Simple Summary
The destructive growth of carcinomas is associated with crossing the border between the epithelial and the connective tissue parts of an organ. One component of this borderline, the basement membrane, is the heterotrimeric laminin 332, which mediates the adhesion of basal epithelial cells. This protein, in particular its gamma 2 chain, is fundamentally reorganized during tumor cell invasion. Specific deposition patterns of laminin 332 are also present in oral squamous cell carcinomas and have been shown to be of high diagnostic and predictive value. Furthermore, laminin 332 restructuring is associated with important tumor biological processes, e.g., stromal activation, the development of a motile phenotype, and tumor spreading. In this review, current knowledge in the field is summarized and the recommendation to consider laminin 332 as a promising grading and monitoring parameter and as a potential therapeutic target is discussed.
Abstract
Invasion of the connective tissue by carcinoma cells is accompanied by disintegration and reorganization of the hemidesmosomes, which connect the basement membrane to the basal epithelial cells. In terms of mediating the basement membrane, i.e., basal cell interactions, the heterotrimeric laminin 332 is the most important bridging molecule. Due to this distinct function, laminin 332, especially its gamma 2 chain, came into the focus of cancer research. Specific de novo synthesis and deposition patterns of laminin 332 are evident upon development and progression of oral squamous cell carcinomas (OSCCs). Loss from the basement membrane, cytoplasmic accumulation, and extracellular deposition are associated with crucial processes such as stromal activation and immune response, epithelial to mesenchymal transition, and tumor cell budding. In networks with components of the tumor microenvironment, altered expression of laminin 332 chains, proteolytic processing, and interaction with integrin receptors seem to promote cancer cell migration. Indeed, reorganization patterns are shown to have a high diagnostic and prognostic value. Here, we summarize the current knowledge on laminin 332 reorganization in OSCCs with special focus on its gamma 2 chain and provide, based on the current literature, evidence on its promising role as a grading and monitoring parameter and as a potential therapeutic target.
1. Introduction
Oral squamous cell carcinomas (OSCCs) represent the most abundantly occurring tumor type among head and neck carcinomas. As reported by the Global Cancer Observatory (GCO), there were 377,713 new cases and 177,757 deaths related to lip and oral cavity tumors worldwide in 2020 [1]. Although there are strong variations in the incidence among different regions and countries, oral cancer remains a big challenge for health care systems. Recently, great efforts have been made to further basic biological tumor research in order to understand the processes of invasion, progression, and metastasis formation, as well as to improve strategies for targeted and individualized therapies. Nevertheless, there have only been minimal changes in overall prognosis and survival rates, which have remained at appr. 40–50% [2].
The latter aspect is especially related to the lack of reliable markers for early diagnosis, prognosis, or therapy surveillance. In addition, there are no established molecular targets for individualized treatment approaches [2]. Therefore, there is an urgent need to establish such new diagnostic and prognostic biomarkers, as well as related molecular therapies.
The loss of the sessile epithelial phenotype and the gain of migratory capability is the first step in cancer cell invasion and a prerequisite for the formation of a metastatic behavior. This is necessarily associated with reorganization of the epithelial basement membrane to enable keratinocyte motility and phenotype transition. Loss of E-cadherin mediated cell–cell contacts and the disintegration of hemidesmosomes are known to play a pivotal role within this process. In hemidesmosomes of squamous epithelia, the integrity of the epidermal–dermal junction zone is mainly mediated by the interaction of laminin 332 (Ln332) and α6β4 integrin. Therefore, modulation of these hemidesmosomal components is strictly associated with squamous cell carcinoma development and progression. Furthermore, the subepithelial extracellular matrix has to be reorganized to allow cancer cell movement into the connective tissue [3,4].
With respect to the crucial role of the extracellular matrix (ECM) microenvironment for tumor cell behavior, understanding the ECM modulating processes at the invasive front may help to develop targeted pharmaceutical strategies to prevent cancer cells from spreading and encourage the improvement of therapeutic concepts for head and neck carcinomas [5,6,7]. Therefore, the aim of this review is to summarize current knowledge on the tumor biological importance of Ln332 and its interaction with fibronectin (Fn) and tenascin-C (Tn-C) as relevant components of the ECM in the development and progression of OSCCs.
2. ECM Reorganization in the OSCC Invasive Front
Invasion of carcinoma cells is a complex and multifactorial process including reorganization of cell–cell contacts, the acquirement of a mesenchymal-like phenotype, the degradation of the basement membrane, and a directed migration into the inherent surrounding tissue [8,9]. Interaction between the carcinoma cells and the components of the tumor microenvironment (TME), also known as tumor–stroma cross-talk, is essential for the initiation and maintenance of this process and includes the mutual activation of carcinoma cells themselves as well as stromal fibroblasts, endothelial cells, and inflammatory cells [10]. Additionally, in OSCCs, cytokine-mediated tumor–stroma cross-talk leads to the development of a desmoplastic stroma reaction dominated by activated fibroblasts which, among others, are responsible for the deposition of abundant amounts of collagens [11,12]. In that context, resident or attracted fibroblast precursor cells gain the alpha smooth muscle actin positive (aSMA+) myofibroblastic phenotype. In the last two decades, tumor myofibroblasts were frequently designated as cancer-associated fibroblasts (CAFs), which seems to be a simplification in the light of current knowledge [13,14,15]. Currently, it is widely accepted that CAFs are a phenotypically and functionally heterogeneous cell population depending on carcinoma type and exact localization [16,17]. Therefore, aSMA positive CAFs should better be designated as myCAFs to take their functionally important diversity into account [18,19].
Besides tumor–stroma cross-talk, the local reorganization of the ECM is another crucial step of OSCC invasion. The process of ECM reorganization includes proteolysis of the adjacent matrix structures, de novo synthesis of migration promoting matrix proteins, and a structural 3D organization of this novel quality ECM, which will be described in detail later [20,21]. As far as we know, both carcinoma cells and the cells of the TME contribute to this ECM reorganization within the basement membrane (BM) structures and the invasion front [22]. During the last three decades, evidence has been given that the reorganized ECM exhibits a lot of similarities to physiological and pathological tissue modulating processes, e.g., embryogenesis, wound healing, and fibrosis [23,24,25,26,27,28]. Therefore, this special ECM composition is also referred to as an “oncofetal” or “provisional” ECM (oncfECM or pECM) [29,30,31,32]. This ECM composition is characterized by the re-occurrence of certain molecular variants of extracellular adhesion proteins, which can be generated by alternative splicing of the pre-mRNA, alternative de novo glycosylation or chain assembly, and changes in the deposition pattern. These variants are expressed in early development but are virtually absent in healthy adult tissues [23]. With respect to cancer cell behavior in the invasion front, those extracellular adhesion proteins, in particular fibronectins, laminins, and tenascins, as well as their oncofetal variants seem to play a critical role within the modulation of cell–matrix interactions during the development of an invasive cell phenotype [33,34]. Re-expression of the oncofetal isoforms of these proteins modulates the ECM properties to enable and promote migration via new integrin-mediated cell–matrix contacts, new interactions with other ECM proteins, and the formation of guiding structures for cancer cell invasion [35].
In the 1990s, our group could already demonstrate a differential expression and reorganization of laminin isoforms and fibronectin variants in OSCCs associated with neoplastic transformation and invasion [24,36]. Since then, there has been growing evidence that reorganized oncofetal adhesion proteins may be of high prognostic and therapeutic importance in head and neck cancer. As discussed in detail later in this review, not only the occurrence of the newly formed matrix proteins themselves but also the molecular interaction and formation of multiprotein complexes, as visualized by colocalization studies [37], seem to influence the biological behavior of both oral cancer cells and oral CAFs. Furthermore, matrix reorganization modulates the inflammatory host response in HNSCC and therefore represents a putative target for immune therapy [38,39].
3. Laminin and Laminin Isoforms
Laminins (Lns) are constitutive components of the BM. The laminin (Ln) molecule is a heterotrimeric ECM adhesion protein, consisting of a large α chain and two smaller chains, β and γ. Up to now, eleven Ln chains encoded in the human genome have been described. Among them, there are five α chains (α1, α2, α3, α4, and α5), three β chains (β1, β2, and β3), and three γ chains (γ1, γ2, and γ3). Alternative splicing of the α3 chain leads to two transcript variants, the shorter α3A and the longer α3B chain. Because the α3A variant is primarily incorporated in the epithelial BM and the α3B chain seems to be preferentially expressed in the vascular BM of healthy tissue and has no association with cancer events [40], in the following we refer to α3Aβ3γ2 Ln as Ln332.
Tissue and developmental specific gene expression, as well as molecular interactions, lead to intracellular heterotrimerization. While the α1 chain is expressed during epithelial morphogenesis and is only marginally detectable in few adult organs, the α2 chain is a component of the muscle basal lamina, the α3 chain is located in the BM of stratified epithelia, and the α4 chain is located in the vascular BM [41]. Until 2005, laminins were numbered in the order of their discovery [42]. The currently defined nomenclature of laminins is based on their chain composition [43]. The prototype of the Ln family proteins is Ln-111, composed of the α1, β1, and γ1 chain. It is the first described and, up to now, the best characterized Ln isoform [44].
Lns are organized in a cross-shaped manner. The C-terminus of the long alpha chain folds in five globular domains (L1–L5) responsible for the interaction of the molecule with the plasma membrane as a link between the BM and the intracellular filament system. Interaction is mediated by a high affinity to integrins, dystroglycans, and other proteins. The N-termini of the three Ln chains are organized separately. They include repetitive amino acid sequences, which are homologous to the epidermal growth factor (EGF) (also known as EGF-like repeats or LE motifs) with interspaced globular domains. The N-termini of most chains fold in a globular domain, the so-called Ln N-terminal domain LN. The N-terminus is involved in molecular interaction with the collagen IV network in the BM. Furthermore, Lns with three full-length short arms are capable of self-polymerization. These polymers are connected, via perlecan and nidogen (entactin), to collagen IV polymers forming large networks crucial for BM formation. For detailed information concerning the molecular structure and general characteristics of laminins, see the excellent reviews by M. Aumailley and H. Colognato [41,45,46].
