therapeutic agent can be chosen based on circumstances in that specific patient and as the catalog of CPPs grows. For example, a synthetic CPP RLWMRWYSPRTRAYGC has been shown to disrupt tumor progression in lung cancers [5,139]. The innate anti-cancer effects of specific CPPs can be combined with the appropriate locating sequence and a chosen therapeutic to create a truly powerful tool against cancer.
Given that some CPPs lack tumor-specificity which could lead to drug delivery to cells in healthy tissues, there have been several attempts to enhance the specificity of CPPs-based delivery by taking advantage of the natural targeting abilities of antibodies and antigens [140,141,142,143,144]. For instance, by linking the antibody against tumorous cells’ highly expressed antigens to a CPP-siRNA complex, siRNA gene-drug delivery can be achieved with high levels of specificity and little cytotoxicity [145]. While some CPPs have intrinsic tumor-targeting capabilities, the CPP–antibody conjugation allows for more general CPP use by utilizing the highly overexpressed antigens present on the surface of cancer cells [145,146]. This principle was tested in a preclinical prostate cancer animal model, in which there is almost universal overexpression of the prostate-specific membrane antigen (PMSA) [147]. By linking the PMSA antibody to a CPP bound with an siRNA, specifically TRIM24, a significant reduction in proliferation and colony formation was achieved [147]. However, antibody-based targeting does come with a challenge, which is controlling the release of the siRNA therapeutic once the complex has reached the desired destination [145]. Despite this drawback, there is still promise for antibody-CPP based drug delivery.
In this section, we summarized the different types of CPP-based therapeutic and diagnostic strategies. CPPs have immense potential in both cancer diagnostic and therapeutic applications. CPPs are promising tools to improve cellular uptake which is one of the major contributions to developing an effective cancer treatment. Further basic/translational studies and clinical trials will provide a better understanding of the mechanisms involved in developing CPP-based cancer treatment.
5. Clinical Trials Utilizing CPPs
Previous preclinical studies suggest promising results for CPP-based treatments and diagnostics, not just in the domain of cancer, but across various types of disease treatment. Trials utilizing CPPs have been conducted to test the efficacy of CPP-based treatments for cancer therapeutics and diagnostics, and have yielded positive results, further showing the wide range of possibilities for future treatments. Here, we highlight CPPs in the clinical studies (Table 2).

Table 2.
CPPs in clinical trials.
5.1. RI-TAT-p53C’ Trial
RI-TAT-p53C’ is a complex made up of a transduction agent, TAT, and the therapeutic which is selected to reactivate dormant p53 anti-tumor peptides. Tumors have been shown to possess mutant or wild-type p53 alterations and can result in a malfunction of the cell cycle causing tumor growth and proliferation. In general, in vivo attempts are limited due to some CPPs having a short half-life, but RI-TAT is designed utilizing the properties of the D-isomer to prolong its half-life [152]. The in vitro studies compared colon cancer and lung carcinoma cells, which were treated with D-isomer RI-TAT-p53C’, L-Isomer RI-TAT-p53C’, and an untreated control [152]. After 7 days, the cell cycle was inhibited in these cancer cells treated with the D-isomer. Moreover, the in vivo studies provided promising results, showing that the mice that were treated with RI-TAT-p53C’ had an average tumor volume of 268 mm3 compared to the 573 mm3 average of the control group [152]. While reducing the average solid tumor volume by more than 50%, it extended the survival of the treated groups with an average survival period of 70 days compared to the untreated control, with an average of 11 days [152].
5.2. DTS-108 Trial
As described in Section 3, an important characteristic of CPPs is their ability to more efficiently and specifically deliver the therapeutic cargo to its specified location better than the anticancer drug alone. The utilization of CPPs has shown a significant reduction in toxicity due to the increased targeting capabilities of the CPPs. This was demonstrated in a pre-clinical trial using CPP DTS-108 to deliver an activatable cytotoxic drug called SN38 [156]. The DTS-108 SN38 conjugate was developed to bypass the hepatic activation required for SN38 to be released from other prodrugs, namely Irinotecan [156]. While Irinotecan is effective in treating colorectal cancers, it has a very low conversion rate from Irinotecan to SN38, resulting in high amounts of waste and difficulty in deciding dosage [156]. When combined with DTS-108, an increase from as low as 2% converted SN38 from Irinotecan to up to 29% free SN38 when combined with DTS-108 CPP, as well as a significant reduction in gastrointestinal cytotoxicity [156].
5.3. p28 Trial
As described above, p28 has both properties of tumor-preferential localization and intrinsic anti-tumor effects. p28 can activate p53 and inhibit the cancer cell cycle and induce apoptosis. Preclinical pharmacological studies of p28 provided significant evidence for efficacy without apparent toxicity or immunogenicity and prompted its entry into a phase I clinical trial. The primary objective of the first-in-class, first-in-human dose acceleration study was to determine the No Observed Adverse Effect Level (NOAEL) and maximum tolerated dose (MTD) of p28 in adult patients with advanced solid tumors. These patients had advanced tumors, which were unresponsive to traditional forms of treatment, and were also predicted to have around 6 months to live in their condition [148,149]. A total of 15 patients were administered p28 i.v. under an accelerated titration 3+3 dose escalation design. p28 was well tolerated with no significant adverse events and appeared to have anti-tumor activity in patients with advanced tumors.
Another phase I clinical trial of p28 as a single agent was conducted in pediatric patients with central nervous system (CNS) tumors [150]. Children with recurrent or progressive CNS tumors received p28 i.v. at 4.16 mg/kg/dose (the adult recommended phase II dose) using a rolling-6 study design. Similar results were found, although the adult p28 dose was tolerated in the adolescents, further showing that p28-based treatments can be handled among all age groups. The results of these trials have established that p28 is safe and well-tolerated at the recommended phase II dose (RP2D). Although p28 showed preliminary efficacy, the further development of this agent with other agents may prove more promising.
