Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment
Abstract
1. Introduction
2. Results
2.1. Patients
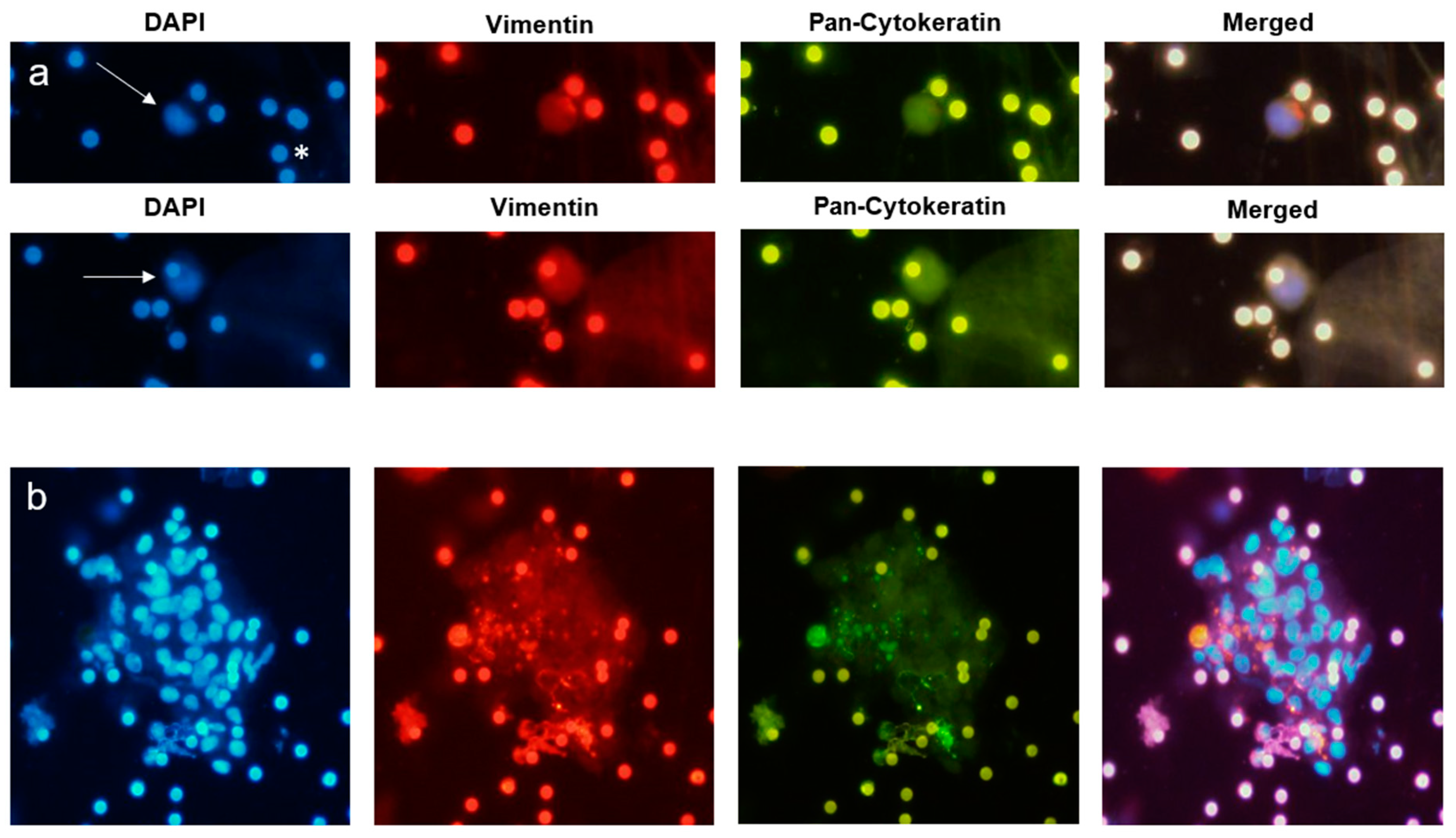
2.2. CTC Detection
2.3. Tissue Analysis
2.4. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. CTC Analysis
4.3. Tissue Analysis
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Giuliano, M.; Giordano, A.; Jackson, S.; de Giorgi, U.; Mego, M.; Cohen, E.N.; Gao, H.; Anfossi, S.; Handy, B.C.; Ueno, N.T.; et al. Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 2014, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Lurje, G.; Schiesser, M.; Claudius, A.; Schneider, P.M. Circulating tumor cells in gastrointestinal malignancies: Current techniques and clinical implications. J. Oncol. 2010, 2010, 392652. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, K.; Takeuchi, H.; Hasegawa, H.; Saikawa, Y.; Suda, K.; Ando, T.; Kumagai, K.; Irino, T.; Yoshikawa, T.; Matsuda, S.; et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann. Surg. Oncol. 2008, 15, 3092–3100. [Google Scholar] [CrossRef]
- Reeh, M.; Effenberger, K.E.; Koenig, A.M.; Riethdorf, S.; Eichstadt, D.; Vettorazzi, E.; Uzunoglu, F.G.; Vashist, Y.K.; Izbicki, J.R.; Pantel, K.; et al. Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients with Esophageal Cancer. Ann. Surg. 2015, 261, 1124–1130. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Peeters, D.; Benoy, I.H.; Elst, H.J.; van Laere, S.J.; Prove, A.; Maes, H.; Huget, P.; van Dam, P.; Vermeulen, P.B.; et al. Circulating tumour cell detection: A direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br. J. Cancer 2010, 102, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Backen, A.; Sloane, R.; Menasce, L.; Ryder, D.; Krebs, M.; Board, R.; Clack, G.; Hughes, A.; Blackhall, F.; et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br. J. Cancer 2012, 106, 508–516. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Yang, X.-R.; Zhou, J.; Qiu, S.-J.; Fan, J.; Xu, Y. Circulating tumor cells: Advances in detection methods, biological issues, and clinical relevance. J. Cancer Res. Clin. Oncol. 