Fused Filament Fabrication (FFF) for Manufacturing of Microfluidic Micromixers: An Experimental Study on the Effect of Process Variables in Printed Microfluidic Micromixers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
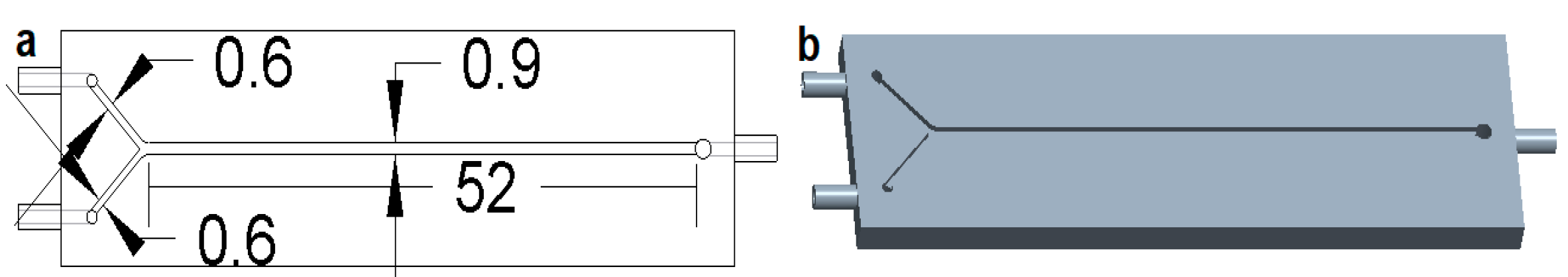
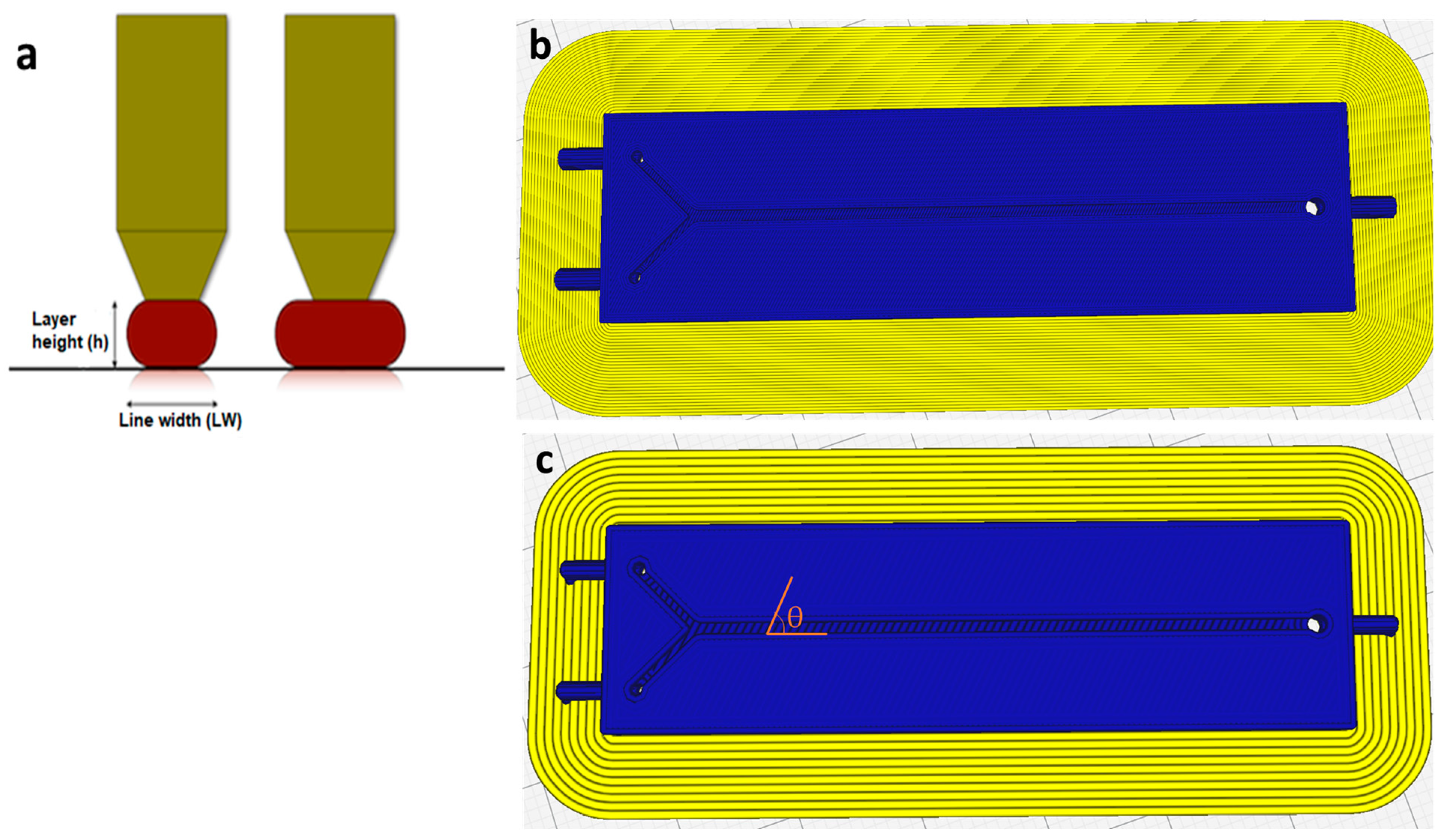
