Graphene-Based Gas Sensors: State-of-the-Art Developments for Gas Sensing Applications
Abstract
1. Introduction
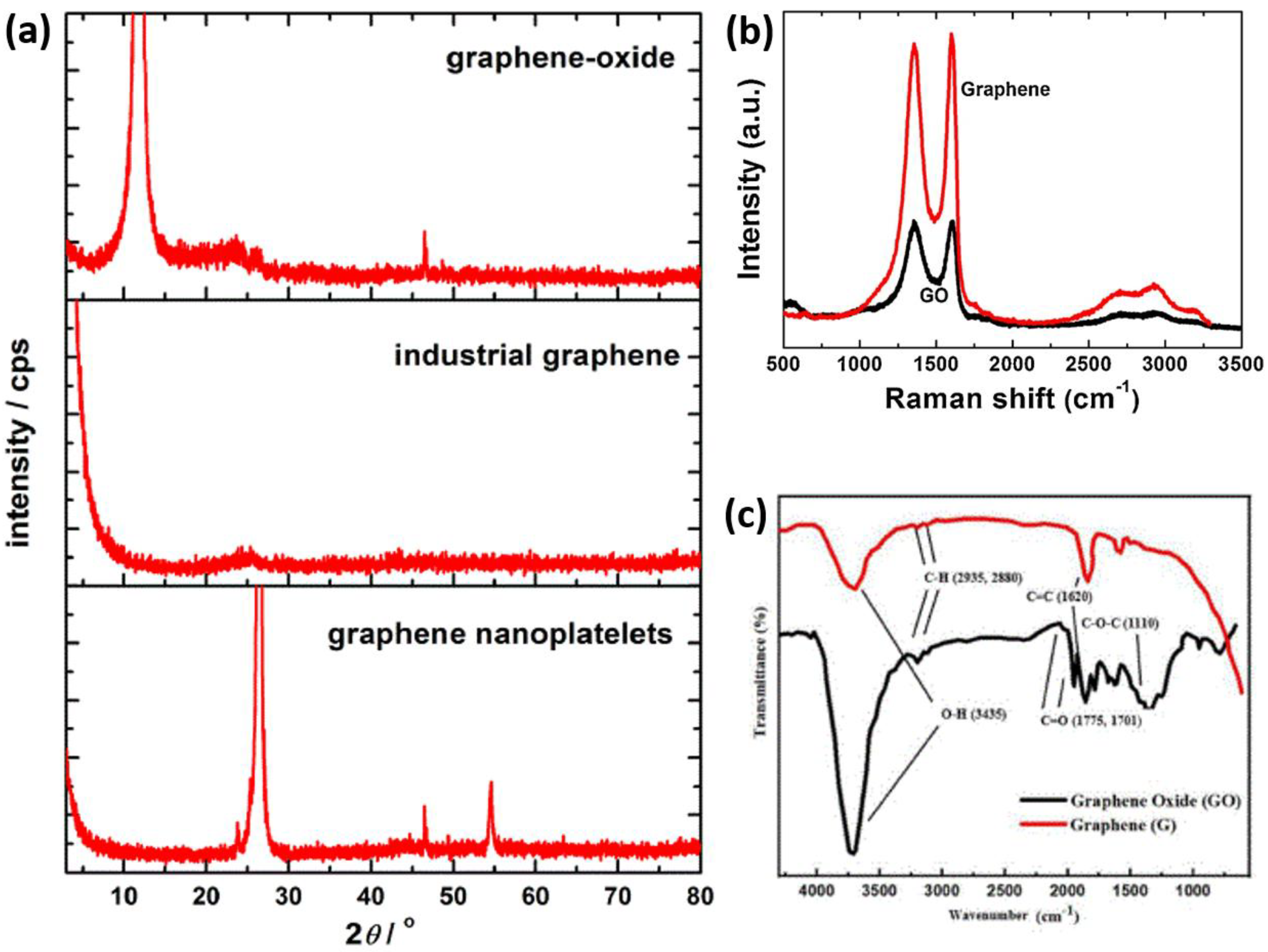
2. Structural Characterizations
3. Gas Sensing Parameters
3.1. Sensitivity
3.2. Selectivity
3.3. Response and Recovery Time
3.4. Detection Limit
3.5. Stability
3.6. Reproducibility
3.7. Operating Temperature
4. Functionalization Strategies: Material Design and Sensing Implications
4.1. NH3 Gas Sensing
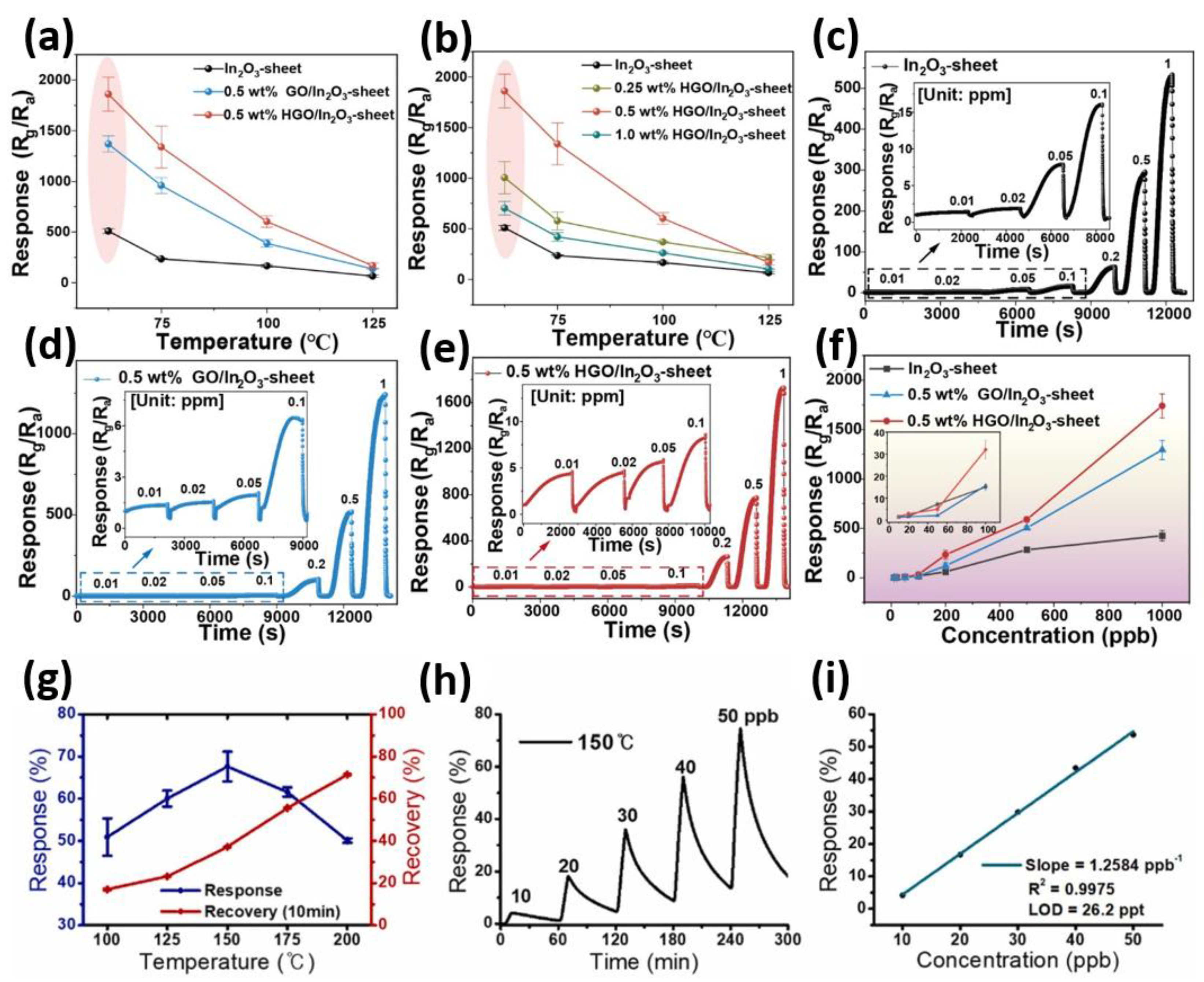
4.2. NO2 Gas Sensing
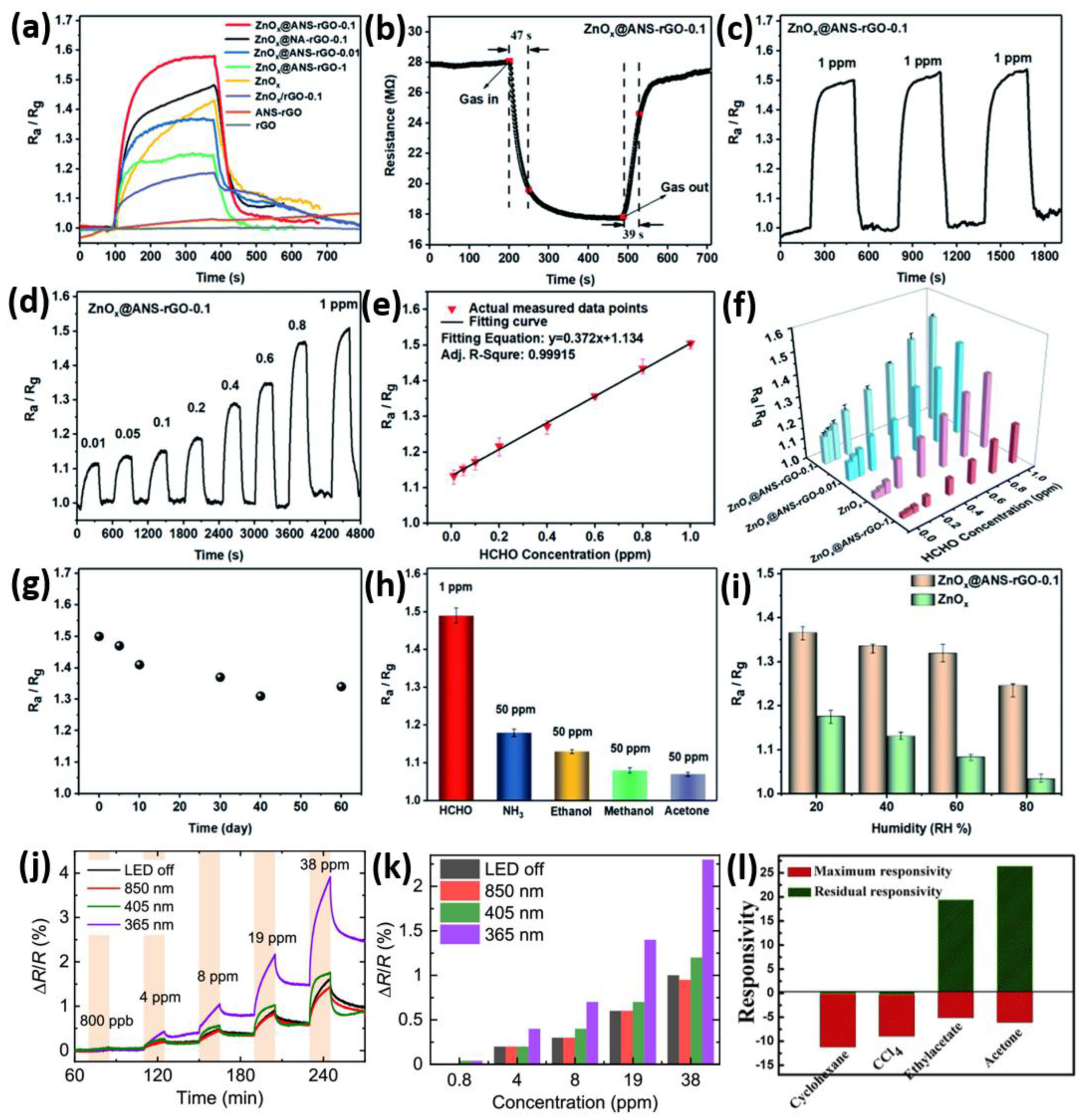
4.3. VOCs Gas Sensing
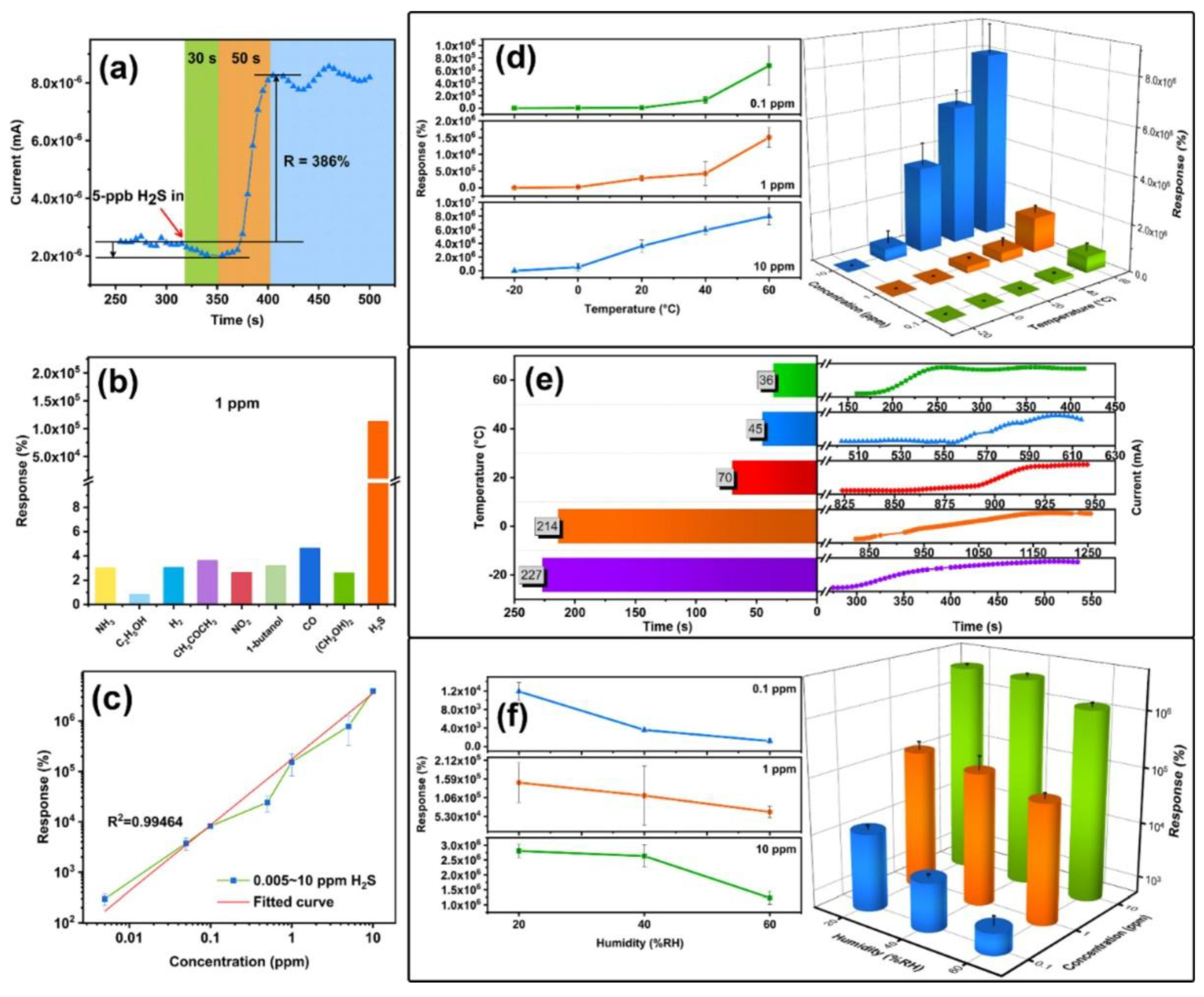
4.4. H2S Gas Sensing
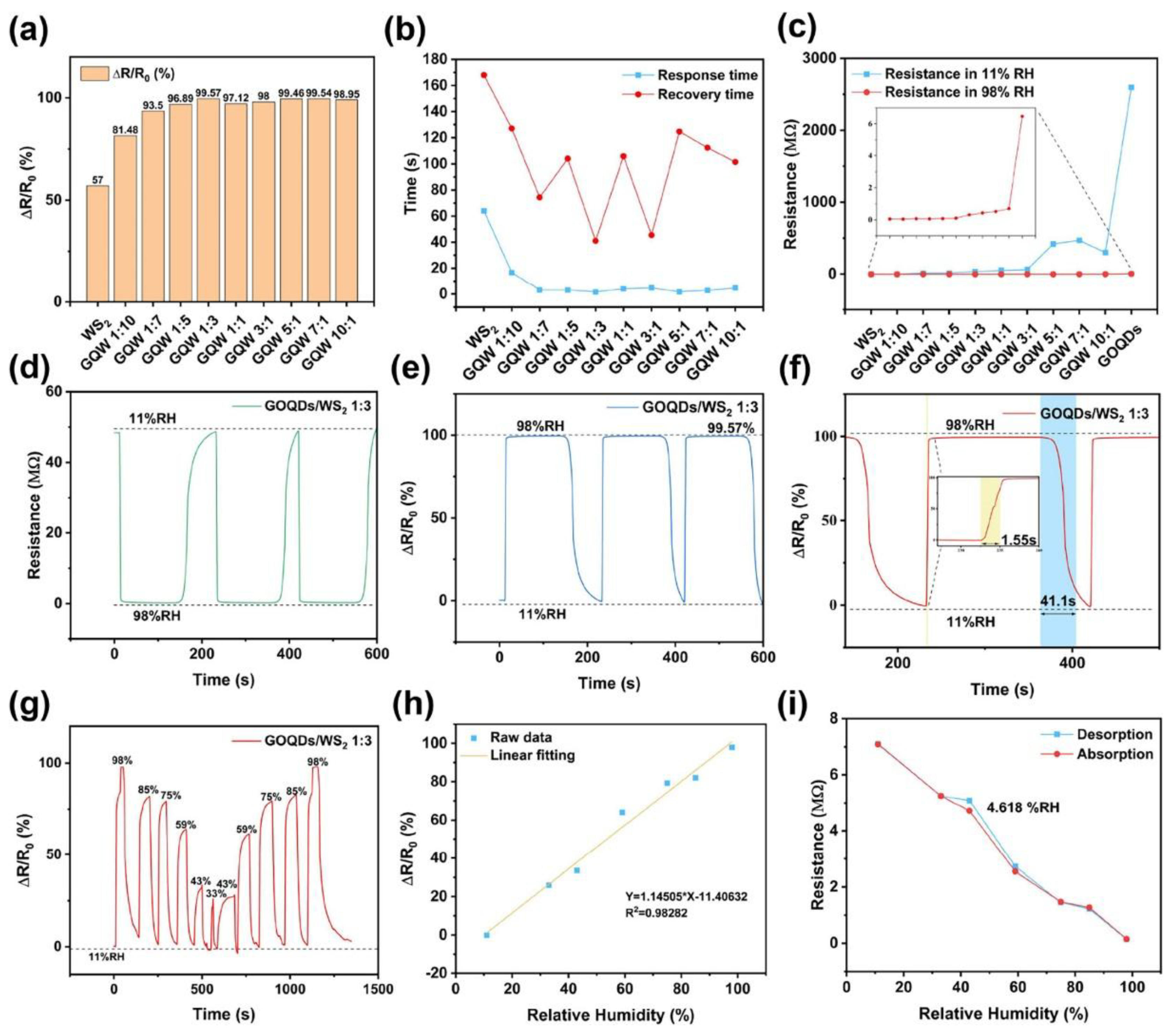
4.5. Humidity Sensing
5. Challenges and Opportunities
5.1. Long-Term Stability and Robustness
5.2. Large-Scale and Cost-Effective Fabrication
5.3. Enhancing Selectivity
6. Future Perspectives
6.1. Next-Generation Sensor Arrays Combined with Graphene Derivatives
6.2. Multi-Analyte Sensing and Selective Recognition
6.3. Towards Real-World Deployment: Flexible, Wearable, and Self-Powered Sensors
6.4. Commercialization Challenges
6.5. Innovative Pathways Forward
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, C.; Wang, Y.; Yang, Z.; Hu, N. Review of Recent Progress on Graphene-Based Composite Gas Sensors. Ceram. Int. 2021, 47, 16367–16384. [Google Scholar] [CrossRef]
- Helwig, A.; Müller, G.; Garrido, J.A.; Eickhoff, M. Gas Sensing Properties of Hydrogen-Terminated Diamond. Sens. Actuators B Chem. 2008, 133, 156–165. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Eickhoff, M. Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials 2015, 8, 6570–6588. [Google Scholar] [CrossRef]
- Tang, J.; Shen, L.; Zhao, K.; Peng, J.; Chen, Q.; Yu, C.; Li, Y.; Abbas, A.; Wang, S.; Liu, Z. An Ultra-Sensitively Ammonia-Responsive Gas Sensor Based on Ag@sulfur Nanosheets. Appl. Surf. Sci. 2024, 643, 158574. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Kumar, V.; Yadav, V.K.; Patel, A.; Singh, K. Graphene-Metal Sulfide Composite Based Gas Sensors for Environmental Sustainability: A Review. Sens. Int. 2024, 5, 100269. [Google Scholar] [CrossRef]
- Li, W.; Qiao, Z.; Liu, Z. Behind the Gas Sensors: Revealing Sensing Mechanisms with Advanced Magnetic Resonance Technology. J. Mater. Chem. A 2023, 11, 19281–19297. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, Z.; Li, C.Y.; Sun, Y. Recent Progress in Multifunctional Gas Sensors Based on 2D Materials. Chemosensors 2023, 11, 483. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Sastikumar, D.; Manivannan, S. Effect of Functional Groups on Dielectric, Optical Gas Sensing Properties of Graphene Oxide and Reduced Graphene Oxide at Room Temperature. RSC Adv. 2015, 5, 10816–10825. [Google Scholar] [CrossRef]
- Krstev, I.; Helwig, A.; Müller, G.; Garrido, J.; Stutzmann, M. Detection of Random Vapour Concentrations Using an Integrating Diamond Gas Sensor. Sens. Actuators B Chem. 2014, 195, 603–608. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-Based Gas Sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Helwig, A.; Beer, S.; Müller, G. Breathing Mode Gas Detection. Sens. Actuators B Chem. 2013, 179, 131–139. [Google Scholar] [CrossRef]
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-Dimensional Layered Nanomaterials for Gas-Sensing Applications. Inorg. Chem. Front. 2016, 3, 433–451. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Cao, H.; Xie, D.; Li, C.; Yang, H.; Yao, W.; Cheetham, A.K. Ultratough Hydrogen-Bond-Bridged Phosphorene Films. Adv. Mater. 2022, 34, 202203332. [Google Scholar] [CrossRef]
- Johnson, R.; Zafar, M.A.; Thomas, S.; Jacob, M.V. A Critical Review on Vacuum and Atmospheric Microwave Plasma-Based Graphene Synthesis. FlatChem 2025, 50, 100812. [Google Scholar] [CrossRef]
- Materón, E.M.; de Azevedo, L.M.L.; Dias, J.M.; Pereira, K.C.R.; Sousa, G.M.; Dias, M.S.; Maroneze, C.M.; Dias, D.; Silva, C.d.C.C. Advancing Biomedical Analysis: Harnessing Laser-Induced Graphene for next-Gen of Low-Cost Sensor Technology. J. Pharm. Biomed. Anal. Open 2025, 5, 100077. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Schöche, S.; Hong, N.; Khorasaninejad, M.; Ambrosio, A.; Orabona, E.; Maddalena, P.; Capasso, F. Optical Properties of Graphene Oxide and Reduced Graphene Oxide Determined by Spectroscopic Ellipsometry. Appl. Surf. Sci. 2017, 421, 778–782. [Google Scholar] [CrossRef]
- Ando, T. The Electronic Properties of Graphene and Carbon Nanotubes. NPG Asia Mater. 2009, 1, 17–21. [Google Scholar] [CrossRef]
