Abstract
Mycotoxin contamination is a crucial issue in food safety. However, the removal of trace amounts of mycotoxins from complex food and feed matrices without significant loss of nutritional and flavor quality remains a significant challenge. The integrated adsorption–catalysis strategy involves immobilizing catalytic modules onto adsorption materials, enabling in situ degradation while enriching the mycotoxins. This approach can significantly reduce the dosage of detoxification agents and achieve efficient removal of trace mycotoxins in food. This review provides an overview of adsorbents with enrichment capabilities and their applications in the targeted removal of mycotoxins from food. The adsorption–degradation coupled systems are categorized into the following two main types: adsorption–photocatalysis coupled systems and adsorption–biocatalysis coupled systems. The review introduces recent advances in the design of bifunctional catalysts, focusing on their synergistic mechanisms and practical applications for detoxifying various mycotoxins in food matrices. Finally, the review discusses current industrial challenges and offers insights into future directions for this field.
Key Contribution:
This review highlights recent advances in the design of adsorption–degradation integrated detoxification catalysts and their applications in efficiently removing mycotoxins from complex food systems.
1. Introduction
Mycotoxins are highly toxic secondary metabolites produced by fungi, which are major contaminants in food and agricultural commodities [1,2]. Genera such as Aspergillus, Fusarium, and Penicillium frequently contaminate cereals, nuts, and dried fruits, producing mycotoxins including aflatoxins (AFs), patulin (PAT), ochratoxin A (OTA), zearalenone (ZEN), deoxynivalenol (DON), and fumonisin B (FBs) [3,4,5]. These persistent contaminants permeate the entire food production chain from pre-harvest to storage and processing, causing substantial economic losses [6,7]. The Food and Agriculture Organization (FAO) estimates that about 25% of global agricultural production (roughly 1 billion metric tons) is adversely affected by fungal contamination annually [8]. In the United States, annual economic losses attributed to crop damage reach approximately USD 932 million [9]. Due to their potent carcinogenic, teratogenic, and mutagenic properties, mycotoxins pose serious health risks in chronic dietary exposure even at trace level [10,11,12,13]. To reduce the risk of mycotoxin contamination, strategies including physical (adsorption, radiation, ultrasound, etc.) [14,15], chemical (uses of oxidants and fungicides, ozone treatment, etc.) [16,17], and biological (microbial and enzymatic degradation) methods have been developed [18,19,20]. Contemporary detoxification approaches for food applications can be primarily classified into the following two categories: adsorption-based [21] and degradation-based strategies [22,23].
Adsorption approaches employ engineered nanoporous and magnetic adsorbents [24,25,26], particularly metal–organic frameworks (MOFs) and their derivatives [27], biochar [28,29], and covalent organic frameworks (COFs), that leverage high specific surface area, tunable porosity, structural flexibility, abundant active sites, and facile functionalization to optimize mycotoxin–adsorbent interactions [30,31]. Particularly, molecularly imprinted polymers (MIPs), designed with molecular-level complementarity to target toxins, provide superior binding selectivity and enhanced removal efficiency, enabling precise enrichment and source-specific mitigation [32,33]. These advanced materials are widely applied in liquid food matrices such as fruit juices, beer, and milk. The degradation approaches include cold plasma and photocatalysis, which generate reactive oxygen species (ROSs) that disrupt mycotoxin structures [34,35]. Nano-photocatalysts are engineered (including binary, ternary, and hybrid photocatalytic nanomaterials) to promote surface-mediated formation of potent oxidizing radicals for efficient toxin degradation [36,37].
However, it is a great challenge to remove trace-level mycotoxins from complex food and feed matrices while preserving their original nutritional and flavor quality. Conventional adsorption-based strategies are constrained by finite adsorption capacity, and overdosing adsorbents can compromise the nutritional and sensory qualities of foods [38,39,40]. For degradation techniques, the typically low mycotoxin level, often near regulatory limits, combined with the compositional complexity of food matrices hinder reaction efficiency and control [41,42,43]. Together, these limitations underscore a persistent challenge: developing robust, highly selective methods for the precise and efficient removal of trace mycotoxins from complex food systems.
Recent advances in hybrid nanomaterials enable in situ adsorption–degradation systems that immobilize catalytic moieties within adsorption carriers via adsorption, encapsulation, or covalent coupling strategies [44,45,46]. The adsorption–degradation integrated approaches reduce the required dosage of detoxifying agents while enabling precise, efficient mycotoxin removal from complex food matrices. The carriers of these hybrid nanomaterials serve dual critical functions. On the one hand, they create protective microenvironments that enhance catalyst specificity, stability, and durability without sacrificing activity [47,48]. On the other hand, they locally concentrate mycotoxins near active sites, significantly improving mass transfer and accelerating reaction kinetics [49,50]. Compared with monofunctional catalysts, these bifunctional adsorption–degradation hybrids achieve superior extraction and conversion of trace mycotoxins through synergistic interactions between catalysts and carriers [51,52]. Moreover, the potential application scope of the hybrid nanomaterials can be broadened by rationally engineering architectures to enable targeted molecular interactions in heterogeneous food systems.
This review systematically summarizes recent advances in bifunctional hybrids for mycotoxin removal in food matrices. The structural designs of targeted adsorbents such as MOFs, COFs, MIPs are summarized. The review also classifies and discusses current bifunctional catalytic systems for food applications, including adsorption–photocatalysis coupled and adsorption–biocatalysis coupled systems, focusing on the synergy mechanisms of the in situ adsorption–degradation systems for mycotoxin removal in food matrices (Figure 1). Finally, challenges to industrial implementation and future research directions are highlighted.
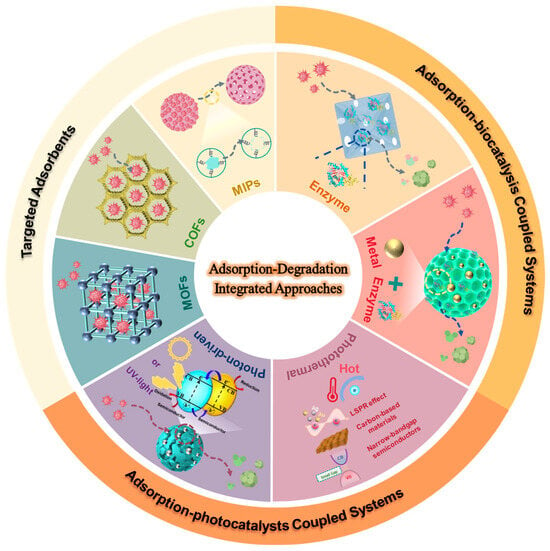
Figure 1.
Adsorption–degradation integrated systems for mycotoxin removal in food.
2. Targeted Adsorbents
Targeted adsorbents are a cutting-edge class of precision-engineered materials tailored to bind specific molecules through techniques such as molecular imprinting and biofunctional modification [27,53]. They feature precisely functionalized surfaces and architecturally optimized pore structures that enable highly selective capture of mycotoxins in complex matrices [26,54]. Unlike conventional adsorbents (e.g., activated carbon, silica gel, zeolites, polymeric resins, and clays), that rely largely on nonspecific interactions such as van der Waals forces and electrostatic interaction forces, targeted adsorbents achieve exceptional specificity by incorporating tailored binding cavities and bio-affinity modules (e.g., aptamers and antibodies) or rationally engineered porous frameworks [24,55,56,57]. In the adsorption–degradation coupled catalytic systems, the most widely used targeted adsorbents include MOFs, COFs, MIPs, and surface-modified derivatives of traditional adsorbents. The following sections elucidate the design strategies and interaction mechanisms by which these materials recognize and capture mycotoxins in food safety applications.
2.1. Metal–Organic Frameworks (MOFs)
Formed through the coordination of metal ions or clusters with organic bridging ligands, MOFs are a type of highly ordered crystalline porous materials [58]. With their exceptionally high surface areas, tunable pore architectures, and controllable topologies, MOFs can be structurally engineered to achieve selective adsorption of mycotoxins in foods [59]. Three principal strategies underpin this selectivity [24,59,60,61,62,63], they are as follows: (1) π-π stacking interactions between aromatic linkers and mycotoxin molecules, (2) introducing unsaturated metal sites (Zr4+, Fe3+, and Cu2+) as high-affinity coordination centers, and (3) installing functional groups (-NH2, -SH, and -COOH) to tailor interfacial chemistry. These strategies significantly improve the adsorption efficiency and molecular recognition specificity of MOFs by exploiting specific interactions with target mycotoxin structures, enabling highly selective capture in complex matrices.
Ma et al. [64] developed a copper-based porous carbonaceous MOF (C-Cu-BTC MOF) for efficient aflatoxin B1 (AFB1) removal from vegetable oils. Using copper-based MOF as a precursor, carbonized porous derivatives were synthesized via calcination at three distinct temperatures (400 °C, 600 °C, and 800 °C). The surface-modified hybrid carbon network featuring a conjugated π-structure, provided abundant chemical binding sites for AFB1 while improving stability in humid environments and preserving porosity (Figure 2a). Remarkably, C-Cu-BTC MOF-600 showed optimal performance, achieving 90% AFB1 removal from vegetable oils within 30 min. Biosafety assessments and edible oil quality evaluations indicated substantially reduced cytotoxicity in detoxified oils, with preservation of quality attributes.
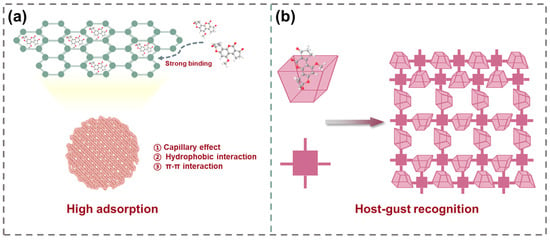
Figure 2.
(a) Schematic illustration for the design and preparation of Cu-BTC MOF with high adsorption efficiency derived porous carbonaceous materials for removal of AFB1. (b) The synthetic procedure of CX4-Tph-COF and adsorption scheme of host–guest recognition with mycotoxins.