2011, 137, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.G.; Chianese, D.; Doyle, G.V.; Miller, M.C.; Russell, T.; Sanders, R.A., Jr.; Terstappen, L.W.M.M. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int. J. Oncol. 2005, 27, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Rose, L.; Zollner, T.M.; Krahn, T.; von Ahsen, O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Kulemann, B.; Rosch, S.; Seifert, S.; Timme, S.; Bronsert, P.; Seifert, G.; Martini, V.; Kuvendjiska, J.; Glatz, T.; Hussung, S.; et al. Pancreatic cancer: Circulating Tumor Cells and Primary Tumors show Heterogeneous KRAS Mutations. Sci. Rep. 2017, 7, 4510. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Muller, V.; Aktas, B.; Janni, W.; Schneeweiss, A.; Stickeler, E.; Lattrich, C.; Lohberg, C.R.; Solomayer, E.; Rack, B.; et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: A prospective, multicenter trial. Breast Cancer Res. Treat. 2010, 124, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [PubMed]
- Hoeppner, J.; Zirlik, K.; Brunner, T.; Bronsert, P.; Kulemann, B.; Sick, O.; Marjanovic, G.; Hopt, U.T.; Makowiec, F. Multimodal treatment of locally advanced esophageal adenocarcinoma: Which regimen should we choose? Outcome analysis of perioperative chemotherapy versus neoadjuvant chemoradiation in 105 patients. J. Surg. Oncol. 2014, 109, 287–293. [Google Scholar] [CrossRef]
- Pachmann, K.; Camara, O.; Kavallaris, A.; Schneider, U.; Schunemann, S.; Hoffken, K. Quantification of the response of circulating epithelial cells to neodadjuvant treatment for breast cancer: A new tool for therapy monitoring. Breast Cancer Res. 2005, 7, R975–R979. [Google Scholar] [CrossRef] [PubMed]
- Camara, O.; Rengsberger, M.; Egbe, A.; Koch, A.; Gajda, M.; Hammer, U.; Jorke, C.; Rabenstein, C.; Untch, M.; Pachmann, K. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 1484–1492. [Google Scholar] [CrossRef]