- Li, W.; Cheng, G.; Liang, Y.; Tian, B.; Liang, X.; Peng, L.; Hight Walker, A.R.; Gundlach, D.J.; Nguyen, N.V. Broadband Optical Properties of Graphene by Spectroscopic Ellipsometry. Carbon 2016, 99, 348–353. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, S.; Zhou, P.; Sun, Q.; Wang, P.; Wan, L.; Li, J.; Chen, L.; Wang, X.; Ding, S.; et al. Evolution of the Band-Gap and Optical Properties of Graphene Oxide with Controllable Reduction Level. Carbon 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Cong, H.P.; Chen, J.F.; Yu, S.H. Graphene-Based Macroscopic Assemblies and Architectures: An Emerging Material System. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef]
- Chung, H.C.; Chang, C.P.; Lin, C.Y.; Lin, M.F. Electronic and Optical Properties of Graphene Nanoribbons in External Fields. Phys. Chem. Chem. Phys. 2016, 18, 7573–7616. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Perreault, F.; Fonseca De Faria, A.; Elimelech, M. Environmental Applications of Graphene-Based Nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Chen, Z.; Umar, A.; Wang, S.; Wang, Y.; Tian, T.; Shang, Y.; Fan, Y.; Qi, Q.; Xu, D.; Jiang, L. Supramolecular Fabrication of Multilevel Graphene-Based Gas Sensors with High NO2 Sensibility. Nanoscale 2015, 7, 10259–10266. [Google Scholar] [CrossRef]
- Das, G.S.; Tripathi, V.K.; Dwivedi, J.; Jangir, L.K.; Tripathi, K.M. Nanocarbon-Based Sensors for the Structural Health Monitoring of Smart Biocomposites. Nanoscale 2023, 16, 1490–1525. [Google Scholar] [CrossRef]
- Tienne, L.G.P.; Candido, L.D.S.; Da Cruz, B.D.S.M.; Gondim, F.F.; Ribeiro, M.P.; Simão, R.A.; Marques, M.D.F.V.; Monteiro, S.N. Reduced Graphene Oxide Synthesized by a New Modified Hummer’s Method for Enhancing Thermal and Crystallinity Properties of Poly(Vinylidene Fluoride). J. Mater. Res. Technol. 2022, 18, 4871–4893. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Molten Salt Synthesis of CrMnFeNi Alloy Nanopowder Passivated by TiOx-ZrOy Shell Used as a Superior Catalyst Support in Liquid-Phase Hydrogenation. RSC Adv. 2023, 13, 10790–10799. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; He, Y.S.; Zhang, W.; Wang, J.; Yang, X.; Liao, X.Z.; Ma, Z.F. An Experimental Insight into the Advantages of in Situ Solvothermal Route to Construct 3D Graphene-Based Anode Materials for Lithium-Ion Batteries. Nano Energy 2015, 16, 235–246. [Google Scholar] [CrossRef]
- Wu, C.Y.; Zeleke Melaku, A.; Chuang, W.T.; Cheng, C.C. Manipulating the Self-Assembly Behavior of Graphene Nanosheets via Adenine-Functionalized Biodegradable Polymers. Appl. Surf. Sci. 2022, 572, 151437. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Mariatti, M. Printed Graphene and Hybrid Conductive Inks for Flexible, Stretchable, and Wearable Electronics: Progress, Opportunities, and Challenges. J. Sci. Adv. Mater. Devices 2022, 7, 100435. [Google Scholar] [CrossRef]
- Van Hazendonk, L.S.; Pinto, A.M.; Arapov, K.; Pillai, N.; Beurskens, M.R.C.; Teunissen, J.P.; Sneck, A.; Smolander, M.; Rentrop, C.H.A.; Bouten, P.C.P.; et al. Printed Stretchable Graphene Conductors for Wearable Technology. Chem. Mater. 2022, 34, 8031–8042. [Google Scholar] [CrossRef] [PubMed]
- Ngidi, N.P.D.; Ollengo, M.A.; Nyamori, V.O. Tuning the Properties of Boron-Doped Reduced Graphene Oxide by Altering the Boron Content. New J. Chem. 2020, 44, 16864–16876. [Google Scholar] [CrossRef]
- Butt, N.; Kubra, K.T.; Ali, G.; Butt, A.; Shahid, A.; Hayder, U.; Iqbal, F.; Salman, A. Electrochemical Study of Nanoneedle Arrays of Quaternary Metal (Ni-Co-Cu-Ce) -Based Oxides Composite Hybridized with Nitrogen-Doped Graphene Oxide and Polyaniline for Supercapacitor Applications. J. Alloys Compd. 2025, 1013, 178549. [Google Scholar] [CrossRef]
- Uruc, S.; Gorduk, O.; Sahin, Y. Construction of Nonenzymatic Flexible Electrochemical Sensor for Glucose Using Bimetallic Copper Ferrite/Sulfur-Doped Graphene Oxide Water-Based Conductive Ink by Noninvasive Method. ACS Appl. Bio Mater. 2025, 8, 1451–1465. [Google Scholar] [CrossRef]
- Wang, M.; Guo, H.; Peng, L.; Hui, Y.; Yan, R.; Yang, W. A Novel Electrochemical Sensor Based on N,P-Doped Porous Carbon Materials Derived from Covalent Organic Frameworks for Highly Sensitive Determination of 4-Aminophenol and Acetaminophen. New J. Chem. 2024, 49, 2660–2664. [Google Scholar] [CrossRef]
- Naghani, M.E.; Neghabi, M.; Zadsar, M.; Ahangar, H.A. Synthesis and Characterization of Linear/Nonlinear Optical Properties of Graphene Oxide and Reduced Graphene Oxide-Based Zinc Oxide Nanocomposite. Sci. Rep. 2023, 13, 1496. [Google Scholar]
