Abstract
Sodium-ion batteries have emerged as a promising alternative to lithium-ion systems, due to the abundance and low cost of sodium resources. However, the demand for higher performance is always increasing, and developing new electrode materials and optimizing their behavior in full cells is necessary. Their electrochemical performance remains limited by challenges related to the anode materials. A fundamental understanding of electrode materials is essential to advance their practical application, for example, by designing strategies to minimize irreversible processes and enhance the reversible capacity. Thus, the properties of metals, including nanoparticles and clusters, are critical for various types of sodium batteries, such as sodium-ion microbatteries. Additionally, metallic nanoparticles exhibiting special properties are generated in situ at the negative electrode during the electrochemical cycling of certain batteries. This review focuses on their formation mechanisms, structural and electrochemical effects, and strategies to control their distribution and size. Particular attention is given to the interaction between metallic particles and carbon matrices, as well as their influence on capacity. Finally, current limitations and future perspectives for optimizing the properties of the metallic particles in advanced sodium battery anodes are highlighted.
1. Introduction
Developing new batteries for renewable energy storage is a serious need. Since sodium is abundant and not costly, SIBs can be handy for a modern and sustainable society [1,2]. On the other hand, since Li can form alloys with Al, but Na does not, SIBs are compatible with low-cost aluminum foils as current collectors, compared to lithium ones that typically employ expensive copper foil at the negative electrode. Although sodium metal has a very high theoretical capacity (1166 mAh g−1) and low redox potential (−2.71 V vs. SHE), it is not usually employed in its elemental state as a negative electrode in a battery. The main drawbacks of metallic Na as an anode are an unstable surface, uneven Na deposition, and the risk of dendrite growth, which can lead to capacity fade and the battery’s failure. However, sodium atoms can be chemically combined with metallic and non-metallic elements to provide a more suitable electrode material. The reactions for sodium storage in electrode materials can be classified into three types: intercalation, alloying, and conversion. The last two of those involve metals and/or alloys. Table 1 summarizes the most relevant electrode materials for SIBs, categorized according to their respective reaction mechanisms as discussed in this review. SIBs are most often based on the intercalation of sodium cations into oxides and other compounds. However, metals and alloys are also intimately linked to many of the most promising SIBs. Recent advances in metallic particles for SIBs, along with strategies for their optimization, are summarized in this review. Pure metals, intermetallics, and alloys can be initially employed as electrode materials in SIBs. The sodiophilic metallic elements that have been typically employed in SIBs are in Groups 14, 15, and 16 of the Periodic Table: Sn, Sb, Te, Pb, and Bi. Interestingly, metallic nanoparticles can be in situ formed during the charge/discharge of the battery. For example, controlling the formation of clusters of sodium atoms, with quasimetallic character, in sodiated carbon is highly relevant for developing SIBs.

Table 1.
Summary of the most significant anode materials for SIBs involving metallic nanoparticles.
Since the ionic radius of Na+ (102 pm) is relatively large (76 pm for Li+), the diffusion of sodium in the solid state is sluggish. Then, compounds in the form of nanoparticles can reduce the path length and improve the kinetics. It is worth noting that the nanosize effect can generate anomalous behavior in properties of metal nanomaterials, such as melting point, crystal structure, and miscibility [43]. On the other hand, the sluggish diffusion kinetics of sodium ions represent a critical challenge for achieving a well-optimized SEI; therefore, rational design and optimization of the SEI are essential. Moreover, the relatively large ionic radius of sodium induces substantial volume variations during the sodiation/desodiation processes, which can lead to severe electrode pulverization and, consequently, rapid battery degradation or failure. To solve this problem, a strategy is to encapsulate the nanoparticles within a stable matrix.
Particularly, the development of sodium-ion microbatteries with 2D or 3D architectures is challenging to achieve [44,45,46,47]. Before the engineering of microelectrodes and microbatteries, it is essential to attain a thorough understanding of the electrochemical mechanisms and associated challenges governing the charge/discharge processes. For example, the repetition of conversion reactions, as well as (de)alloying processes, over many charge/discharge cycles can be detrimental to the structural integrity of electrodes, necessitating the design of strategies to make advanced high-performance microelectrodes.
2. Sodium and Interface
Maintaining a stable anode of metallic Na, working properly and safely during many charge/discharge cycles, is challenging [48]. The properties of the SEI built on Na should be electronic insulation, good sodium-ion conductivity, and high mechanical strength. Typically, the main challenges involve the SEI instability and the formation of dendrites, particularly under high current densities. Nevertheless, sodium is less prone to dendrite formation than lithium, and the resulting sodium dendrites are generally softer and pose a lower safety risk. Mechanical stress and strain within the SEI can induce cracking, thereby exposing fresh sodium surfaces to the electrolyte. This exposure accelerates electrolyte decomposition and contributes to the gradual thickening of the SEI layer over repeated cycling. A very thick SEI is impractical for the rapid diffusion of sodium ions. Sodium carbonate and sodium oxide are the main components of the SEI on Na, and NaPF6 residue present on the Na surface can form NaxPFy and NaxPFyOz [3]. Using theoretical calculations allows us to select solvents with high LUMO energy levels. For example, FEC with a LUMO value of 1.03 eV is very convenient. Many electrolyte solutions contain F-compounds to induce the formation of a more stable SEI [49,50]. Thus, FEC is a very convenient layer-forming additive. Typically, NaF is formed in the SEI. However, the Na+-conductivity of NaF is not very high. Sodium fluoride can create a protective interface on Na and inhibit dendrite formation. For example, Zhou et al. found that nanoparticles of CoF2 react in situ with Na, forming NaF and Co on the sodium surface (CoF2 + 2 Na → 2NaF + Co), and the NaF/Co hybrid layer is about 15 µm in thickness [4]. The protective layer enhances ion diffusion kinetics and promotes uniform and stable sodium electrodeposition. A minimal NaF content in the SEI and excessive Na2O may not effectively protect the Na electrode [3]. It has been suggested that a NaI-rich SEI exhibits a lower energy barrier for sodium diffusion than a NaF-rich SEI, and the use of the CTAI electrolyte has been proposed to achieve such an SEI composition [51]. CTAI helps to create a more stable SEI with a thickness of ca. 40 nm and prevents the formation of dendrites. It was suggested that the upper SEI on Na consists of carbonates, and the inner layer consists mainly of NaF and NaI.
Sodiophilic elements or compounds deposited on metallic Na can create an artificial and more stable SEI. Xie et al. employed silver perchlorate in an organic solvent for creating a layer of Ag crystals on the surface of Na, through the following reaction [5]:
where the thickness of the artificial layer can be controlled by the soaking time of Na in the silver perchlorate solution.
Na + AgClO4 → NaClO4 + Ag
As a novel strategy to stabilize the Na/electrolyte interface and improve the properties of the SEI, it has been proposed to introduce lithium compounds into the SEI of Na by employing certain additives [52]. The SEI contains Li-based compounds (Li3N, LiF, and Li2CO3) formed by the reaction of Na with electrolyte additives such as LiNO3 and LiTFSI. With an average thickness of ~887 nm, the SEI remains intact without visible cracking after repeated plating/stripping, while also reducing the polarization during sodium deposition. The challenges and strategies of Na and SEI are summarized in Figure 1.
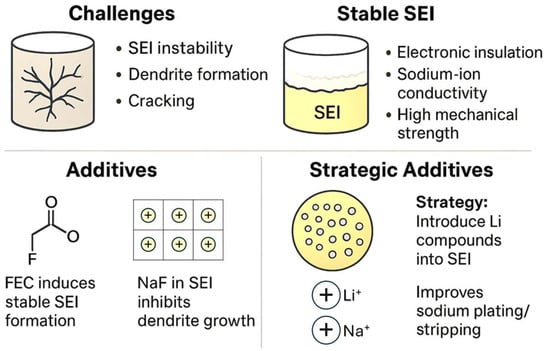
Figure 1.
Main issues and strategies of the sodium electrode.
It has recently been proposed that sodium metal batteries exhibit improved performance over a wide temperature range (−20 °C to 55 °C) when formulated with carefully designed low-concentration electrolytes, for instance, 0.3 M NaPF6 in PC, FEC, and TTE [53]. Forming a flexible interphase with a low shear modulus, capable of accommodating the expansion of sodium metal, can contribute to the improvement of sodium plating/stripping. The architecture of gel polymer electrolytes can offer stable interfaces for homogeneous Na plating/stripping [6]. Coating the surface of Na nanoparticles with a layer of tin-sodium alloy can be a sort of interfacial protection engineering to achieve non-dendritic and long-life batteries [54]. Through the careful selection of the solvents in the electrolyte solution, the interface can be modified. For example, a mixture of THF and 2-MeTHF facilitates the cleavage of the P-F bond in P and promotes the formation of a NaF-rich SEI, and improves the cycling stability of microsized Sn electrode [9]. Addition of octamethyltrisiloxane to the electrolyte solution also weakens the P-F bond and facilitates the formation of a NaF-rich SEI [55].
Sodium-potassium alloys exist as a liquid phase over a wide range of compositions (9.2–58.2 wt.% Na at room temperature), and this state can be advantageous for self-healing properties [56]. Anode-less SIBs have also been proposed to reduce the economic cost and to enhance cycling stability. For example, the hard-carbon-derived interphase on an aluminum current collector enables crack-free plating [57].
Bearing in mind that silver is sodiophilic and that TiO2 nanotubes exhibit a large surface area, a 3D conductive scaffold of vertically aligned nanotubes of TiO2 embedded with Ag nanoparticles can reduce the nucleation barrier for Na plating and prevent dendrite growth, compared to the planar Ti foils [58]. In fact, TiO2 nanotubes may be very useful for future microbatteries.
Considering the limitations of experimental studies on clusters of sodium atoms (NaN), Huwig et al. computed the most stable isomers for N = 2–150 [59]. These cluster sizes are not sufficiently large to exhibit bulk properties and adopt several atomic arrangements. According to their calculations, the stable structures in the small clusters are not bcc, and a more compact arrangement of atoms (fcc) was suggested. Magic clusters with enhanced stability were identified at N = 74, 83, 87, 95, 104, 131, and 137. Interestingly, through theoretical calculations on the clusters Na39, Na39−, and Na39+, it was concluded that the electrical charge in the clusters changes their thermodynamic properties, such as specific heat capacities and melting temperatures [60]. It is worth noting that clusters Na150 would have a diameter of about 2 nm and can be formed in situ at the discharge of SIBs.
3. Sodium Clusters in the Carbon Electrode
Certain sodium properties differ from those of lithium and potassium, for example, the energy of substrate binding, and these different properties affect the ion-intercalation [61]. DFT calculations show that Na–graphite compounds (e.g., NaC6, NaC8) have positive formation energies and are therefore thermodynamically unstable, unlike their Li or K analogues. The instability arises from the unusually weak substrate/binding energy of Na, which reaches a minimum among alkali metals. This weak binding is a general phenomenon, not specific to graphite, but extended to carbon and other substrates. As a consequence, Na insertion in graphitic materials is intrinsically unfavorable, explaining the low capacity and poor performance of Na-ion batteries with graphite anodes.
On the other hand, hard carbons exhibit different behavior when interacting with alkali metals. According to DFT calculations, the Na-C interaction is substantially weaker than that of the Na-Na interaction, and adsorption of Na on the graphene layer is thermodynamically unstable [62]. In contrast, Li-C and K-C interactions are stronger. This could be due to the relatively higher ionization energy of Na. Consequently, Na has a large tendency to form clusters of atoms with quasimetallic character in the sodiated hard carbon, which is a very common anode material in SIBs. Reduction of sodium ions to form pseudometallic sodium does not occur only at the surface of the electrode exposed to the electrolyte solution, but also within the micropores following the sodium diffusion process [63,64,65], and the pore-filling mechanism is reversible. Thus, the carbonaceous material can act like a sort of matrix that efficiently envelops metallic nanoparticles of Na.