4. Ln332 and Hemidesmosomes
The functionally most important laminin within the dermal–epidermal junction is the laminin variant 332 (Ln332), formerly called laminin-5 [42,43]. Along with its molecular interaction partners and the integrin receptor α6β4, it represents the main component of the extracellular part of the hemidesmosome (HD). HDs are crucial for the maintenance of tissue integrity at the mesenchymal–epidermal contact zone. There are two types of HDs, type I (HD I) and type II (HD II), with HD I as the most common one in stratified and pseudostratified epithelia, including the epidermis and the airway epithelium. Besides Ln332, HD I is composed of α6β4 integrin, the bullous pemphigoid antigen (BP) 180 (also known as collagen type XVII), the tetraspanin CD151, and, inside the cell, BP230 and plectin [47] (Figure 1).
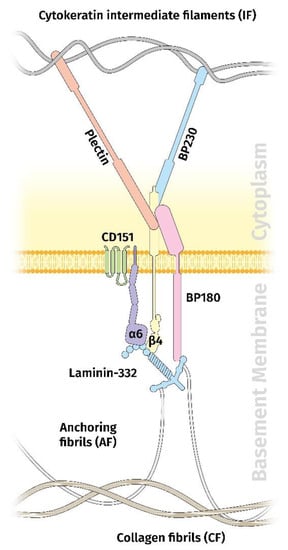
Figure 1.
Schematic diagram showing the organization of the hemidesmosme in the dermal–epidermal junction. Besides laminin 332, the hemidesmosome contains the integrin α6β4 receptor, BP180, CD151, BP230, and plectin.
HD dynamics are crucial for tissue modulating processes in the dermal–epidermal junction, as seen in embryogenesis, organogenesis, wound healing, and, finally, in carcinoma cell invasion and migration. HD dynamics and related cell signaling is mainly regulated by α6β4 [48]. Interestingly, Ln332 plays a critical role in the formation and turnover of HDs. It is synthesized by keratinocytes and secreted in its unprocessed form. Extracellular proteolytic processing of its α3 chain occurs and enables the interaction with α6β4 integrin in stable HDs of quiescent epidermis. Additional processing of the γ2 chain is known to generate a 105 kDa product. When keratinocytes or carcinoma cells become motile (e.g., skin wounding or invasion), unprocessed Ln332 (α3 chain) seems to be secreted at the leading edge, enabling cell movement through the α3β1 integrin [49]. In this case, further processing of the γ2 chain is also known and will be described later.
With respect to its function as a binding partner to α6β4 or α3β1 integrins and the extracellular matrix network, Ln332 is involved, first, in HD formation and, second, in both inside-out and outside-in signaling. On the other hand, HD remodeling must be also reflected by altered Ln332 synthesis, secretion, and deposition pattern [49]. Therefore, Ln332 came into the focus of cancer invasion and metastasis research once the molecule was described and characterized. Indeed, BM modulation, as detected mainly by Ln332 immunohistochemistry, was reported for many carcinoma types and was characterized by loss of Ln332 from the BM zone accompanied by a cytoplasmic accumulation, as well as by extracellular deposition outside the BM region [50,51]. The tumor-promoting role of Ln332 was also described for different squamous cell carcinomas, and the molecular background behind this was recently summarized by P. Marinkovich [52]. Such changes were also detected in HNSCC, especially in oral squamous cell carcinomas, both in vitro and in vivo, which will be now described in more detail.
5. Laminin Reorganization in OSCCs
In the 1980s, Ln expression was already being investigated in OSCCs in relation to BM structure, showing intact BM staining in normal and hyperplastic epithelium but a loss of laminin in dysplastic oral mucosa and, especially, in invasive tumor regions [53]. Attenuation or loss of Ln staining in the BM zone was shown to be associated with a higher grade of malignancy [54] and, later, it became evident that loss of BM material (as shown by Ln immunohistochemistry) was also related to the neoplastic transformation process [55] and to an enhanced risk of regional lymph node metastasis [56]. Furthermore, immunohistochemical assessment of ECM molecule expression including Ln was postulated to be helpful in improving the grading of OSCCs [57,58]. Also at this time, several studies demonstrated that Ln BM reorganization was accompanied by a restructuring of the ECM proteins collagen type IV, fibronectin, and tenascin in the invasion front [57,59]. Additionally, during the late 1980s and the 1990s, several members of the Ln family were discovered and specific antibodies became available, such that investigations concerning Ln in the BM of oral mucosae were more and more focused on different Ln isoforms. In 1992, Epiligrin, currently known as Ln332, was described as the major keratinocyte integrin ligand involved in bullous skin disease as a target for autoantibodies and it became evident that this Ln isoform was the major BM Ln in squamous epithelia [60].
Years before, homologue proteins were described by several groups and named as BM600/nicein, GB3 antigen, kalinin, and ladsin (for review see [50]), which, in the end, mean the same molecule, which is Ln5 according to the unified nomenclature as suggested by Burgeson and coworkers [42]. According to the fact that ECM proteins have a crucial impact on cellular differentiation, proliferation, and migration [23], and that Ln5 was the major laminin of the squamous epithelial BM, research became focused on elucidating differential expression and changes in spatial distribution during neoplastic transformation, invasion, and tumor progression in OSCCs. Altered synthesis and deposition of Ln5 was already described in 1997 by Kainulainen and coworkers, demonstrating increased cytoplasmic immunostaining along with Ln γ2 chain (Lng2) mRNA detection in carcinoma cells at the invasion border [61]. In 1999, our group published, for the first time, a comprehensive analysis of the immunohistochemical expression of different Ln chains in oral mucosae and OSCCs [24]. Staining was performed using chain-specific antibodies on shock-frozen tumor samples. According to the results, the BM of normal adult oral epithelia comprises the α3, α5, β1, β3, γ1, and γ2 chain. In hyperproliferative conditions, and during neoplastic transformation, the α2 and the β2 chain were re-expressed. An increased expression of the β3 and γ2 chain at the invasive front was associated with cytoplasmic accumulation in budding carcinoma cells, as well as deposits outside the basement membrane region. Based on these findings, we suggested that Ln5 is an immunohistochemical marker for invasion and OSCC cell invasion, which is guided by the reorganized Ln5 matrix. Furthermore, α2 and β2 chain re-expression hints to a more embryonal state of the BM zone during the epithelial reorganization process. In line with increased cytoplasmic accumulation in tumor cells and extracellular depositions, there was a total loss of Ln5 from the tumorous BM. Quantification of Ln5 loss by confocal laser scanning microscopy correlated with the malignancy grade [62].
A BM-independent reorganization of Ln5 could also be proved in 3D cell culture models. In 1989, Matsumoto and coworkers presented, for the first time, a collagen gel-based in vitro model for OSCCs, demonstrating that gel-incorporated fibroblasts are necessary for the invasive behavior of tumor cells [63]. Accordingly, our group and others could immunohistochemically demonstrate a deposition of at least the Ln α3 and γ2 chains in the tumor–gel interface without the formation of a structural BM or hemidesmosomes [36,64].
OSCC invasion and progression is not only accompanied by a modulation of Ln chain expression in the tumor BM, but also in the tumor microenvironment. myCAF occurrence and endothelial activation seem to be associated with a decrease in α2 and an increase in α3, α4, α5, and γ2 chain expression in the stromal compartment, as well as modulation of α3 chain expression in tumor vessels. Interestingly, γ2 chain positivity was also seen in some vascular structures in well-differentiated OSCCs [65].
6. Laminin γ2 Chain Reorganization in the OSCC Invasive Front: Tumor Biological Implications
As stated before, the γ2 chain is unique for Ln5/Ln332. Therefore, most investigations concerning the Ln332 reorganization in OSCCs that have been performed considered this chain as an immunohistochemical surrogate for Ln332, using different antibodies specific for the molecule. Indeed, there are several reports on the diagnostic and predictive value of Lng2 immunohistochemistry in OSCCs. Among others, a correlation has been shown between a diffuse expression of Lng2 in disseminating and infiltrating tumor cells, with higher grades of malignancy, poor prognosis, and a higher risk for nodal metastasis observed in patients with tongue cancer [66,67,68,69]. Furthermore, shorter life expectancy in OSCCs with an increased number of Lng2-positive tumor cells [70], an elevated risk for transformation in Lng2-positive pre-neoplastic mucosal lesions [71,72], as well as a higher risk for nodal metastasis [20,73,74] could be evidenced. Interestingly, expression of Lng2 in invading human carcinoma cells was already described as a common phenomenon in 1994 by C. Pyke and colleagues [75,76] and has been repeatedly demonstrated for several other epithelial tumor entities. Table 1 provides an overview of the relevant literature describing the impact of Lng2 assessment on OSCC diagnosis and prognosis.

Table 1.
Overview of studies conducted to assess the diagnostic and prognostic value of the laminin γ2 chain expression pattern in oral squamous cell carcinomas.
Against the background of these clinico-pathological findings, a link between altered Lng2 synthesis/deposition and the tumor biological behavior of neoplastic oral keratinocytes must be assumed. Although it cannot be completely excluded that the tumor expression pattern of Lng2 is merely a secondary phenomenon following dysregulation of laminin turnover during neoplastic transformation, disturbed BM formation, and/or phenotype transition, there are credible arguments for a direct protumorigenic influence of this laminin chain on cellular activity, which now will be discussed.