- Gupta, S.; Narajczyk, M.; Sawczak, M.; Bogdanowicz, R. Perspectives on Electron Transfer Kinetics across Graphene-Family Nanomaterials and Interplay of Electronic Structure with Defects and Quantum Capacitance. Sci. Rep. 2025, 15, 19722. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible Graphene-Based Wearable Gas and Chemical Sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Song, W. Physical and Chemical Sensors on the Basis of Laser-Induced Graphene: Mechanisms, Applications, and Perspectives. ACS Nano 2021, 15, 18708–18741. [Google Scholar] [CrossRef]
- Cui, J.; Du, X.; Wang, Y.; Yu, H.; Feng, X.; Lou, Z.; Shan, W.; Xiong, Y. Redox-Active Graphene Dispersant and Its Ability to Improve the Conductivity and Pseudo-Capacitance of Carbon Film. J. Colloid. Interface Sci. 2025, 694, 137657. [Google Scholar] [CrossRef] [PubMed]
- Bandeira de Souza, Z.S.; Araújo do Nascimento, P.L.; Samara, M.; David, É.; Macedo Fechine, G.J.; Alves da Motta Sobrinho, M.; Demarquette, N.R. Influence of Graphene Functionalization on the Curing Kinetics, Dynamical Mechanical Properties and Morphology of Epoxy Nanocomposites. Polymer 2025, 320, 128067. [Google Scholar] [CrossRef]
- Mohammadipour, E.; Ghorbani, M. The Effect of Calcium and Phosphorus Base Compounds on the Characteristic and Morphology of in Situ Synthesized Hydroxyapatite-Reduced Graphene Oxide Nanocomposite. Results Mater. 2025, 25, 128067. [Google Scholar] [CrossRef]
- Cichomski, M.; Spilarewicz, K.; Borkowska, E.; Kisielewska, A.; Stanecka-Badura, R.; Dudek, M.; Piwoński, I. Influence of Self-Assembled Perfluoroalkylsilane Monolayers on Wettability and Tribological Properties of Graphene Derivatives Films. Appl. Surf. Sci. 2025, 704, 163466. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.; Byeon, M.; Hong, T.E.; Park, H.; Lee, C.Y. Graphene-Based Gas Sensors with High Sensitivity and Minimal Sensor-to-Sensor Variation. ACS Appl. Nano Mater. 2020, 3, 2257–2265. [Google Scholar] [CrossRef]
- Li, J.; Shi, W.; Yang, L.; Yu, X.; Tang, J.; Wang, J.; Zhao, J.; Kang, X.; Zhang, L.; Xing, C.; et al. Oxidation-Resistant Boron-Linked Strengthened Graphene-Based Composite. Appl. Surf. Sci. 2025, 702, 163376. [Google Scholar] [CrossRef]
- Gutić, S.; Dobrota, A.S.; Gavrilov, N.; Baljozović, M.; Pašti, I.A.; Mentus, S.V. Surface Charge Storage Properties of Selected Graphene Samples in PH-Neutral Aqueous Solutions of Alkali Metal Chlorides—Particularities and Universalities. Int. J. Electrochem. Sci. 2016, 11, 8662–8682. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Mehmood, A.; Mubarak, N.M.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Graphene/PVA Buckypaper for Strain Sensing Application. Sci. Rep. 2020, 10, 20106. [Google Scholar] [CrossRef]
- Du, Z.; Wang, S.; Chen, B.; Sun, J.; Ai, W. Polymer-Contraction-Enabled Topological Engineering of Graphene for 2D/3D Hybridized Li2S Deposition in High-Performance Li-S Batteries. ACS Sustain. Chem. Eng. 2025, 13, 10119–10128. [Google Scholar] [CrossRef]
- Veríssimo, M.I.S. A Critical Review of the Analytical Performance of the Most Recent MOS-Based Gas Sensors for Indoor Air Quality Monitoring of WHO Priority Pollutants. TrAC Trends Anal. Chem. 2024, 178, 117813. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.H.; Shin, H.; Cho, H.J.; Koo, W.T.; Choi, S.J.; Park, C.; Ahn, J.; Güntner, A.T.; Penner, R.M.; et al. Selectivity in Chemiresistive Gas Sensors: Strategies and Challenges. Chem. Rev. 2025, 125, 4111–4183. [Google Scholar] [CrossRef] [PubMed]
- Malepe, L.; Ndinteh, D.; Mamo, M.A. The Effect of Measurement Parameters on the Performance of the Sensors in the Detection of Organic Compound Vapours. Chem. Phys. Impact 2022, 4, 100068. [Google Scholar] [CrossRef]
- Odebowale, A.A.; Abdulghani, A.; Berhe, A.M.; Somaweera, D.; Akter, S.; Abdo, S.; As’ham, K.; Saadabad, R.M.; Tran, T.T.; Bishop, D.P.; et al. Emerging Low Detection Limit of Optically Activated Gas Sensors Based on 2D and Hybrid Nanostructures. Nanomaterials 2024, 14, 1521. [Google Scholar] [CrossRef]
- Sharma, A.; Eadi, S.B.; Noothalapati, H.; Otyepka, M.; Lee, H.D.; Jayaramulu, K. Porous Materials as Effective Chemiresistive Gas Sensors. Chem. Soc. Rev. 2024, 53, 2530–2577. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Ghaemi, A. Chemiresistor Gas Sensors: Design, Challenges, and Strategies: A Comprehensive Review. Chem. Eng. J. 2024, 498, 154999. [Google Scholar] [CrossRef]