6. Current Limitations of CPPs
Despite the many advantages of CPPs, it should be noted that there are limitations of CPPs similar to any other agents. Cell selectivity, penetrating efficacy, and in vivo stability are considered as the major challenges for current CPPs. Many CPPs have low cell selectivity, probably due to their chemical characteristics (e.g., cationic amino acids). These types of CPPs need to be administrated directly to target tumors to avoid adverse effects. When CPPs are used in vivo, immunogenicity induced by CPPs may also limit their applications in clinics, similar to many other drug delivery carriers [157,158,159]. For CPPs as delivery carriers [160], not as peptide antigen vaccines [161,162], the assessment of immunogenicity is a critical step toward the characterization of clinical applicability.
Although it depends on the modes of entry and intracellular trafficking, endosomal uptake is another issue to increase efficacy and stability. The endosomal escape of CPPs can be improved by the addition of peptides that disrupt membranes at acidic pH as the pH in endosomes becomes acidic during endosomal maturation. Moreover, CPPs can be stabilized by chemical modifications to avoid inactivation by proteases. Recent advances in peptide chemistry will overcome such limitations in creating the next generation of CPPs.
7. Conclusions
To date, a major hurdle for cancer treatment is administering the desired drug efficiently while also leaving surrounding cells unharmed. Traditional treatments such as chemotherapy induce high rates of unwanted toxicity, because of their low specificity, and CPPs offer a new way of infiltrating the cell biome in a more effective and less toxic way. As described in this review, CPPs come in a large range of conformations, chemical properties, and bonding capabilities, each offering its own set of advantages when treating certain diseases. We discussed how these CPP complexes are either isolated naturally or synthesized, as well as their potential uses in diagnostics or therapeutics. The control over components of the cell that CPP-based treatments offer can be further optimized by the inclusion of organelle targeting sequences, granting us the ability to interact with the cells at an even more precise level. Several clinical trials show encouraging and promising results on how effective CPPs can be when combatting disease. In general, the selective delivery of cargoes (e.g., therapeutic and diagnostic agents) into an organ is also one of the major challenges for current drug development [163,164]. For instance, the central nervous system (CNS) is highly protected by several barrier structures, of which the blood-brain barrier (BBB) is the most critical one. This makes it very difficult to deliver drugs to the brain effectively. Some of CPPs successfully cross the BBB and deliver cargo molecules to the target site [59,165,166]. While more research is required to unlock the full potential of CPPs, CPP-based new approaches can ultimately lead to next-generation technologies as finely tuned vehicles for intracellular targeted delivery for cancer treatment.
Author Contributions
R.A.B. and T.Y. writing—original draft preparation, R.A.B. and T.Y. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH/National Cancer Institute (R21CA252370), NIH/National Institute of Biomedical Imaging and Bioengineering (R01EB023924), and Michael Reese Pioneer in Research Award to TY.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Sonia, A.; Abderraouf, K. Cell-Penetrating Peptides: A Challenge for Drug Delivery. In Cheminformatics and Its Applications; Amalia, S., Azhar, R., Ghulam, H., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. 11. [Google Scholar]
- Skotland, T.; Iversen, T.G.; Torgersen, M.L.; Sandvig, K. Cell-penetrating peptides: Possibilities and challenges for drug delivery in vitro and in vivo. Molecules 2015, 20, 13313–13323. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, H.; Li, T.; Pan, H.; Ren, S.; Luo, G.; Jiang, J.; Yu, L.; Chen, B.; Zhang, Y.; et al. Efficient intracellular delivery of proteins by a multifunctional chimaeric peptide in vitro and in vivo. Nat. Commun. 2021, 12, 5131. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Erazo-Oliveras, A.; Muthukrishnan, N.; Baker, R.; Wang, T.-Y.; Pellois, J.-P. Improving the Endosomal Escape of Cell-Penetrating Peptides and Their Cargos: Strategies and Challenges. Pharmaceuticals 2012, 5, 1177–1209. [Google Scholar] [CrossRef]
- Zorko, M.; Langel, Ü. Studies of cell-penetrating peptides by biophysical methods. Q. Rev. Biophys. 2022, 55, e3. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.S.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2015, 44, D1098–D1103. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Pooga, M.; Hällbrink, M.; Zorko, M.; Langel, U. Cell penetration by transportan. Faseb J. 1998, 12, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.; Gallet, X.; Soomets, U.; Hällbrink, M.; Bråkenhielm, E.; Pooga, M.; Brasseur, R.; Langel, U. Translocation properties of novel cell penetrating transportan and penetratin analogues. Bioconjugate Chem. 2000, 11, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Magzoub, M.; Kilk, K.; Eriksson, L.E.; Langel, U.; Gräslund, A. Interaction and structure induction of cell-penetrating peptides in the presence of phospholipid vesicles. Biochim. Biophys. Acta 2001, 1512, 77–89. [Google Scholar] [CrossRef]