Storage of sodium in carbonaceous materials can proceed through different mechanisms. The sodium initially inserted into the carbon at a high voltage possesses ionic character. Below 0.1 V vs. Na+/Na, formation of quasi-metallic clusters of Na has been observed in disordered carbons possessing microporosity [66,67]. For the intercalation of sodium ions between graphene layers in hard carbon, the interlayer spacing must be slightly larger than 3.6 Å [68], whereas “intercalation” for graphene interlayer spacings greater than 4.0 Å should be considered an adsorption process [69]. The presence of quasimetallic Na on the electrodes can be confirmed through a straightforward chemical test: immersing the reduced carbon electrode in ethanol with phenolphthalein results in a red color change and the evolution of H2 gas, indicative of reactive sodium species [70]. Pair distribution function analysis of neutron scattering data unveiled a diameter for the sodium clusters of approximately 13–15 Å [71]. Hard carbons with abundant closed pores exhibit a larger capacity for filling these pores with sodium in a quasi-metallic state (Figure 2) [72,73,74,75].
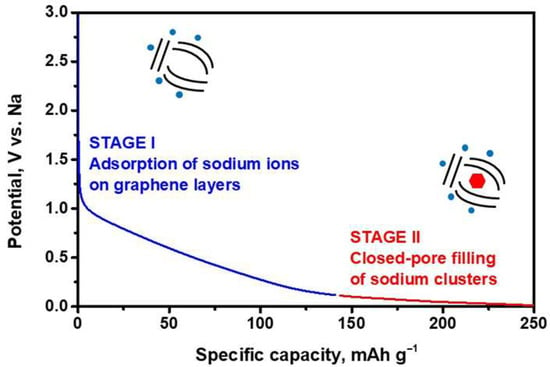
Figure 2.
Voltage-capacity curve and schematic diagram of the sodium storage mechanism of a hard-carbon [72,73].
To study the formation of sodium clusters with quasimetallic character in a carbon electrode, several spectroscopic techniques can be employed, for example, EPR [62,75,76,77]. EPR measurements by Kukeva et al. revealed the presence of sodium clusters with diameters between ~2 and 15 nm, whose size distribution depends on the carbon microstructure [78]. The change from ionic sodium to quasimetallic Na near the particle surface can be followed by XPS of the Na 1s core level [79]. The observed shift in the 23Na NMR peak during the low-voltage plateau implies that the metallic character of sodium increases [71,80]. In the 23Na MAS NMR spectra of sodiated hard carbon, the peak of quasimetallic Na clusters (length > 10 Å) at low voltage is shifted to ca. 660 ppm, while ionic sodium intercalated at higher voltages is detected in the spectra near 0 ppm [71]. Other authors observed the Knight shift of semimetallic sodium clusters at 960 ppm [81,82]. Using in situ XRD, Song et al. detected the reversible formation of Na clusters during filling of closed pores as low-intensity reflections [83]. Pore filling with Na atoms can be followed as an attenuation in the SAXS signal at around 0.01 V, and a reduction in electron density contrast between carbon walls and pore interior [84].
Compared to pure graphite, the interlayer spacing is wider, and the carbon layers are less aligned in hard carbon. A true intercalation of sodium is more favorable, up to the theoretical capacity of 140 mAh g−1 (NaC16). DFT calculations have found that this intercalation could contribute to the low-voltage plateau (0–0.1 V), although the total capacity of this plateau cannot be explained only by the intercalation [85]. Nanometer-scale Na clusters (3–6 atomic layers thick) lodged between graphene sheets in model micropores are energetically stable, in addition to classical adsorption and intercalation mechanisms. Thus, these nanometer-sized Na clusters stored in carbon micropores can significantly enhance the plateau capacity. The computed formation energies and insertion potentials confirm that such metallic-like Na clustering can account for the unusually high reversible capacity (more than the typical ca. 300 mAh g−1 value) observed experimentally (e.g., 478 mAh g−1 in MgO-templated hard carbon). Their quasimetallic nature, verified by the Na NMR chemical shifts, facilitates their growth and pore filling, thereby giving rise to the excess capacity already discussed. Meanwhile, the slope capacity (0.1–1.5 V) arises from Na adsorption on defective graphene layers, and the sodium–carbon interaction can be tracked using Raman spectroscopy. The process of sodiation through adsorption on the surface and defective sites can be followed by a decrease in the relative intensity of the Raman band ascribed to disordered regions in carbon, and a red shift in the band ascribed to graphitic regions (transfer of electrons from sodium to graphene sheets) [83,86].
Both the size of the sodium clusters and the careful tuning of the pore size must be considered to optimize sodium storage. It is believed that the smaller the sodium clusters, the higher the carbon capacity. Xie et al. reported that Na clusters forming the plateau close to 0 V are about 1.3–1.5 nm in size, and that smaller pores do not contribute to this low-voltage capacity [86]. Youn et al. found that pores larger than 1.1 nm cannot be completely filled by Na [85]. This voltage region displays diffusion-controlled redox behavior. One key benefit is that closed pores do not increase the carbon–electrolyte contact area, yet their filling with quasi-metallic sodium provides additional capacity near 0 V [87,88,89]. Many micropores that can accommodate Na clusters are closed and not accessible to N2 adsorption analysis, making CO2 adsorption isotherms necessary for their characterization.
Theoretical simulations of sodium storage in disordered carbon, performed with the ReaxFF force field on a model containing 65,000 carbon atoms, support the notion that the filling of micropores with quasi-metallic sodium occurs during the low-voltage plateau [7]. In this model, the total carbon capacity is 220 mAh g−1, and 105 mAh g−1 of that capacity corresponds to the filling of the pores with quasi-metallic sodium, which happens after surface adsorption and sodium intercalation. This result highlights the importance of tailoring the pores. A maximum of 6200 atoms of sodium can be accommodated, which is equivalent to Na0.54C6. Probably, experimental capacities higher than these values would involve the storage of sodium ions through other mechanisms. The simulations also prelude the formation of small Na clusters around vacancies and defect sites.
Xiao et al. studied the relationship between pore size and the voltage at which sodium fills the pores [90]. Closed micropores exhibit a higher potential for Na-filling and reduce the possibility of Na-plating, particularly at high current intensity. The capacity of the plateau near 0 V (formation of quasimetallic Na) does not increase monotonically with the total volume of the closed pores. The ideal size of the closed pores is 1.6 nm. Wang et al., using DFT calculations, elucidated the critical role of pore engineering in sodium storage. The models reveal that a closed pore size of 0.45 nm yields the maximum sodium adsorption energy of −4.87 eV, identifying it as the thermodynamically optimal site for sodium cluster storage. This specific geometry is clearly better than that of both smaller (−0.91 eV) and larger pore configurations (−2.53 eV). On the other hand, optimized architecture features an expanded interlayer spacing of ca. 0.39 nm, which lowers the energy barrier for Na+ intercalation and facilitates the ion transport compared to graphite [91].
There are several strategies to create closed pores. Phenolic resins obtained from resorcinol and aldehydes have been employed to create an abundance of closed pores after carbonization and to enhance the storage of quasimetallic sodium [92,93]. To control carbon porosity and enhance the formation of sodium clusters, adding fullerene, C60, to the phenolic resin can increase the total volume of the closed pores and generate more sites for sodium storage (plateau capacity) [94]. It seems that C60 induces the curvature of graphitic microdomains and thus favors the disordered structure and closed porosity in the hard carbon, while the specific surface area is reduced. Metal oxide particles can act like templates to create pores in the carbons [95,96,97,98,99]. Using in situ template-etching, with zinc gluconate as a templating agent, can generate more closed pores and a large plateau capacity (192 mAh g−1) [98]. Zinc gluconate and glucose are carbonized, and nanometric particles of ZnO are formed and then washed off with acid. Zinc gluconate generates closed pores, inhibits the graphitization degree, and expands the interlayer spacing.
It has been discussed that the coexistence of high-potential sloping capacity and low-potential plateau capacity can be detrimental for excellent performance at high rates [100,101], and it has been proposed to integrate materials with sloping voltage curves, such as N/P/S-doped hard carbon embedded with amorphous SnPx/SnSx composites [101]. It was observed that it results in an impressive 90% capacity retention over 10,000 cycles. Adding PTCDA to lignin, during the pyrolysis of this mixture to obtain hard carbon, PTCDA molecules connect the lignin-derived carbon skeleton [102]. The resulting hard carbon has an enlarged interlayer spacing (0.397 nm) between graphene layers, and the closed pore structure is reduced. Consequently, sodium storage is dominated by intercalation between the graphene layers, instead of the formation of pseudo-metallic Na clusters. With large interlayer spacing and fewer closed pores, sodium diffusion in hard carbon is more rapid, the contribution of the surface to the capacity is large, and the storage of sodium in the low-voltage plateau is limited. Thus, this type of material is more adequate for a sodium-ion hybrid capacitor [102].
Crystallinity, interlayer spacing, and porosity can be regulated and optimized in carbon materials derived from industrial wastes. Low-temperature (850 °C) pyrolysis and sulfur-doping of carbon derived from petroleum coke, using thiophenic sulfur, enable the development of hierarchical pores [103]. Covalent C-S bonds suppress excessive graphitization, expand the interlayer spacing (0.373 nm), generate defect sites, and facilitate rapid diffusion of sodium towards internal or closed micropores (<2 nm) for storage of quasi-metallic sodium in the voltage plateau. Higher pyrolysis temperatures (e.g., 950 °C) and larger surface area induce carbon framework collapse during cycling, causing pore blockage.
Sequential sulfur-oxygen doping of hard carbon, derived from asphalt, allows a more precise regulation of its microstructure and an extended plateau region [104]. First, sulfur-treatment constructs a 3D disordered network, and then oxygen-treatment promotes denser cross-linking, resulting in the formation of closed-pore microstructures during pyrolysis. SAXS analysis shows that the smallest closed pores have a radius of 1.165 nm, and helium pycnometry indicates a minimum density of 1.68 g cm−3. In contrast, pyrolysis without constraints leads to an architecture devoid of closed pores. P4O10 serves a dual function, acting both as a template for pore-mouth design and closed-nanopore formation, as well as a dopant source [81]. Yang et al. have proposed creating defects (carbon vacancies) in the walls of the closed micropores to increase the plateau capacity and the sloping capacity [8]. To increase carbon vacancies, H2 thermal reduction was employed. The reversible capacity is 470 mAh g−1, including a plateau region with a capacity of 299 mAh g−1.
These results demonstrate that the carbon-based matrix plays a crucial role in controlling the nucleation and growth of sodium nanoparticles and clusters within the anode of sodium-ion batteries (SIBs). Nevertheless, a serious safety issue has been recently published. Using NMR, Cui et al. found that the sodium clusters exhibit significant metallic character, with even more conduction electrons at the Fermi energy level than bulk Na, making them highly reactive and accelerating thermal runaway [105].
4. Sodium-Alloying
4.1. Tin
Silicon and germanium have relatively low ability to form alloys with sodium and/or very sluggish kinetics for sodium diffusion, and consequently, tin is the lightest element in group 14 with a significant theoretical capacity in a sodium cell (847 mAh g−1). To illustrate their different dimensions, the crystal structures of the main intermetallic sodium-tin compounds are shown in Figure 3. The total electrochemical reaction can be written as:
4Sn + 15Na → Na15Sn4
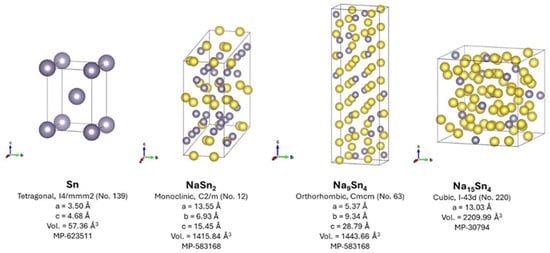
Figure 3.
Crystal structures for tin and sodium-tin intermetallics obtained from the Materials Project database [106].
The substantial volume expansion (about 420% for Na15Sn4 relative to Sn) that occurs during charge/discharge cycling induces severe mechanical stress, resulting in electrode pulverization and eventual battery failure [107]. One strategy to mitigate the volume changes occurring during the formation of tin-sodium alloys is to mix, or alloy, tin with other elements such as cobalt or carbon. Another approach involves reducing the initial particle size (typically to around 10 nm); however, smaller particles exhibit a larger surface area in contact with the electrolyte, which promotes irreversible electrolyte decomposition during SEI formation and consequently reduces the coulombic efficiency. Interestingly, recent studies have shown that, in ether-based electrolytes, micrometric Sn exhibits better cycling stability than its nanometric counterpart [10]. A high Sn-charge (80%) in tin-carbon composites is better when diglyme-based electrolytes (676 mAh g−1 after 70 cycles at 0.1 A g−1), and the reason for that can be the formation of an extended coral-like framework by Sn that enables connectivity during the cycling.