- Robbiani, S.; Lotesoriere, B.J.; Dellacà, R.L.; Capelli, L. Physical Confounding Factors Affecting Gas Sensors Response: A Review on Effects and Compensation Strategies for Electronic Nose Applications. Chemosensors 2023, 11, 514. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Vigueras-Zuniga, M.O.; Shi, H.; Mashruk, S.; Alnajideen, M.; Alnasif, A.; Davies, J.; Wang, Y.; Zhu, X.; Yang, W.; et al. Ammonia Combustion in Furnaces: A Review. Int. J. Hydrogen Energy 2024, 49, 1597–1618. [Google Scholar] [CrossRef]
- Jang, H.; Mujeeb-Ahmed, M.P.; Wang, H.; Park, C.; Hwang, I.; Jeong, B.; Zhou, P.; Papadakis, A.; Giannakis, A.; Sykaras, K. Safety Evaluation on Ammonia-Fueled Ship: Gas Dispersion Analysis through Vent Mast. Int. J. Hydrogen Energy 2024, 83, 1060–1077. [Google Scholar] [CrossRef]
- Aligayev, A.; Jabbarli, U.; Samadova, U.; Dominguez–Gutierrez, F.J.; Papanikolaou, S.; Huang, Q. Dissociative Mechanism from NH3 and CH4 on Ni-Doped Graphene: Tuning Electronic and Optical Properties. Appl. Surf. Sci. 2025, 686, 162022. [Google Scholar] [CrossRef]
- Raza, A.; Abid, R.; Murtaza, I.; Fan, T. Room Temperature NH3 Gas Sensor Based on PMMA/RGO/ZnO Nanocomposite Films Fabricated by in-Situ Solution Polymerization. Ceram. Int. 2023, 49, 27050–27059. [Google Scholar] [CrossRef]
- Yao, B.; Wu, Y.; Cheng, Y.; Zhang, A.; Gong, Y.; Rao, Y.J.; Wang, Z.; Chen, Y. All-Optical Mach-Zehnder Interferometric NH3 Gas Sensor Based on Graphene/Microfiber Hybrid Waveguide. Sens. Actuators B Chem. 2014, 194, 142–148. [Google Scholar] [CrossRef]
- Yu, C.; Wu, Y.; Liu, X.; Fu, F.; Gong, Y.; Rao, Y.J.; Chen, Y. Miniature Fiber-Optic NH3 Gas Sensor Based on Pt Nanoparticle-Incorporated Graphene Oxide. Sens. Actuators B Chem. 2017, 244, 107–113. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, T.T.; Chen, T.; He, W.J.; Chen, M.M.; Cao, D. The Naked-Eye NH3 Sensor Based on Fluorinated Graphene. Sens. Actuators B Chem. 2019, 281, 789–794. [Google Scholar] [CrossRef]
- Matatagui, D.; López-Sánchez, J.; Peña, A.; Serrano, A.; del Campo, A.; de la Fuente, O.R.; Carmona, N.; Navarro, E.; Marín, P.; del Carmen Horrillo, M. Ultrasensitive NO2 Gas Sensor with Insignificant NH3-Interference Based on a Few-Layered Mesoporous Graphene. Sens. Actuators B Chem. 2021, 335, 129657. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Lei, X.; Sun, H.; Ai, T.; Ma, F.; Chu, P.K. Edge-Enriched SnS2 Nanosheets on Graphene for Chemiresistive Room Temperature NH3 Sensors. Sens. Actuators B Chem. 2025, 433, 137565. [Google Scholar] [CrossRef]
- Lee, C.T.; Wang, Y.S. High-Performance Room Temperature NH3 Gas Sensors Based on Polyaniline-Reduced Graphene Oxide Nanocomposite Sensitive Membrane. J. Alloys Compd. 2019, 789, 693–696. [Google Scholar] [CrossRef]
- Shi, J.; Liu, C.; Lin, M.; Fu, Y.; Wang, D.; Song, J.; Zhang, G.; Liu, H.; Hou, L. Graphene Oxide Nanosheets Modified with TiO2 Nanoparticles for Highly Sensitive NH3 Detection at Room Temperature. J. Alloys Compd. 2024, 1002, 175245. [Google Scholar] [CrossRef]
- Gao, C.; Wang, T.; Wang, X. A NH3 Gas Sensor Based on Flexible Copper(II) Isonicotinate MOF/Reduced Graphene Oxide Composite Modified Interdigital Electrode. Int. J. Electrochem. Sci. 2022, 17, 220763. [Google Scholar] [CrossRef]
- Esmaeili, C.; Ashtiani, S.; Regmi, C.; Laposa, A.; Voves, J.; Kroutil, J.; Friess, K.; Povolny, V.; Lotfian, S. Preparation and Characterisation of NH3 Gas Sensor Based on PANI/Fe-Doped CeO2 Nanocomposite. Heliyon 2024, 10, e34801. [Google Scholar] [CrossRef]
- Srivastava, S.; Jain, S.K.; Gupta, G.; Senguttuvan, T.D.; Gupta, B.K. Boron-Doped Few-Layer Graphene Nanosheet Gas Sensor for Enhanced Ammonia Sensing at Room Temperature. RSC Adv. 2019, 10, 1007–1014. [Google Scholar] [CrossRef]
- Falak, A.; Tian, Y.; Yan, L.; Zhang, X.; Xu, L.; Song, Z.; Dong, F.; Chen, P.; Zhao, M.; Wang, H.; et al. Simultaneous Achievement of Superior Response and Full Recovery of Titanium Dioxide/Graphene Hybrid FET Sensors for NH3 through p-to n-Mode Switch. Phys. Chem. Chem. Phys. 2020, 22, 16701–16711. [Google Scholar] [CrossRef] [PubMed]
- Rodner, M.; Icardi, A.; Kodu, M.; Jaaniso, R.; Schütze, A.; Eriksson, J. Metal Oxide Nanolayer-Decorated Epitaxial Graphene: A Gas Sensor Study. Nanomaterials 2020, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Huang, Y.; Lei, Z.; Liu, N.; Huang, C.; Qi, F.; Zhao, N.; Zhou, Y.; Cao, J.; Ouyang, X. UV-Promoted NH3 Sensor Based on Ti3C2Tx/TiO2/Graphene Sandwich Structure with Ultrasensitive RT Sensing Performances for Human Health Detection. Sens. Actuators B Chem. 2024, 410, 135681. [Google Scholar] [CrossRef]
- Du, X.; Gu, S.; Wang, X.; Zhang, S.; Zhang, B.; Yu, G.; Wang, Z.; Chen, W.; Li, Q. The Preparation of SiO2/GO/PVA Based Hydrogel Sensor and Its Application for Rapid and Sensitive Detection of NH3. Sens. Actuators B Chem. 2025, 424, 136885. [Google Scholar] [CrossRef]