- Pachmann, K.; Camara, O.; Kavallaris, A.; Krauspe, S.; Malarski, N.; Gajda, M.; Kroll, T.; Jorke, C.; Hammer, U.; Altendorf-Hofmann, A.; et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1208–1215. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Zhou, J.; Kenny, L.; Papautsky, I.; Punyadeera, C. Capture of Circulating Tumour Cell Clusters Using Straight Microfluidic Chips. Cancers 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Chen, K.; Che, J.; Hang, J.; Li, H. Detection of Epithelial-Mesenchymal Transition Status of Circulating Tumor Cells in Patients with Esophageal Squamous Carcinoma. Biomed Res. Int. 2018, 2018, 7610154. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, D.; Uenosono, Y.; Arigami, T.; Yanagita, S.; Nishizono, Y.; Hagihara, T.; Hirata, M.; Haraguchi, N.; Arima, H.; Kijima, Y.; et al. Clinical Significance of Circulating Tumor Cells in Peripheral Blood of Patients with Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 22, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Kenny, L.; Perry, C.; Thiery, J.-P.; Jovanovic, L.; Vela, I.; Nelson, C.; Punyadeera, C. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget 2016, 7, 71223–71234. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, D.; Barr, J.; Beeson, J.; Beddow, E.; McGonigle, N.; Rice, A.; Nicholson, A.; Anikin, V. Detection of Circulating Tumour Cells and Survival of Patients with Non-small Cell Lung Cancer. Anticancer Res. 2017, 37, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, J.; Lordick, F.; Brunner, T.; Hopt, U.T.; Siewert, J.R. ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016, 16, e503. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Chichester/West Sussex, UK; Hoboken, NJ, USA, 2010. [Google Scholar]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Bottcher, K.; Siewert, J.R.; Hofler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef] [PubMed]

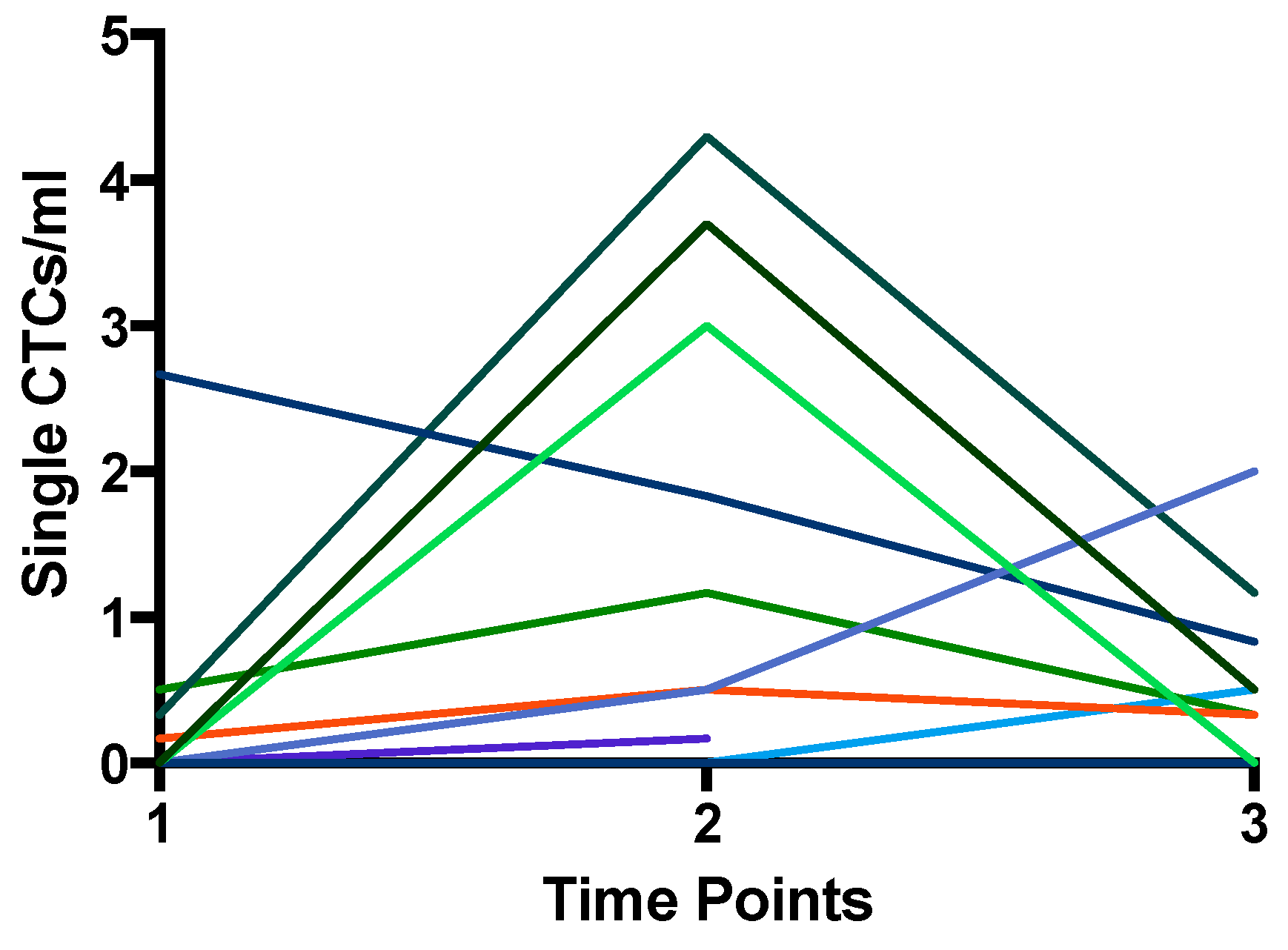


| Variables | n | CTC Positive (n) | ||
|---|---|---|---|---|
| TP1 | TP2 | TP3 | ||
| All | 20 | 6 | 9 | 8 |
| Sex | ||||
| Male | 18 | 2 | 1 | 1 |
| Female | 2 | 4 | 8 | 7 |
| Resection | ||||