Within the strategies for high-performance tin-based electrodes, metallic substrates with non-planar geometry can help to achieve electrodes with higher areal capacities. Thus, Co-Sn alloy can be electrodeposited on nickel-foam [108]. Ultrafast heating of fructose and SnO2 can form nanoparticles of Sn surrounded by a carbon matrix with good electrochemical cycling [109]. Rapid heating is critical for obtaining small Sn particles and good electrochemical properties. A hierarchical yolk-shell structure composed of Sn-yolk and carbon/silicon oxycarbide bilayer shell stabilizes the electrode/electrolyte interface [110]. Void interspaces inside the yolk-shell nanohybrid accommodate the volume change of the Sn yolk during cycling. Another interesting microstructure is tin nanospheres anchored on and embedded in carbon nanofibers [111]. Alloys may also be incorporated as electrode additives to enhance electrochemical performance. To increase the initial coulombic efficiency of the SIB, the intermetallic compound Na15Sn4, mixed with hard carbon, has been proposed as a sodium reservoir to compensate for the irreversible sodiation in the first cycle of the carbon [112].
Examination of the electrode particles after electrochemical cycling can unveil surprising behaviors. It has been found that optimizing cell configuration, for example, by using a double nanoporous separator as a physical barrier, Sn powder exhibits a self-healing phenomenon during cycling [113]. Although the Sn powder undergoes pulverization during the initial (de)sodiation cycle, the in situ formed Sn particles with a porous coral-like morphology exhibit mechanical stability against volume fluctuations and preserve electrical contact. The MWCNT conductive additive establishes efficient conductive pathways between Sn particles, outperforming Super P carbon.
4.2. Antimony
The formation of Na3Sb delivers a high theoretical capacity of 660 mAh g−1, presenting significant potential for sodium-ion battery applications. Nevertheless, the substantial volume expansion of about 390% compared to pure Sb remains a critical limitation, motivating the development of advanced synthesis approaches to produce micro- or nanoscale particles. In contrast to Sb nanoparticles, atomically dispersed Sb species act as superior nucleation centers for the reversible growth of Na–Sb alloy phases such as Na3Sb [11]. Via electrospinning, the metallic Sb core was encapsulated by the MoS2 shell (Sb@MoS2), and the Sb@MoS2 nanoparticles were embedded in carbon nanofibers [12]. Dispersing nanoparticles of a compound with Na-alloying elements (SnSb of about 10 nm) in a carbon matrix can provide an electrode with a very high capacity (544 mAh g−1) [13]. Nanoparticles of Sn-Ge-Sb alloy (10–15 nm) can also deliver exceptionally high capacity and cycling stability (662 mAh g−1 after 50 cycles) [14]. The hollow structure of carbon nanotubes can encapsulate nanoparticles of Sb [114] and Sb2O3 and accommodate the large volume changes [115]. Electrospinning was employed to fabricate a mosaic distribution of Sb/P nanospheres, with sizes ranging from 100 to 200 nm, embedded within a carbon fiber matrix. During sodiation, the formation of amorphous Na3Sb and Na3P was assumed to occur [116].
The theoretical capacity of Sb2O3 in a conversion reaction is as high as 1109 mAh g−1. Zheng et al. confirmed the transformation of Sb2O3 into Na2O and Na3Sb by TEM, XRD, and XPS [115]. During desodiation, Na3Sb is converted back into crystalline Sb and Sb2O3. Regulating the crystallographic facets exposed to the electrolyte solution can be employed to enhance the electrochemical performance. Zheng et al. prepared nanobelts of Sb2O3 with exposed (010) facets [117]. The polymer PVP promotes the anisotropic growth of Sb2O3 particles with a nanobelt morphology and the exposure of the (010) crystallographic plane, which has a large spacing. This nanoarchitecture decreases the Na-driven volume expansion and improves sodium storage capability. It was proposed that starting from a mixture of Na3Sb and Na2O, during the charge process, firstly Na3Sb dealloys to Sb, and then further charging and desodiation transform the mixture Sb-Na2O into Sb2O3.
4.3. Tellurium
Tellurium has an excellent electronic conductivity (2 × 102 S cm−1), high density (6.2 g cm−3), and a high theoretical specific capacity (420 mAh g−1), resulting in an outstanding theoretical volumetric capacity for SIB (2621 mAh cm−3) [15,16]. These intrinsic properties make Te-based materials highly attractive as high-energy-density anodes. However, the practical application of pure Te is hindered by several critical challenges. Like other chalcogen-based materials, elemental tellurium is prone to polychalcogenide shuttle behavior during cycling, which leads to active material loss and poor Coulombic efficiency. In addition, Te undergoes substantial volume expansion upon sodiation, which can cause particle pulverization, loss of electrical contact, and rapid capacity decay. To overcome these limitations, researchers have increasingly focused on metal tellurides and carbon–telluride composites, which offer improved structural stability and optimized reaction kinetics. Metal tellurides, such as FeTe2, NiTe2, or CoTe2, typically exhibit stronger metal–Te bonding and more favorable conversion or alloying mechanisms, leading to reduced shuttle effects and enhanced cycling durability. Likewise, integrating Te or metal tellurides within conductive carbon frameworks—such as carbon nanofibers, graphene, or porous carbons—provides mechanical buffering, improved electron/ion transport pathways, and confinement of telluride species. For example, FeTe2 nanoparticles embedded in carbon nanofibers, and prepared using the electrospinning technique, have demonstrated remarkable electrochemical resilience, delivering stable performance over 1000 cycles [17]. This behavior is attributed to the synergistic combination of the robust Fe-Te structure and the flexible, conductive carbon matrix, which effectively buffers volume changes and preserves electrode integrity during repeated sodiation/desodiation processes.
4.4. Bismuth
The maximum theoretical capacity of bismuth is relatively modest (385 mAh g−1 for Na3Bi) due to its high atomic mass. In recent years, the economic cost of Bi has risen significantly, making it less competitive compared to tin. Nevertheless, several research groups have continued to investigate its potential. In the Bi-Na system, intermediate compositions (tetragonal NaBi) and several structures (cubic and hexagonal Na3Bi) can be formed [18]. The sodiation process and structure evolution involve dramatic volume changes. Nanoparticles of Bi reduce the diffusion path length of sodium but tend to agglomerate during electrochemical cycling.
Like other Na-alloying elements, encapsulation of Bi nanoparticles in carbon matrices is being explored. For example, carbon fibers [19]. Hollow carbon spheres are highly effective, even over 20,000 cycles [20], and Bi nanoparticles were observed inside the hollow carbon spheres after electrochemical cycling. Very often, the behavior of SIBs at very low temperatures is very poor. However, Bai et al. reported that Bi nanoparticles embedded in carbon nanorods have a capacity of 238 mAh g−1 at 2 A g−1 and −40 °C [21]. Since Sb is lighter than Bi, the Sb-Bi alloys can have a higher gravimetric capacity than pure Bi. Although Sb can be dissolved and shuttled, Bi crystals tend to fix Sb atoms. Porous carbon can attenuate the issues of the Sb-Bi alloy [22]. The ultrafast high-temperature shock method has been employed to prepare Bi nanoparticles (average particle size ca. 26 nm) embedded in carbon nanorods, [21]. The activation of the Bi electrode through high-rate cycling at low temperature was found to increase the specific surface area, generate new electrochemically active sites, and consequently improve the capacity. As an alternative to encapsulating Bi nanoparticles, it has been proposed to create Bi nanotubes. For this purpose, Pu et al. employed iodine-assisted galvanic replacement, BiI3 as a bismuth precursor, and copper nanowires as templates and reducing agents [23]. The formation of the Bi nanotubes was based on the Kirkendall effect. The cycling stability was impressive: 355 mAh g−1 after 15,000 cycles at 20 A g−1. Bi nanoparticles (35–75 nm) dispersed in a silica matrix are formed by heat-treatment of CuO-Bi2O3-SiO2 glass in a reducing atmosphere of H2, and this material serves as an electrode for sodium batteries [24]. The silica matrix inhibits the dissolution of metallic particles.
Bismuth has a layer structure and can be exfoliated into 2D nanosheets, forming a few-layer structure and even a monolayer structure. Analogous to graphene, the monolayer of bismuth is named bismuthene, with a theoretical thickness of 0.395 nm [118]. Bismuthene/graphene heterostructure can exhibit improved properties [119]. According to theoretical calculations, increasing the concentration of Na in bismuthene involves clustering Na atoms together, and it becomes energetically more favorable than binding to bismuthene, which increases the risk of dendritic growth. However, the heterostructure graphene/bismuthene inhibits the growth of dendrites and improves the electronic conductivity.
Bismuth oxide can work like a conversion-type material, and the Na-alloying capacity of the in situ-formed Bi metal particles can increase the overall capacity (theoretically up to 670 mAh g−1). Mu et al. have proposed to use a composite electrode material containing Bi2O3 and reduced graphene oxide [120]. The flexibility of the graphene structure accommodates the volume changes. The Bi/Bi2O3 heterojunction spontaneously generates internal electric fields that facilitate the transfer of electrons and ions.
4.5. Binders for Metals
Binders play a critical role in enhancing the electrochemical performance and mechanical integrity of sodium-ion batteries. In particular, they help mitigate the substantial volume changes associated with alloy-type anode materials, thereby improving electrode stability, adhesion, and cycling durability. It has been reported that the most popular polymer binder for LIBs, PVDF, is not very suitable for the anodes of SIBs, particularly Na-alloying anodes such as Sn, Sb, and Bi; therefore, alternative binders should be used [121,122]. For example, cross-linking of PAA with GLY has been proposed. The tensile resistance of PAA-GLY surpasses that of PAA and PVDF, and tin electrodes using PAA-GLY as a binder exhibit enhanced stability. The cross-linking of PAA-GLY increases the Young’s modulus of the electrode. Notably, this binder enables the efficient utilization of Bi microparticles even in the absence of dispersion within the carbon material.
5. Formation of Metallic Particles Through Conversion-Type Electrodes
Conversion-type electrodes, including metal oxides and other reducible compounds, undergo reduction by sodium to yield metallic phases and sodium compounds. The sodium stored within the electrode can subsequently be released through oxidation. While the multivalence of the metal contributes to high theoretical capacities, the pronounced lattice-volume variation during redox cycling often leads to structural degradation. Gaining insight into these reaction mechanisms is crucial for designing strategies to improve long-term cycling stability.
5.1. Transition-Metal Oxides
Bimetallic oxides are particularly promising, as their capacities can exceed those of hard carbon, making them suitable for use in SIBs (Figure 4). For the electrochemical reaction between NiCo2O4 and sodium down to ca. 0.0 V vs. Na+/Na, a mechanism mainly based on conversion reactions was first proposed in the year 2002 and later studied in more depth [25,26,123,124,125,126,127,128,129,130,131,132]:
where reaction (3) is irreversible, and NiCo2O4 is not recuperated, while reaction (4) is reversible with x ≅ 1.0 and y ≅ 1.0. On the other hand, reaction (3) could be divided into three sodiation steps:
NiCo2O4 + 8Na → Ni + 2Co + 4Na2O
Ni + 2Co + 4Na2O ⇌ NiOx + 2CoOy + (2x + 4y)Na + (4 − x −2y)Na2O
- –
- Step 1. Veritable insertion of sodium ions into the host structure of the spinel in the range between ca. 3.0 and ca. 0.8 V. This step remains little explored.
- –
- Step 2. Destruction of the spinel structure and formation of NiO, CoO, and Na2O, at around 0.8–0.2 V. This intermediate step was particularly corroborated by Zhu et al. [127], using in situ TEM and electron diffraction patterns.
- –
- Step 3. Reduction of the oxides into metallic nickel and metallic cobalt, and further formation of sodium oxide near 0.0 V. This reduction process has been reported by several authors [25,123,124,125]. Thus, Zhu et al. observed the diffraction rings of metallic Co, Ni, and Na2O in the electron diffraction images of the fully sodiated product [123].