- Taha, S.S.; Idoudi, S.; Alhamdan, N.; Ibrahim, R.H.; Surkatti, R.; Amhamed, A.; Alrebei, O.F. Comprehensive Review of Health Impacts of the Exposure to Nitrogen Oxides (NOx), Carbon Dioxide (CO2), and Particulate Matter (PM). J. Hazard. Mater. Adv. 2025, 19, 100771. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, H.; Yin, K.; Wu, Z.; Xu, F.; Gao, T.; Yue, Y.; Chen, J.; Feng, Q.; Yang, Y.; et al. Tuning the Electronic and Optical Properties of Graphene via Doping to Realize Nitrogen Dioxide Sensing: A Computational Study. ACS Omega 2025, 10, 1486–1492. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedoseeva, Y.V.; Lavrukhina, S.A.; Sysoev, V.I.; Maksimovskii, E.A.; Makarova, A.A.; Okotrub, A.V. Role of Graphene Substrate in the Formation of MoS2-Based Nanoparticles with Improved Sensitivity to NO2 Gas. Appl. Surf. Sci. 2025, 679, 161104. [Google Scholar] [CrossRef]
- Yin, L.; Chu, X.; Chen, H.; Liu, B.; Zhang, P.; Du, L.; Cui, G.; Lv, L. Room Temperature NO2 Sensing with a ZIF-67/RGO Nanocomposite: A Highly Sensitive Approach. J. Alloys Compd. 2025, 1021, 179621. [Google Scholar] [CrossRef]
- Yang, W.; Huo, Y.; Wang, T.; Liu, X.; Li, D.; Yu, H.; Dong, X.; Yang, Y. RGO@In2O3 Based Flexible Gas Sensor: Efficient Monitoring of Trace NO2 Gas at Room Temperature. Sens. Actuators B Chem. 2025, 430, 137359. [Google Scholar] [CrossRef]
- Drozdowska, K.; Smulko, J. Selective Light-Activation of Sensing Regions in Hybrid Au-Graphene-TiO2 Chemiresistive Gas Sensor. Sens. Actuators B Chem. 2025, 437, 137764. [Google Scholar] [CrossRef]
- Yang, C.R.; Huang, J.G.; Huang, M.J.; Shen, H.Y.; Tseng, S.F. High-Performance NO2 Gas Sensors Based on Vanadium Metal Organic Frameworks (V-MOFs) on Flexible Graphene Electrodes. J. Alloys Compd. 2024, 1008, 176675. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Cui, H.; Yu, J.; Sun, S.; Bai, J. First-Principles Insights into Au- and B-Embedded Graphene for Detections of NO2 and O3 in Air Insulated Switchgears. Results Phys. 2024, 58, 107496. [Google Scholar] [CrossRef]
- Jung, W.T.; Jang, H.S.; Lee, S.M.; Hong, W.G.; Bae, Y.J.; Lee, H.S.; Kim, B.H. High-Response Room-Temperature NO2 Gas Sensor Fabricated with Thermally Reduced Graphene Oxide-Coated Commercial Cotton Fabric. Heliyon 2024, 10, e24425. [Google Scholar] [CrossRef]
- Lee, H.Y.; Wu, M.J.; Chu, S.Y.; Chang, T.C.; Tung, Y.F.; Yeh, T.H.; Lee, C.T. Highly Sensitive NO2 Gas Sensors Based on Heterostructured P-RGO/n-Ga2O3 Nanorods. Appl. Surf. Sci. Adv. 2025, 25, 100679. [Google Scholar] [CrossRef]
- Solanki, K.; Verma, S.; Das, P.K.; Paltani, P.P.; Majumder, M.K. Ab Initio Modeling of Doped/Undoped ArGNR Sensors for NO2 Detection. IEEE Trans. Nanotechnol. 2024, 23, 567–577. [Google Scholar] [CrossRef]
- Long, J.; Li, X.; Wang, W.; Wu, W.; Zu, X.; Guo, Y. Ambiently Rapid Response and High Selectivity to NO Enabled by Pd-RGO Composite Film-Coated Surface Acoustic Wave Sensor. IEEE Sens. J. 2024, 24, 23649–23657. [Google Scholar] [CrossRef]
- Khaleghiabbasabadi, M.; Taghavian, H.; Gholami, P.; Khodabakhshi, S.; Gheibi, M.; Wacławek, S.; Černík, M.; Silvestri, D.; Raczak, K.B.; Moezzi, R. A Novel Organic–Inorganic-Nanocomposite-Based Reduced Graphene Oxide as an Efficient Nanosensor for NO2 Detection. Nanomaterials 2024, 14, 1983. [Google Scholar] [CrossRef]
- Sayago, I.; Sánchez-Vicente, C.; Santos, J.P. Highly Sensitive and Selective SnO2-Gr Sensor Photoactivated for Detection of Low NO2 Concentrations at Room Temperature. Nanomaterials 2024, 14, 1994. [Google Scholar] [CrossRef] [PubMed]
- Buiculescu, V.; Dinu, L.A.; Veca, L.M.; Pârvulescu, C.; Mihai, M.; Brîncoveanu, O.; Comănescu, F.; Brașoveanu, C.; Stoian, M.; Baracu, A.M. The Development of Sensitive Graphene-Based Surface Acoustic Wave Sensors for NO2 Detection at Room Temperature. Microchim. Acta 2024, 191, 323. [Google Scholar] [CrossRef] [PubMed]
- Santos-Betancourt, A.; Santos-Ceballos, J.C.; Alouani, M.A.; Malik, S.B.; Romero, A.; Ramírez, J.L.; Vilanova, X.; Llobet, E. ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday. Sensors 2024, 24, 1431. [Google Scholar] [CrossRef]
- Berholts, A.; Kodu, M.; Rubin, P.; Kahro, T.; Alles, H.; Jaaniso, R. Layered Heterostructure of Graphene and TiO2 as a Highly Sensitive and Stable Photoassisted NO2 Sensor. ACS Appl. Mater. Interfaces 2024, 16, 43827–43837. [Google Scholar] [CrossRef]
- Trinh, V.; Xu, K.; Yu, H.; Ha, N.; Hu, Y.; Khan, M.W.; Ou, R.; Luan, Y.; Zhang, J.; Ma, Q.; et al. Upcycled Graphene Oxide Nanosheets for Reversible Room Temperature NO2 Gas Sensor. Chemosensors 2024, 12, 108. [Google Scholar] [CrossRef]