2.2. Instrumentation
2.3. Design of Experiments (DoE)
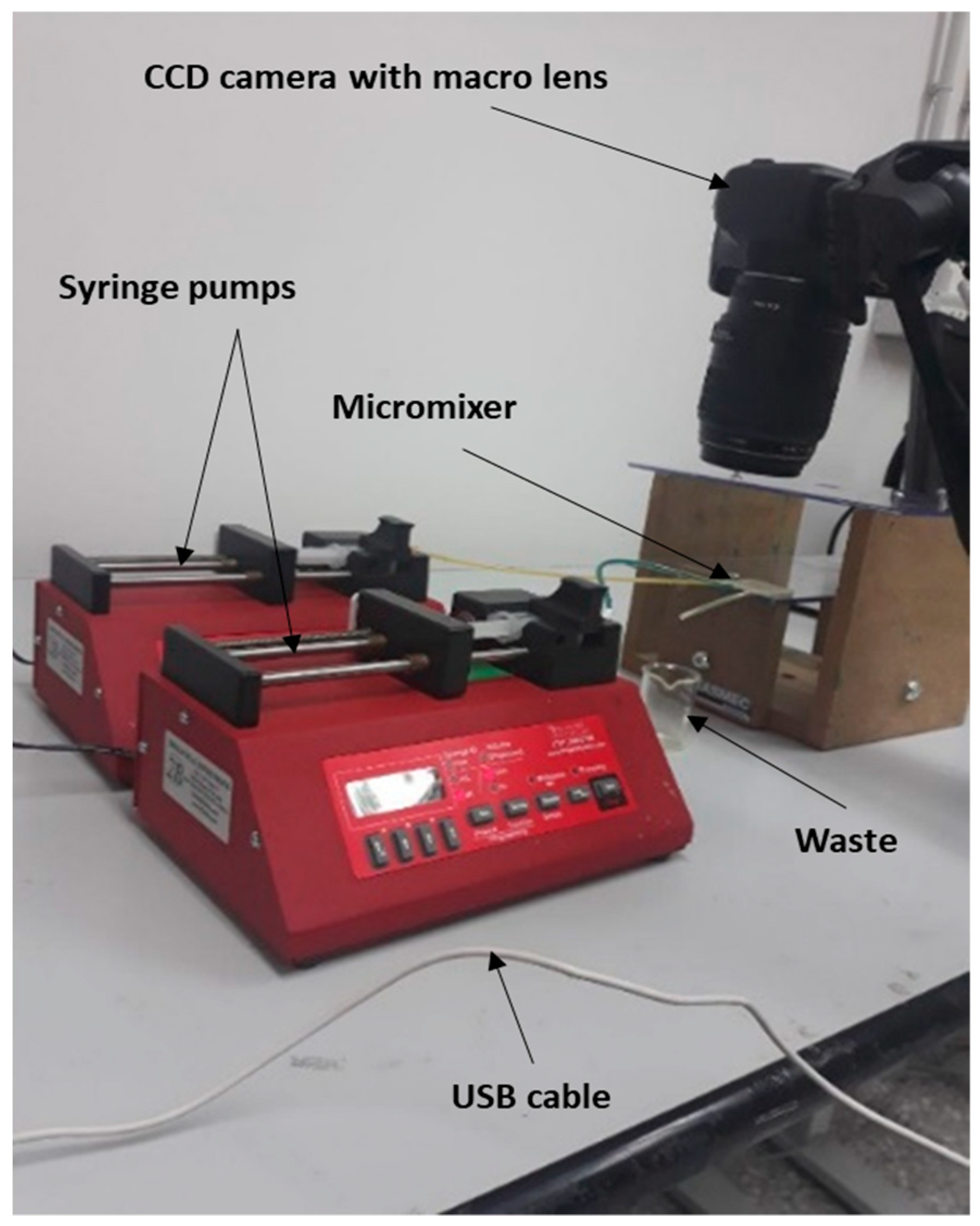
2.4. Experimental Setup
3. Results and Discussion
- (1)
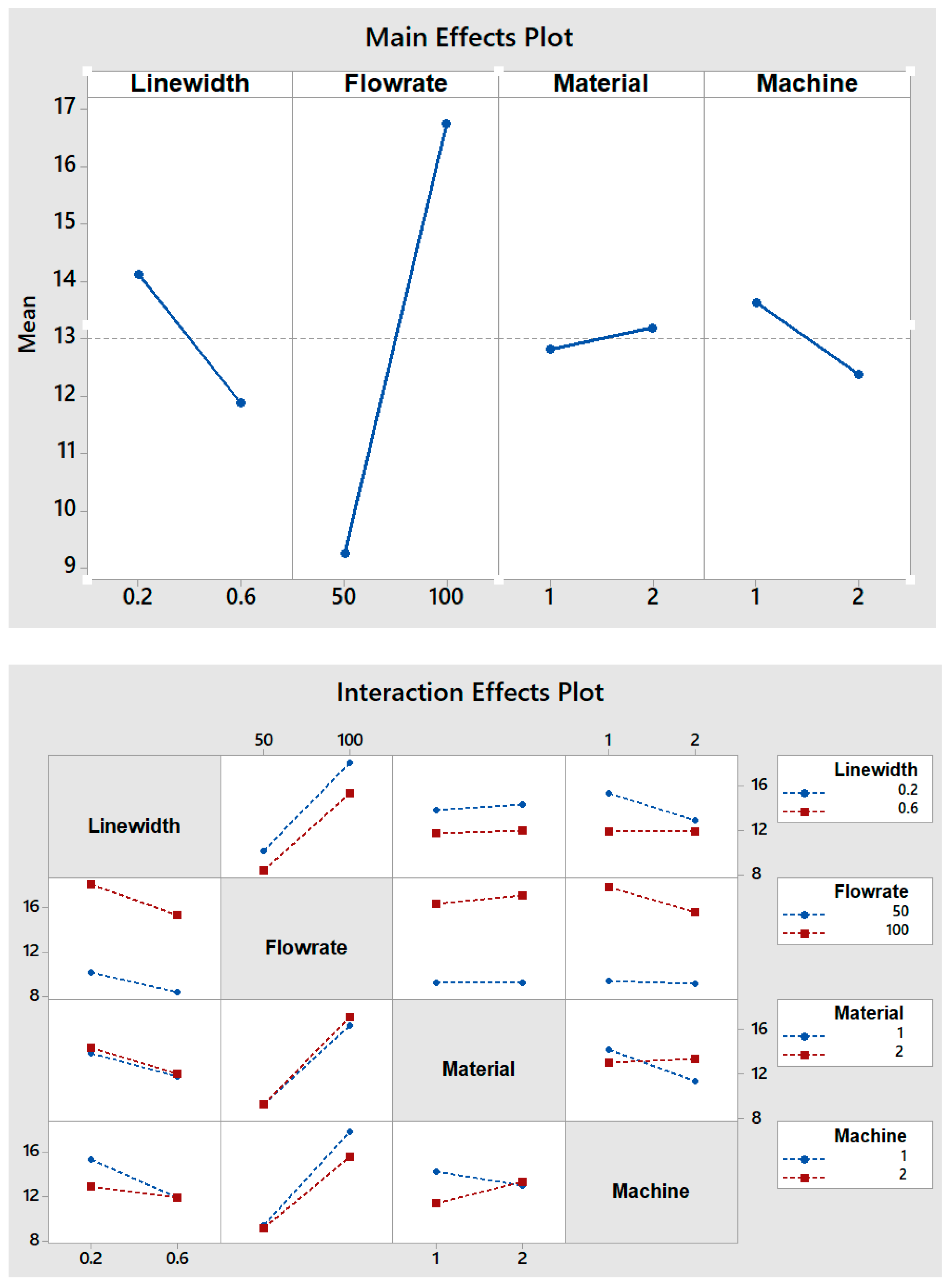
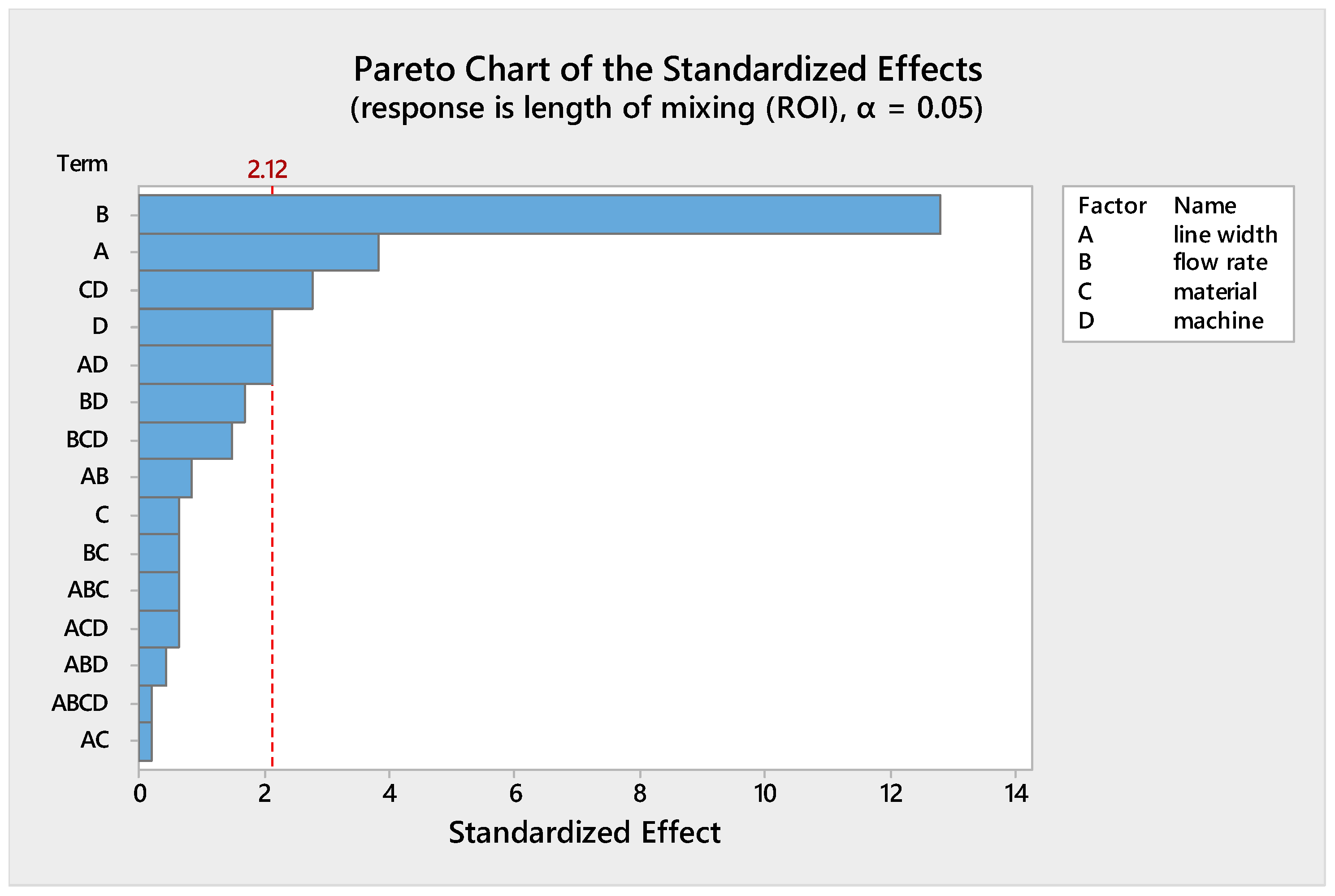
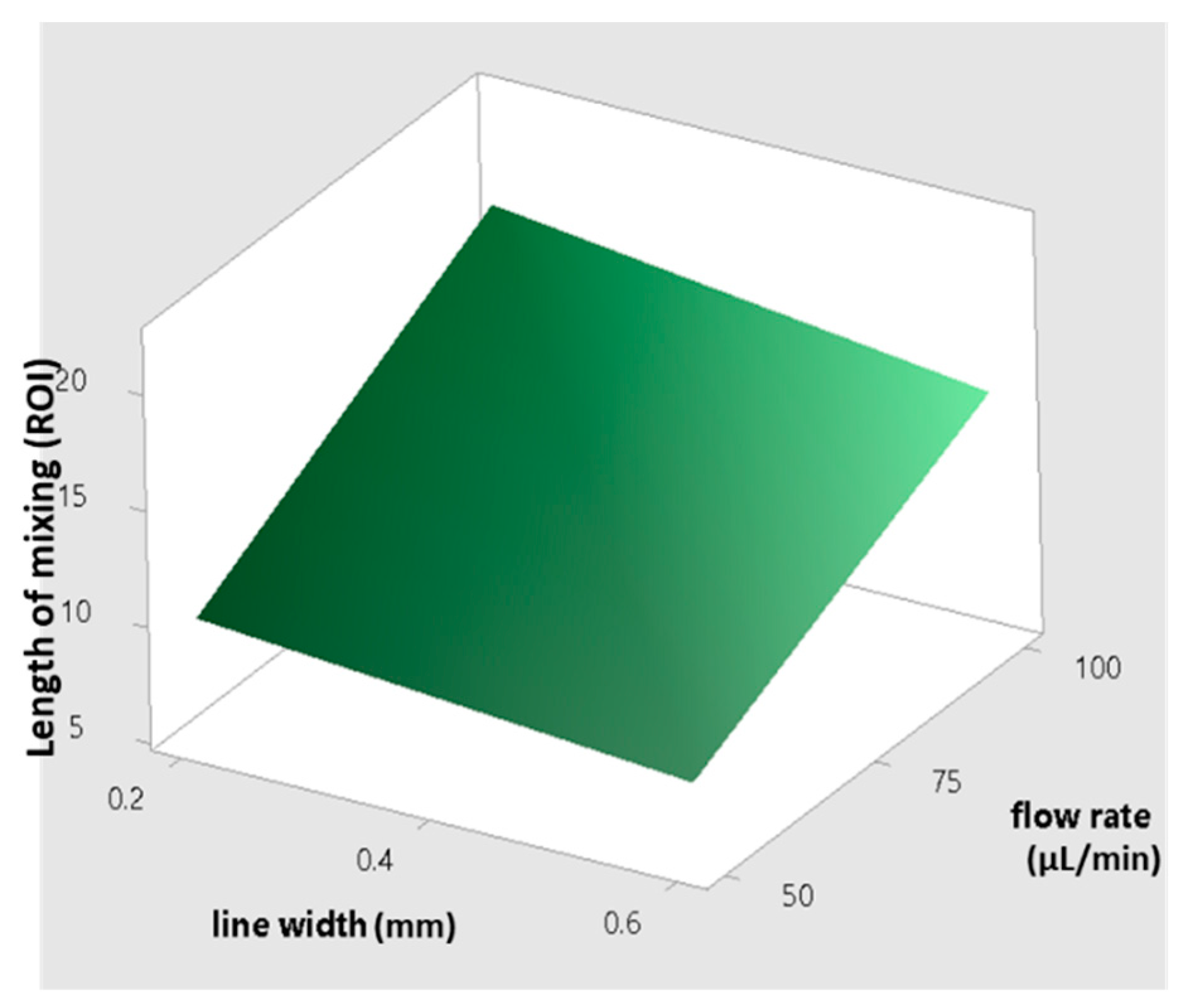
- The flow rate is the factor with the greatest effect on the length of mixing. With an increase in the flow rate from 50 to 100 µL/min, the required length for complete mixing was increased in all fabricated micromixers, which is consistent with other studies [29,30]. As shown in the Pareto chart and Table 4, the interaction effects of this factor are insignificant. Figure 6 shows the Pareto chart of the standardized effects, while in Table 4, the effects (non-standardized) and respective T-values and p-values (α = 0.05) for each factor and their interaction effects are summarized.