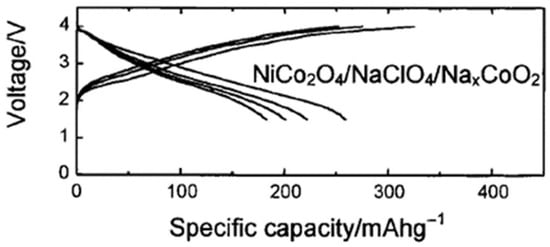
Figure 4.
Galvanostatic charge/discharge cycles for a full SIB NiCo2O4/NaxCoO2 [106]. Reproduced under ACS License No. 6147051118158.
The primary challenge of this electrode material is that the substantial volume changes of the crystal lattice can adversely affect electrochemical cycling. Since the ionic radius of sodium is larger than that of lithium, this limitation is even more pronounced in SIBs. A typical strategy to overcome this drawback is to employ nanoparticles and special nanostructures that could buffer the volume changes and alleviate the strain during the charge/discharge cycle [26,128,129,130,131]. Lee et al. grew NiCo2O4 directly on Ni foam and studied the conversion mechanism in both lithium and sodium cells [132]. After the formation of Ni and Co, it was found that Co3O4 was reformed during the oxidation in the Li cell, but CoO was formed in the Na cell.
After NiCo2O4, many other transition-metal oxides have been reported in the literature as conversion-type anodes for sodium-ion batteries. For example, in a paper by López et al. Na3V2(PO4)3 was employed as a positive electrode, and the negative electrode was a thin film of NiO and Fe2O3 [133]. It has been assumed that during the reaction of CoFe2O4 in a sodium cell, Fe and Co are first in situ formed and then Fe3+ and Co2+ are regenerated (in the form of CoO and Fe2O3) during the desodiation process, although the direct evidence of that still has not been checked [27,28,29]. Strong surface capacitance was detected by magnetometry in the reduced metallic nanoparticles, and this charge on the particle surface can be a source of extra capacity in the battery [134,135]. This effect can justify that the decrease in particle size increases the initial capacity. The formation of metallic Ni and Na2O after the conversion reaction of NiO was corroborated by using ex situ XRD, XPS, and magnetization measurements [30].
The morphology of the particles strongly influences the reaction mechanism with Na and the formation of metallic particles. Thus, Vincent et al. demonstrated that Co3O4, composed of nanoparticles with a spherical morphology, exhibits capacitive-type charge storage rather than a conversion-based mechanism [31]. These particles could facilitate surface charge storage, which is not limited by ion diffusion. This contrasts with diffusion-controlled conversion, for example, in Co3O4 with nanosheet morphology, which undergoes a conversion reaction. Defective oxides can exhibit modified properties. The spinel-type Fe3O4 was prepared with cation vacancies through Mo4+-doping [136]. Iron vacancies are created to balance the electrical charge. This defective anode Fe3−4x/3□x/3MoxO4 (x ≤ 0.20) exhibits improved capacity retention and rate capability compared to defect-free Fe3O4. The cation vacancies enhance sodium intercalation, and the conversion process is partial even after 150 cycles. The theoretical capacity of CoNiO2 is 716 mAh g−1, and in the form of 3D rose-like morphology, it possesses an outstanding electrochemical reaction kinetics (343.5 mAh g−1 following 100 cycles at 0.5 A g−1) [32].
5.2. Transition-Metal Phosphides
Transition metal phosphides (FeP, Ni2P, Cu3P…) react with sodium and sodium phosphides are formed (Ni2P + 3Na = Na3P + 2Ni). NaP is produced by the transformation of Na3P (Na3P = 2Na + NaP). Composite electrodes containing several phosphides have been studied [137]. Special morphologies and a carbon matrix can also contribute to buffer volume changes during discharge/charge of the phosphides. In the sodiated FeP@carbon nanocomposite, the formation of metallic Fe and Na3P phases was corroborated by SAED [33]. Their capacity is limited, while phosphides of Na-alloying elements (e.g., Sn) can deliver higher capacities than transition-metal phosphides [34] and can be classified into another category.
5.3. Metal Sulfides
Ferric pyrite could be a cheap and non-toxic substance for a conversion-type anode with high capacity, following these reactions:
with the intermediate formation of NaxFeS2 intercalation compound. During the charge process, the discharge products, Na2S and Fe, are not reconverted into FeS2. Soluble polysulfides (Na2Sx) are formed, and these lead to the polysulfide effect and electrode failure [138]. Then, strategies have been adopted to suppress the polysulfide effect. Nitrogen-doped carbon nanosheet has been employed as an optimized substrate to support FeS2 nanoparticles, and a remarkable cycling stability (2200 cycles) was achieved [139]. A metal–organic framework was employed as a precursor and template to prepare pyrrhotite (Fe1−xS) encapsulated by N, S-co-doped carbon [140]. The carbon matrix functions as an adsorbent for polysulfides, thereby reducing the shuttling effect, and also acts as a physical shield that protects the copper foil (current collector) from corrosion. The aluminum foil current collector also experiences corrosion because of the shuttle effect [138]. A Mott-Schottky heterojunction formed between FeS2 and N-doped carbon nanofibers could enable fast electronic/ionic transfer [35]. The junction establishes a built-in electric field that drives charge redistribution at the interface, promoting efficient charge transfer during (de)sodiation. After the intercalation of sodium into MoS2, the conversion reaction and formation of Na2S and metallic Mo have been proposed [36,37,38,39]. The method of synthesis and, particularly, the source of sulfur, influence the properties of the cobalt sulfides CoSx, and it seems that the compound CoS2 has better electrochemical performance and more efficient conversion-reaction [40]. Cobalt sulfide nanoparticles embedded in carbon layers, forming a core–shell structure (Co9S8@Co/C), can be reduced by sodium reversibly forming metallic Co and Na2S [41]. During charging, the reaction rate of metallic Co with Na2S is slower than the conversion of sodium sulfide into polysulfides, resulting in the shuttle effect (migration of polysulfides within the electrolyte). Fortunately, the carbon shell mitigates sulfide and polysulfide dissolution in the electrolyte, thereby suppressing the shuttle effect.
FeS2 + xNa → NaxFeS2
NaFeS2 + Na → Na2S + Fe
Sulfides of Na-alloying metals, such as Sn and Zn, could have larger capacities. In fact, monolayer tin disulfide SnS2 is an intrinsically appealing anode material for rechargeable sodium-ion batteries due to its high theoretical capacity and layered structure. However, its practical application is fundamentally hindered by two major drawbacks: severe volumetric expansion during sodiation and a wide electronic band gap that limits charge transfer kinetics. These drawbacks can be overcome with SnS2/graphene heterostructures [141,142,143]. DFT calculations were used to model the sodiation thermodynamics and kinetics in a monolayer of SnS2 and in a SnS2/graphene heterostructure. The pristine monolayer exhibited a high theoretical capacity of 586 mA h g−1 and a remarkably low Na+ diffusion barrier of 0.13 eV, but it was constrained by a ca. 20% area expansion and a large electronic band gap (1.54 eV). The formation of the SnS2/graphene heterostructure increased the binding energy of Na at the interface, simultaneously resulting in a metallic character and effectively decreasing the volume expansion to 2.4%. Although the interface diffusion barrier slightly increased to 0.26 eV, the resulting composite is computationally predicted to be a high-performance, mechanically stable, and conductive anode material for SIBs [142].
Another closely related approach is to encapsulate nanoparticles of SnS2 by carbon nanofibers. These materials can maintain a capacity of ca. 650 mAh g−1 [141]. DFT calculations were again performed to elucidate the synergistic role of the SnS2/C heterostructure in sodium storage. Interfacial charge density difference maps confirmed significant electronic coupling, predicting enhanced electronic conductivity due to favorable charge redistribution between the SnS2 nanoparticles and the conductive carbon matrix. DFT calculations further revealed a low energy barrier for Na+ diffusion (0.25 eV, a similar value to that computed when using graphene [142]) within the SnS2 lattice, confirming the rapid Na+ ion kinetics in this material. The strong SnS2/C interfacial binding energy theoretically stabilizes the conversion/alloying intermediates and suggests that the carbon encapsulation effectively mitigates the strong volume expansion stress inherent to the sodiation process [142].
The formation of a Zn, Na2S, and NaxZn alloy, through the conversion of ZnS, has been confirmed [144]:
ZnS + 2 Na → Na2S + Zn (0.5 V)
Zn + xNa → NaxZn (0.5–0.01 V)
When certain types of biomass are used as a carbon precursor to form a carbon-ZnS composite, a nitrogen-doped carbon can be formed. The highly electronegative nitrogen atoms can irreversibly trap sodium atoms, thereby decreasing the columbic efficiency [144].
Another strategy is the use of a high-entropy conversion-type electrode, such as the sulfoselenide Cu0.88Sn0.02Sb0.02Bi0.02Mn0.02S0.9Se0.1, with a reversible capacity of 325 mAh g−1 after 10,000 cycles at 30 A g−1 [42]. The resulting particle morphology suggests that the high-entropy configuration reduces the energy for surface formation. Many metal elements can undergo sequential electrochemical reactions and prevent the instantaneous collapse of the structure. The experimental results confirmed the conversion reaction and formation of Cu, Mn, Na2S, Na2Se, Na9Sn4, Na3Sb, and Na3Bi.
6. Innovative Directions
A conversion-reaction has been employed in a light-driven rechargeable battery, combining energy harvesting with energy storage in a cell made of an exfoliated MoSe2 electrode and a Na metal electrode [145]. The discharge/charge reactions are given below.
First reduction:
xNa + MoSe2 → NaxMoSe2 (intercalation at ca. 0.6 V vs. Na)
NaxMoSe2 → Na2Se + Mo (conversion at ca. 0.3 V vs. Na)
Oxidation:
2Na2Se + Mo → MoSe2 + 4Na
Second reduction: from Se to Na2Sen (4 < n < 8) and then to Na2Se2. Second oxidation: from Na2Se to Se.
The peak current in the cyclic voltammograms increases under illumination. The light generates charge carriers that assist the charge reaction. Electron holes are photogenerated in the valence band, and these holes repulse the sodium intercalated into the electrolyte, desodiating and generating MoSe2.
As an alternative to conventional alloys, high-entropy alloys can be employed. This type of alloy contains at least five elements randomly distributed in the same crystal structure, to provide high capacity for Na-alloying and to exhibit improved mechanical properties. A high-entropy BixSnySbyCuxAlx nanoalloy embedded in porous carbon fibers, and fabricated by the electrospinning method, was reported by Liu et al. [146]. The metallic nanoparticles, with diameters around 50–100 nm, were dispersed in carbon fibers with diameters of 200–300 nm. The concept of high entropy could be applied to other types of battery materials, not only alloys, for example, oxides.
It is worth noting that hard carbon can even be suitable as an anode for all-stretchable SIB [147]. However, Na-alloys would require sophisticated microengineering and innovative three-dimensional microarchitecture.
A 3D carbon architecture can mitigate the volume changes of the Na-alloying microelectrode, and the semiconductor industry’s technology can be applied to the fabrication of microelectrodes. Interestingly, Yue et al. first fabricated a square Si micro-rods array using photolithographic technique and etching, then employed plasma-enhanced chemical vapor deposition (PECVD) to grow graphene on the Si rods, and finally, an Sb shell was deposited by radio frequency magnetron sputtering (RF-MS) [148]. The resulting 3D silicon/graphene/antimony microrod array has excellent cycle stability in SIB. Additionally, 3D printing fabrication could be more convenient for future SIB microbatteries, although this area remains scarcely explored, and the observed capacities are moderate [149,150,151].