To simultaneously remove aflatoxins and zearalenone from peanut oils, Du et al. [65] synthesized an Fe-based MOF (MOF-235). The topology structure is established by basic triangular prism topological building blocks, which are formed by interconnected oxygen-centered trinuclear iron clusters with benzene dicarboxylates. Remarkably, this optimized adsorbent achieved removal efficiencies of 96.1% for aflatoxins and 83.3% for zearalenone within 30 min, while maintaining structural integrity and reusability. X-ray photoelectron spectroscopy (XPS) elucidated the underlying adsorption mechanisms, in which aflatoxins and zearalenone were adsorbed through interaction with the active site of MOF-235. The increased π–π signals of MOF-235 after adsorption of mycotoxins implicated π–π stacking between the benzene-carboxylate-modified framework and aromatic toxin rings of aflatoxin and zearalenone molecules. Additionally, decreases in the Fe 2p3/2 and Fe 2p1/2 binding energies indicated potential chelation of Fe sites with the dicarbonyl groups of mycotoxins or electrostatic interactions with the ionic dipole of the ketone carbonyl group in toxin molecules. For industrial applicability assessment, MOF-235 was packed into purification columns, demonstrating a rapid reduction in five mycotoxin contaminants from initial concentrations of 50 μg/kg to below 10.5 μg/kg. This work establishes a practical platform for engineering MOFs for the removal of multiple mycotoxins in vegetable oils. Liu et al. [66] constructed a UiO-66(NH2)-derived composite which distinguishably augmented active sites through strategic functionalization with cysteine and co-immobilization of gold nanoparticles (UiO-66(NH2)@Au-Cys) for PAT removal from apple juice. The amine, hydroxyl, and carboxyl groups from Cys enhanced the targeted binding with PAT, compensating for the shortcomings of UiO-66(NH2). Structural characterization via scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FT-IR), and X-ray diffraction (XRD) confirmed the successful Au nanoparticle integration and cysteine functionalization in UiO-66(NH2)@Au-Cys. The composite demonstrated an adsorption capacity of 4.38 µg/mg with low cytotoxicity, which was 10 times higher than inactivated microorganisms, while reducing adsorption time from 24 h to 3 h. In a greener design, vitamin B3 is used as an organic linker coordinating with cobalt to prepare MOFs for extracting aflatoxins in commercial soy milk [67]. This approach achieved high adsorption capacity for aflatoxins (AFB1, AFB2, AFG1, and AFG2), with extraction recoveries of 64–75% within acceptable analytical ranges.
2.2. Covalent Organic Frameworks (COFs)
While MOFs offer high surface areas and tunable porosity, their practical use in foods can be limited by aqueous instability, potential metal leaching, and matrix interference [68]. In contrast, COFs are crystalline porous materials built from precisely designed organic monomers linked by robust covalent bonds, conferring superior structural integrity and chemical stability across wide pH and temperature ranges [69,70]. COFs combine ultrahigh surface areas with molecularly tunable pore architectures [71,72], and their adsorption performance can be enhanced through molecular-level design [73,74], such as installing specific functional groups and tailoring pore size/shape to optimize multiple interaction mechanisms (hydrogen bonding, π–π stacking, electrostatic interactions) [75]. The chemical diversity of COF building units also facilitates the integration of chemical and biological catalytic sites, enabling sophisticated adsorption–catalysis coupling within a single framework and addressing key limitations of conventional porous adsorbents [68,71].
Although the porosity and active sites of COFs can be engineered via building-unit and framework-network modulation, selective guest molecule recognition in food matrices imposes stringent design requirements for COFs adsorbents. To realize both host–guest recognition functionality and multiscale pore-size distribution, Xie et al. [76] incorporated dual macrocycles with defined cavities into a single framework, creating a double-macrocyclic COFs with hierarchical pores (CX4-Tph-COF) (Figure 2b). In detail, an aldehyde-functionalized macrocycle CX4-CHO was synthesized from hexamethylenetetramine. The assembly of CX4-CHO and nitrogen-rich tetrakis (4-aminophenyl) porphyrin (Tph) building units into spherical crystals was achieved through a Schiff base reaction. High-magnification transmission electron microscopy (TEM) and SEM confirmed the uniform spherical morphology of CX4-Tph-COF. Brenner–Emmett–Taylor (BET) analysis revealed a bimodal pore-size distribution. These results demonstrated the successful construction of double macrocycles with hierarchical porosity. Adsorption size-screening test and dye-adsorption studies indicated that precise size matching is pivotal for target recognition of CX4-Tph-COF. Target molecules exhibiting precise size complementarity demonstrate optimal binding affinity, driven by synergistic contributions from electrostatic interactions, hydrogen bonding, and π–π stacking, all of which collectively enhance adsorption performance. The dual-macrocyclic architecture provides abundant recognition sites, increases binding energy, and improves size-selective capture of diverse hazardous compounds in complex matrices, thereby promoting efficient and targeted enrichment of mycotoxins. For example, the maximum adsorption capacity for ZEN reached 298.6 mg/g, consistent with the model prediction and more than 30% higher than that of other reported adsorbents, which exhibits significant potential for toxin enrichment in complex food matrices.
A practical hurdle for COFs in food applications is material recovery, owing to their low density and dispersibility. To address this limitation, strategies involving magnetic functionalization and membrane fabrication have been developed. For instance, Li et al. [77] fabricated a flower-patterned COFs fiber membrane (PAN@COF FM). Firstly, polyacrylonitrile (PAN) fibers containing 2,5-divinylterephthalaldehyde (DVA) were prepared by electrospinning. Subsequently, COFs were in situ synthesized on the membrane via immersion in 3,5-tris(4-aminophenyl) benzene (TPB) solution for AFB1 removal. Simulations and characterization indicated that adsorption is dominated by hydrogen bonding and π-π stacking. In real water samples, the membrane maintained an AFB1 absorption rate above 98% after ten consecutive regeneration cycles, demonstrating excellent reusability relevant to food detoxification processes. In a complementary approach, a magnetically responsive COFs (M-COF) was developed for extracting aflatoxins (AFs) from milk, rice, and edible oils [78]. Fe3O4 coating on the COF surface formed a core–shell structure for magnetic dispersive solid-phase extraction (MDSPE), achieving extraction efficiencies ranging from 82.8% to 103.6%, while maintaining operational stability for over eight reuse cycles.
2.3. Molecularly Imprinted Polymers (MIPs)
MIPs have gained significant attention as a class of promising enrichment materials for mycotoxin purification. In molecular imprinting, functional monomers and cross-linkers are polymerized around a template molecule, followed by template removal which leaves three-dimensional recognition cavities whose size, shape, and functional group orientation are complementary to the target analyte [32,33]. These selective binding sites endow MIPs with high affinity and specificity for particular mycotoxins in complex matrices. However, compared to COFs, MOFs, and conventional adsorbents, MIPs suffer from several intrinsic limitations including restricted accessibility of active sites and slow mass transfer, which hinder their implementation in food systems [79]. Consequently, current research focuses on strategies such as surface imprinting and sacrificial support techniques to bring recognition sites to the exterior, increase porosity, and accelerate diffusion [80].
Surface-imprinted MIPs confine recognition sites exclusively at the particle interface, markedly reducing the mass transfer distance for target analytes. As illustrated in Figure 3a, a MIPs layer was grafted onto the UiO-66-NH2 surface to synthesize a surface-imprinted polymer (UiO-66-NH2@MIP), which was subsequently employed as a sorbent for aflatoxins (AFs) extraction from cereals [81]. Initiator dosage, reaction temperature, and cross-linker type were optimized to balance imprinting fidelity and site accessibility. The resulting core–shell nanocomposites formed via precipitation polymerization exhibited selective adsorption of AFs.
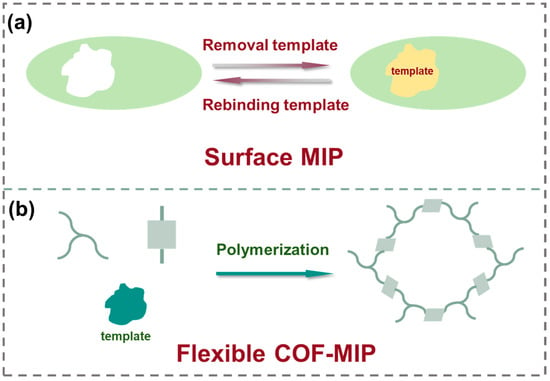
Figure 3.
(a) Scheme for the fabrication of UiO-66-NH2@MIP. (b) Schematic diagram for the synthesis of MI-FCOF.
In a related advancement, Su et al. [82] designed a molecularly imprinted flexible covalent organic framework (MI-FCOF) for selective extraction of aflatoxins from spiked rice, corn, wheat, and peanut samples. Incorporating flexible chain segments into the porous framework increased rotational and conformational freedom, enabling dynamic cavity adjustment and stronger host–guest interactions during imprinting. The MI-FCOF was synthesized using a one-step approach involving flexible building blocks, functional monomers, and template molecules, which enabled dynamic structural transformations during the formation of COFs (Figure 3b). Therefore, the MI-FCOF exhibited strong self-adaptive abilities, making it more flexible in interacting with guest molecules and maximizing the host–guest interactions. Moreover, the combination of large surface area and accessible imprinted sites in MI-FCOF resulted in significantly enhanced adsorption capacity and selectivity for mycotoxins. Specifically, adsorption equilibrium was achieved within 3 min in a 30 mg/L aflatoxin (AFT) solution when using 1 mL of adsorbents. The maximum adsorption capacity, calculated by the Langmuir model, reached 258.4 mg/g, which is three times higher than that of the non-imprinted FCOF (NI-FCOF). Selectivity studies confirmed superior affinity for AFs over other mycotoxins.
Anene et al. [83] fabricated an MIP thin film on silica supports for PAT adsorption. The silica was prefunctionalized with surface polymerizable groups via co-condensation of tetraethoxysilane (TEOS) and γ-methacryloxypropyltrimethoxysilane (γ-MPTS), enabling subsequent MIP grafting using PAT as the template. The MIP films exhibited uptake capacities which were four times higher than those of the corresponding non-imprinted materials (NIPs) and achieved complete adsorption saturation within 20 min. These findings provide a theoretical foundation and practical basis for the application of MIPs in the efficient removal of mycotoxins from food products.
