Abstract
Breast cancer cells produce stimulators of bone resorption known as interleukins (ILs). However, data on the functional roles of ILs in the homing of metastatic breast cancer to bone are still fragmented. A systematic search was carried out in three databases (PubMed, Scopus, Web of Science Core Collection) to identify preclinical reports, and in three clinical registers (ClinicalTrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform, European Union (EU) Clinical Trials Register) to identify clinical trials, from 2008 to 2019. Sixty-seven preclinical studies and 11 clinical trials were recognized as eligible. Although preclinical studies identified specific key ILs which promote breast cancer bone metastases, which have pro-metastatic effects (e.g., IL-6, IL-8, IL-1β, IL-11), and whose inhibition also shows potential preclinical therapeutic effects, the clinical trials focused principally on ILs (IL-2 and IL-12), which have an anti-metastatic effect and a potential to generate a localized and systemic antitumor response. However, these clinical trials are yet to post any results or conclusions. This inconsistency indicates that further studies are necessary to further develop the understanding of cellular and molecular relations, as well as signaling pathways, both up- and downstream of ILs, which could represent a novel strategy to treat tumors that are resistant to standard care therapies for patients affected by breast cancer bone disease.
1. Introduction
Breast cancer represents the most recurrent malignancy among women, contributing to over 25% of the total number of new diagnosed cancers, and the second most frequent cause of cancer death [1,2]. Between 20% and 30% of patients with breast cancer develop metastases [3], with bone metastases occurring in about 15% of these patients [4]; about 50% of metastases involve bone as the primary metastatic site, while 80% involve bone as a secondary and/or recurring site [5,6,7]. Breast cancer bone metastatic patients have an increased morbidity, mainly due to hypercalcemia, bone fractures, spinal cord compression, impaired mobility, and pain [8]. This last factor is commonly caused by the mechanical pressure carried out by the tumor mass and/or by the release of inflammatory cytokines from tumor cells and/or from the bone microenvironment, thus causing an alteration of the bone homeostasis. All these observations emphasize the importance of understanding the mechanism(s) through which breast cancer cells grow and colonize the bone. Variances in anatomical sites and clinical pathology, together with the heterogeneity of molecular and cellular factors, make it difficult to predict susceptibility to bone metastases.
Breast cancer bone metastasis is mainly an osteolytic disease, but osteoblastic and mixed lesions can also occur [9,10,11]. During osteolytic bone metastases, osteoclast activity and/or the inhibition of osteoblast differentiation are stimulated by tumor cells, bringing an increase in bone resorption and/or a decrease in bone formation, respectively, leading to bone disease and higher risk of fractures [9,10,11]. As already well documented, the tumor microenvironment plays a central role in breast cancer progression. Alteration in the crosstalk between bone cells (osteoblasts or osteoclasts) and tumor cells promotes the release of secreted factors responsible for pre-metastatic niche formation [9,12,13,14,15,16]. In this complex network of molecules and factors, interleukins (ILs) were identified as key actors able to act on bone homeostasis and tumor cells stimulating tumor growth and bone destruction [12,17]. ILs are a family of small proteins implicated in cell signaling, originally isolated from leukocytes; however, it is now known that ILs are produced by nearly all cell types including osteoclasts, osteoblasts, osteocytes, and mesenchymal stem cells. ILs are modulators of immune response and are implicated in the regulation of cell functions, activation, differentiation, and proliferation, as well as cell–cell communication. Thus, it is evident that ILs can have a pivotal role in bone metastasis development. They can also be produced by tumor cells with a variety of functions, and they may act as tumor-promoting or tumor-inhibiting factors. To date, numerous ILs were investigated; however, available literature data do not provide a complete overview of IL role(s), function(s), and mechanism(s) of action in breast cancer bone metastasis pathogenesis and therapy. Having a complete framework of these aspects would provide specific indication of the role of ILs in breast cancer bone metastasis, and it could give specific evidence for the evaluation of new potential therapeutic IL-targeted approaches. Thus, we performed a systematic review of the latest evidence on the roles and functions of ILs that act in the breast cancer bone metastatic process. Finally, we also evaluated IL-based therapies that could be employed for the treatment of breast cancer bone metastases.
2. Method
Literature Search
A systematic search of preclinical articles published in English between May 2008 and October 2019 was conducted in three electronic databases, PubMed, Scopus, and Web of Science Core Collection, using the keywords (interleukin OR interleukins) AND (breast cancer bone metastasis OR breast cancer to bone OR breast cancer in bone OR breast cancer skeletal metastasis OR metastatic breast cancer to bone). Subsequently, a public reference manager (Mendeley 1.17.11, Mendeley Ltd, London, UK) was used to eliminate duplicates. Two reviewers screened titles and abstracts, in addition to the full text, against the pre-specified criteria.
In order to evaluate current or concluded clinical trials, some of the major clinical registry websites were also checked (ClinicalTrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform, European Union (EU) Clinical Trials Register). The search was applied with the following string: “(breast cancer) and (interleukin)”, “(breast cancer), and (anti-IL)”.
Detailed inclusion and exclusion criteria are reported in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart shown in Figure 1. This article does not contain any study performed by any of the authors with human participants or animals.
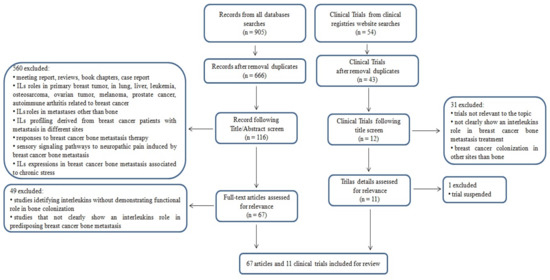
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of search criteria.
3. Results
3.1. Literature Search
An initial literature search found 905 references, of which 151 articles were recognized using the PubMed database, 481 articles were recognized using Scopus, and 273 articles were found in the Web of Science Core Collection. Subsequently, the resulting references were submitted to a public reference manager (Mendeley 1.17.11) to eliminate duplicate articles, obtaining 666 articles. From the 666 articles obtained, after exclusion, 116 full-text articles were evaluated, of which 67 met the inclusion criteria. The 560 studies excluded from this review were excluded because they were meeting reports, reviews, book chapters, and case reports, and/or because they reported IL roles in primary breast tumor, as well as in lung, liver, and pulmonary metastasis, leukemia, osteosarcoma, ovarian tumor, melanoma, prostate cancer, autoimmune arthritis related to breast cancer, and other pathological conditions; however, they did not reveal the favorable development of bone metastases from breast cancer. In addition, we excluded studies where IL profiling was derived from breast cancer metastatic patients that were not grouped/analyzed based on the site of metastasis, and where breast cancer bone metastases were evaluated, but no IL roles/functions were examined. Finally, we also eliminated studies where responses to specific therapy (not IL-based) for breast cancer bone metastasis were analyzed, studies that evaluated the contributions of the sensory signaling pathways to neuropathic pain induced by breast cancer bone metastases, and studies on chronic stress and IL expression in breast cancer bone metastasis (Figure 1).
Fifty-four clinical trials were found in the clinical registry websites (ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, EU Clinical Trials Register). The results from registries were collected, assessed for relevance, and matched to eliminate any duplicates. After the screening process, 11 clinical trials were selected that focused on the topic of the present review.
Data were extracted to associate, arrange, compare, and combine the results. The retrieved data for preclinical studies (in vitro and in vivo) were as follows: author and publication date, interleukin(s) identified, interleukin(s) source, aims, and experimental approaches (Table 1). Extracted data for clinical trials were as follows: trial number of registries, aim, arms of the study, identified ILs and dosage, IL function, and summary of main findings (Table 2).

Table 1.