6.1. Laminin γ2 Chain Expression Is Related to the Migration of Normal and Neoplastic Keratinocytes
Lng2, probably in its monomeric form, seems to play an important role in guiding keratinocytes during wound re-epithelialization [81,82]. For a review of the contributions of an ECM to skin wound healing, see the excellent reviews of P. Rousselle [83,84]. This process was also proven to be crucial in an in vitro wound healing assay using rat oral epithelial cells. Here, accumulation of Lng2 was detected in the peripheral cytoplasm of cells at the wound edge, with migration mediated via the integrin α3 chain [85]. Ln332–α3β1 integrin interaction has been proven to direct the stabilization of polarized lamellipodia in epithelial cells via activation of Rac1 [86,87]. Alternatively, Decline and Rousselle described an α2β1-dependent mechanism of keratinocyte migration in human foreskin keratinocytes based on interaction with the short arm of Lng2 in unprocessed Ln332 [88]. The putative role of Lng2 in guiding keratinocyte migration during physiologic morphogenesis is also supported by the fact that the molecule shows a differential expression pattern and developmental change in early human embryonic and fetal tissue [89].
Although there are some differences in the deposition and proteolytic processing of Lng2 between normal and neoplastic tissue, squamous carcinoma cell migration seems to mirror keratinocyte movement during wound healing and vice versa. Not surprisingly, there are also several reports on the migration promoting activity of Ln332 or Lng2 for carcinoma cells in vitro and in vivo. This seems to be a common phenomenon that is detectable in cell lines of different carcinoma entities and is communicated as being associated with epithelial to mesenchymal transition (EMT) and the EGFR pathway [90,91,92]. Additionally, for OSCCs, a relation between Lng2 expression and cancer cell motility, along with a regulatory effect of different miRNAs or long non-coding RNAs, has been assessed [93,94]. Additionally, there seems to be a relation between Lng2 expression in carcinoma cells, EGFR signaling, and EMT. Although we were not able to immunohistochemically evidence a correlation between activation of EGFR downstream signaling (detected by using phosphor-specific antibodies against p44/42MAP kinase, p38 MAP kinase, and Akt) and invasion-associated accumulation of Lng2, a relation to EGFR expression could be proven by others for esophageal SCC in situ and in vitro [95,96]. Accordingly, we were able to show that stimulation of OSCC cells in vitro with EGF and TGFβ1 causes mesenchymal trans-differentiation accompanied by increased synthesis and deposition of Lng2, as well as raised invasive capability [97]. This goes in line with the findings of Ono and colleagues which showed a correlation between EGFR amplification and Lng2 protein expression in OSCC cell lines [98]. Additionally, Lng2 protein expression seems to be associated with a migratory EMT phenotype of carcinoma cells in situ [99]. Interestingly, the full EMT phenotype, as induced by SNAIL transfection of OSCC cells, is associated with a lack of Ln332 expression, probably caused by an inhibition of α3 chain synthesis but unchanged γ2 production. Therefore, accumulation of Lng2 seems to be a marker for the ongoing mesenchymal transition of OSCC cells [100].
6.2. Proteolytic Processing of Laminin γ2 Generates Migration Promoting Matrikines
As stated above, the biological activity of Ln332 and its single chains is strictly associated with and determined by proteolytic processing. Proteolytic degradation of ECM structures in the invasive front is a precondition for carcinoma cell invasion into preexisting healthy subepithelial tissue; however, it is more than a pure destruction [22,101]. Thus, ECM degradation prepares the grounds for an embryonal extracellular reorganization and re-expression of the oncofetal variants of different matrix proteins, providing the necessary microenvironmental flexibility for tissue remodeling. Furthermore, proteolytic processing leads to newly structured 3D protein networks with altered binding characteristics, the solubilization of growth factors, and the liberation of bioactive fragments. With respect to Ln332, matrix metalloproteinases (MMP) play a pivotal role [51]. Lng2 processing during tumor progression and the induction of migration seems to be mostly associated with MMP2 and MT1-MMP [102,103], but MMP-3, -12, -13, -19, and -20 may also be of importance [51]. In humans, MT1-MMP cleavage leads to the generation of a 100 kDa fragment, γ2′, and a 85 kDa fragment, γ2x, as well as two smaller DIII fragments [104]. Comparable cleavage patterns have also been observed in mice [105]. The generation of further, functionally relevant Lng2-derived fragments during keratinocyte or carcinoma cell migration cannot be excluded. In addition to the Lng2 chain, proteolytical processing of the β3 chain by MT1-MMP and/or Matrilysin 1 also seems to be associated with increased cell motility [106,107] (Figure 2).
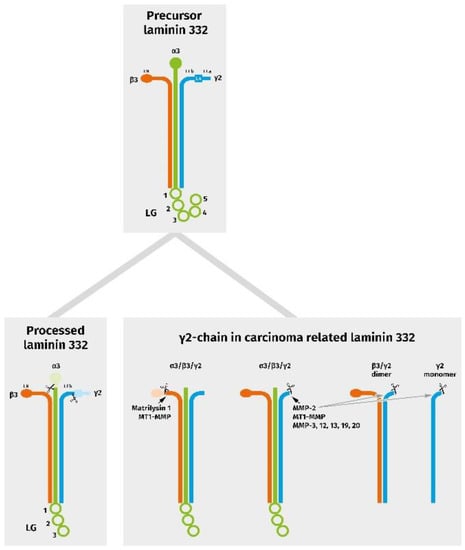
Figure 2.
Schematic diagram representing chain assembly and proteolytic events in physiologically processed, as well as in carcinoma-related, laminin 332 variants. In tumors, the γ2 chain can be present in association with the α3 chain and a truncated β3 chain, as well as with a further specific cleavage in a heterotrimeric, dimeric, and/or monomeric form.
Additionally, for OSCCs, an association between Lng2 cleavage and the presence of MMP-2 and MT1-MMP, and their regulation via extracellular calcium, could be shown in vitro [108,109]. This is in line with reports on a correlation between immunohistochemical MMP2 and/or MT1-MMP expression and OSCC progression and metastasis [110,111] (for review see [112]). Furthermore, combined immunohistochemical evaluation of Lng2 and MT1-MMP has a predictive value for cervical lymph node metastasis in squamous cell carcinomas of the tongue and floor of the mouth [74]. Interestingly, although it was originally thought that MMPs were synthesized by the tumor cells, it becomes evident that these enzymes are also abundantly expressed by surrounding CAFs. In line with this, we were able to demonstrate coordinated immunohistochemical and mRNA expression of Lng2, as well as a spatial association with TGFβ1 and MT1-MMP/BMP1 in the stroma of the OSCC invasive front. Here, the stromal Lng2-positive cells displayed a mesenchymal phenotype, as shown by vimentin positivity, suggesting that either activated CAF or EMT-like OSCC cells contribute to the deposition of processed promigratory Lng2 [99]. However, it should be mentioned that plasmin may also have an impact on degradation and impair BM assembly of Ln332 [113].
Recently, our understanding of ECM–cell interaction has changed from a passive role towards a more dynamic mutual interaction, whereby ECM components exert signals strongly influencing cell differentiation, proliferation, and tumor biological behavior [114,115]. Besides the common haptokine signaling via integrin/adhesion receptors, ECM proteins can also harbor “cryptic” ligands that are exposed or released during conformational changes after proteolytic processing (also known as matrikines or matricryptins) [116]. Prototypical matrikines involved in epithelial/cutaneous processes such as wound healing or tumor cell invasion include the epidermal growth factor-like repeats (EGF-L) of tenascin-C and laminin/Lng2. In contrast to normal growth factors, EGF-L binds to the EGFR with a low affinity/high dissociation constant [117]. It is suggested that these matrikines signal to various cells in a distinctly promigratory way without inducing significant proliferation [118]. Although this hypothesis is proven for several epithelial tumors in vitro, there are nearly no reports for oral cancer. However, supporting this hypothesis, Fullar and colleagues showed that carcinoma cell–CAF interaction regulates MMP and tissue inhibitor of metalloproteinase (TIMP) synthesis in OSCCs [119]. We recently demonstrated that the secretome of activated fibroblasts is able to induce MMP activity in the membrane of OSCC cells, thereby releasing ligands (so far not defined in detail) that activate EGFR and subsequently induce EGFR upregulation. Furthermore, there is a stimulation of the highly oncogenic hetero-dimerization between HER3 and p95HER2 [120]. These results give further strong evidence that the TME influences the regulation of Ln332 reorganization and modulation of its tumor biological activity.
Besides this more local paracrine regulation of growth factor receptor activity, another route of Lng2 action has to be taken into account here. Thus, it could be shown that Lng2 can also be delivered via OSCC cell-derived extracellular vesicles inducing in vitro lymphangiogenesis by integrin-dependent uptake in lympho-endothelial cells [121], supporting a potential mechanism of mediating nodular metastasis.
6.3. Modulation of Laminin γ2 Expression Pattern Is a Result of Tumor–Stroma Cross-Talk and EMT
It is well known that the interaction of carcinoma cells with TME cells is a prerequisite of cancer cell invasion and tumor progression [122]. Using in vitro 3D invasion models, it could be impressively shown that the presence of fibroblasts is mandatory for the development of an invasive OSCC cell phenotype, and that this is also accompanied by Lng2 synthesis and deposition [36]. This is in line with several reports postulating the presence of myCAF in OSCC tissue as a marker for more aggressive tumor behavior and thereby as a predictor of cancer progression and prognosis [123,124,125]. The tumor-promoting activity of myCAFs seems to be associated with the induction of EMT. This could be shown in vitro for OSCCs, as well as tongue SCCs [126,127], and was evidenced by comparative immunohistochemical analyses of the expression of aSMA and EMT markers in situ in tongue SCCs [128]. Furthermore, EMT seems to also be related to the phenomenon of tumor budding. Tumor budding is defined as the presence of single cancer cells or clusters up to <5 cells in the invasive front. Budding can be scored according to the guidelines of the International Tumor Budding Consensus Conference [129], and is also reported as a prognostic indicator for OSCC [130,131,132]. The quantification of Lng2-positive buds was considered as a prognostic and predictive marker in different carcinoma entities, including HNSCC [133]. With respect to these findings, the direct influence of myofibroblastic stroma on Lng2 reorganization and associated EMT must be assumed. As far as we know, the molecular basis of this interrelationship is currently not investigated and understood in detail. The supply of enzymes for proteolytic Ln332 processing, the delivery of growth factors influencing tumor cell phenotype and metabolism, and active ECM organization seem to play a central role. Supporting this hypothesis, it was recently be shown that there is indeed a relation between the extent of budding, the presence of myCAFs, and Lng2 expression in OSCCs [79]. Examples of different immunohistochemical Lng2 expression patterns in relation to the presence of myCAFs are given in Figure 3.