7. Conclusions
A schematic illustration of the principal aspects discussed above is shown in Figure 5. This study highlights that metals offer several advantages as components in sodium-ion batteries (SIBs). The conventional copper current collector can be replaced with more corrosion-resistant metals such as aluminum or titanium, while tuning particle size and morphology enables the tailoring of electrochemical behavior and the controlled generation of metallic species within electrodes. The main reaction mechanisms that lead to the in situ formation of metallic particles include (i) pore filling by quasi-metallic sodium in hard carbon through an intercalation reaction, (ii) conversion reactions of metal oxides, and (iii) alloying with other metals such as tin. At present, the use of hard carbon as an anode remains the most realistic, economical, and straightforward approach. However, recent progress regarding the other two mechanisms suggests that they could become viable alternatives if the synthesis routes for these emerging materials become more accessible. Moreover, metallic nanoparticles formed during conversion reactions can contribute additional capacity through surface charge accumulation, and the design of hard carbons with closed pores provides a means to regulate the formation of quasimetallic sodium clusters. Much effort is devoted to designing carbon materials with optimized pores capable of storing sodium clusters. The use of cross-linked polymer binders also represents a promising strategy to mitigate the mechanical degradation of Na-alloying particles. DFT calculations are essential for guiding the design of high-performance SIB materials, from electrolyte additives and SEI chemistry to hard-carbon anodes and metal-sulfide nanostructures. By revealing sodium’s unique interactions at the atomic scale, DFT enables the prediction of optimal pore sizes, interlayer spacings, and composite architectures. The theoretical insights not only clarify the mechanisms of Na storage but also accelerate the development of safer, more efficient SIB technologies and advanced metal-based nanomaterials. Nevertheless, significant challenges remain, including the large irreversible capacity loss during the first cycle, the pronounced volume changes associated with conversion reactions, and the heavy nature of Na-alloying elements. Additional concerns involve parasitic reactions such as shuttle effects in metal sulfide electrodes, the limited suitability of common LIB binders for metallic SIB electrodes, and potential safety risks related to the instability of quasimetallic sodium clusters that may trigger thermal runaway. Future research should focus on exploring the in situ formation and evolution of metallic nanoparticles during cycling, controlling exposed crystallographic facets to enhance electrochemical performance, and developing composite electrodes that exploit synergistic effects between different materials. One critical factor for achieving remarkable progress is the design of binders that can accommodate reversible metallic particle formation while buffering volume variations. Promising directions also include the design of self-healing electrodes, the application of high-entropy alloys, and the use of advanced 3D-printing techniques to create novel architectures for high-performance sodium-ion microbatteries.
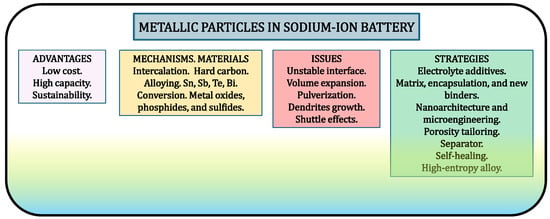
Figure 5.
Schematic overview of the key aspects of metallic particles in SIBs.
Author Contributions
Writing—original draft preparation, R.A.; writing—review and editing, R.A., C.P.-V. and R.R.; funding acquisition, C.P.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Junta de Andalucía (ProyExcel_0001020 and research group PAIDI FQM288.
Data Availability Statements
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SIBs | Sodium-ion batteries |
| SHE | Standard hydrogen electrode |
| LIBs | Lithium-ion batteries |
| SEI | Solid electrolyte interface |
| FEC | Fluoroethylene carbonate |
| LUMO | Lowest unoccupied molecular orbital |
| CTAI | Cetyltrimethylammonium iodide |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| PC | Propylene carbonate |
| TTE | 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether |
| THF | Tetrahydrofuran |
| 2-MeTHF | 2-methyltetrahydrofuran |
| DFT | Density Functional Theory |
| EPR | Electron Paramagnetic Resonance |
| XPS | X-ray Photoelectron Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| MASNMR | Magic Angle Spinning Nuclear Magnetic Resonance |
| XRD | X-Ray Diffraction |
| SAXS | Small-Angle X-Ray Scattering |
| ReaxFF | Reactive Force Field |
| PTCDA | Perylene-3,4,9,10-tetracarboxylic dianhydride |
| PAA | Poly (acrylic acid) |
| GLY | Glycerin |
| PVP | Polyvinylpyrrolidone |
| PVDF | Poly (vinylidene fluoride) |
| MWCNT | Multi-walled carbon nanotubes |
| TEM | Transmission Electron Microscopy |
| SAED | Selected area electron diffraction |
| PECVD | Plasma-enhanced Chemical Vapor Deposition |
References
- Tarascon, J.-M. Na-ion versus Li-ion Batteries: Complementarity Rather than Competitiveness. Joule 2020, 4, 1616–1620. [Google Scholar] [CrossRef]
- Rojo, T.; Hu, Y.-S.; Forsyth, M.; Li, X. Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800880. [Google Scholar] [CrossRef]
- Xu, Z.; You, J.; Zhang, B.; Li, Y.; Wang, W. Enhancing carbonate ester electrolyte selection for sodium-metal batteries through simulation and experimental validation. J. Energy Storage 2025, 116, 116068. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, F.; Wang, Y.; Yao, Y.; Shao, Y.; Rui, X.; Wu, F.; Yu, Y. Heterogeneous Interfacial Layers Derived from the In Situ Reaction of CoF2 Nanoparticles with Sodium Metal for Dendrite-Free Na Metal Anodes. Adv. Energy Mater. 2022, 12, 2202323. [Google Scholar] [CrossRef]
- Xie, J.; Li, Z.; Zheng, X.; Tian, F.; Lei, D.; Wang, C. Built-In Electric Field of In Situ Formed Artificial Interface Layer Induces Fast and Uniform Sodium-Ions Transmission to Achieve a Long-Term Stable Sodium Metal Battery Under Harsh Conditions. Adv. Funct. Mater. 2024, 34, 2315309. [Google Scholar] [CrossRef]
- Mittal, N.; Tien, S.; Lizundia, E.; Niederberger, M. Hierarchical Nanocellulose-Based Gel Polymer Electrolytes for Stable Na Electrodeposition in Sodium Ion Batteries. Small 2022, 18, 2107183. [Google Scholar] [CrossRef]
- Li, J.; Peng, C.; Li, J.; Wang, J.; Zhang, H. Insight into Sodium Storage Behaviors in Hard Carbon by ReaxFF Molecular Dynamics Simulation. Energy Fuels 2022, 36, 5937–5952. [Google Scholar] [CrossRef]
- Yang, H.; Yang, C.; Zhang, Y.; Li, Q.; Zhang, J.; Lu, Z.; Chu, Y.; Yang, Q.-H.; Tao, Y. Carbon vacancies boost plateau sodium storage in sieving carbon anodes. Carbon 2025, 243, 120506. [Google Scholar] [CrossRef]
- Lv, H.; Yao, Q.; Zheng, C.; Sun, Y.; Qian, Z.; Wei, Z.; Xie, F.; Ahuja, R.; Yang, J. Anion-Dipole Repulsions Improve Low-Temperature Performance of μ-Sn for Advanced Sodium-Ion Batteries. Small 2025, 21, 2502038. [Google Scholar] [CrossRef]
- Albenga, C.; Gott, J.A.; Skurtveit, A.; Warnett, J.M.; Maddar, F.M.; Koposov, A.Y.; Pinzon, G.; West, G.; Hasa, I. Assessing the role of morphological changes as the origin of improved cycling stability of Sn-based anodes for sodium-ion batteries. J. Mater. Chem. A 2025, 13, 30967. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Wang, Y.; Hong, Z.; Zheng, L.; Zhang, Y.; Wei, M.; Lu, J. Highly Reversible Sodium Metal Batteries Enabled by Extraordinary Alloying Reaction of Single-Atom Antimony. Adv. Energy Mater. 2024, 15, 2403432. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, Y. Encapsulating Sb/MoS2 into Carbon Nanofibers via Electrospinning Towards Enhanced Sodium Storage. ChemistrySelect 2024, 9, e202400172. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Xiao, J.; Wang, W.; Kovarik, L.; Nie, Z.; Liu, J. High capacity, reversible alloying reactions in SnSb/C nanocomposites for Na-ion battery applications. Chem. Commun. 2012, 48, 3321. [Google Scholar] [CrossRef]
- Farbod, B.; Cui, K.; Kalisvaart, W.P.; Kupsta, M.; Zahiri, B.; Kohandehghan, A.; Lotfabad, E.M.; Li, Z.; Luber, E.J.; Mitlin, D. Anodes for Sodium Ion Batteries Based on Tin–Germanium–Antimony Alloys. ACS Nano 2014, 8, 4415–4429. [Google Scholar] [CrossRef]
- Fan, H.; Mao, P.; Sun, H.; Wang, Y.; Mofarah, S.S.; Koshy, P.; Arandiyan, H.; Wang, Z.; Liu, Y.; Shao, Z. Recent advances of metal telluride anodes for high-performance lithium/sodium–ion batteries. Mater. Horiz. 2022, 9, 524–546. [Google Scholar] [CrossRef]
- Hu, H.; Zhong, J.; Jian, B.; Zheng, C.; Zeng, Y.; Kou, C.; Xiao, Q.; Luo, Y.; Wang, H.; Guo, Z.; et al. In-Situ Construction of Anti-Aggregation Tellurium Nanorods/Reduced Graphene Oxide Composite to Enable Fast Sodium Storage. Nanomaterials 2024, 14, 118. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, H.; Yang, C.; Liu, Z.; Wen, D.; Peng, X.; Li, S.; Wu, X. Constructing FeTe2 nanoparticles embedded in N-doped carbon nanofiber composites as a long-life and high-rate anode material for sodium-ion batteries. Sustain. Energy Fuels 2024, 8, 934–941. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Bismuth: A new anode for the Na-ion battery. Nano Energy 2015, 12, 88–95. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, S.; Zhang, H.; Yan, Y.; Peng, Z.; Zhao, H. Bismuth nanoparticles embedded in carbon fibers as flexible and free-standing anodes for efficient sodium ion batteries. RSC Adv. 2024, 14, 39921–39926. [Google Scholar] [CrossRef]
- Xu, L.T.; Yuan, Y.F.; Shen, S.H.; Zhu, M.; Wang, B.X.; Huang, X.H.; Guo, S.Y. Metallic bismuth nanoparticles encapsulated within nanoporous hollow carbon sphere/polydopamine-derived carbon shell for stable and rapid sodium storage. J. Power Sources 2025, 629, 236066. [Google Scholar] [CrossRef]
- Bai, J.; Jia, J.H.; Wang, Y.; Yang, C.C.; Jiang, Q. Ideal Bi-Based Hybrid Anode Material for Ultrafast Charging of Sodium-Ion Batteries at Extremely Low Temperatures. Nano Micro Lett. 2025, 17, 60. [Google Scholar] [CrossRef]
- Wang, X.; Feng, B.; Huang, L.; Fu, Q.; Li, W.; Zhu, C.; Chen, P.; Yang, C.; Ding, Y.-L. Superior electrochemical performance of Sb-Bi alloy for sodium storage: Understanding from alloying element effects and new cause of capacity attenuation. J. Power Sources 2021, 520, 230826. [Google Scholar] [CrossRef]
- Pu, B.; Liu, Y.; Bai, J.; Chu, X.; Zhou, X.; Qing, Y.; Wang, Y.; Zhang, M.; Ma, Q.; Xu, Z.; et al. Iodine-Ion-Assisted Galvanic Replacement Synthesis of Bismuth Nanotubes for Ultrafast and Ultrastable Sodium Storage. ACS Nano 2022, 16, 18746–18756. [Google Scholar] [CrossRef]