Preclinical studies on interleukins (ILs) and breast cancer bone metastases.

Table 2.
Clinical trials on ILs and breast cancer bone metastases.
3.2. Findings from Preclinical Studies
As reported in Table 1 and references therein, in the 67 preclinical articles extracted in this review, several ILs, i.e., IL-1β, IL-2, IL-6, IL-8, IL-10, IL-11, IL-15, IL-17, IL-18, and IL-20 were linked with breast cancer cells homing to bone. Forty-one studies (61%) were in vitro and, of them, 33 used established human (MDA-MB-231, MDA-231B, T-47D, ZR-75-1, 435/BRMS1, 231/BRMS1, MDA-MB-435, MCF-7, BT474, SKBR-3, and Hs578T) or murine (MRMT-1, 13762 MAT B III, 4T1, 4T1.2, and 67NR) breast cancer cells lines, normal or modified (i.e., transfected), cultured alone and/or in direct or indirect co-culture with bone cells (osteoblasts, osteoclasts, fibroblasts, bone marrow-derived cells) and/or bone fragments, while the remaining studies (n = 8) used peripheral blood and tissue from breast cancer bone metastatic patients. Twenty-six studies (39%) were in vivo or both in vitro and in vivo, and they used intracardiac, intratibial, and subcutaneous injection of breast cancer cell lines (MDA-MB-231 variants, 4T1, 4T1.2, MDA-P, MDA-MET, NT2.5, MCF-7), normal or transfected, into mice or rats.
The examined papers mainly focused on (1) evaluation of the upregulation or downregulation of the expression of ILs during breast cancer bone metastases, (2) inhibition, blockade, and/or neutralization of Is signaling, by using IL dual-selective antagonists, anti-IL, anti-IL receptor, and IL monoclonal antibodies (mAb) in breast cancer bone metastases, and (3) definition of the role of ILs as potential biomarkers during breast cancer bone metastases. Although focused on different IL functions and roles in breast cancer bone metastases, almost all the examined studies supported the vicious cycle of breast cancer metastasis to bone that is driven by four main contributors: tumor cells, bone-forming osteoblasts, bone-destroying osteoclasts, and the organic bone matrix. However, this is an oversimplification of the breast cancer bone metastasis mechanism, and a more complex crosstalk between cells, cytokines, and growth factors is present. In fact, in this review, several studies (n = 21, 31%) evaluated new and unexplored mechanisms of action mediated by ILs in breast cancer bone metastasis. Findings of these mechanisms are schematically illustrated in Figure 2 and detailed in the subsequent paragraphs.

Figure 2.
Mechanisms that regulate the interactions between breast cancer cells and bone. Black lines indicate established interactions of interleukins (ILs) within the vicious cycle. Red lines indicate potential additional interactions reviewed in this paper [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
3.2.1. IL-1
IL-1, a prototypic pro-inflammatory cytokine that presents itself in two forms, i.e., IL-1α and IL-Iβ, seems to be involved in different molecular mechanisms underlying primary breast cancer development and the formation of metastasis in bone. IL-1β involvement in breast cancer bone metastases was highlighted by its high expression in metastatic breast cancer cell lines, in serum from mice-bearing bone metastatic tumors and also in tissue samples from patients with breast cancer bone metastases [39,40]. Increased levels of IL-1β were also detected using three-dimensional (3D) in vitro models of breast cancer bone metastases where different breast cancer cell lines were cultured with bone tissue fragments from non-osteoporotic [41,42] and osteoporotic patients [43]; this last study also showed a higher expression of IL-1β in comparison to non-osteoporotic patients [43]. Increased IL-1β levels in a 3D model of breast cancer bone metastases were also associated with increased expression of adipokine/cytokine leptin, underling not only the critical role of IL-1β in the breast cancer bone metastatic niche but also in bone marrow adipose tissue [41]. A positive correlation between IL-1β expression and osteoprotegerin (OPG) was also found, revealing a potential role for OPG in the invasion-promoting effects of IL-1β and showing that IL-1β led to an increase in OPG production, via the p38 and p42/22 mitogen-activated protein kinase (MAPK) signaling pathway, independent of breast cancer cell subtype [18,19]. Since breast cancer cells express elevated levels of not only IL-1β but also other pro-inflammatory cytokines, Safina et al. showed, using an in vitro model showed that IL1-β and TNF-α cooperate with TGF-β in the production of MMP-9 by breast cancer cells and TGF-β activated protein kinase 1 (TAK1) is required for this process [30]. Additionally, co-culturing breast cancer cells with mice osteoblasts, it was seen that breast cancer cells were attached to the matrix, produced by osteoblasts, but grew slowly or not at all until TNF-α and IL-β addition [44]. Stimulation of cell proliferation by these cytokines was suppressed with indomethacin, an inhibitor of cyclooxygenase and of prostaglandin production, or a PGE2 receptor antagonist, showing that IL-1β and TNFα activate the arachidonic acid pathway [44]. Since the above mentioned studies underlined that IL-1β directly or through specific molecular mechanisms impacts on tumor aggressiveness and bone metastatic potential, several studies hypothesized that the blocking of IL-1β activity should have the potential to be an effective anti-cancer therapy. In fact, blocking of IL-1R signaling with the clinically licensed antagonist, i.e. anakinra, before injection of tumor cells in mice inhibited the development of metastases [45]. When the IL-1β dual selective antagonist was injected 1 week after tumour cell inoculation the existing bone metastases stopped growing [45]. Additionally, it was observed that in tumour-naïve mice, a single dose of IL-1β dual selective antagonist reduces osteoclast and osteoblast activity and IL-1β expression, also following continuous administration of IL-1β dual selective antagonist for more than 21 days [45]. Similarly, Tulotta et al. [46] using patient samples with stage II/III breast cancer, humanized mouse models of spontaneous breast cancer bone metastasis, genetic manipulation of breast cancer cells (MDA-MB-231-IL-1B+, T47D-IL-1B+ and MCF7-IL-1B+) and in vitro models (co-culture between HS5 or OB1 cells and MDA-MB-231 or T47D cells) demonstrated that not only the IL-1R antagonist, anakinra, but also the anti that a IL-1β antibody, canakinumab, inhibited breast tumor growth and progression to bone metastasis. Additionally, the production of IL-1β by tumor cells promoted epithelial–mesenchymal transition, invasion, migration, and bone colonization. Contact between tumor and osteoblasts or bone marrow cells increased IL-1β secretion from all three cell types [46].
Finally, only one study evaluated IL-1α expression using an in vitro model where breast cancer cells were treated with an endothelin-1 (ET-1) receptor dual antagonist, showing a local increase in breast cancer cell secretions of IL-1α [47].
3.2.2. IL-2
IL-2, a master activation factor for helper/regulatory T-cell and natural killer (NK) cell proliferation and differentiation, acting as a mediator for pro- and anti-inflammatory immune responses, is also associated with breast cancer bone metastases. According to the paper of Iakovou et al., who assessed the levels of serum cytokines in women with bone metastases treated with radionuclide palliative therapy (RTP), responders to therapy showed higher levels of IL-2, a cytokine released by T-helper leucocytes, which have a role in the antitumor immune response, but no prognostic value [48].