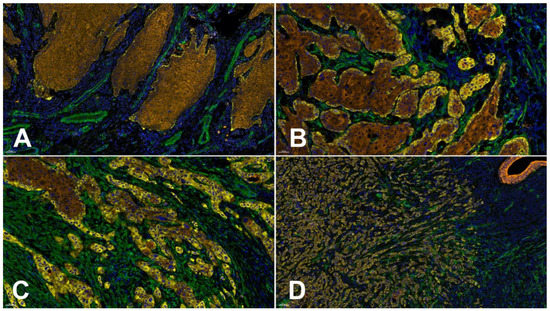
Figure 3.
Different laminin gamma 2 chain (Lng2) deposition patterns in relation to the mode of invasion and the presence of myCAFs demonstrated by 4plex immunofluorescence staining using the Opal Multiplex Detection System from AKOYA Biosciences (full protocol available on request). The antibodies used were: clone B2 against Lng2 (Sc-25341, Santa Cruz Biotechnology, Inc., Heidelberg, Germany, yellow), clone 1A4 against alpha smooth muscle actin (aSMA) (IR611, Agilent Technologies Germany GmbH & Co. KG/DAKO, Hamburg, Germany, green), and clone AE1/AE3 against pan Cytokeratin (IR053, Agilent Technologies Germany GmbH & Co. KG/DAKO, Hamburg, Germany, brown). DAPI was used for nuclei counterstaining (blue). (A) OSCC with pushing well delineated infiltrating borders,, no aSMA-positive stromal fibroblasts (myCAFs), histopathological grade G2, a budding score of 1 according to the International Tumor Budding Consensus Conference (ITBCC 2021 [129]), and minimal basement membrane (BM) decoration for Lng2. (B) An OSCC showing infiltrating solid cords, clusters of myCAFs, histopathological grade G2, a budding score of 3 according to the ITBCC, and cytoplasmic accumulation of Lng2 in a few invasive disseminated OSCC cells in addition to positive marginal cells in more central parts of the tumor. (C) An OSCC with small groups or cords of infiltrating cells, myCAFs present in the whole invasive front, histopathological grade G3, a budding score of 3 according to the ITBCC 2021, and up to 50% Lng2-positive OSCC cells. (D) An OSCC showing a widespread dissemination of single tumor cells or small groups, an abundant presence of myCAFs, histopathological grade G3, a budding score of 3 according to the ITBCC 2021, and predominant Lng2-positive OSCC cells. Bar = 50 µm.
6.4. Laminin γ2 Interacts with Oncofetal Fibronectin and Tenascin-C
As we know from re-epithelialization during wound healing, migration of keratinocytes is also regulated by an orchestrated remodulation of the ECM composition within the wound bed in space and time [84]. This includes not only a reorganization of laminin itself, but also the reoccurrence of special variants of cellular fibronectin (Fn), tenascin-C (Tn-C), and others. Isoforms of cellular Fn and Tn-C generated by alternative splicing of so-called extra domains, or by de novo glycosylation, are re-expressed during tissue remodeling processes and are only rarely detectable in healthy adult tissues. Cellular Fn variants containing the extra domains (ED) A and B (EDA-Fn, EDB-Fn), as well as domains in the IIICS region, are known to be associated with embryogenesis, wound healing, angioneogenesis, and carcinoma invasion [134,135]. Therefore, they are also known as oncofetal Fn variants (oncfFn). It has been shown that stromal fibroblasts and endothelial cells are mainly responsible for the synthesis of this molecular variant in OSCCs [24,136]. Interestingly, cellular Fn is able to modulate migration modalities (collective versus single cell) of OSSC cells in vitro in a differentiation-dependent manner [137]. Additionally, the hexameric glycoprotein Tn-C, a member of a protein family comprising at least four different molecules in humans, is a multidomain protein with up to nine fibronectin type III-like repeats (FNIII), which can be omitted or included by alternative splicing of pre-mRNA. As with Fn, large Tn-C isoforms are also known to be re-expressed during tissue modulating processes such as wound healing, inflammation and fibrosis, angioneogenesis, and in the carcinoma invasive front (oncfTn-C) [138].
Dependent on the individual splicing pattern, oncfTn-C is able to develop various effects in relation to tumor development and progression [139]. It is well known that oncfTn-C is able to modulate tumor cell behavior and inflammatory reaction in a tumor-type-dependent manner [140]. There are also several reports on the tumor biological, diagnostic, and prognostic relevance of oncfTn-C in OSCCs [68,141,142,143]. Hindermann and coworkers were able to show that the main sources of large unspliced Tn-C variants in OSCCs are invasive cancer cells. Stromal fibroblasts also contribute to its mRNA synthesis [144]. It has been shown that the protein is deposited in the stroma in close proximity to the invasive front, and that the extension of mRNA synthesis correlates to the grade of malignancy. With respect to the overlapping expression and deposition patterns of Lng2, oncfFn, and oncfTn-C, a structural interaction of these molecules may be assumed. In 1998, Ramos and coworkers had already shown in vitro that extracellular fibrillary organization of TnC depends on the presence of fibroblasts and fibrillary Fn, indicating a direct interaction between these matrix proteins [145]. Later, our group were able to prove that OSCC cell-produced Lng2 is also fibrillary organized in vitro in the presence of fibroblasts and is colocalized with fibrillary Fn and Tn-C [37]. This colocalization pattern could also be confirmed in situ in the invasive tumor front [146]. Although the functional relevance of the structural 3D organization of these matrix multiprotein complexes is not yet fully understood, it seems to play a crucial role in tumor biology. It may be assumed that the oncofetal restructuring of extracellular multiprotein complexes at least modulates physical properties for tissue modulation and morphogenesis. This hypothesis is supported by results from laser scanning microscope studies on OSCCs, demonstrating increased oncfTn-C incorporation and colocalization with Lng2 in the invasive front of the BM region with rising malignancy grade [147]. This may represent a step toward the higher flexibility of the BM at the tumor–stroma interface and a prerequisite for BM disintegration during OSCC progress. Furthermore, it was suggested that fibrillary multiprotein complexes containing oncfTnC, oncfFn, and Lng2 form tracks guiding cancer cell invasion and the movement of endothelial and inflammatory cells, as well as fibroblasts [148]. In line with these hypotheses, it could be shown that stromal co-expression of Fn and TnC is a strong prognostic marker in tongue carcinomas [149]. The biological importance of oncofetal extracellular multiprotein complexes for tissue remodeling and morphogenesis is additionally supported by the fact that, during tumor angioneogenesis, sprouting new vessels are also surrounded by a stratified oncfFn and onfTnC matrix [150].
7. Conclusions
In this review, we have summarized the current knowledge on the remodeling of Ln332 during OSCC development, invasion, and progression. Reorganization of hemidesmosomal contacts in the invasive front of OSCCs leads to aberrant synthesis, processing, and deposition of Ln332 and its chains, mainly caused by tumor-specific proteolytic patterns and tumor–stroma cross-talk.
Remodeling in the invasive front shows a variety of similarities to re-epithelialization during wound healing. Ln332 reorganization is critically modulated by OSCC–myCAF interaction, whereas cancer fibroblasts are responsible for the proteolytic landscape, synthesis, and deposition of further oncofetal matrix proteins, as well as 3D organization of the invasive front of an ECM.
The most remarkable cancer-associated deposition pattern is the abundant accumulation of Lng2 in the cytoplasm of invading tumor cells in the invasive front. This pattern is associated with an EMT-like phenomenon and is of relevant diagnostic and prognostic value. Cytoplasmic Lng2 accumulation is furthermore associated with tumor cell budding, which is a potential additional grading parameter. Up to now, invasive front grading has not yet been introduced in the WHO grading system for OSCCs; however, there is increasing evidence that the distribution pattern of Lng2-positive OSCC cells predicts patient outcome and may play a key role in optimizing therapy and the development of novel therapeutic strategies. In line with this, Lng2 immunohistochemistry has been reported to improve diagnosis in tissue sections, as well as the value of oral brush cytology [77,78]. Furthermore, serum concentrations of Lng2 could be shown to serve as an indicator for monitoring disease activity in patients with OSCCs and other epithelial tumors [80,151].
To understand the complex circuit of Lng2-mediated OSCC progression, prospective clinical studies are warranted in order to investigate the relation between Lng2 reorganization, the structure of the invasive front, stromal activation, the expression and organization of extracellular oncofetal adhesion proteins, and EGFR downstream signaling in relation to the clinical outcome. This could be facilitated by the recent laboratory developments relating to spatial phenotyping and multiplex immunofluorescence staining. This could also pave the way for the development of novel Ln332-based targeted treatment strategies. Although there have been a few encouraging results using Ln332 functional blocking approaches, established laminin-based treatment options are not yet available [38].