- (2)
- Fabricated micromixers with an extrusion line width of 600 µm showed a better mixing performance than devices printed with a line width of 200 µm, where the average required length for complete mixing is decreased. However, the interaction effects of line width are negligible (see Figure 6 and Table 4).
- (3)
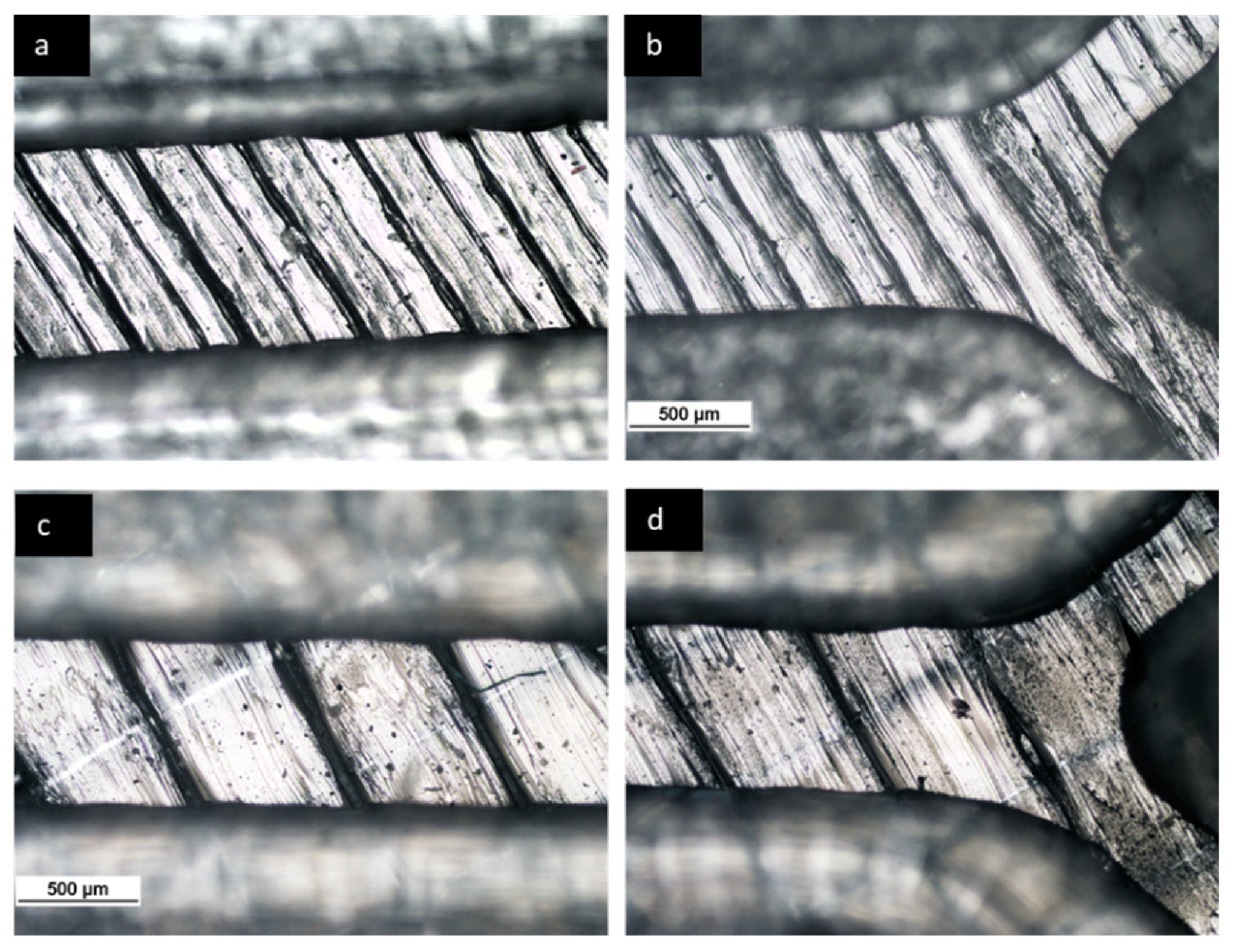
- The effect of material on the length of mixing is negligible due to the equal level of hydrophobicity for the materials used. The surface of PLA is strongly hydrophobic [47], and the use of hydrophobic surfaces is one of the topics of interest for mixing enhancement in microfluidic systems [48,49]. Although the effect of the material was insignificant on the mixing process, the transparency of printed devices is another vital point in the fabrication of microfluidic devices. Examples of the printed micromixers with translucent and transparent PLA are shown in Figure 7. The devices printed with translucent PLA were often opaque, rendering optical analysis challenging. The semi-transparency of the printed device with the transparent PLA allows imaging with back illumination [29].
