Simple Summary
This meta-analysis compares the treatment results of partial-breast radiotherapy to those of whole-breast radiotherapy after breast conserving surgery in early-stage breast cancer. The results show that the tumor is slightly more likely to recur in the operated breast after partial radiotherapy compared to radiation therapy to the whole breast. These additional recurrences are located away from the original tumor bed. The technique by which partial-breast radiotherapy is applied also appears to affect the likeliness of tumor regrowth. Intraoperative radiation, given during the removal of the tumor, might lead to more relapses compared to other techniques. Partial-breast treatment also led to more lymph node recurrences in a very small number of patients. However, rates of distant relapses were not increased. We were unable to identify a specific subgroup that was most suitable for partial-breast irradiation. The differences between treatment of partial- and whole-breast radiotherapy are small when the patient groups and the radiation technique are appropriately selected.
Abstract
Purpose/Objective: The standard treatment for localized low-risk breast cancer is breast-conserving surgery, followed by adjuvant radiotherapy and appropriate systemic therapy. As the majority of local recurrences occur at the site of the primary tumor, numerous trials have investigated partial-breast irradiation (PBI) instead of whole-breast treatment (WBI) using a multitude of irradiation techniques and fractionation regimens. This meta-analysis addresses the impact on disease-specific endpoints, such as local and regional control, as well as disease-free survival of PBI compared to that of WBI in published randomized trials. Material and Methods: We conducted a systematic literature review and searched for randomized trials comparing WBI and PBI in early-stage breast cancer with publication dates after 2009. The meta-analysis was based on the published event rates and the effect sizes for available oncological endpoints of at least two trials reporting on them. We evaluated in-breast tumor recurrences (IBTR), local recurrences at the primary site and elsewhere in the ipsilateral breast, regional recurrences (RR), distant metastasis-free interval (DMFI), disease-free survival (DFS), contralateral breast cancer (CBC), and second primary cancer (SPC). Furthermore, we aimed to assess the impact of different PBI techniques and subgroups on IBTR. We performed all statistical analyses using the inverse variance heterogeneity model to pool effect sizes. Results: For the intended meta-analysis, we identified 13 trials (overall 15,561 patients) randomizing between PBI and WBI. IBTR was significantly higher after PBI (OR = 1.66; CI-95%: 1.07–2.58; p = 0.024) with an absolute difference of 1.35%. We detected significant heterogeneity in the analysis of the PBI technique with intraoperative radiotherapy resulting in higher local relapse rates (OR = 3.67; CI-95%: 2.28–5.90; p < 0.001). Other PBI techniques did not show differences to WBI in IBTR. Both strategies were equally effective at the primary tumor site, but PBI resulted in statistically more IBTRs elsewhere in the ipsilateral breast. IBTRs after WBI were more likely to be located at the primary tumor bed, whereas they appeared equally distributed within the breast after PBI. RR was also more frequent after PBI (OR = 1.75; CI-95%: 1.07–2.88; p < 0.001), yet we did not detect any differences in DMFI (OR = 1.08; CI-95%: 0.89–1.30; p = 0.475). DFS was significantly longer in patients treated with WBI (OR = 1.14; CI-95%: 1.02–1.27; p = 0.003). CBC and SPC were not different in the test groups (OR = 0.81; CI-95%: 0.65–1.01; p = 0.067 and OR = 1.09; CI-95%: 0.85–1.40; p = 0.481, respectively). Conclusion: Limiting the target volume to partial-breast radiotherapy appears to be appropriate when selecting patients with a low risk for local and regional recurrences and using a suitable technique.
1. Introduction
Whole-breast irradiation (WBI) and adequate systemic therapy are the two standard treatments after breast conserving surgery of early-stage breast cancer. This multimodal approach has been shown to be the oncological equivalent to mastectomy in numerous randomized trials [1,2,3,4,5]. Both adjuvant treatment modalities have been shown to reduce recurrence rates and improve overall survival [6,7]. With the advent of more sophisticated radiological modalities, standardized pathological testing, low-morbidity surgery, and effective systemic therapies, attempts have been made to de-escalate the treatment in early-stage breast cancer. Omission of adjuvant whole-breast irradiation was studied in multiple randomized trials of low-risk breast cancer patients [8,9,10,11,12,13,14,15]. Two meta-analyses demonstrated that omission of WBI had no negative impact on overall survival in selected patients but led to a significant loss in local control [16,17].
Numerous reports showed that the majority of local recurrences occur at the primary tumor bed after WBI [2,18,19,20,21,22,23,24,25,26,27,28]. Furthermore, histopathological analyses of mastectomy specimen showed that the highest density of tumor tissue was found within the first 2 cm around the tumor [29]. These observations led to the introduction of the de-escalation approach of partial-breast irradiation (PBI). Here, the treated breast volume is restricted to the tumor bed and the directly surrounding tissue.
The concept of PBI aimed to achieve non-inferior or equivalent control rates, improve cosmetic results, and reduce toxicities. As PBI treats a smaller volume of breast tissue, it has also been assumed that an acceleration of the treatment schedule might be possible to optimize convenience for the patients. However, initial randomized trials showed higher recurrence rates, and the differences between the treatments seemed to be highly dependent on the included risk groups [30,31].
We attempted to comprehensively review the current efficacy data, comparing WBI to PBI in terms of oncological outcome with special emphasis on local and systemic control. The analysis of the survival data has already been published [32]. The assessment of the adverse outcome data, including cosmesis and quality of life, will be reported separately.
2. Material and Methods
A systematic literature review in PubMed formed the basis of the analysis. This was carried out in accordance with the published PRISMA guideline and completed on 1 May 2021. In addition, we screened the major meetings (e.g., ASTRO, ESTRO, ESMO, and ASCO annual meetings) for published abstracts. The chosen keywords were “radiation therapy” or “radiotherapy” or “irradiation” AND “breast cancer” or “carcinoma of the breast” AND “partial” or “targeted” AND “randomized” OR “randomised” OR “randomly”. The inclusion criteria were randomized controlled trials including patients diagnosed with invasive breast cancer or carcinoma in situ comparing PBI to WBI. The trials were considered eligible when published after December 2009 in order to include comparable techniques and a homogeneous study population.
To allow an estimation of the effect sizes comparing WBI to PBI, we extracted the published hazard and odds ratios as well as the event numbers from the identified trials. When no hazard ratios were reported, we estimated the hazard ratios and their corresponding 95% confidence intervals by reconstructing all events from the published survival curves or using the method published by Parmar and Tierney and colleagues [33,34]. When hazard ratios were neither reported nor estimable, we used the absolute number of events and calculated the odds ratio and the corresponding confidence interval.
The aim of the present study was to evaluate the endpoints of ipsilateral breast tumor recurrence or local recurrence (IBTR), local recurrence-free survival (LRFS), local recurrences at the primary site (LRPS) and elsewhere in the ipsilateral breast (LREB), regional recurrences (RR), distant metastasis-free interval (DMFI), disease-free survival (DFS), contralateral breast cancer (CBC), and second primary cancer (SPC). The definition of disease-free survival was any first breast cancer-related event or death as defined in the included trials. For the analysis of the location of a local recurrence in the ipsilateral breast, we used the following definitions: recurrences at the primary site were those that originated within the margin of the tumor bed, and recurrences elsewhere were located in a different quadrant. The rates of cumulative incidence of a given endpoint were calculated for the full length of the available follow-up.
We used the inverse variance heterogeneity model (ivhet) to pool effect sizes, as this model uses a more conservative estimation of the confidence limits, produces lesser observed variances, and favors larger trials compared to the commonly used random effects model [35]. Zero-event correction was applied where appropriate [36]. The statistically significant limit was set at p-values lower than 0.05. Heterogeneity within the meta-analysis was obtained with Cochran’s Q-test with the corresponding p-values. Furthermore, we also described the I2 statistics where we defined values above 25% as considerable heterogeneity that triggered a subgroup analysis by the PBI technique as described in [37]. Funnel plots were created to assess publication bias. For statistical analysis, we used the Microsoft Excel add-in MetaXL 5.3 (EpiGear International, Sunrise Beach, Australia). Plots were created using Microsoft Excel for Microsoft Office 365 Pro Plus (Redmond, WA, USA). In order to obtain pooled event rates over the full course of follow-up or at the five-year time point, we calculated prevalence with the function embedded in MetaXL with the continuity correction set at 0.5.
The analysis of available subgroups on IBTR was also performed when there were at least two trials reported on this patient group. In order to compare the effect of different radiation techniques, the effect of PBI vs. WBI was analyzed separately for trials using external beam radiation, intraoperative radiotherapy with electrons or photons, and for any form of brachytherapy. We acknowledge that this approach ignores the detailed differences between each individual techniques, which have their own characteristics. However, creating a subgroup for each technique used in the trials makes a general comparison impossible and ignores the basic approaches of each treatment. For the assessment of IBTR by the PBI technique described in Figure 2, we divided the results from the NSABP B-39 trial into the external beam technique and any form of brachytherapy.
When trials reported on the same endpoint in different publications, we attempted to include the most recent data in order to allow for the longest possible follow-up. For this reason, we have included the separate publications of the stratified prepathology and postpathology subgroups in the TARGIT trials [38,39,40,41].
3. Results
The results of the systematic literature search are presented in Figure A1, which revealed 40 publications reporting on thirteen different trials including a total number of 15,561 patients. Table 1 describes the details of the included studies with the respective inclusion criteria, treated patient cohorts, and interventions. In short, the trials included patients older than 40 years diagnosed with early-stage breast cancer with primary tumors up to 3 cm in size. Tumor biology consisted mainly of estrogen receptor-positive (83%) and node-negative disease (91.2%). Adjuvant endocrine therapy was the principal adjuvant systemic therapy (63.7%), whereas chemotherapy was applied less regularly (15.3%). A total of 16.8% of participants were younger than 50 years of age, and 9.8% had non-invasive tumors (DCIS). The median follow-up ranged between 2 and 17 years (median 8.6 years).