Interestingly, in 1993, Noguchi and coworkers had already reported a comparative immunohistochemical study of Ln and CD3 positivity in OSCCs, demonstrating a relationship between Ln alterations, decreased T-cell tumor infiltration, and resistance to chemotherapy [152]. Recently, evidence has been provided supporting the ability of Lng2 to prevent T-cell infiltration and to attenuate the response to immune checkpoint inhibitors in non-small cell lung esophageal cancer. This effect depends on the upregulation of Lng2 synthesis by TGFβ1, which is, in turn, provided by stromal myCAF, emphasizing the impact of the TME on tumor biology and potential treatment strategies [39]. Therefore, Lng2-targeted therapeutic interventions must also be taken into consideration as part of combination therapies targeting EGFR signaling, immune checkpoint molecules, and others.
Author Contributions
Conceptualization, A.B., N.G. and M.F. Writing—Original Draft Preparation, A.B. Writing—Review and Editing, M.F. and N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Dagmar Samsel and Carolin Berg for excellent technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 3D | 3-dimensional |
| aSMA | alpha smooth muscle actin |
| BM | basement membrane |
| CAF | cancer-associated fibroblast |
| ECM | extracellular matrix |
| EDA-Fn | EDA domain containing fibronectin |
| EDB-Fn | EDB domain containing fibronectin |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| Fn | fibronectin |
| FNIII | fibronectin type III-like repeats |
| HD | hemidesmosome |
| HNSCC | head and neck squamous cell carcinoma |
| Ln | laminin |
| Ln332 | laminin 332 |
| Ln5 | laminin 5 |
| Lng2 | laminin gamma 2 chain |
| MMP | matrix metalloproteinase |
| mRNA | messenger RNA |
| myCAF | cancer-associated fibroblast with a myofibroblast phenotype |
| oncf | oncofetal |
| oncfECM | oncofetal extracellular matrix |
| oncfFn | oncofetal fibronectin |
| oncfTn-C | oncofetal tenascin-C |
| OSCC | oral squamous cell carcinoma |
| RNA | ribonucleic acid |
| TGFβ1 | transforming growth factor beta 1 |
| TIMP | tissue inhibitor of metalloproteinases |
| TME | tumor microenvironment |
| Tn-C | tenascin-C |
References
- Observatory, T.G.C. GLOBOCAN 2020/Lip, Oral Cavity. 2022. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/1-Lip-oral-cavity-fact-sheet.pdf (accessed on 1 September 2022).
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Grand Challenges in Oral Cancers. Front. Oral Health 2020, 1, 3. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, B.; Tao, X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int. J. Cancer 2021, 148, 1548–1561. [Google Scholar] [CrossRef]
- Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 2015, 112, 273–300. [Google Scholar] [CrossRef]
- Van den Bossche, V.; Zaryouh, H.; Vara-Messler, M.; Vignau, J.; Machiels, J.P.; Wouters, A.; Schmitz, S.; Corbet, C. Microenvironment-driven intratumoral heterogeneity in head and neck cancers: Clinical challenges and opportunities for precision medicine. Drug Resist. Updates 2022, 60, 100806. [Google Scholar] [CrossRef]
- Qin, Y.; Zheng, X.; Gao, W.; Wang, B.; Wu, Y. Tumor microenvironment and immune-related therapies of head and neck squamous cell carcinoma. Mol. Ther. Oncolytics 2021, 20, 342–351. [Google Scholar] [CrossRef]
- Kaspar, M.; Zardi, L.; Neri, D. Fibronectin as target for tumor therapy. Int. J. Cancer 2006, 118, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Te Boekhorst, V.; Friedl, P. Plasticity of Cancer Cell Invasion-Mechanisms and Implications for Therapy. Adv. Cancer Res. 2016, 132, 209–264. [Google Scholar] [CrossRef]
- Mughees, M.; Sengupta, A.; Khowal, S.; Wajid, S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol. Biol. Rep. 2021, 48, 1773–1786. [Google Scholar] [CrossRef]
- Nisar, S.; Yousuf, P.; Masoodi, T.; Wani, N.A.; Hashem, S.; Singh, M.; Sageena, G.; Mishra, D.; Kumar, R.; Haris, M.; et al. Chemokine-Cytokine Networks in the Head and Neck Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4584. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef]
- Rasanen, K.; Vaheri, A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Gibbons, D.L.; Zong, C.; Fradette, J.J.; Bota-Rabassedas, N.; Kurie, J.M. Fibroblast heterogeneity and its impact on extracellular matrix and immune landscape remodeling in cancer. Matrix Biol. 2020, 91–92, 8–18. [Google Scholar] [CrossRef]
- Augsten, M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 2014, 4, 62. [Google Scholar] [CrossRef]
- Bienkowska, K.J.; Hanley, C.J.; Thomas, G.J. Cancer-Associated Fibroblasts in Oral Cancer: A Current Perspective on Function and Potential for Therapeutic Targeting. Front. Oral Health 2021, 2, 686337. [Google Scholar] [CrossRef]
- Mezawa, Y.; Orimo, A. Phenotypic heterogeneity, stability and plasticity in tumor-promoting carcinoma-associated fibroblasts. FEBS J. 2022, 289, 2429–2447. [Google Scholar] [CrossRef]
- Tanis, T.; Cincin, Z.B.; Gokcen-Rohlig, B.; Bireller, E.S.; Ulusan, M.; Tanyel, C.R.; Cakmakoglu, B. The role of components of the extracellular matrix and inflammation on oral squamous cell carcinoma metastasis. Arch. Oral Biol. 2014, 59, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Patankar, S.R.; Wankhedkar, D.P.; Tripathi, N.S.; Bhatia, S.N.; Sridharan, G. Extracellular matrix in oral squamous cell carcinoma: Friend or foe? Indian J. Dent. Res. 2016, 27, 184–189. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Kosmehl, H.; Berndt, A.; Katenkamp, D. Molecular variants of fibronectin and laminin: Structure, physiological occurrence and histopathological aspects. Virchows Arch. 1996, 429, 311–322. [Google Scholar] [CrossRef]
- Kosmehl, H.; Berndt, A.; Strassburger, S.; Borsi, L.; Rousselle, P.; Mandel, U.; Hyckel, P.; Zardi, L.; Katenkamp, D. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br. J. Cancer 1999, 81, 1071–1079. [Google Scholar] [CrossRef]
- Kosmehl, H.; Berndt, A.; Katenkamp, D.; Mandel, U.; Bohle, R.; Gabler, U.; Celeda, D. Differential expression of fibronectin splice variants, oncofetal glycosylated fibronectin and laminin isoforms in nodular palmar fibromatosis. Pathol. Res. Pract. 1995, 191, 1105–1113. [Google Scholar] [CrossRef]
- Franz, M.; Grun, K.; Richter, P.; Brehm, B.R.; Fritzenwanger, M.; Hekmat, K.; Neri, D.; Gummert, J.; Figulla, H.R.; Kosmehl, H.; et al. Extra cellular matrix remodelling after heterotopic rat heart transplantation: Gene expression profiling and involvement of ED-A+ fibronectin, alpha-smooth muscle actin and B+ tenascin-C in chronic cardiac allograft rejection. Histochem. Cell Biol. 2010, 134, 503–517. [Google Scholar] [CrossRef]
- Franz, M.; Jung, C.; Lauten, A.; Figulla, H.R.; Berndt, A. Tenascin-C in cardiovascular remodeling: Potential impact for diagnosis, prognosis estimation and targeted therapy. Cell Adhes. Migr. 2015, 9, 90–95. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Tawara, I.; Yoshida, T. Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am. J. Physiol. Cell Physiol. 2020, 319, C781–C796. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Engler, A.J. The provisional matrix: Setting the stage for tissue repair outcomes. Matrix Biol. 2017, 60–61, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.J.; Bateman, A.C.; Spedding, A.; Primrose, J.N.; Mandel, U. Oncofetal fibronectin and oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2001, 39, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Hakomori, S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: Its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc. Natl. Acad. Sci. USA 1985, 82, 6517–6521. [Google Scholar] [CrossRef]
- Matsuura, H.; Takio, K.; Titani, K.; Greene, T.; Levery, S.B.; Salyan, M.E.; Hakomori, S. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J. Biol. Chem. 1988, 263, 3314–3322. [Google Scholar] [CrossRef]
- Ziober, A.F.; Falls, E.M.; Ziober, B.L. The extracellular matrix in oral squamous cell carcinoma: Friend or foe? Head Neck 2006, 28, 740–749. [Google Scholar] [CrossRef]
- Fromme, J.E.; Zigrino, P. The Role of Extracellular Matrix Remodeling in Skin Tumor Progression and Therapeutic Resistance. Front. Mol. Biosci. 2022, 9, 864302. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.J.; Jones, J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int. J. Oral Maxillofac. Surg. 2007, 36, 671–679. [Google Scholar] [CrossRef]
- Berndt, A.; Hyckel, P.; Konneker, A.; Katenkamp, D.; Kosmehl, H. Oral squamous cell carcinoma invasion is associated with a laminin-5 matrix re-organization but independent of basement membrane and hemidesmosome formation. clues from an in vitro invasion model. Invasion Metastasis 1997, 17, 251–258. [Google Scholar]