3. Adsorption–Photocatalysis Coupled Systems
Photocatalysis, which utilizes renewable light sources to drive targeted chemical reactions, has emerged as a widely adopted approach for water pollution control and air purification and is increasingly explored for food detoxification [84,85]. The photocatalytic detoxification process involves the following critical steps [37]: When a solid photocatalyst absorbs photons (hν) with energy equal to or greater than its bandgap (Eg), electrons (e−) are promoted from the valence band (VB) to the conduction band (CB) while generating holes (h+) in the VB. The photogenerated charge carriers then migrate to the catalyst surface, where the reductive electrons facilitate the formation of superoxide anions (·O2−) and the oxidative holes oxidize water or hydroxide to generate hydroxyl radicals (·OH). These reactive oxygen species (ROSs) subsequently initiate oxidative degradation of mycotoxins in aqueous solutions, ultimately mineralizing them into small molecules such as carbon dioxide (CO2) and water (H2O).
Due to environmental friendliness, minimal secondary pollution, and cost-effectiveness, photocatalysis shows great potential for mycotoxin degradation in food systems [86,87]. The catalytic performance of photocatalysts can be enhanced by increasing active site density, intensifying light absorption, accelerating charge separation, suppressing electron–hole recombination, facilitating charge transport, and optimizing interfacial reaction pathways [88,89]. However, the photogenerated reactive radicals, which play pivotal roles in breaking down mycotoxins, are easily quenched during mass transfer from the heterogeneous photocatalyst to the reaction system. In addition, compared with purely aqueous applications, implementing photocatalysts in foods necessitates careful attention to safety, light absorption/penetration, and matrix compatibility [90,91,92].
Combining the adsorption function with photocatalysis to construct a system with dual functions, which can concentrate toxins at the active sites and activate multiple synergistic mechanisms, is an important approach for achieving precise photocatalytic removal of mycotoxins in food matrix [93]. Based on the mechanisms of light energy utilization employed in food systems, adsorption–photocatalysis synergistic systems can be distinctly classified into the following two primary categories: (1) purely photon-driven systems encompassing ultraviolet-light-activated and visible-light-activated systems, and (2) photothermal-assisted catalysis systems.
3.1. Purely Photon-Driven Systems
3.1.1. Ultraviolet-Light-Activated Systems
Ultraviolet (UV) light, with its typically high photon energy, can directly decompose a wide range of recalcitrant organic contaminants and toxic substances by generating highly reactive oxygen species [94]. Particularly, for wide-bandgap semiconductors such as TiO2, UV irradiation ensures strong oxidative performance [94,95]. Xu et al. [96] synthesized rGO-doped TiO2 via hydrothermal treatment (HT-rGO/TiO2) to degrade DON in beer. LC-MS analysis revealed that the detoxification of DON resulted from the disruption of key structural motifs in DON, including the epoxy group at C12-C13, the hydroxyl at C3, and the C9–C10 double bond.
The unique organic–inorganic hybrid nature of MOFs promotes spatial separation of photoinduced charge carriers [97]. Their inherent substrate enrichment capability enhances photocatalytic degradation efficiency through synergistic effects. Furthermore, incorporating magnetic nanoparticles addresses the critical challenge of recovering heterogeneous photocatalysts, such as graphene oxide-based composites, from complex food matrices. For example, Samuel et al. [98] designed a recyclable GO/Cu3(BTC)2/Fe3O4 hybrid nanocomposite that achieved 99% of AFB1 removal under UV irradiation, demonstrating a versatile approach for food-applicable photocatalytic composites.
Featuring a tri-modal synergistic mechanism combining adsorption, nanozyme catalysis, and photocatalysis, Zhu et al. [93] developed a zirconium-based MOFs for OTA removal in edible oils. Utilizing the high toxin-adsorption surface area (172.29 m2/g) of PCN-222, the broadband light absorption of porphyrin ligand, and strong photoelectronic properties derived from π-electron conjugation, this system achieved efficient photocatalytic and laccase-mimetic co-degradation of OTA in edible oils without the need for hydrogen peroxide (Figure 4a). To improve recoverability, PCN-222(Mn) was immobilized on edible fungal mycelial membranes. SEM revealed a dense biofilm structure with rough surfaces and protruding features, and elemental mapping confirmed the uniform distribution of Mn and Zr, collectively verifying successful composite membrane fabrication. In OTA-contaminated edible oils, PCN-222(Mn) achieved removal rates of 75.8% to 90.25% after 60 min of UV irradiation. When incorporated into the biofilm, the combined adsorption and molecular sieving capabilities of the mycelial matrix slightly enhanced degradation performance. Notably, the treated oils showed no significant changes in key physicochemical parameters.
Figure 4.
(a) Synergistic scheme for the removal of OTA from edible oil of PCN-222(Mn)/biomembrane composite under visible-light. (b) Schematic illustration of the photocatalytic mechanism of AFB1 by MGO/TiO2-aptamer photocatalyst under UV light.3.2. Photothermal-assisted catalysis systems.
For enhancing selective identification of target molecules in the adsorption–photocatalysis coupled systems, molecular recognition elements such as MIPs, aptamers, and antibodies, are incorporated [82,99,100]. This approach is expected to improve the efficiency of capturing and degrading mycotoxins in complex food matrices. Nisa et al. [101] developed a magnetic imprinted photocatalyst (Fe3O4@SiO2@TiO2@MIP) by sequentially depositing a TiO2 semiconductor layer onto silica-coated iron oxide nanoparticles, followed by MIP synthesis using coumarin (DMC) as a dummy template. TEM images revealed a progressive increase in particle size with each functionalized shell layer, culminating in a thick outer shell with evident agglomeration. Three-dimensional atomic force microscope (3D-AFM) analysis confirmed the successful modification of MIP.
The intermediate silica layer, a wide-bandgap nonconductor, effectively suppressed unfavorable heterojunction formation between Fe3O4 and TiO2. To optimize the capping layer, the following four configurations were evaluated for AFB1 photodegradation: Fe3O4@SiO2@TiO2@MIP, Fe3O4@SiO2@TiO2@NIP, Fe3O4@SiO2@MIP@TiO2, and Fe3O4@SiO2@NIP@TiO2. Several key parameters, including light source (UV/visible), UV intensity, irradiation duration (0–5 h), catalyst dosage (5–20 mg), and initial AFB1 concentration (50–200 μg/L), were systematically investigated. The optimal degradation conditions were found to be UV light, 45 W, 10 mg catalyst, 100 μg/L AFB1, and 5 h of irradiation. In spiked chili oil (12 μg/kg AFB1), Fe3O4@SiO2@TiO2@MIP achieved 95.51% removal within 50 min under UV light, with negligible degradation of capsaicin or ascorbic acid, confirming matrix selectivity. The catalyst retained about 100% efficiency over seven cycles without metal leaching, demonstrating exceptional stability for food safety applications.
3.1.2. Visible-Light-Activated Systems
The adoption of milder visible-light irradiation, as opposed to UV-based excitation, meets the growing demand for more sustainable industrial processes, while significantly reducing damage to food matrices by eliminating the disruption of ROS produced by UV light [102]. From the perspective of catalyst design, various studies reported to enhance the excitation effect of visible-light, including exposing more active sites and enhancing the separation of charge carriers [103,104]. Thus, exploring novel photocatalysts that can leverage visible-light is of great importance.
Hu et al. [105] constructed a band-aligned heterojunction between graphitic carbon nitride (g-C3N4) and titanium dioxide (TiO2) to suppress charge carrier recombination under visible-light. The as-synthesized TiO2/SiO2 microspheres (TiSiMs) were coupled with tubular g-C3N4 (TCN) using a hydrothermal method for OTA removal in wine. Nitrogen adsorption–desorption isotherms revealed that the TiSiMs-TCN possessed a markedly increased specific surface area (53.4 m2/g) and well-defined mesoporosity (12 nm) compared with pristine g-C3N4 and SiO2, with this hierarchical pore architecture facilitating efficient toxin adsorption. Photoelectrochemical characterization confirmed superior optoelectronic properties, as follows: UV-Vis DRS and photoluminescence (PL) spectra revealed a narrowed bandgap (2.52 eV) and quenched PL intensity, indicating enhanced charge separation. Transient absorption spectroscopy showed a 3.2-fold increase in charge-carrier lifetime, attributable to the heterojunction and TCN tubular morphology, which together promoted electron transfer and improved light utilization. In real samples, OTA concentration was 100 μg/L under TiSiMs-TCN, close to the real contamination concentration, and the removal rate was more than 89.8% after 120 min of visible-light illumination. The safety of its intermediate products and final treated solution was verified by cellular activity and toxicology.
To address the difficulty in recycling photocatalyst in practical applications while effectively remediating toxins, a flexible PCL-g-C3N4/CQDs electrospun membrane was constructed by incorporating g-C3N4/CQDs heterojunctions into the membrane [106]. SEM and TEM characterizations confirmed the successful construction of the membrane. High-resolution XPS C 1s spectra identified graphitic carbon (C=C) from CQDs and sp2-hybridized carbon (N-C=N) in g-C3N4, indicating the linkage configuration of g-C3N4/CQDs within the membrane. The PCL-g-C3N4/CQDs electrospun membrane exhibited excellent activity in the removal of AFB1. The adsorption equilibrium was achieved within 30 min under dark conditions with 58.6% of AFB1 was adsorbed. Subsequently, AFB1 was degraded under visible-light irradiation. The membrane maintained above 96% AFB1 degradation efficiency across five consecutive cycles, showing good reusability.
Single-stranded nucleic acid ligands possess inherently high affinity for their complementary strands, enabling versatile target binding [107,108,109]. The programmability and conformational flexibility of aptamers endow them with enhanced stability during synthesis and superior nanomaterial-loading capacity [108]. Additionally, the structures and sequences of aptamer can be rationally designed in vitro to recognize target-specific characteristics, eliminating the need for conditional selection processes [100]. These advantages make aptamers more suitable than antibodies for real-matrix applications. To address the challenge of degrading trace aflatoxins (e.g., in peanut oil, typically <20 μg/kg), an aptamer-functionalized photocatalyst (MGO/TiO2-aptamer) was engineered by conjugating amine-rich AFB1 aptamers to carboxyl-activated magnetic graphene oxide-TiO2 composites [100]. This design boosted the AFB1 degradation rate constant from 0.0075 min−1 to 0.0096 min−1 (a 1.3-fold enhancement) in spiked peanut oil, displaying significant improvement in toxin capture and photocatalytic efficiency at low concentrations. Combined experimental and theoretical analyses elucidated the underlying mechanism. Frontier molecular orbital (FMO) calculations revealed that Ti/Fe incorporation reduced the bandgap of GO from 1.878 eV to 1.714 eV, with a further 0.028 eV decrease upon aptamer conjugation, enhancing UV–visible light excitation without compromising activity. The proposed degradation pathway involves aptamer-mediated AFB1 enrichment, followed by ·OH radical attack on the C8=C9 double bond via photogenerated holes and conduction band electrons (Figure 4b). This work establishes a paradigm for integrating aptamer recognition with photocatalytic degradation in food safety control.