3.2.3. IL-6
IL-6, a multifunctional cytokine that was originally characterized as acting in immune and inflammatory responses, appears to play a key role in breast cancer growth and bone metastasis. Its role in breast cancer growth and bone metastasis was confirmed by evaluating blood samples from healthy, breast cancer, or metastatic breast cancer patients, where the higher levels of IL-6 were detected in patients with metastatic bone disease [49,50]. This higher expression of IL-6 in blood samples from patients with metastatic bone disease also correlated with high levels of Y-box-binding protein 1 expression, a multifunctional cold-shock protein [51], but not with cystatin C (Cyst C), an endogenous inhibitor of cysteine proteinase cathepsin K [52]. Increased levels of IL-6 were also highlighted in sera of mice with bone metastases [40,53], and by using a humanized 3D breast cancer bone metastasis model in which the higher levels of IL-6 were found in the presence of an osteoporotic microenvironment [43]. However, in order to evaluate the main contributors involved in IL-6 expression during breast cancer bone metastases, numerous in vitro and in vivo studies analyzed tumor cells, bone cells, immune cells, and the organic bone matrix. Several preclinical studies showed that metastatic breast cancer cells, directly [54,55,56,57,58,59] or through Jagged1-expressing tumor cells [58], induced osteoblasts to express high levels of IL-6 [54,55,56,57,58,59,60,61], while they seemed to not affect IL-6 production by osteoclasts [62,63]. The high expression of IL-6 by osteoblasts, particularly activated throughout the bone marrow [60], completely suppresses osteoblast functions [55] and stimulates osteoclastogenesis in the presence or absence of the receptor activator of nuclear factor kappa-Β (RANK)/RANK ligand (RANKL) pathway [56,59]. IL-6 induces the expression of RANK by breast cancer cells, which sensitizes the tumor to RANKL and enhances cancer IL-6 release [64]. RANKL and IL-6 mediate direct paracrine/autocrine signaling between cells of the osteoblast lineage and cancer cells, enhancing the growth of metastatic breast cancers within bone [59,64]. The disruption of this crosstalk by knockdown of IL-6 or RANK in breast cancer cells, or via treatment with anti-IL-6 receptor antibodies or an antagonist molecule, i.e., TB-2-081, significantly reduced breast cancer growth in bone [27,28,64,65], as well as in the presence of hormonal therapy (HT)-resistant metastatic disease where the anti-IL-6 receptor would block the IL-6hi/estrogen (ER)lo feed-forward loop [26]. Additionally, the blockade of IL-6 induced with TB-2-081 increased phosphorylation of the signal transducer and activator of transcription 3 (pSTAT3) in breast cancer cells and reduced osteolytic bone remodeling [28]. In fact, it was observed that STAT3-dependent upregulation of Notch-3, Jagged-1, and carbonic anhydrase IX correlates with growth and invasion of breast cancer cells to bone [25]. Neutralization of IL-6 was also sufficient to limit senescent-induced reactive osteoblasts which are responsible for the increased osteoclastogenesis via increased IL-6 production [66]. Moreover, the in vivo knockdown of oncostatin M (OSM), a pleiotropic IL-6 family cytokine, seems to decrease breast cancer bone metastasis [35,38]. OSM also induced osteoclast differentiation via an amphiregulin, an uncharacterized OSM-regulated bone metastasis factor, through an autocrine loop [35]. In addition, OSM skews macrophages toward an M2 polarized phenotype via the mTOR (mammalian target of rapamycin) signaling complex 2 (mTORC2) which relays signals through PKC-α (protein kinase C-α) and Akt (protein kinase B) kinases [38]. However, in addition to the effect of IL-6 antagonists, anti-IL-6 receptors, and knockdown of OSM on IL-6 expression, a reduced level of IL-6 was also observed in blood samples of women with breast cancer bone metastases treated with radionuclide palliative therapy (RPT) [48] and in breast cancer cell lines treated with the miR-520/373 family [24], or where the ABL (Abelson murine leukemia) family of non-receptor tyrosine kinases, ABL1 (also known as c-Abl) and ABL2 (also known as Arg), was depleted [36]. The decrease in IL-6 secretion by depleting ABL kinases was accompanied by an enhanced OPG expression and reduced overall RANKL/OPG ratio, thereby decreasing osteoclast differentiation and bone destruction [36]. Other potential targets for bone metastatic breast cancer could be the calcium sensing receptor (CaSR) and the bioactive lipids lysophosphatidic acid (LPA) and sphingosine 1-phospahte (S1P) [27,34]. The role of CaSR as an inhibitor of the constitutive secretion of various cytokines was detected both in cancer cells and in normal breast cells. In breast cancer cells, CaSR inhibited IL-6 secretion [27], while LPA and S1P enhanced IL-6 expression [34]. On the other hand, based on the key role of low-molecular-weight protein tyrosine phosphatase slow isoform (LMW-PTPsi) in the interplay between tumor cells and osteoclasts during bone metastases, it was seen that the knockdown of LMW-PTP and its slow isoform did not decrease IL-6 expression in breast cancer cells [67].
3.2.4. IL-8
IL-8, a prototypical member of a superfamily of small, inducible, secreted CXCs (chemokines) or α-chemokines originally identified as monocyte-derived factors capable of attracting and activating neutrophils, is expressed by a number of cancer cell lines in vitro. Numerous correlations were observed between breast cancer tumor cell IL-8 expression, plasma levels of IL-8, and metastatic potential to bone [54,68,69], also evaluating how chemical and physical properties of specific biomineralized culture platforms can alter breast cancer cell growth and secretion of tumorigenic IL-8 [70]. Increased levels of IL-8 were also highlighted in sera of mice with bone metastases and, together with IL-6, it might be responsible for the attraction of cancer stem-like cells to bone and might support the phenotypic switch [53]. Also, by using a humanized breast cancer bone metastasis model, increased levels of IL-8 were seen, in particular under osteoporotic conditions [43]. Metastatic breast cancer cells directly induce osteoblasts to express increased levels of IL-8 [54,56,68,71,72,73], especially under osteoporotic conditions [71], in the presence or in absence of the RANK/RANKL pathway [56]. Osteoblasts release several growth factors and, among them, TGF-β1 (Transforming Growth Factor – β1) is an important contributor to extracellular signal-related kinase (ERK), p38, and c-Jun N-terminal kinase (JNK) activation [72], which lead to the activation of activator protein 1(AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) on the IL-8 promoter, and which initiate IL-8 release, thus promoting breast cancer cell migration and osteoclastogenesis [21,54,69,72]. In addition, IL-8 and other pro-inflammatory cytokines also cooperate with TGF-β1 in the production of matrix metalloproteinase-9 (MMP-9) by breast cancer cells, and TGF-β-activated protein kinase 1 is required for this response, contributing to the metastatic potential of breast cancer cells [30]. By using measurements of osteoblast and osteoclast differentiation and function in vitro and a mouse model of skeletal metastasis, it was demonstrated that both soluble Sema4D (semaphoring 4D)and protein, an immune semaphore expressed by T-lymphocytes and eosinophils and to a lesser extent by dendritic cells and B-lymphocytes, produced by the breast cancer cell line, inhibit the differentiation of osteoblast cells, while Sema4D-mediated induction of IL-8 and LIX/CXCL5 (C-X-C motif chemokine 5), the murine homologue of IL-8, increases osteoclast numbers and activity [37]. Using in vitro assays of osteoclastogenesis and bone resorption, it was shown that enhanced osteoclastogenesis also requires the presence of syndecan-1, a proteoglycan whose shedding from tumor cell surfaces is enhanced by the expression of heparanase and protein agents, such as IL-8 [31]. Others showed that breast cancer cells can induce the release of lysophosphatidic acid (LPA) from activated platelets which, in turn, promotes tumor cell proliferation and the LPA-dependent secretion of IL-8, thereby also enhancing tumor growth and osteolysis [33,34]. Despite several factors, proteins, and mechanisms that lead to high expression of IL-8, it was also seen that metastatic breast cancer cells treated with the miR-520/373 family [24] or knockdown for LMW-PTP and its slow isoform [67] showed a decrease level of IL-8 expression in breast cancer cells [24,67]. In addition, similar results were observed in vivo using a nude mice model injected into the tibia with metastatic breast cancer cells and treated via neutralizing IL-8 [52] or knockdown of osterix [29], a zinc finger-containing transcription factor essential for osteoblast differentiation and bone formation, suggesting a novel and attractive target for the control of bone metastasis of breast cancers [29].
