Abstract
Background: The use of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) for biochemical recurrent prostate cancer (BRPCa) is increasing worldwide. Recently, 18F-labeled PSMA agents have become available. We performed a systematic review and meta-analysis regarding the detection rate (DR) of 18F-labeled PSMA PET/CT in BRPCa to provide evidence-based data in this setting. Methods: A comprehensive literature search of PubMed/MEDLINE, EMBASE, and Cochrane Library databases through 23 April 2019 was performed. Pooled DR was calculated on a per-patient basis, with pooled proportion and 95% confidence interval (95% CI). Furthermore, pooled DR of 18F-PSMA PET/CT using different cut-off values of prostate-specific antigen (PSA) was obtained. Results: Six articles (645 patients) were included in the meta-analysis. The pooled DR of 18F-labeled PSMA PET/CT in BRPCa was 81% (95% CI: 71–88%). The pooled DR was 86% for PSA ≥ 0.5 ng/mL (95% CI: 78–93%) and 49% for PSA < 0.5 ng/mL (95% CI: 23–74%). Statistical heterogeneity was found. Conclusions: 18F-labeled PSMA PET/CT demonstrated a good DR in BRPCa. DR of 18F-labeled PSMA PET/CT is related to PSA values with significant lower DR in patients with PSA < 0.5 ng/mL. Prospective multicentric trials are needed to confirm these findings.
1. Introduction
The recent development of metabolic imaging methods has been aimed at improving diagnosis of prostate cancer (PCa), both at staging and in biochemical recurrent prostate cancer (BRPCa) when an increase of prostate-specific antigen (PSA) serum values is detected following curative primary treatments as radical prostatectomy or radiation therapy [1,2]. In patients with low but rising PSA serum values after definitive local therapy, it is important to identify the sites of recurrence early to maximize the effects of treatment; localizing the PCa recurrence can impact treatment decisions as local recurrence can be treated with focal radiation therapy, whereas distant metastases require more systemic therapies [1]. To this regard, radiolabeled prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) is emerging as a very useful imaging method for detecting tumor lesions in BRPCa patients, with higher DR compared to other imaging modalities [1,2,3,4,5].
The PSMA is overexpressed in the majority of PCa cells but its overexpression has not been found in benign prostatic diseases; however, PSMA is not prostate specific and this protein may be expressed in other tissues and tumors beyond PCa [3,4,5].
Several PSMA ligands, differing slightly in chemical structure, are commercially available and they may be radiolabeled with different positron-emitters isotopes as Gallium-68 (68Ga), Fluorine-18 (18F), or Copper-64 (64Cu) to obtain PET radiopharmaceuticals which could be used in clinical practice [4,5,6,7,8]. 68Ga-labeled PSMA tracers are currently the most used PSMA agents for PET imaging of BRPCa patients. More recently, PSMA ligands had been labeled with other isotopes with more favorable physical characteristics, such as 18F or 64Cu [6,7,8]. Several 18F-labeled PSMA agents have become available (18F-PSMA-1007, 18F-DCFPyL, and 18F-DCFBC). Labeling of PSMA agents with 18F may offer numerous advantages, including longer half-life and improved image resolution. Due to the lower positron energy, the theoretical achievable resolution of 18F is slightly better in comparison to 68Ga [7,8]. To date, several evidence-based articles evaluated the detection rate (DR) of 68Ga-labeled PSMA PET/CT in BRPCa patients [9,10,11,12,13,14,15]. Conversely, we aimed to perform a meta-analysis about the DR of 18F-labeled PSMA PET/CT in BRPCa patients to add evidence-based data in this setting.
2. Methods
Reporting of this systematic review and meta-analysis conforms to the “Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies” (PRISMA-DTA statement) which describes an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses of diagnostic studies [16,17].
2.1. Search Strategy
Three authors (G.T., S.A., D.A.P.) performed a comprehensive computer literature search of PubMed/MEDLINE, EMBASE and Cochrane library databases to find relevant published articles on the DR of PET/CT using 18F-labeled PSMA-agents in patients with BRPCa.
