In Vivo Evaluation of Dual-Targeted Nanoparticles Encapsulating Paclitaxel and Everolimus
Abstract
1. Introduction
2. Results
2.1. Preparation and Characterization of Dual-NPs
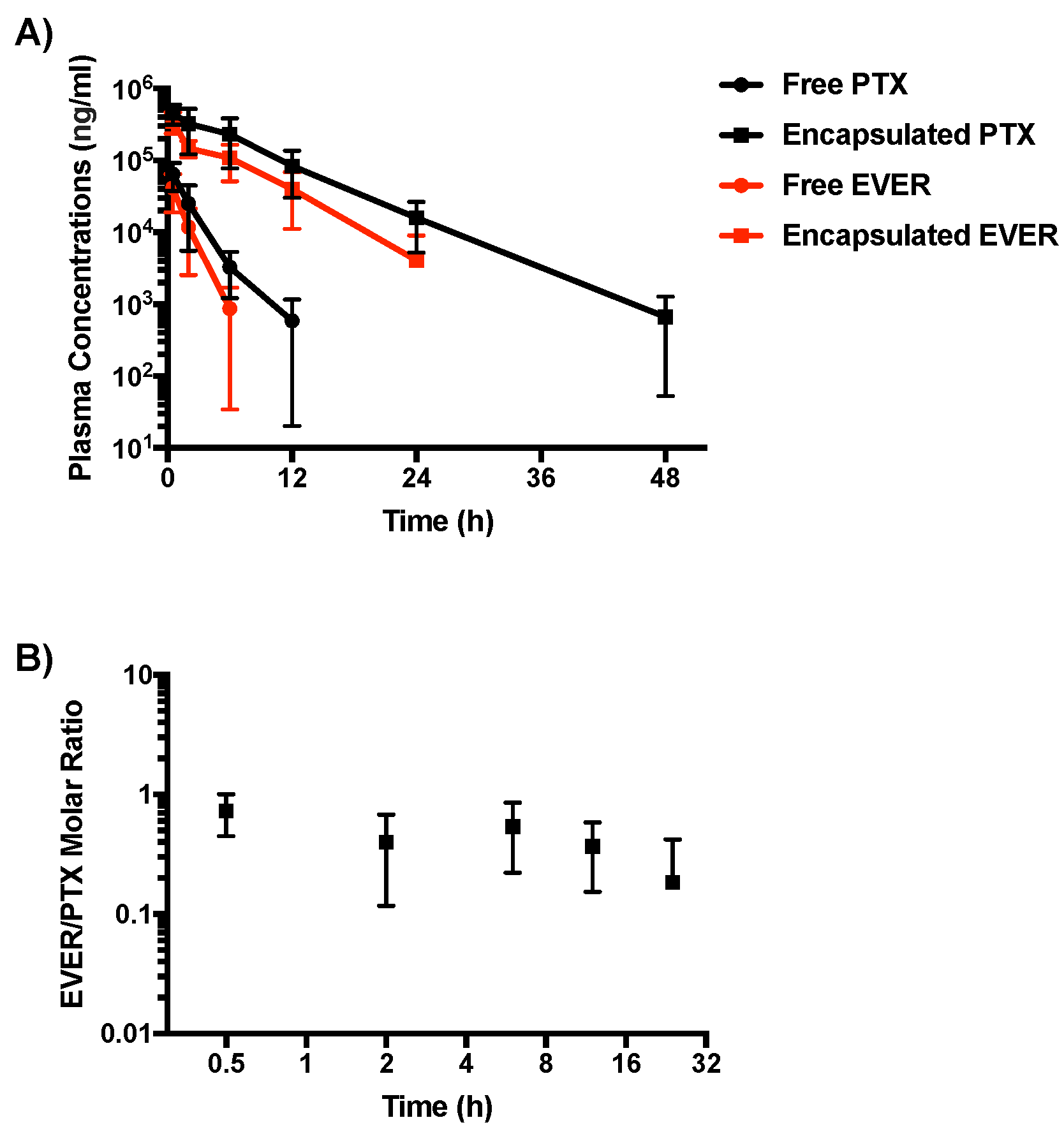
2.2. Pharmacokinetic Study
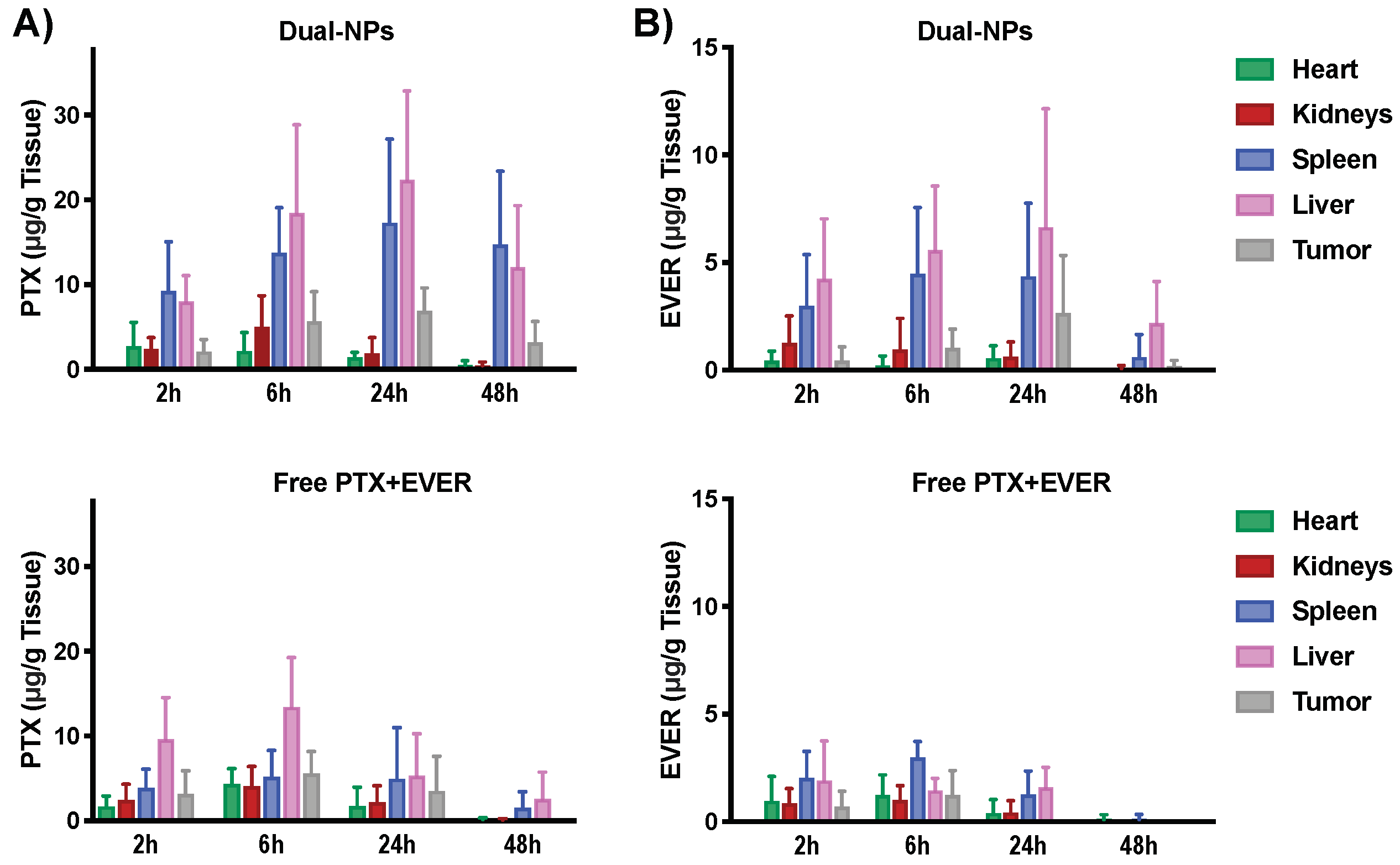
