Simple Summary
Meningiomas are mainly benign intracranial tumors. Nevertheless, risk of recurrence exists in long-term follow-up, so new prognostic markers are still need to be identified. MIB-1 is no diagnostic criterion in WHO classification of meningiomas by now. This retrospective study shows that MIB-1 as well as mitotic count are good predictors for progression-free survival in skull-base meningiomas. The implantation of MIB-1 may enable an improved classification of meningiomas regarding progression-free survival. Moreover, this analysis of skull-base meningiomas shows that current cut-offs may have to be adjusted for meningioma location.
Abstract
Although meningiomas are mainly non-aggressive and slow-growing tumors, there is a remarkable recurrence rate in a long-term follow-up. Proliferative activity and progression-free survival (PFS) differs significantly among the anatomic location of meningiomas. The aim of the present study was to investigate the predictive power of MIB-1 labeling index and mitotic count (MC) regarding the probability of PFS in the subgroup of skull-base meningiomas. A total of 145 patients were included in this retrospective study. Histopathological examinations and follow-up data were collected. Ideal cut-off values for MIB-1 and MC were ≥4.75 and ≥6.5, respectively. MIB-1 as well as MC were good predictors for PFS in skull-base meningiomas. Time-dependent analysis of MIB-1 and MC in prediction of recurrence of skull-base meningioma showed that their prognostic values were comparable, but different cut-offs for MC should be considered regarding the meningioma’s location. As the achievement of a gross total resection can be more challenging in skull-base meningiomas and second surgery implies a higher risk profile, the recurrence risk could be stratified according to these findings and guide decision-making for follow-ups vs. adjuvant therapies.
1. Introduction
Although meningiomas—especially World Health Organization (WHO) grade 1 and 2—are mainly non-aggressive and slow-growing tumors, there is a remarkable recurrence rate in a long-term follow-up [1,2]. First-line treatment is complete resection to prevent tumor recurrence [3]. There is growing evidence for increased cellular proliferative potential as an important mechanism of oncogenesis [4]. Recent studies emphasized the importance of Molecular Immunology Borstel 1 (MIB-1) labeling index and mitotic count (MC) as independent predictors of progression-free survival (PFS) [5,6,7,8,9]. However, proliferative activity differs significantly among the anatomic location (non-skull base, skull base, spinal) of meningiomas. Hence, tumor biology and cytoreductive treatment significantly influences the probability of PFS [10]. The aim of the present study was to investigate the predictive power of MIB-1 labeling index and MC regarding the probability of PFS in the subgroup of skull-base meningiomas
2. Materials and Methods
2.1. Study Design and Patient Characteristics
A total of 880 patients were surgically treated for WHO grade 1 and 2 meningioma between 01/2009 and 07/2019 at our neurosurgical department. Patient data were retrospectively reviewed. Institutional review board approval had been obtained. Histopathologically confirmed meningioma, intracranial localization, age equal or greater than 18 years, the availability of the MIB-1 index and/or MC, and neurosurgical resection were inclusion criteria for this study. Meningiomas outside of skull-base or associated with neurofibromatosis type 2 were excluded due to differences regarding histopathology and proliferation potential [10,11]. Patients with macroscopic residual tumor as patients who underwent a Simpson grade > III resection (constituting a subtotal or partial resection/biopsy) were excluded because those resected tumors do not necessarily contain the “hotspot region”, which reflects the area of maximum proliferative activity [12]. Moreover, patients lost to follow-up were also excluded. In all, 145 patients were included for data analysis (Figure 1).
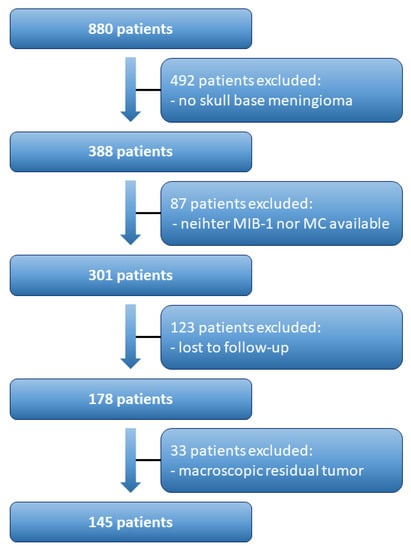
Figure 1.