Table 1.
Overview of the included trials with relevant patient characteristics.
External beam radiotherapy (n = 9), intraoperative radiotherapy (IORT) (n = 3), and interstitial (single-entry catheter and multicatheter) brachytherapy (n = 3) were the PBI techniques used within the trials. The NSABP B-39 and the Budapest trials used EBRT and brachytherapy techniques. The different PBI schedules using EBRT were conventionally fractionated RT (n = 1) [42], once-daily hypofractionated RT (n = 5) [43,44,45,46,47], or twice-daily accelerated hypofractionated RT schedules (n = 4) [45,48,49,50]. The TARGIT-A trials used additional WBI in cases of prespecified risk factors in the PBI arms [38,39,40,41,51]. The funnel plots did not detect any publication bias.
3.1. Local Control
First, we evaluated cumulative IBTR, as depicted in Figure 1 and Figure A2. The comparison showed a statistical difference between PBI and WBI when comparing odds ratios (OR = 1.66; CI-95%: 1.07–2.58; p = 0.024; I2 = 53.61) and pooled IBTR + LRFS (HR = 1.31; CI-95%: 0.96–1.78; p = 0.086). The comparison of the absolute cumulative incidence of IBTR in the PBI arms showed a difference of 1.35% (PBI rate: 3.4% vs. WBI rate: 2.05%) after median follow-up of 8.6 years (range 2–17 years). As we detected a significant heterogeneity in the analysis, we further examined the different PBI techniques. Here, we found a significant inferior local control rate for IORT (OR = 3.67; CI-95%: 2.28–5.90; p < 0.001). The other PBI techniques of EBRT/BT (OR = 1.25; CI-95%: 0.93–1.69; p = 0.146), EBRT (OR = 1.25; CI-95%: 0.85–1.84; p = 0.256), and BT (OR = 1.58; CI-95%: 0.52–4.73; p = 0.418) showed no differences in IBTR compared to WBI. After exclusion of the trials using IORT, the heterogeneity in the analysis was no longer present (p = 0.625).
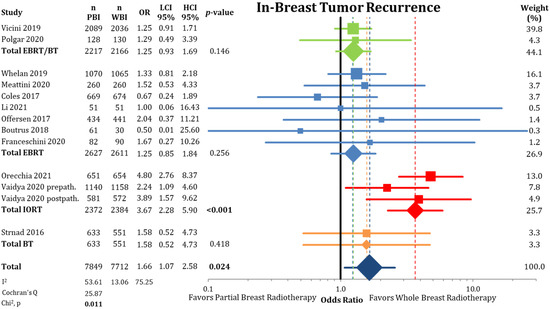
Figure 1.
Comparison of in-breast tumor recurrences between partial-breast radiotherapy and whole-breast radiotherapy. The odds ratios for each trial grouped by radiation technique and the pooled effect sizes with corresponding 95% confidence intervals are reported. The squares represent the effect sizes of the individual trials, while the center of the diamonds indicate the pooled odds ratios for the individual techniques and the overall effect. Heterogeneity analysis is shown using the I2 with 95% confidence intervals and Cochran’s Q analysis. Bold p-values indicate statistically significant results.
An overview of the cumulative local recurrence rate over the published follow-up time in the different trials is shown in Figure A3.
Controlling for different lengths of follow-up, Table 2 shows the five-year IBTR rates separated by the PBI technique. Overall, at the five-year IBTR time point, PBI was not statistically different to WBI (2.47% vs. 1.46%; OR = 1.61 CI-95%: 0.97–2.66; p = 0.066). As shown, the analysis detected significant heterogeneity between the trials (p = 0.028). PBI using IORT showed significantly worse IBTR rates at five years (3.07% vs. 0.90%; OR = 3.39 CI-95%: 1.64–7.00; p = 0.001), comparing unfavorably to the other PBI techniques where no differences between PBI and WBI were detectable (EBRT/BT: 2.76% vs. 2.32%; EBRT: 1.7% vs. 1.41%; BT: 1.57% vs. 1.02%).

Table 2.
Analysis of in-breast tumor recurrences after a five-year follow-up period between partial-breast irradiation and whole-breast irradiation for the whole group and by technique. The prevalence rates by treatment arm and the statistical comparison using the odds ratios with the corresponding 95% confidence intervals are given. The bold p-values are considered statistically significant.
The following analysis, depicted in Figure 2, shows a comparison between PBI and WBI for all available data for the entire follow-up period. Here, the difference with Figure 1 is the splitting of the NSABP B-39 results separated by the PBI method. This modification still leads to higher pooled IBTR rates for PBI (OR = 1.71; CI-95%: 1.17–2.50; p = 0.005; I2 = 58.84) for all trials. In this analysis, IORT and all BT techniques (single catheter device, multicatheter technique) show higher statistically significant IBTR rates (IORT: HR = 3.67; CI-95%: 2.28–5.90; p < 0.001 and BT: HR = 2.03; CI-95%: 1.36–3.02; p = 0.001). The pooled raw numbers by technique showed a difference in cumulative IBTR rate between PBI and WBI for EBRT/BT: 1.65%; EBRT: 0.21%; BT: 0.62%; and IORT: 3.06%.
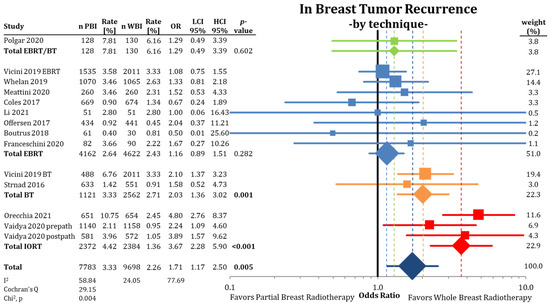
Figure 2.
Analysis of in-breast tumor recurrence by radiation technique using odds ratios with a 95% confidence interval. The squares represent the effect sizes of the individual trials, while the center of the diamonds indicate the pooled odds ratios for the individual techniques and the overall effect. Because of the splitting of the NSABP B-39 trial [53], the WBI arm is counted twice in the comparison. The pooled estimate should be interpreted accordingly. Bold p-values are statistically significant.
When separating IBTRs by location within the ipsilateral breast (Figure 3), we found no differences between PBI and WBI at the primary tumor site (LRPS: 1.36% vs. 1.34%; OR = 1.01; CI-95%: 0.65–1.59; p = 0.951; I2 = 32.68). The pooled estimate showed a higher IBTR rate for IORT (OR = 3.51; CI-95%: 1.36–9.11; p = 0.010). Recurrences elsewhere in the ipsilateral breast were more likely after treatment with PBI (LREB: 1.17% vs. 0.53%; OR = 2.21; CI-95%: 1.53–3.20; p < 0.001) (Figure 4) with a numerical difference of 0.64%.
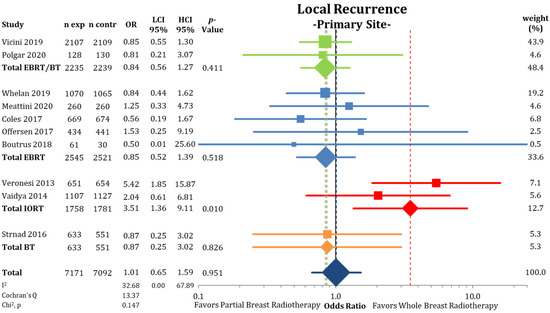
Figure 3.
Evaluation of the local recurrences located at the primary site or marginally to the primary tumor using odds ratios with squares representing individual trials and diamonds the pooled effect sizes for technique and overall analysis.
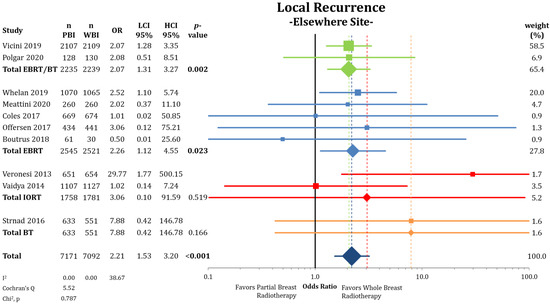
Figure 4.
Evaluation of the local recurrences elsewhere in the ipsilateral breast using odds ratios with squares representing indi-vidual trials and diamonds the pooled effect sizes for technique and overall analysis. Bold p-values indicate statistically significant results.
According to Figure 5, tumor recurrences were equally distributed between LRPS and LREB in the trial arms applying PBI (OR = 1.00; CI-95%: 0.70–1.43; p = 0.986; I2 = 14.89). This is in contrast to the analysis in the WBI arms, where IBTRs were more likely at the primary site than elsewhere in the breast (OR = 2.20; CI-95%: 1.52–3.18; p < 0.001; I2 = 0).
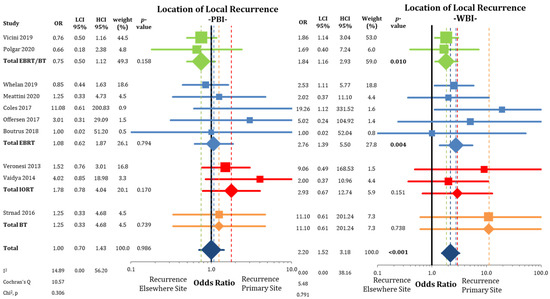
Figure 5.