Bio-based photocatalytic nanomaterials, created by integrating biomaterials with photocatalytic components, improve the safety of adsorption–photocatalysis coupled systems [110]. For example, Qiu et al. [111] linked graphitic carbon nitride to konjac glucomannan and introduced thiol (-SH) to fabricate a separation-free composite aerogel (g-C3N4-SH(Gl)@KG) that couples adsorption and photocatalysis for patulin (PAT) removal from apple juice. The -SH grafting treatment of graphite-phase carbon nitride (g-C3N4) increased the adsorption capacity of PAT and also facilitated photocatalytic efficiency. After five consecutive cycles, the aerogel remained 83% of its initial PAT adsorption capacity (0.92 mg/g). When applied to real apple juice samples, the adsorbent represented remarkable performance, achieving 53-fold and 46-fold higher adsorption capacities than unmodified g-C3N4 within 24 h and retaining 81% of its initial capacity after three adsorption–regeneration cycles. Moreover, no adverse effects on juice quality were observed.
3.2. Photothermal-Assisted Catalysis Systems
Thermally assisted adsorption–photocatalysis coupled systems represent a promising advancement in the field of photocatalysis, offering new avenues to enhance performance across a range of applications. The integration of materials such as plasmonic metal nanoparticles (e.g., Ag, Au) [112], narrow-bandgap semiconductors [113], transition metal compounds [114], and carbon-based materials into photocatalytic systems can significantly improve efficiency by harnessing photothermal effects [115]. By generating localized heat, these materials can promote enhanced charge-carrier separation [116], exciton generation, and reactant adsorption, boosting photocatalytic efficiency.
Lu et al. [117] explored the use of calcined nickel foam (NiO/Ni) as a substrate for immobilizing photocatalysts, effectively preventing their dispersion in solution. The modified Ni substrate not only exhibited excellent conductivity, adsorption capacity, and strong photothermal conversion. By loading recyclable ZnIn2S4 onto this substrate, an S-scheme heterojunction was constructed, achieving a remarkable H2 production rate of 92.3 μmol/h under light irradiation. This work provides valuable insights into the potential application of photothermal-assisted effects for detoxification in food.
Luo et al. [118] addressed challenges in copper sulfide (CuS) photocatalysis, such as rapid charge recombination and limited light absorption by integrating CuS with graphitic carbon nitride (g-C3N4) to fabircate a hybrid nanomaterial with dual photothermal and photocatalytic functionalities, along with the enrichment effect of g-C3N4. Their work on minimizing thermal losses and improving photothermal-assisted photocatalytic efficiency paves the way for environmental remediation. The work of Sun and his team further illustrated the dual functionality of photothermal and photocatalytic materials. Silver nanoparticles (Ag) possess intrinsic disinfection properties and localized surface plasmon resonance (LSPR) effects that enhance visible-light absorption and accelerate photogenerated charge separation, significantly improving the photocatalytic performance of composite materials [119]. By incorporating Ag-AgCl nanoparticles onto tetrahedral α-Fe2O3, they created a plasmonic composite that significantly improved photocatalytic disinfection performance, showing an impressive protection rate against Aspergillus spores. Through photoelectrochemical characterization, density functional theory calculations, and finite-difference time-domain simulations, the disinfection capability, electron sink behavior, and localized surface plasmon resonance effects of the photocatalyst were demonstrated. Under visible-light irradiation for 50 min, the protection rate against Aspergillus spores reached 93.65 ± 1.53%. Furthermore, the Ag-AgCl/α-Fe2O3 composite also exhibited high protective activity (90.52 ± 1.26%) during peanut storage. This work opens up avenues for food safety and antifungal applications, such as controlling fungal toxins in food storage.
These studies, particularly those exploring the application of thermally assisted photocatalysis in food safety, represent an exciting new frontier. With photocatalytic materials already showing promise in environmental remediation, further research into their use in food safety could revolutionize mycotoxin contamination control and prevention methods, contributing to public health and food security.
4. Adsorption–Biocatalysis Coupled Systems
Although current single-adsorption catalysts and adsorption–photocatalysis coupled systems have shown promising efficacy in mycotoxin detoxification, their practical implementation in the food industry remains hindered by several key challenges. These include ambiguous degradation pathways, potential nutrient loss, and excessive energy requirements [37]. In contrast, adsorption–biocatalysis coupled systems overcome these limitations by integrating enzymes that are capable of executing highly selective degradation [120]. Biocatalysis can mediate mycotoxins transformation via specific biochemical pathways and obtaining clearly structured conversion products [121]. Operating under mild conditions, enzymatic detoxification ensures both safety and sustainability. Therefore, the engineering of integrated platforms that couple enzymatic specificity with advanced adsorption materials offers a paradigm shift in mycotoxin management and presents considerable commercial potential for sustainable food safety solutions.
4.1. Enzyme for Mycotoxins Removal
Specific microbial-derived enzymes have been isolated, purified, and genetically engineered for the precise recognition and detoxification of mycotoxin, thereby transforming these hazardous compounds into benign or minimally toxic metabolites [120,121]. This biocatalytic decontamination strategy demonstrates unparalleled molecular precision, superior catalytic performance, and negligible secondary pollution. Given these distinctive merits, the direct implementation of these engineered enzymes to contaminated food matrices presents an ideal solution for mycotoxin mitigation, enabling comprehensive toxin breakdown without compromising the nutritional value of treated products. The currently common detoxification enzymes for mycotoxins mainly target toxins such as AFs, ZEN, DON, OTA, and PAT. However, these enzymes encounter problems such as low activity (requiring several days for the reaction) and poor stability, which limit their applications [22,57].
4.2. Adsorption–Enzymatic Catalysis Systems
The use of enzyme immobilization technology with adsorptive supports has become a significant advancement in the field of food detoxification, offering a promising alternative for improving the effectiveness of enzymes in food safety applications. By utilizing interfacial deposition, matrix entrapment, or covalent tethering methods, enzymes are immobilized onto engineered substrates, which improves both structural integrity and functional competence [57,122]. The resultant adsorption–enzymatic catalysis systems exploit the tailored physicochemical properties of supporting materials to reduce the enzyme–substrate spatial gap, dramatically enhancing mass transfer efficiency and overall catalytic performance while ensuring selective molecular recognition [123]. Furthermore, rational design enables these systems to establish specific reaction interfaces within multiphase food matrices, substantially broadening operational scope of enzymes [124]. Recent studies have validated the effectiveness of immobilized enzyme systems for removal of mycotoxins in food [52,125], highlighting the transformative potential of bifunctional adsorption–biocatalytic platforms for industrial implementation.
Porous adsorbents with hierarchical mesoporous structures, particularly COFs and MOFs, have emerged as promising matrices for enzyme immobilization due to their extraordinary specific surface areas and multifunctional surface chemistries [69,126]. To enhance reusability and facilitate the recovery of enzyme catalysts in complex food matrices, various strategies such as magnetic nanoparticle integration and hydrogel network fabrication have been developed. For example, Yan and coworkers co-immobilized cysteine and porcine pancreatic lipase into a hierarchical mesoporous zirconium MOF (CMC@HMMOF-Cys/PPL) [127]. The immobilized enzyme was then doped on an aerogel through the ex situ method and self-assembly strategy. This system proved highly effective in removing PAT from apple juice. The Zr-OH group on the HMMOFs interacted with the carboxyl group of biomolecules to achieve the co-immobilization of Cys and PPL without cross-linking reagents. This strategy demonstrated exceptional loading capacities, which were 16-fold and 4-fold higher than those of other carriers, respectively. The strong adsorption of HMMOFs, confirmed by BET, toward PAT was 38.41 μg/mg. The biodegradation of PPL was 28.68 μg/mg at 10 μg/mL PAT, attributable to the continuous increase in removal capacity over time. The synergistic effect of adsorption and degradation is demonstrated in Figure 5a. Additionally, excellent reusability, storage stability, and improved selectivity in simulated apple juice were also observed. To achieve continuous removal of PAT from real apple juice, CMC@HMMOF-Cys/PPL was subsequently filled into a continuous flow reactor. Moreover, the biologically derived components exhibited excellent biosafety profiles without significantly affecting the quality of apple juice.
In another example, polydopamine-coated membranes with reactive catechol and quinone groups were employed for enzyme immobilization. These groups facilitate spontaneous conjugation with thiol- or amine-containing biomolecules, enabling catalyst immobilization without exogenous coupling agents. An ultrasound-assisted polydopamine-functionalized magnetic porous chitosan (MPCTS@PDA@pancreatin) was designed for reducing OTA in wine [128]. Compared with the free enzyme, the detoxification rate of OTA was significantly enhanced, along with enhanced thermal stability (17–47 °C) and acid resistance (pH 3.0–7.0). In the presence of magnetic separation, MPCTS@PDA@pancreatin remained highly catalytic activity after eight cycles. Zhang et al. [129] developed a co-immobilization system by linking an aldo-keto reductase to a nanocarrier for degrading PAT in fresh pear juice. The nanocarrier was synthesized by imparting magnetite nanoparticles to cellulose nanocrystals (CNCs), and then functionalizing dopamine (DA) and polyethyleneimine (PEI) (DA/PEI@Fe3O4/CNCs). Using NADPH as a coenzyme, this system achieved a 98% removal of PAT in pear juice, with no impact on the quality of juice. In addition, due to the large surface area, high magnetization value, and oxygen/amine functionality, the stability and reusability of reductase were increased, with a detoxification rate over 87% after five cycles, retaining 62% of its activity after 14 days of storage at 4 °C. However, the use of coenzymes in food systems remains a problem to be solved in practical applications.