- Hällbrink, M.; Florén, A.; Elmquist, A.; Pooga, M.; Bartfai, T.; Langel, U. Cargo delivery kinetics of cell-penetrating peptides. Biochim. Biophys. Acta 2001, 1515, 101–109. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int. J. Nanomed. 2019, 14, 6497–6517. [Google Scholar] [CrossRef]
- Pereira, M.; Vale, N. Two Possible Strategies for Drug Modification of Gemcitabine and Future Contributions to Personalized Medicine. Molecules 2022, 27, 291. [Google Scholar] [CrossRef]
- Traykovska, M.; Otcheva, L.A.; Penchovsky, R. Targeting TPP Riboswitches Using Chimeric Antisense Oligonucleotide Technology for Antibacterial Drug Development. ACS Appl. Bio Mater. 2022, 5, 4896–4902. [Google Scholar] [CrossRef]
- Ziegler, A.; Nervi, P.; Dürrenberger, M.; Seelig, J. The cationic cell-penetrating peptide CPP(TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: Optical, biophysical, and metabolic evidence. Biochemistry 2005, 44, 138–148. [Google Scholar] [CrossRef]
- Gonçalves, E.; Kitas, E.; Seelig, J. Binding of oligoarginine to membrane lipids and heparan sulfate: Structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry 2005, 44, 2692–2702. [Google Scholar] [CrossRef]
- Futaki, S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv. Drug Deliv. Rev. 2005, 57, 547–558. [Google Scholar] [CrossRef]
- Allen, J.; Pellois, J.P. Hydrophobicity is a key determinant in the activity of arginine-rich cell penetrating peptides. Sci. Rep. 2022, 12, 15981. [Google Scholar] [CrossRef]
- Lavogina, D.; Nasirova, N.; Sõrmus, T.; Tähtjärv, T.; Enkvist, E.; Viht, K.; Haljasorg, T.; Herodes, K.; Jaal, J.; Uri, A. Conjugates of adenosine mimetics and arginine-rich peptides serve as inhibitors and fluorescent probes but not as long-lifetime photoluminescent probes for protein arginine methyltransferases. J. Pept. Sci. 2022, e3456. [Google Scholar] [CrossRef] [PubMed]
- Verdurmen, W.P.; Brock, R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol. Sci. 2011, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Elmquist, A.; Lindgren, M.; Bartfai, T.; Langel, U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res. 2001, 269, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef]
- Nakamura, S.; Ando, N.; Ishihara, M.; Sato, M. Development of Novel Heparin/Protamine Nanoparticles Useful for Delivery of Exogenous Proteins In Vitro and In Vivo. Nanomaterials 2020, 10, 1584. [Google Scholar] [CrossRef]
- Sakaue, Y.; Kim, J.; Miyamoto, Y. Effects of TAT-conjugated platinum nanoparticles on lifespan of mitochondrial electron transport complex I-deficient Caenorhabditis elegans, nuo-1. Int. J. Nanomed. 2010, 5, 687–695. [Google Scholar]
- Derossi, D.; Chassaing, G.; Prochiantz, A. Trojan peptides: The penetratin system for intracellular delivery. Trends Cell Biol. 1998, 8, 84–87. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kiyoshi, M.; Hashii, N.; Fujita, M.; Kurohara, T.; Ishii-Watabe, A.; Fukuhara, K.; Misawa, T.; Demizu, Y. Development of a penetratin-conjugated stapled peptide that inhibits Wnt/β-catenin signaling. Bioorganic Med. Chem. 2022, 73, 117021. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, E.; Mumin, S.; Cheung, D.; Fridman, D.; Lin, B.; Wong, L.; Rosal, R.; Rudolph, R.; Frenkel, M.; Thadi, A.; et al. PNC-27, a Chimeric p53-Penetratin Peptide Binds to HDM-2 in a p53 Peptide-like Structure, Induces Selective Membrane-Pore Formation and Leads to Cancer Cell Lysis. Biomedicines 2022, 10, 945. [Google Scholar] [CrossRef]
- Jiao, C.Y.; Sachon, E.; Alves, I.D.; Chassaing, G.; Bolbach, G.; Sagan, S. Exploiting Benzophenone Photoreactivity To Probe the Phospholipid Environment and Insertion Depth of the Cell-Penetrating Peptide Penetratin in Model Membranes. Angew. Chem. (Int. Ed. Engl.) 2017, 56, 8226–8230. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Kar, R.K.; Mondal, S.; Pahan, K.; Bhunia, A. Structural Elucidation of the Cell-Penetrating Penetratin Peptide in Model Membranes at the Atomic Level: Probing Hydrophobic Interactions in the Blood-Brain Barrier. Biochemistry 2016, 55, 4982–4996. [Google Scholar] [CrossRef] [PubMed]
- Masman, M.F.; Rodríguez, A.M.; Raimondi, M.; Zacchino, S.A.; Luiten, P.G.; Somlai, C.; Kortvelyesi, T.; Penke, B.; Enriz, R.D. Penetratin and derivatives acting as antifungal agents. Eur. J. Med. Chem. 2009, 44, 212–228. [Google Scholar] [CrossRef]
- Rádis-Baptista, G. Cell-Penetrating Peptides Derived from Animal Venoms and Toxins. Toxins 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Fajloun, Z.; Kharrat, R.; Chen, L.; Lecomte, C.; Di Luccio, E.; Bichet, D.; El Ayeb, M.; Rochat, H.; Allen, P.D.; Pessah, I.N.; et al. Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca(2+) release channel/ryanodine receptors. FEBS Lett. 2000, 469, 179–185. [Google Scholar] [CrossRef]
- Ponnappan, N.; Chugh, A. Cell-penetrating and cargo-delivery ability of a spider toxin-derived peptide in mammalian cells. Eur. J. Pharm. Biopharm. 2017, 114, 145–153. [Google Scholar] [CrossRef]
- Tansi, F.L.; Filatova, M.P.; Koroev, D.O.; Volpina, O.M.; Lange, S.; Schumann, C.; Teichgräber, U.K.; Reissmann, S.; Hilger, I. New generation CPPs show distinct selectivity for cancer and noncancer cells. J. Cell. Biochem. 2019, 120, 6528–6541. [Google Scholar] [CrossRef]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef]
- Gros, E.; Deshayes, S.; Morris, M.C.; Aldrian-Herrada, G.; Depollier, J.; Heitz, F.; Divita, G. A non-covalent peptide-based strategy for protein and peptide nucleic acid transduction. Biochim. Et Biophys. Acta 2006, 1758, 384–393. [Google Scholar] [CrossRef]