Assessment of the location of the local recurrences (in-breast tumor recurrences) after partial-breast irradiation and whole-breast irradiation with the use of odds ratios. Squares represent individual trials and diamonds the pooled effect sizes for technique and overall analysis. Bold p-values indicate statistically significant results.
3.2. Other Endpoints
Regional recurrences rates were higher in the PBI arms of the randomized trials than in the WBI arms as presented in Figure 6 (0.58% vs. 0.33%; OR = 1.75; CI-95%: 1.07–2.88; p < 0.001) with a numerical difference of 0.25%. Conversely, DMFI was not decreased in the PBI groups (97.2% vs. 97.4%; OR = 1.08; CI-95%: 0.89–1.30; p = 0.475) (Figure A4). The comparison shown in Figure A5 of DFS indicates significantly higher failure-free survival (OR = 1.16; CI-95%: 1.05–1.28; p = 0.003) after WBI. During follow-up, the calculated DFS rate was 86.4% after PBI and 88.3% after WBI, resulting in a numerical difference of 1.9%.
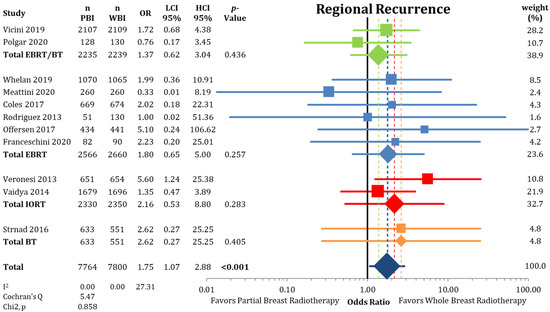
Figure 6.
Assessment of regional recurrences between partial-breast radiotherapy and whole-breast radiotherapy. The odds ratios for each trial (squares) grouped by radiation technique and the pooled effect sizes (diamonds) with corresponding 95% confidence intervals are displayed. Bold p-values indicate statistically significant results.
The incidence of contralateral breast cancer was not different between the groups (2% vs. 2.46%; OR = 0.81; CI-95%: 0.65–1.01; p = 0.067), as shown in Figure A6. The frequency of second primary cancers (Figure A7) others than breast cancers was equally distributed between PBI and WBI (5.51% vs. 5.04%; OR = 1.09; CI-95%: 0.85–1.40; p = 0.481).
Table 3 compares the anticipated absolute effects based on the relative effects and the risk per 100 for PBI and WBI.

Table 3.
Overview of investigated endpoints comparing partial- to whole-breast radiotherapy and showing the relative effect (odds ratio) and the anticipated absolute effects per 100 patients.
The subgroup analysis of IBTR is shown in Table 4. Because of the detected heterogeneity in the IBTR analysis, this assessment was influenced by the different trials and PBI methods. None of the investigated subgroups showed a significant effect on the comparison of PBI vs. WBI, as demonstrated by the non-significant interaction tests. Statistically inferior IBTR in the PBI arms was detected in tumors of a size between 11 and 20 mm (HR = 2.53; CI-95%: 1.22–5.28; p = 0.013), primary tumor of a size greater than 1.5 cm (HR = 2.71; CI-95%: 1.28–5.72; p = 0.009), N1 disease (HR = 2.82; CI-95%: 1.41–5.62; p = 0.003), and Her2-negative status (HR = 3.92; CI-95%: 1.15–13.40; p = 0.029).

Table 4.
Subgroup comparison of PBI and WBI regarding in-breast tumor recurrences. Hazard ratios and the corresponding 95% confidence intervals as well as the p-values for the interaction test of the subgroups are reported. Hazard ratios below 1 favor PBI and those above 1 favor WBI. Bold p-values represent statistically significant results.
The statistical comparison did not find any differences in IBTR in the subgroups of DCIS; hormone receptor-negative cancers; grade III, high-risk criteria according to ASTRO consensus; or younger women under the age of 50 years.
4. Discussion
4.1. Main Results
Numerous randomized trials using various techniques of partial-breast irradiation have attempted to demonstrate non-inferior local control rates in early-stage breast cancer. Some were able to confirm this hypothesis [39,41,43,49,50,60,69,78], whereas others could not conclude non-inferiority between PBI and WBI [38,62]. Our meta-analysis shows that the pooled PBI trials using different techniques are formally inferior in local control rates compared to whole-breast irradiation with a statistically significant numerical difference of 1.35% after a follow-up ranging between 3 and 17 years. After a period of 5 years, the numerical difference was 1.01%. Additionally, we found strong suggestions that selecting patients according to risk group and utilizing a suitable method influence the efficacy of PBI. When analyzed by the location in the breast, the treatments were similarly effective at the primary site (LRPS rates: 1.47% vs. 1.34%), showing that the remaining microscopic disease at the tumor bed is adequately treated by PBI. However, PBI led to more recurrences elsewhere in the breast, with raw incidences of 1.26% after PBI compared to 0.53% after WBI. This finding demonstrates that WBI is superior in treating microscopic tumor foci that are distant from the resected primary tumor. Furthermore, this confirms the past analysis of the START-trials, where WBI reduced the rate of ipsilateral new primary tumors [81].
The RAPID investigators first reported on differences in the IBTR distribution within the ipsilateral breast after PBI and WBI [49]. In the present meta-analysis. we can confirm this observation with the ratio of primary site vs. other ipsilateral recurrences of 1.5% to 1.4% (~1:1) after PBI and 1.4% to 0.6% (~2.3:1) after WBI. An explanation for this phenomenon could be that other foci beyond the original tumor site were controlled with WBI, while the cells at the original site might be more resistant due to higher tumor cell density, more hypoxia, or other post-therapeutic changes.
The results from several prospective randomized trials demonstrated that in-breast failures are distributed approximately 75% to the primary site and 25% to elsewhere in the surrounding tissue [25,26,27,28,82]. In this analysis, we found a similar ratio in the patients receiving WBI. In contrast, recurrences after PBI were equally distributed across the breast. Ipsilateral breast recurrences can originate from cells located far from the original tumor bed and occur at a clinically and statistically detectable rate. Earlier opinions questioned whether PBI could reach non-inferior efficacy, because with an assumed 10% local failure rate after 10-year follow-up, the proportion of other in-breast recurrences would reach around 2.5%. However, in this report, the cumulative incidence of ipsilateral local failures outside the tumor bed region was only 0.6–1.4%, mirroring the rapid advances in breast cancer treatment.
The detectable increase in regional nodal recurrences rates after PBI reinforces the role of the incidental radiation dose in the lower axillary levels or the internal mammary nodes. The incidental dose applied to the axillary lymph nodes during whole-breast radiotherapy has been postulated to affect axillary relapses since the publication of the ACOSOG Z0011 trial [83,84]. The recently published dosimetric data from the prospective randomized INSEMA trial showed that at least 25–50% of the patients received an unintended therapeutic dose to the axillary level I lymph nodes [85]. Whether regional failures occurred more frequently in patients with positive lymph nodes or a higher risk profile is unknown.
The absolute increase in local and regional relapses with PBI was small (0.3–0.8%), which questions the clinical relevance of this observation. It is reassuring that PBI did not affect systemic control, as shown by the similar occurrence of distant metastases of both treatments. This is in accordance with our recently published assessment of mortality, which confirmed no differences in overall, breast cancer-specific-, and non-breast cancer mortality between PBI and WBI [32].
The presumed lower doses to organs at risk currently did not translate into a reduction in the total number of cases of contralateral or second non-breast cancers. In the analysis of the EBCTCG data by Taylor and colleagues, the contralateral breast cancer risk associated with WBI was detectable with more than five years of fol-low-up, while the incidence of lung cancers started becoming evident only after more than 10 years [86]. Longer follow-up will have to determine whether PBI can reduce the risk of second cancers.
The rate at which patients relapse elsewhere in the breast or regionally should be similar to the risk factors for a CBC, which typically include biological risk groups, such as lobular histology, lack of an estrogen receptor, a higher proliferation index, or a history of receipt of radiation therapy. Conversely, systemic therapies, such as endocrine-, chemo-, and Her2-targeted therapies are protective agents [87,88]. Therefore, the logical conclusion is to select low-risk patients for partial-breast treatments where the risk of relapse in the remaining glandular tissue and regional lymph nodes is either negligible or is adequately treated by adjuvant systemic therapy [62,89]. The role of systemic agents in controlling residual disease elsewhere in the breast instead of WBI will be interesting, as we know that endocrine therapy alone cannot substitute for WBI in controlling disease at the primary site [16]. Hopefully, future investigations will further examine PBI in subgroups receiving adjuvant systemic therapies or no adjuvant therapy. Especially in Her2-positive patients, the use of very effective systemic treatments might lower the need for radiation therapy to treat the microscopic remnant cancer cells.
4.2. Techniques
Our analysis of IBTR revealed a significant heterogeneity in the comparison, which might be attributable to either difference in risk groups in the selected patients or PBI techniques. The analysis by PBI methods suggests that PBI by EBRT achieved similar local control, while techniques such as IORT and BT may be associated with higher recurrence rates. In the included trials, BT was performed as a multicatheter approach (NSABP B-39, GEC-ESTRO, Budapest) or a single-catheter treatment (NSABP B-39). The long-term analysis of the NSABP B-39 trial suggested that the failure of achieving equivalence in IBTR was driven by the patients treated with BT. Notably, the majority of these patients (~80%) were treated with single-device brachytherapy, which is a highly debated approach due to its concentric rigid dose distribution. The largest registry series reported an actuarial LR rate of 3.8% after five years, which compares unfavorable to other PBI techniques [90]. However, both BT techniques reported similar effect sizes compared to that of WBI (HR multicatheter: 2.21 CI-95%: 1.10–4.46; HR single-catheter: 2.15 CI-95%: 1.34–3.44; 10 y cumulative incidence: 7.7% and 7.8%, respectively). Furthermore, the primary endpoint analysis was not stratified by the irradiation technique, and the subgroup analysis was reported per protocol, which means that imbalances in the subgroups could also explain this finding. In contrast, the GEC-ESTRO trial reported equivalent IBTR with multicatheter BT to WBI. This difference could be due to a shorter follow-up period, a higher level of expertise of the treating centers, the sole use of multicatheter BT, or the inclusion of lower risk patients in the GEC-ESTRO trial.