For removing mycotoxins from edible oils, Lu et al. recently developed an amphiphilic laccase–inorganic hybrid nanoflower (Lac NF-P) [130]. Laccase was immobilized within the inorganic nanoflowers (Lac NF) by the co-precipitation of copper sulfate pentahydrate and laccase-containing phosphate buffer. Subsequently, the amphiphilic polymer Pluronic F127 (PEO-PPO-PEO) was conjugated onto Lac NF using the cross-linking agent concanavalin A (ConA), endowing the hybrid system with exceptional dispersibility and stability in both aqueous and oil phases. With this operation, efficient toxin adsorption was facilitated while maintaining localized aqueous microenvironment around the enzyme to preserve its catalytic activity. Confocal microscopy images confirmed the distribution of Lac and ConA, while BET surface area and pore diameter displayed the expected minimal mass transfer resistance (Figure 5b). Furthermore, tannic acid (TA) served as a redox mediator, where its enzymatically oxidized cationic radicals further degraded AFB1, significantly raising the catalytic efficiency of laccase (Figure 5c). The results demonstrated that Lac NF-P exhibited a remarkable 134-fold and 3.2-fold increase in AFB1 degradation efficiency compared to free laccase and Lac NF, respectively. Notably, the treatment did not compromise peanut oil quality, and no catalyst leakage was detected. Toxicological analysis illustrated that the degradation product was almost non-toxic to human liver cells.
This work highlights that the rational design of amphiphilic immobilized enzyme catalysts can maintain the high activity of natural enzymes while enabling efficient mycotoxin removal in edible oils and dairy products. Furthermore, the suggested integration of a laccase–redox mediator system significantly improves the enzymatic degradation efficiency for AFB1.
Chen et al. [131] developed a zearalenone lactonase–inorganic hybrid nanoflower (InHNF-ZHD518) using a split intein moiety, enabling rapid and site-specific immobilization of ZHD518 directly from crude cell lysates without the need for organic solvents. The immobilized biocatalyst demonstrated a 40–60% increase in specific activity compared to the free enzyme, maintained structural stability across a wide pH range (3–11), and exhibited remarkable operational durability, retaining over 70% of its initial activity after eight reuse cycles. In practical ZEN detoxification assays using beer samples, InHNF-ZHD518 achieved more than 50% degradation of ZEN, whereas the free enzyme was mostly inactivated under the same conditions. The InHNF strategy combines several benefits, including environmentally friendly immobilization, purification-free processing, and enhanced catalytic performance, showcasing its strong potential for applications in food enzyme engineering and industrial biocatalysis.
4.3. Adsorption–Chemoenzymatic Catalysis Coupled Systems
Owing to the limited substrate spectrum available for enzymatic reactions, the integration of enzymatic catalysis with chemical catalysis to establish chemoenzymatic cascade systems exhibiting both high activity and stability has attracted increasing attention in recent years [120]. Through synergistic cascade reactions and colocalization strategies, spatial confinement effects and synergistic interactions can be achieved, thereby accelerating reaction kinetics [132,133]. However, research on the application of biochemical cascade reactions coupled with enrichment strategies for mycotoxin decontamination in food systems remains scarce.
A representative study in this area is the work by Fu et al. [134], in which GOx and Fe3O4 NPs were co-immobilized on an amphipathic covalent organic framework (COFs), forming a hybrid catalyst denoted as PL-GOx-Fe3O4@COF. During the synthesis process, iron ions (Fe3+) were in situ reduced within the pores of COF. GOx was subsequently immobilized on the surface through a Schiff base reaction (Figure 5d). This design enabled the separate yet confined loading of GOx and Fe NPs within one compartment, enhancing intermediate diffusion efficiency. Furthermore, an aldehyde-functionalized triblock copolymer was grafted to improve the mass transfer efficiency for applications in edible oils.
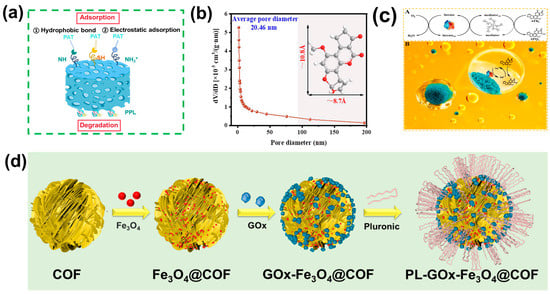
Figure 5.
(a) Removal mechanism of PAT by CMC@HMMOF-Cys/PPL aerogel. (b) Pore-size distributions of Lac NF-P. (c) (A) Reaction mechanism of AFB1 oxidation catalyzed by the laccase–TA system. (B) Schematic illustration for the biodegradation of AFB1 in peanut oil catalyzed by Lac NF-P. (b,c) Reproduced with permission (Lu et al., 2023) [130] Copyright © 2023, American Chemical Society. (d) Preparation process of PL-GOx-Fe3O4@COF metal–biological hybrid catalyst. Reproduced with permission (Fu et al., 2024) [134] Copyright © 2024, American Chemical Society.
This hybrid catalyst successfully combined the Fenton reaction with enzymatic catalysis, achieving a metal–biological cascade system for mycotoxin removal. The targeted detoxification of mycotoxins can be realized due to the π-π stacking interaction between COFs and mycotoxins (especially AFB1), and convenient separation in edible oils. The flower-like morphology of the hybrid catalysts was characterized by TEM and fluorescence microscopy, which also confirmed the successful co-immobilization of enzymes and Fe3O4 NPs. Mechanistically, continuous hydrogen peroxide generation by GOx triggered the Fenton reaction, producing reactive oxygen radicals, while the gluconic acid formed in the oxidation process helped regulate the microenvironment, thereby stabilizing Fe2+/Fe3+ redox cycling. As a result, the cascade system achieved a detoxification efficiency against AFB1 that was six times higher than that of a simple mixture of free GOx and Fe3O4. Moreover, the catalytic activity was maintained after six reuse cycles without significant morphological changes. Importantly, no notable deterioration in the nutritional composition of peanut oil, and almost no toxicity of the degradation products and treated oil in human liver cells, was observed, underscoring the potential of this strategy for mycotoxin detoxification in vegetable oils.
5. Conclusions and Prospects
The adsorption–degradation coupled systems integrate the advantages of adsorptive support materials and catalytic components, enabling a seamless cascade of adsorption and degradation processes with high catalytic efficiency. Systems composed of molecular recognition materials and catalysts in the adsorption–photocatalyst system achieve directional mycotoxin detoxification in complex food matrices through synergistic interactions, while featuring facile synthesis and strong material specificity. This review summarizes enrichment-capable adsorbents used in the development of such bifunctional systems, along with their individual roles in mitigating fungal toxin contamination in food. Furthermore, the existing synergistic adsorption–photocatalytic and adsorption–biocatalytic systems were summarized for the first time, elucidating their design principles, bifunctional mechanisms, application performances, and potential toxicological aspects in the food matrices.
However, due to the complexity of food matrices, diversity of toxins, trace-level contamination, and stringent food safety standards, the available range of enrichment supports and catalysts for constructing systems that combine precision, efficiency, and biocompatibility remains limited. Future research is expected to focus on designing synergistic and directional adsorption–degradation systems and developing novel cascade reactions to enhance catalytic performance for efficient mycotoxin removal. These systems aim to eliminate trace and ultra-trace mycotoxins in food. Smart materials that are capable of conformational responses to temperature, pH, and other stimuli may regulate toxin-binding capacity, providing an ideal structure for adsorbents. In addition, technologies such as machine learning-assisted aptamer screening, may serve as essential tools in synthesizing the bifunctional system. Despite promising laboratory outcomes, the application of integrated enrichment–degradation materials in large-scale food processing remains limited. Thus, the development of non-toxic, cost-effective, and scalable industrial synthesis methods represents a key direction for future applied research, potentially advancing the practical implementation of these innovative adsorption–degradation coupled systems.