A search algorithm based on a combination of these terms was used: (A) “PSMA” AND (B) “DCFPyL” OR “DCFBC” OR “1007”. No beginning date limit and language restrictions were used, and the literature search was updated until 23 April 2019. To expand our search, references of the retrieved articles were also screened for additional studies.
2.2. Study Selection
Studies or subsets of studies investigating the DR of 18F-labeled PSMA PET/CT in patients with BRPCa were eligible for inclusion in the qualitative (systematic review) and quantitative analysis (meta-analysis). The exclusion criteria for the systematic review were: (a) articles not within the field of interest of this review; (b) review articles, editorials or letters, comments, conference proceedings; (c) case reports or small case series. For the meta-analysis, articles with possible patient data overlap were excluded; in this case, articles with more complete information were included in the meta-analysis.
Titles and abstracts were independently reviewed by three researchers applying the selected inclusion and exclusion criteria. Disagreements were solved in a consensus meeting.
2.3. Data Extraction
For each eligible article, information was collected concerning basic study (authors, year of publication, country of origin, study design), patient characteristics (type and number of patients evaluated, mean age, Gleason score, mean/median PSA serum values, and PSA doubling time before 18F-PSMA PET/CT), technical aspects (radiotracer used, hybrid imaging modality, mean radiotracer injected activity, time interval between radiotracer injection and image acquisition, image analysis and other imaging modalities performed for comparison). For articles included in the meta-analysis, information was collected about DR values of 18F-PSMA PET/CT (overall and at different PSA cut-off values) on a per patient-based analysis, mean PSA serum values in patients with positive and negative 18F-PSMA PET/CT, percentage of change of management by using 18F-PSMA PET/CT in BRPCa.
2.4. Quality Assessment
The overall quality of the studies included in the meta-analysis was critically appraised based on the revised “Quality Assessment of Diagnostic Accuracy Studies” tool (QUADAS-2) [18]. This tool comprises four domains (patient selection, index test, reference standard, and flow and timing) and each domain was assessed in terms of risk of bias, and the first three domains were also assessed in terms of concerns regarding applicability [18].
2.5. Statistical Analysis
The DR of 18F-PSMA PSMA PET/CT was defined as the ratio between the number of patients with at least one suspected lesion detected by PET/CT and the total number of BRPCa patients who underwent the scan. Pooled analyses about DR of 18F-PSMA PET/CT were performed using data retrieved from the selected studies and subgroup analyses taking into account different PSA serum values or different radiotracers were planned. Furthermore, a pooled analysis about the mean difference of PSA serum values in patients with positive and negative 18F-PSMA PET/CT was carried out.
A random-effects model was used for statistical pooling of the data, taking into account the heterogeneity between studies. The different weight of each study in the pooled analysis was related to the different sample size. Pooled data were presented with their respective 95% confidence interval (95% CI) values, and data were displayed using plots. Heterogeneity was estimated using the I-square index (I2), which describes the percentage of variation across studies that was due to the heterogeneity rather than chance [19], whereas the publication bias was assessed through the Egger’s test [20]. Statistical analyses were performed using the StatsDirect software version 3 (StatsDirect Ltd., Cambridge, UK).
3. Results
3.1. Literature Search
Literature search results are reported in Figure 1.
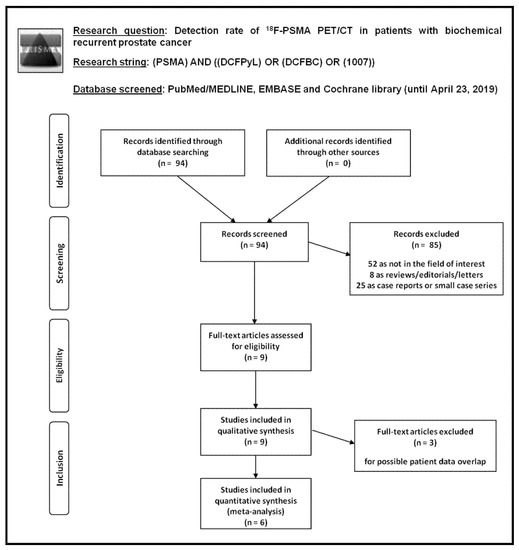
Figure 1.
Flow chart of the search for eligible studies on the detection rate of 18F-PSMA PET/CT in patients with biochemical recurrent prostate cancer.