Similarly, trials with PBI using the IORT technique also showed inferior local control rates. This was also the case when only low-risk patients (ELIOT trial) were selected or IORT was restricted to immediate IORT during lumpectomy only (TARGIT prepath.) compared to other techniques. There are several details to be considered here. The TARGIT trials included patients treated with IORT during the initial lumpectomy (prepathology) or during a second surgery where the initial scar was reopened (postpathology). The different approaches introduced considerable differences in the risk groups between the strata. It should also be noted that the TARGIT-A trial did not purely test partial- vs. whole-breast radiotherapy but included a risk-adapted approach of IORT with additional WBI in the presence of certain risk factors, resulting in a proportion of 26.8% in the prepathology and 5.7% in the postpathology stratum receiving both treatments [38,41]. We were unable to analyze the long-term local control rate in the TARGIT trials beyond five years, as the numbers were reported as the pooled endpoint of LRFS. Moreover, the technical differences of both IORT techniques using photon and electron irradiation must also be considered. Electron treatment covers larger intraoperative volumes with a homogenous dose distribution, while KV-based IORT applies heterogeneously distributed energy.
It is unclear whether the observed tendencies of a higher IBTR risk for IORT and BT are due to a higher risk of missing residual tumor cells at the tumor bed or an inclusion of higher risk patients. The analysis of the GEC-ESTRO trial does not indicate any evidence of missing microscopic disease at the tumor bed, as equal numbers of IBTRs at the primary site were reported for PBI and WBI. This is in contrast to the trials using IORT, were we detected higher recurrence rates at the primary tumor site (Figure 3).
4.3. Subgroups
At present, the leading radiation oncology societies recommend the use of partial-breast radiation in selected low-risk patients [91,92]. The presented subgroup analysis was unable to identify a specific subgroup unsuitable for PBI given by the non-significant interaction tests. However, as we detected heterogeneity in the included trials in the main analysis, the assessment of the different subgroups might be biased by the PBI techniques.
Nonetheless, multiple exploratory subgroups showed numerically higher recurrence rates when using PBI in patients with known risk factors for a local relapse, such as tumor size and nodal positivity. Therefore, it is difficult to make firm conclusions, as the sample size and different techniques limited this analysis. We await the planned individual patient data analysis by the early breast cancer trialist collaborative group (EBCTCG).
According to the ASTRO consensus, DCIS is included in the “suitable group” when the lesion was screening detected, had low to intermediate nuclear grade and a size of ≤2.5 cm, and was resected with negative margins ≥ 3 mm [92,93], as multiple prospective single arm trials reported favorable outcomes [94,95,96]. The appropriateness of PBI in women with DCIS has not been generally established, as randomized trials included only a small number of patients with non-invasive tumors, because the pattern of recurrence as well as the spread within the breast might differ to those in invasive cancers [60,69]. The NSABP B-39 and RAPID trials were the first to include a larger sample of DCIS patients. The presented subgroup analysis strengthens the argument to expand the use of PBI, as we showed no differences in IBTR between 1412 prerandomization-stratified patients (HR = 1.21; CI-95%: 0.69–2.11).
When comparing different techniques of partial-breast RT, one has to acknowledge that clinical target volume and dose application differ substantially. IORT and brachytherapy deliver (by nature of the technique) an inhomogeneous dose within the target volume. The TARGIT applicator reaches single-fraction surface doses from 20 to 31 Gy with a reduction to 4–8 Gy at a depth of 1 cm [41,97,98]. In contrast, IORT using electrons delivers a more homogenously distributed dose over a larger area. The IMPORT LOW trial applied EBRT using “mini-tangents” with much larger treated volumes and a homogenous dose distribution. Furthermore, the definition of a clinical target volume is complex and has to account for the surgical technique, including oncoplastic methods, the preoperative tumor location, the surgical path and scar, and the RT technique. The ideal target volume is concentric around the original tumor, which usually does not correlate well with the surgical tumor cavity since the surgical resection defect is rarely uniformly around the tumor. When comparing the absolute numbers, it is notable that the only techniques that reduced local failures are the partial tangents used in the IMPORT LOW trial [43].
The IBTR rates after PBI have to be weighed against the number reported by studies testing the omission of radiotherapy in low-risk breast cancer. The randomized PRIME II trial calculated an actuarial 5- and 10-year local control of ET alone of 4.1% and 9.8% compared to 1.3% and 0.9%, respectively, after ET and WBI [8,99]. Despite the higher risk population included in the PBI trials, we did not observe local recurrence rates reaching 10% after 10 years follow-up, demonstrating that the indirect comparison between partial-breast treatment combined with systemic therapy and endocrine therapy alone suggests that the former is superior.
4.4. Limitations of Analysis
Our analysis is based on pooled, published data that does not regard individual patients, which would be generally preferable and might allow a more comprehensive subgroup analysis. The fractionation schedules in the control arm using WBI where both normofractionated RT (fraction sizes between 1.8 and 2 Gy per fraction) and hypofractionated RT (>2.5 Gy per fraction). In some trials, hypofractionated schedules were associated with a reduction in IBTR; this might have introduced heterogeneity into the control arms of this analysis, which we could not statistically control [100]. Using an alpha/beta ratio of 3.7 for breast cancer [101], the dose comparison of some WBI regimes results in a higher dose compared to that of most of the PBI regimes included in this analysis. Additionally, many trials applied tumor bed boosts to their WBI arms, which are known to further reduce the local recurrence rates [75,102,103]. The similar failure rate at the primary tumor site questions the use of a tumor bed boost (dose escalation) in the included trial population. Another factor adding heterogeneity is the different follow-up times, which range from 2 to 17 years. However, the majority of the studies reported on an observation period of at least five years, which is usually considered long enough to assess the effect of radiotherapy on local recurrences [26,104].
Numerous meta-analyses and systematic reviews have been published on this subject since 2010 [105,106,107,108,109,110,111,112,113,114]. The number of included patients and follow-up time limited earlier analyses [105,106,107,108,109,110,111]. Newer publications either lack the inclusion of larger recently published trials [112] or excluded the three randomized trials performing PBI with IORT [114]. The report published by Viani and colleagues reported similar results to ours; however, a strength of our analysis is the detailed assessment of local relapse with localization and distribution in addition to a high number of included patients (n = 15,661) [113].
5. Conclusions
Partial-breast radiotherapy achieves equivalent oncological outcomes to those of whole-breast radiotherapy when selecting low-risk patients and using appropriate techniques. The appropriateness of limiting the target volume to a partial treatment of the breast depends on the individual risk profile and the margin of acceptance for non-inferiority.
Author Contributions
W.B., C.M. and J.H. conceived and designed the analysis. W.B., K.K. and J.H. collected the data. J.H. and K.K. performed the statistical analysis. V.S. contributed data to the analysis. J.H. prepared the graphs and tables. W.B., V.S., S.C., D.K., L.S., B.T., I.S. and C.M. significantly contributed to the interpretation of the results. J.H., W.B., V.S., E.B. and C.M. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This work analyzed and pooled data from published trials, and, thus, no institutional review board statement was needed.
Informed Consent Statement
This work analyzed and pooled data from published trials, and, thus, no informed consent statement was needed.
Data Availability Statement
All data used in the analysis are available from the cited published trials.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 3DCRT | 3D conventional radiation therapy |
| BCS | breast-conserving surgery |
| BID | twice daily |
| BT | brachytherapy |
| CTx | chemotherapy |
| DCIS | ductal carcinoma in situ |
| DMFI | distant metastasis-free interval |
| e- | electrons |
| EBRT | external beam radiotherapy |
| EP | endpoint |
| ER | estrogen receptor |
| ET | endocrine therapy |
| FU | follow-up |
| IBC | invasive breast cancer |
| IDC | invasive ductal cancer |
| IBTR | in-breast tumor recurrence |
| IMRT | intensity-modulated radiation therapy |
| IORT | intraoperative radiotherapy |
| ivhet | inverse variance heterogeneity mode |
| HR+ | hormone receptor positive |
| LR | local recurrence |
| LREB | local recurrence elsewhere in the ipsilateral breast |
| LRFS | local recurrence-free survival |
| LRPS | local recurrence at the primary site |
| Med. | median |
| n | number |
| N+ | nodal positive |
| Noninf | non-inferiority |
| n.r. | not reported |
| PBI | partial-breast irradiation |
| Pop | population |
| QD | once daily |
| RT | radiotherapy |
| q.o.d. | every other day |
| SIB | simultaneous integrated boost |
| Stat. | statistical |
| Strat. | stratification |
| WBI | whole-breast irradiation |
| X | photons |
| y | years |
Appendix A
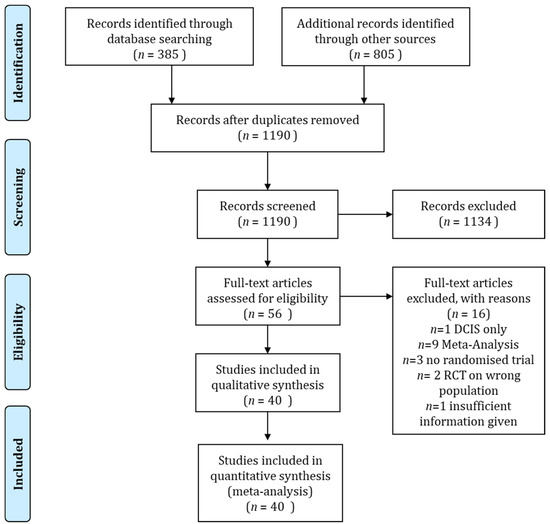
Figure A1.