Author Contributions
Conceptualization, X.Y. and X.L.; data curation, X.Y. and X.L.; writing—original draft preparation, X.Y.; writing—review and editing, X.L., X.Y. and M.Y.; visualization, M.Y. and W.L.; supervision, X.L.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFF1102800), the National Natural Science Foundation of China (Grant No. 22308143, 22168024), and the Jiangxi Provincial Natural Science Foundation (Grant No. 20232ACB215008, 20252BAC250148, jxsq2023101073).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adeyeye, S.A. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Azam, M.S.; Ahmed, S.; Islam, M.N.; Maitra, P.; Islam, M.M.; Yu, D. Critical Assessment of Mycotoxins in Beverages and Their Control Measures. Toxins 2021, 13, 323. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef] [PubMed]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Munirah, A.; Norfarizan-Hanoon, N. Interrelated of food safety, food security and sustainable food production. Food Res. 2022, 6, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Shahbazi, F.; Shahbazi, S.; Nadimi, M.; Paliwal, J. Losses in agricultural produce: A review of causes and solutions, with a specific focus on grain crops. J. Stored Prod. Res. 2025, 111, 102547. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Bai, X.; Ran, Y. Recent Development of Methods and Techniques in the Detection of Mycotoxins in Agricultural Products. J. Agric. Food Chem. 2025, 73, 20530–20546. [Google Scholar] [CrossRef]
- Lemée, P.; Fessard, V.; Habauzit, D. Prioritization of mycotoxins based on mutagenicity and carcinogenicity evaluation using combined in silico QSAR methods. Environ. Pollut. 2023, 323, 121284. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ toxicological mechanisms involving humans, livestock and their associated health concerns: A review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Gurikar, C.; Shivaprasad, D.; Sabillón, L.; Gowda, N.N.; Siliveru, K. Impact of mycotoxins and their metabolites associated with food grains. Grain Oil Sci. Technol. 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Sebastià, A.; Calleja-Gómez, M.; Pallarés, N.; Castagnini, J.M.; Ferrer, E.; Berrada, H. Combination of pulsed electric fields and ultrasounds technologies for mycotoxins mitigation in grape juice. Appl. Food Res. 2025, 5, 100963. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Liu, Y.; Bian, K. Structures of Reaction Products and Degradation Pathways of Aflatoxin B1 by Ultrasound Treatment. Toxins 2019, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, N.; Bhavana, B.K.; Mudliar, S.N.; Bhatt, P.; Vasu, P.; Debnath, S. Comparative efficacy of cold plasma and ozone treatments in mitigating Aspergillus flavus and Aflatoxin B1 in Byadagi chili. Food Control 2025, 177, 111408. [Google Scholar] [CrossRef]
- da Luz, S.R.; Pazdiora, P.C.; Dallagnol, L.J.; Dors, G.C.; Chaves, F.C. Mycotoxin and fungicide residues in wheat grains from fungicide-treated plants measured by a validated LC-MS method. Food Chem. 2017, 220, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Han, Y.; Hou, M.; Gao, Z. Novel strategies for efficiently detoxifying mycotoxins in plant-derived foods inspired by non-thermal technologies and biological resources: A review. Crit. Rev. Food Sci. Nutr. 2025, 65, 5929–5955. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, L.; Xie, Y.; Li, X.; Zhao, R.; Yang, Y.; Sun, S.; Li, Q.; Ma, W.; Jia, H. Characterization, Mechanism, and Application of Dipeptidyl Peptidase III: An Aflatoxin B1-Degrading Enzyme from Aspergillus terreus HNGD-TM15. J. Agric. Food Chem. 2024, 72, 15998–16009. [Google Scholar] [CrossRef]
- Murtaza, B.; Guo, L.-l.; Wang, L.; Li, X.; Zeb, L.; Jin, B.; Li, J.-b.; Xu, Y. Innovative probiotic fermentation approach for zearalenone detoxification in dried distiller’s grains. Front. Microbiol. 2025, 16, 1533515. [Google Scholar] [CrossRef]
- Acosta, E.R.; Lima da Silva, J.; Lopes de Moura da Costa, Y.; Roberto Sant’Anna Cadaval Júnior, T.; Garda-Buffon, J. Adsorption techniques for mycotoxin mitigation in food: A review. Food Addit. Contam. Part A 2025, 42, 1091–1120. [Google Scholar] [CrossRef]
- Fang, J.; Sheng, L.; Ye, Y.; Ji, J.; Sun, J.; Zhang, Y.; Sun, X. Recent advances in biosynthesis of mycotoxin-degrading enzymes and their applications in food and feed. Crit. Rev. Food Sci. Nutr. 2025, 65, 1465–1481. [Google Scholar] [CrossRef]
- Song, C.; Yang, J.; Wang, Y.; Ding, G.; Guo, L.; Qin, J. Mechanisms and transformed products of aflatoxin B1 degradation under multiple treatments: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2263–2275. [Google Scholar] [CrossRef]
- Cui, X.; Sun, Y.; Song, C.; Hu, Y.; Man, Y.; Li, J.; Zhao, R.; He, L. Removal of Mycotoxins in Food by Emerging Porous Materials: Advances, Mechanisms and Prospects. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70182. [Google Scholar] [CrossRef]
- Hu, G.; Wang, X.; Zhuang, Y.; Yu, P.; Gao, S.; Hao, J. Advances in the application of porous organic framework materials for adsorption and detection of mycotoxins and marine biotoxins. Talanta 2026, 298, 128966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, W.; Huang, J.; Tao, X.; Xu, H. Porous framework materials for mycotoxin detection: From synthesis to application. TrAC Trends Anal. Chem. 2025, 189, 118249. [Google Scholar] [CrossRef]
- Song, C.; Qin, J. High-performance fabricated nano-adsorbents as emerging approach for removal of mycotoxins: A review. Int. J. Food Sci. Technol. 2022, 57, 5781–5789. [Google Scholar] [CrossRef]
- Ahmadou, A.; Napoli, A.; Durand, N.; Montet, D. High physical properties of cashew nut shell biochars in the adsorbtion of mycotoxins. Int. J. Food Res. 2019, 6, 18–28. [Google Scholar]
- Li, P.; Wang, S.; Lv, B.; Zhang, M.; Xing, C.; Sun, X.; Fang, Y. Magnetic rice husk-based biochar for removal of aflatoxin B1 from peanut oil. Food Control 2023, 152, 109883. [Google Scholar] [CrossRef]
- Fang, J.; Li, D.; Li, J.; Wang, Q.; Wang, J.; Gao, Y.; He, Y.; Wang, C. High efficient adsorption of aflatoxins in peanuts by novel covalent organic framework through multiple interactions. Food Chem. 2025, 481, 143919. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Hu, Y.; Zhong, Q. Host-guest mediated recognition and rapid extraction of Fusarium mycotoxins in cereals by nickel ferrite magnetic calix [4] arene-derived covalent organic framework fabricated in room-temperature. Food Chem. 2025, 464, 141887. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.C.; Sánchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutiérrez, T.J. Molecularly imprinted polymers for food applications: A review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Hua, Y.; Ahmadi, Y.; Sonne, C.; Kim, K.-H. Progress and challenges in sensing of mycotoxins using molecularly imprinted polymers. Environ. Pollut. 2022, 305, 119218. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, A.; Yu, B.; Sun, X. Recent Advances in Non-Contact Food Decontamination Technologies for Removing Mycotoxins and Fungal Contaminants. Foods 2024, 13, 2244. [Google Scholar] [CrossRef]
- Mirza Alizadeh, A.; Hashempour-Baltork, F.; Mousavi Khaneghah, A.; Hosseini, H. New perspective approaches in controlling fungi and mycotoxins in food using emerging and green technologies. Curr. Opin. Food Sci. 2021, 39, 7–15. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Li, J.; Liu, N.; Zhang, M.; Le, T. Advances in photocatalysis for mycotoxins elimination: Engineering strategies in photocatalyst designing, practical applications and future prospects. J. Alloys Compd. 2023, 955, 170234. [Google Scholar] [CrossRef]
- Murugesan, P.; Brunda, D.K.; Moses, J.A.; Anandharamakrishnan, C. Photolytic and photocatalytic detoxification of mycotoxins in foods. Food Control 2021, 123, 107748. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, J.; Wang, G.; Song, A.; Li, C.; Zheng, S. Hydrothermal fabrication of rectorite based biocomposite modified by chitosan derived carbon nanoparticles as efficient mycotoxins adsorbents. Appl. Clay Sci. 2020, 184, 105373. [Google Scholar] [CrossRef]
- Ji, J.; Xie, W. Detoxification of Aflatoxin B1 by magnetic graphene composite adsorbents from contaminated oils. J. Hazard. Mater. 2020, 381, 120915. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, L.; Wang, B.; Han, Y.; Shi, H.; Wei, L.; Guo, X.; Zhang, Y. Recent progress of separation and sensing applications of metal-organic framework-based membranes. Chem. Eng. J. 2025, 506, 160371. [Google Scholar] [CrossRef]
- Iqbal, S.Z. Mycotoxins in food, recent development in food analysis and future challenges: A review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Recent advances in coupled green assisted extraction techniques for foodstuff analysis. TrAC Trends Anal. Chem. 2023, 169, 117411. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, G.; Liu, P.; Li, Z.; Yu, B. Recent Development of Aptamer Sensors for the Quantification of Aflatoxin B1. Appl. Sci. 2019, 9, 2364. [Google Scholar] [CrossRef]
- Khalid, N.; Kalsoom, U.; Ahsan, Z.; Bilal, M. Non-magnetic and magnetically responsive support materials immobilized peroxidases for biocatalytic degradation of emerging dye pollutants—A review. Int. J. Biol. Macromol. 2022, 207, 387–401. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated metal nanoparticles for catalysis. Chem. Rev. 2020, 121, 834–881. [Google Scholar] [CrossRef]
- Garcia-Toral, D.; Báez, R.M.; Sánchez, S.J.I.; Flores-Riveros, A.; Cocoletzi, G.H.; Rivas-Silva, J. Encapsulation of pollutant gaseous molecules by adsorption on boron nitride nanotubes: A quantum chemistry study. ACS Omega 2021, 6, 14824–14837. [Google Scholar] [CrossRef]
- Pragya; Sharma, K.K.; Kumar, A.; Singh, D.; Kumar, V.; Singh, B. Immobilized phytases: An overview of different strategies, support material, and their applications in improving food and feed nutrition. Crit. Rev. Food Sci. Nutr. 2023, 63, 5465–5487. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Meng, Q.; Zhao, Y.; Yang, D.; Rao, L.; Liao, X. Bacterial spore surface display system for enzyme stabilization in food industry: Principles, applications and efficiency optimization strategies. Trends Food Sci. Technol. 2025, 164, 105276. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, M.; Zhang, Y.; Pan, J.; Zhang, W.; Guo, L.; Zhang, X.; Jiang, Y. Enzyme immobilization based on reticular framework materials: Strategy, food applications, and prospect. Adv. Colloid Interface Sci. 2025, 344, 103589. [Google Scholar] [CrossRef]