- (4)
- Using different printers can cause different results due to the different accuracy of the printers. The two printers used in this study had a slight effect on the required length of mixing (see Figure 5). In general, the printed channels in the FFF process are consistently smaller than the CAD model, due to the spreading of the polymer as it is extruded [30]. For this, the mixers printed with the Ultimaker S5 had a better mixing performance, as its higher accuracy allowed the printing channels to be closer to the designed model. Among the different interaction effects, the effect of C*D (material*machine) is significant (see Table 4). A probable explanation is related to the ability of the machines to print different materials. While the results for the printed mixers with the translucent PLA were somewhat similar in two printers, the fabricated mixers with the transparent PLA had different performances when printed with two different printers (see interaction effects plot in Figure 5).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, R.-J.; Liu, C.-C.; Wang, Y.-N.; Hou, H.-H.; Fu, L.-M. A comprehensive review of micro-distillation methods. Chem. Eng. J. 2017, 313, 1509–1520. [Google Scholar] [CrossRef]
- Hasegawa, K.; Matsumoto, M.; Hosokawa, K.; Maeda, M. Detection of methylated DNA on a power-free microfluidic chip with laminar flow-assisted dendritic amplification. Anal. Sci. 2016, 32, 603–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.-J.; Hou, H.-H.; Wang, Y.-N.; Fu, L.-M. Micro-magnetofluidics in microfluidic systems: A review. Sens. Actuators B Chem. 2016, 224, 1–15. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrigno, R.; Stroock, A.D.; Clark, T.D.; Mayer, M.; Whitesides, G.M. Membraneless vanadium redox fuel cell using laminar flow. J. Am. Chem. Soc. 2002, 124, 12930–12931. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Kjeang, E. Co-laminar flow cells for electrochemical energy conversion. J. Power Sources 2014, 260, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Burns, M.A.; Johnson, B.N.; Brahmasandra, S.N.; Handique, K.; Webster, J.R.; Krishnan, M.; Sammarco, T.S.; Man, P.M.; Jones, D.; Heldsinger, D. An integrated nanoliter DNA analysis device. Science 1998, 282, 484–487. [Google Scholar] [CrossRef] [Green Version]
- Fu, A.Y.; Chou, H.-P.; Spence, C.; Arnold, F.H.; Quake, S.R. An integrated microfabricated cell sorter. Anal. Chem. 2002, 74, 2451–2457. [Google Scholar] [CrossRef]
- Zhang, A.C.; Gu, Y.; Han, Y.; Mei, Z.; Chiu, Y.-J.; Geng, L.; Cho, S.H.; Lo, Y.-H. Computational cell analysis for label-free detection of cell properties in a microfluidic laminar flow. Analyst 2016, 141, 4142–4150. [Google Scholar] [CrossRef] [Green Version]
- Pihl, J.; Sinclair, J.; Sahlin, E.; Karlsson, M.; Petterson, F.; Olofsson, J.; Orwar, O. Microfluidic gradient-generating device for pharmacological profiling. Anal. Chem. 2005, 77, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Seong, G.H.; Crooks, R.M. Efficient mixing and reactions within microfluidic channels using microbead-supported catalysts. J. Am. Chem. Soc. 2002, 124, 13360–13361. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, S.; Hattori, K.; Kanamori, T. Microfluidic serial dilution cell-based assay for analyzing drug dose response over a wide concentration range. Anal. Chem. 2010, 82, 8278–8282. [Google Scholar] [CrossRef] [PubMed]
- Losey, M.W.; Schmidt, M.A.; Jensen, K.F. Microfabricated multiphase packed-bed reactors: Characterization of mass transfer and reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Watanabe, S.; Tanaka, H.; Miyahara, M.T.; Mae, K. Synthesis and adsorption properties of ZIF-8 nanoparticles using a micromixer. Chem. Eng. J. 2013, 227, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Guichardon, P.; Falk, L. Characterisation of micromixing efficiency by the iodide–iodate reaction system. Part I: Experimental procedure. Chem. Eng. Sci. 2000, 55, 4233–4243. [Google Scholar] [CrossRef]
- Song, Y.; Hormes, J.; Kumar, C.S. Microfluidic synthesis of nanomaterials. Small 2008, 4, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef]
- Kevin, W.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar]
- Tiboni, M.; Tiboni, M.; Pierro, A.; Del Papa, M.; Sparaventi, S.; Cespi, M.; Casettari, L. Microfluidics for nanomedicines manufacturing: An affordable and low-cost 3D printing approach. Int. J. Pharm. 2021, 599, 120464. [Google Scholar] [CrossRef]