Consort diagram of the literature search according to the PRISMA guideline.
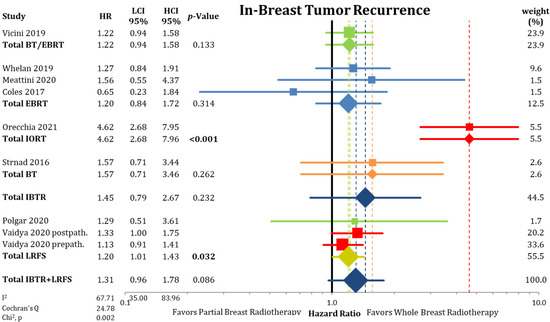
Figure A2.
Analysis of in-breast tumor recurrences and local recurrence-free survival between partial- and whole-breast radiation using hazard ratios in a forest plot separated by radiation techniques with corresponding 95% confidence intervals. Bold p-values signify statistical difference.
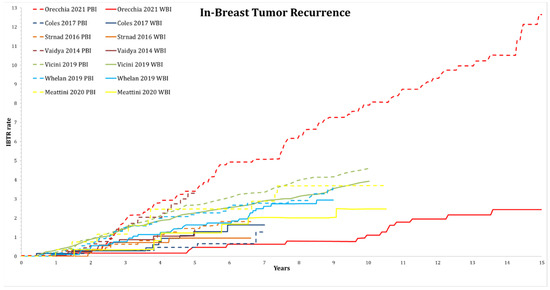
Figure A3.
Analysis of in-breast tumor recurrences (rate in %) of randomized trials, where long-term graphical assessment was available. Trials are grouped by color. Solid lines represent whole-breast irradiation arms and dashed lines represent partial-breast irradiation arms. Graphs were extracted from the original publications.
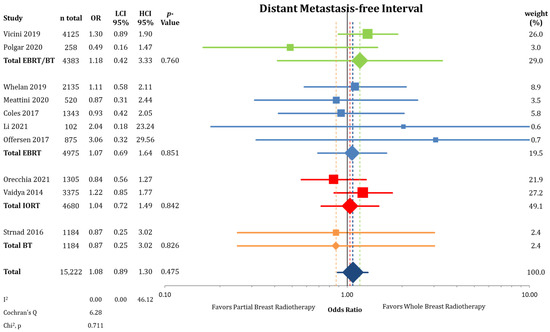
Figure A4.
Assessment of distant metastasis-free interval between partial-breast radiotherapy and whole-breast radiotherapy. The odds ratios for each trial (squares) grouped by radiation technique and the pooled effect sizes (diamonds) with corresponding 95% confidence intervals are displayed.
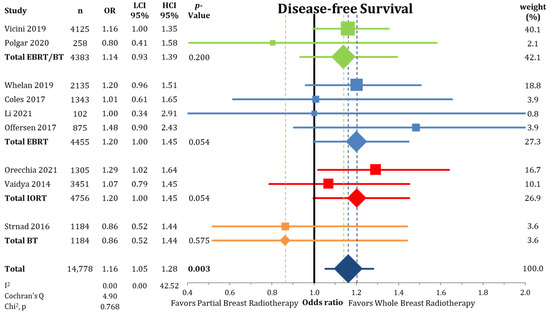
Figure A5.
Forest plot analysis of disease-free survival between partial-breast radiotherapy and whole-breast radiotherapy. The odds ratios for each trial (squares) grouped by radiation technique and the pooled effect sizes (diamonds) with corresponding 95% confidence intervals are displayed. Bold p-values signify statistical difference.
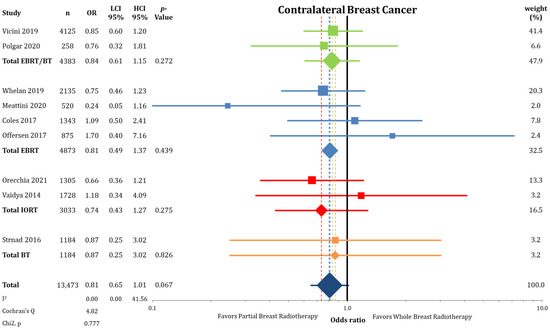
Figure A6.
Analysis of contralateral breast cancer between partial-breast radiotherapy and whole-breast radiotherapy. The odds ratios for each trial (squares) grouped by radiation technique and the pooled effect sizes (diamonds) with corresponding 95% confidence intervals are displayed.
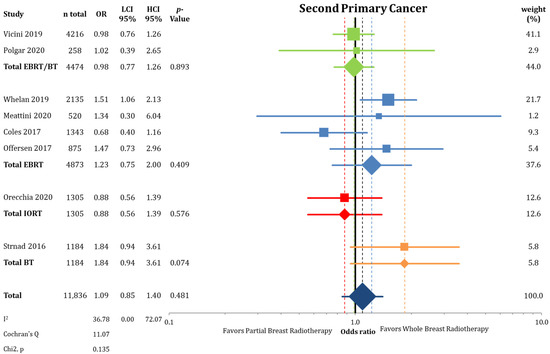
Figure A7.
Comparison of the endpoint second primary cancer between partial-breast radiotherapy and whole-breast radiotherapy using a forest plot. The odds ratios for each trial (squares) are grouped by radiation technique and the pooled effect sizes (diamonds) with corresponding 95% confidence intervals are displayed.
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Veronesi, U.; Marubini, E.; Mariani, L.; Galimberti, V.; Luini, A.; Salvadori, B.; Zucali, R. Radiotherapy after breast-conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann. Oncol. 2001, 12, 997–1003. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Voogd, A.C.; Fentiman, I.S.; Legrand, C.; Sylvester, R.J.; Tong, D.; Van Der Schueren, E.; Helle, P.A.; Van Zijl, K.; Bartelink, H. Long-Term Results of a Randomized Trial Comparing Breast-Conserving Therapy with Mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef]
- Blichert-Toft, M.; Nielsen, M.; Düring, M.; Møller, S.; Rank, F.; Overgaard, M.; Mouridsen, H.T. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008, 47, 672–681. [Google Scholar] [CrossRef]
- Lichter, A.S.; Lippman, M.E.; Danforth, D.N.; D’Angelo, T.; Steinberg, S.M.; Demoss, E.; Macdonald, H.D.; Reichert, C.M.; Merino, M.; Swain, S.M. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J. Clin. Oncol. 1992, 10, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; McGale, P.; Correa, C.R.; Taylor, C.A.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- Williams, L.; Kunkler, I.; King, C.; Jack, W.; Van Der Pol, M. A randomised controlled trial of post-operative radiotherapy following breast-conserving surgery in a minimum-risk population. Quality of life at 5 years in the PRIME trial. Clin. Gov. Int. J. 2011, 16. [Google Scholar] [CrossRef]
- Fyles, A.W.; McCready, D.R.; Manchul, L.A.; Trudeau, M.E.; Merante, P.; Pintilie, M.; Weir, L.M.; Olivotto, I.A. Tamoxifen with or without Breast Irradiation in Women 50 Years of Age or Older with Early Breast Cancer. N. Engl. J. Med. 2004, 351, 963–970. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy Plus Tamoxifen with or Without Irradiation in Women Age 70 Years or Older With Early Breast Cancer: Long-Term Follow-Up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Schnaper, L.A.; Berry, D.; Cirrincione, C.; McCormick, B.; Shank, B.; Wheeler, J.; Champion, L.A.; Smith, T.J.; Smith, B.L. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N. Eng. J. Med. 2004, 351, 971–977. [Google Scholar] [CrossRef]
- Fisher, B.; Bryant, J.; Dignam, J.J.; Wickerham, D.L.; Mamounas, E.P.; Fisher, E.R.; Margolese, R.G.; Nesbitt, L.; Paik, S.; Pisansky, T.M.; et al. Tamoxifen, Radiation Therapy, or Both for Prevention of Ipsilateral Breast Tumor Recurrence after Lumpectomy in Women with Invasive Breast Cancers of One Centimeter or Less. J. Clin. Oncol. 2002, 20, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.-J.; Sauerbrei, W.; Braun, M.; Liersch, T.; Dunst, J.; Guski, H.; Schumacher, M. Radiation therapy and tamoxifen after breast-conserving surgery: Updated results of a 2 × 2 randomised clinical trial in patients with low risk of recurrence. Eur. J. Cancer 2010, 46, 95–101. [Google Scholar] [CrossRef]
- Blamey, R.; Bates, T.; Chetty, U.; Duffy, S.; Ellis, I.; George, D.; Mallon, E.; Mitchell, M.; Monypenny, I.; Morgan, D.; et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur. J. Cancer 2013, 49, 2294–2302. [Google Scholar] [CrossRef]
- Matuschek, C.; Bölke, E.; Haussmann, J.; Mohrmann, S.; Nestle-Krämling, C.; Gerber, P.A.; Corradini, S.; Orth, K.; Kammers, K.; Budach, W. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer—A meta-analysis of randomized trials. Radiat. Oncol. 2017, 12, 60. [Google Scholar] [CrossRef]
- Chesney, T.R.; Yin, J.X.; Rajaee, N.; Tricco, A.C.; Fyles, A.W.; Acuna, S.A.; Scheer, A.S. Tamoxifen with radiotherapy compared with Tamoxifen alone in elderly women with early-stage breast cancer treated with breast conserving surgery: A systematic review and meta-analysis. Radiother. Oncol. 2017, 123, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Wilkinson, R.H.; Miceli, P.N.; Macdonald, W.D. Breast Cancer Experiences with Conservation Therapy. Am. J. Clin. Oncol. 1987, 10, 461–468. [Google Scholar] [CrossRef]
- The Uppsala-Orebro Breast Cancer Study Group Sector Resection with or without Postoperative Radiotherapy for Stage I Breast Cancer: A Randomized Trial. J. Natl. Cancer Inst. 1990, 82, 277–282. [CrossRef]
- Goldstein, N.S.; Kestin, L.; Vicini, F.; Goldstein, N.S.; Kestin, L. Factors Associated with Ipsilateral Breast Failure and Distant Metastases in Patients with Invasive Breast Carcinoma Treated with Breast-Conserving Therapy: A Clinicopathologic Study of 607 Neoplasms From 583 Patients. Am. J. Clin. Pathol. 2003, 120, 500–527. [Google Scholar] [CrossRef]
- Faverly, D.; Holland, R.; Burgers, L. An original stereomicroscopic analysis of the mammary glandular tree. Virchows Archiv 1992, 421, 115–119. [Google Scholar] [CrossRef]
- Haffty, B.G.; Carter, D.; Flynn, S.D.; Fischer, D.B.; Brash, D.E.; Simons, J.; Ziegler, A.; Fischer, J.J. Local recurrence versus new primary: Clinical analysis of 82 breast relapses and potential applications for genetic fingerprinting. Int. J. Radiat. Oncol. 1993, 27, 575–583. [Google Scholar] [CrossRef]
- Imamura, H.; Haga, S.; Shimizu, T.; Watanabe, O.; Kinoshita, J.; Nagumo, H.; Kajiwara, T.; Aiba, M. Relationship between the morphological and biological characteristics of intraductal components accompanying invasive ductal breast carcinoma and patient age. Breast Cancer Res. Treat. 2000, 62, 177–184. [Google Scholar] [CrossRef]