- Miwa, A.; Kamiya, K. Control of Enzyme Reaction Initiation inside Giant Unilamellar Vesicles by the Cell-Penetrating Peptide-Mediated Translocation of Cargo Proteins. ACS Synth. Biol. 2022. [Google Scholar] [CrossRef]
- Phambu, N.; Almarwani, B.; Alwadai, A.; Phambu, E.N.; Faciane, N.; Marion, C.; Sunda-Meya, A. Calorimetric and Spectroscopic Studies of the Effects of the Cell Penetrating Peptide Pep-1 and the Antimicrobial Peptide Combi-2 on Vesicles Mimicking Escherichia coli Membrane. Langmuir ACS J. Surf. Colloids 2017, 33, 12908–12915. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jeon, S.; Kim, S.; Chang, Y.K.; Kim, Y.C. Development of a pVEC peptide-based ribonucleoprotein (RNP) delivery system for genome editing using CRISPR/Cas9 in Chlamydomonas reinhardtii. Sci. Rep. 2020, 10, 22158. [Google Scholar] [CrossRef] [PubMed]
- Alaybeyoglu, B.; Sariyar Akbulut, B.; Ozkirimli, E. Insights into membrane translocation of the cell-penetrating peptide pVEC from molecular dynamics calculations. J. Biomol. Struct. Dyn. 2016, 34, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Alaybeyoglu, B.; Sariyar Akbulut, B.; Ozkirimli, E. pVEC hydrophobic N-terminus is critical for antibacterial activity. J. Pept. Sci. 2018, 24, e3083. [Google Scholar] [CrossRef]
- Elmquist, A.; Langel, U. In vitro uptake and stability study of pVEC and its all-D analog. Biol. Chem. 2003, 384, 387–393. [Google Scholar] [CrossRef]
- Kerth, A.; Erbe, A.; Dathe, M.; Blume, A. Infrared reflection absorption spectroscopy of amphipathic model peptides at the air/water interface. Biophys. J. 2004, 86, 3750–3758. [Google Scholar] [CrossRef]
- Silva, S.; Kurrikoff, K.; Langel, Ü.; Almeida, A.J.; Vale, N. A Second Life for MAP, a Model Amphipathic Peptide. Int. J. Mol. Sci. 2022, 23, 8322. [Google Scholar] [CrossRef]
- Oehlke, J.; Scheller, A.; Wiesner, B.; Krause, E.; Beyermann, M.; Klauschenz, E.; Melzig, M.; Bienert, M. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Et Biophys. Acta 1998, 1414, 127–139. [Google Scholar] [CrossRef]
- Oehlke, J.; Wallukat, G.; Wolf, Y.; Ehrlich, A.; Wiesner, B.; Berger, H.; Bienert, M. Enhancement of intracellular concentration and biological activity of PNA after conjugation with a cell-penetrating synthetic model peptide. Eur. J. Biochem. FEBS 2004, 271, 3043–3049. [Google Scholar] [CrossRef]
- Moghal, M.M.R.; Islam, M.Z.; Hossain, F.; Saha, S.K.; Yamazaki, M. Role of Membrane Potential on Entry of Cell-Penetrating Peptide Transportan 10 into Single Vesicles. Biophys. J. 2020, 118, 57–69. [Google Scholar] [CrossRef]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Heldt, M.; Serocki, M.; Stupak, A.; Martynow, D.; Dębowski, D.; Gitlin-Domagalska, A.; Lica, J.; et al. Conjugates of Ciprofloxacin and Levofloxacin with Cell-Penetrating Peptide Exhibit Antifungal Activity and Mammalian Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 4696. [Google Scholar] [CrossRef] [PubMed]
- Rittner, K.; Benavente, A.; Bompard-Sorlet, A.; Heitz, F.; Divita, G.; Brasseur, R.; Jacobs, E. New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2002, 5, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Crombez, L.; Divita, G. A non-covalent peptide-based strategy for siRNA delivery. Methods Mol. Biol. 2011, 683, 349–360. [Google Scholar] [CrossRef]
- Deshayes, S.; Konate, K.; Aldrian, G.; Crombez, L.; Heitz, F.; Divita, G. Structural polymorphism of non-covalent peptide-based delivery systems: Highway to cellular uptake. Biochim. Et Biophys. Acta 2010, 1798, 2304–2314. [Google Scholar] [CrossRef]
- Wang, T.Y.; Sun, Y.; Muthukrishnan, N.; Erazo-Oliveras, A.; Najjar, K.; Pellois, J.P. Membrane Oxidation Enables the Cytosolic Entry of Polyarginine Cell-penetrating Peptides. J. Biol. Chem. 2016, 291, 7902–7914. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Grundmann, C.; Sauter, M.; Storck, P.; Tursch, A.; Özbek, S.; Leotta, K.; Roth, R.; Witzigmann, D.; Kulkarni, J.A.; et al. Coating of PLA-nanoparticles with cyclic, arginine-rich cell penetrating peptides enables oral delivery of liraglutide. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102132. [Google Scholar] [CrossRef] [PubMed]
- Komin, A.; Bogorad, M.I.; Lin, R.; Cui, H.; Searson, P.C.; Hristova, K. A peptide for transcellular cargo delivery: Structure-function relationship and mechanism of action. J. Control. Release 2020, 324, 633–643. [Google Scholar] [CrossRef]
- Mander, S.; Naffouje, S.A.; Gao, J.; Li, W.; Christov, K.; Green, A.; Bongarzone, E.R.; Das Gupta, T.K.; Yamada, T. Tumor-targeting cell-penetrating peptide, p28, for glioblastoma imaging and therapy. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Naffouje, S.A.; Goto, M.; Coward, L.U.; Gorman, G.S.; Christov, K.; Wang, J.; Green, A.; Shilkaitis, A.; Das Gupta, T.K.; Yamada, T. Nontoxic Tumor-Targeting Optical Agents for Intraoperative Breast Tumor Imaging. J. Med. Chem. 2022, 65, 7371–7379. [Google Scholar] [CrossRef]
- Taylor, B.N.; Mehta, R.R.; Yamada, T.; Lekmine, F.; Christov, K.; Chakrabarty, A.M.; Green, A.; Bratescu, L.; Shilkaitis, A.; Beattie, C.W.; et al. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 2009, 69, 537–546. [Google Scholar] [CrossRef]