2.3. Biodistribution Study
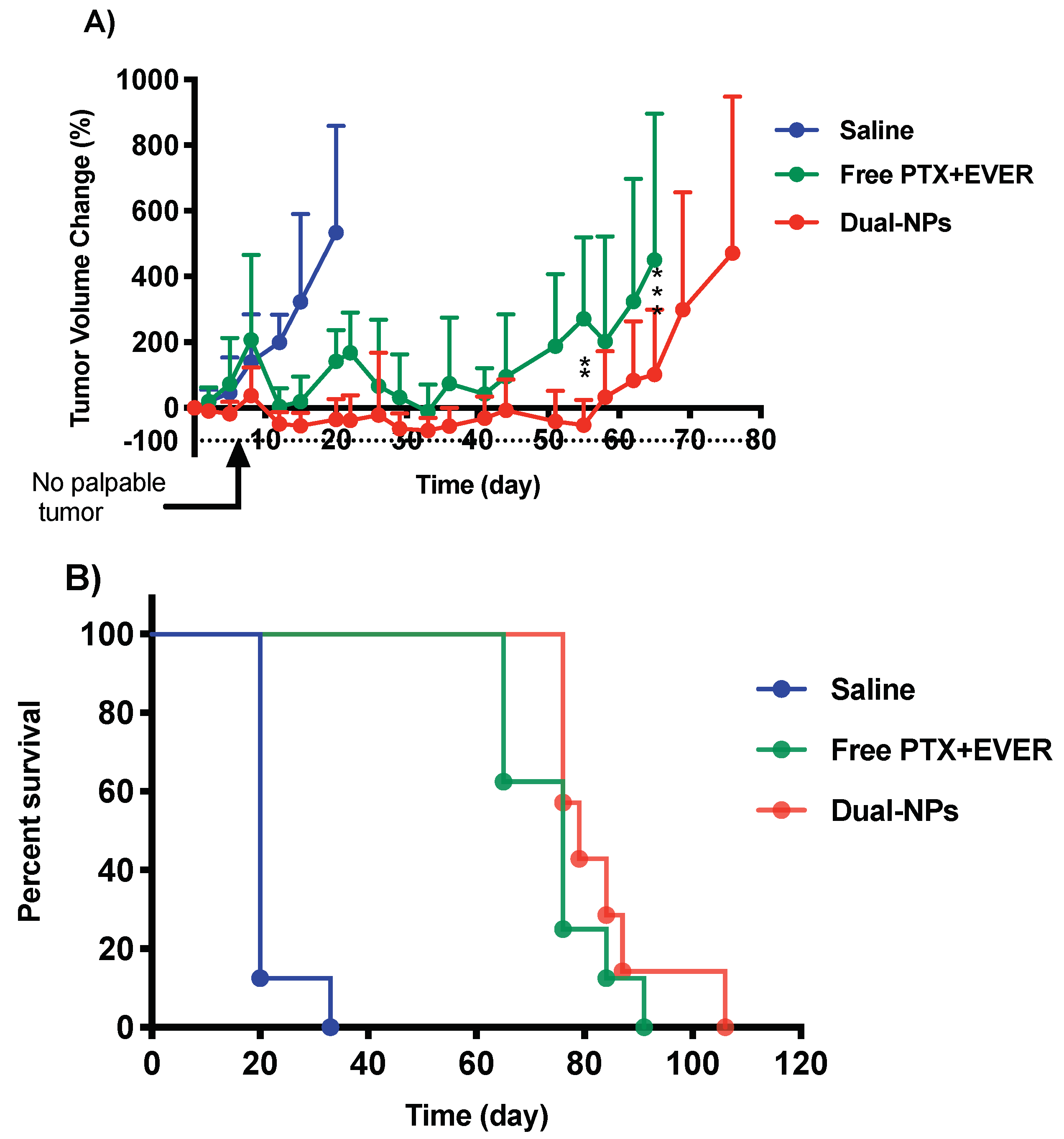
2.4. Efficacy Studies
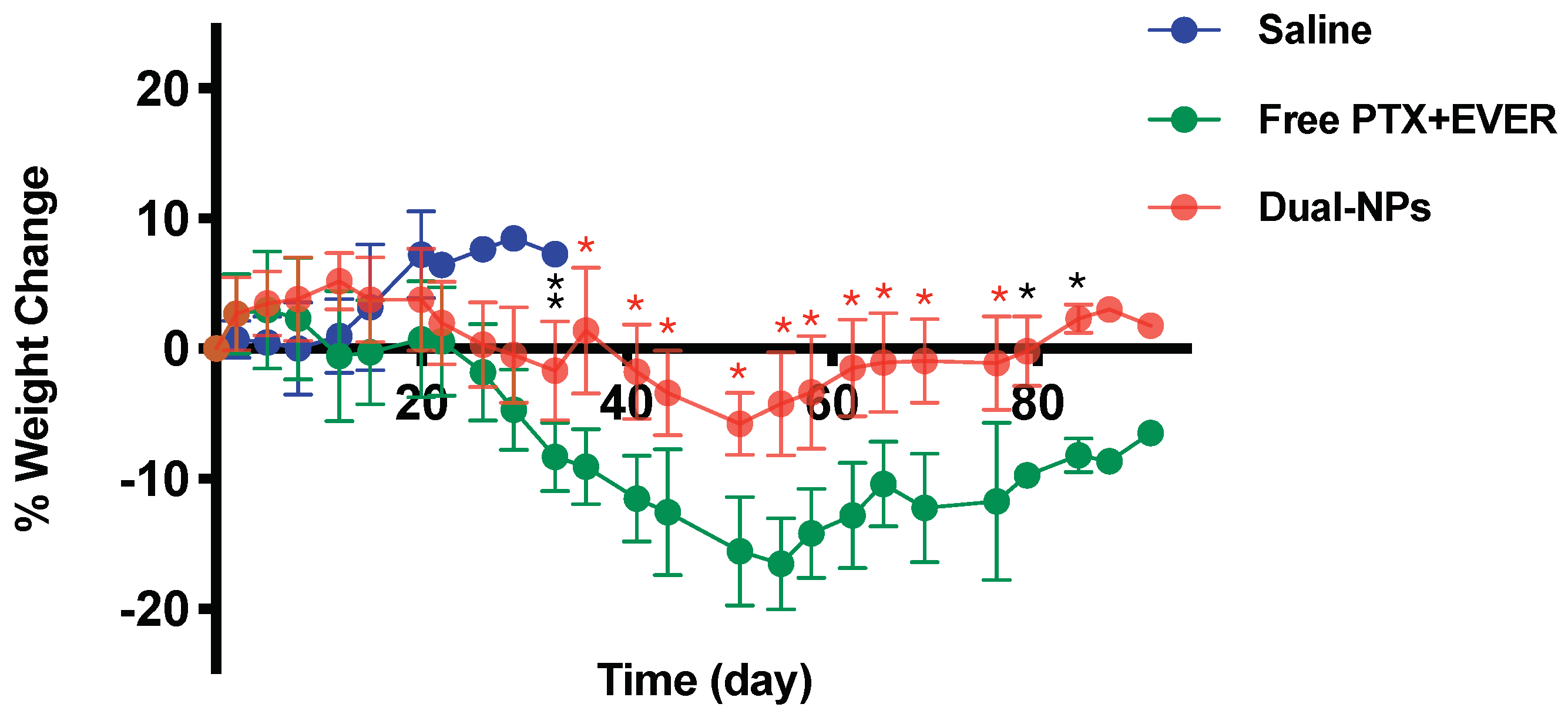
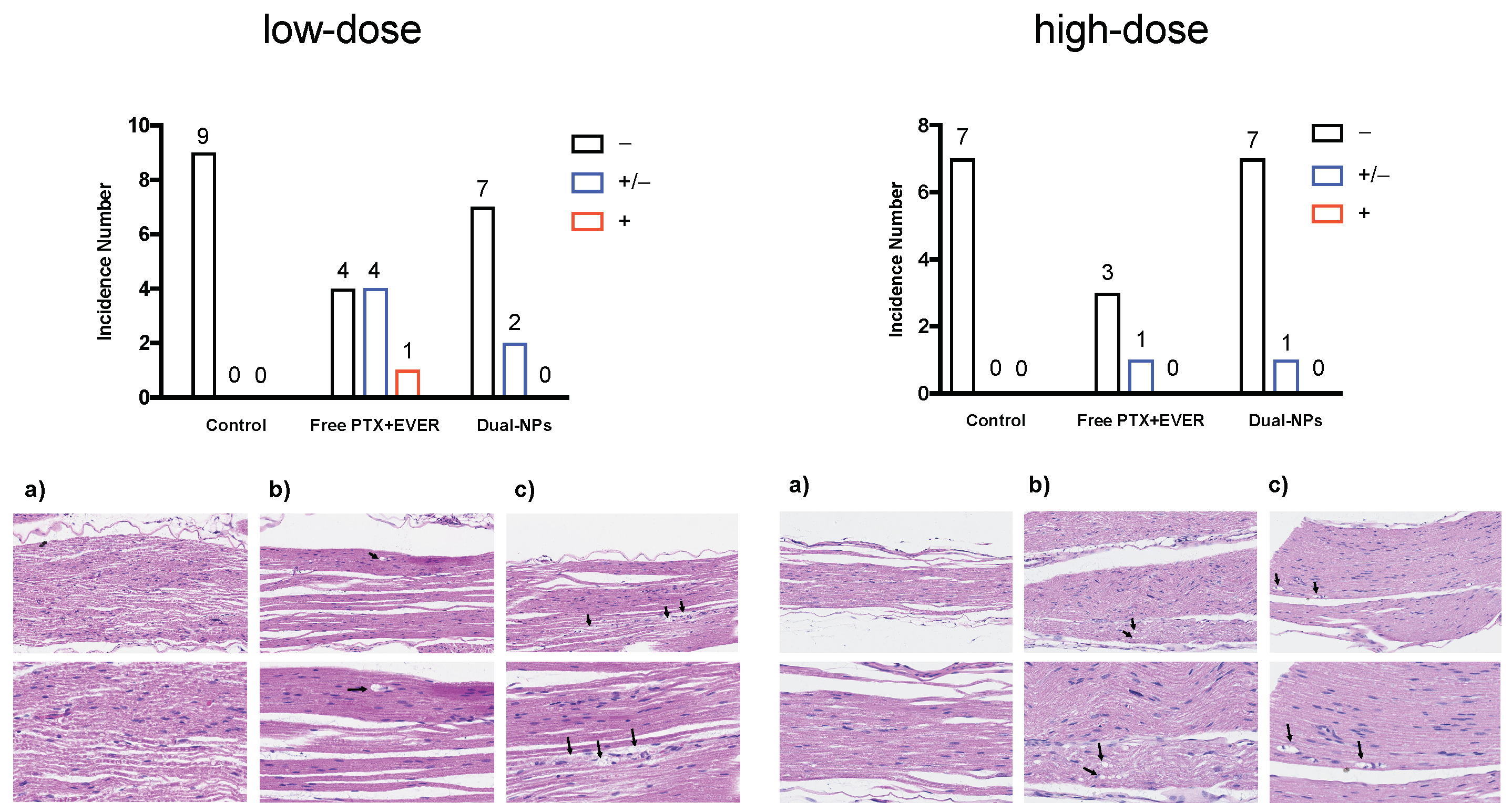
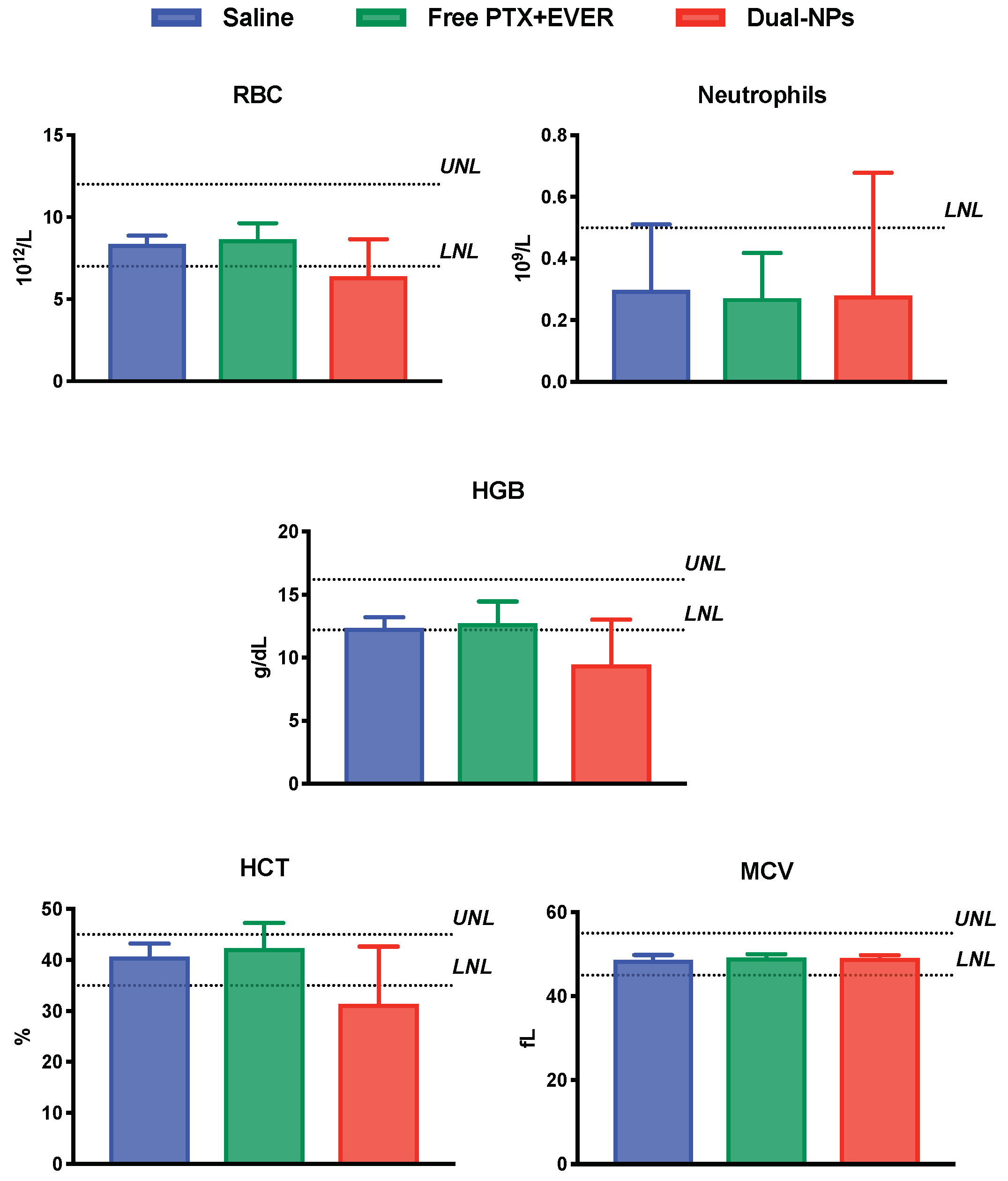
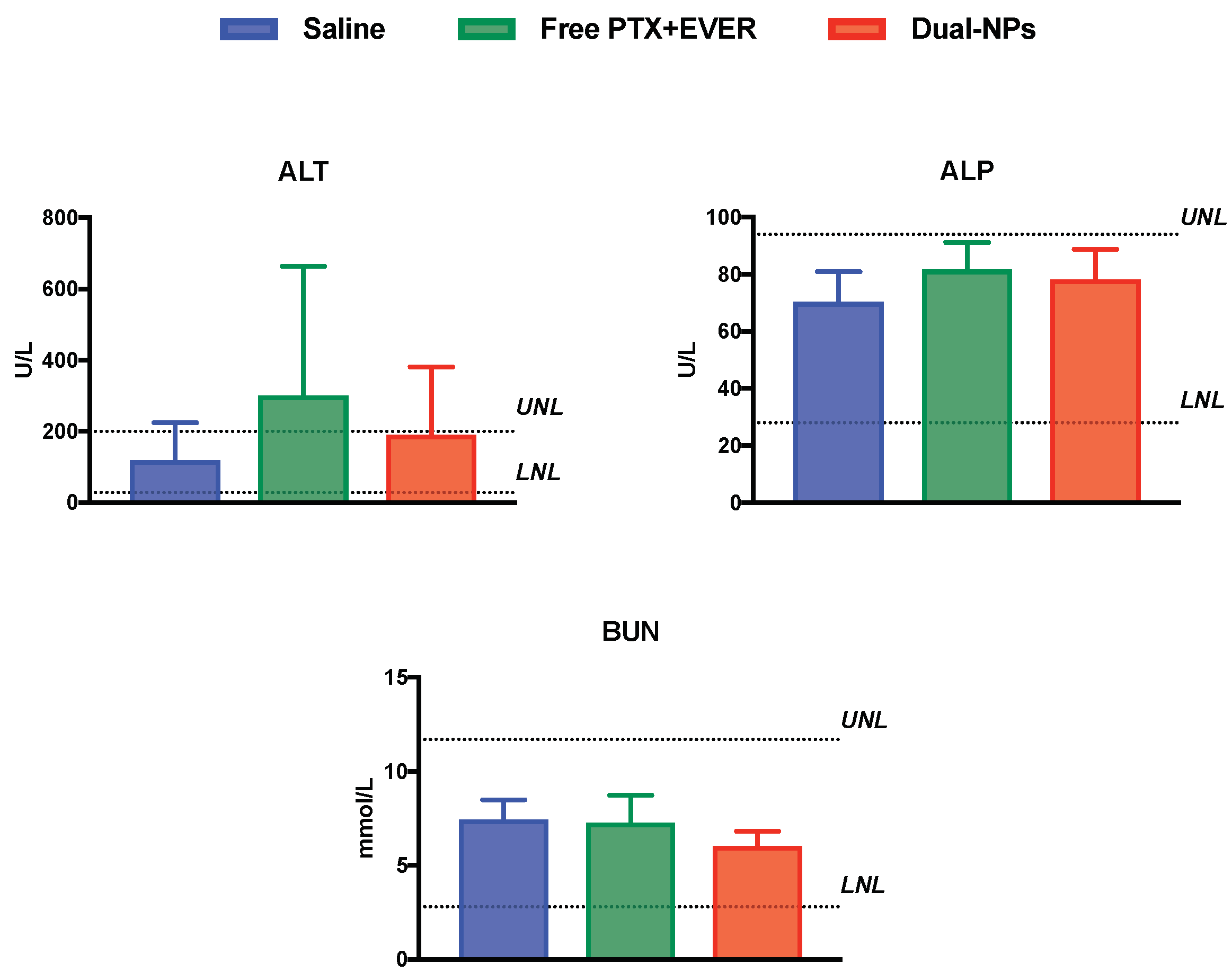
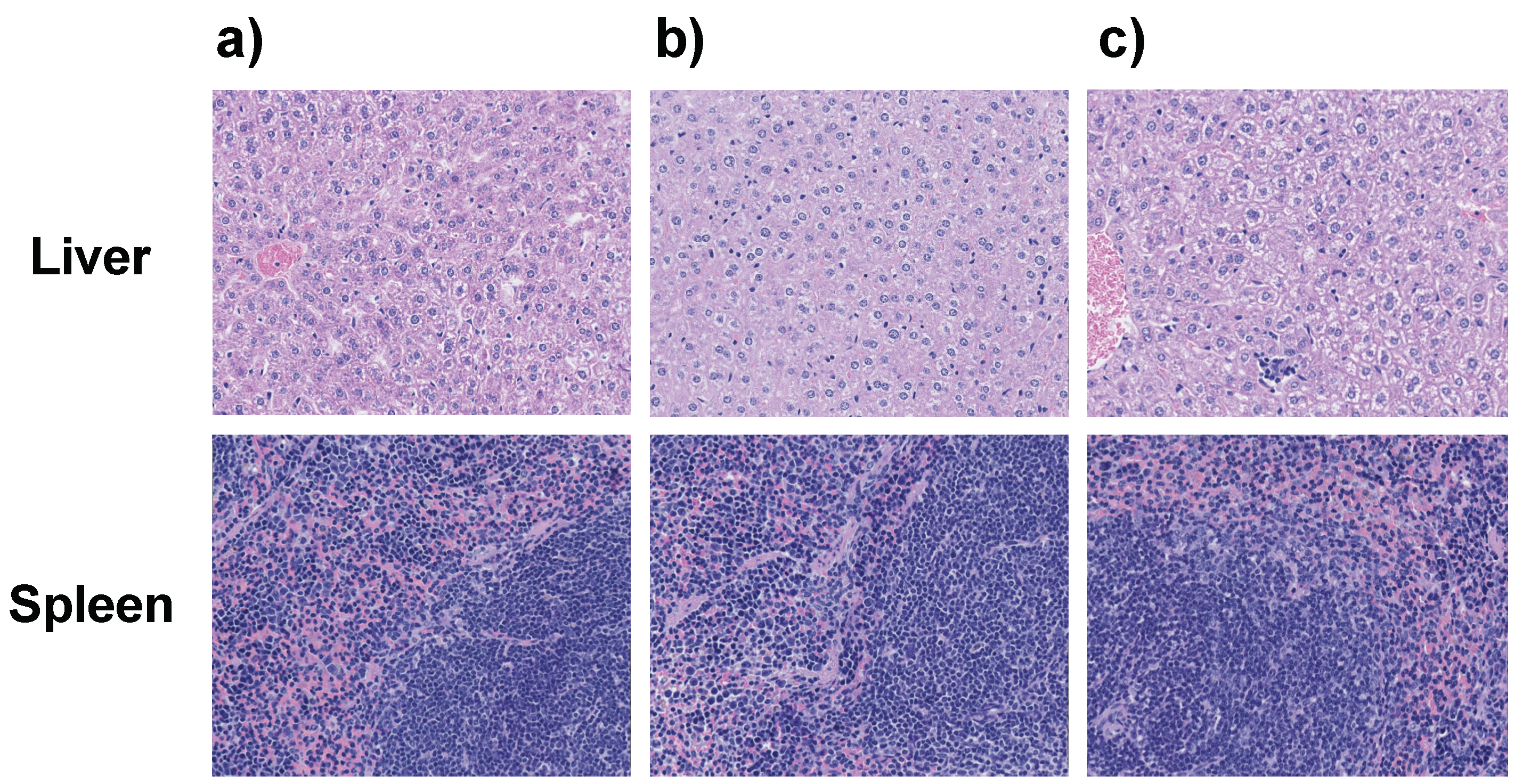
2.5. Toxicity Studies
3. Discussion
4. Methods
4.1. Tumor Cells
4.2. Animals and Tumor Model
4.3. Preparation of Dual-NPs
4.4. Pharmacokinetic Study
4.5. Tumor Inoculation
4.6. Biodistribution Study
4.7. Efficacy Studies
4.8. Toxicity Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Hidalgoa, M.; Sánchez-Morenob, C.; Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Chou, T.; Talalay, P. Quantitative Dose-effect relationships: The combined effects of multiple drug or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Mignani, S.; Bryszewska, M.; Klajnert-Maculewicz, B.; Zablocka, M.; Majoral, J.P. Advances in combination therapies based on nanoparticles for efficacious cancer treatment: An analytical report. Biomacromolecules 2015, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jadia, R.; Scandore, C.; Rai, P. Nanoparticles for Effective Combination Therapy of Cancer. Int. J. Nanotechnol. Nanomed. 2016, 1. Available online: http://www.opastonline.com/wp-content/uploads/2016/10/nanoparticles-for-effective-combination-therapy-of-cancer-ijnn-16-003.pdf. (accessed on 12 November 2017).