- Ohtake, T.; Abe, R.; Kimijima, I.; Fukushima, T.; Tsuchiya, A.; Hoshi, K.; Wakasa, H. Intraductal extension of primary invasive breast carcinoma treated by breast conservative surgery. Computer graphic three-dimensional reconstruction of the mammary duct-lobular systems. Cancer 1995, 76, 32–45. [Google Scholar] [CrossRef]
- Fisher, E.R.; Sass, R.; Fisher, B.; Gregorio, R.; Brown, R.; Wickerham, L. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). II. Relation of local breast recurrence to multicentricity. Cancer 1986, 57, 1717–1724. [Google Scholar] [CrossRef]
- Liljegren, G.; Holmberg, L.; Bergh, J.; Lindgren, A.; Tabár, L.; Nordgren, H.; Adami, H. 10-Year Results After Sector Resection with or without Postoperative Radiotherapy for Stage I Breast Cancer: A Randomized Trial. J. Clin. Oncol. 1999, 17, 2326. [Google Scholar] [CrossRef]
- Clark, R.M.; Whelan, T.; Levine, M.; Roberts, R.; Willan, A.; McCulloch, P.; Lipa, M.; Wilkinson, R.H.; Mahoney, L.J. Randomized Clinical Trial of Breast Irradiation Following Lumpectomy and Axillary Dissection for Node-Negative Breast Cancer: An Update. J. Natl. Cancer Inst. 1996, 88, 1659–1664. [Google Scholar] [CrossRef]
- Malmström, P.; Holmberg, L.; Anderson, H.; Mattsson, J.; Jönsson, P.-E.; Tennvall-Nittby, L.; Balldin, G.; Lovén, L.; Svensson, J.-H.; Ingvar, C.; et al. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: A randomised clinical trial in a population with access to public mammography screening. Eur. J. Cancer 2003, 39, 1690–1697. [Google Scholar] [CrossRef]
- Holland, R.; Veling, S.H.J.; Mravunac, M.; Hendriks, J.H.C.L. Histologic multifocality of tis, T1–2 breast carcinomas implications for clinical trials of breast-conserving surgery. Cancer 1985, 56, 979–990. [Google Scholar] [CrossRef]
- Magee, B.; Swindell, R.; Harris, M.; Banerjee, S. Prognostic factors for breast recurrence after conservative breast surgery and radiotherapy: Results from a randomised trial. Radiother. Oncol. 1996, 39, 223–227. [Google Scholar] [CrossRef]
- Ribeiro, G.; Magee, B.; Swindell, R.; Harris, M.; Banerjee, S. The christie hospital breast conservation trial: An update at 8 years from inception. Clin. Oncol. 1993, 5, 278–283. [Google Scholar] [CrossRef]
- Haussmann, J.; Budach, W.; Corradini, S.; Krug, D.; Tamaskovics, B.; Bölke, E.; Djiepmo-Njanang, F.-J.; Simiantonakis, I.; Kammers, K.; Matuschek, C. No Difference in Overall Survival and Non-Breast Cancer Deaths after Partial Breast Radiotherapy Compared to Whole Breast Radiotherapy—A Meta-Analysis of Randomized Trials. Cancers 2020, 12, 2309. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.; Barendregt, J.J.; Khan, S.; Thalib, L.; Williams, G. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp. Clin. Trials 2015, 45, 130–138. [Google Scholar] [CrossRef]
- Friedrich, J.O.; Adhikari, N.K.J.; Beyene, J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Methodol. 2007, 7, 5–6. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Saunders, C.; Flyger, H.; Tobias, J.S.; Corica, T.; Massarut, S.; Wenz, F.; Pigorsch, S.; Alvarado, M.; et al. Effect of Delayed Targeted Intraoperative Radiotherapy vs Whole-Breast Radiotherapy on Local Recurrence and Survival: Long-term Results From the TARGIT-A Randomized Clinical Trial in Early Breast Cancer. JAMA Oncol. 2020, 6, e200249. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Keshtgar, M.; Flyger, H.L.; Massarut, S.; Alvarado, M.; Saunders, C.; et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT—A randomised trial. Lancet 2014, 383, 603–613. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Saunders, C.; Brew-Graves, C.; Potyka, I.; Morris, S.; Vaidya, H.J.; et al. An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol. Assess. 2016, 20, 1–188. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT—A randomised clinical trial. BMJ 2020, 370, m2836. [Google Scholar] [CrossRef]
- Polgár, C.; Major, T.; Takácsi-Nagy, Z.; Fodor, J. Breast-Conserving Surgery Followed by Partial or Whole Breast Irradiation: Twenty-Year Results of a Phase 3 Clinical Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef]
- Offersen, B.; Nielsen, H.; Thomsen, M.; Jacobsen, E.; Nielsen, M.; Stenbygaard, L.; Pedersen, A.; Krause, M.; Jensen, M.; Overgaard, J. SP-0315: Partial breast radiotherapy after breast conservation for breast cancer: Early results from the randomised DBCG PBI trial. Radiother. Oncol. 2017, 123, S163–S164. [Google Scholar] [CrossRef]
- Boutrus, R.; El Hossieny, H.; El Sherif, S.; Amin, A.; Bayomy, M.; Hashim, T.; Farahat, A.; El-Sebaie, M. Two-Year Results of Once Daily Accelerated Partial Breast Irradiation: A Randomized Controlled Study. Int. J. Radiat. Oncol. 2018, 102, S81. [Google Scholar] [CrossRef]
- Meattini, I.; Marrazzo, L.; Saieva, C.; Desideri, I.; Scotti, V.; Simontacchi, G.; Bonomo, P.; Greto, D.; Mangoni, M.; Scoccianti, S.; et al. Accelerated Partial-Breast Irradiation Compared with Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 2020, 38, 4175–4183. [Google Scholar] [CrossRef]
- Franceschini, D.; Loi, M.; Chiola, I.; Arculeo, S.; Marzo, M.; Fernandes, B.; Masci, G.; Torrisi, R.; Tinterri, C.; Testori, A.; et al. Preliminary Results of a Randomized Study on Postmenopausal Women with Early Stage Breast Cancer: Adjuvant Hypofractionated Whole Breast Irradiation Versus Accelerated Partial Breast Irradiation (HYPAB Trial). Clin. Breast Cancer 2020. [Google Scholar] [CrossRef]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; Ganz, P.A.; Parda, D.S.; Scheier, M.F.; et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef]
- Whelan, T.J.; Julian, J.A.; Berrang, T.S.; Kim, D.-H.; Germain, I.; Nichol, A.M.; Akra, M.; Lavertu, S.; Germain, F.; Fyles, A.; et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet 2019, 394, 2165–2172. [Google Scholar] [CrossRef]
- Rodríguez, N.; Sanz, X.; Dengra, J.; Foro, P.; Membrive, I.; Reig, A.; Quera, J.; Fernández-Velilla, E.; Pera, Ó.; Lio, J.; et al. Five-Year Outcomes, Cosmesis, and Toxicity With 3-Dimensional Conformal External Beam Radiation Therapy to Deliver Accelerated Partial Breast Irradiation. Int. J. Radiat. Oncol. 2013, 87, 1051–1057. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Joseph, D.J.; Tobias, J.S.; Bulsara, M.; Wenz, F.; Saunders, C.; Alvarado, M.; Flyger, H.L.; Massarut, S.; Eiermann, W.; et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): An international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010, 376, 91–102. [Google Scholar] [CrossRef]
- Vicini, F.; Cecchini, R.; White, J.; Julian, T.; Arthur, D.; Rabinovitch, R.; Kuske, R.; Parda, D.; Ganz, P.; Scheier, M.; et al. Abstract GS4-04: Primary results of NSABP B-39/RTOG 0413 (NRG Oncology): A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. General Session Abstracts 2019, 79, GS4-04. [Google Scholar] [CrossRef]
- White, J.; Winter, K.; Cecchini, R.; Vicini, F.; Arthur, D.; Kuske, R.; Rabinovitch, R.; Sehkon, A.; Khan, A.; Chmura, S.; et al. Cosmetic Outcome from Post Lumpectomy Whole Breast Irradiation (WBI) Versus Partial Breast Irradiation (PBI) on the NRG Oncology/NSABP B39-RTOG 0413 Phase III Clinical Trial. Int. J. Radiat. Oncol. 2019, 105, S3–S4. [Google Scholar] [CrossRef]
- Olivotto, I.A.; Whelan, T.J.; Parpia, S.; Kim, D.-H.; Berrang, T.; Truong, P.T.; Kong, I.; Cochrane, B.; Nichol, A.; Roy, I.; et al. Interim Cosmetic and Toxicity Results From RAPID: A Randomized Trial of Accelerated Partial Breast Irradiation Using Three-Dimensional Conformal External Beam Radiation Therapy. J. Clin. Oncol. 2013, 31, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.; Truong, P.T.; Parpia, S.; Olivotto, I.A.; Berrang, T.; Kim, D.-H.; Kong, I.; Germain, I.; Nichol, A.; Akra, M.; et al. Predictors of Adverse Cosmetic Outcome in the RAPID Trial: An Exploratory Analysis. Int. J. Radiat. Oncol. 2015, 91, 968–976. [Google Scholar] [CrossRef]