- Yamada, T.; Fialho, A.M.; Punj, V.; Bratescu, L.; Gupta, T.K.; Chakrabarty, A.M. Internalization of bacterial redox protein azurin in mammalian cells: Entry domain and specificity. Cell. Microbiol. 2005, 7, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Signorelli, S.; Cannistraro, S.; Beattie, C.W.; Bizzarri, A.R. Chirality switching within an anionic cell-penetrating peptide inhibits translocation without affecting preferential entry. Mol. Pharm. 2015, 12, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Mehta, R.R.; Lekmine, F.; Christov, K.; King, M.L.; Majumdar, D.; Shilkaitis, A.; Green, A.; Bratescu, L.; Beattie, C.W.; et al. A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol. Cancer 2009, 8, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Das Gupta, T.K.; Beattie, C.W. p28, an anionic cell-penetrating peptide, increases the activity of wild type and mutated p53 without altering its conformation. Mol. Pharm. 2013, 10, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Das Gupta, T.K.; Beattie, C.W. p28-Mediated Activation of p53 in G2–M Phase of the Cell Cycle Enhances the Efficacy of DNA Damaging and Antimitotic Chemotherapy. Cancer Res. 2016, 76, 2354–2365. [Google Scholar] [CrossRef]
- Yamada, T.; Goto, M.; Punj, V.; Zaborina, O.; Chen, M.L.; Kimbara, K.; Majumdar, D.; Cunningham, E.; Das Gupta, T.K.; Chakrabarty, A.M. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 14098–14103. [Google Scholar] [CrossRef]
- Punj, V.; Bhattacharyya, S.; Saint-Dic, D.; Vasu, C.; Cunningham, E.A.; Graves, J.; Yamada, T.; Constantinou, A.I.; Christov, K.; White, B.; et al. Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene 2004, 23, 2367–2378. [Google Scholar] [CrossRef]
- Cantini, F.; Gianni, P.; Savarin, P.; Bizzarri, A.R.; Sette, M. Solution structure of the anticancer p28 peptide in biomimetic medium. J. Pept. Sci. 2021, 27, e3357. [Google Scholar] [CrossRef]
- Suyama, K.; Shimizu, M.; Maeda, I.; Nose, T. Flexible customization of the self-assembling abilities of short elastin-like peptide Fn analogs by substituting N-terminal amino acids. Biopolymers 2022, 113, e23521. [Google Scholar] [CrossRef]
- Chen, T.H.; Bae, Y.; Furgeson, D.Y. Intelligent biosynthetic nanobiomaterials (IBNs) for hyperthermic gene delivery. Pharm. Res. 2008, 25, 683–691. [Google Scholar] [CrossRef]
- Raucher, D.; Chilkoti, A. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition. Cancer Res. 2001, 61, 7163–7170. [Google Scholar] [PubMed]
- Bae, Y.; Buresh, R.A.; Williamson, T.P.; Chen, T.H.; Furgeson, D.Y. Intelligent biosynthetic nanobiomaterials for hyperthermic combination chemotherapy and thermal drug targeting of HSP90 inhibitor geldanamycin. J. Control. Release 2007, 122, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- Eriste, E.; Kurrikoff, K.; Suhorutšenko, J.; Oskolkov, N.; Copolovici, D.M.; Jones, S.; Laakkonen, P.; Howl, J.; Langel, Ü. Peptide-Based Glioma-Targeted Drug Delivery Vector gHoPe2. Bioconjugate Chem. 2013, 24, 305–313. [Google Scholar] [CrossRef]
- Goto, M.; Ryoo, I.; Naffouje, S.; Mander, S.; Christov, K.; Wang, J.; Green, A.; Shilkaitis, A.; Das Gupta, T.K.; Yamada, T. Image-guided surgery with a new tumour-targeting probe improves the identification of positive margins. EBioMedicine 2022, 76, 103850. [Google Scholar] [CrossRef] [PubMed]
- Naffouje, S.; Goto, M.; Ryoo, I.; Green, A.; Das Gupta, T.K.; Yamada, T. A Method of Tumor In Vivo Imaging with a New Peptide-Based Fluorescent Probe. Methods Mol. Biol. 2022, 2394, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Christov, K.; Shilkaitis, A.; Bratescu, L.; Green, A.; Santini, S.; Bizzarri, A.R.; Cannistraro, S.; Gupta, T.K.; Beattie, C.W. p28, a first in class peptide inhibitor of cop1 binding to p53. Br. J. Cancer 2013, 108, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; Bizzarri, A.R.; Cannistraro, S. Modelling the interaction between the p53 DNA-binding domain and the p28 peptide fragment of Azurin. J. Mol. Recognit. JMR 2011, 24, 1043–1055. [Google Scholar] [CrossRef]
- Hazrati, F.; Saidijam, M.; Ahmadyousefi, Y.; Nouri, F.; Ghadimipour, H.; Moradi, M.; Haddadi, R.; Soleimani, M. A novel chimeric protein with enhanced cytotoxic effects on breast cancer in vitro and in vivo. Proteins 2022, 90, 936–946. [Google Scholar] [CrossRef]
- Raber, H.F.; Heerde, T.; El Din, S.N.; Flaig, C.; Hilgers, F.; Bitzenhofer, N.; Jäger, K.E.; Drepper, T.; Gottschalk, K.E.; Bodenberger, N.E.; et al. Azulitox-A Pseudomonas aeruginosa P28-Derived Cancer-Cell-Specific Protein Photosensitizer. Biomacromolecules 2020, 21, 5067–5076. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Khazaei, M.; Avan, A.; Hasanian, S.M.; Cho, W.C.; Soleimanpour, S. p28 Bacterial Peptide, as an Anticancer Agent. Front. Oncol. 2020, 10, 1303. [Google Scholar] [CrossRef]
- Yamada, T.; Goto, M.; Punj, V.; Zaborina, O.; Kimbara, K.; Das Gupta, T.K.; Chakrabarty, A.M. The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect. Immun. 2002, 70, 7054–7062. [Google Scholar] [CrossRef]
- Juretić, D. Designed Multifunctional Peptides for Intracellular Targets. Antibiotics 2022, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef] [PubMed]
- Lucana, M.C.; Arruga, Y.; Petrachi, E.; Roig, A.; Lucchi, R.; Oller-Salvia, B. Protease-Resistant Peptides for Targeting and Intracellular Delivery of Therapeutics. Pharmaceutics 2021, 13, 2065. [Google Scholar] [CrossRef]
