COVID-19 Vaccination Campaign in Cancer Patients and Healthcare Workers-Results from a French Prospective Multicenter Cohort (PAPESCO-19)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Analysis of Antibody Serum Titers
2.4. Statistical Analysis
3. Results
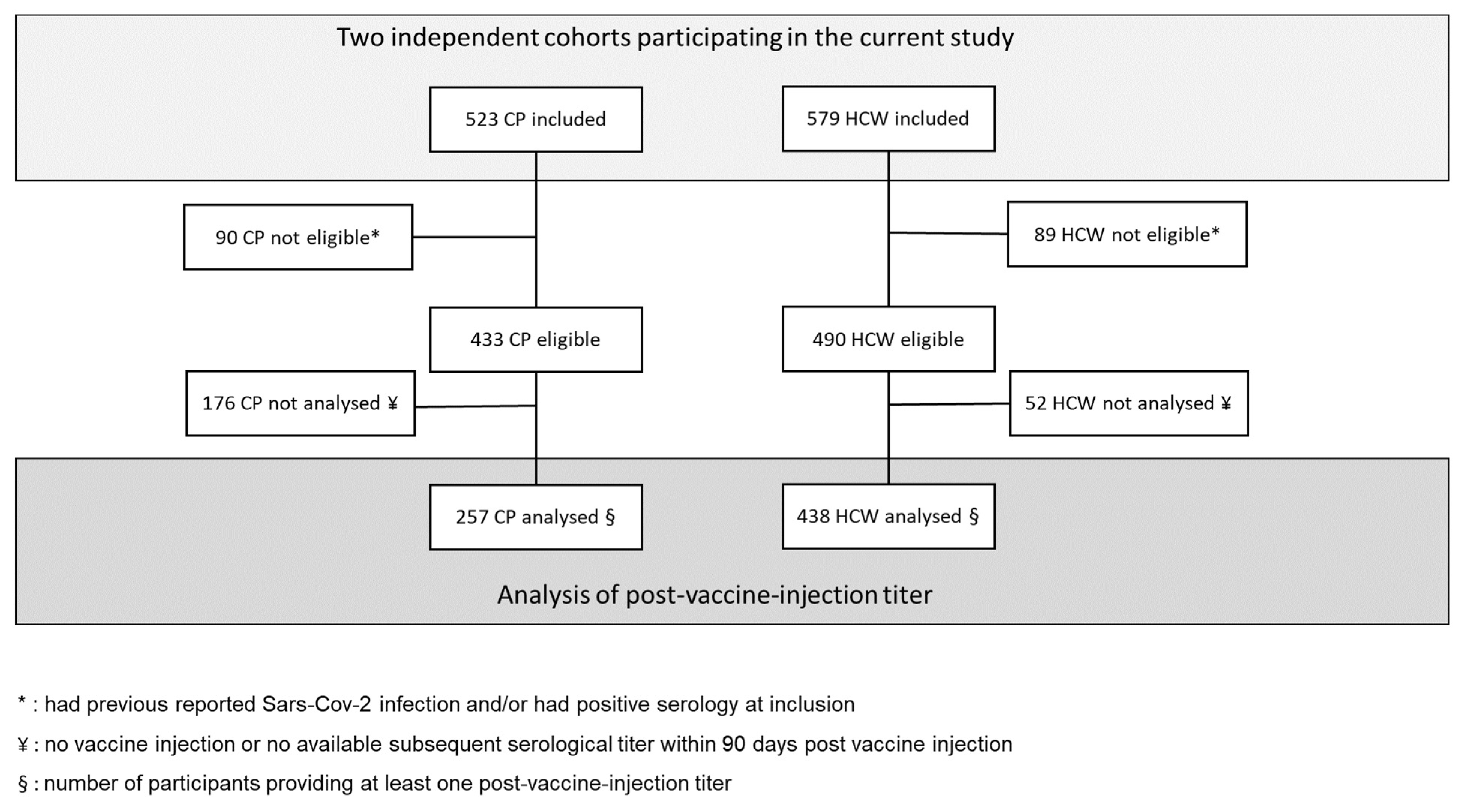
3.1. Participants
3.2. COVID Vaccination
3.3. SARS-CoV-2 Infection in Participants
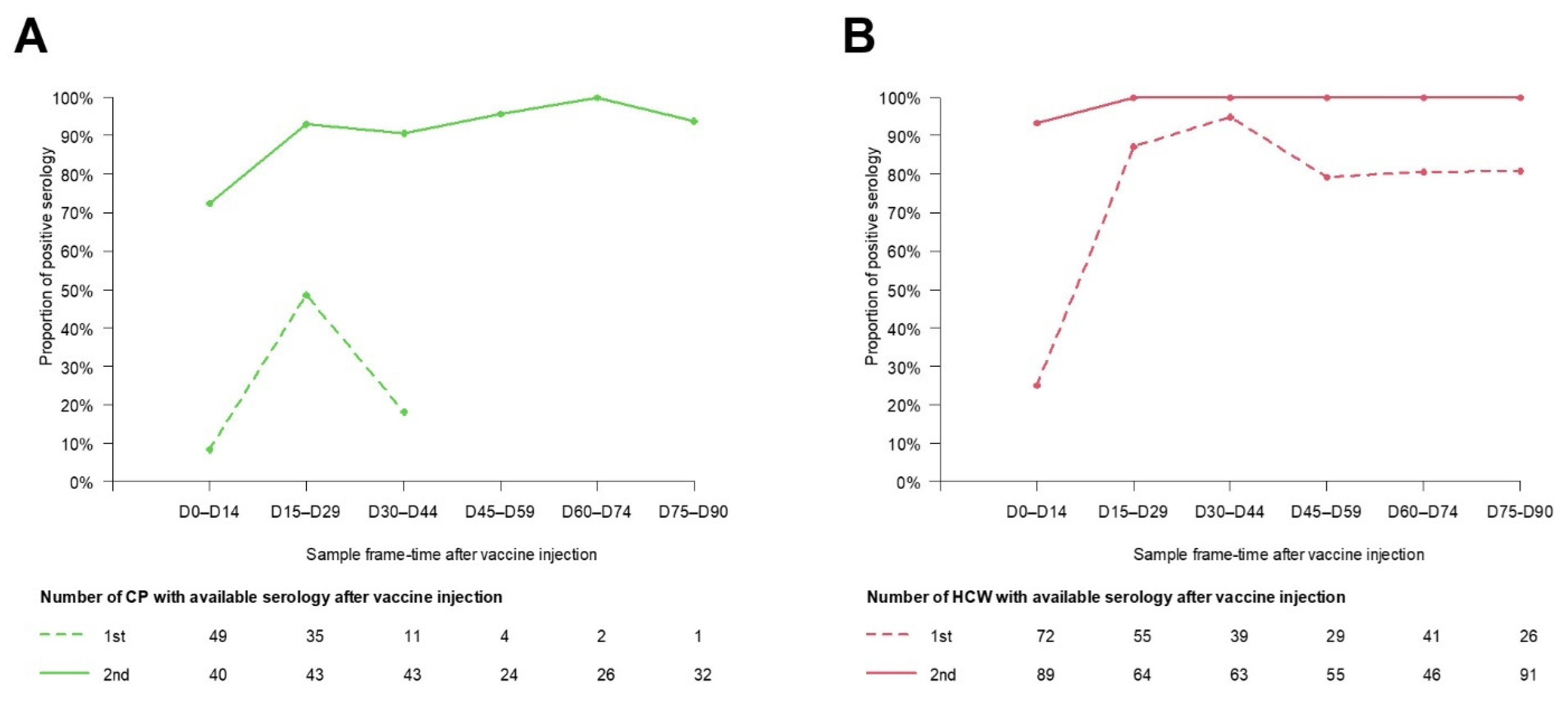
3.4. Seroconversion Rates
3.5. Antibody Response following Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statements
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T. COVID-19 Prevalence and Mortality in Patients with Cancer and the Effect of Primary Tumour Subtype and Patient Demographics: A Prospective Cohort Study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Giesen, N.; Sprute, R.; Ruthrich, M. Update of the AGIHO Guideline on Evidence-Based Management of COVID-19 in Patients with Cancer Regarding Diagnostics, Viral Shedding, Vaccination and Therapy. Eur. J. Cancer 2021, 147, 154–160. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Segal, B.H. Immune Responses to COVID-19 Vaccines in Patients with Cancer: Promising Results and a Note of Caution. Cancer Cell 2021, 39, 1045–1047. [Google Scholar] [CrossRef]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Munoz-Ruiz, M. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Oosting, S.F.; Veldt, A.A.M.; Fehrmann, R.S.N. Immunogenicity after Second and Third MRNA-1273 Vaccination Doses in Patients Receiving Chemotherapy, Immunotherapy, or Both for Solid Tumours. Lancet Oncol. 2022, 23, 833–835. [Google Scholar] [CrossRef]
- Fendler, A.; Vries, E.G.E.; GeurtsvanKessel, C.H. COVID-19 Vaccines in Patients with Cancer: Immunogenicity, Efficacy and Safety. Nat. Rev. Clin. Oncol. 2022, 19, 385–401. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, D.; Jiang, Q.; Guo, Y.; Chen, C. The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis. Front. Public Health 2022, 10, 940956. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Kliker, L.; Lustig, Y. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Denis, K.J.; Hoelzemer, A. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef]