- Whelan, T.; Julian, J.; Levine, M.; Berrang, T.; Kim, D.-H.; Gu, C.; Germain, I.; Nichol, A.; Akra, M.; Lavertu, S.; et al. Abstract GS4-03: RAPID: A randomized trial of accelerated partial breast irradiation using 3-dimensional conformal radiotherapy (3D-CRT). Gen. Sess. Abstr. 2019, 79, GS4-03. [Google Scholar] [CrossRef]
- Livi, L.; Buonamici, F.B.; Simontacchi, G.; Scotti, V.; Fambrini, M.; Compagnucci, A.; Paiar, F.; Scoccianti, S.; Pallotta, S.; Detti, B.; et al. Accelerated Partial Breast Irradiation with IMRT: New Technical Approach and Interim Analysis of Acute Toxicity in a Phase III Randomized Clinical Trial. Int. J. Radiat. Oncol. 2010, 77, 509–515. [Google Scholar] [CrossRef]
- Livi, L.; Meattini, I.; Marrazzo, L.; Simontacchi, G.; Pallotta, S.; Saieva, C.; Paiar, F.; Scotti, V.; Cardillo, C.D.L.; Bastiani, P.; et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur. J. Cancer 2015, 51, 451–463. [Google Scholar] [CrossRef]
- Meattini, I.; Saieva, C.; Miccinesi, G.; Desideri, I.; Francolini, G.; Scotti, V.; Marrazzo, L.; Pallotta, S.; Meacci, F.; Muntoni, C.; et al. Accelerated partial breast irradiation using intensity modulated radiotherapy versus whole breast irradiation: Health-related quality of life final analysis from the Florence phase 3 trial. Eur. J. Cancer 2017, 76, 17–26. [Google Scholar] [CrossRef]
- Meattini, I.; Saieva, C.; Lucidi, S.; Russo, M.L.; Scotti, V.; Desideri, I.; Marrazzo, L.; Simontacchi, G.; Mangoni, M.; Becherini, C.; et al. Abstract GS4-06: Accelerated partial breast or whole breast irradiation after breast conservation surgery for patients with early breast cancer: 10-year follow up results of the APBI IMRT Florence randomized phase 3 trial. Gen. Sess. Abstr. 2020, 80, GS4-06. [Google Scholar] [CrossRef]
- Orecchia, R.; Veronesi, U.; Maisonneuve, P.; Galimberti, V.E.; Lazzari, R.; Veronesi, P.; Jereczek-Fossa, B.A.; Cattani, F.; Sangalli, C.; Luini, A.; et al. Intraoperative irradiation for early breast cancer (ELIOT): Long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021, 22, 597–608. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef]
- Andersen, K.G.; Gärtner, R.; Kroman, N.; Flyger, H.; Kehlet, H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: A randomized trial. Breast 2012, 21, 46–49. [Google Scholar] [CrossRef]
- Sperk, E.; Welzel, G.; Keller, A.; Kraus-Tiefenbacher, U.; Gerhardt, A.; Sütterlin, M.; Wenz, F. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: Results from the randomized phase III trial TARGIT A. Breast Cancer Res. Treat. 2012, 135, 253–260. [Google Scholar] [CrossRef]
- Welzel, G.; Boch, A.; Sperk, E.; Hofmann, F.; Kraus-Tiefenbacher, U.; Gerhardt, A.; Suetterlin, M.; Wenz, F. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: Results from the randomized phase III trial TARGIT-A. Radiat. Oncol. 2013, 8, 9. [Google Scholar] [CrossRef]
- Keshtgar, M.R.S.; Williams, N.R.; Bulsara, M.; Saunders, C.; Flyger, H.; Cardoso, J.S.; Corica, T.; Bentzon, N.; Michalopoulos, N.V.; Joseph, D.J. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: Results from a randomised controlled trial. Breast Cancer Res. Treat. 2013, 140, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Corica, T.; Nowak, A.K.; Saunders, C.M.; Bulsara, M.; Taylor, M.; Vaidya, J.S.; Baum, M.; Joseph, D.J. Cosmesis and Breast-Related Quality of Life Outcomes After Intraoperative Radiation Therapy for Early Breast Cancer: A Substudy of the TARGIT-A Trial. Int. J. Radiat. Oncol. 2016, 96, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Corica, T.; Nowak, A.K.; Saunders, C.M.; Bulsara, M.K.; Taylor, M.; Williams, N.R.; Keshtgar, M.; Joseph, D.J.; Vaidya, J.S. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-A Trial. Radiat. Oncol. 2018, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar] [CrossRef]
- Polgár, C.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Strnad, V.; Polgár, C.; Uter, W.; Hildebrandt, G.; Ott, O.J.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; et al. Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol. 2018, 19, 834–844. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Haviland, J.S.; Kirby, A.M.; Kirwan, C.C.; Hopwood, P.; Yarnold, J.R.; Bliss, J.; Coles, C.E.; IMPORT Trialists. Patient-Reported Outcomes Over 5 Years After Whole- or Partial-Breast Radiotherapy: Longitudinal Analysis of the IMPORT LOW (CRUK/06/003) Phase III Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 305–317. [Google Scholar] [CrossRef]
- Bhattacharya, I.S.; Haviland, J.S.; Hopwood, P.; Coles, C.E.; Yarnold, J.R.; Bliss, J.M.; Kirby, A.M.; Trialists, I. Can patient-reported outcomes be used instead of clinician-reported outcomes and photographs as primary endpoints of late normal tissue effects in breast radiotherapy trials? Results from the IMPORT LOW trial. Radiother. Oncol. 2019, 134, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Haviland, J.S.; Perotti, C.; Eaton, D.; Gulliford, S.; Harris, E.; Coles, C.E.; Kirwan, C.C.; Bliss, J.M.; Kirby, A.M.; et al. Is breast seroma after tumour resection associated with patient-reported breast appearance change following radiotherapy? Results from the IMPORT HIGH (CRUK/06/003) trial. Radiother. Oncol. 2019, 136, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Polgár, C.; Major, T.; Fodor, J.; Németh, G.; Orosz, Z.; Sulyok, Z.; Udvarhelyi, N.; Somogyi, A.; Takácsi-Nagy, Z.; Lövey, K.; et al. High-dose-rate brachytherapy alone versus whole breast radiotherapy with or without tumor bed boost after breast-conserving surgery: Seven-year results of a comparative study. Int. J. Radiat. Oncol. 2004, 60, 1173–1181. [Google Scholar] [CrossRef]
- Polgár, C.; Fodor, J.; Major, T.; Németh, G.; Lövey, K.; Orosz, Z.; Sulyok, Z.; Takácsi-Nagy, Z.; Kásler, M. Breast-Conserving Treatment with Partial or Whole Breast Irradiation for Low-Risk Invasive Breast Carcinoma—5-Year Results of a Randomized Trial. Int. J. Radiat. Oncol. 2007, 69, 694–702. [Google Scholar] [CrossRef]
- Lövey, K.; Fodor, J.; Major, T.; Szabó, É.; Orosz, Z.; Sulyok, Z.; Jánváry, L.; Fröhlich, G.; Kásler, M.; Polgár, C. Fat Necrosis after Partial-Breast Irradiation with Brachytherapy or Electron Irradiation Versus Standard Whole-Breast Radiotherapy—4-Year Results of a Randomized Trial. Int. J. Radiat. Oncol. 2007, 69, 724–731. [Google Scholar] [CrossRef]
- Polgár, C.; Fodor, J.; Major, T.; Sulyok, Z.; Kásler, M. Breast-conserving therapy with partial or whole breast irradiation: Ten-year results of the Budapest randomized trial. Radiother. Oncol. 2013, 108, 197–202. [Google Scholar] [CrossRef]
- Polgár, C.; Major, T.; Sulyok, Z.; Takácsi-Nagy, Z.; Fodor, J. Long-Term Toxicity and Cosmetic Results of Partial Versus Whole Breast Irradiation: 10-Year Results of a Phase III APBI Trial. Int. J. Radiat. Oncol. 2014, 90, S133–S134. [Google Scholar] [CrossRef]
- Li, X.; Sanz, J.; Foro, P.; Martínez, A.; Zhao, M.; Reig, A.; Liu, F.; Huang, Y.; Membrive, I.; Algara, M.; et al. Long-term results of a randomized partial irradiation trial compared to whole breast irradiation in the early stage and low-risk breast cancer patients after conservative surgery. Clin. Transl. Oncol. 2021. [Google Scholar] [CrossRef]
- Gujral, D.M.; Sumo, G.; Owen, J.R.; Ashton, A.; Bliss, J.M.; Haviland, J.; Yarnold, J.R. Ipsilateral Breast Tumor Relapse: Local Recurrence Versus New Primary Tumor and the Effect of Whole-Breast Radiotherapy on the Rate of New Primaries. Int. J. Radiat. Oncol. 2011, 79, 19–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mannino, M.; Yarnold, J. Accelerated partial breast irradiation trials: Diversity in rationale and design. Radiother. Oncol. 2009, 91, 16–22. [Google Scholar] [CrossRef]