- Fu, C.; Lu, T.; Dai, X.; Ding, P.; Xiong, Y.; Ge, J.; Li, X. Co-immobilization of enzymes and metals on the covalent-organic framework for the efficient removal of mycotoxins. ACS Appl. Mater. Interfaces 2023, 15, 6859–6867. [Google Scholar] [CrossRef]
- Sun, Z.; Song, A.; Wang, B.; Wang, G.; Zheng, S. Adsorption behaviors of aflatoxin B1 and zearalenone by organo-rectorite modified with quaternary ammonium salts. J. Mol. Liq. 2018, 264, 645–651. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, D.; Li, X.; Li, W.; Ren, J.; Zhong, H. Covalent organic frameworks assisted for food safety analysis. Crit. Rev. Food Sci. Nutr. 2024, 64, 11006–11025. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Z.; Zhang, Q.; Li, P. Mycotoxin determination in foods using advanced sensors based on antibodies or aptamers. Toxins 2016, 8, 239. [Google Scholar] [CrossRef]
- Conrad, M.; DeRosa, M.C. Assay formats and target recognition strategies in lateral flow assays for the detection of mycotoxins. TrAC Trends Anal. Chem. 2025, 190, 118273. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-J.; Wu, C.; Fang, M.; Ding, B.; Liu, P.-P.; Zhou, M.-X.; Gong, Z.-Y.; Ma, D.-L.; Leung, C.-H. Application of metal–organic framework for the adsorption and detection of food contamination. TrAC Trends Anal. Chem. 2021, 143, 116384. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wang, L.; Huang, Q.; Bu, L.; Wang, Q. Metal-organic frameworks for food contaminant adsorption and detection. Front. Chem. 2023, 11, 1116524. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, Z.; Wang, R.; Zhao, Y.; Jia, Q. Research advances of porous organic framework materials on enrichment and detection of mycotoxins. Chin. J. Chromatogr. 2023, 41, 891–900. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Gao, Y.; Cai, Y.; Liu, J.; Ramachandraiah, K.; Mao, J.; Ke, F. Functional modification engineering of metal–organic frameworks for the contaminants detection in food. Coord. Chem. Rev. 2024, 516, 215990. [Google Scholar] [CrossRef]
- Han, D.; Liu, X.; Wu, S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem. Soc. Rev. 2022, 51, 7138–7169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Song, C.G.; Ding, G.; Yang, J.; Wu, J.R.; Wu, G.; Zhang, M.Z.; Song, C.; Guo, L.P.; Qin, J.C. High-performance functional Fe-MOF for removing aflatoxin B1 and other organic pollutants. Adv. Mater. Interfaces 2022, 9, 2102480. [Google Scholar] [CrossRef]
- Ma, F.; Cai, X.; Mao, J.; Yu, L.; Li, P. Adsorptive removal of aflatoxin B1 from vegetable oils via novel adsorbents derived from a metal-organic framework. J. Hazard. Mater. 2021, 412, 125170. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, W.; Xu, N.; Jiang, X.; Cheng, J.; Wang, R.; Wang, P. Efficient and simultaneous removal of aflatoxin B1, B2, G1, G2, and zearalenone from vegetable oil by use of a metal–organic framework absorbent. Food Chem. 2023, 418, 135881. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Yang, Q.; Hu, N.; Zhang, W.; Zhu, W.; Wang, R.; Suo, Y.; Wang, J. Patulin removal from apple juice using a novel cysteine-functionalized metal-organic framework adsorbent. Food Chem. 2019, 270, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, A.; Nemati, M.; Afshar Mogaddam, M.R.; Farajzadeh, M.A.; Lotfipour, F. Dispersive micro–solid–phase extraction of aflatoxins from commercial soy milk samples using a green vitamin–based metal–organic framework as an efficient sorbent followed by high performance liquid chromatography–tandem mass spectrometry determination. J. Chromatogr. A 2022, 1673, 463099. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Aguila, B.; Ma, S. Opportunities of Covalent Organic Frameworks for Advanced Applications. Adv. Sci. 2019, 6, 1801410. [Google Scholar] [CrossRef]
- Qiao, S.; Jin, H.; Zuo, A.; Chen, Y. Integration of Enzyme and Covalent Organic Frameworks: From Rational Design to Applications. Acc. Chem. Res. 2023, 57, 93–105. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Yang, C.; Cheng, K.; Tan, T.; Lv, Y.; Liu, Y. Hybrid Porous Crystalline Materials from Metal Organic Frameworks and Covalent Organic Frameworks. Adv. Sci. 2021, 8, 2101883. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Fang, Q.; Qiu, S. Covalent Organic Frameworks for Catalysis. EnergyChem 2020, 2, 100035. [Google Scholar] [CrossRef]
- Tan, K.T.; Ghosh, S.; Wang, Z.; Wen, F.; Rodríguez-San-Miguel, D.; Feng, J.; Huang, N.; Wang, W.; Zamora, F.; Feng, X.; et al. Covalent organic frameworks. Nat. Rev. Methods Primers 2023, 3, 1. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Zhang, J.; He, P.; Fan, Z.; He, N.; Li, X.; Li, Y.; Ma, L. Cooperative Steric Modulation of Flexibility, Disorder, and Pore Size in Two-Dimensional Covalent Organic Framework Membranes for Enhanced Selective Ion Sieving. J. Am. Chem. Soc. 2025, 147, 32580–32590. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, H.; Ji, J.; Chen, F.; Wang, Y.; Suo, J.; Song, J.; Zhao, D.; Valtchev, V.; Qiu, S.; et al. Topological Derivative Strategy for Large-Pore Three-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2025, 147, 39223–39231. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Wei, W.; Liu, Y.; Chen, S.; Li, M.; Li, J.; Gao, Z. Advances in the application of covalent organic frameworks as solid phase microextraction coating materials for environmental organic pollutant detection. Chem. Eng. Sci. 2025, 313, 121758. [Google Scholar] [CrossRef]
- Xie, Z.; Lin, J.; Hu, Y.; Li, G.; Zhong, Q. Rapid construction of double macrocycles, hierarchical covalent organic framework with size-sieving and host-guest recognition for selective adsorption and targeted analysis of mycotoxins in cereals. Chem. Eng. J. 2024, 493, 152464. [Google Scholar] [CrossRef]
- Li, S.; Qin, K.; Fu, Y.; He, D.; Han, D.; Li, S.; Wang, Y.; Ren, S.; Peng, Y.; Gao, Z. Highly efficient removal of Aflatoxin B1 employing a flower-like covalent organic framework-based fiber membrane. J. Environ. Chem. Eng. 2023, 11, 111382. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Guo, W.; Zhang, Y.; Feng, X.; Zhang, F. Synthesis of a magnetic covalent organic framework as sorbents for solid-phase extraction of aflatoxins in food prior to quantification by liquid chromatography-mass spectrometry. Food Chem. 2022, 387, 132821. [Google Scholar] [CrossRef]
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Molecularly imprinted polymers as highly selective sorbents in sample preparation techniques and their applications in environmental water analysis. Trends Environ. Anal. Chem. 2023, 37, e00193. [Google Scholar] [CrossRef]
- Xie, D.; Kuang, Y.; Yuan, B.; Zhang, Y.; Ye, C.; Guo, Y.; Qiu, H.; Ren, J.; Alshammari, S.O.; Alshammari, Q.A.; et al. Convenient and highly efficient adsorption of diosmetin from lemon peel by magnetic surface molecularly imprinted polymers. J. Mater. Sci. Technol. 2025, 211, 159–170. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Huang, Z.; Li, H.; Zhang, Y.; Wang, H.; Rui, C.; Li, Y.; You, L.; Li, K.; et al. An amino-functionalized zirconium-based metal-organic framework of type UiO-66-NH2 covered with a molecularly imprinted polymer as a sorbent for the extraction of aflatoxins AFB1, AFB2, AFG1 and AFG2 from grain. Microchim. Acta 2019, 187, 32. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-H.; Qian, H.-L.; Yang, C.; Wang, C.; Wang, Z.; Yan, X.-P. Integrating molecular imprinting into flexible covalent organic frameworks for selective recognition and efficient extraction of aflatoxins. J. Hazard. Mater. 2024, 467, 133755. [Google Scholar] [CrossRef]
- Anene, A.; Kalfat, R.; Chevalier, Y.; Hbaieb, S. Molecularly imprinted polymer-based materials as thin films on silica supports for efficient adsorption of Patulin. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 293–303. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef]
- Jing, G.; Wang, Y.; Wu, M.; Liu, W.; Xiong, S.; Yu, J.; Li, W.; Liu, W.; Jiang, Y. Photocatalytic Degradation and Pathway from Mycotoxins in Food: A Review. Food Rev. Int. 2024, 40, 276–292. [Google Scholar] [CrossRef]
- Raesi, S.; Mohammadi, R.; Khammar, Z.; Paimard, G.; Abdalbeygi, S.; Sarlak, Z.; Rouhi, M. Photocatalytic detoxification of aflatoxin B1 in an aqueous solution and soymilk using nano metal oxides under UV light: Kinetic and isotherm models. LWT 2022, 154, 112638. [Google Scholar] [CrossRef]
- Huang, Q.-Q.; Li, N.; Han, M.-S.; Liu, J.; Lan, Y.-Q. Conductive Knitting of Covalent Organic Framework Manipulates Spin Density, Orbital Reorganization, and Charge Mobility for Outstanding Photoreactivity. Angew. Chem. Int. Ed. 2025, 64, e202513848. [Google Scholar] [CrossRef]
- Balapure, A.; Dutta, J.R.; Ganesan, R. Recent advances in semiconductor heterojunctions: A detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Huang, X.; Xie, W.; Xu, T.; Weng, W.; Zhou, T.; Guo, J. Enantioselective Immobilization of Nonprecious Metal Complexes on Chiral Covalent Organic Frameworks for Improved Single-Site Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2025, 64, e202509095. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lu, L.; Zhang, Y.; Yuan, Z.; Yang, L.; Wang, L.; Rao, Y. A bioinspired cercosporin/polymethylmethacrylate photocatalyst with high efficiency for decontamination of pharmaceuticals and pathogens. J. Hazard. Mater. 2021, 419, 126555. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Riaz Rajoka, M.S.; Albahi, A.; Abid, M.; Ranjha, M.M.A.N.; El-Seedi, H.R.; Xie, F.; et al. Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, J.; Xu, S.; Zhu, Y.; Shen, W.; Wu, L. Metal-organic framework incorporated fungal mycelium membrane for synergistic mycotoxin degradation via adsorption, oxidation, and photocatalysis. Food Chem. 2025, 480, 143861. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, R.; Lo Porto, C.; Lotito, S.; Ingrosso, C.; Comparelli, R.; Curri, M.L.; Barucca, G.; Fracassi, F.; Palumbo, F.; Milella, A. Atmospheric Pressure Plasma Deposition of Hybrid Nanocomposite Coatings Containing TiO2 and Carbon-Based Nanomaterials. Molecules 2023, 28, 5131. [Google Scholar] [CrossRef]