Ninety-four records were identified from the literature search of PubMed/MEDLINE, EMBASE, and Cochrane library databases. Screening titles and abstracts, 85 records were excluded: 52 because they were not in the field of interest; 8 as they were reviews, editorials or letters; and 25 as they were case reports. Nine articles were selected and retrieved [21,22,23,24,25,26,27,28,29]. No additional records were found screening the references of these articles. Therefore, 9 articles were eligible for the qualitative analysis (systematic review). Three articles were excluded from the meta-analysis for possible patient data overlap [21,25,26]; finally, 6 articles including 645 patients with BRPCa were included in the quantitative analysis (meta-analysis) [22,23,24,27,28,29]. The characteristics of the studies selected for the systematic review are presented in Table 1 and Table 2. The main findings of the articles included in the meta-analysis are shown in Table 3, whereas the overall quality assessment of the studies is reported in Figure 2.

Table 1.
Basic study and patient characteristics.

Table 2.
Technical aspects of 18F-PSMA PET/CT in the included studies.

Table 3.
Main findings of the included studies about 18F-PSMA PET/CT in patients with biochemical recurrence of prostate cancer.
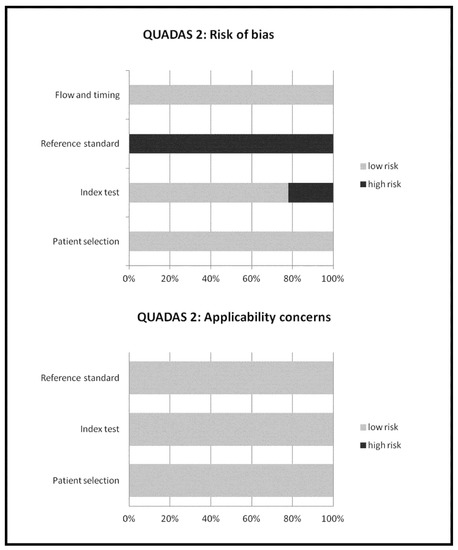
Figure 2.
Overall quality assessment of the studies included in the systematic review according to the QUADAS-2 tool.
3.2. Qualitative Analysis (Systematic Review)
3.2.1. Basic Study and Patient Characteristics
Nine articles evaluating the DR of 18F-PSMA PET/CT in BRPCa patients were selected (Table 1) [21,22,23,24,25,26,27,28,29]. The selected articles were published in the last five years by researchers from Europe and America; only two out of nine studies were prospective studies (22%). Mean and median age of the patients included in these studies ranged from 64 to 70 years. The Gleason score was 7 in 43–56%, ≤6 in 5–13%, and ≥8 in 28–42% of cases. Mean and median PSA serum values before PET/CT among the included BRPCa patients ranged from 0.6 to 5.2 ng/mL.
3.2.2. Technical Aspects
Technical aspects of the included studies are reported in Table 2. The 18F-labeled PSMA agent used was 18F-PSMA-1007 in four studies, 18F-DCFPyL in four studies, and 18F-DCFBC in one study only. The hybrid imaging modality was always PET/CT, mainly performed without CT contrast media injection. The injected radiopharmaceutical activity and the time between radiotracer injection and image acquisition were quite heterogeneous; in four studies a dual time point PET/CT imaging was performed. Analysis of PET images was performed using qualitative criteria (visual analysis) in all the studies and additional semi-quantitative criteria, i.e., calculating the maximal standardized uptake values (SUVmax), in most of the studies. Areas of increased radiopharmaceutical uptake greater than the surrounding tissue that could not be explained by physiological activity were judged as positive findings at visual analysis. A clear reference standard was not specified in the included studies.
3.2.3. Main Findings
Most of the included studies demonstrated a good DR of 18F-PSMA PET/CT in BRPCa patients which was dependent on PSA serum values: the proportion of positive scans increased with PSA levels [21,22,23,24,25,26,27,28,29]. Conversely, no significant correlation between PSA doubling time and DR of 18F-PSMA PET/CT was found [24]. The higher DR values were obtained using 18F-PSMA-1007 or 18F-DCFPyL as radiotracers (Table 3).