- Abolhasani, M.; Oskooei, A.; Klinkova, A.; Kumacheva, E.; Günther, A. Shaken, and stirred: Oscillatory segmented flow for controlled size-evolution of colloidal nanomaterials. Lab A Chip 2014, 14, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, H.; Chen, B.; Wu, J. Alternating current electrothermal micromixer with thin film resistive heaters. Adv. Mech. Eng. 2016, 8, 1687814016646264. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.; Kwon, K.; Lee, S.J.; Kim, D.; Park, J. Non-equilibrium electrokinetic micromixer with 3D nanochannel networks. Lab A Chip 2015, 15, 1794–1798. [Google Scholar] [CrossRef]
- Hejazian, M.; Nguyen, N.-T. A rapid magnetofluidic micromixer using diluted ferrofluid. Micromachines 2017, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Frommelt, T.; Kostur, M.; Wenzel-Schäfer, M.; Talkner, P.; Hänggi, P.; Wixforth, A. Microfluidic mixing via acoustically driven chaotic advection. Phys. Rev. Lett. 2008, 100, 034502. [Google Scholar] [CrossRef]
- Chung, C.; Chen, Y.-J.; Chen, P.-C.; Chen, C.-Y. Fabrication of PDMS passive micromixer by lost-wax casting. Int. J. Precis. Eng. Manuf. 2015, 16, 2033–2039. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab A Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Zeraatkar, M.; Filippini, D.; Percoco, G. On the Impact of the Fabrication Method on the Performance of 3D Printed Mixers. Micromachines 2019, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing microfluidic performance of three-dimensional (3D) printing platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Chen, Y.; Rios, C.O.; Imeri, A.; Russell, N.A.; Fidan, I. Investigation of the tensile properties in fibre-reinforced additive manufacturing and fused filament fabrication. Int. J. Rapid Manuf. 2020, 9, 251–267. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, transparent, sub-100 µm microfluidic channels with fused deposition modeling 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Romanov, V.; Samuel, R.; Chaharlang, M.; Jafek, A.R.; Frost, A.; Gale, B.K. FDM 3D printing of high-pressure, heat-resistant, transparent microfluidic devices. Anal. Chem. 2018, 90, 10450–10456. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Marschewski, J.; Jung, S.; Ruch, P.; Prasad, N.; Mazzotti, S.; Michel, B.; Poulikakos, D. Mixing with herringbone-inspired microstructures: Overcoming the diffusion limit in co-laminar microfluidic devices. Lab A Chip 2015, 15, 1923–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.J.; Ross, D.; Locascio, L.E. Rapid microfluidic mixing. Anal. Chem. 2002, 74, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Iovenitti, P.; Harvey, E.; Masood, S. Numerical investigation of mixing in microchannels with patterned grooves. J. Micromech. Microeng. 2003, 13, 801. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Yim, C.; Lin, M.; Cao, X. Evaluation of floor-grooved micromixers using concentration-channel length profiles. Micromachines 2010, 1, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Kwak, T.J.; Nam, Y.G.; Najera, M.A.; Lee, S.W.; Strickler, J.R.; Chang, W.-J. Convex grooves in staggered herringbone mixer improve mixing efficiency of laminar flow in microchannel. PLoS ONE 2016, 11, e0166068. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, T.; Kothapalli, C.R.; Fodor, P.S. Mixing Optimization in Grooved Serpentine Microchannels. Micromachines 2020, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Cattafesta, L.; Fan, Z.H. Deconvolution microscopy for flow visualization in microchannels. Anal. Chem. 2007, 79, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Lim, G.; Kang, K.H.; Suh, Y.K. Geometric effects on lateral transport induced by slanted grooves in a microchannel at a low Reynolds number. Chem. Eng. Sci. 2013, 104, 82–92. [Google Scholar] [CrossRef]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Using printing orientation for tuning fluidic behavior in microfluidic chips made by fused deposition modeling 3D printing. Anal. Chem. 2017, 89, 12805–12811. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Okuducu, M.B.; Aral, M.M. Performance analysis and numerical evaluation of mixing in 3-D T-shape passive micromixers. Micromachines 2018, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.; Kweon, J.-H.; Kwon, S.; Song, J.; Lee, S. Representation of surface roughness in fused deposition modeling. J. Mater. Process. Technol. 2009, 209, 5593–5600. [Google Scholar] [CrossRef]