3.2.5. IL-10
Salamanna et al. found that osteoporosis modulates the release of cytokines as the anti-inflammatory mediator IL-10, in a 3D in vitro model where bone samples were cultivated with MCF-7 [43].
3.2.6. IL-11
IL-11, a member of the IL-6 family, is an osteolytic factor produced by breast cancer cells, and its expression seems to be associated with the development of bone metastases. Mendoza et al. showed that IL-11 expression is regulated by runt-related transcription factor 2 (Runx2) in human breast cancer cells [74]. Induction of IL-11 in metastatic breast cancer cells is controlled by TGF-β in a dose-dependent manner [75] through different signaling pathways, such as SMAD (small mother against decapentaplegic) and p38 MAPK [21]. TGF-β-induced IL-11 expression can also be regulated by specific microRNAs (miRNAs). Pollari et al. [22,23] identified miR-204, -211, and -379, which downregulate the TGF-β-induced expression of IL-11. Additionally, miR-124 negatively regulates IL-11 expression in vitro and in vivo, representing a key pathogenetic process in breast cancer metastasis [76]. A key mechanism in the induction of IL-11 is represented by the JAK1/STAT3 (Janus kinase/signal transducer and activator of transcription protein) pathway. Patients with breast cancer bone metastases showed higher levels of serum IL-11, tumor tissue IL-11 messenger RNA (mRNA), and IL-11 immunohistochemical staining in comparison with patients with primary breast cancer only, associated with a higher expression of p-p38, p-c-JUN, and p-STAT3, suggesting that IL-11 plays an important role in regulating bone metastasis in breast cancer, and that its level in serum may be used to assess the risk of bone metastasis from primary breast cancer [77]. Liang et al. also found that IL-11 is capable of inducing osteoclastogenesis by activating the JAK1/STAT3 signaling pathway, which induces the expression of c-Myc, a necessary factor required for osteoclastogenesis, independent of RANKL [32]. This represents a controversial issue, as McCoy and colleagues reported that IL-11 promotes RANKL-induced osteoclast (OC) differentiation [78]. A different signaling pathway can be involved, as the synthesis of IL-11 by breast cancer cells is induced by LPA through a specific PKC delta subtype, in the enhancement of breast cancer cell-mediated OC development [33]. Finally, Irawam et al. described a direct significant association between the expression of IL-11-RA and bone metastasis incidence in patients with advanced breast cancer [79].
3.2.7. IL-12
One paper evaluated the in vitro synthesis of the anti-inflammatory cytokine IL-12, reporting that it is increased by the treatment of 4T1 mouse breast cancer cells with endothelin-1 [47].
3.2.8. IL-15
Bottos et al. showed that the JAK/STAT pathway is activated in breast cancer bone metastasis. In fact, the blocking of the JAK/STAT pathway with JAK inhibitors (JAKi) increased the metastatic burden. The treatment of tumor-bearing mice with IL-15, a cytokine crucial for natural killer (NK), NK and memory (m) CD8+ (cluster of differentiation 8) T-cell function and homeostasis, in addition to JAKi, caused an increase in NK cell population and a reduction in metastases, preventing the JAKi-mediated increase in metastases [80].
3.2.9. IL-17
Some papers focused on IL-17, a pro-inflammatory cytokine produced by breast cancer cells, hBMSCs (human bone marrow stem cells), and T-cells. Jewell et al. reported a decrease in the synthesis of IL-17 in a 4T1 mouse metastasis model upon in vivo treatment with an endothelin-1 antagonist, associated with a reduction in tumor mass and bone metastasis [47]. Goldstein evaluated the role of hBMSCs in the process of bone metastases and found that IL-17B can drive migration and metastasis. The in vitro treatment of breast cancer cells with hBMSC-derived IL-17B increased the migration of metastatic breast cancer cells, with no effect on non-metastatic breast cancer cells (MCF-7). An increase in the expression of IL-17B receptor was also observed in mouse bone metastases when compared to primary mammary tumor, as confirmed by the in vitro study in which the over-expression of IL-17BR in breast cancer cells caused an increase in migration [81]. Finally, Monteiro et al. showed that T-cells establish a pre-metastatic niche and that metastatic breast cancer cells induce the synthesis of osteclastogenic cytokines such as IL-17F and RANKL by CD4+ T-cells [40].
3.2.10. IL-18
In the work by Nayir et al., the serum levels of pro-inframammary IL-18, a member of the IL-1 superfamily, were compared among breast cancer women with or without bone metastases and healthy controls, showing a decrease in IL-18 levels in response to decreased skeletal tumor burden [82].
3.2.11. IL-20
One paper evaluated the function of IL-20, a cytokine with a pro-inflammatory effect structurally related to the IL-10 family, and high expression levels of IL-20 were found in primary breast tumor tissue and bone-metastatic tissue [83]. IL-20 expression in breast cancer tissue was associated with a higher mitotic rate, advanced tumor stage, and bone metastasis. IL-20 determines an increase in breast cancer cell growth, migration, and protease expression in vitro. In a mouse model of bone metastases, treatment with anti-IL-20 mAb 7E was able to reduce tumor growth, bone colonization, and osteolysis (see in Table 1) [83].
3.3. Findings from Clinical Trials
The 11 clinical trials included in the review studied six ILs, i.e., IL-1, IL-2, IL-3, IL-7, IL-12, and anti-IL-6R (Table 2). The most studied ILs were IL-2 (n = 4) and IL-12 (n = 3).
IL-2 and IL-2 variants were used alone or in association with monoclonal antibodies, drugs to treat low blood neutrophils, and proteins for the evaluation of safety, tolerability, toxicity, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity in treating patients with metastatic breast cancer that did not respond to previous therapy.
In women with metastatic breast cancer, IL-12 was studied to evaluate the efficacy of high-dose chemotherapy and peripheral stem-cell transplantation or to determine its activity as defined by the percentage of patients who did not progress after six months of therapy. IL-12 may eradicate tumor cells by blocking blood flow to the tumor and by soliciting patient white blood cells to destroy breast cancer cells. An additional trial on IL-12 evaluated the toxicity and maximum tolerated dose of intratumoral injection of the adenovirus-mediated human IL-12 gene in women with metastatic breast cancer.
Trials on the administration of IL-1, IL-3, anti-IL-6R, and IL-7 principally evaluated the toxicity, in terms of maximal tolerated dose of these ILs in metastatic breast cancer patients. However, it is important to underline that, although the majority of the trials were completed (8/11) no study results were posted in the clinical registries. The results of these clinical trials are awaited with interest because they may provide valuable information on the use of these ILs alone or in combination with other therapeutic regimes to treat patients with breast cancer bone metastasis.
4. Discussion
In this systematic review, we presented an overview on ILs that have an already known or putative role, function, and mechanism of action in breast cancer cells homing to bone and that were used or are actually used in therapeutic regimes for breast cancer bone metastasis management.