Most frequent sites of lesions detected by 18F-PSMA PET/CT in BRPCa were regional and distant lymph nodal metastases, local relapse, and bone metastases [21,22,23,24,25,26,27,28,29].
In three studies, a statistically significant difference of PSA serum values in patients with positive 18F-PSMA PET/CT compared to patients with negative 18F-PSMA PET/CT was found, but with a large overlap in PSA values across these two categories [24,28,29].
In studies performing a dual time point 18F-PSMA PET/CT, a significant increased lesion uptake and higher lesion-to-background uptake ratios were observed at a second time point (120 or 180 min after radiotracer injection) compared to the first time point (60 min after radiotracer injection) [23,24,25,26].
No significant adverse effects of 18F-PSMA PET/CT were reported [21,24,27,28,29]. The change of management by using 18F-PSMA PET/CT in BRPCa ranged from 50 to 87% of cases [24,28].
Two articles compared the DR of 18F-DCFPyL PET/CT with 18Ga-PSMA-11 PET/CT in BRPCa patients. The 18F-DCFPyL PET/CT detected additional lesions compared to 18Ga-PSMA-11 PET/CT (in particular for PSA values between 0.5 and 3.5 ng/mL) and the mean SUVmax of 18F-DCFPyL PSMA-positive lesions was significantly higher as compared to 18Ga-PSMA-11 positive lesions [21,22].
Several discordant findings were found when 18F-PSMA PET/CT was compared to multi-parametric MRI, demonstrating the complementary role of these imaging methods in BRPCa patients [24].
3.3. Quantitative Analysis (Meta-Analysis)
Six studies (645 BRPCa patients) were selected for the pooled analysis [22,23,24,27,28,29]. The overall DR of 18F-PSMA PET/CT on a per patient-based analysis ranged from 60% to 95%, with a pooled value of 81% (95% CI: 71–88%) (Figure 3 and Table 3). We have detected a significant heterogeneity among the selected studies (I2 = 86%), whereas a publication bias was not revealed (p = 0.16).
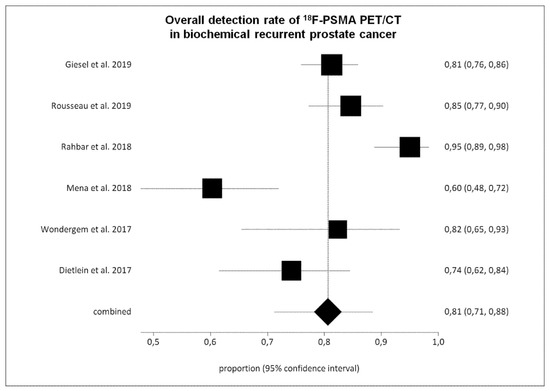
Figure 3.
Plots of individual studies and pooled detection rate of 18F-PSMA PET/CT in biochemical recurrent prostate cancer on a per patient-based analysis, including 95% confidence intervals (95% CI). The size of the squares indicates the weight of each study.
Performing a sub-group analysis taking into account different PSA cut-off values (Table 3 and Figure 4), we found a statistical significant difference in DR of 18F-PSMA PET/CT in BRPCa patients with PSA ≥ 0.5 ng/mL (pooled DR: 86%; 95% CI: 78–93%) compared to patients with PSA < 0.5 ng/mL (pooled DR: 49%; 95% CI: 23–74%).
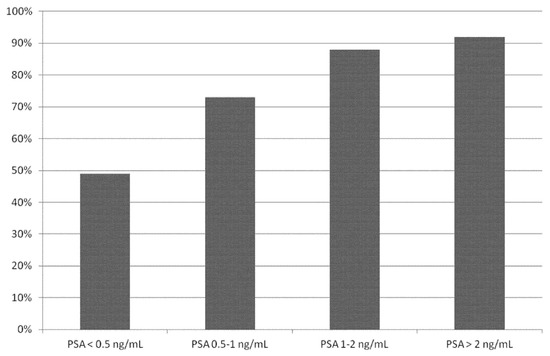
Figure 4.
Bar graph showing the pooled detection rate of 18F-PSMA PET/CT in biochemical recurrent prostate cancer on a per patient-based analysis, according to different PSA serum values.