- Houdaihed, L.; Evans, J.C.; Allen, C. Codelivery of Paclitaxel and Everolimus at the Optimal Synergistic Ratio: A Promising Solution for the Treatment of Breast Cancer. Mol. Pharm. 2018, 15, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Akcakanat, A.; Liu, S.; Green, M.C.; Murray, J.L.; Chen, H.; Palla, S.L.; Koenig, K.B.; Brewster, A.M.; Valero, V.; et al. Open-label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor-negative breast cancer. Ann. Oncol. 2014, 25, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; Levy, V.; Bourbouloux, E.; Berton Rigaud, D.; Bootle, D.; Dutreix, C.; Zoellner, U.; Shand, N.; Calvo, F.; Raymond, E. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br. J. Cancer 2009, 100, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Approved Drugs—FDA Approves Liposome-Encapsulated Combination of Daunorubicin-Cytarabine for Adults with Some Types of Poor Prognosis AML. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm569950.htm (accessed on 23 October 2017).
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.; Allen, C. Dual-Targeted Delivery of Nanoparticles Encapsulating Paclitaxel and Everolimus: A Novel Strategy to Overcome Breast Cancer Receptor Heterogeneity. 2019. under review. [Google Scholar]
- Van der Meel, R.; Vehmeijer, L.J.C.; Kok, R.J.; Storm, G.; van Gaal, E.V.B. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Lux, M.; Nabieva, N.; Hartkopf, A.D.; Huober, J.; Volz, B.; Taran, F.-A.; Overkamp, F.; Kolberg, H.-C.; Hadji, P.; Tesch, H.; et al. Therapy Landscape in Patients with Metastatic HER2-Positive Breast Cancer: Data from the PRAEGNANT Real-World Breast Cancer Registry. Cancers 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanna, M.P.; Stern, D.F.; Edgerton, S.M.; Whalen, S.G.; Moore, D.; Thor, A.D. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J. Clin. Oncol. 2005, 23, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Remick, D.; Nemzek, J.A.; Bolgos, G.L.; Williams, B.A.; Remick, D.G. Differences in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflamm. Res. 2001, 50, 523–527. [Google Scholar]
- Quimby, F.H.; Goff, L.G. Effect of Source of Blood Sample on Total White Cell Count of the Rat. Am. J. Physiol. 1952, 170, 196–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Musacchio, T.; Laquintana, V.; Latrofa, A.; Trapani, G.; Torchilin, V. PEG-PE micelles loaded with paclitaxel and surface-modified by a PBR-ligand: Synergistic anticancer effect. Mol. Pharm. 2009, 6, 468–479. [Google Scholar] [CrossRef]
- Abouzeid, A.H.; Patel, N.R.; Torchilin, V.P. Polyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin e micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancer. Int. J. Pharm. 2014, 464, 178–184. [Google Scholar] [CrossRef]
- Averineni, R.; Shavi, G.V.; Gurram, A.K.; Deshpande, P.B.; Arumugam, K.; Maliyakkal, N.; Meka, S.R.; Nayanabhirama, U. PLGA 50:50 nanoparticles of paclitaxel: Development, in vitro anti-tumor activity in BT-549 cells and in vivo evaluation. Bull. Mater. Sci. 2010, 35, 319–326. [Google Scholar] [CrossRef]
- Hrkach, J.; Von Hoff, D.; Mukkaram Ali, M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Kim, S.C.; Kima, D.W.; Shima, Y.H.; Banga, J.S.; Oh, H.S.; Kim, S.W.; Seoa, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control Release 2001, 72, 191–202. [Google Scholar] [CrossRef]
- Chen, H.; Kim, S.W.; He, W.; Wang, H.F.; Low, P.S.; Park, K.; Cheng, J.X. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo Forster resonance energy transfer imaging. Langmuir 2008, 24, 5213–5217. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a Cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Beletsi, A.; Panagi, Z.; Avgoustakis, K. Biodistribution properties of nanoparticles based on mixtures of PLGA with PLGA-PEG diblock copolymers. Int. J. Pharm. 2005, 298, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Koopaei, M.N.; Khoshayand, M.R.; Mostafavi, S.H.; Amini, M.; Khorramizadeh, M.R.; Tehrani, M.J.; Atyabi, F.; Dinarvanda, R. Docetaxel Loaded PEG-PLGA Nanoparticles: Optimized Drug Loading, In-vitro Cytotoxicity and In-vivo Antitumor Effect. Iran. J. Pharm. Res. 2014, 13, 819–833. [Google Scholar]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Nielsen, U.B.; Kirpotin, D.B.; Poul, M.; Marks, J.D.; Benz, C.C. Biological Effects of Anti-ErbB2 Single Chain Antibodies Selected for Internalizing Function. Biochem. Biophys. Res. Commun. 2001, 280, 274–279. [Google Scholar] [CrossRef]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef]