Flow chart illustrating the selection process of consecutive meningioma patients.
2.2. Data Recording
Clinical information including age, sex, comorbidities, Karnofsky performance status (KPS), body mass index (BMI), peritumoral edema, tumor growth pattern, WHO grading based on postoperative histopathological examination, immunohistochemical examinations, extent of tumor resection based on the Simpson grading system according to the European Association of Neuro-Oncology (EANO), surgical and medical complications according to the classification of Landriel Ibañez [13], and postoperative follow-up data were collected and entered into a computerized database (SPSS, Version 27 for Windows, IBM Corp., Armonk, NY, USA) [14,15]. Meningioma location was subdivided into three groups: medial skull-base, lateral skull-base, and occipital fossa [10].
2.3. Histopathology
The following histopathological investigations were previously described [15,16,17]: Histopathological grading was performed based on the 2016 WHO criteria [1]. All pathology reports underwent renewed review to confirm that diagnosis was in keeping with these requirements. Immunohistochemistry was performed in a similar fashion as described before for paraffin-embedded biopsy tissue specimens [18,19]. MIB-1 labeling index was determined using an anti-Ki67 antibody (Clone Ki-67P, dilution 1:1000, DAKO, Glostrup, Denmark). Diaminobenzidine was used for visualization. An expert neuropathologist carried out the investigations. The MIB-1 index was assessed in randomly selected high-power microscopic fields as proportion of stained and unstained nuclei in the neoplastic cells. MC were regularly investigated as “number of mitotic figures/10 high power fields” as a given diagnostic criteria of atypical meningioma [1].
2.4. Follow-Up
As institutional routine, clinical and imaging follow-up consisted of MRI scans at 3 months after surgery as well as on an annual basis for the following years [15]. Earlier or shorter intervals of clinical and imaging follow-up were strongly recommended in case of new or worsened neurological deficits as well as radiological signs of meningioma progression or recurrence [15]. Recurrence of tumors were considered for analysis only if tumors occurred at the same location as the initial surgery. The time to recurrence was defined as the time between surgery and radiological recurrence (i.e., date of MRI). Radiological recurrence of tumors without clinical or functional implications, thus not requiring any subsequent therapy, were not included in the analysis [20].
2.5. Statistical Analysis
Data were organized and analyzed using SPSS (Version 27, IBM Corp, Armonk, NY, USA). Descriptive statistics of baseline characteristics included mean and standard deviation (SD) for continuous variables and absolute and relative frequencies for categorical variables. Receiver-operating characteristic (ROC) analysis was performed to evaluate how MIB-1 index and MC discriminate between meningioma recurrence. The selection of cut-off points was based on the Youden index [16]. Kaplan–Meier estimates were used to visualize the probability of PFS in either MIB-1 and MC groups. To evaluate the time-varying performance of MIB-1 and MC on PFS time-dependent ROC analysis was conducted using the R package “risksetROC” of the R software (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria) [21,22].
3. Results
3.1. Patient Characteristics
In all, 145 patients were included in the present study. The mean age was 59.9 years and the female/male ratio was 2.6:1. The mean KPS before surgery was 90.9. Tumor grading according to the WHO classification criteria [1] classified 126 (86.9%) tumors as grade 1 and 19 (13.1%) tumors as grade 2. Meningiomas were predominantly located at lateral skull-base (49%), followed by medial skull-base (30.3%) and occipital fossa (20.7%). Substantial peritumoral edema was caused by 73 (50.3%) tumors, and 8 (5.5%) patients suffered multiple meningiomas. Simpson grade I/II resections were achieved in 135 (93.1%) patients, whereas 10 (6.9%) patients underwent Simpson grade III resections. MIB-1 index was available in all 145 patients and MC in 117 patients. Those 28 meningiomas in whom MC were not available showed no tumor recurrence. These results are summarized in Table 1.