- Berndt, A.; Borsi, L.; Hyckel, P.; Kosmehl, H. Fibrillary co-deposition of laminin-5 and large unspliced tenascin-C in the invasive front of oral squamous cell carcinoma in vivo and in vitro. J. Cancer Res. Clin. Oncol. 2001, 127, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Meireles Da Costa, N.; Mendes, F.A.; Pontes, B.; Nasciutti, L.E.; Ribeiro Pinto, L.F.; Palumbo Junior, A. Potential Therapeutic Significance of Laminin in Head and Neck Squamous Carcinomas. Cancers 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, J.R.; Dong, J.; Lin, Q.G.; Tang, H.; Jia, Y.X.; Tan, W.; Chen, Q.Y.; Zeng, T.T.; Xing, S.; et al. Laminin gamma2-mediating T cell exclusion attenuates response to anti-PD-1 therapy. Sci. Adv. 2021, 7, eabc8346. [Google Scholar] [CrossRef]
- Kariya, Y.; Mori, T.; Yasuda, C.; Watanabe, N.; Kaneko, Y.; Nakashima, Y.; Ogawa, T.; Miyazaki, K. Localization of laminin alpha3B chain in vascular and epithelial basement membranes of normal human tissues and its down-regulation in skin cancers. J. Mol. Histol. 2008, 39, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M. Laminins and interaction partners in the architecture of the basement membrane at the dermal-epidermal junction. Exp. Dermatol. 2021, 30, 17–24. [Google Scholar] [CrossRef]
- Burgeson, R.E.; Chiquet, M.; Deutzmann, R.; Ekblom, P.; Engel, J.; Kleinman, H.; Martin, G.R.; Meneguzzi, G.; Paulsson, M.; Sanes, J.; et al. A new nomenclature for the laminins. Matrix Biol. 1994, 14, 209–211. [Google Scholar] [CrossRef]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.; et al. A simplified laminin nomenclature. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin—A glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar] [CrossRef]
- Aumailley, M. The laminin family. Cell Adhes. Migr. 2013, 7, 48–55. [Google Scholar] [CrossRef]
- Colognato, H.; Yurchenco, P.D. Form and function: The laminin family of heterotrimers. Dev. Dyn. 2000, 218, 213–234. [Google Scholar] [CrossRef]
- Walko, G.; Castanon, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef]
- Te Molder, L.; de Pereda, J.M.; Sonnenberg, A. Regulation of hemidesmosome dynamics and cell signaling by integrin alpha6beta4. J. Cell Sci. 2021, 134, jcs259004. [Google Scholar] [CrossRef]
- Litjens, S.H.; de Pereda, J.M.; Sonnenberg, A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006, 16, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Guess, C.M.; Quaranta, V. Defining the role of laminin-332 in carcinoma. Matrix Biol. 2009, 28, 445–455. [Google Scholar] [CrossRef]
- Rousselle, P.; Scoazec, J.Y. Laminin 332 in cancer: When the extracellular matrix turns signals from cell anchorage to cell movement. Semin. Cancer Biol. 2020, 62, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Marinkovich, M.P. Tumour microenvironment: Laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer 2007, 7, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.R.; Silverman, S., Jr.; Daniels, T.E.; Kramer, R.H.; Greenspan, J.S. Distribution of fibronectin and laminin in oral leukoplakia and carcinoma. J. Oral Pathol. 1985, 14, 247–255. [Google Scholar] [CrossRef]
- Noguchi, M.; Kohama, G.; Hiratsuka, H.; Miyakawa, A.; Yamaguchi, A.; Nagai, I.; Kyogoku, J.; Odajima, T. Immunohistochemical localization of laminin and its relation to the grade of histological malignancy in oral cancer. Gan No Rinsho 1989, 35, 880–885. [Google Scholar] [PubMed]
- Tosios, K.I.; Kapranos, N.; Papanicolaou, S.I. Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology 1998, 33, 261–268. [Google Scholar] [CrossRef]
- Kumagai, S.; Kojima, S.; Imai, K.; Nakagawa, K.; Yamamoto, E.; Kawahara, E.; Nakanishi, I. Immunohistologic distribution of basement membrane in oral squamous cell carcinoma. Head Neck 1994, 16, 51–57. [Google Scholar] [CrossRef]
- Shinohara, M.; Nakamura, S.; Harada, T.; Shimada, M.; Oka, M. Mode of tumor invasion in oral squamous cell carcinoma: Improved grading based on immunohistochemical examination of extracellular matrices. Head Neck 1996, 18, 153–159. [Google Scholar] [CrossRef]
- Yellapurkar, S.; Natarajan, S.; Boaz, K.; Manaktala, N.; Baliga, M.; Shetty, P.; Prasad, M.; Ravi, M. Expression of Laminin in Oral Squamous Cell Carcinomas. Asian Pac. J. Cancer Prev. 2018, 19, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Shinohara, M.; Nakamura, S.; Oka, M. An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Arch. 1994, 424, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Domloge-Hultsch, N.; Gammon, W.R.; Briggaman, R.A.; Gil, S.G.; Carter, W.G.; Yancey, K.B. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J. Clin. Investig. 1992, 90, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, T.; Autio-Harmainen, H.; Oikarinen, A.; Salo, S.; Tryggvason, K.; Salo, T. Altered distribution and synthesis of laminin-5 (kalinin) in oral lichen planus, epithelial dysplasias and squamous cell carcinomas. Br. J. Dermatol. 1997, 136, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.M.; Berndt, A.; Stiller, K.J.; Hyckel, P.; Kosmehl, H. A comparative quantitative analysis of laminin-5 in the basement membrane of normal, hyperplastic, and malignant oral mucosa by confocal immunofluorescence imaging. J. Histochem. Cytochem. 2001, 49, 1261–1268. [Google Scholar] [CrossRef]
- Matsumoto, K.; Horikoshi, M.; Rikimaru, K.; Enomoto, S. A study of an in vitro model for invasion of oral squamous cell carcinoma. J. Oral Pathol. Med. 1989, 18, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Kulasekara, K.K.; Lukandu, O.M.; Neppelberg, E.; Vintermyr, O.K.; Johannessen, A.C.; Costea, D.E. Cancer progression is associated with increased expression of basement membrane proteins in three-dimensional in vitro models of human oral cancer. Arch. Oral Biol. 2009, 54, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Wolheim, A.; Richter, P.; Umbreit, C.; Dahse, R.; Driemel, O.; Hyckel, P.; Virtanen, I.; Kosmehl, H.; Berndt, A. Stromal laminin chain distribution in normal, hyperplastic and malignant oral mucosa: Relation to myofibroblast occurrence and vessel formation. J. Oral Pathol. Med. 2010, 39, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Nakanishi, Y.; Ino, Y.; Niki, T.; Yamada, T.; Yoshimura, K.; Saikawa, M.; Nakajima, T.; Hirohashi, S. Clinocopathologic significance of laminin-5 gamma2 chain expression in squamous cell carcinoma of the tongue: Immunohistochemical analysis of 67 lesions. Cancer 1999, 85, 2315–2321. [Google Scholar] [CrossRef]
- Kuratomi, Y.; Kumamoto, M.; Kidera, K.; Toh, S.; Masuda, M.; Nakashima, T.; Inokuchi, A. Diffuse expression of laminin gamma2 chain in disseminating and infiltrating cancer cells indicates a highly malignant state in advanced tongue cancer. Oral Oncol. 2006, 42, 73–76. [Google Scholar] [CrossRef]
- Thangaraj, S.V.; Shyamsundar, V.; Krishnamurthy, A.; Ramshankar, V. Deregulation of extracellular matrix modeling with molecular prognostic markers revealed by transcriptome sequencing and validations in Oral Tongue squamous cell carcinoma. Sci. Rep. 2021, 11, 250. [Google Scholar] [CrossRef]
- Xin, Z.; Yamaguchi, A.; Sakamoto, K. Aberrant expression and altered cellular localization of desmosomal and hemidesmosomal proteins are associated with aggressive clinicopathological features of oral squamous cell carcinoma. Virchows Arch. 2014, 465, 35–47. [Google Scholar] [CrossRef]
- Gasparoni, A.; Della Casa, M.; Milillo, L.; Lorenzini, G.; Rubini, C.; Urso, R.; Lo Muzio, L. Prognostic value of differential expression of Laminin-5 gamma2 in oral squamous cell carcinomas: Correlation with survival. Oncol. Rep. 2007, 18, 793–800. [Google Scholar]
- Nordemar, S.; Hogmo, A.; Lindholm, J.; Auer, G.; Munck-Wikland, E. Laminin-5 gamma 2: A marker to identify oral mucosal lesions at risk for tumor development? Anticancer Res. 2003, 23, 4985–4989. [Google Scholar]
- Silva, E.M.R.; Freitas, V.M.; Bautz, W.G.; de Barros, L.A.P.; da Gama de Souza, L.N. Immunohistochemical Study of Laminin-332 gamma2 Chain and MMP-9 in High Risk of Malignant Transformation Oral Lesions and OSCC. J. Oral Maxillofac. Res. 2018, 9, e3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zanaruddin, S.N.; Saleh, A.; Yang, Y.H.; Hamid, S.; Mustafa, W.M.; Khairul Bariah, A.A.; Zain, R.B.; Lau, S.H.; Cheong, S.C. Four-protein signature accurately predicts lymph node metastasis and survival in oral squamous cell carcinoma. Hum. Pathol. 2013, 44, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Kawano, K.; Yanagisawa, S. Predictive value of laminin-5 and membrane type 1-matrix metalloproteinase expression for cervical lymph node metastasis in T1 and T2 squamous cell carcinomas of the tongue and floor of the mouth. Head Neck 2006, 28, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Pyke, C.; Romer, J.; Kallunki, P.; Lund, L.R.; Ralfkiaer, E.; Dano, K.; Tryggvason, K. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am. J. Pathol. 1994, 145, 782–791. [Google Scholar]