- Carstens, M.G.; de Jonga, P.; van Nostrum, C.F.; Kemmink, J.; Verrijk, R.; de Leede, L.; Crommelin, D.; Hennink, W.E. The effect of core composition in biodegradable oligomeric micelles as taxane formulations. Eur. J. Pharm. Biopharm. 2008, 68, 596–606. [Google Scholar] [CrossRef]
- Harrington, K.J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective Targeting of Solid Tumors in Patients With Locally Advanced Cancers by Radiolabeled Pegylated Liposomes Effective Targeting of Solid Tumors in Patients With Locally Advanced Cancers by Radiolabeled Pegylated Liposomes. Clin. Cancer Res. 2001, 7, 243–254. [Google Scholar]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Scripture, C.D.; Figg, W.D.; Sparreboom, A. Peripheral Neuropathy Induced by Paclitaxel: Recent Insights and Future Perspectives. Curr. Neuropharmacol. 2006, 4, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Waseem, A.; Rao, R.R.; Agarwal, A.; Saha, R.; Bajpai, P.; Qureshi, S.; Mittal, A. Incidence of Neuropathy with Weekly Paclitaxel and Role of Oral Glutamine Supplementation for Prevention of Paclitaxel Induced Peripheral Neuropathy Randomized Controlled Trial. Indian J. Med. Paediatr. Oncol. 2018, 39, 339–348. [Google Scholar]
- Houdaihed, L.; Evans, J.C.; Allen, C. Overcoming the Road Blocks: Advancement of Block Copolymer Micelles for Cancer Therapy in the Clinic. Mol. Pharm. 2017, 14, 2503–2517. [Google Scholar] [CrossRef] [PubMed]
- Razumienko, E.; Dryden, L.; Scollard, D.; Reilly, R.M. MicroSPECT/CT Imaging of Co-Expressed HER2 and EGFR on Subcutaneous Human Tumor Xenografts in Athymic Mice Using 111In-Labeled Bispecific Radioimmunoconjugates. Breast Cancer Res. Treat. 2013, 138, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Reilly, R.M.; Allen, C. Block Copolymer Micelles Target Auger Electron Radiotherapy to the Nucleus of HER2-Positive Breast Cancer Cells. Biomacromolecules 2012, 13, 455–465. [Google Scholar] [CrossRef]
- Gao, J.; Kou, G.; Wang, H.; Chen, H.; Li, B.; Lu, Y.; Zhang, D.; Wang, S.; Hou, S.; Qian, W.; et al. PE38KDEL-loaded anti-HER2 nanoparticles inhibit breast tumor progression with reduced toxicity and immunogenicity. Breast Cancer Res. Treat. 2009, 115, 29–41. [Google Scholar] [CrossRef]
- Sparreboom, A.; van Tellingen, O.; Nooijen, W.J.; Beijnen, J.H. Nonlinear Pharmacokinetics of Paclitaxel in Mice Results from the Pharmaceutical Vehicle Cremophor EL. Cancer Res. 1996, 56, 2112–2115. [Google Scholar]

| Formulation | PTX:EVER LC (wt%) a | PTX (mg/mL) | EVER (mg/mL) | PTX:EVER Molar Ratio | Size (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| Dual-NPs | 5.6 ± 1.1 | 1.2 ± 0.2 | 0.5 ± 0.1 | 1:0.37 | 101.3 ± 6.3 | −11.3 ± 2.1 |
| Treatment | Cmax (µg/mL) | T1/2z (h) | AUC0–t (µg h mL−1) | AUC0–inf. (µg h mL−1) | CLtot (mL h kg−1) | Vd (mL kg−1) | |
|---|---|---|---|---|---|---|---|
| Free Combination | PTX | 64.91 | 1.88 | 170.49 a | 172.09 | 81.35 | 222.52 |
| EVER | 38.41 | 1.02 | 83.6 b | 84.88 | 88.35 | 131.73 | |
| Dual-NPs | PTX | 458.03 | 5.17 | 3666.65 c | 3671.59 | 3.81 | 28.47 |
| EVER | 351.25 | 3.78 | 1792.52 d | 1814.83 | 4.13 | 22.85 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houdaihed, L.; Evans, J.C.; Allen, C. In Vivo Evaluation of Dual-Targeted Nanoparticles Encapsulating Paclitaxel and Everolimus. Cancers 2019, 11, 752. https://doi.org/10.3390/cancers11060752
Houdaihed L, Evans JC, Allen C. In Vivo Evaluation of Dual-Targeted Nanoparticles Encapsulating Paclitaxel and Everolimus. Cancers. 2019; 11(6):752. https://doi.org/10.3390/cancers11060752
Chicago/Turabian StyleHoudaihed, Loujin, James Christopher Evans, and Christine Allen. 2019. "In Vivo Evaluation of Dual-Targeted Nanoparticles Encapsulating Paclitaxel and Everolimus" Cancers 11, no. 6: 752. https://doi.org/10.3390/cancers11060752
APA StyleHoudaihed, L., Evans, J. C., & Allen, C. (2019). In Vivo Evaluation of Dual-Targeted Nanoparticles Encapsulating Paclitaxel and Everolimus. Cancers, 11(6), 752. https://doi.org/10.3390/cancers11060752