- Odone, A.; Vigezzi, G.P.; Baldanti, F. Implications of COVID-19 Vaccine Effectiveness Waning for Public Health. Lancet Infect. Dis. 2022, 22, 918–919. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 Vaccine Effort: Viruses, Vaccines and Variants versus Efficacy, Effectiveness and Escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Heudel, P.; Favier, B.; Solodky, M.-L.; Assaad, S.; Chaumard, N.; Tredan, O.; Bachelot, T.; Ray-Coquard, I.; Russias, B.; Fournier, M.-L.; et al. Survival and Risk of COVID-19 after SARS-COV-2 Vaccination in a Series of 2391 Cancer Patients. Eur. J. Cancer 2022, 165, 174–183. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Starkey, T.; Ionescu, M.C.; Little, M.; Tilby, M.; Tripathy, A.R.; Mckenzie, H.S.; Al-Hajji, Y.; Barnard, M.; Benny, L.; et al. Vaccine Effectiveness against COVID-19 Breakthrough Infections in Patients with Cancer (UKCCEP): A Population-Based Test-Negative Case-Control Study. Lancet Oncol. 2022, 23, 748–757. [Google Scholar] [CrossRef]
- Wu, J.T.-Y.; La, J.; Branch-Elliman, W.; Huhmann, L.B.; Han, S.S.; Parmigiani, G.; Tuck, D.P.; Brophy, M.T.; Do, N.V.; Lin, A.Y.; et al. Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients with Cancer: A US Nationwide Veterans Affairs Study. JAMA Oncol. 2022, 8, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 Vaccine Waning and Effectiveness and Side-Effects of Boosters: A Prospective Community Study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Shrotri, M.; Fragaszy, E.; Nguyen, V.; Navaratnam, A.M.D.; Geismar, C.; Beale, S.; Kovar, J.; Byrne, T.E.; Fong, W.L.E.; Patel, P.; et al. Spike-Antibody Responses to COVID-19 Vaccination by Demographic and Clinical Factors in a Prospective Community Cohort Study. Nat. Commun. 2022, 13, 5780. [Google Scholar] [CrossRef]
- Zhou, K.; Blanc-Lapierre, A.; Seegers, V. Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients. First Results from the PAPESCO-19 Cohort Study. Cancers 2021, 13, 3389. [Google Scholar] [CrossRef]
