The Clinical Impact of Methylated Homeobox A9 ctDNA in Patients with Non-Resectable Biliary Tract Cancer Treated with Erlotinib and Bevacizumab
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility
2.2. Treatment
2.3. Endpoints
2.4. Efficacy
2.5. Analysis of Methylated HOXA9
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Patient Characteristics
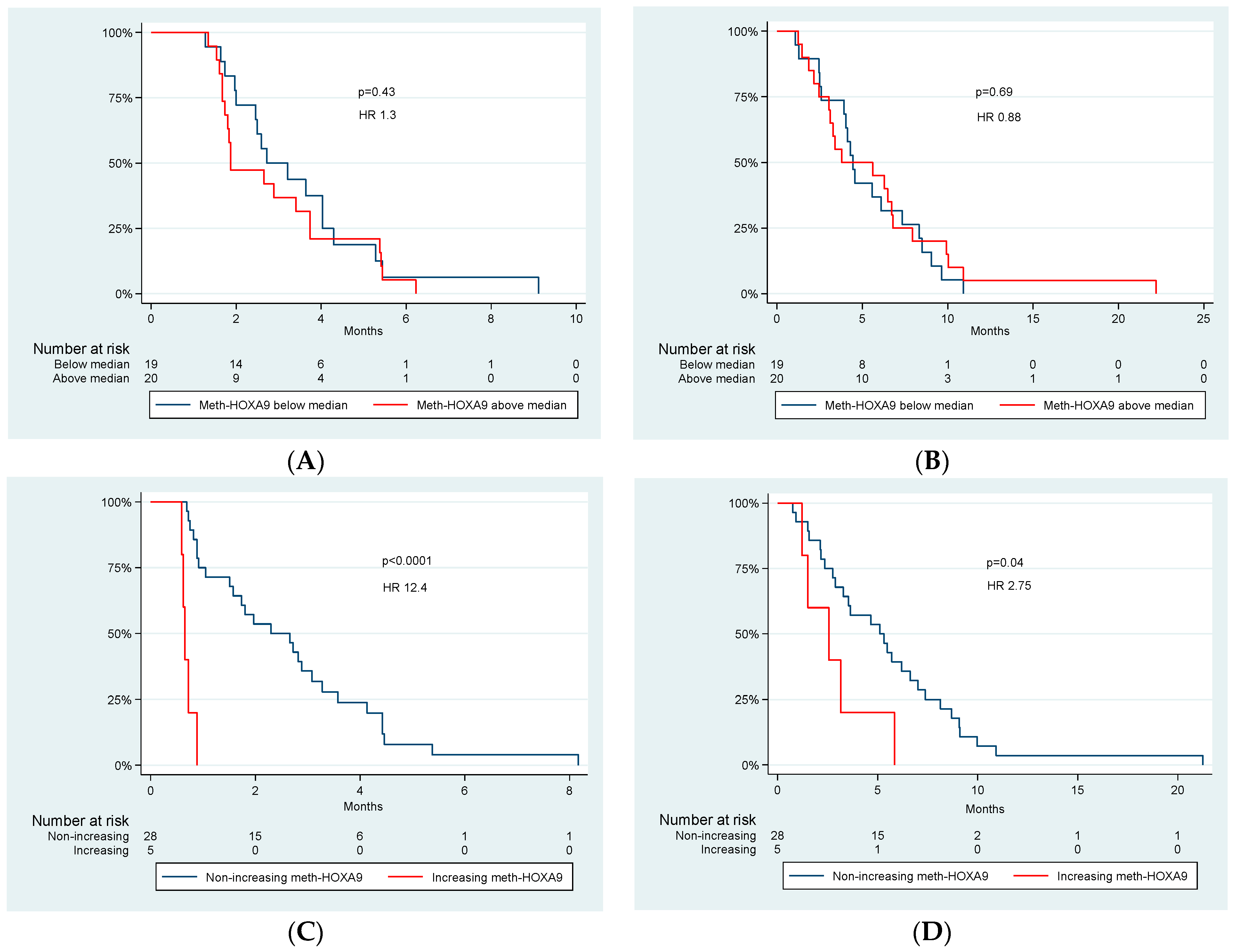
3.2. Meth-HOXA9—Dynamics and Survival
3.3. Response
3.4. Adverse Events and Meth-HOXA9
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, L.A.; Soares, H.P. Quality of life and symptom management in advanced biliary tract cancers. Cancers 2021, 13, 5074. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.; Pape, U.F.; Utku, N. A review of systemic therapy in biliary tract carcinoma. J. Gastrointest. Oncol. 2020, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Brieau, B.; Dahan, L.; De Rycke, Y.; Boussaha, T.; Vasseur, P.; Tougeron, D.; Lecomte, T.; Coriat, R.; Bachet, J.B.; Claudez, P.; et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015, 121, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Hubner, R.A.; David Ryder, W.; Valle, J.W. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef]
- Valle, J.W.; Wasan, H.; Palmer, D.; Cunningham, D.; Anthoney, A. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Kliniske Retningslinjer: Udredning og Behandling af Cholangiokarcinom. Available online: https://www.dmcg.dk/siteassets/kliniske-retningslinjer---skabeloner-og-vejledninger/kliniske-retningslinjer-opdelt-pa-dmcg/dlgcg/dlgcg_cc_admgodk271020.pdf (accessed on 7 January 2022).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Jensen, L.H. Clinical aspects and perspectives of erlotinib in the treatment of patients with biliary tract cancer. Expert Opin. Investig. Drugs 2016, 25, 359–365. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; LoConte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.P.; Horvath, L.; et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: A phase II consortium study. J. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef]
- Burotto, M.; Manasanch, E.E.; Wilkerson, J.; Fojo, T. Gefitinib and Erlotinib in Metastatic Non-Small Cell Lung Cancer: A Meta-Analysis of Toxicity and Efficacy of Randomized Clinical Trials. Oncologist 2015, 20, 400–410. [Google Scholar] [CrossRef]
- Wei, F.; Shin, D.; Cai, X. Incidence, risk and prognostic role of anti-epidermal growth factor receptor-induced skin rash in biliary cancer: A meta-analysis. Int. J. Clin. Oncol. 2018, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Haworth, L.; Sherry, R.; Hwu, P.; Schwartzentruber, D. A Randomized Trial of Bevacizumab, an Anti-Vascular Endothelial Growth Factor Antibody for Metastatic Renal Cancer. N. Engl. J. Med. 2003, 349, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Giantonio, B.J.; Levy, D.E.; O’Dwyer, P.J.; Meropol, N.J.; Catalano, P.J.; Benson, A.B. A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: Results from the eastern cooperative oncology group study E2200. Ann. Oncol. 