- Junnan, Q.; Huimin, L.; Guihong, L.; Yao, C. Innovation of TiO2-x Nanomaterials in the Biomedical Field: Synthesis, Properties, and Application Prospects. Chem. Eng. J. 2024, 491, 151773. [Google Scholar] [CrossRef]
- Xu, J.; Su, S.; Song, X.; Luo, S.; Ye, S.; Situ, W. A simple nanocomposite photocatalyst HT-rGO/TiO2 for deoxynivalenol degradation in liquid food. Food Chem. 2023, 408, 135228. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.-H.; Chen, F.-Y.; Geng, W.-Y.; Lu, X.-X.; Zhang, D.-E. Enhancing the spatial separation of photogenerated charges on Fe-based MOFs via structural regulation for highly-efficient photocatalytic Cr (VI) reduction. J. Hazard. Mater. 2023, 441, 129875. [Google Scholar] [CrossRef]
- Samuel, M.S.; Mohanraj, K.; Chandrasekar, N.; Balaji, R.; Selvarajan, E. Synthesis of recyclable GO/Cu3(BTC)2/Fe3O4 hybrid nanocomposites with enhanced photocatalytic degradation of aflatoxin B1. Chemosphere 2022, 291, 132684. [Google Scholar] [CrossRef]
- Van Dorst, B.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; De Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent advances in recognition elements of food and environmental biosensors: A review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef]
- Ku, M.; Li, J.; Zhang, W.; Sun, S.; Zhang, Y.; Xie, Y. Degradation of AFB1 in edible oil by aptamer-modified TiO2 composite photocatalytic materials: Selective efficiency, degradation mechanism and toxicity. Food Chem. 2025, 470, 142674. [Google Scholar] [CrossRef]
- Nisa, M.U.; Bilhod, W.; Insin, N. Selective photodegradation of aflatoxin B1 in chili oil using titania-silica-iron oxide nanocomposites with molecularly imprinted technology. Inorg. Chem. Commun. 2025, 173, 113883. [Google Scholar] [CrossRef]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Kumar, D.P.; Nollen, L.-M.; Rangappa, A.P.; Kim, T.K. Effective dye degradation by an environment-friendly porous few-layered carbon nitride photocatalyst developed using sequential molecule self-assembly. Environ. Res. 2022, 204, 112362. [Google Scholar] [CrossRef]
- Bariki, R.; Pradhan, S.K.; Panda, S.; Nayak, S.K.; Pati, A.R.; Mishra, B.G. Hierarchical UiO-66(−NH2)/CuInS2 S-Scheme Photocatalyst with Controlled Topology for Enhanced Photocatalytic N2 Fixation and H2O2 Production. Langmuir 2023, 39, 7707–7722. [Google Scholar] [CrossRef]
- Hu, C.; Yang, C.; Li, B.; Peng, B. Removal of ochratoxin A from wine by adsorption-photocatalytic synergy of tubular TiO2/SiO2/g-C3N4: Mechanistic insights and degradation pathways. Food Chem. 2025, 471, 142758. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Enhancement of AFB1 Removal Efficiency via Adsorption/Photocatalysis Synergy Using Surface-Modified Electrospun PCL-g-C3N4/CQDs Membranes. Biomolecules 2023, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Alnaser-Almusa, O.; Mahmoud, M.; Ilyas, M.; Adwan, R.; Rub, F.A.; Alnaser-Almusa, N.; Mustafa, F.; Ahmed, S.; Alzhrani, A.; Mir, T.A. Recent advances in aptamer-based biosensing technology for isolation and detection of extracellular vesicles. Front. Cell Dev. Biol. 2025, 13, 1555687. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Ding, M.; Wu, K.; Wang, Z.; Wu, S.; Duan, N. Spatially confined multivalent aptamers in the cavity of a DNA nanocage against bacterial superantigens infection. Nano Today 2026, 66, 102899. [Google Scholar] [CrossRef]
- Hayat, M.; Bukhari, S.A.R.; Raza, M.; Rafia; Aslam, A.; Liu, Z. Nanostructured aptasensors for ricin detection and tumor therapy: Exploring aptamer-protein interactions and conformational stability in biological complexities. Int. J. Biol. Macromol. 2025, 310, 143282. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Nguyen, N.H.; Nguyen, D.H.; Nguyen, D.T.C.; Tran, T.V. Green synthesis of ZnFe2O4@ZnO nanocomposites using Chrysanthemum spp. floral waste for photocatalytic dye degradation. J. Environ. Manag. 2023, 326, 116746. [Google Scholar] [CrossRef]
- Qiu, Y.; Yan, J.; Liu, X.; Pang, Y.; Ding, Y.; Lyu, F. A novel g-C3N4-SH@konjac glucomannan composite aerogel for patulin removal from apple juice and its photocatalytic regeneration. Food Chem. 2024, 451, 139421. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two Different Roles of Metallic Ag on Ag/AgX/BiOX (X = Cl, Br) Visible Light Photocatalysts: Surface Plasmon Resonance and Z-Scheme Bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Xia, W.-X.; Zhou, Y.; Zhang, Q.-R.; Chen, X.-B.; Ma, L.; Ding, S.-J. Efficient photothermal conversion and Z-scheme charge transfer in narrow-gap semiconductor heterojunction for photothermal-assisted photocatalysis. J. Environ. Chem. Eng. 2025, 13, 115147. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal nanomaterials: A powerful light-to-heat converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Kuang, L.; Chen, Z.; Yan, Y.; Guo, F.; Shi, W. Research progress of g–C3N4–based materials for photothermal-assisted photocatalysis. Int. J. Hydrogen Energy 2024, 87, 20–49. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Li, Z.; Yin, Z.; Wang, Y.; Gao, S.; Yang, Z.; Qiu, J.; Song, Z. Synergizing piezoelectric field and photothermal effect modulated charge migration behavior in Bi4O5Br2/Bi2S3 S-scheme heterojunction for enhanced near-infrared-light-driven photocatalytic activity. Chem. Eng. J. 2025, 519, 165096. [Google Scholar] [CrossRef]
- Lu, J.; Shan, P.; Chen, F.; Lu, C.; Su, N.; Xiong, B.; Hou, J.; Liu, Z.; Sun, Y.; Shi, W. Construction of a recyclable foam photocatalyst for boosted photothermal-assisted photocatalytic H2 production. J. Alloys Compd. 2025, 1014, 178819. [Google Scholar] [CrossRef]
- Luo, M.; Tian, J.; Liu, S.; Zhang, W. An integrated photothermal-photocatalytic materials for efficient photocatalytic performance boosting by synergistic photothermally. Appl. Surf. Sci. 2022, 593, 153382. [Google Scholar] [CrossRef]
- Sun, D.; Mao, J.; Wei, H.; Zhang, Q.; Cheng, L.; Yang, X.; Li, P. Efficient Prevention of Aspergillus flavus Spores Spread in Air Using Plasmonic Ag-AgCl/α-Fe2O3 under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2022, 14, 28021–28032. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X.; Ge, J. Enzyme Catalyst Engineering toward the Integration of Biocatalysis and Chemocatalysis. Trends Biotechnol. 2021, 39, 1173–1183. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Mulè, G. Mycotoxin Biotransformation by Native and Commercial Enzymes: Present and Future Perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of Immobilized Enzymes in Food Industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef]
- Sha, M.; Xu, W.; Fang, Q.; Wu, Y.; Gu, W.; Zhu, C.; Guo, S. Metal-organic-framework-involved nanobiocatalysis for biomedical applications. Chem. Catal. 2022, 2, 2552–2589. [Google Scholar] [CrossRef]
- Mao, S.; Jiang, J.; Xiong, K.; Chen, Y.; Yao, Y.; Liu, L.; Liu, H.; Li, X. Enzyme engineering: Performance optimization, novel sources, and applications in the food industry. Foods 2024, 13, 3846. [Google Scholar] [CrossRef]
- Wu, W.; Lu, S.; Jiang, S.; Chen, J.; Zheng, Z.; Jiang, S.; Yang, P. Immobilization of recombinant Trametes versicolor aflatoxin B1-degrading enzyme (TV-AFB1D) with montmorillonite for absorption and in situ degradation of aflatoxin B1. Mycotoxin Res. 2024, 40, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Lu, Y.; Chen, L.; He, M.; Deng, Z. Design, Synthesis, and Application of Immobilized Enzymes on Artificial Porous Materials. Adv. Sci. 2025, 12, 2500345. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Dong, X.; Zhao, Q.; Du, G.; Guo, Q.; Yuan, Y.; Yue, T. Continuous flow removal of patulin by cysteine and porcine pancreatic lipase-modified hierarchical mesoporous zirconium metal–organic framework aerogel for apple juice treatment. Chem. Eng. J. 2023, 475, 146472. [Google Scholar] [CrossRef]
- Wang, S.; Guo, T.; Mei, X.; Zhong, X.; Gao, L.; Cai, R.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Immobilization of pancreatin based on ultrasound-assisted polydopamine functionalized magnetic porous chitosan for the detoxification of ochratoxin A in wine. Food Chem. 2024, 451, 139496. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhao, Q.; Gurusamy, S.; Lu, Y.; Chen, X.; Yang, Q.; Zeng, K.; Li, Y.; Liu, X.; et al. Immobilization of aldo-keto reductase on dopamine/polyethyleneimine functionalized magnetic cellulose nanocrystals to enhance the detoxification of patulin in fresh pear juice. Int. J. Biol. Macromol. 2024, 278, 134689. [Google Scholar] [CrossRef]
- Lu, T.; Fu, C.; Xiong, Y.; Zeng, Z.; Fan, Y.; Dai, X.; Huang, X.; Ge, J.; Li, X. Biodegradation of Aflatoxin B1 in Peanut Oil by an Amphipathic Laccase–Inorganic Hybrid Nanoflower. J. Agric. Food Chem. 2023, 71, 3876–3884. [Google Scholar] [CrossRef]
- Zhou, C.; He, N.; Lin, X.; Liu, H.; Lu, Z.; Zhang, G. Site-directed display of zearalenone lactonase on spilt-intein functionalized nanocarrier for green and efficient detoxification of zearalenone. Food Chem. 2024, 446, 138804. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.A.; Ladeira, Y.F.; França, A.D.; Souza, R.O.; Moraes, A.H.; Wojcieszak, R.; Itabaiana, I.; Miranda, A.S. Multicatalytic Hybrid Materials for Biocatalytic and Chemoenzymatic Cascades—Strategies for Multicatalyst (Enzyme) Co-Immobilization. Catalysts 2021, 11, 936. [Google Scholar] [CrossRef]
- Chen, T.; Lu, Y.; Xiong, X.; Xu, Z. Co-immobilization of enzymes and chemocatalysts for one-pot chemoenzymatic cascades: Scaffold engineering toward more efficient catalysis. Chem. Catal. 2024, 4, 100894. [Google Scholar] [CrossRef]
- Fu, C.; Hou, L.; Chen, D.; Huang, T.; Yin, S.; Ding, P.; Liao, Q.; Huang, X.; Xiong, Y.; Ge, J.; et al. Targeted Detoxification of Aflatoxin B1 in Edible Oil by an Enzyme–Metal Nanoreactor. J. Agric. Food Chem. 2024, 72, 5966–5974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).