Performing a sub-group analysis taking into account different radiotracers, the pooled DR of 18F-PSMA-1007, 18F-DCFPyL, and 18F-DCFBC PET/CT were 89% (95% CI: 72–98%), 81% (95% CI: 74–87%) and 60% (95% CI: 48–72%), respectively.
Weighted mean difference of PSA values among patients with positive 18F-PSMA PET/CT and patients with negative 18F-PSMA PET/CT was 4.5 (95% CI: 3.3–5.7) without significant heterogeneity (I2 = 0%).
4. Discussion
Recently, some studies have evaluated the diagnostic performance of 18F-PSMA PET/CT in BRPCa patients [21,22,23,24,25,26,27,28,29]; as these studies have limited power, due to the relatively small number of patients enrolled and assessed, we have pooled data reported in published studies to derive more robust estimates on the DR of 18F-PSMA PET/CT in this setting.
Overall, our systematic review and meta-analysis indicates a good DR of 18F-PSMA PET/CT in BRPCa patients, in particular using 18F-PSMA-1007 and 18F-DCFPyL. The DR was dependent on PSA serum values [21,22,23,24,25,26,27,28,29]: using the PSA cut-off of 0.5 ng/mL, the pooled DR of 18F-PSMA PET/CT was 86% in BRPCa patients with PSA ≥ 0.5 ng/mL and 49% in patients with PSA < 0.5 ng/mL. Therefore, accurate timing of 18F-PSMA PET/CT, based on PSA values, substantially affects its diagnostic value in BRPCa patients, and monitoring of PSA values could be useful for accurate timing of 18F-PSMA PET/CT.
Beyond the PSA serum values, low PSMA expression (i.e., due to the tumor heterogeneity) might cause false-negative 18F-PSMA PET/CT findings in some PCa patients [21,22,23,24,25,26,27,28,29]. About the pooled DR of 18F-PSMA PET/CT in BRPCa, we found similar results compared to those reported in the literature with 68Ga-labeled PSMA PET/CT [9,10,11,12,13,14,15]. Compared to PET/CT with 68Ga-labeled PSMA, the longer half-life and higher injected activities of 18F-PSMA allow high-quality delayed images, higher lesion uptake, and superior clearance of background activity [21,22,23,24,25,26,27,28,29]. Two studies only reported a comparison of 18F-PSMA and 68Ga-PSMA PET/CT in BRPCa patients with a trend towards a higher DR of 18F-PSMA compared to 68Ga-PSMA PET/CT, but the acquisition protocols used in these studies included different tracer uptake time periods for 18F-PSMA (120 min) and 68Ga-PSMA (60 min) before image acquisition and different activity used for these radiopharmaceuticals (18F-PSMA > 68Ga-PSMA), which could explain these results [21,22]. Assuming similar DR, the real additional value of 18F-PSMA tracers might be the possibility of large-scale batch production in a cyclotron and satellite-center supply due to the longer half-life [21,22,23,24,25,26,27,28,29].
As a significant increased 18F-PSMA uptake over time was demonstrated in PCa lesions with a higher contrast at delayed PET/CT time points compared to early PET/CT time points [23,24,25,26], it is not recommended to perform 18F-PSMA PET/CT at 60 min after radiotracer injection (which is the common imaging time point for 68Ga-PSMA PET/CT). However, imaging at late time points may be a logistic challenge for PET/CT centers.
Only two articles assessed the change of management that can be obtained by using 18F-PSMA PET/CT in patients with BRPCa [24,28], reporting a significant change of management ranging from 50 to 87% of cases, in line with literature data about the change of management obtained by using 18Ga-PSMA PET/CT in this setting [30].
Some limitations and biases of our meta-analysis should be taken into account. First of all, a limited number of studies were available for the meta-analysis. The major limitation of the included studies was that not all positive PET/CT findings were confirmed by histology (verification bias). Confirmation was impaired by the small volume of individual lesions and the high number of biopsy-inaccessible lesions. In absence of histological validation, it cannot be excluded that some lesions detected by 18F-PSMA PET/CT may represent false-positive findings. Nevertheless, if modern imaging methods are performed in BRPCa patients, then confirmation of positive findings are needed only in highly selected cases and with a biopsy when findings are equivocal [1]. Even in the absence of histological confirmation, clinical follow-up or decline of PSA after therapy can be helpful.