The reviewed preclinical studies identified several ILs linked to breast cancer metastases to bone, i.e., IL-1β, IL-2, IL-6, IL-8, IL-10, IL-11, IL-15, IL-17, IL-18, and IL-20. Of them, IL-6, IL-8, IL-1β, and IL-11 were the ILs that showed a more critical bone tropic role. These ILs support the vicious cycle of breast cancer bone metastasis and promote bone metastasis when over-expressed or downregulated. However, the review also recognized proof for additional and potential roles and mechanisms of action of these ILs. IL-1β seems to be stimulated by OPG that acts in invasion and promotion, in the regulation of IL-11 and IL-8 production from TGF-β signaling pathways, for specific miRNAs that control TGF-β-induced IL-11 expression and for a series of mechanisms of action and roles related to IL-6 (e.g., STAT3 and its downstream effectors, Jagged1, calcium-sensing receptor, and IL6/Notch3 signaling). Most of these preclinical studies were in vitro and, in order to evaluate the abovementioned roles, functions, and mechanisms of ILs in breast cancer bone metastases pathogenesis and therapy, used different approaches. These approaches can be categorized as monoculture, co-culture, and two-dimensional (2D) and 3D cultures. This high number of in vitro studies is probably due to the fact that in vitro models are essential for high-performance assay execution. While in vivo models better mimic the native bone microenvironment in comparison to in vitro models, they frequently do not consent a methodical analysis of individual stimuli. Thus, in vitro models were probably favorable in the study of ILs in breast cancer bone metastasis, since they permitted evaluating signaling pathways, specific roles, and mechanisms of ILs critical for the metastatic colonization of bone. In this review, the analyzed in vivo studies evaluated specific IL level/expression, and, above all, the IL-based treatments after breast cancer cell injection (i.e., into blood circulation, intracardiac, intraosseous). Only small animal models were used in these studies. Several reports studied human breast cancer cell metastasis in a non-human host, using immunodeficient mice, but they did not have an immune response that could be of key importance for bone metastasis. On the other hand, some other studies used immunocompetent mice with immune response factors, but murine breast tumors basically differ from human ones. Another important issue in the preclinical studies examined in this review concerns the cell lines used, both in the in vitro and the in vivo studies that were not always completely able to mimic the intricate scenario of the clinical breast cancer bone metastasis. In detail, in the evaluated studies, the most used cell lines were human MDA-MB-231 and murine 4T1 breast cancer cells. These two cell lines are estrogen receptor-negative (ER−), although bone metastases develop more frequently from ER+ breast cancers. However, in the majority, but not all, of the studies, these cells were compared to ER+ breast cancer cells lines, such as T47D, MCF-7, ZR-75-1, and BT-474, among others. Despite many times neglected, these factors should be considered when interpreting research findings because the possibility to use ER+ breast cancer cells allows better modeling the natural processes of breast cancer metastasis to bone. Nine in vitro studies also used patient peripheral venous blood and/or tumor tissue samples. Half of these studies [49,51,77,79] reported detailed demographic and clinical–pathological information such as age of primary diagnosis of breast cancer and bone metastasis, primary breast cancer histology, hormone receptor (estrogen, progesterone) and HER2 condition, breast cancer molecular subtype, sites of metastases at breast cancer presentation, if metastatic, or at time of recurrence, date and site of disease recurrence, date of bone progression, and date of skeletal-related events (SREs), while the other half reported less or no information on derived patient fluids or tissues. However, all the studies reported the patient number and the median age, and almost all (5/8) had a control group (healthy patients or breast cancer patients without bone metastases). The presence of these clinical–pathological variables allows a more complete and accurate association between the expression of specific biomarkers (ER, Progesterone Receptor, Human Epidermal Growth Factor Receptor 2-HER2-, molecular subtypes) and ILs with breast cancer bone metastasis incidence.
Although most of the preclinical studies identified specific key ILs which promote breast cancer metastases to bone, which have a pro-metastatic effects (IL-6, IL-8, IL-1β, IL-11), and whose inhibition also shows potential preclinical therapeutic effects, the ongoing clinical trials seemed to focus principally on ILs that have anti-metastatic effects and a potential to generate a localized and systemic antitumor response. In fact, only one trial evaluated the dose-limiting toxicity of the anti-IL-6R monoclonal antibody tocilizumab in combination with trastuzumab and pertuzumab in subjects with metastatic HER2-positive breast cancer. Most of the trials focused on IL-2 and IL-12, used alone or associated with other drugs, to eradicate tumor cells by blocking blood flow to the tumor and by soliciting patient white blood cells to destroy breast cancer cells. IL-2 was characterized as a T-cell growth factor, which stimulates a broad range of effector cells, including T- and B-lymphocytes, monocytes, macrophages, and NK cells, while IL-12 causes the production of IFN-γ (interferon – γ), promotes the differentiation of helper T-cells (Th1), and establishes a connection between adoptive immunity and innate resistance. Our results show that the few preclinical literature data dealt with IL-2 or IL-12 as a response to therapies, rather than the evaluation of their contribution to the antitumor effect. Additionally, a low number of preclinical data were also present prior to 2008 and demonstrated that IL-2 and IL-12 have potent antitumor and antimetastatic activities in several murine tumor models through an immune-mediated, T-cell-dependent mechanism [84]. The trials focused on the immunotherapy principle that works to augment the cellular immune responses toward differentiation, thus achieving IL concentrations adequate to incite effector cells to induce antitumor responses in the tumor microenvironment. Despite the importance that these trials could have for the scientific community, the trial design or the information reported on the clinical registries was almost always not well structured, with the majority of the trials without randomization and with an inadequate or unclear concealment of treatment allocation. Most importantly, none of these trials posted the results and, unfortunately, without accessible and usable reports, the trials fail to help patients and their clinicians.
However, despite the limits related to the clinical trials, the main emergent question from this IL overview is as follows: “Why are preclinical studies predominantly focused on pro-inflammatory ILs that have a pro-metastatic effect, while clinical trials are mainly oriented toward anti-inflammatory IL-based therapy that has an anti-metastatic effect?” The most correct answer could be found in popular wisdom that “most things in life are a double-edged sword”. However, this is not a scientifically correct answer, but it actually holds some truth. ILs represent a considerable number of soluble factors, and they are considered as key targets in anti-cancer immunotherapy. This review showed that a large number of pro-inflammatory ILs (IL-1, IL-6, IL-8, IL-11, and others) generated by host immune cells and/or tumor cells are linked with tumor aggressiveness, as confirmed by numerous preclinical studies. On the contrary, anti-inflammatory ILs (IL-2, IL-12, IL-10, and others) frequently activate antitumor immunity, thus interfering with tumor growth. These demonstrations could offer a promising therapeutic approach [85]. Although many IL functions are already well known, others still remain to be better evaluated, while others are yet to be discovered; thus, the understanding of the basic biological mechanisms of this complex immune microenvironment and the recognition of the effector functions should be augmented and potentiated to effectively target and destroy breast cancer bone metastasis.
5. Conclusions and Implications for Future Research
Bone is the preferred site for breast cancer metastasis. In vitro studies suggest that some pro-inflammatory ILs firmly correlate with augmented breast cancer cell aggressiveness. Even though in vivo reports are in their primitive stages, those performed up to now confirmed these findings. In particular, one review highlighted that some IL signaling is associated with breast cancer bone metastasis, and the inhibition of these ILs leads to a reduction in bone metastases. Conversely, other clinical trials focused on anti-inflammatory IL therapies that should correlate with reduced aggressiveness of breast cancer cell lines through the killing of tumor cells, by stopping the blood flow to the tumor and stimulating patient white blood cells to eradicate breast cancer cells. However, no conclusion can be finalized regarding clinical trials since none of them reported results on the safety, tolerability, toxicity, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of these therapies.
Further preclinical studies are specifically necessary to augment the understanding of cellular and molecular relations and signaling pathways, both up- and downstream of ILs recognized in this review, which have or could have critical roles in breast cancer metastases to bone. Additionally, clinical trials with detailed specifications on randomization, concealment of treatment allocation, blinding, co-interventions, compliance to the interventions and timing of outcome assessment for intervention, and posted results are mandatory, since they may be able to identify supplementary essential factors and/or therapies for breast cancer metastases to bone.