Table 1.
Baseline characteristics (n = 145).
3.2. MIB-1 Labeling Index and Mitotic Count in Prediction of Recurrence of Skull-Base Meningioma
Mean MIB-1 labeling index was 4.9 ± 2.3% and 1.3 ± 2.2 for MC. The AUC of MIB-1 labeling index and MC for intracranial recurrent meningioma were 0.71 (95% CI: 0.58–0.84, p = 0.06) and 0.58 (95% CI: 0.38–0.75, p = 0.44), respectively. Sensitivity and specificity of MIB-1 labeling index at the threshold of 4.75 (according to Youden index) were estimated to 89% and 46%, respectively. For MC, sensitivity and specificity at the threshold of 6.5 were 22% and 98%, respectively. Figure 2 shows the ROC curves of MIB-1 labeling index and MC, respectively.
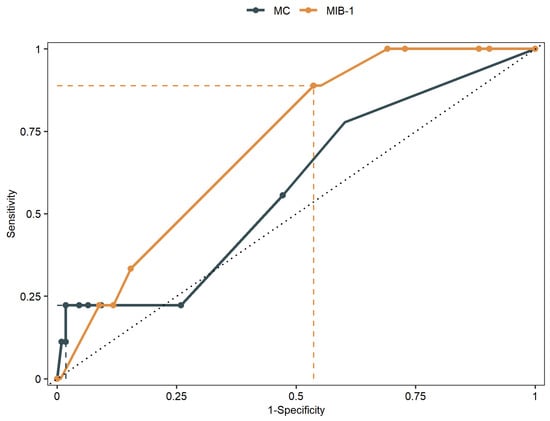
Figure 2.
Receiver-operating characteristic curves illustrating MIB-1 labeling index and MC in prediction of PFS of skull-base meningiomas.
Patients with an MIB-1 labeling index of <4.75% had a median time to progression of 456 weeks (95% CI: 431–480) and patients with an MIB-1 labeling index of ≥4.75% had a median PFS time of 402 weeks (95% CI: 358–445). Patients with an MC of <6.5% had a median PFS time of 406 weeks (95% CI: 376–436) and patients with an MC of ≥6.5% had a median PFS time of 250 weeks (95% CI: 71–429) (Figure 3).
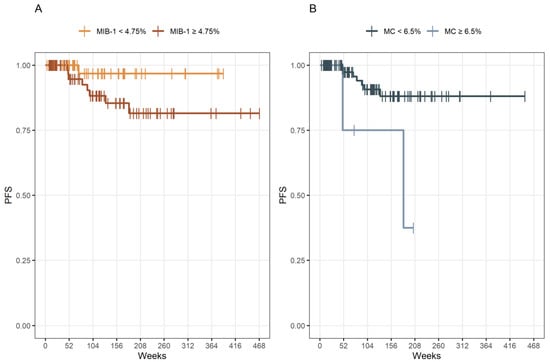
Figure 3.
(A) Kaplan–Meier analysis of PFS stratified by “MIB-1 ≥ 4.75%” (dark yellow line) and “MIB-1 < 4.75%” (yellow line), p = 0.083. (B) Kaplan–Meier analysis of PFS stratified by “MC ≥ 6.5” (blue line) and “MC < 6.5” (dark blue line), p = 0.014. Vertical dashes indicate censored data (here: progression-free at last follow-up) within the PFS curves.
3.3. Time-Dependent Analysis of MIB-1 Labeling Index and Mitotic Count in Prediction of Recurrence of Skull-Base Meningioma
Results of the time-dependent ROC analysis of MIB-1 and MC are shown in Table 2 as well as Figure 4. The C-index that represents the probability that MIB-1 or MC in a patient with a shorter time to tumor progression after surgery is high, was estimated to 0.61 (95% CI 0.51–0.77) for MIB-1 and 0.60 (95% CI 0.50–0.81) for MC, respectively.