- Kuroiwa, M.; Sato, F.; Honma, T.; Daiko, Y. Synthesis of highly dispersed metallic bismuth nanoparticles in CuO–Bi2O3–SiO2 glass-ceramics for sodium ion battery anode. J. Solid State Chem. 2024, 338, 124838. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Liao, H.; Zheng, Y.; Fu, X.; Lu, J.; Cheng, S.; Gao, Y. Progress and Prospect of Bimetallic Oxides for Sodium-Ion Batteries: Synthesis, Mechanism, and Optimization Strategy. ACS Nano 2024, 18, 7796–7824. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y.; Yu, D.; Li, L.; Ramakrishna, S.; Peng, S. Electrospun NiCo2O4 Nanotubes as Anodes for Li- and Na-Ion Batteries. J. Alloys Compd. 2019, 777, 1286–1293. [Google Scholar] [CrossRef]
- Muhamad, S.U.; Idris, N.H.; Yusoff, H.M.; Majid, S.R.; Din, M.F.M.; Noerochim, L. Enhanced reversible sodium storage performance in CoFe2O4 nanoparticles as an anode material for sodium-ion batteries. Ionics 2025, 31, 2487–2500. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, J.; Yang, Y.; Zhao, J. Template-Assisted Synthesis of Honeycomb-Like CoFe2O4/CNTs/rGO Composite as Anode Material for Li/Na-Ion Batteries. ChemElectroChem 2019, 6, 3468. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Zhu, G.; Lu, T.; Pan, L. Porous CoFe2O4 nanocubes derived from metal-organic frameworks as high-performance anode for sodium ion batteries. J. Colloid Interface Sci. 2017, 499, 145–150. [Google Scholar] [CrossRef]
- Iturrondobeitia, A.; Goñi, A.; Gil de Muro, I.; Lezama, L.; Rojo, T. Physico-Chemical and Electrochemical Properties of Nanoparticulate NiO/C Composites for High Performance Lithium and Sodium Ion Battery Anodes. Nanomaterials 2017, 7, 423. [Google Scholar] [CrossRef]
- Vincent, M.; Sajeev, S.; Srivastava, M.; Kowalska, E.; Srinivasan, S.; Kowalski, D. Hierarchical Co3O4 anode for high-performance Na-ion battery. Electrochim. Acta 2025, 509, 145309. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, W.; Yao, J.; Chen, H.; Li, Y.; Lei, C. Sodium storage properties and mechanism of three-dimensional rose-like CoNiO2 as anode for sodium-ion batteries. J. Power Sources 2025, 648, 237342. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational Design of Three-Dimensional Graphene Encapsulated with Hollow FeP@Carbon Nanocomposite as Outstanding Anode Material for Lithium Ion and Sodium Ion Batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Chen, H.; Lv, X.; Jiang, Y.; Feng, Y.; Rui, X.; Yu, Y. Advances in metal phosphides for sodium-ion batteries. SusMat 2021, 1, 359–392. [Google Scholar] [CrossRef]
- Dou, Y.; Yue, L.; Song, W.; Feng, Y.; Zhang, L.; Shi, R.; Jiang, L.; Song, J.; Qu, Y.; Qi, G.; et al. Interfacial engineering via Mott-Schottky heterojunction enables high-rate capability and cycling life in FeS2 anodes for sodium-ion batteries. J. Alloys Compd. 2025, 1035, 181492. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-S.; Park, J.-W.; Nam, T.-H.; Kim, K.-W.; Ahn, J.-H.; Wang, G.; Ahn, H.-J. Discharge mechanism of MoS2 for sodium ion battery: Electrochemical measurements and characterization. Electrochim. Acta 2013, 92, 427–432. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/Graphene Composite Paper for Sodium-Ion Battery Electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef]
- González, J.R.; Alcántara, R.; Tirado, J.L.; Fielding, A.J.; Dryfe, R.A.W. Electrochemical Interaction of Few-Layer Molybdenum Disulfide Composites vs Sodium: New Insights on the Reaction Mechanism. Chem. Mater. 2017, 29, 5886–5895. [Google Scholar] [CrossRef]
- Arcon, D.; Zorko, A.; Cevc, P.; Mrzel, A.; Remskar, M.; Dominko, R.; Gaberscek, M.; Mihailović, D. Electron spin resonance of doped chalcogenide nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 125423. [Google Scholar] [CrossRef]
- Bi, H.; Ma, L.; Li, Y.; Hu, J.; Ma, H.; Li, R.; Ma, X.; Chen, J.; Huang, H.; Wang, X.; et al. The influence of crystal growth mechanism based on various sulfur sources on the morphology, component and electrochemical performance of cobalt sulfide as anode material for sodium-ion batteries. J. Alloys Compd. 2022, 907, 164483. [Google Scholar] [CrossRef]
- Song, J.-X.; Deng, D.-R.; Cai, S.-L.; Huang, H.; Li, G.-F.; Zeng, Y.; Weng, J.-C.; Fan, X.-H.; Li, Y.; Wu, Q.-H. Effective protection for cobalt sulfide constructed using a three-layer core-shell structure in biomass carbon for sodium ion batteries. J. Power Sources 2025, 629, 236056. [Google Scholar] [CrossRef]
- Zhang, S.; Zuo, W.; Fu, X.; Li, J.; Zhang, Q.; Yang, W.; Chen, H.; Zhang, J.; Xiao, X.; Amine, K.; et al. High-entropy sulfoselenide as negative electrodes with fast kinetics and high stability for sodium-ion batteries. Nat. Commun. 2025, 16, 4052. [Google Scholar] [CrossRef]
- Kusada, K.; Kitagawa, H. Phase Control in Monometallic and Alloy Nanomaterials. Chem. Rev. 2025, 125, 599–659. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Das, P.; Lu, P.; Yu, Y.; Wu, Z. Sodium Ion Microscale Electrochemical Energy Storage Device: Present Status and Future Perspective. Small Struct. 2020, 1, 2000053. [Google Scholar] [CrossRef]
- Cabello, M.; Ortiz, G.F.; López, M.C.; Alcántara, R.; González, J.R.; Tirado, J.L.; Stoyanova, R.; Zhecheva, E. Self-organized sodium titanate/titania nanoforest for the negative electrode of sodium-ion microbatteries. J. Alloys Compd. 2015, 646, 816–826. [Google Scholar] [CrossRef]
- Moghadami, M.; Massoudi, A.; Nangir, M. Unveiling the recent advances in micro-electrode materials and configurations for sodium-ion micro-batteries. J. Mater. Chem. A 2024, 12, 17923–17957. [Google Scholar] [CrossRef]
- Chen, F.; Xu, Z.L. Design and manufacture of high-performance microbatteries: Lithium and beyond. Microstructures 2022, 2, 2022012. [Google Scholar] [CrossRef]
- Huang, F.; Xu, P.; Fang, G.; Liang, S. In-Depth Understanding of Interfacial Na+ Behaviors in Sodium Metal Anode: Migration, Desolvation, and Deposition. Adv. Mater. 2024, 36, 2405310. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated Ethylene Carbonate as Electrolyte Additive for Rechargeable Na Batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]
- Pérez-Vicente, C.; Alcántara, R. New perspectives on the multianion approach to adapt electrode materials for lithium and post-lithium batteries. Phys. Chem. Chem. Phys. 2023, 25, 15600–15623. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, S.; Ali, M.; Aman, S.; Chishti, A.N.; Zhang, J.; Shen, Z.; Huang, H.; Yousaf, M.; Jiang, Y. Tailoring the NaI-rich solid electrolyte interphase for enhanced stability in sodium metal batteries. J. Power Sources 2025, 640, 236733. [Google Scholar] [CrossRef]
- Xia, L.; Lin, L.; Li, J.; Zhang, Y.; Zheng, H.; Chang, X.; Liang, J.; Sa, B.; Wang, L.; Lin, J.; et al. Lithium-containing hybrid SEI layer enabling high mass loading and anode-less sodium metal batteries. Angew. Chem. Int. Ed. 2025, 64, e202423090. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Li, H.; Qiao, R. Low-Concentration Electrolyte Design for Wide-Temperature Operation in Sodium Metal Batteries. J. Electrochem. Soc. 2025, 172, 010501. [Google Scholar] [CrossRef]
- You, S.; Ye, M.; Xiong, J.; Hu, Z.; Zhang, Y.; Yang, Y.; Li, C.C. Interfacial Protection Engineering of Sodium Nanoparticles toward Dendrite-Free and Long-Life Sodium Metal Battery. Small 2021, 17, 2102400. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, C.; Yao, Q.; Lv, H.; Ma, H.; Yang, J. Rational Screening of Siloxane Molecules as Electrolyte Additives for High-Temperature Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2025, 64, e202420573. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Chen, Z.; Zhong, Y.; Wang, X.; Tu, J. Semi-Solid Na-K Alloy Anode with Enhanced Stabilities for Advanced Sodium-Ion Batteries. Adv. Funct. Mater. 2025, 35, 2502682. [Google Scholar] [CrossRef]
- Ruan, J.; Hu, J.; Li, Q.; Luo, S.; Yang, J.; Liu, Y.; Zheng, S.; Sun, D.; Fang, F.; Wang, F. Current collector interphase design for high-energy and stable anode-less sodium batteries. Nat. Sustain. 2025, 8, 530–541. [Google Scholar] [CrossRef]
- Chen, C.; Yang, R.; Zhu, J.; Yao, W.; Tang, Y. Superior Sodium Metal Anodes Enabled by 3D Hierarchical Metallic Scaffolds with Enhanced Sodiophilicity. Adv. Sci. 2025, 12, 2500756. [Google Scholar] [CrossRef] [PubMed]
- Huwig, K.; Grigoryan, V.G.; Springborg, M. Global Optimization of Li and Na Clusters: Application of a Modified Embedded Atom Method. J. Clust. Sci. 2020, 31, 769–790. [Google Scholar] [CrossRef]
- Ghazi, S.M.; Mahmoudi, M. Statistical and data visualization techniques to study the role of one-electron in the energy of neutral and charged clusters of Na39. Sci. Rep. 2025, 15, 1739. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Merinov, B.V.; Goddard, W.A. Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals. Proc. Natl. Acad. Sci. USA 2016, 113, 3735. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Zhong, L.; Geng, F.; Tao, Y.; Geng, C.; Li, S.; Hu, B.; Yang, Q.-H. Unraveling the Key Atomic Interactions in Determining the Varying Li/Na/K Storage Mechanism of Hard Carbon Anodes. Adv. Energy Mater. 2022, 12, 2201734. [Google Scholar] [CrossRef]
- Eren, E.O.; Esen, C.; Scoppola, E.; Song, Z.; Senokos, E.; Zschiesche, H.; Cruz, D.; Lauermann, I.; Tarakina, N.V.; Kumru, B.; et al. Microporous Sulfur–Carbon Materials with Extended Sodium Storage Window. Adv. Sci. 2024, 11, 2310196. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.O.; Senokos, E.; Scoppola, E.; Song, Z.; Antonietti, M.; Giusto, P. An enhanced three-stage model for sodium storage in hard carbons. Energy Environ. Sci. 2025, 18, 7859–7868. [Google Scholar] [CrossRef] [PubMed]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015, 15, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, H.; Wang, X.; Xie, X.; Li, Y.; Ma, G.; Lei, Z. Micropore filling and sodium cluster formation in optimized hard carbon for robust sodium storage. J. Energy. Chem. 2025, 108, 118. [Google Scholar] [CrossRef]
- Morikawa, Y.; Nishimura, S.; Hashimoto, R.; Ohnuma, M.; Yamada, A. Mechanism of Sodium Storage in Hard Carbon: An X-Ray Scattering Analysis. Adv. Energy Mater. 2020, 10, 1903176. [Google Scholar] [CrossRef]