- Zhou, K.; Raoul, J.L.; Blanc-Lapierre, A. COVID-19 Infections in Cancer Patients Were Frequently Asymptomatic: Description From a French Prospective Multicenter Cohort (PAPESCO-19). Clin. Med. Insights Oncol. 2022, 16, 11795549221090187. [Google Scholar] [CrossRef]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 Serological Tests: Towards Harmonization of Anti-Spike Assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Shioda, K.; Lau, M.S.Y.; Kraay, A.N.M.; Nelson, K.N.; Siegler, A.J.; Sullivan, P.S.; Collins, M.H.; Weitz, J.S.; Lopman, B.A. Estimating the Cumulative Incidence of SARS-CoV-2 Infection and the Infection Fatality Ratio in Light of Waning Antibodies. Epidemiology 2021, 32, 518–524. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M. Efficacy of a Fourth Dose of Covid-19 MRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Martins-Branco, D.; Nader-Marta, G.; Tecic Vuger, A.; Debien, V.; Ameye, L.; Brandão, M.; Punie, K.; Loizidou, A.; Willard-Gallo, K.; Spilleboudt, C.; et al. Immune Response to Anti-SARS-CoV-2 Prime-Vaccination in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Cancer Res. Clin. Oncol. 2022, 1–6. [Google Scholar] [CrossRef]
- Terpos, E.; Liontos, M.; Fiste, O. SARS-CoV-2 Neutralizing Antibodies Kinetics Postvaccination in Cancer Patients under Treatment with Immune Checkpoint Inhibition. Cancers 2022, 14, 2796. [Google Scholar] [CrossRef] [PubMed]
- Janzic, U.; Bidovec-Stojkovic, U.; Mohorcic, K. Solid Cancer Patients Achieve Adequate Immunogenicity and Low Rate of Severe Adverse Events after SARS-CoV-2 Vaccination. Futur. Oncol. 2022, 18, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Wu, M.; Harvey, R. AZD1222-Induced Neutralising Antibody Activity against SARS-CoV-2 Delta VOC. Lancet 2021, 398, 207–209. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R. Neutralising Antibody Activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 Vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
| CP n = 523 | HC n = 579 | |
|---|---|---|
| Age: mean (sd) | 61 (12) | 41 (10) |