Table 2.
Time-dependent AUCs for PFS skull-base meningiomas.
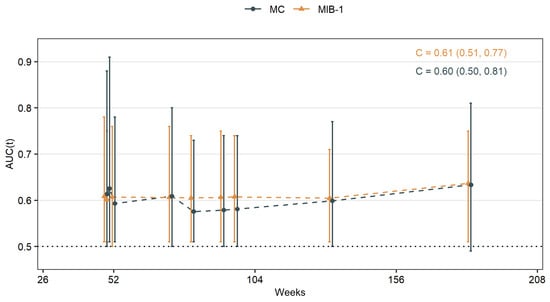
Figure 4.
Time-dependent AUCs for MIB-1 labeling index and MC in skull-base meningiomas.
4. Discussion
Primary treatment of cranial meningioma is a maximum cytoreductive approach, that mostly results to be curative [3]. In certain cases tumor recurrence is observed if the follow-up is long enough [2]. Current follow-up involves yearly MRI scans and sometimes even shorter intervals, if tumor was incompletely removed or recurred [3]. Therefore, it is important to identify predictors for meningioma recurrence, so that either follow-up could be optimized or adjuvant therapy could be advocated. WHO grading aims to classify the risk of recurrence. In the current as well as previous version of WHO classification MC and brain invasion are major diagnostic criteria of atypical meningioma and therefore predictors [1,23].
Nevertheless, there is still an incongruence between the WHO grade and clinical course, so new prognostic markers are still need to be identified [24]. MIB-1 and MC are already known predictors for tumor recurrence, but only MC is integrated in the current classification as described above [5,6,7,8,9,14,23].
MIB-1 stains nuclear protein Ki67 which is expressed in proliferation phases of the cell cycles [25]. It’s bearing as an additional criterion for meningioma grading is controversial [24]. By now, use of MIB-1 is more common in breast cancer, but there are differences in procedure leading to inter- and intralaboratory variability [26]. Some laboratories or investigators use hot spot analysis, where number of positive nuclei were divided by the total number of nuclei within the hot spot, other laboratories use global scoring, where entire tumor area was analyzed [26]. In addition, cut-offs are not standardized, so laboratory-specific cut-offs are maybe necessary [27]. This inter- and intralaboratory variability causes that no generalized recommendation can be made for the use of MIB-1 in meningiomas. MC were defined as “number of mitotic figures/10 high power fields” as stated above [1]. These mitotic figures were usually counted by a neuropathologist on hematoxylin and eosin-stained slides just like in our cohort. This requires a neuropathologist’s expertise and carries therefore poor intra- and interobserver reproducibility [28]. An additional staining with phosphohistone H3 could improve reproducibility, but the threshold of MC should be adopted when utilizing new methods [28]. Therefore, both MIB-1 and MC are limited in validity due to their different determinations. One possibility to overcome this issue would be a multicentric trial investigating the predictive power of the MIB-1 labeling index regarding PFS. Moreover, a future implementation in the classification system necessitates a homogenous approach regarding the determination of MIB-1 labeling index and has to be investigated in such a multicentric trial (e.g., hotspot method, digital image analysis, average method).
The static allocation of all meningiomas regardless of their origins—e.g., skull-base, non-skull-base and spinal—appears to be inaccurate as the location has considerable impact on meningioma PFS, especially in skull-base meningiomas [29,30]. In the present study, we compared MIB-1 and MC for their predictive power in skull-base meningiomas.
Intuitively, MC was associated with meningioma recurrence but with a slightly higher cut-off as in the WHO classification [1]. MIB-1 was associated with meningioma recurrence with a cut-off similar to previous studies [16,17,24,31]. So both MC and MIB-1 are valuable predictors for PFS in our cohort. However, other studies demonstrated MIB-1 to be a marker for time to recurrence rather than a predictor of recurrence [24]. We therefore analyzed time-dependent ROC curves to reveal time differences. AUCs reached their maximum on week 183 with 0.63 for MC and 0.64 for MIB-1, respectively. Time-dependent AUCs did not differ significantly inter- and intraindividually, while MC of ≥6.5% had a median PFS time of 250 weeks in contrast to 402 weeks for MIB-1 of ≥4.75%.