2006, 17, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. J. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Maleki, Z.; Tsay, J.C.J.; Goparaju, C.; Brait, M.; Turaga, N.; Nam, H.S.; Rom, W.N.; Pass, H.I.; Sidransky, D.; et al. A panel of novel detection and prognostic methylated DNA markers in primary non–small cell lung cancer and serum DNA. Clin. Cancer Res. 2017, 23, 7141–7152. [Google Scholar] [CrossRef]
- Hulbert, A.; Jusue-Torres, I.; Stark, A.; Chen, C.; Rodgers, K.; Lee, B. Early Detection of Lung Cancer Using DNA Promoter Hypermethylation in Plasma and Sputum. Clin Cancer Res 2017, 15, 1998–2005. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, W.; Sun, L.; Zhou, H.; Zhou, B.; Hu, Y. DNA methylation analysis of selected genes for the detection of early-stage lung cancer using circulating cell-free DNA. Adv. Clin. Exp. Med. 2019, 28, 361–366. [Google Scholar] [CrossRef]
- Sandoval, J.; Mendez-Gonzalez, J.; Nadal, E.; Chen, G.; Carmona, F.J.; Sayols, S.; Moran, S.; Heyn, H.; Vizoso, M.; Gomez, A.; et al. A prognostic DNA methylation signature for stage I non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 4140–4147. [Google Scholar] [CrossRef]
- Balgkouranidou, I.; Chimonidou, M.; Milaki, G.; Tsaroucha, E.; Kakolyris, S.; Georgoulias, V.; Lianidou, E. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin. Chem. Lab. Med. 2016, 54, 1385–1393. [Google Scholar] [CrossRef]
- Spindler, K.L.G.; Pallisgaard, N.; Vogelius, I.; Jakobsen, A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin. Cancer Res. 2012, 18, 1177–1185. [Google Scholar] [CrossRef]
- Thomsen, C.B.; Hansen, T.F.; Andersen, R.F.; Lindebjerg, J.; Jensen, L.H.; Jakobsen, A. Early identification of treatment benefit by methylated circulating tumor DNA in metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2020, 12, 1–7. [Google Scholar] [CrossRef]
- Yu, S.L.; Lee, D.C.; Sohn, H.A.; Lee, S.Y.; Jeon, H.S.; Lee, J.H.; Park, C.G.; Lee, H.Y.; Yeom, Y., II; Son, J.W.; et al. Homeobox A9 directly targeted by miR-196b regulates aggressiveness through nuclear Factor-kappa B activity in non-small cell lung cancer cells. Mol. Carcinog. 2016, 55, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.C.; Andersen, R.F.; Hansen, T.F.; Nyhus, C.H.; Hager, H.; Hilberg, O.; Jakobsen, A. The prognostic impact of circulating homeobox A9 methylated DNA in advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Filho, J.; Mallisetty, A.; Villani, C.; Kottorou, A.; Rodgers, K. Detection of promotor DNA methylation in urine and plasma aids the detection of non-small lung cancer. Clin Cancer Res 2020, 15, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Rusan, M.; Andersen, R.F.; Jakobsen, A.; Steffensen, K.D. Circulating HOXA9-methylated tumour DNA: A novel biomarker of response to poly (ADP-ribose) polymerase inhibition in BRCA-mutated epithelial ovarian cancer. Eur. J. Cancer 2020, 125, 121–129. [Google Scholar] [CrossRef]