- Escher, I.; Ferrero, G.A.; Goktas, M.; Adelhelm, P. In Situ (Operando) Electrochemical Dilatometry as a Method to Distinguish Charge Storage Mechanisms and Metal Plating Processes for Sodium and Lithium Ions in Hard Carbon Battery Electrodes. Adv. Mater. Interfaces 2022, 9, 2100596. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, X.; Bai, Y.; Yang, H.; Dong, R.; Wang, X.; Xu, H.; Wang, Q.; Li, H.; Gao, H.; et al. Probing the energy storage mechanism of quasi-metallic Na in hard carbon for sodium-ion batteries. Adv. Energy Mater. 2021, 11, 2003854. [Google Scholar] [CrossRef]
- Stratford, J.M.; Allan, P.K.; Pecher, O.; Chater, P.A.; Grey, C.P. Mechanistic Insights into Sodium Storage in Hard Carbon Anodes Using Local Structure Probes. Chem. Commun. 2016, 52, 12430–12433. [Google Scholar] [CrossRef]
- Qiu, C.; Li, A.; Qiu, D.; Wu, Y.; Jiang, Z.; Zhang, J.; Xiao, J.; Yuan, R.; Jiang, Z.; Liu, X.; et al. One-Step Construction of Closed Pores Enabling High Plateau Capacity Hard Carbon Anodes for Sodium-Ion Batteries: Closed-Pore Formation and Energy Storage Mechanisms. ACS Nano 2024, 18, 11941–11954. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rubio, S.; Lavela, P.; Tirado, J.T.; Alcántara, R. From acorn to microporous carbon for sustainable sodium-ion battery. J. Electroanal. Chem. 2025, 980, 118988. [Google Scholar] [CrossRef]
- Cheng, M.-G.; Xie, L.-J.; Yi, Z.L.; Mao, Y.-X.; Su, F.-Y.; Wey, X.-X.; Sun, G.-H.; Chen, C.-M. Preparation of Enriched Closed-Pores Hard Carbon for High-Performance Sodium-Ion Batteries by the Poly (vinyl butyral) Template Method. ACS Appl. Energy Mater. 2024, 7, 3452–3461. [Google Scholar] [CrossRef]
- Huang, Q.; You, S.; Yang, C. Surface Porousization of Hard Carbon Anode Materials for Sodium-Ion Batteries. Micromachines 2025, 16, 771. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Li, Y.; Sun, Y.; Fielding, A.J.; Iamprasertkun, P.; Yang, J.; Wang, B.; Li, Q.; Wu, M.; et al. Evaluation of the Impact of Conductive Additives on the EPR Spectra of Hard Carbon Anodes. Small Methods 2025, 2500786. [Google Scholar] [CrossRef]
- Wang, B.; Fitzpatrick, J.R.; Brookfield, A.; Fielding, A.J.; Reynolds, E.; Entwistle, J.; Tong, J.; Spencer, B.F.; Baldock, S.; Hunter, K.; et al. Electron paramagnetic resonance as a tool to determine the sodium charge storage mechanism of hard carbon. Nat. Commun. 2024, 15, 3013. [Google Scholar] [CrossRef]
- Kukeva, R.R.; Kalapsazova, M.; Harizanova, S.; Uzunov, I.M.; Markov, P.; Spassova, I.; Stoyanova, R. Electron Paramagnetic Resonance Monitoring of Sodium Clustering and Its Effect on the Sodium Storage of Biowaste-Derived Carbons. ACS Appl. Energy Mater. 2024, 7, 9543–9550. [Google Scholar] [CrossRef]
- Cui, J.; Li, W.; Su, P.; Song, X.; Ye, W.; Zhang, Y.; Chen, Z. Repair Surface Defects on Biomass Derived Hard Carbon Anodes with N-Doped Soft Carbon to Boost Performance for Sodium-Ion Batteries. Adv. Energy Mater. 2025, 15, 2502082. [Google Scholar] [CrossRef]
- Morita, R.; Gotoh, K.; Kubota, K.; Komaba, S.; Hashi, K.; Shimizu, T.; Ishida, H. Correlation of Carbonization Condition with Metallic Property of Sodium Clusters Formed in Hard Carbon Studied Using 23Na Nuclear Magnetic Resonance. Carbon 2019, 145, 712–715. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Xie, X.; Li, Y.; Peng, H.; Ma, G.; Lei, Z. Dual-regulation of pore confinement and mouth size for enhanced sodium storage in hard carbon. J. Energy Chem. 2026, 112, 1–12. [Google Scholar] [CrossRef]
- Shan, P.; Chen, J.; Tao, M.; Zhao, D.; Lin, H.; Fu, R.; Yang, Y. The applications of solid-state NMR and MRI techniques in the study of rechargeable sodium-ion batteries. J. Magn. Reson. 2023, 353, 107516. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Di, M.; Zhang, X.; Wang, Z.; Chen, S.; Zhang, Q.; Bai, Y. Nanoconfined Strategy Optimizing Hard Carbon for Robust Sodium Storage. Adv. Energy Mater. 2024, 14, 2401763. [Google Scholar] [CrossRef]
- Yu, X.; Yang, D.; Qiu, X.; Xiong, X.; Yi, C.; Lou, H.; Liu, W.; Zhang, W. Self-activated micropores tailor carbon layer stacking and graphitic microstructures for high-performance sodium storage. J. Energy Chem. 2025, 107, 660–670. [Google Scholar] [CrossRef]
- Youn, Y.; Gao, B.; Kamiyama, A.; Kubota, K.; Komaba, S.; Tateyama, Y. Nanometer-size Na cluster formation in micropore of hard carbon as origin of higher-capacity Na-ion battery. NPJ Comput. Mater. 2021, 7, 48. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Xie, S.; Zhang, Y.; Fan, Z.; Chen, Y.; Zhu, J.; Dou, Q.; Yan, X. Molecular-level biomass composition and crosslinking regulation towards hard carbon with high initial Coulombic efficiency for sodium-ion battery. Sci. China Mater. 2025, 68, 2408–2418. [Google Scholar] [CrossRef]
- Xie, F.; Niu, Y.; Zhang, Q.; Guo, Z.; Hu, Z.; Zhou, Q.; Xu, Z.; Li, Y.; Yan, R.; Lu, Y.; et al. Screening Heteroatom Configurations for Reversible Sloping Capacity Promises High-Power Na-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202116394. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, X.; Hu, X.; Li, Y.; Wang, K.; Tu, H.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Rationally designing closed pore structure by carbon dots to evoke sodium storage sites of hard carbon in low-potential region. Adv. Funct. Mater. 2024, 34, 2308392. [Google Scholar] [CrossRef]
- Medina, A.; Alcántara, R.; Lavela, P.; Tirado, J.L. A facile method to transform pickled olive wastes into sulfur-doped carbon for sodium-ion battery electrode. ChemSusChem 2024, 17, e202400708. [Google Scholar] [CrossRef]
- Xiao, S.; Guo, Y.-J.; Chen, H.-X.; Liu, H.; Lei, Z.-Q.; Huang, L.-B.; Jin, R.-X.; Su, X.-C.; Zhang, Q.; Guo, Y.-G. Insight into the Role of Closed-Pore Size on Rate Capability of Hard Carbon for Fast-Charging Sodium-Ion Batteries. Adv. Mater. 2025, 37, 2501434. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Tang, Y.; Wang, J.; Zhong, Y.; Guo, C.; Shi, L.; Hu, M.; Ding, J. Regulating closed pores structure of hard carbon anodes to boost plateau storage for advanced sodium-ion batteries. J. Energy Storage 2025, 129, 117379. [Google Scholar] [CrossRef]
- Guo, L.; Qiu, C.; Liu, H.; Yuan, R.; Li, X.; Li, X.; Li, K.; Zhu, W.; Tabish, M.; Liu, X.; et al. Spatial configuration engineering of phenolic resins to tune the closed pores of hard carbon for enhanced plateau-capacity sodium storage. Chem. Eng. J. 2025, 512, 162478. [Google Scholar] [CrossRef]
- Alcántara, R.; Lavela, P.; Ortiz, G.F.; Tirado, J.T. Carbon microspheres obtained from resorcinol-formaldehyde as high-capacity electrodes for sodium-ion batteries. Electrochem. Solid State Lett. 2005, 8, A222. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, J.; Guo, L.; Zhang, H.; Yuan, R.; Liu, H.; Li, A.; Chen, X.; Song, H. C60 to Modulate the Closed Pore Structures of Hard Carbon for High-Performance Sodium-Ion Batteries. ACS Nano 2025, 19, 14829–14838. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Tsumura, T.; Toyoda, M.; Przepiorski, J.; Morawski, A.W.; Konno, H.; Inagaki, M. A review of the control of pore structure in MgO-templated nanoporous carbons. Carbon 2010, 48, 2690–2707. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. A general and facile synthesis strategy towards highly porous carbons: Carbonization of organic salts. J. Mater. Chem. A 2013, 1, 13738–13741. [Google Scholar] [CrossRef]
- Kamiyama, A.; Kubota, K.; Igarashi, D.; Youn, Y.; Tateyama, Y.; Ando, H.; Gotoh, K.; Komaba, S. MgO-Template Synthesis of Extremely High Capacity Hard Carbon for Na-Ion Battery. Angew. Chem. Int. Ed. 2021, 60, 5114–5120. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Lu, Z.; Tang, B. Method for Preparing Porous Carbon Material for Lithium/Sodium-Ion Battery. CN106654268A. 9 December 2016. Available online: https://worldwide.espacenet.com/patent/search/family/058824758/publication/CN106654268A?q=pn%3DCN106654268A (accessed on 1 December 2025).
- Chen, X.; Tian, J.; Li, P.; Fang, Y.; Fang, Y.; Liang, X.; Feng, J.; Dong, J.; Ai, X.; Yang, H.; et al. An Overall Understanding of Sodium Storage Behaviors in Hard Carbons by an “Adsorption-Intercalation/Filling” Hybrid Mechanism. Adv. Energy Mater. 2022, 12, 2200886. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Wang, X.; Peng, Y.; Liu, Y.; Ullah, S.; Duan, Z.; Gao, W.; Song, B.; Wei, M.; et al. Evidence of Quasi-Na Metallic Clusters in Sodium Ion Batteries Through in Situ X-Ray Diffraction. Adv. Mater. 2025, 37, 2410673. [Google Scholar] [CrossRef]
- Jia, Z.; Duan, Y.; Chen, X.; Sun, Z.; Liu, L.; Fu, L.; Chen, Y.; Wang, F.; Wang, T.; Wu, Y. Active Component Design of Amorphous SnPx/SnSx and Interfacial Bonding Engineering in N/P/S-Doped Hard Carbon for High-Rate Sodium-Ion Hybrid Capacitors. Adv. Sci. 2025, 12, e06532. [Google Scholar] [CrossRef]
- Zhong, L.; Qiu, X.; Hao, S.; Jiang, Z.; Zhang, W. Molecular pillaring driven microcrystalline structure engineering of hard carbon for high-rate sodium storage. Chem. Eng. J. 2025, 516, 164007. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, L.; Zhong, Y. Construction of Porous Sulfur-Doped Petroleum Coke-Derived Carbon Materials with Adjustable Interlayer Spacing via a Low-Temperature Sulfurization Strategy and Its Efficient Sodium Storage Mechanism. Ind. Eng. Chem. Res. 2025, 64, 17732–17749. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, G.; Wang, C.; Wan, F.; Qiu, L.; Xiao, Y.; Wu, Z.; Guo, X. Closed-Pore Engineering Based on Spatiotemporally Partitioned Radical Editing for High-Performance Hard Carbon Anodes in Sodium-Ion Batteries. Adv. Funct. Mater. 2025, e15405. [Google Scholar] [CrossRef]
- Niu, J.; Dong, J.; Zhang, X.; Huang, L.; Guoli, L.; Han, X.; Wang, J.; Gong, T.; Chen, Z.; Zhao, J.; et al. Sodium cluster-driven safety concerns of sodium-ion batteries. Energy Environ. Sci. 2025, 18, 2474–2484. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Wang, J.W.; Liu, X.H.; Huang, J.Y. Microstructural Evolution of Tin Nanoparticles during In Situ Sodium Insertion and Extraction. Nano Lett. 2012, 12, 5897–5902. [Google Scholar] [CrossRef]
- González, J.R.; Nacimiento, F.; Alcántara, R.; Ortiz, G.F.; Tirado, J.L. Electrodeposited CoSn2 on nickel open-cell foam: Advancing towards high power lithium ion and sodium ion batteries. CrystEngComm. 2013, 15, 9196. [Google Scholar] [CrossRef]
- Kempf, A.; Zajac, M.G.; Riedel, R. High-Capacity C/Sn-Composites as Next-Generation Anodes for Sodium-Ion Batteries. ACS Mater. Lett. 2025, 7, 275–285. [Google Scholar] [CrossRef]
- Lim, H.; Yu, S.; Chang, W.; Yoon, K.; Chung, K.Y.; Choi, W.; Kim, S.-O. Boosting the Sodiation Kinetics of Sn Anode Using a Yolk-Shell Nanohybrid Structure for High-Rate and Ultrastable Sodium-Ion Batteries. Adv. Sci. 2024, 11, 2408450. [Google Scholar] [CrossRef]