- Alouani, M.A.; Casanova-Chafer, J.; de Bernardi-Martín, S.; García-Gómez, A.; Vilanova, X.; Llobet, E. A NO2 Sensitive MnO2/Graphene Oxide Composite Based Gas Sensor. Chemosensors 2025, 13, 96. [Google Scholar] [CrossRef]
- Goodwin, D.M.; Carta, M.; Ali, M.M.; Gillard, D.; Guy, O.J. Enhanced Nitrogen Dioxide Detection Using Resistive Graphene-Based Electronic Sensors Modified with Polymers of Intrinsic Microporosity. ACS Sens. 2025, 10, 1378–1386. [Google Scholar] [CrossRef]
- Walleni, C.; Malik, S.B.; Missaoui, G.; Alouani, M.A.; Nsib, M.F.; Llobet, E. Selective NO2 Gas Sensors Employing Nitrogen- and Boron-Doped and Codoped Reduced Graphene Oxide. ACS Omega 2023, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Soydan, G.; Ergenc, A.F.; Alpas, A.T.; Solak, N. Development of an NO2 Gas Sensor Based on Laser-Induced Graphene Operating at Room Temperature. Sensors 2024, 24, 3217. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Q.; He, Z.; Gao, X.; Wu, E.; Guo, J.; Zhou, C.; Feng, Z. Epitaxial Graphene Gas Sensors on SiC Substrate with High Sensitivity. J. Semicond. 2020, 41, 032101. [Google Scholar] [CrossRef]
- Niavol, S.S.; Budde, M.; Papadogianni, A.; Heilmann, M.; Moghaddam, H.M.; Aldao, C.M.; Ligorio, G.; List-Kratochvil, E.J.W.; Lopes, J.M.J.; Barsan, N.; et al. Conduction Mechanisms in Epitaxial NiO/Graphene Gas Sensors. Sens. Actuators B Chem. 2020, 325, 128797. [Google Scholar] [CrossRef]
- Han, J.; Gu, G.; Gao, Y.; Yu, N.; Zhou, W.; Wang, Y.; Kong, D.; Gao, Y.; Lu, G. Prototype Alarm Integrating Pulse-Driven Nitrogen Dioxide Sensor Based on Holey Graphene Oxide/In2O3. ACS Sens. 2024, 9, 5425–5435. [Google Scholar] [CrossRef]
- Yang, M.S.; Bae, S.K.; Seo, D.B.; Lee, K.; Son, Y.; Kim, S.H.; Jung, J.H.; An, K.S.; Park, I.; Seo, J.H. Facile Formation of Nanoporous Reduced Graphene Oxide via Epoxy-Based Negative Photoresist Laser Irradiation for Highly Sensitive and Selective Gas Detection. Sens. Actuators B Chem. 2025, 426, 137073. [Google Scholar] [CrossRef]
- Lim, Y.M.; Swamy, V.; Ramakrishnan, N.; Chan, E.S.; Kesuma, H.P. Volatile Organic Compounds (VOCs) in Wastewater: Recent Advances in Detection and Quantification. Microchem. J. 2023, 195, 109537. [Google Scholar] [CrossRef]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis the Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef]
- Madvar, H.R.; Moayedi, M.; Kordrostami, Z. Impact of Reduced-Graphene-Oxide Functionalization of Flower-like Zinc-Oxide Nanostructures on Sensing Performance of Resistive Ethanol Gas Sensor. Thin Solid Film. 2024, 804, 140498. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, M.; Wang, S.; Song, C.; Xiao, J. Ultra-Fast Responding and Recovering Ethanol Sensors Based on CdS Nanospheres Doped with Graphene. Appl. Surf. Sci. 2018, 453, 513–519. [Google Scholar] [CrossRef]
- Jia, L.P.; Wang, H.S. Preparation and Application of a Highly Sensitive Nonenzymatic Ethanol Sensor Based on Nickel Nanoparticles/Nafion/Graphene Composite Film. Sens. Actuators B Chem. 2013, 177, 1035–1042. [Google Scholar] [CrossRef]
- Inyawilert, K.; Wisitsoraat, A.; Sriprachaubwong, C.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Rapid Ethanol Sensor Based on Electrolytically-Exfoliated Graphene-Loaded Flame-Made In-Doped SnO2 Composite Film. Sens. Actuators B Chem. 2015, 209, 40–55. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, R. Selective Determination of Ammonia, Ethanol and Acetone by Reduced Graphene Oxide Based Gas Sensors at Room Temperature. Sens. Biosensing Res. 2020, 28, 100336. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, D.; Li, H.; Xie, C. Room Temperature Formaldehyde Sensors with Enhanced Performance, Fast Response and Recovery Based on Zinc Oxide Quantum Dots/Graphene Nanocomposites. Nanoscale 2012, 4, 5651–5658. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H.; Xia, B. Room-Temperature High-Performance Acetone Gas Sensor Based on Hydrothermal Synthesized SnO2-Reduced Graphene Oxide Hybrid Composite. RSC Adv. 2015, 5, 3016–3022. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, F.; Zha, L.; Wang, X.; Zhang, G. A Sensitive Cataluminescence-Based Sensor Using a SrCO3/Graphene Composite for n-Propanol. RSC Adv. 2015, 5, 57482–57489. [Google Scholar] [CrossRef]
- Su, Y.; Xie, G.; Chen, J.; Du, H.; Zhang, H.; Yuan, Z.; Ye, Z.; Du, X.; Tai, H.; Jiang, Y. Reduced Graphene Oxide-Polyethylene Oxide Hybrid Films for Toluene Sensing at Room Temperature. RSC Adv. 2016, 6, 97840–97847. [Google Scholar] [CrossRef]