| Yes | 15 | |||
| No | 4 | |||
| unknown | 1 | |||
| T-Stage | ||||
| ypT0 | 3 | 0 | 1 | 1 |
| ypT1 | 4 | 2 | 3 | 3 |
| ypT2 | 3 | 1 | 2 | 2 |
| ypT3 | 4 | 2 | 2 | 2 |
| ypT4 | 1 | 0 | 0 | 0 |
| Missing | 5 | |||
| N-Stage | ||||
| ypN0 | 7 | 3 | 5 | 5 |
| ypN1 | 4 | 1 | 1 | 2 |
| ypN2 | 2 | 1 | 1 | 1 |
| ypN3 | 2 | 0 | 1 | 0 |
| Missing | 5 | |||
| Regression status | ||||
| complete | 2 | 0 | 1 | 1 |
| subtotal | 6 | 3 | 4 | 4 |
| partial | 3 | 1 | 1 | 1 |
| minimal | 4 | 1 | 2 | 2 |
| Patient | Treatment Regimen | Resection | TP1 | TP2 | TP3 | pTNM | Pathology | Tumor | 1-Year Follow-up | 2-Year Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC/mL | Single CTC/mL | CTC/mL | Single CTC/mL | CTC/mL | Single CTC/mL | T | N | M | Regression | Vimentin Expression | No Relapse | Tumor Relapse | Death | No Relapse | Tumor Relapse | Death | |||
| 1 | FLOT | yes | 0 | 0 | 3.7 | 3.7 | 0.5 | 0.5 | 1 | 0 | 0 | subtotal | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 2 | FLOT | yes | 0 | 0 | 8.7 | 3 | 0 | 0 | 3 | 3 | 0 | minimal | ? | 0 | 1 | 1 | / | / | / |
| 3 | CROSS | yes | 15.3 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | partial | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 4 | FLOT | yes | 0 | 0 | 0 | 0 | /* | * | 4 | 3 | 1 | minimal | 3 | 0 | 1 | 1 | / | / | / |
| 5 | FLOT | unknown | 0 | 0 | /* | /* | /* | /* | / | / | / | / | / | / | / | / | / | / | / |
| 6 | FLOT | yes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | complete | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| 7 | FLOT | yes | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | subtotal | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 8 | FLOT | yes | 0 | 0 | 0.5 | 0.5 | 2 | 2 | 0 | 1 | 0 | complete | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 9 | CROSS | no | 0 | 0 | / | / | / | / | / | / | / | / | / | 0 | 1 | 1 | / | / | / |
| 10 | FLOT | no | 0 | 0 | 0.17 | 0.17 | / | / | / | / | / | / | / | 0 | 1 | 1 | / | / | / |