A possible limitation of our meta-analysis is the detected heterogeneity, likely due to the different characteristics of patients, methods, and quality of included studies. We tried to explain this heterogeneity performing sub-group analyses taking into account different PSA cut-off values and different radiotracers. Some differences of DR were found using various 68F-PSMA agents, but studies performing a head-to-head comparison of these tracers in BRPCa setting are not available yet. We found a lower DR value using 18F-DCFBC compared with second-generation 68F-PSMA agents (18F-PSMA-1007 and 18F-DCFPyL), likely because of the higher background signal of 18F-DCFBC due to the considerable blood-pool activity, which could limit the DR of pelvic and retroperitoneal lymph node metastases [24]. In a recent pilot prospective study comparing 18F-PSMA-1007 and 18F-DCFPyL in the setting of PCa staging, similar DR were found using these radiopharmaceuticals [31]. Non-urinary excretion of 18F-PSMA-1007 might present some advantages with regard to delineation of local recurrence or pelvic lymph node metastases in selected patients; the lower hepatic background might favor 18F-DCFPyL in late stages, when rare cases of liver metastases can occur [31].
Diagnostic accuracy of a test is not a measure of clinical effectiveness and high DR values do not necessarily result in improved patient outcomes. Other factors beyond the DR should influence the choice of an imaging modality in patients with BRPCa (i.e., availability, radiation dose, safety, examination time, legal, organization, economic aspects, and cost-effectiveness). Overall, our systematic review and meta-analysis demonstrated a good DR of 18F-PSMA PET/CT in patients with BRPCa, but large prospective multi-center studies, and in particular, cost-effectiveness analyses comparing 18F-PSMA to other PET radiopharmaceuticals are warranted.
5. Conclusions
- 18F-labeled PSMA PET/CT demonstrated a good DR in BRPCa, in particular using 18F-PSMA-1007 and 18F-DCFPyL, with similar results compared to those reported in the literature with 68Ga-labeled PSMA PET/CT.
- The DR of 18F-labeled PSMA PET/CT is related to PSA values with significant lower DR in patients with PSA < 0.5 ng/mL.
- Prospective multicentric trials are needed to confirm these findings; nevertheless, 18F-labeled PSMA PET/CT seems to be a promising cost-effective alternative to 68Ga-labeled PSMA PET/CT in BRPCa.
Author Contributions
Conceptualization, G.T. and L.C.; methodology, G.T.; software, G.T.; validation, J.O.P. and L.C., formal analysis, G.T., S.A. and D.A.P.; resources, G.T.; data curation, G.T., S.A. and D.A.P.; writing—original draft preparation, G.T.; writing—review and editing, S.A., D.A.P.; supervision, J.O.P., L.C. and L.G.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fanti, S.; Minozzi, S.; Antoch, G.; Banks, I.; Briganti, A.; Carrio, I.; Chiti, A.; Clarke, N.; Eiber, M.; De Bono, J.; et al. Consensus on molecular imaging and theranostics in prostate cancer. Lancet Oncol. 2018, 19, e696–e708. [Google Scholar] [CrossRef]
- Tangel, M.R.; Rastinehad, A.R. Advances in prostate cancer imaging. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Castellucci, P.; Fanti, S. Current application and future perspectives of PSMA PET imaging in prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 7–18. [Google Scholar] [CrossRef]
- Giovacchini, G.; Giovannini, E.; Riondato, M.; Ciarmiello, A. PET/CT With (68)Ga-PSMA in Prostate Cancer: Radiopharmaceutical Background and Clinical Implications. Curr. Radiopharm. 2018, 11, 4–13. [Google Scholar] [CrossRef]
- Gourni, E.; Henriksen, G. Metal-Based PSMA Radioligands. Molecules 2017, 22, 523. [Google Scholar] [CrossRef]
- Czarniecki, M.; Mena, E.; Lindenberg, L.; Cacko, M.; Harmon, S.; Radtke, J.P.; Giesel, F.; Turkbey, B.; Choyke, P.L. Keeping up with the prostate-specific membrane antigens (PSMAs): An introduction to a new class of positron emission tomography (PET) imaging agents. Transl. Androl. Urol. 2018, 7, 831–843. [Google Scholar] [CrossRef]