| Sex: F (%) | 377 (72.1%) | 482 (83.2%) |
| Vaccination reported during follow-up | ||
| No | 147 (28.1%) | 68 (11.8%) |
| 1 injection | 34 (6.5%) | 50 (8.8%) |
| 2 | 290 (55.4%) | 444 (76.7%) |
| 3 | 52 (9.9%) | 17 (2.9%) |
| Confirmed SARS-CoV-2 infection (self-reported) | 36 (6.9%) | 55 (9.5%) |
| never vaccinated | 10 | 10 |
| before vaccination | 18 | 31 |
| after vaccination | 8 | 14 |
| Positive Serology * without or before vaccination | 83 (22%) | 76 (14.9%) |
| Previous test-confirmed infection | 21 | 28 |
| Negative Serology * before vaccination | 293 (78%) | 503 (86.9%) |
| Previous test-confirmed infection | 7 | 13 |
| Hospitalization due to COVID | 16 (3%) | 3 (%) |
| ICU | 2 | 0 |
| Death (considered as related to COVID) | 0 | 0 |
| D0–D14 | D15–D29 | D30–D44 | D45–D59 | D60–D74 | D75–D90 | |
|---|---|---|---|---|---|---|
| All CP | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/49 | 32 (<4.81, 187)/35 | 18 (4, 30)/11 | 97 (62, 341)/4 | n = 2 | n = 1 |
| 2nd injection | 138 (32, 450)/40 | 1600 (505, >2080)/43 | 1560 (485, >2080)/43 | 1270 (364, 2070)/24 | 728 (358, 1538)/26 | 730 (423, 1383)/32 |
| CP with chemotherapy | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/26 | 23 (<4.81, 81)/18 | 22 (17, 30)/7 | n = 1 | n = 1 | n = 1 |
| 2nd injection | 103 (26, 520)/28 | 1910 (703, >2080)/29 | 1835 (656, >2080)/26 | 1670 (760, 2015)/11 | 782 (315, 1840)/15 | 810 (494, 1255)/19 |
| CP with immunotherapy | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/7 | 9.8 (<4.81, 77)/6 | n = 2 | n = 1 | No serology | No serology |
| 2nd injection | 79 (8, 172)/4 | 528 (296, 1371.5)/6 | 2030 (716, >2080)/7 | 980 (472, 1810)/7 | 142 (93, 300)/3 | 817 (481, 1290)/6 |
| Women CP | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/31 | 6.8 (<4.81, 264.5)/26 | 16.6 (6.4, 29.7)/8 | n = 2 | n = 2 | n = 1 |
| 2nd injection | 174 (46.2, 565)/24 | 1890 (716.8, >2080)/34 | 1860 (634, >2080)/30 | 1270 (467, 1992.5)/20 | 776 (421, 1667.5)/20 | 1010 (492, 1430)/21 |
| Men CP | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/18 | <4.81 (<4.81, 13.1)/9 | 21.7 (10.8, 47.9)/3 | n = 2 | No serology | No serology |