- Zorko, M.; Jones, S.; Langel, Ü. Cell-penetrating peptides in protein mimicry and cancer therapeutics. Adv. Drug Deliv. Rev. 2022, 180, 114044. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Navarro, M. Advances in peptide-mediated cytosolic delivery of proteins. Adv. Drug Deliv. Rev. 2021, 171, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, C.P.; Langel, Ü. An update on cell-penetrating peptides with intracellular organelle targeting. Expert Opin. Drug Deliv. 2022, 19, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Nigatu, A.S.; Vupputuri, S.; Flynn, N.; Ramsey, J.D. Effects of cell-penetrating peptides on transduction efficiency of PEGylated adenovirus. Biomed. Pharmacother. 2015, 71, 153–160. [Google Scholar] [CrossRef]
- Lightowlers, R.N.; Taylor, R.W.; Turnbull, D.M. Mutations causing mitochondrial disease: What is new and what challenges remain? Science 2015, 349, 1494–1499. [Google Scholar] [CrossRef]
- Appiah Kubi, G.; Pei, D. Chapter Thirteen—Cell-penetrating and mitochondrion-targeting molecules. In Methods in Enzymology; Chenoweth, D.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 641, pp. 311–328. [Google Scholar]
- Batista da Cunha, D.; Pupo Silvestrini, A.V.; Gomes da Silva, A.C.; Maria de Paula Estevam, D.; Pollettini, F.L.; de Oliveira Navarro, J.; Alves, A.A.; Remédio Zeni Beretta, A.L.; Annichino Bizzacchi, J.M.; Pereira, L.C.; et al. Mechanistic insights into functional characteristics of native crotamine. Toxicon 2018, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Battogtokh, G.; Choi, Y.S.; Kang, D.S.; Park, S.J.; Shim, M.S.; Huh, K.M.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm. Sinica B 2018, 8, 862–880. [Google Scholar] [CrossRef]
- Sousa, M.L.; Ribeiro, T.; Vasconcelos, V.; Linder, S.; Urbatzka, R. Portoamides A and B are mitochondrial toxins and induce cytotoxicity on the proliferative cell layer of in vitro microtumours. Toxicon Off. J. Int. Soc. Toxinology 2020, 175, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhu, Z.; Tian, Y.; Liang, L.; Wu, W.; Cao, J.; Cheng, B.; Liu, W.; Tang, Y. A TAT peptide-based ratiometric two-photon fluorescent probe for detecting biothiols and sequentially distinguishing GSH in mitochondria. Talanta 2020, 218, 121127. [Google Scholar] [CrossRef]
- Parodi, A.; Corbo, C.; Cevenini, A.; Molinaro, R.; Palomba, R.; Pandolfi, L.; Agostini, M.; Salvatore, F.; Tasciotti, E. Enabling cytoplasmic delivery and organelle targeting by surface modification of nanocarriers. Nanomed. (Lond. Engl.) 2015, 10, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Capitani, M.; Sallese, M. The KDEL receptor: New functions for an old protein. FEBS Lett. 2009, 583, 3863–3871. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Hill, R.A. High efficacy gold-KDEL peptide-siRNA nanoconstruct-mediated transfection in C2C12 myoblasts and myotubes. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 329–337. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.L.Y.; Rennick, J.J.; Yuen, D.; Al-Wassiti, H.; Johnston, A.P.R.; Pouton, C.W. Unravelling cytosolic delivery of cell penetrating peptides with a quantitative endosomal escape assay. Nat. Commun. 2021, 12, 3721. [Google Scholar] [CrossRef] [PubMed]
- Lönn, P.; Kacsinta, A.D.; Cui, X.-S.; Hamil, A.S.; Kaulich, M.; Gogoi, K.; Dowdy, S.F. Enhancing Endosomal Escape for Intracellular Delivery of Macromolecular Biologic Therapeutics. Sci. Rep. 2016, 6, 32301. [Google Scholar] [CrossRef] [PubMed]
- Hajimolaali, M.; Mohammadian, H.; Torabi, A.; Shirini, A.; Khalife Shal, M.; Barazandeh Nezhad, H.; Iranpour, S.; Baradaran Eftekhari, R.; Dorkoosh, F. Application of chloroquine as an endosomal escape enhancing agent: New frontiers for an old drug. Expert Opin. Drug Deliv. 2021, 18, 877–889. [Google Scholar] [CrossRef]
- Angeles-Boza, A.M.; Erazo-Oliveras, A.; Lee, Y.J.; Pellois, J.P. Generation of endosomolytic reagents by branching of cell-penetrating peptides: Tools for the delivery of bioactive compounds to live cells in cis or trans. Bioconjugate Chem. 2010, 21, 2164–2167. [Google Scholar] [CrossRef]
- Wharton, S.A.; Martin, S.R.; Ruigrok, R.W.; Skehel, J.J.; Wiley, D.C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J. Gen. Virol. 1988, 69 Pt 8, 1847–1857. [Google Scholar] [CrossRef]
- Li, W.; Nicol, F.; Szoka, F.C. GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 967–985. [Google Scholar] [CrossRef]
- Juliano, R.L.; Alam, R.; Dixit, V.; Kang, H.M. Cell-targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. WIREs Nanomed. Nanobiotechnol. 2009, 1, 324–335. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; van Driel, P.; Baatenburg de Jong, R.J.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Y.; Li, X.; Chen, H.; Wang, Z.; Zhang, H. Peptide-functionalized NaGdF(4) nanoparticles for tumor-targeted magnetic resonance imaging and effective therapy. RSC Adv. 2019, 9, 17093–17100. [Google Scholar] [CrossRef]
- Ding, C.; Wu, K.; Wang, W.; Guan, Z.; Wang, L.; Wang, X.; Wang, R.; Liu, L.; Fan, J. Synthesis of a cell penetrating peptide modified superparamagnetic iron oxide and MRI detection of bladder cancer. Oncotarget 2017, 8, 4718–4729. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, L.; Liu, F.; Yin, L.; Yan, S.; Zhao, H.; Ding, X.; Guo, Y.; Cao, Y.; Li, P.; et al. Cell penetrating peptide-modified nanoparticles for tumor targeted imaging and synergistic effect of sonodynamic/HIFU therapy. Int. J. Nanomed. 2019, 14, 5875–5894. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.S.; Jiang, T.; Aguilera, T.A.; Nguyen, Q.T.; Ellies, L.G.; Scadeng, M.; Tsien, R.Y. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. USA 2010, 107, 4311–4316. [Google Scholar] [CrossRef] [PubMed]