- Pyke, C.; Salo, S.; Ralfkiaer, E.; Romer, J.; Dano, K.; Tryggvason, K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995, 55, 4132–4139. [Google Scholar]
- Driemel, O.; Dahse, R.; Hakim, S.G.; Tsioutsias, T.; Pistner, H.; Reichert, T.E.; Kosmehl, H. Laminin-5 immunocytochemistry: A new tool for identifying dysplastic cells in oral brush biopsies. Cytopathology 2007, 18, 348–355. [Google Scholar] [CrossRef]
- Zargaran, M.; Eshghyar, N.; Vaziri, P.B.; Mortazavi, H. Immunohistochemical evaluation of type IV collagen and laminin-332 gamma2 chain expression in well-differentiated oral squamous cell carcinoma and oral verrucous carcinoma: A new recommended cut-off. J. Oral Pathol. Med. 2011, 40, 167–173. [Google Scholar] [CrossRef]
- Marangon Junior, H.; Rocha, V.N.; Leite, C.F.; de Aguiar, M.C.; Souza, P.E.; Horta, M.C. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J. Oral Pathol. Med. 2014, 43, 199–204. [Google Scholar] [CrossRef]
- Kuratomi, Y.; Sato, S.; Monji, M.; Shimazu, R.; Tanaka, G.; Yokogawa, K.; Inoue, A.; Inokuchi, A.; Katayama, M. Serum concentrations of laminin gamma2 fragments in patients with head and neck squamous cell carcinoma. Head Neck 2008, 30, 1058–1063. [Google Scholar] [CrossRef]
- Masaoka, T.; Hashimoto, S.; Kinumatsu, T.; Muramatsu, T.; Jung, H.S.; Yamada, S.; Shimono, M. Immunolocalization of laminin and integrin in regenerating junctional epithelium of mice after gingivectomy. J. Periodontal Res. 2009, 44, 489–495. [Google Scholar] [CrossRef]
- Chang, Y.C.; Gordon, M.K.; Gerecke, D.R. Expression of Laminin 332 in Vesicant Skin Injury and Wound Repair. Clin. Dermatol. 2018, 2, 115. [Google Scholar]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, M.; Masaoka, T.; Enokiya, Y.; Muramatsu, T.; Hashimoto, S.; Yamada, S.; Shimono, M. Expression and function of laminin and integrins on adhesion/migration of primary culture cells derived from rat oral epithelium. J. Periodontal Res. 2010, 45, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Choma, D.P.; Milano, V.; Pumiglia, K.M.; DiPersio, C.M. Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J. Investig. Dermatol. 2007, 127, 31–40. [Google Scholar] [CrossRef]
- Choma, D.P.; Pumiglia, K.; DiPersio, C.M. Integrin alpha3beta1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J. Cell Sci. 2004, 117, 3947–3959. [Google Scholar] [CrossRef]
- Decline, F.; Rousselle, P. Keratinocyte migration requires alpha2beta1 integrin-mediated interaction with the laminin 5 gamma2 chain. J. Cell Sci. 2001, 114, 811–823. [Google Scholar] [CrossRef]
- Lu, W.; Miyazaki, K.; Mizushima, H.; Nemoto, N. Immunohistochemical distribution of laminin-5 gamma2 chain and its developmental change in human embryonic and foetal tissues. Histochem. J. 2001, 33, 629–637. [Google Scholar] [CrossRef]
- Pei, Y.F.; Liu, J.; Cheng, J.; Wu, W.D.; Liu, X.Q. Silencing of LAMC2 Reverses Epithelial-Mesenchymal Transition and Inhibits Angiogenesis in Cholangiocarcinoma via Inactivation of the Epidermal Growth Factor Receptor Signaling Pathway. Am. J. Pathol. 2019, 189, 1637–1653. [Google Scholar] [CrossRef]
- Huang, C.; Chen, J. Laminin332 mediates proliferation, apoptosis, invasion, migration and epithelialtomesenchymal transition in pancreatic ductal adenocarcinoma. Mol. Med. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Kirtonia, A.; Pandey, A.K.; Ramachandran, B.; Mishra, D.P.; Dawson, D.W.; Sethi, G.; Ganesan, T.S.; Koeffler, H.P.; Garg, M. Overexpression of laminin-5 gamma-2 promotes tumorigenesis of pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR cascade. Cell Mol. Life. Sci. 2022, 79, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Yao, Y.L.; Liu, W.; Shen, X.M.; Shi, L.J.; Wu, L. MicroRNA-134 inhibits tumor stem cell migration and invasion in oral squamous cell carcinomas via downregulation of PI3K-Akt signaling pathway by inhibiting LAMC2 expression. Cancer Biomark. 2020, 29, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shi, W.; Chen, M. Long non-coding RNA BBOX1-antisense RNA 1 enhances cell proliferation and migration and suppresses apoptosis in oral squamous cell carcinoma via the miR-3940-3p/laminin subunit gamma 2 axis. Bioengineered 2022, 13, 11138–11153. [Google Scholar] [CrossRef]
- Richter, P.; Bohmer, F.D.; Hindermann, W.; Borsi, L.; Hyckel, P.; Schleier, P.; Katenkamp, D.; Kosmehl, H.; Berndt, A. Analysis of activated EGFR signalling pathways and their relation to laminin-5 gamma2 chain expression in oral squamous cell carcinoma (OSCC). Histochem. Cell Biol. 2005, 124, 151–160. [Google Scholar] [CrossRef]
- Fukai, Y.; Masuda, N.; Kato, H.; Fukuchi, M.; Miyazaki, T.; Nakajima, M.; Sohda, M.; Kuwano, H.; Nakajima, T. Correlation between laminin-5 gamma2 chain and epidermal growth factor receptor expression in esophageal squamous cell carcinomas. Oncology 2005, 69, 71–80. [Google Scholar] [CrossRef]
- Richter, P.; Umbreit, C.; Franz, M.; Berndt, A.; Grimm, S.; Uecker, A.; Bohmer, F.D.; Kosmehl, H.; Berndt, A. EGF/TGFbeta1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J. Oral Pathol. Med. 2011, 40, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Nakanishi, Y.; Gotoh, M.; Sakamoto, M.; Hirohashi, S. Epidermal growth factor receptor gene amplification is correlated with laminin-5 gamma2 chain expression in oral squamous cell carcinoma cell lines. Cancer Lett. 2002, 175, 197–204. [Google Scholar] [CrossRef]
- Franz, M.; Richter, P.; Geyer, C.; Hansen, T.; Acuna, L.D.; Hyckel, P.; Bohmer, F.D.; Kosmehl, H.; Berndt, A. Mesenchymal cells contribute to the synthesis and deposition of the laminin-5 gamma2 chain in the invasive front of oral squamous cell carcinoma. J. Mol. Histol. 2007, 38, 183–190. [Google Scholar] [CrossRef]
- Takkunen, M.; Grenman, R.; Hukkanen, M.; Korhonen, M.; Garcia de Herreros, A.; Virtanen, I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J. Histochem. Cytochem. 2006, 54, 1263–1275. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Giannelli, G.; Falk-Marzillier, J.; Schiraldi, O.; Stetler-Stevenson, W.G.; Quaranta, V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 1997, 277, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Giannelli, G.; Cirulli, V.; Miyazaki, K.; Quaranta, V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000, 148, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Minegishi, T.; Sharabi, A.; Quaranta, V.; Seiki, M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J. Biol. Chem. 2005, 280, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wang, J.D.; Chang, H.Y.; Zhou, P.; Hahn, R.A.; Gordon, M.K.; Laskin, J.D.; Gerecke, D.R. Expression of Laminin gamma2 Proteolytic Fragments in Murine Skin Following Exposure to Sulfur Mustard. Anat. Rec. 2020, 303, 1642–1652. [Google Scholar] [CrossRef]
- Udayakumar, T.S.; Chen, M.L.; Bair, E.L.; Von Bredow, D.C.; Cress, A.E.; Nagle, R.B.; Bowden, G.T. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003, 63, 2292–2299. [Google Scholar]
- Remy, L.; Trespeuch, C.; Bachy, S.; Scoazec, J.Y.; Rousselle, P. Matrilysin 1 influences colon carcinoma cell migration by cleavage of the laminin-5 beta3 chain. Cancer Res. 2006, 66, 11228–11237. [Google Scholar] [CrossRef]
- Oku, N.; Sasabe, E.; Ueta, E.; Yamamoto, T.; Osaki, T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006, 66, 5251–5257. [Google Scholar] [CrossRef]
- Munshi, H.G.; Wu, Y.I.; Ariztia, E.V.; Stack, M.S. Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J. Biol. Chem. 2002, 277, 41480–41488. [Google Scholar] [CrossRef]
- Kurahara, S.; Shinohara, M.; Ikebe, T.; Nakamura, S.; Beppu, M.; Hiraki, A.; Takeuchi, H.; Shirasuna, K. Expression of MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of the oral cavity: Correlations with tumor invasion and metastasis. Head Neck 1999, 21, 627–638. [Google Scholar] [CrossRef]
- Myoung, H.; Kim, M.J.; Hong, S.D.; Lee, J.I.; Lim, C.Y.; Hong, S.P. Expression of membrane type I-matrix metalloproteinase in oral squamous cell carcinoma. Cancer Lett. 2002, 185, 201–209. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Matrisian, L.M. Matrix metalloproteases in head and neck cancer. Head Neck 2006, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Matsunaga, Y.; Nishiyama, T.; Amano, S. Plasmin induces degradation and dysfunction of laminin 332 (laminin 5) and impaired assembly of basement membrane at the dermal-epidermal junction. Br. J. Dermatol. 2008, 159, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; Lamb, P.; Deng, J.S. Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. J. Dermatol. Sci. 2005, 40, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Swindle, C.S.; Tran, K.T.; Johnson, T.D.; Banerjee, P.; Mayes, A.M.; Griffith, L.; Wells, A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J. Cell Biol. 2001, 154, 459–468. [Google Scholar] [CrossRef]