Although MIB-1 is not a classification criterion by now, many neuropathologists determine MIB-1 already as a routine. This study should not induce to replace MC by MIB-1 but to use it in addition. We suggest that in the modern era of tailored neuro-oncological care a maximum of potential molecular markers may be used to achieve a most efficient and reliable individual treatment as well as follow-up scheduling. The additional assessment of MIB-1 regarding tumor recurrence risk could improve stratification of patients to specific follow-up. Nevertheless, this study also emphasizes that different cut-offs for both, MIB-1 and MC, may be necessary in order to comply with the different pathophysiology depending on meningioma location.
5. Conclusions
MIB-1 as well as MC are good predictors for PFS in skull-base meningiomas. Their prognostic value is more or less equal. We do not want to substitute MC with MIB-1, rather emphasize the possible benefit of MIB-1 as an additional histopathological factor. Moreover, higher cut-off for MC should be applied in predicting PFS among skull-base meningiomas, but further studies are needed.
As the achievement of a gross total resection can be more challenging in skull-base meningiomas and second surgery implies a higher risk profile, the recurrence risk could be stratified according to these findings and guide decision-making for follow-ups vs. adjuvant therapies.
Author Contributions
Conceptualization E.G. and T.L.; methodology E.G. and T.L.; data curation T.L. and J.W.; writing—original draft preparation T.L. and E.G.; writing—review and editing J.W., M.-T.S., Á.G., H.V. and E.G.; visualization M.-T.S., T.L. and J.W.; supervision Á.G., H.V. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical Faculty and the University Hospital of Bonn (code: 228/21).
Informed Consent Statement
Patient informed consent was not necessary according to the retrospective character of this study and after approval of our Ethics Committee Approval.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Pettersson-Segerlind, J.; Orrego, A.; Lönn, S.; Mathiesen, T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011, 76, 564–571. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Gogou, P.; Markoula, S.; Kyritsis, A.P. Management of meningiomas. Clin. Neurol. Neurosurg. 2010, 112, 177–182. [Google Scholar] [CrossRef]
- van Diest, P.J.; Brugal, G.; Baak, J.P. Proliferation markers in tumours: Interpretation and clinical value. J. Clin. Pathol. 1998, 51, 716–724. [Google Scholar] [CrossRef]
- Olar, A.; Wani, K.M.; Sulman, E.P.; Mansouri, A.; Zadeh, G.; Wilson, C.D.; DeMonte, F.; Fuller, G.N.; Aldape, K.D. Mitotic Index is an Independent Predictor of Recurrence-Free Survival in Meningioma. Brain Pathol. 2015, 25, 266–275. [Google Scholar] [CrossRef]
- Oya, S.; Kawai, K.; Nakatomi, H.; Saito, N. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J. Neurosurg. 2012, 117, 121–128. [Google Scholar] [CrossRef]
- Vranic, A.; Popovic, M.; Cör, A.; Prestor, B.; Pizem, J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: A study of 86 patients. Neurosurgery 2010, 67, 1124–1132. [Google Scholar] [CrossRef]
- Liu, N.; Song, S.-Y.; Jiang, J.-B.; Wang, T.-J.; Yan, C.-X. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine 2020, 99, e18644. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.H.; Lee, E.H.; Lee, Y.M.; Lee, S.-H.; Kim, H.D.; Kim, Y.Z. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J. Neurosurg. 2014, 121, 1189–1200. [Google Scholar] [CrossRef]
- Maiuri, F.; Mariniello, G.; Guadagno, E.; Barbato, M.; Corvino, S.; Del Basso De Caro, M. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir. 2019, 161, 2553–2561. [Google Scholar] [CrossRef]