Based on the preclinical results and on the initial clinical results, the translation potential of IL-based therapies could represent a novel therapeutic approach for breast cancer patients at risk of progressing to bone metastasis. However, despite these preliminary results, to date, IL-based therapies still require more extensive analyses to confirm and extend their use through the design of new and advanced preclinical studies and prospective randomized trials.
Author Contributions
F.S. and M.F. designed the review. F.S. and V.B. performed the literature search. F.S., V.B., and D.C. analyzed the obtained articles. F.S., V.B., D.C., and M.F. wrote the paper. F.S., D.C., and G.G. collected and assembled the data. G.G. and V.C. revised the manuscript critically. F.S., M.F., D.C., and V.B. finally approved the article. All authors read and approved the submitted manuscript version.
Funding
This work was supported by grants from IRCCS Istituto Ortopedico Rizzoli (Ricerca Corrente), by the 5 × 1000 Project, year 2017, and by the Project “Oncologia di Precisione e Nuove Terapie Antitumorali (ONCOPENTA); Sviluppo di modelli preclinici avanzati per il trattamento locale di tumori primitivi e metastatici”.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this article.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Liede, A.; Cai, M.; Crouter, T.F.; Niepel, D.; Callaghan, F.; Evans, D.G. Risk-reducing mastectomy rates in the US: A closer examination of the Angelina Jolie effect. Breast Cancer Res. Treat. 2018, 171, 435–442. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The science and practice of bone health in oncology: Managing bone loss and metastasis in patients with solid tumors. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. S7), S1–S29. [Google Scholar] [CrossRef]
- Selvaggi, G.; Scagliotti, G.V. Management of bone metastases in cancer: A review. Crit. Rev. Oncol. Hematol. 2005, 56, 365–367. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef]
- Phadke, P.A.; Mercer, R.R.; Harms, J.F.; Jia, Y.; Frost, A.R.; Jewell, J.L.; Bussard, K.M.; Nelson, S.; Moore, C.; Kappes, J.C.; et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin. Cancer Res. 2006, 12, 1431–1440. [Google Scholar] [CrossRef]
- Weinberg, R.A. Coming full circle-from endless complexity to simplicity and back again. Cell 2014, 157, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Chirgwin, J.M.; Mohammad, K.S.; Guise, T.A. Tumor-bone cellular interactions in skeletal metastases. J. Musculoskelet. Neuronal Interact. 2004, 4, 308–318. [Google Scholar] [PubMed]
- Guise, T.A.; Kozlow, W.M.; Heras-Herzig, A.; Padalecki, S.S.; Yin, J.J.; Chirgwin, J.M. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer 2005, 5 (Suppl. S2), S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Vargas, G.; Pape, F.L.; Clézardin, P. Cancer Cell Colonisation in the Bone Microenvironment. Int. J. Mol. Sci. 2016, 17, E1674. [Google Scholar] [CrossRef] [PubMed]
- Ottewell, P.D. The role of osteoblasts in bone metastasis. J. Bone Oncol. 2016, 5, 124–127. [Google Scholar] [CrossRef]
- Virk, M.S.; Lieberman, J.R. Tumor metastasis to bone. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S5. [Google Scholar] [CrossRef]
- Kapoor, P.; Suva, L.J.; Welch, D.R.; Donahue, H.J. Osteoprotegrin and the bone homing and colonization potential of breast cancer cells. J. Cell Biochem. 2008, 103, 30–41. [Google Scholar] [CrossRef]
- Chung, S.T.; Geerts, D.; Roseman, K.; Renaud, A.; Connelly, L. Osteoprotegerin mediates tumor-promoting effects of Interleukin-1 beta in breast cancer cells. Mol. Cancer 2017, 16, 27. [Google Scholar] [CrossRef]
- Fong, Y.C.; Maa, M.C.; Tsai, F.J.; Chen, W.C.; Lin, J.G.; Jeng, L.B.; Yang, R.S.; Fu, W.M.; Tang, C.H. Osteoblast-derived TGF-beta1 stimulates IL-8 release through AP-1 and NF-kappaB in human cancer cells. J. Bone Min. Res. 2008, 23, 961–970. [Google Scholar] [CrossRef]
- Gupta, J.; Robbins, J.; Jilling, T.; Seth, P. TGFβ-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol. Ther. 2011, 11, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Pollari, S.; Käkönen, R.S.; Mohammad, K.S.; Rissanen, J.P.; Halleen, J.M.; Wärri, A.; Nissinen, L.; Pihlavisto, M.; Marjamäki, A.; Perälä, M.; et al. Heparin-like polysaccharides reduce osteolytic bone destruction and tumor growth in a mouse model of breast cancer bone metastasis. Mol. Cancer Res. 2012, 10, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Pollari, S.; Leivonen, S.K.; Perälä, M.; Fey, V.; Käkönen, S.M.; Kallioniemi, O. Identification of microRNAs inhibiting TGF-β-induced IL-11 production in bone metastatic breast cancer cells. PLoS ONE 2012, 7, e37361. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglou, I.; Koerner, C.; Schmidt, C.; Zhang, J.D.; Heckmann, D.; Shavinskaya, A.; Allgayer, H.; Gückel, B.; Fehm, T.; Schneeweiss, A.; et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene 2012, 31, 4150–4163. [Google Scholar] [CrossRef] [PubMed]
- Studebaker, A.W.; Storci, G.; Werbeck, J.L.; Sansone, P.; Sasser, A.K.; Tavolari, S.; Huang, T.; Chan, M.W.; Marini, F.C.; Rosol, T.J.; et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008, 68, 9087–9095. [Google Scholar] [CrossRef]
- Sansone, P.; Ceccarelli, C.; Berishaj, M.; Chang, Q.; Rajasekhar, V.K.; Perna, F.; Bowman, R.L.; Vidone, M.; Daly, L.; Nnoli, J.; et al. Self-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat. Commun. 2016, 7, 10442. [Google Scholar] [CrossRef]
- Hernández-Bedollaì, M.A.; Carretero-Ortega, J.; Valadez-Sánchez, M.; Vázquez-Prado, J.; Reyes-Cruz, G. Chemotactic and proangiogenic role of calcium sensing receptor is linked to secretion of multiple cytokines and growth factors in breast cancer MDA-MB-231 cells. Biochim. Biophys. Acta 2015, 1853, 166–182. [Google Scholar] [CrossRef]
- Remeniuk, B.; King, T.; Sukhtankar, D.; Nippert, A.; Li, N.; Li, F.; Cheng, K.; Rice, K.C.; Porreca, F. Disease modifying actions of interleukin-6 blockade in a rat model of bone cancer pain. Pain 2018, 159, 684–698. [Google Scholar] [CrossRef]
- Yao, B.; Wang, J.; Qu, S.; Liu, Y.; Jin, Y.; Lu, J.; Bao, Q.; Li, L.; Yuan, H.; Ma, C. Upregulated osterix promotes invasion and bone metastasis and predicts for a poor prognosis in breast cancer. Cell Death Dis. 2019, 10, 28. [Google Scholar] [CrossRef]
- Safina, A.; Sotomayor, P.; Limoge, M.; Morrison, C.; Bakin, A.V. TAK1-TAB2 signaling contributes to bone destruction by breast carcinoma cells. Mol. Cancer Res. 2011, 9, 1042–1053. [Google Scholar] [CrossRef]