- Kovalska, E.; Lesongeur, P.; Hogan, B.T.; Baldycheva, A. Multi-Layer Graphene as a Selective Detector for Future Lung Cancer Biosensing Platforms. Nanoscale 2019, 11, 2476–2483. [Google Scholar] [CrossRef]
- Liang, H.; Guo, L.; Cao, N.; Hu, H.; Li, H.; de Rooij, N.F.; Umar, A.; Algarni, H.; Wang, Y.; Zhou, G. Practical Room Temperature Formaldehyde Sensing Based on a Combination of Visible-Light Activation and Dipole Modification. J. Mater. Chem. A Mater 2021, 9, 23955–23967. [Google Scholar] [CrossRef]
- Park, J.; Rautela, R.; Alzate-Carvajal, N.; Scarfe, S.; Scarfe, L.; Alarie, L.; Luican-Mayer, A.; Ménard, J.M. UV Illumination as a Method to Improve the Performance of Gas Sensors Based on Graphene Field-Effect Transistors. ACS Sens. 2021, 6, 4417–4424. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Yang, X.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, X.; Guo, J.; Guo, Z. Organic Vapor Sensing Behaviors of Conductive Thermoplastic Polyurethane-Graphene Nanocomposites. J. Mater. Chem. C 2016, 4, 4459–4469. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Fabrication of Water-Dispersible and Highly Conductive PSS-Doped PANI/Graphene Nanocomposites Using a High-Molecular Weight PSS Dopant and Their Application in H2S Detection. Nanoscale 2014, 6, 15181–15195. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, A.; Chinh, N.D.; Jeong, Y.J.; Hou, T.F.; Kim, D.S.; Kim, D.; Kim, Y.B.; Lee, D.W. Hierarchical Nanohybrids of B- and N-Codoped Graphene/Mesoporous NiO Nanodisks: An Exciting New Material for Selective Sensing of H2S at near Ambient Temperature. J. Mater. Chem. A 2019, 7, 9263–9278. [Google Scholar] [CrossRef]
- Salmankhani, A.; Karami, Z.; Mashhadzadeh, A.H.; Ganjali, M.R.; Vatanpour, V.; Esmaeili, A.; Habibzadeh, S.; Saeb, M.R.; Fierro, V.; Celzard, A. New Insights into H2S Adsorption on Graphene and Graphene-Like Structures: A Comparative DFT Study. C 2020, 6, 74. [Google Scholar] [CrossRef]
- Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Park, I.; Van Hieu, N. Excellent Detection of H2S Gas at Ppb Concentrations Using ZnFe2O4 Nanofibers Loaded with Reduced Graphene Oxide. Sens. Actuators B Chem. 2019, 282, 876–884. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile Synthesis of Reduced Graphene Oxide/Hexagonal WO3 Nanosheets Composites with Enhanced H2S Sensing Properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.S.; Tsymbalenko, O.; Lan, P.T.; Moon, M.W.; Lee, K.B. Highly Sensitive Chemiresistive H2S Gas Sensor Based on Graphene Decorated with Ag Nanoparticles and Charged Impurities. Sens. Actuators B Chem. 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yang, Y.; Yu, H.; Dong, X. Highly Sensitive H2S Sensors Based on Metal-Organic Framework Driven γ-Fe2O3 on Reduced Graphene Oxide Composites at Room Temperature. Sens. Actuators B Chem. 2020, 325, 128804. [Google Scholar] [CrossRef]
- Hingangavkar, G.M.; Kadam, S.A.; Ma, Y.R.; Bandgar, S.S.; Mulik, R.N.; Patil, V.B. MoS2-GO Hybrid Sensor: A Discerning Approach for Detecting Harmful H2S Gas at Room Temperature. Chem. Eng. J. 2023, 472, 144789. [Google Scholar] [CrossRef]
- Bai, S.; Chen, C.; Luo, R.; Chen, A.; Li, D. Synthesis of MoO3/Reduced Graphene Oxide Hybrids and Mechanism of Enhancing H2S Sensing Performances. Sens. Actuators B Chem. 2015, 216, 113–120. [Google Scholar] [CrossRef]
- Balasubramani, V.; Sureshkumar, S.; Rao, T.S.; Sridhar, T.M. Impedance Spectroscopy-Based Reduced Graphene Oxide-Incorporated ZnO Composite Sensor for H2S Investigations. ACS Omega 2019, 4, 9976–9982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, J.; Wang, T.; Liu, H.; Chen, J.; Sun, Y.; Zhang, P.; Cui, G. Ultrasensitive H2S Sensor Based on Cu2O/Graphene Heterostructures at Room Temperature. Appl. Surf. Sci. 2025, 702, 163339. [Google Scholar] [CrossRef]
- Chen, R.; Liu, S.; Zhang, C.; Jiang, C.; Zhou, W.; Chen, P.; Wu, D.; Li, D.; Zhang, J.; Luo, T. Laser Fabrication of Humidity Sensors on Ethanol-Soaked Polyimide for Fully Contactless Respiratory Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 45252–45264. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, C.; Liu, X.; Wang, L.; Wang, L.J. A Highly Responsive Graphene Oxide Humidity Sensor Based on PVA Nanofibers. Langmuir 2024, 40, 16361–16366. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, H.; He, C.; Wang, X.; Ding, J.; Zhang, W.; Fan, X. Humidity Sensing Properties of Different Atomic Layers of Graphene on the SiO2/Si Substrate. ACS Appl. Mater. Interfaces 2024, 16, 55955–55963. [Google Scholar] [CrossRef]