| 2nd injection | 93.9 (4.9, 215.8)/16 | 455 (380, 1190)/9 | 814 (386, 1170)/13 | 1251.5 (307.8, 2100)/4 | 526 (219.8, 1027.2)/6 | 576 (308, 834.5)/11 |
| CP aged 60 y and over | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/24 | 11.9 (<4.81, 77.6)/24 | 18 (8.6, 29.3)/5 | n = 2 | n = 1 | No serology |
| 2nd injection | 101.5 (9.6, 258.5)/24 | 1500 (464.5, >2080)/22 | 1140 (303, >2080)/23 | 989 (123.1, 1937.5)/14 | 599 (304, 1170)/17 | 576 (241, 1015)/19 |
| CP younger than 60 y | ||||||
| 1st injection | <4.81 (<4.81, <4.81)/25 | 85.6 (49.2, 252)/11 | 18.5 (3.8, 28.4)/6 | n = 2 | n = 1 | n = 1 |
| 2nd injection | 230.5 (46.2, 616.2)/16 | 1910 (550, >2080)/21 | 1800 (778.8, >2080)/20 | 1745 (660.8, >2080)/10 | 1240 (685, 2050)/9 | 1210 (810, 1430)/13 |
| D0–D14 | D15–D29 | D30–D44 | D45–D59 | D60–D74 | D75–D90 | |
|---|---|---|---|---|---|---|
| All HCW | ||||||
| 1st injection | <4.81 (<4.81, 31)/72 | 236 (70, 573)/55 | 168 (75, 267)/39 | 84 (36, 133)/29 | 77.8 (37.5, 122)/41 | 74 (39, 117)/26 |
| 2nd injection | 1950 (219, >2080)/89 | >2080 (2038, >2080)/64 | >2080 (1880, >2080)/63 | >2080 (1570, >2080)/55 | 1475 (889, >2080)/46 | 1400 (876, 1925)/91 |
| Women HCW | ||||||
| 1st injection | <4.81 (<4.81, 26.5)/59 | 236 (53.6, 583.5)/47 | 168 (87.9, 358)/33 | 95.4 (36.7, 133)/25 | 70.6 (37.4, 131)/35 | 79.4 (55.1, 136)/22 |
| 2nd injection | 1655 (175.8, >2080)/76 | >2080 (2037, >2080)/52 | >2080 (1880, >2080)/52 | >2080 (1585, >2080)/44 | 1520 (905, >2080)/40 | 1450 (884, 1960)/76 |
| Men HCW | ||||||
| 1st injection | 6.9 (<4.81, 137)/13 | 259.5 (159.2, 418.2)/8 | 126.8 (61.4, 185.5)/6 | 42 (31, 82.9)/4 | 91.1 (88.8, 100.4)/6 | 32.4 (30.8, 33.6)/4 |
| 2nd injection | >2080 (776, >2080)/13 | >2080 (1973, >2080)/12 | 2030 (1785, >2080)/11 | 1780 (1475, >2080)/11 | 1250 (929, 1922.5)/6 | 1060 (801.5, 1690)/15 |
| HCW aged 40 y and over | ||||||
| 1st injection | <4.81 (<4.81, 31.7)/38 | 236 (93, 449)/33 | 183 (112.7, 243.2)/20 | 38 (34.2, 95.4)/9 | 74.2 (43.9, 143.8)/14 | 73.5 (41.1, 80.6)/9 |