- Cancer.net, Bile Duct Cancer (Cholangiocarcinoma): Statistics. 2021. Available online: https://www.cancer.net/cancer-types/bile-duct-cancer-cholangiocarcinoma/statistics (accessed on 7 January 2022).
- National Cancer Institute. Common Terminology Criteria for Adverse Events. NCI Thesaurus 2017, 2009, C49704. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Pallisgaard, N.; Spindler, K.L.G.; Andersen, R.F.; Brandslund, I.; Jakobsen, A. Controls to validate plasma samples for cell free DNA quantification. Clin. Chim. Acta 2015, 446, 141–146. [Google Scholar] [CrossRef]
- BIO-RAD, Droplet Digital PCR Applications Guide. Bulletin 6407 Ver B. Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf (accessed on 7 January 2022).
- Rizzo, A.; Ricci, A.D.; Tavolari, S.; Brandi, G. Circulating tumor DNA in biliary tract cancer: Current evidence and future perspectives. Cancer Genom. Proteom. 2020, 17, 441–452. [Google Scholar] [CrossRef]
- Jensen, L.H.; Andersen, R.F.; Byriel, L.; Fernebro, E.; Jakobsen, A.; Lindebjerg, J.; Nottelmann, L.; Ploen, J.; Hansen, T.F. Phase II study of gemcitabine, oxaliplatin and capecitabine in patients with KRAS exon 2 mutated biliary tract cancers. Acta Oncol. 2020, 59, 298–301. [Google Scholar] [CrossRef]
- Steffensen, K.D.; Madsen, C.V.; Andersen, R.F.; Waldstrøm, M.; Adimi, P.; Jakobsen, A. Prognostic importance of cell-free DNA in chemotherapy resistant ovarian cancer treated with bevacizumab. Eur. J. Cancer 2014, 50, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Wasenang, W.; Chaiyarit, P.; Proungvitaya, S.; Limpaiboon, T. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin. Epigenetics 2019, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.B.; Andersen, R.F.; Steffensen, K.D.; Adimi, P.; Jakobsen, A. Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol. Res. 2019, 141, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.; Andersen, R.F.; Hansen, T.F.; Jensen, L.H.; Faaborg, L.; Steffensen, K.D.; Thomsen, C.B.; Wen, S.W.C. Early ctDNA response to chemotherapy. A potential surrogate marker for overall survival. Eur. J. Cancer 2021, 149, 128–133. [Google Scholar] [CrossRef]
- Rohrberg, K.S.; Olesen, R.K.; Pfeiffer, P.; Ladekarl, M.; Pappot, H.; Christensen, I.J.; Høyer-Hansen, G.; Sørensen, M.; Skov, B.G.; Buysschaert, I.; et al. Phase II trial of erlotinib and bevacizumab in patients with advanced upper gastrointestinal cancers. Acta Oncol. 2012, 51, 234–242. [Google Scholar] [CrossRef]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II study of erlotinib in patients with advanced biliary cancer. J. Clin. Oncol. 2006, 24, 3069–3074. [Google Scholar] [CrossRef]