- Eiber, M.; Fendler, W.P.; Rowe, S.P.; Calais, J.; Hofman, M.S.; Maurer, T.; Schwarzenboeck, S.M.; Kratowchil, C.; Herrmann, K.; Giesel, F.L. Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J. Nucl. Med. 2017, 58, 67S–76S. [Google Scholar] [CrossRef]
- Tan, N.; Bavadian, N.; Calais, J.; Oyoyo, U.; Kim, J.; Turkbey, I.B.; Mena, E.; Davenport, M.S. Imaging of PSMA-targeted Radiotracers for the Detection of Prostate Cancer Biochemical Recurrence After Definitive Therapy: A Systematic Review and Meta-analysis. J. Urol. 2019. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2019. [Google Scholar] [CrossRef]
- Pereira Mestre, R.; Treglia, G.; Ferrari, M.; Pascale, M.; Mazzara, C.; Azinwi, N.C.; Llado’, A.; Stathis, A.; Giovanella, L.; Roggero, E. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: A meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13063. [Google Scholar] [CrossRef]
- Hope, T.A.; Goodman, J.Z.; Allen, I.E.; Calais, J.; Fendler, W.P.; Carroll, P.R. Meta-analysis of (68)Ga-PSMA-11 PET Accuracy for the Detection of Prostate Cancer Validated by Histopathology. J. Nucl. Med. 2018. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Butaney, M.; Konety, B.R. The utility of PET-based imaging for prostate cancer biochemical recurrence: A systematic review and meta-analysis. World J. Urol. 2018. [Google Scholar] [CrossRef]
- Eissa, A.; Elsherbiny, A.; Coelho, R.F.; Rassweiler, J.; Davis, J.W.; Porpiglia, F.; Patel, V.R.; Prandini, N.; Micali, S.; Sighinolfi, M.C.; et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol. Nefrol. 2018, 70, 462–478. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. (68)Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 686–693. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomäcker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of [(18)F]DCFPyL and [(68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef]
- Dietlein, F.; Kobe, C.; Neubauer, S.; Schmidt, M.; Stockter, S.; Fischer, T.; Schomäcker, K.; Heidenreich, A.; Zlatopolskiy, B.D.; Neumaier, B.; et al. PSA-Stratified Performance of (18)F- and (68)Ga-PSMA PET in Patients with Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2017, 58, 947–952. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.M.; Knol, R.J.J.; Lazarenko, S.V.; Pruim, J.; de Jong, I.J. (18)F-DCFPyL PET/CT in the Detection of Prostate Cancer at 60 and 120 Minutes: Detection Rate, Image Quality, Activity Kinetics, and Biodistribution. J. Nucl. Med. 2017, 58, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Lindenberg, M.L.; Shih, J.H.; Adler, S.; Harmon, S.; Bergvall, E.; Citrin, D.; Dahut, W.; Ton, A.T.; McKinney, Y.; et al. Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Afshar-Oromieh, A.; Bögemann, M.; Wagner, S.; Schäfers, M.; Stegger, L.; Weckesser, M. (18)F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: Biodistribution, tumour detection and activity kinetics. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Will, L.; Kesch, C.; Freitag, M.; Kremer, C.; Merkle, J.; Neels, O.C.; Cardinale, J.; Hadaschik, B.; Hohenfellner, M.; et al. Biochemical Recurrence of Prostate Cancer: Initial Results with [(18)F]PSMA-1007 PET/CT. J. Nucl. Med. 2018, 59, 632–635. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Seifert, R.; Wagner, S.; Schäfers, M.; Bögemann, M.; Weckesser, M. Diagnostic performance of (18)F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2055–2061. [Google Scholar] [CrossRef]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Bénard, F. A Prospective Study on (18)F-DCFPyL PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of (18)F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of (68)Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Haberkon, U.; Kopka, K.; et al. Intraindividual Comparison of (18)F-PSMA-1007 and (18)F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).