- Lertphirun, K.; Srikulkit, K. Properties of Poly(Lactic Acid) Filled with Hydrophobic Cellulose/SiO2 Composites. Int. J. Polym. Sci. 2019, 2019, 7835172. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Moss, G.R.; Rothstein, J.P. Enhanced mixing in laminar flows using ultrahydrophobic surfaces. Phys. Rev. E 2007, 76, 016304. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Perot, B.; Rothstein, J.P. Laminar drag reduction in microchannels using ultrahydrophobic surfaces. Phys. Fluids 2004, 16, 4635–4643. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chen, T. Simulation and optimization of chaotic micromixer using lattice Boltzmann method. Sens. Actuators B Chem. 2005, 106, 871–877. [Google Scholar]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Neuta, I.; Neumann, F.; Brightmeyer, J.; Ba Tis, T.; Madaboosi, N.; Wei, Q.; Ozcan, A.; Nilsson, M. Smartphone-based clinical diagnostics: Towards democratization of evidence-based health care. J. Intern. Med. 2019, 285, 19–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Printing Parameter | Nozzle 0.25 mm | Nozzle 0.4 mm |
|---|---|---|
| Printing temperature (°C) | 190 | 200 |
| Printing speed (mm/s) | 30 | 70 |
| Layer height (μm) | 100 | 100 |
| Filament orientation (°) | 60 | 60 |
| Flow (%) | 100 | 100 |
| Infill line distance (mm) | 0.2 | 0.6 |
| Build temperature (°C) | 60 | 60 |
| Factor | Level | |
|---|---|---|
| −1 | +1 | |
| Line width (LW) | 200 μm | 600 μm |
| Flow rate | 50 µL/min | 100 µL/min |
| Material | Transparent PLA | Translucent PLA |
| Machine | Ultimaker 3 | Ultimaker S5 |
| Run Number | A (Line Width) | B (Flow Rate) | C (Material) | D (Machine) | Run Label | Length of Mixing (ROI) | |
|---|---|---|---|---|---|---|---|
| n1 | n2 | ||||||
| 1 | −1 | −1 | −1 | −1 | (1) | 12 | 10 |
| 2 | +1 | −1 | −1 | −1 | a | 10 | 7 |
| 3 | −1 | +1 | −1 | −1 | b | 22 | 19 |
| 4 | +1 | +1 | −1 | −1 | ab | 18 | 16 |
| 5 | −1 | −1 | +1 | −1 | c | 11 | 10 |
| 6 | +1 | −1 | +1 | −1 | ac | 8 | 7 |
| 7 | −1 | +1 | +1 | −1 | bc | 21 | 18 |
| 8 | +1 | +1 | +1 | −1 | abc | 16 | 13 |
| 9 | −1 | −1 | −1 | +1 | d | 9 | 10 |
| 10 | +1 | −1 | −1 | +1 | ad | 7 | 9 |
| 11 | −1 | +1 | −1 | +1 | bd | 13 | 16 |
| 12 | +1 | +1 | −1 | +1 | abd | 14 | 13 |
| 13 | −1 | −1 | +1 | +1 | cd | 9 | 10 |
| 14 | +1 | −1 | +1 | +1 | acd | 9 | 10 |
| 15 | −1 | +1 | +1 | +1 | bcd | 20 | 16 |
| 16 | +1 | +1 | +1 | +1 | abcd | 15 | 18 |
| Factor/n Way Interactions | Effect | T-Value | p-Value |
|---|---|---|---|
| A | −2.25 | −3.84 | 0.001 |
| B | 7.50 | 12.79 | 0.000 |
| C | 0.375 | 0.64 | 0.531 |
| D | −1.25 | −2.13 | 0.049 |
| A*B | −0.50 | −0.85 | 0.406 |
| A*C | −0.125 | −0.21 | 0.834 |
| A*D | 1.25 | 2.13 | 0.049 |
| B*C | 0.375 | 0.64 | 0.531 |
| B*D | −1.00 | −1.71 | 0.107 |
| C*D | 1.625 | 2.77 | 0.014 |
| A*B*C | −0.375 | −0.64 | 0.531 |
| A*B*D | 0.25 | 0.43 | 0.675 |
| A*C*D | 0.375 | 0.64 | 0.531 |
| B*C*D | 0.875 | 1.49 | 0.155 |
| A*B*C*D | −0.125 | −0.21 | 0.834 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeraatkar, M.; de Tullio, M.D.; Percoco, G. Fused Filament Fabrication (FFF) for Manufacturing of Microfluidic Micromixers: An Experimental Study on the Effect of Process Variables in Printed Microfluidic Micromixers. Micromachines 2021, 12, 858. https://doi.org/10.3390/mi12080858
Zeraatkar M, de Tullio MD, Percoco G. Fused Filament Fabrication (FFF) for Manufacturing of Microfluidic Micromixers: An Experimental Study on the Effect of Process Variables in Printed Microfluidic Micromixers. Micromachines. 2021; 12(8):858. https://doi.org/10.3390/mi12080858
Chicago/Turabian StyleZeraatkar, Mojtaba, Marco D. de Tullio, and Gianluca Percoco. 2021. "Fused Filament Fabrication (FFF) for Manufacturing of Microfluidic Micromixers: An Experimental Study on the Effect of Process Variables in Printed Microfluidic Micromixers" Micromachines 12, no. 8: 858. https://doi.org/10.3390/mi12080858
APA StyleZeraatkar, M., de Tullio, M. D., & Percoco, G. (2021). Fused Filament Fabrication (FFF) for Manufacturing of Microfluidic Micromixers: An Experimental Study on the Effect of Process Variables in Printed Microfluidic Micromixers. Micromachines, 12(8), 858. https://doi.org/10.3390/mi12080858