- Veal, M.; Dias, G.; Kersemans, V.; Sneddon, D.; Faulkner, S.; Cornelissen, B. A Model System to Explore the Detection Limits of Antibody-Based Immuno-SPECT Imaging of Exclusively Intranuclear Epitopes. J. Nucl. Med. 2021, 62, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Liu, T.; Li, Z.; Zhang, X.; Chen, X. Peptide-based imaging agents for cancer detection. Adv. Drug Deliv. Rev. 2017, 110–111, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Vallis, K.; Cornelissen, B. Development of a Tat-conjugated anti-γH2AX Fab fragment SPECT probe to image DNA damage. J. Nucl. Med. 2012, 53, 1086. [Google Scholar]
- Hao, G.; Zhou, J.; Guo, Y.; Long, M.A.; Anthony, T.; Stanfield, J.; Hsieh, J.-T.; Sun, X. A cell permeable peptide analog as a potential-specific PET imaging probe for prostate cancer detection. Amino Acids 2011, 41, 1093–1101. [Google Scholar] [CrossRef]
- Knight, J.C.; Koustoulidou, S.; Cornelissen, B. Imaging the DNA damage response with PET and SPECT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1065–1078. [Google Scholar] [CrossRef]
- Chen, X. Multimodality imaging of tumor integrin alphavbeta3 expression. Mini Rev. Med. Chem. 2006, 6, 227–234. [Google Scholar] [CrossRef]
- Huang, C.W.; Li, Z.; Conti, P.S. In vivo near-infrared fluorescence imaging of integrin α2β1 in prostate cancer with cell-penetrating-peptide-conjugated DGEA probe. J. Nucl. Med. 2011, 52, 1979–1986. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Shao, Q.; Min, Y.; Costa, M.; Yeow, E.K.; Xing, B. Enzyme-responsive cell-penetrating peptide conjugated mesoporous silica quantum dot nanocarriers for controlled release of nucleus-targeted drug molecules and real-time intracellular fluorescence imaging of tumor cells. Adv. Healthc. Mater. 2014, 3, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.P.; Arami, H.; Banga, I.; Gupta, J.; Gandhi, S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 2018, 9, 37252–37267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Chang, L.; Liu, H.; Song, J.; Liu, Y.; Bao, H.; Liu, B.; Wang, R.; Ni, J. Design of a new pH-activatable cell-penetrating peptide for drug delivery into tumor cells. Chem. Biol. Drug Des. 2019, 94, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.S.; Aguilera, T.A.; Jiang, T.; Ellies, L.G.; Nguyen, Q.T.; Wong, E.H.; Gross, L.A.; Tsien, R.Y. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr. Biol. Quant. Biosci. Nano Macro 2009, 1, 382–393. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. Harnessing the power of cell-penetrating peptides: Activatable carriers for targeting systemic delivery of cancer therapeutics and imaging agents. WIREs Nanomed. Nanobiotechnol. 2013, 5, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872. [Google Scholar] [CrossRef]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.-X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef]
- Scorilas, A.; Karameris, A.; Arnogiannaki, N.; Ardavanis, A.; Bassilopoulos, P.; Trangas, T.; Talieri, M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: A potential favourable indicator in node-negative patients. Br. J. Cancer 2001, 84, 1488–1496. [Google Scholar] [CrossRef]
- de Jong, H.; Bonger, K.M.; Löwik, D. Activatable cell-penetrating peptides: 15 years of research. RSC Chem. Biol. 2020, 1, 192–203. [Google Scholar] [CrossRef]
- Stiltner, J.; McCandless, K.; Zahid, M. Cell-Penetrating Peptides: Applications in Tumor Diagnosis and Therapeutics. Pharmaceutics 2021, 13, 890. [Google Scholar] [CrossRef]
- Huang, R.; Li, J.; Kebebe, D.; Wu, Y.; Zhang, B.; Liu, Z. Cell penetrating peptides functionalized gambogic acid-nanostructured lipid carrier for cancer treatment. Drug Deliv. 2018, 25, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Coppari, E.; Yamada, T.; Bizzarri, A.R.; Beattie, C.W.; Cannistraro, S. A nanotechnological, molecular-modeling, and immunological approach to study the interaction of the anti-tumorigenic peptide p28 with the p53 family of proteins. Int. J. Nanomed. 2014, 9, 1799–1813. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Santini, S.; Coppari, E.; Bucciantini, M.; Di Agostino, S.; Yamada, T.; Beattie, C.W.; Cannistraro, S. Interaction of an anticancer peptide fragment of azurin with p53 and its isolated domains studied by atomic force spectroscopy. Int. J. Nanomed. 2011, 6, 3011–3019. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Cannistraro, S. Time-Resolved Fluorescence and Essential Dynamics Study on the Structural Heterogeneity of p53DBD Bound to the Anticancer p28 Peptide. J. Phys. Chem. B 2020, 124, 9820–9828. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Moscetti, I.; Cannistraro, S. Interaction of the anticancer p28 peptide with p53-DBD as studied by fluorescence, FRET, docking and MD simulations. Biochim. Biophys. Acta. Gen. Subj. 2019, 1863, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Santini, S.; Yamada, T.; Bizzarri, A.R.; Beattie, C.W.; Cannistraro, S. Binding of Amphipathic Cell Penetrating Peptide p28 to Wild Type and Mutated p53 as studied by Raman, Atomic Force and Surface Plasmon Resonance spectroscopies. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 910–921. [Google Scholar] [CrossRef]
- Yang, W.; Xia, Y.; Fang, Y.; Meng, F.; Zhang, J.; Cheng, R.; Deng, C.; Zhong, Z. Selective Cell Penetrating Peptide-Functionalized Polymersomes Mediate Efficient and Targeted Delivery of Methotrexate Disodium to Human Lung Cancer In Vivo. Adv. Healthc. Mater. 2018, 7, e1701135. [Google Scholar] [CrossRef] [PubMed]