- Jagsi, R.; Chadha, M.; Moni, J.; Ballman, K.; Laurie, F.; Buchholz, T.A.; Giuliano, A.; Haffty, B.G. Radiation Field Design in the ACOSOG Z0011 (Alliance) Trial. J. Clin. Oncol. 2014, 32, 3600–3606. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.; Stachs, A.; Gerber, B.; Potenberg, J.; Krug, D.; Wolter, K.; Kühn, T.; Zierhut, D.; Sedlmayer, F.; Kaiser, J.; et al. Central Review of Radiation Therapy Planning Among Patients with Breast-Conserving Surgery: Results from a Quality Assurance Process Integrated into the INSEMA Trial. Int. J. Radiat. Oncol. 2020, 107, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Ramin, C.; Withrow, D.; Lynn, B.D.; Gierach, G.; De González, A.B. Contralateral breast cancer risk according to first breast cancer characteristics among United States women from 1992 to 2015. J. Clin. Oncol. 2019, 37, 1549. [Google Scholar] [CrossRef]
- Cheung, K.J.; Davidson, N.E. Double Trouble: Contralateral Breast Cancer Risk Management in the Modern Era. J. Natl. Cancer Inst. 2019, 111, 641–643. [Google Scholar] [CrossRef]
- Kirby, A.M. Updated ASTRO guidelines on accelerated partial breast irradiation (APBI): To whom can we offer APBI outside a clinical trial? Br. J. Radiol. 2018, 91, 20170565. [Google Scholar] [CrossRef]
- Shah, C.; Badiyan, S.; Ben Wilkinson, J.; Vicini, F.; Beitsch, P.; Keisch, M.; Arthur, D.; Lyden, M. Treatment Efficacy with Accelerated Partial Breast Irradiation (APBI): Final Analysis of the American Society of Breast Surgeons MammoSite® Breast Brachytherapy Registry Trial. Ann. Surg. Oncol. 2013, 20, 3279–3285. [Google Scholar] [CrossRef]
- Strnad, V.; Major, T.; Polgar, C.; Lotter, M.; Guinot, J.-L.; Gutierrez-Miguelez, C.; Galalae, R.; Van Limbergen, E.; Guix, B.; Niehoff, P.; et al. ESTRO-ACROP guideline: Interstitial multi-catheter breast brachytherapy as Accelerated Partial Breast Irradiation alone or as boostx—GEC-ESTRO Breast Cancer Working Group practical recommendations. Radiother. Oncol. 2018, 128, 411–420. [Google Scholar] [CrossRef]
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract. Radiat. Oncol. 2017, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Vicini, F.; Shaitelman, S.F.; Hepel, J.; Keisch, M.; Arthur, D.; Khan, A.J.; Kuske, R.; Patel, R.; Wazer, D.E. The American Brachytherapy Society consensus statement for accelerated partial-breast irradiation. Brachytherapy 2018, 17, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Ciervide, R.; Dhage, S.; Guth, A.; Shapiro, R.L.; Axelrod, D.; Roses, D.F.; Formenti, S.C. Five Year Outcome of 145 Patients with Ductal Carcinoma In Situ (DCIS) After Accelerated Breast Radiotherapy. Int. J. Radiat. Oncol. 2012, 83, e159–e164. [Google Scholar] [CrossRef]
- Shah, C.; McGee, M.; Ben Wilkinson, J.; Berry, S.; Grills, I.; Wallace, M.; Mitchell, C.; Vicini, F. Clinical Outcomes Using Accelerated Partial Breast Irradiation in Patients with Ductal Carcinoma In Situ. Clin. Breast Cancer 2012, 12, 259–263. [Google Scholar] [CrossRef]
- Vicini, F.; Shah, C.; Ben Wilkinson, J.; Keisch, M.; Beitsch, P.; Lyden, M. Should Ductal Carcinoma-in-situ (DCIS) Be Removed from the ASTRO Consensus Panel Cautionary Group for Off-protocol Use of Accelerated Partial Breast Irradiation (APBI)? A Pooled Analysis of Outcomes for 300 Patients with DCIS Treated with APBI. Ann. Surg. Oncol. 2013, 20, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Schneider, F.; Ma, L.; Wenz, F.; Herskind, C. Relative Biologic Effectiveness (RBE) of 50 kV X-rays Measured in a Phantom for Intraoperative Tumor-Bed Irradiation. Int. J. Radiat. Oncol. 2013, 85, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.G.F.; Bekerat, H.; Papaconstadopoulos, P.; Davis, S.; Seuntjens, J. An investigation into the INTRABEAM miniature x-ray source dosimetry using ionization chamber and radiochromic film measurements. Med Phys. 2018, 45, 4274–4286. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.; Cameron, D.A.; Dixon, M. Abstract GS2-03: Prime 2 randomised trial (postoperative radiotherapy in minimum-risk elderly): Wide local excision and adjuvant hormonal therapy +/- whole breast irradiation in women =/> 65 years with early invasive breast cancer: 10 year results. Gen. Ses. Abstr. 2021, 81, GS2-03. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Brunt, A.M.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.-C.; et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar] [CrossRef]
- Romestaing, P.; Lehingue, Y.; Carrie, C.; Coquard, R.; Montbarbon, X.; Ardiet, J.M.; Mamelle, N.; Gérard, J.P. Role of a 10-Gy boost in the conservative treatment of early breast cancer: Results of a randomized clinical trial in Lyon, France. J. Clin. Oncol. 1997, 15, 963–968. [Google Scholar] [CrossRef]
- Killander, F.; Karlsson, P.; Anderson, H.; Mattsson, J.; Holmberg, E.; Lundstedt, D.; Malmström, P. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen-year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur. J. Cancer 2016, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Polgár, C.; Strnad, V.; Kovács, G. Partial-Breast Irradiation or Whole-Breast Radiotherapy for Early Breast Cancer: A Meta-Analysis of Randomized Trials. Strahlenther. Onkol. 2010, 186, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Mauri, D.; Polyzos, N.P.; Mavroudis, D.; Georgoulias, V.; Casazza, G. Partial Breast Irradiation or Whole Breast Radiotherapy for Early Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Breast J. 2010, 16, 245–251. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Wenz, F.; Coombs, N.; Singer, J.; Ebbs, S.; Massarut, S.; Saunders, C.; Douek, M.; Williams, N.R.; et al. Reduced Mortality with Partial-Breast Irradiation for Early Breast Cancer: A Meta-Analysis of Randomized Trials. Int. J. Radiat. Oncol. 2016, 96, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hickey, B.; Lehman, M.; Francis, D.P.; See, A.M. Partial breast irradiation for early breast cancer. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Marta, G.N.; Macedo, C.R.; Carvalho, H.D.A.; Hanna, S.A.; Da Silva, J.L.F.; Riera, R. Accelerated partial irradiation for breast cancer: Systematic review and meta-analysis of 8653 women in eight randomized trials. Radiother. Oncol. 2015, 114, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Cheng, J.; Ding, X.; Li, B.; Zhang, J.; Li, H.; Huang, W.; Zhou, T.; Sun, H. Efficacy and Safety of Accelerated Partial Breast Irradiation after Breast-conserving Surgery: A Meta-analysis of Published Comparative Studies. Breast J. 2013, 20, 116–124. [Google Scholar] [CrossRef]
- Ye, X.-P.; Bao, S.; Guo, L.-Y.; Wang, C.-H.; Ma, Y.-P.; Wei, Z.; Chun-Hua, W.; Yan-Fang, Z.; Zhi, F.; Gao, Y.; et al. Accelerated Partial Breast Irradiation for Breast Cancer: A Meta-Analysis. Transl. Oncol. 2013, 6, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Korzets, Y.; Fyles, A.; Shepshelovich, D.; Amir, E.; Goldvaser, H. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 175, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Viani, G.A.; Arruda, C.V.; Faustino, A.C.; De Fendi, L.I. Partial-breast irradiation versus whole-breast radiotherapy for early breast cancer: A systematic review and update meta-analysis. Brachytherapy 2020, 19, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Jia, X.; Hobbs, B.P.; Tendulkar, R.D.; Sittenfeld, S.M.C.; Al-Hilli, Z.; Arthur, D.W.; Keisch, M.E.; Khan, A.J.; Shaitelman, S.F.; et al. Outcomes with Partial Breast Irradiation vs. Whole Breast Irradiation: A Meta-Analysis. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).