- Antinheimo, J.; Haapasalo, H.; Haltia, M.; Tatagiba, M.; Thomas, S.; Brandis, A.; Sainio, M.; Carpen, O.; Samii, M.; Jääskeläinen, J. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J. Neurosurg. 1997, 87, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Coons, S.W.; Johnson, P.C. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J. Neuropathol. Exp. Neurol. 1993, 52, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Landriel Ibañez, F.A.; Hem, S.; Ajler, P.; Vecchi, E.; Ciraolo, C.; Baccanelli, M.; Tramontano, R.; Knezevich, F.; Carrizo, A. A new classification of complications in neurosurgery. World Neurosurg. 2011, 75, 709–715; discussion 604-11. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Becker, A.J.; Hölzel, M.; Toma, M.; Güresir, E. Combining FORGE Score and Histopathological Diagnostic Criteria of Atypical Meningioma Enables Risk Stratification of Tumor Progression. Diagnostics 2021, 11, 2011. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Schuss, P.; Vatter, H.; Herrlinger, U.; Becker, A.; Hölzel, M.; Toma, M.; Güresir, E. FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas. Cancers 2021, 13, 3643. [Google Scholar] [CrossRef]
- Schneider, M.; Borger, V.; Güresir, Á.; Becker, A.; Vatter, H.; Schuss, P.; Güresir, E. High Mib-1-score correlates with new cranial nerve deficits after surgery for frontal skull base meningioma. Neurosurg. Rev. 2021, 44, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Majores, M.; Schick, V.; Engels, G.; Fassunke, J.; Elger, C.E.; Schramm, J.; Blümcke, I.; Becker, A.J. Mutational and immunohistochemical analysis of ezrin-, radixin-, moesin (ERM) molecules in epilepsy-associated glioneuronal lesions. Acta Neuropathol. 2005, 110, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Majores, M.; von Lehe, M.; Fassunke, J.; Schramm, J.; Becker, A.J.; Simon, M. Tumor recurrence and malignant progression of gangliogliomas. Cancer 2008, 113, 3355–3363. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Güresir, Á.; Borger, V.; Schuss, P.; Becker, A.; Coch, C.; Schmitz, M.-T.; Hölzel, M.; Toma, M.; Herrlinger, U.; et al. Elevated baseline C-reactive protein levels predict poor progression-free survival in sporadic vestibular schwannoma. J. Neurooncol. 2022, 156, 365–375. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Lumley, T.; Pepe, M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000, 56, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Mirian, C.; Skyrman, S.; Bartek, J.; Jensen, L.R.; Kihlström, L.; Förander, P.; Orrego, A.; Mathiesen, T. The Ki-67 Proliferation Index as a Marker of Time to Recurrence in Intracranial Meningioma. Neurosurgery 2020, 87, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar]
- Robertson, S.; Acs, B.; Lippert, M.; Hartman, J. Prognostic potential of automated Ki67 evaluation in breast cancer: Different hot spot definitions versus true global score. Breast Cancer Res. Treat. 2020, 183, 161–175. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Duregon, E.; Cassenti, A.; Pittaro, A.; Ventura, L.; Senetta, R.; Rudà, R.; Cassoni, P. Better see to better agree: Phosphohistone H3 increases interobserver agreement in mitotic count for meningioma grading and imposes new specific thresholds. Neuro Oncol. 2015, 17, 663–669. [Google Scholar] [CrossRef]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Meningiomas: Skull base versus non-skull base. Neurosurg. Rev. 2019, 42, 163–173. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Herrlinger, U.; Becker, A.; Cases-Cunillera, S.; Hölzel, M.; Toma, M.; Güresir, E. Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location-From Convexity to Skull Base and Spine. Cancers 2022, 14, 1033. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Kanungo, I.; Sudhir, S.; Chen, J.-S.; Raleigh, D.R.; Magill, S.T.; McDermott, M.W.; Aghi, M.K. WHO Grade I Meningioma Recurrence: Identifying High Risk Patients Using Histopathological Features and the MIB-1 Index. Front. Oncol. 2020, 10, 1522. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).