- Rosell, R.; Dafni, U.; Felip, E.; Curioni-Fontecedro, A.; Gautschi, O.; Peters, S.; Massutí, B.; Palmero, R.; Aix, S.P.; Carcereny, E.; et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): An international, multicentre, single-arm, phase 2 trial. Lancet Respir. Med. 2017, 5, 435–444. [Google Scholar] [CrossRef]
- Shepherd, F.; Pereira, J.R.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S. Erlotinib in Previously Treated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef]
- Sozzi, G.; Roz, L.; Conte, D.; Mariani, L.; Andriani, F.; Verderio, P.; Pastorino, U. Effects of prolonged storage of whole plasma or isolated plasma DNA on the results of circulating DNA quantification assays. J. Natl. Cancer Inst. 2005, 97, 1848–1850. [Google Scholar] [CrossRef]
- Whale, A.S.; Devonshire, A.S.; Karlin-Neumann, G.; Regan, J.; Javier, L.; Cowen, S.; Fernandez-Gonzalez, A.; Jones, G.M.; Redshaw, N.; Beck, J.; et al. International interlaboratory digital PCR study demonstrating high reproducibility for the measurement of a rare sequence variant. Anal. Chem. 2017, 89, 1724–1733. [Google Scholar] [CrossRef]
- Wiencke, J.K.; Bracci, P.M.; Hsuang, G.; Zheng, S.; Hansen, H.; Wrensch, M.R.; Rice, T.; Eliot, M.; Kelsey, K.T. A comparison of DNA methylation specific droplet digital PCR (ddPCR) and real time qPCR with flow cytometry in characterizing human T cells in peripheral blood. Epigenetics 2014, 9, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | All Patients n = 39 | Increasing Meth-HOXA9 after 1st Cycle (n = 5) | Non-Increasing Meth-HOXA9 after 1st Cycle (n = 28) | p-Value |
|---|---|---|---|---|
| Age at first treatment, years Median Range | 62 25–80 | 71 55–73 | 64 25–80 | 0.24 |
| Sex Male Female | 19 (49%) 20 (51%) | 2 (40%) 3 (60%) | 14 (50%) 14 (50%) | 0.53 |
| Performance status 0 1 2 | 10 (25%) 26 (67%) 3 (8%) | 4 (80%) 1 (20%) 0 (0%) | 4 (14%) 22 (79% 2 (7%) | 0.01 |
| Metastatic or localized disease Metastatic Localized | 28 (72%) 11 (28%) | 4 (80%) 1 (0%) | 20 (71%) 8 (29% | 0.58 |
| Tumorlocalization Intrahepatic Extrahepatic Gall bladder Unknown | 23 (59%) 6 (15%) 1 (3%) 9 (23%) | 3 (60%) 2 (40%) 0 (0%) 0 (0%) | 17 (61%) 3 (10%) 1 (4%) 7 (25%) | 0.28 |
| Curative surgery previous Yes No | 1 (3%) 38 (97%) | 0 (0%) 5 (100%) | 1 (4%) 27 (96%) | 0.85 |
| Type of previous therapy Gemcitabine Oxaliplatin Capecitabine Cisplatin Panitumumab Bevacizumab | 39 (100%) 30 (77%) 28 (72%) 8 (21%) 5 (13%) 10 (26%) | 5 (100%) 3 (60%) 3 (60%) 2 (40%) 0 (0%) 2 (40%) | 28 (100%) 21 (75%) 21 (75%) 6 (21%) 4 (14%) 6 (21%) | 0.46 |
| HOXA9 Detectable Undetectable | 37 (95%) 2 (5%) | 5 (100%) 0 (0%) | 26 (93%) 2 (7%) | 0.71 |
| Tumorlocalization | Median Meth-HOXA9 Copies/mL Plasma at Baseline |