| 2nd injection | 1800 (269.2, >2080)/44 | >2080 (1620, >2080)/37 | >2080 (1620, >2080)/41 | 1740 (1420, >2080)/27 | 976 (577, 1605)/19 | 1150 (763, 1800)/41 |
| HCW younger than 40 y | ||||||
| 1st injection | <4.81 (<4.81, 25.1)/34 | 370.5 (52.4, 931.8)/22 | 143 (59, 310)/19 | 106 (40.5, 187.2)/20 | 82.1 (37.4, 101.5)/27 | 78.3 (36.9, 139)/17 |
| 2nd injection | 1950 (175, >2080)/45 | >2080 (>2080, >2080)/27 | >2080 (2048, >2080)/22 | >2080 (1710, >2080)/28 | 1890 (1435, >2080)/27 | 1550 (986.8, >2080)/50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seegers, V.; Rousseau, G.; Zhou, K.; Blanc-Lapierre, A.; Bigot, F.; Mahammedi, H.; Lambert, A.; Moreau-Bachelard, C.; Campone, M.; Conroy, T.; et al. COVID-19 Vaccination Campaign in Cancer Patients and Healthcare Workers-Results from a French Prospective Multicenter Cohort (PAPESCO-19). Cancers 2022, 14, 5547. https://doi.org/10.3390/cancers14225547
Seegers V, Rousseau G, Zhou K, Blanc-Lapierre A, Bigot F, Mahammedi H, Lambert A, Moreau-Bachelard C, Campone M, Conroy T, et al. COVID-19 Vaccination Campaign in Cancer Patients and Healthcare Workers-Results from a French Prospective Multicenter Cohort (PAPESCO-19). Cancers. 2022; 14(22):5547. https://doi.org/10.3390/cancers14225547
Chicago/Turabian StyleSeegers, Valérie, Guillaume Rousseau, Ke Zhou, Audrey Blanc-Lapierre, Frédéric Bigot, Hakim Mahammedi, Aurélien Lambert, Camille Moreau-Bachelard, Mario Campone, Thierry Conroy, and et al. 2022. "COVID-19 Vaccination Campaign in Cancer Patients and Healthcare Workers-Results from a French Prospective Multicenter Cohort (PAPESCO-19)" Cancers 14, no. 22: 5547. https://doi.org/10.3390/cancers14225547
APA StyleSeegers, V., Rousseau, G., Zhou, K., Blanc-Lapierre, A., Bigot, F., Mahammedi, H., Lambert, A., Moreau-Bachelard, C., Campone, M., Conroy, T., Penault-Llorca, F., Boisdron-Celle, M., Bellanger, M., & Raoul, J.-L. (2022). COVID-19 Vaccination Campaign in Cancer Patients and Healthcare Workers-Results from a French Prospective Multicenter Cohort (PAPESCO-19). Cancers, 14(22), 5547. https://doi.org/10.3390/cancers14225547