| 11 | FLOT | yes | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | subtotal | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 12 | CROSS | yes | 0.2 | 0.2 | 2.8 | 2.3 | 0.3 | 0.3 | 2 | 0 | 0 | minimal | 0 | 0 | 1 | 1 | / | / | / |
| 13 | CROSS | yes | 44.3 | 2.7 | 6.7 | 1.8 | 0.8 | 0.8 | 1 | 0 | 0 | subtotal | 2 | 1 | 0 | 0 | 1 | 0 | 0 |
| 14 | FLOT | no | 0 | 0 | / | / | / | / | / | / | / | / | / | 1 | 0 | 0 | 1 | 0 | 0 |
| 15 | FLOT | yes | 0 | 0 | 0.5 | 0.5 | 11 | 0.3 | 2 | 0 | 0 | partial | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 16 | FLOT | yes | 0.3 | 0.3 | 4.3 | 4.3 | 1.2 | 1.2 | 3 | 2 | 0 | subtotal | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 17 | FLOT | no | 0.2 | 0.2 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| 18 | CROSS | yes | 8 | 0.5 | 12.8 | 1.2 | 0.3 | 0.3 | 1 | 0 | 0 | subtotal | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 19 | FLOT | yes | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 3 | 1 | 0 | minimal | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 20 | FLOT | yes | 0 | 0 | /* | /* | /* | /* | 1 | 2 | 1 | partial | 0 | 0 | 1 | 1 | / | / | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuvendjiska, J.; Bronsert, P.; Martini, V.; Lang, S.; Pitman, M.B.; Hoeppner, J.; Kulemann, B. Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment. Cancers 2019, 11, 397. https://doi.org/10.3390/cancers11030397
Kuvendjiska J, Bronsert P, Martini V, Lang S, Pitman MB, Hoeppner J, Kulemann B. Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment. Cancers. 2019; 11(3):397. https://doi.org/10.3390/cancers11030397
Chicago/Turabian StyleKuvendjiska, Jasmina, Peter Bronsert, Verena Martini, Sven Lang, Martha B. Pitman, Jens Hoeppner, and Birte Kulemann. 2019. "Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment" Cancers 11, no. 3: 397. https://doi.org/10.3390/cancers11030397
APA StyleKuvendjiska, J., Bronsert, P., Martini, V., Lang, S., Pitman, M. B., Hoeppner, J., & Kulemann, B. (2019). Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment. Cancers, 11(3), 397. https://doi.org/10.3390/cancers11030397