- Kelly, T.; Suva, L.J.; Nicks, K.M.; MacLeod, V.; Sanderson, R.D. Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis. J. Bone Min. Res. 2010, 25, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ma, Q.; Ding, N.; Luo, F.; Bai, Y.; Kang, F.; Gong, X.; Dong, R.; Dai, J.; Dai, Q.; et al. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Jagga, S.; Lee, Y.H.; Sharma, G.; Lee, S.S. Lysophosphatidic acid enhances breast cancer cells-mediated osteoclastogenesis. Korean J. Physiol. Pharmacol. 2018, 22, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Boucharaba, A.; Guillet, B.; Menaa, F.; Hneino, M.; van Wijnen, A.J.; Clézardin, P.; Peyruchaud, O. Bioactive lipids lysophosphatidic acid and sphingosine 1-phosphate mediate breast cancer cell biological functions through distinct mechanisms. Oncol. Res. 2009, 18, 173–184, Erratum in: Oncol. Res. 2009, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Bolin, C.; Tawara, K.; Sutherland, C.; Redshaw, J.; Aranda, P.; Moselhy, J.; Anderson, R.; Jorcyk, C.L. Oncostatin m promotes mammary tumor metastasis to bone and osteolytic bone degradation. Genes Cancer 2012, 3, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rouse, C.; Jasper, J.S.; Pendergast, A.M. ABL kinases promote breast cancer osteolytic metastasis by modulating tumor-bone interactions through TAZ and STAT5 signaling. Sci. Signal. 2016, 9, ra12. [Google Scholar] [CrossRef]
- Yang, Y.H.; Buhamrah, A.; Schneider, A.; Lin, Y.L.; Zhou, H.; Bugshan, A.; Basile, J.R. Semaphorin 4D Promotes Skeletal Metastasis in Breast Cancer. PLoS ONE 2016, 11, e0150151. [Google Scholar] [CrossRef]
- Shrivastava, R.; Asif, M.; Singh, V.; Dubey, P.; Ahmad Malik, S.; Lone, M.U.; Tewari, B.N.; Baghel, K.S.; Pal, S.; Nagar, G.K.; et al. M2 polarization of macrophages by Oncostatin M in hypoxic tumor microenvironment is mediated by mTORC2 and promotes tumor growth and metastasis. Cytokine 2019, 118, 130–143. [Google Scholar] [CrossRef]
- Nutter, F.; Holen, I.; Brown, H.K.; Cross, S.S.; Evans, C.A.; Walker, M.; Coleman, R.E.; Westbrook, J.A.; Selby, P.J.; Brown, J.E.; et al. Different molecular profiles are associated with breast cancer cell homing compared with colonisation of bone: Evidence using a novel bone-seeking cell line. Endocr. Relat. Cancer 2014, 21, 327–341. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Leal, A.C.; Gonçalves-Silva, T.; Mercadante, A.C.; Kestelman, F.; Chaves, S.B.; Azevedo, R.B.; Monteiro, J.P.; Bonomo, A. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 2013, 8, e68171. [Google Scholar] [CrossRef]
- Templeton, Z.S.; Lie, W.R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; Maloney, W.J.; Contag, C.H.; et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia 2015, 17, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Nutter, F.; Wilkinson, J.M.; Evans, C.A.; Avgoustou, P.; Ottewell, P.D. Human breast cancer bone metastasis in vitro and in vivo: A novel 3D model system for studies of tumour cell-bone cell interactions. Clin. Exp. Metastasis 2015, 32, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Borsari, V.; Brogini, S.; Torricelli, P.; Cepollaro, S.; Cadossi, M.; Fini, M. A Human 3D In Vitro Model to Assess the Relationship Between Osteoporosis and Dissemination to Bone of Breast Cancer Tumor Cells. J. Cell Physiol. 2017, 232, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Sosnoski, D.M.; Norgard, R.J.; Grove, C.D.; Foster, S.J.; Mastro, A.M. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin. Exp. Metastasis 2015, 32, 335–344. [Google Scholar] [CrossRef]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef]
- Tulotta, C.; Lefley, D.V.; Freeman, K.; Gregory, W.M.; Hanby, A.M.; Heath, P.R.; Nutter, F.; Wilkinson, J.M.; Spicer-Hadlington, A.R.; Liu, X.; et al. Endogenous Production of IL1B by Breast Cancer Cells Drives Metastasis and Colonization of the Bone Microenvironment. Clin. Cancer Res. 2019, 25, 2769–2782. [Google Scholar] [CrossRef]
- Jewell, A.N.; Swamydas, M.; Castillo, C.I.; Wyan, H.; Allen, L.D.; McDermott, K.A.; Eddy, J.M.; Dréau, D. The endothelin axis stimulates the expression of pro-inflammatory cytokines and pro-migratory molecules in breast cancer. Cancer Investig. 2010, 28, 932–943. [Google Scholar] [CrossRef]
- Iakovou, I.; Doumas, A.; Badiavas, K.; Mpalaris, V.; Frangos, S.; Farmakis, G. Pain palliative therapy in women with breast cancer osseous metastatic disease and the role of specific serum cytokines as prognostic factors. Cancer Biother. Radiopharm. 2014, 29, 116–123. [Google Scholar] [CrossRef]
- Dean-Colomb, W.; Hess, K.R.; Young, E.; Gornet, T.G.; Handy, B.C.; Moulder, S.L.; Ibrahim, N.; Pusztai, L.; Booser, D.; Valero, V.; et al. Elevated serum P1NP predicts development of bone metastasis and survival in early-stage breast cancer. Breast Cancer Res. Treat. 2013, 137, 631–636. [Google Scholar] [CrossRef][Green Version]
- Noman, A.S.; Uddin, M.; Chowdhury, A.A.; Nayeem, M.J.; Raihan, Z.; Rashid, M.I.; Azad, A.K.; Rahman, M.L.; Barua, D.; Sultana, A.; et al. Serum sonic hedgehog (SHH) and interleukin-(IL-6) as dual prognostic biomarkers in progressive metastatic breast cancer. Sci. Rep. 2017, 7, 1796. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Bettencourt, M.; Alho, I.; Costa, A.L.; Sousa, A.R.; Mansinho, A.; Abreu, C.; Pulido, C.; Macedo, D.; Vendrell, I.; et al. Serum YB-1 (Y-box binding protein 1) as a biomarker of bone disease progression in patients with breast cancer and bone metastases. J. Bone Oncol. 2017, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tumminello, F.M.; Badalamenti, G.; Incorvaia, L.; Fulfaro, F.; D’Amico, C.; Leto, G. Serum interleukin-6 in patients with metastatic bone disease: Correlation with cystatin C. Med. Oncol. 2009, 26, 10–15. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Patanè, S.; Grange, C.; Bussolati, B.; Isella, C.; Fontani, L.; Godio, L.; Cilli, M.; D’Amelio, P.; Isaia, G.; et al. Primary breast cancer stem-like cells metastasise to bone, switch phenotype and acquire a bone tropism signature. Br. J. Cancer 2013, 108, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Kinder, M.; Chislock, E.; Bussard, K.M.; Shuman, L.; Mastro, A.M. Metastatic breast cancer induces an osteoblast inflammatory response. Exp. Cell Res. 2008, 314, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Dhurjati, R.; Krishnan, V.; Shuman, L.A.; Mastro, A.M.; Vogler, E.A. Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clin. Exp. Metastasis 2008, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Venzon, D.J.; Mastro, A.M. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J. Cell Biochem. 2010, 111, 1138–1148. [Google Scholar] [CrossRef]
- Krishnan, V.; Shuman, L.A.; Sosnoski, D.M.; Dhurjati, R.; Vogler, E.A.; Mastro, A.M. Dynamic interaction between breast cancer cells and osteoblastic tissue: Comparison of two- and three-dimensional cultures. J. Cell Physiol. 2011, 226, 2150–2158. [Google Scholar] [CrossRef]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef]