|---|---|
| Intrahepatic (n = 23) | 206 |
| Extrahepatic (n = 6) | 12 |
| Gall bladder (n = 1) | 32 |
| Unknown (n = 9) | 19 |
| CTCAE Grade,N (%) | ||||||
|---|---|---|---|---|---|---|
| AdverseEvent | 0 | 1 | 2 | 3 | 4 | Total 1–4 |
| Erlotinib | ||||||
| Nausea | 21 (57%) | 13 (35%) | 2 (5%) | 1 (3%) | 0 (0%) | 16 (43%) |
| Vomiting | 29 (78%) | 5 (14%) | 1 (3%) | 2 (5%) | 0 (0%) | 8 (22%) |
| Loss of appetite | 14 (38%) | 19 (51%) | 4 (11%) | 0 (0%) | 0 (0%) | 23 (62%) |
| Diarrhea | 21 (57%) | 9 (24%) | 6 (16%) | 1 (3%) | 0 (0%) | 16 (43%) |
| Cough | 30 (81%) | 6 (16%) | 1 (3%) | 0 (0%) | 0 (0%) | 7 (19%) |
| Dyspnea | 32 (86%) | 4 (11%) | 1 (3%) | 0 (0%) | 0 (0%) | 5 (14%) |
| Fatigue | 5 (14%) | 21 (57%) | 9 (24%) | 2 (5%) | 0 (0%) | 32 (86%) |
| Stomatitis | 30 (81%) | 7 (19%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (19%) |
| Pain | 11 (30%) | 12 (32%) | 10 (27%) | 4 (11%) | 0 (0%) | 26 (70%) |
| Rash | 14 (38%) | 12 (32%) | 11 (30%) | 0 (0%) | 0 (0%) | 23 (62%) |
| Conjunctivitis | 35 (95%) | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (5%) |
| Infection | 18 (48%) | 6 (16%) | 4 (11%) | 7 (19%) | 2 (5%) | 19 (51%) |
| Interstitiel lung disease | 36 (97%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) | 1 (3%) |
| Bevacizumab | ||||||
| Bleeding | 29 (78%) | 6 (16%) | 0 (0%) | 2 (5%) | 0 (0%) | 8 (21%) |
| GI perforation | 37 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Arterial Thrombosis | 37 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Wound | 35 (95%) | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, L.B.; Mahler, M.S.K.; Andersen, R.F.; Jensen, L.H.; Raunkilde, L. The Clinical Impact of Methylated Homeobox A9 ctDNA in Patients with Non-Resectable Biliary Tract Cancer Treated with Erlotinib and Bevacizumab. Cancers 2022, 14, 4598. https://doi.org/10.3390/cancers14194598
Andersen LB, Mahler MSK, Andersen RF, Jensen LH, Raunkilde L. The Clinical Impact of Methylated Homeobox A9 ctDNA in Patients with Non-Resectable Biliary Tract Cancer Treated with Erlotinib and Bevacizumab. Cancers. 2022; 14(19):4598. https://doi.org/10.3390/cancers14194598
Chicago/Turabian StyleAndersen, Line Bechsgaard, Marit Sofie Kjær Mahler, Rikke Fredslund Andersen, Lars Henrik Jensen, and Louise Raunkilde. 2022. "The Clinical Impact of Methylated Homeobox A9 ctDNA in Patients with Non-Resectable Biliary Tract Cancer Treated with Erlotinib and Bevacizumab" Cancers 14, no. 19: 4598. https://doi.org/10.3390/cancers14194598
APA StyleAndersen, L. B., Mahler, M. S. K., Andersen, R. F., Jensen, L. H., & Raunkilde, L. (2022). The Clinical Impact of Methylated Homeobox A9 ctDNA in Patients with Non-Resectable Biliary Tract Cancer Treated with Erlotinib and Bevacizumab. Cancers, 14(19), 4598. https://doi.org/10.3390/cancers14194598