- Ke, N.; Si, F.; Ma, H.; Gao, Q.; Ge, G.; Liu, W.; Ding, J.; Zhang, W.; Fan, X. Fully Flexible Humidity Sensor with Fast Response and High Responsivity Based on RGO/MoS2 for Human Respiration Monitoring and Nontouch Switches. ACS Appl. Mater. Interfaces 2025, 17, 2317–2326. [Google Scholar] [CrossRef]
- Yu, H.W.; Kim, H.K.; Kim, T.; Bae, K.M.; Seo, S.M.; Kim, J.M.; Kang, T.J.; Kim, Y.H. Self-Powered Humidity Sensor Based on Graphene Oxide Composite Film Intercalated by Poly(Sodium 4-Styrenesulfonate). ACS Appl. Mater. Interfaces 2014, 6, 8320–8326. [Google Scholar] [CrossRef]
- Yue, X.; Yang, J.; Zhu, X.; Wang, X.; Jing, Y.; Li, W.; Dong, L.; Zhang, Y.; Jiang, H.; Li, X. Surface Defects of Electron Irradiation Engineering for Graphene/Polymer Composite-Based Flexible Humidity Sensors. ACS Appl. Nano Mater. 2023, 6, 9257–9267. [Google Scholar] [CrossRef]
- Liu, S.; Chen, R.; Chen, R.; Jiang, C.; Zhang, C.; Chen, D.; Zhou, W.; Chen, S.; Luo, T. Facile and Cost-Effective Fabrication of Highly Sensitive, Fast-Response Flexible Humidity Sensors Enabled by Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2023, 15, 57327–57337. [Google Scholar] [CrossRef]
- Anichini, C.; Aliprandi, A.; Gali, S.M.; Liscio, F.; Morandi, V.; Minoia, A.; Beljonne, D.; Ciesielski, A.; Samorì, P. Ultrafast and Highly Sensitive Chemically Functionalized Graphene Oxide-Based Humidity Sensors: Harnessing Device Performances via the Supramolecular Approach. ACS Appl. Mater. Interfaces 2020, 12, 44017–44025. [Google Scholar] [CrossRef] [PubMed]
- Khattak, Z.J.; Sajid, M.; Javed, M.; Rizvi, H.M.Z.; Awan, F.S. Mass-Producible 2D Nanocomposite-Based Temperature-Independent All-Printed Relative Humidity Sensor. ACS Omega 2022, 7, 16605–16615. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Cao, Y.; Luo, Y.; Pang, K.; Zhang, H.Y. Stable, High-Sensitivity, and Rapid-Response Humidity Sensor Based on Ultrathin Graphene Oxide Film after Vacuum Annealing. ACS Appl. Electron. Mater. 2025, 7, 2741–2750. [Google Scholar] [CrossRef]
- Rathi, K.; Pal, K. Impact of Doping on GO: Fast Response-Recovery Humidity Sensor. ACS Omega 2017, 2, 842–851. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.; Si, F.; Ke, N.; Wang, G.; Zhang, S.; Ding, J.; Zhang, W.; Fan, X. A Flexible Wearable Humidity Sensor with Fast Response and High Responsivity Based on GOQDs/WS2 Heterojunctions. ACS Appl. Nano Mater. 2025, 8, 5193–5206. [Google Scholar] [CrossRef]
- Capman, N.S.S.; Zhen, X.V.; Nelson, J.T.; Chaganti, V.R.S.K.; Finc, R.C.; Lyden, M.J.; Williams, T.L.; Freking, M.; Sherwood, G.J.; Bühlmann, P.; et al. Machine Learning-Based Rapid Detection of Volatile Organic Compounds in a Graphene Electronic Nose. ACS Nano 2022, 16, 19567–19583. [Google Scholar] [CrossRef]
- Lind, M.; Kiisk, V.; Kodu, M.; Kahro, T.; Renge, I.; Avarmaa, T.; Makaram, P.; Zurutuza, A.; Jaaniso, R. Semiquantitative Classification of Two Oxidizing Gases with Graphene-Based Gas Sensors. Chemosensors 2022, 10, 68. [Google Scholar] [CrossRef]
- Agbonlahor, O.G.; Muruganathan, M.; Banerjee, A.; Mizuta, H. Machine Learning Identification of Atmospheric Gases by Mapping the Graphene-Molecule van Der Waals Complex Bonding Evolution. Sens. Actuators B Chem. 2023, 380, 133383. [Google Scholar] [CrossRef]
- Huang, S.; Croy, A.; Bierling, A.L.; Khavrus, V.; Panes-Ruiz, L.A.; Dianat, A.; Ibarlucea, B.; Cuniberti, G. Machine Learning-Enabled Graphene-Based Electronic Olfaction Sensors and Their Olfactory Performance Assessment. Appl. Phys. Rev. 2023, 10. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teli, A.M.; Mane, S.M.; Beknalkar, S.A.; Mishra, R.K.; Jeon, W.; Shin, J.C. Graphene-Based Gas Sensors: State-of-the-Art Developments for Gas Sensing Applications. Micromachines 2025, 16, 916. https://doi.org/10.3390/mi16080916
Teli AM, Mane SM, Beknalkar SA, Mishra RK, Jeon W, Shin JC. Graphene-Based Gas Sensors: State-of-the-Art Developments for Gas Sensing Applications. Micromachines. 2025; 16(8):916. https://doi.org/10.3390/mi16080916
Chicago/Turabian StyleTeli, Aviraj M., Sagar M. Mane, Sonali A. Beknalkar, Rajneesh Kumar Mishra, Wookhee Jeon, and Jae Cheol Shin. 2025. "Graphene-Based Gas Sensors: State-of-the-Art Developments for Gas Sensing Applications" Micromachines 16, no. 8: 916. https://doi.org/10.3390/mi16080916
APA StyleTeli, A. M., Mane, S. M., Beknalkar, S. A., Mishra, R. K., Jeon, W., & Shin, J. C. (2025). Graphene-Based Gas Sensors: State-of-the-Art Developments for Gas Sensing Applications. Micromachines, 16(8), 916. https://doi.org/10.3390/mi16080916