- Krishnan, V.; Vogler, E.A.; Sosnoski, D.M.; Mastro, A.M. In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. J. Cell Physiol. 2014, 229, 453–462. [Google Scholar] [CrossRef]
- Bussard, K.M.; Okita, N.; Sharkey, N.; Neuberger, T.; Webb, A.; Mastro, A.M. Localization of osteoblast inflammatory cytokines MCP-1 and VEGF to the matrix of the trabecula of the femur, a target area for metastatic breast cancer cell colonization. Clin. Exp. Metastasis 2010, 27, 331–340. [Google Scholar] [CrossRef]
- Rajski, M.; Vogel, B.; Baty, F.; Rochlitz, C.; Buess, M. Global gene expression analysis of the interaction between cancer cells and osteoblasts to predict bone metastasis in breast cancer. PLoS ONE 2012, 7, e29743. [Google Scholar] [CrossRef] [PubMed]
- Liverani, C.; Mercatali, L.; Spadazzi, C.; La Manna, F.; De Vita, A.; Riva, N.; Calpona, S.; Ricci, M.; Bongiovanni, A.; Gunelli, E.; et al. CSF-1 blockade impairs breast cancer osteoclastogenic potential in co-culture systems. Bone 2014, 66, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mazaki, A.; Orita, S.; Inage, K.; Suzuki, M.; Abe, K.; Shiga, Y.; Inoue, M.; Norimoto, M.; Umimura, T.; Ohtori, S.; et al. Tumor Necrosis Factor-α Produced by Osteoclasts Might Induce Intractable Pain in a Rat Spinal Metastasis Model of Breast Cancer. Spine Surg. Relat. Res. 2019, 3, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chow, S.O.; Boernert, K.; Basel, D.; Mikuscheva, A.; Kim, S.; Fong-Yee, C.; Trivedi, T.; Buttgereit, F.; Sutherland, R.L.; et al. Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways. J. Bone Min. Res. 2014, 29, 1938–1949. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Hamaguchi, T.; Nagao, N.; Kato, S.; Iino, T.; Nakamura, T.; Sudo, A. Interleukin-6 receptor inhibitor suppresses bone metastases in a breast cancer cell line. Breast Cancer 2018, 25, 566–574. [Google Scholar] [CrossRef]
- Luo, X.; Fu, Y.; Loza, A.J.; Murali, B.; Leahy, K.M.; Ruhland, M.K.; Gang, M.; Su, X.; Zamani, A.; Shi, Y.; et al. Stromal-Initiated Changes in the Bone Promote Metastatic Niche Development. Cell Rep. 2016, 14, 82–92. [Google Scholar] [CrossRef]
- Alho, I.; Costa, L.; Bicho, M.; Coelho, C. Low Molecular Weight Protein Tyrosine Phosphatase Slow Isoform Knockdown in MDA-MB-435 Cells Decreases RAW 264.7 Osteoclastic Differentiation. Anticancer Res. 2016, 36, 2227–2232. [Google Scholar]
- Schwaninger, R.; Rentsch, C.A.; Wetterwald, A.; van der Horst, G.; van Bezooijen, R.L.; van der Pluijm, G.; Löwik, C.W.; Ackermann, K.; Pyerin, W.; Hamdy, F.C.; et al. Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am. J. Pathol. 2007, 170, 160–175. [Google Scholar] [CrossRef]
- Kamalakar, A.; Bendre, M.S.; Washam, C.L.; Fowler, T.W.; Carver, A.; Dilley, J.D.; Bracey, J.W.; Akel, N.S.; Margulies, A.G.; Skinner, R.A.; et al. Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone 2014, 61, 176–185. [Google Scholar] [CrossRef]
- Choi, S.; Coonrod, S.; Estroff, L.; Fischbach, C. Chemical and physical properties of carbonated hydroxyapatite affect breast cancer cell behavior. Acta Biomater. 2015, 24, 333–342. [Google Scholar] [CrossRef]
- Pagani, S.; Fini, M.; Giavaresi, G.; Salamanna, F.; Borsari, V. The active role of osteoporosis in the interaction between osteoblasts and bone metastases. Bone 2015, 79, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, J.; Ji, Y.; Hong, A.; Xie, Q. Cytokines in osteoblast-conditioned medium promote the migration of breast cancer cells. Tumour Biol. 2014, 35, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Castro, N.J.; Cui, H.; Zhou, X.; Boualam, B.; McGrane, R.; Glazer, R.I.; Zhang, L.G. A 3D printed nano bone matrix for characterization of breast cancer cell and osteoblast interactions. Nanotechnology 2016, 27, 315103. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villanueva, D.; Zeef, L.; Shore, P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011, 13, R106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Z.; Gupta, J.; Krimmel, J.D.; Gerseny, H.M.; Berg, A.F.; Robbins, J.S.; Du, H.; Prabhakar, B.; Seth, P. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther. 2012, 19, 630–636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, W.L.; Huang, W.D.; Li, B.; Chen, T.R.; Li, Z.X.; Zhao, C.L.; Li, H.Y.; Wu, Y.M.; Yan, W.J.; Xiao, J.R. microRNA-124 inhibits bone metastasis of breast cancer by repressing Interleukin-11. Mol. Cancer 2018, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, X.; Dong, Z.; Liu, J.; Zhang, S. Bone metastasis from breast cancer involves elevated IL-11 expression and the gp130/STAT3 pathway. Med. Oncol. 2013, 30, 634. [Google Scholar] [CrossRef]
- McCoy, E.M.; Hong, H.; Pruitt, H.C.; Feng, X. IL-11 produced by breast cancer cells augments osteoclastogenesis by sustaining the pool of osteoclast progenitor cells. BMC Cancer 2013, 13, 16. [Google Scholar] [CrossRef]
- Irawan, C.; Atmakusumah, D.; Siregar, N.C.; Tean, T.B.; Kong, L.W.; Kiat, O.C.; Abdulmuthalib, A.; Harahap, A.; Mansyur, M. Expression of Biomarkers CXCR4, IL11-RA, TFF1, MLF1P in Advanced Breast Cancer Patients with Bone Metastatic: A Diagnostic Study. Acta Med. Indones. 2016, 48, 261–268. [Google Scholar]
- Bottos, A.; Gotthardt, D.; Gill, J.W.; Gattelli, A.; Frei, A.; Tzankov, A.; Sexl, V.; Wodnar-Filipowicz, A.; Hynes, N.E. Decreased NK-cell tumour immunosurveillance consequent to JAK inhibition enhances metastasis in breast cancer models. Nat. Commun. 2016, 7, 12258. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reagan, M.R.; Anderson, K.; Kaplan, D.L.; Rosenblatt, M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010, 70, 10044–10050. [Google Scholar] [CrossRef] [PubMed]
- Nayir, E.; Aygün, S.; Ata, A.; Arican, A. Relationship Between IL-18 and Bone Metastasis in Female Breast Cancer. Turk. J. Oncol. 2016, 31, 10–14. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Hsing, C.H.; Li, C.F.; Chan, C.H.; Chang, M.C.; Yan, J.J.; Chang, M.S. Anti-IL-20 monoclonal antibody suppresses breast cancer progression and bone osteolysis in murine models. J. Immunol. 2012, 188, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Brunda, M.J.; Luistro, L.; Warrier, R.R.; Wright, R.B.; Hubbard, B.R.; Murphy, M.; Wolf, S.F.; Gately, M.K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 1993, 178, 1223–1230. [Google Scholar] [CrossRef]
- Setrerrahmane, S.; Xu, H. Tumor-related interleukins: Old validated targets for new anti-cancer drug development. Mol. Cancer 2017, 16, 153. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).