- Jiang, F.; Luo, F.; Fan, Q.; Guo, P.; Liao, Y.; Chen, M.; Liu, C.; Chen, J.; Wang, D.; Zheng, Z. A composite material consisting of tin nanospheres anchored on and embedded in carbon nanofibers for outstanding sodium-ion storage. J. Electroanal. Chem. 2024, 959, 118186. [Google Scholar] [CrossRef]
- Lai, Q.; Wei, R.; Yang, R.; Li, Q.; Mou, K.; Yang, D.; Liu, Z.; Gao, X.-W.; Gu, Q.; Luo, W.-B. Reversible Na15Sn4 alloy compensation for hard carbon anodes to enhance the initial coulombic efficiency in sodium-ion full cells. Chem. Commun. 2025, 61, 8363–8366. [Google Scholar] [CrossRef]
- Kim, C.; Kim, I.; Kim, H.; Sadan, M.K.; Yeo, H.; Cho, G.-B.; Ahn, J.; Ahn, J.-H.; Ahn, H.-J. A self-healing Sn anode with an ultra-long cycle life for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 22809–22818. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Zhou, L.; Mai, L. Bottom-up confined synthesis of nanorod-in-nanotube structured Sb@N-C for durable lithium and sodium storage. Adv. Energy Mater. 2018, 8, 1703237. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Liu, C.; Liao, H.; Li, Z.; Jiang, Y.; Hou, Y.; Sun, L.; Gao, Y. A self-encapsulation hollow structure design for high-capacity and fast-charging sodium-ion batteries at high-temperature. Chem. Eng. J. 2025, 510, 161745. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Zhao, J.; He, P.; Ding, X. Mass produced Sb/P@C composite nanospheres for advanced sodium-ions battery anodes. Electrochim. Acta 2023, 439, 141602. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, C.; Zhang, Z.; Liao, H.; Li, Z.; Jiang, Y.; Hou, Y.; Sun, L.; Su, J.; Gao, Y. Regulating (010) Exposed Facets of a Sb2O3 Anode to Achieve High-Performance Sodium-Ion Batteries. Nano Lett. 2025, 25, 5461–5468. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Zhang, S.; Hu, X.; Zhang, K.; Zhou, W.; Guo, S.; Xu, F.; Zeng, H. Ultrathin Bismuth Nanosheets for Stable Na-Ion Batteries: Clarification of Structure and Phase Transition by in Situ Observation. Nano Lett. 2019, 19, 1118–1123. [Google Scholar] [CrossRef]
- Ali, M.; Sun, J.; Li, Z.; Liang, H.; Liu, C. Mechanisms and Properties of Bismuthene and Graphene/Bismuthene Heterostructures as Anodes of Lithium-/Sodium-Ion Batteries by First-Principles Study. J. Phys. Chem. C 2021, 125, 11391–11401. [Google Scholar] [CrossRef]
- Mu, B.; Wang, G.; Chi, C.; Cao, K.; Li, Z.; Qi, B.; Miao, Q.; Wu, D.; Wei, T.; Fan, Z. Heterostructured Bi@Bi2O3 nanoparticles bonding on reduced graphene oxide with high-rate capability and outstanding cycling stability for sodium-ion batteries. Carbon 2025, 240, 120370. [Google Scholar] [CrossRef]
- Wang, C.; Su, L.; Wang, N.; Lv, D.; Wang, D.; Yang, J.; Qian, Y. Unravelling binder chemistry in sodium/potassium ion batteries for superior electrochemical performances. Mater. Chem. A 2022, 10, 4060–4067. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, Y.; Zheng, C.; Wang, N.; Wang, D.; Tian, F.; Bai, Z.; Yang, J.; Qian, Y.; Dou, S. Intermolecular Cross-Linking Reinforces Polymer Binders for Durable Alloy-Type Anode Materials of Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2202939. [Google Scholar] [CrossRef]
- Alcántara, R.; Jaraba, M.; Lavela, P.; Tirado, J.T. NiCo2O4 Spinel: First Report on a Transition Metal Oxide for the Negative Electrode of Sodium-Ion Batteries. Chem. Mater. 2002, 14, 2847–2848. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Savin, S.L.P.; Fiddy, S.; Alcantara, R.; Fernandez Lisbona, D.; Lavela, P.; Ortiz, G.F.; Tirado, J.L. Formation and Oxidation of Nanosized Metal Particles by Electrochemical Reaction of Li and Na with NiCo2O4: X-ray Absorption Spectroscopic Study. J. Phys. Chem. C 2007, 111, 4636–4642. [Google Scholar] [CrossRef]
- Thissen, A.; Ensling, D.; Madrigal, F.J.F.; Jaegermann, W.; Alcántara, R.; Lavela, P.; Tirado, J.L. Photoelectron Spectroscopic Study of the Reaction of Li and Na with NiCo2O4. Chem. Mater. 2005, 17, 5202. [Google Scholar] [CrossRef]
- Chang, T.-C.; Lu, Y.T.; Lee, C.H.; Gupta, J.K.; Hardwick, L.J.; Hu, C.-C.; Chen, H.T. The Effect of Degrees of Inversion on the Electronic Structure of Spinel NiCo2O4: A Density Functional Theory Study. ACS Omega 2021, 6, 9692–9699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xu, F.; Min, H.; Huang, Y.; Xia, W.; Wang, Y.; Xu, Q.; Gao, P.; Sun, L. Identifying the Conversion Mechanism of NiCo2O4 during Sodiation–Desodiation Cycling by In Situ TEM. Adv. Funct. Mater. 2017, 27, 1606163. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, P.; Zhang, Z.; Zhao, Y.; Zhang, Y.; Li, L.; Yang, K.; Li, X.; Gu, L. Nickel/cobalt metal-organic framework derived 1D hierarchical NiCo2O4/NiO/carbon nanofibers for advanced sodium storage. Chem. Eng. J. 2019, 364, 123–131. [Google Scholar] [CrossRef]
- Chen, J.; Ru, Q.; Mo, Y.; Hu, S.; Hou, X. Design and Synthesis of Hollow NiCo2O4 Nanoboxes as Anodes for Lithium-Ion and Sodium-Ion Batteries. Phys. Chem. Chem. Phys. 2016, 18, 18949–18957. [Google Scholar] [CrossRef]
- Fu, F.; Li, J.; Yao, Y.; Qin, X.; Dou, Y.; Wang, H.; Tsui, J.; Chan, K.Y.; Shao, M. Hierarchical NiCo2O4 Micro- and Nanostructures with Tunable Morphologies as Anode Materials for Lithium- and Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16194–16201. [Google Scholar] [CrossRef]
- Tang, X.; Ren, Q.; Yu, F.-D.; Wang, Z.-B. The improved cycling stability of nanostructured NiCo2O4 anodes for lithium and sodium ion batteries. Ionics 2023, 29, 3943–3954. [Google Scholar] [CrossRef]
- Lee, J.W.; Shin, H.S.; Lee, C.W.; Jung, K.N. Carbon- and Binder-Free NiCo2O4 Nanoneedle Array Electrode for Sodium-Ion Batteries: Electrochemical Performance and Insight into Sodium Storage Reaction. Nanoscale Res. Lett. 2016, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; Aragón, M.J.; Ortiz, G.F.; Lavela, P.; Alcántara, R.; Tirado, J.L. High Performance Full Sodium-Ion Cell Based on a Nanostructured Transition Metal Oxide as Negative Electrode. Chem. Eur. J. 2015, 21, 14879–14885. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, H.; Xia, Q.; Hu, Z.; Zhu, Y.; Yan, S.; Ge, C.; Zhang, Q.; Wang, X.; Shang, X.; et al. Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry. Nat. Mater. 2021, 20, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Edge Sites-Driven Accelerated Kinetics in Ultrafine Fe2O3 Nanocrystals Anchored Graphene for Enhanced Alkali Metal Ion Storage. Chem. Eng. J. 2022, 428, 131204. [Google Scholar] [CrossRef]
- Guo, S.; Ait Tamerd, M.; Li, C.; Shi, X.; Yang, M.; Hou, J.; Liu, J.; Tang, M.; Haw, S.-C.; Chen, C.-T.; et al. Activating Sodium Intercalation in Cation-Deficient Fe3O4 Through Mo Substitution. Small 2025, 21, 2408212. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Q.; Liu, X.; Fan, J.; Ren, L.; Tong, J. Ni2P/Cu3P bimetallic phosphide embedded in nitrogen-doped carbon as SIB anode: Constructed hetero interface promoting fast Na+ diffusion and storage performance. Chem. Eng. J. 2024, 502, 157889. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, J.; Zhang, Y.; Qu, W.; Wang, W.; Yao, M.; Zhang, Y.; Wang, Q.; Tang, Y. Revealing Na+-coordination induced Failure Mechanism of Metal Sulfide Anode for Sodium Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202403463. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Y.; Zhou, H.; Wang, X.; Sun, S.; He, X.; Luo, Y.; Ying, B.; Yao, Y.; Ma, X.; et al. Optimizing Reversible Phase-Transformation of FeS2 Anode via Atomic-Interface Engineering Toward Fast-Charging Sodium Storage: Theoretical Predication and Experimental Validation. Adv. Sci. 2025, 12, 2411884. [Google Scholar] [CrossRef]
- Sahoo, S.; Garlapati, K.K.; Martha, S.K. Design of NH2- MIL-101(Fe) Derived N, S Co-Doped Carbon Encapsulated Iron Pyrrhotite Anode for Sodium-Ion Batteries: Mechanistic Correlation of Degradation Pathways. Small 2025, 21, 2504016. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Wang, J.; Zhu, Z.; Wu, R.; Niu, X.; Jiang, J.; Li, H.; Chen, J.S. Encapsulating tin disulfide nanoparticles in carbon nanofibers for durable sodium storage. J. Solid State Chem. 2025, 346, 125279. [Google Scholar] [CrossRef]
- Samad, A.; Noord-A-Alam, M.; Shin, Y.-H. First principles study of a SnS2/graphene heterostructure: A promising anode material for rechargeable Na ion batteries. J. Mater. Chem. A 2016, 4, 14316. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Wang, C.; Jin, W.; Li, Y.; Wang, H.; Ding, C.; Wang, Z. First-principles insight into SnS2/graphene heterostructure as potential anode materials for rechargeable lithium/sodium ion batteries. Chem. Phys. 2025, 594, 112664. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, F.; Xue, S.; Yu, D.; Jin, Y.; Song, L.; Zhang, M.; Zheng, H. Constructing ZnS@hard carbon nanosheets for high-performance and long-cycle sodium-ion batteries. Chem. Eng. J. 2025, 509, 161551. [Google Scholar] [CrossRef]
- Cheng, G.; Guo, Z.; Goli, N.; Podjaski, F.; Zheng, K.; Jiang, J.; Ramadan, S.; Kerherve, G.; Tagliaferri, S.; Och, M.; et al. Photo-Rechargeable Sodium-Ion Batteries with a Two-Dimensional MoSe2 Crystal Cathode. Nano Lett. 2025, 25, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, M.; Wu, Y.; Ren, R.; Ge, Q.; Fan, L.; Qin, P.; Qian, J.; Ding, X. High-entropy BiSnSbCuAl nanoalloys conformed in carbon fibers as fast-charging and high-capacity anode material for sodium-ion batteries. J. Power Sources 2025, 652, 237600. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Ha, H.; Shi, Y.; Peng, L.; Zhang, X.; Ellison, C.J.; Yu, G. An All-Stretchable-Component Sodium-Ion Full Battery. Adv. Mater. 2017, 29, 1700898. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Li, Y.; Fang, X.; Wang, G.; Hu, F.; Yang, Y. Three-dimensional graphene networks as efficient Sb anode skeleton for high-performance Si-based sodium ion microbatteries. J. Power Sources 2024, 604, 234497. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Chi, L.; Liu, Y.; Zhang, Y.; Wang, K.; Wu, Z.-S. 3D Printing Flexible Sodium-Ion Microbatteries with Ultrahigh Areal Capacity and Robust Rate Capability. Adv. Mater. 2022, 34, 2205569. [Google Scholar] [CrossRef]
- Chen, K.; Deng, Z.; Luo, W.; Wei, S.; Tu, Z.; Wu, X. The Application of 3D Printing in Battery Manufacturing. ChemSusChem 2025, 18, e202500904. [Google Scholar] [CrossRef]
- Ramírez, C.; Osendi, M.I.; Moyano, J.J.; Mosa, J.; Aparicio, M. Electrochemical Response of 3D-Printed Free-Standing Reduced Graphene Oxide Electrode for Sodium Ion Batteries Using a Three-Electrode Glass Cell. Materials 2023, 16, 5386. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).