- Habault, J.; Poyet, J.L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Lim, K.J.; Sung, B.H.; Shin, J.R.; Lee, Y.W.; Kim, D.J.; Yang, K.S.; Kim, S.C. A cancer specific cell-penetrating peptide, BR2, for the efficient delivery of an scFv into cancer cells. PLoS ONE 2013, 8, e66084. [Google Scholar] [CrossRef]
- Shin, M.C.; Zhang, J.; Ah Min, K.; Lee, K.; Moon, C.; Balthasar, J.P.; Yang, V.C. Combination of antibody targeting and PTD-mediated intracellular toxin delivery for colorectal cancer therapy. J. Control Release 2014, 194, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Shin, M.C.; Liang, Q.; He, H.; Yang, V.C. 15 years of ATTEMPTS: A macromolecular drug delivery system based on the CPP-mediated intracellular drug delivery and antibody targeting. J. Control Release 2015, 205, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, X.; Pei, X.; Cao, W.; Ye, J.; Wang, J.; Sun, L.; Yu, F.; Wang, J.; Li, N.; et al. Antibody-siRNA conjugates (ARCs) using multifunctional peptide as a tumor enzyme cleavable linker mediated effective intracellular delivery of siRNA. Int. J. Pharm. 2021, 606, 120940. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, R.; Pei, X.; Chai, M.; Sun, L.; Huang, Y.; Wang, J.; Barth, S.; Yu, F.; He, H. Antibody–siRNA conjugates (ARC): Emerging siRNA drug formulation. Med. Drug Discov. 2022, 15, 100128. [Google Scholar] [CrossRef]
- Shi, S.J.; Wang, L.J.; Han, D.H.; Wu, J.H.; Jiao, D.; Zhang, K.L.; Chen, J.W.; Li, Y.; Yang, F.; Zhang, J.L.; et al. Therapeutic effects of human monoclonal PSMA antibody-mediated TRIM24 siRNA delivery in PSMA-positive castration-resistant prostate cancer. Theranostics 2019, 9, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Warso, M.A.; Richards, J.M.; Mehta, D.; Christov, K.; Schaeffer, C.; Rae Bressler, L.; Yamada, T.; Majumdar, D.; Kennedy, S.A.; Beattie, C.W.; et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br. J. Cancer 2013, 108, 1061–1070. [Google Scholar] [CrossRef]
- Razzak, M. Targeted therapies: One step closer to drugging p53. Nat. Rev. Clin. Oncol. 2013, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J.; et al. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro Oncol. 2016, 18, 1319–1325. [Google Scholar] [CrossRef]
- Nekhotiaeva, N.; Elmquist, A.; Rajarao, G.K.; Hällbrink, M.; Langel, U.; Good, L. Cell entry and antimicrobial properties of eukaryotic cell-penetrating peptides. Faseb J. 2004, 18, 394–396. [Google Scholar] [CrossRef]
- Snyder, E.L.; Meade, B.R.; Saenz, C.C.; Dowdy, S.F. Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS Biol. 2004, 2, E36. [Google Scholar] [CrossRef]
- Malkas, L.H.; Herbert, B.S.; Abdel-Aziz, W.; Dobrolecki, L.E.; Liu, Y.; Agarwal, B.; Hoelz, D.; Badve, S.; Schnaper, L.; Arnold, R.J.; et al. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc. Natl. Acad. Sci. USA 2006, 103, 19472–19477. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Lingeman, R.; Yakushijin, F.; Sun, E.; Cui, Q.; Chao, J.; Hu, W.; Li, H.; Hickey, R.J.; Stark, J.M.; et al. The Anticancer Activity of a First-in-class Small-molecule Targeting PCNA. Clin. Cancer Res. 2018, 24, 6053–6065. [Google Scholar] [CrossRef]
- Srimanee, A.; Arvanitidou, M.; Kim, K.; Hällbrink, M.; Langel, Ü. Cell-penetrating peptides for siRNA delivery to glioblastomas. Peptides 2018, 104, 62–69. [Google Scholar] [CrossRef]
- Meyer-Losic, F.; Nicolazzi, C.l.; Quinonero, J.r.m.; Ribes, F.; Michel, M.; Dubois, V.; de Coupade, C.; Boukaissi, M.; Chéné, A.-S.; Tranchant, I.; et al. DTS-108, A Novel Peptidic Prodrug of SN38: In vivo Efficacy and Toxicokinetic Studies. Clin. Cancer Res. 2008, 14, 2145–2153. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Jiskoot, W.; van Schie, R.M.F.; Carstens, M.G.; Schellekens, H. Immunological Risk of Injectable Drug Delivery Systems. Pharm. Res. 2009, 26, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Dinca, A.; Chien, W.M.; Chin, M.T. Intracellular Delivery of Proteins with Cell-Penetrating Peptides for Therapeutic Uses in Human Disease. Int. J. Mol. Sci. 2016, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, R.; Bahmani, A.; Afshar, S. Investigating the role of peptides in effective therapies against cancer. Cancer Cell Int. 2022, 22, 139. [Google Scholar] [CrossRef]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef]
- Reissmann, S.; Filatova, M.P. New generation of cell-penetrating peptides: Functionality and potential clinical application. J. Pept. Sci. 2021, 27, e3300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, P.; Ma, Z.; Lu, P.; Kebebe, D.; Liu, Z. Combination of cell-penetrating peptides with nanomaterials for the potential therapeutics of central nervous system disorders: A review. J. Nanobiotechnol. 2021, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Al Humaidan, E.L.; Pedersen, S.L.; Burkhart, A.; Rasmussen, C.L.M.; Moos, T.; Fuchs, P.; Fernandes, E.F.A.; Ozgür, B.; Strømgaard, K.; Bach, A.; et al. The Cell-Penetrating Peptide Tat Facilitates Effective Internalization of PSD-95 Inhibitors Into Blood–Brain Barrier Endothelial Cells but less Efficient Permeation Across the Blood–Brain Barrier In Vitro and In Vivo. Front. Drug Deliv. 2022, 2, 854703. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Liu, Y.; Qu, Y.; Wang, K.; Zhang, Y.; Chang, Y.; Yang, Z.; Wan, J.; Liu, J.; et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates. Nat. Biomed. Eng. 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).