- Schenk, S.; Hintermann, E.; Bilban, M.; Koshikawa, N.; Hojilla, C.; Khokha, R.; Quaranta, V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J. Cell Biol. 2003, 161, 197–209. [Google Scholar] [CrossRef]
- Fullar, A.; Kovalszky, I.; Bitsche, M.; Romani, A.; Schartinger, V.H.; Sprinzl, G.M.; Riechelmann, H.; Dudas, J. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp. Cell Res. 2012, 318, 1517–1527. [Google Scholar] [CrossRef]
- Buttner, R.; Berndt, A.; Valkova, C.; Richter, P.; Korn, A.; Kosan, C.; Liebmann, C. Myofibroblasts have an impact on expression, dimerization and signaling of different ErbB receptors in OSCC cells. J. Recept. Signal Transduct. Res. 2017, 37, 25–37. [Google Scholar] [CrossRef]
- Wang, S.H.; Liou, G.G.; Liu, S.H.; Chang, J.S.; Hsiao, J.R.; Yen, Y.C.; Chen, Y.L.; Wu, W.L.; Chang, J.Y.; Chen, Y.W. Laminin gamma2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin alpha3-dependent uptake by lymphatic endothelial cells. Int. J. Cancer 2019, 144, 2795–2810. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Luksic, I.; Suton, P.; Manojlovic, S.; Virag, M.; Petrovecki, M.; Macan, D. Significance of myofibroblast appearance in squamous cell carcinoma of the oral cavity on the occurrence of occult regional metastases, distant metastases, and survival. Int. J. Oral Maxillofac. Surg. 2015, 44, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.R.; Guerra, E.N.S.; Salo, T.; Lambert, D.W.; Coletta, R.D. Prognostic value of the immunohistochemical detection of cancer-associated fibroblasts in oral cancer: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, K.; Girish, H.C.; Murgod, S.; Alshame, A.M.J.; Shyamala, K.; Nayak, V.N. A Comparative Immunohistochemical Study of Presence and Distribution Pattern of Stromal Myofibroblast in Oral Dysplasia and in Different Grades of Oral Squamous Cell Carcinoma. J. Int. Soc. Prev. Community Dent. 2018, 8, 451–456. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, W.L.; Wang, Y.Y.; Lin, Z.Y.; Zhang, D.M.; Fan, S.; Li, J.S. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. J. Oral Pathol. Med. 2014, 43, 585–592. [Google Scholar] [CrossRef]
- Berndt, A.; Buttner, R.; Guhne, S.; Gleinig, A.; Richter, P.; Chen, Y.; Franz, M.; Liebmann, C. Effects of activated fibroblasts on phenotype modulation, EGFR signalling and cell cycle regulation in OSCC cells. Exp. Cell Res. 2014, 322, 402–414. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Z.; Shang, D.; Cheng, J.; Yuan, H.; Wu, Y.; Song, X.; Jiang, H. alpha-Smooth muscle actin-positive myofibroblasts, in association with epithelial-mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J. Oral Pathol. Med. 2014, 43, 335–343. [Google Scholar] [CrossRef]
- Zlobec, I.; Bachli, M.; Galuppini, F.; Berger, M.D.; Dawson, H.E.; Nagtegaal, I.D.; Lugli, A. Refining the ITBCC tumor budding scoring system with a “zero-budding” category in colorectal cancer. Virchows Arch. 2021, 479, 1085–1090. [Google Scholar] [CrossRef]
- Wahab, A.; Onkamo, O.; Pirinen, M.; Almangush, A.; Salo, T. The budding and depth of invasion model in oral cancer: A systematic review and meta-analysis. Oral Dis. 2020, 28, 275–283. [Google Scholar] [CrossRef]
- Almangush, A.; Salo, T.; Hagstrom, J.; Leivo, I. Tumour budding in head and neck squamous cell carcinoma–a systematic review. Histopathology 2014, 65, 587–594. [Google Scholar] [CrossRef]
- Almangush, A.; Pirinen, M.; Heikkinen, I.; Makitie, A.A.; Salo, T.; Leivo, I. Tumour budding in oral squamous cell carcinoma: A meta-analysis. Br. J. Cancer 2018, 118, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Okado, Y.; Aoki, M.; Hamasaki, M.; Koga, K.; Sueta, T.; Shiratsuchi, H.; Oda, Y.; Nakagawa, T.; Nabeshima, K. Tumor budding and laminin5-gamma2 in squamous cell carcinoma of the external auditory canal are associated with shorter survival. Springerplus 2015, 4, 814. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Mandel, U.; Hamilton Therkildsen, M.; Reibel, J.; Sweeney, B.; Matsuura, H.; Hakomori, S.; Dabelsteen, E.; Clausen, H. Cancer-associated changes in glycosylation of fibronectin. Immunohistological localization of oncofetal fibronectin defined by monoclonal antibodies. APMIS 1992, 100, 817–826. [Google Scholar] [CrossRef]
- Berndt, A.; Borsi, L.; Luo, X.; Zardi, L.; Katenkamp, D.; Kosmehl, H. Evidence of ED-B+ fibronectin synthesis in human tissues by non-radioactive RNA in situ hybridization. Investigations on carcinoma (oral squamous cell and breast carcinoma), chronic inflammation (rheumatoid synovitis) and fibromatosis (Morbus Dupuytren). Histochem. Cell Biol. 1998, 109, 249–255. [Google Scholar] [CrossRef]
- Ramos Gde, O.; Bernardi, L.; Lauxen, I.; Sant’Ana Filho, M.; Horwitz, A.R.; Lamers, M.L. Fibronectin Modulates Cell Adhesion and Signaling to Promote Single Cell Migration of Highly Invasive Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0151338. [Google Scholar] [CrossRef]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef]
- Giblin, S.P.; Midwood, K.S. Tenascin-C: Form versus function. Cell Adhes. Migr. 2015, 9, 48–82. [Google Scholar] [CrossRef]
- Yilmaz, A.; Loustau, T.; Salome, N.; Poilil Surendran, S.; Li, C.; Tucker, R.P.; Izzi, V.; Lamba, R.; Koch, M.; Orend, G. Advances on the roles of tenascin-C in cancer. J. Cell Sci. 2022, 135, jcs260244. [Google Scholar] [CrossRef]
- Berndt, A.; Richter, P.; Kosmehl, H.; Franz, M. Tenascin-C and carcinoma cell invasion in oral and urinary bladder cancer. Cell Adhes. Migr. 2015, 9, 105–111. [Google Scholar] [CrossRef]
- Spenle, C.; Loustau, T.; Burckel, H.; Riegel, G.; Abou Faycal, C.; Li, C.; Yilmaz, A.; Petti, L.; Steinbach, F.; Ahowesso, C.; et al. Impact of Tenascin-C on Radiotherapy in a Novel Syngeneic Oral Squamous Cell Carcinoma Model With Spontaneous Dissemination to the Lymph Nodes. Front. Immunol. 2021, 12, 636108. [Google Scholar] [CrossRef] [PubMed]
- Spenle, C.; Loustau, T.; Murdamoothoo, D.; Erne, W.; Beghelli-de la Forest Divonne, S.; Veber, R.; Petti, L.; Bourdely, P.; Morgelin, M.; Brauchle, E.M.; et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Hindermann, W.; Berndt, A.; Borsi, L.; Luo, X.; Hyckel, P.; Katenkamp, D.; Kosmehl, H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J. Pathol. 1999, 189, 475–480. [Google Scholar] [CrossRef]
- Ramos, D.M.; Chen, B.; Regezi, J.; Zardi, L.; Pytela, R. Tenascin-C matrix assembly in oral squamous cell carcinoma. Int. J. Cancer 1998, 75, 680–687. [Google Scholar] [CrossRef]
- Franz, M.; Hansen, T.; Richter, P.; Borsi, L.; Bohmer, F.D.; Hyckel, P.; Schleier, P.; Katenkamp, D.; Zardi, L.; Kosmehl, H.; et al. Complex formation of the laminin-5 gamma2 chain and large unspliced tenascin-C in oral squamous cell carcinoma in vitro and in situ: Implications for sequential modulation of extracellular matrix in the invasive tumor front. Histochem. Cell Biol. 2006, 126, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Hansen, T.; Borsi, L.; Geier, C.; Hyckel, P.; Schleier, P.; Richter, P.; Altendorf-Hofmann, A.; Kosmehl, H.; Berndt, A. A quantitative co-localization analysis of large unspliced tenascin-C(L) and laminin-5/gamma2-chain in basement membranes of oral squamous cell carcinoma by confocal laser scanning microscopy. J. Oral Pathol. Med. 2007, 36, 6–11. [Google Scholar] [CrossRef]
- Spenle, C.; Gasser, I.; Saupe, F.; Janssen, K.P.; Arnold, C.; Klein, A.; van der Heyden, M.; Mutterer, J.; Neuville-Mechine, A.; Chenard, M.P.; et al. Spatial organization of the tenascin-C microenvironment in experimental and human cancer. Cell Adhes. Migr. 2015, 9, 4–13. [Google Scholar] [CrossRef]
- Sundquist, E.; Kauppila, J.H.; Veijola, J.; Mroueh, R.; Lehenkari, P.; Laitinen, S.; Risteli, J.; Soini, Y.; Kosma, V.M.; Sawazaki-Calone, I.; et al. Tenascin-C and fibronectin expression divide early stage tongue cancer into low- and high-risk groups. Br. J. Cancer 2017, 116, 640–648. [Google Scholar] [CrossRef]
- Berndt, A.; Kollner, R.; Richter, P.; Franz, M.; Voigt, A.; Berndt, A.; Borsi, L.; Giavazzi, R.; Neri, D.; Kosmehl, H. A comparative analysis of oncofetal fibronectin and tenascin-C incorporation in tumour vessels using human recombinant SIP format antibodies. Histochem. Cell Biol. 2010, 133, 467–475. [Google Scholar] [CrossRef]
- Katayama, M.; Sanzen, N.; Funakoshi, A.; Sekiguchi, K. Laminin gamma2-chain fragment in the circulation: A prognostic indicator of epithelial tumor invasion. Cancer Res. 2003, 63, 222–229. [Google Scholar]
- Noguchi, M.; Kohama, G.; Hiratsuka, H.; Sekiguchi, T. Clinical significance of laminin deposition and T-cell infiltration in oral cancer. Head Neck 1993, 15, 125–132. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).