Deoxynivalenol and Its Metabolites: Contamination, Metabolism, and Toxicity
Abstract
1. Introduction
2. Contamination in Food and Feed
2.1. Regulatory Maximum Limits for DON in Food and Feed
2.2. DON Contamination
2.3. Transmission in the Food Chain
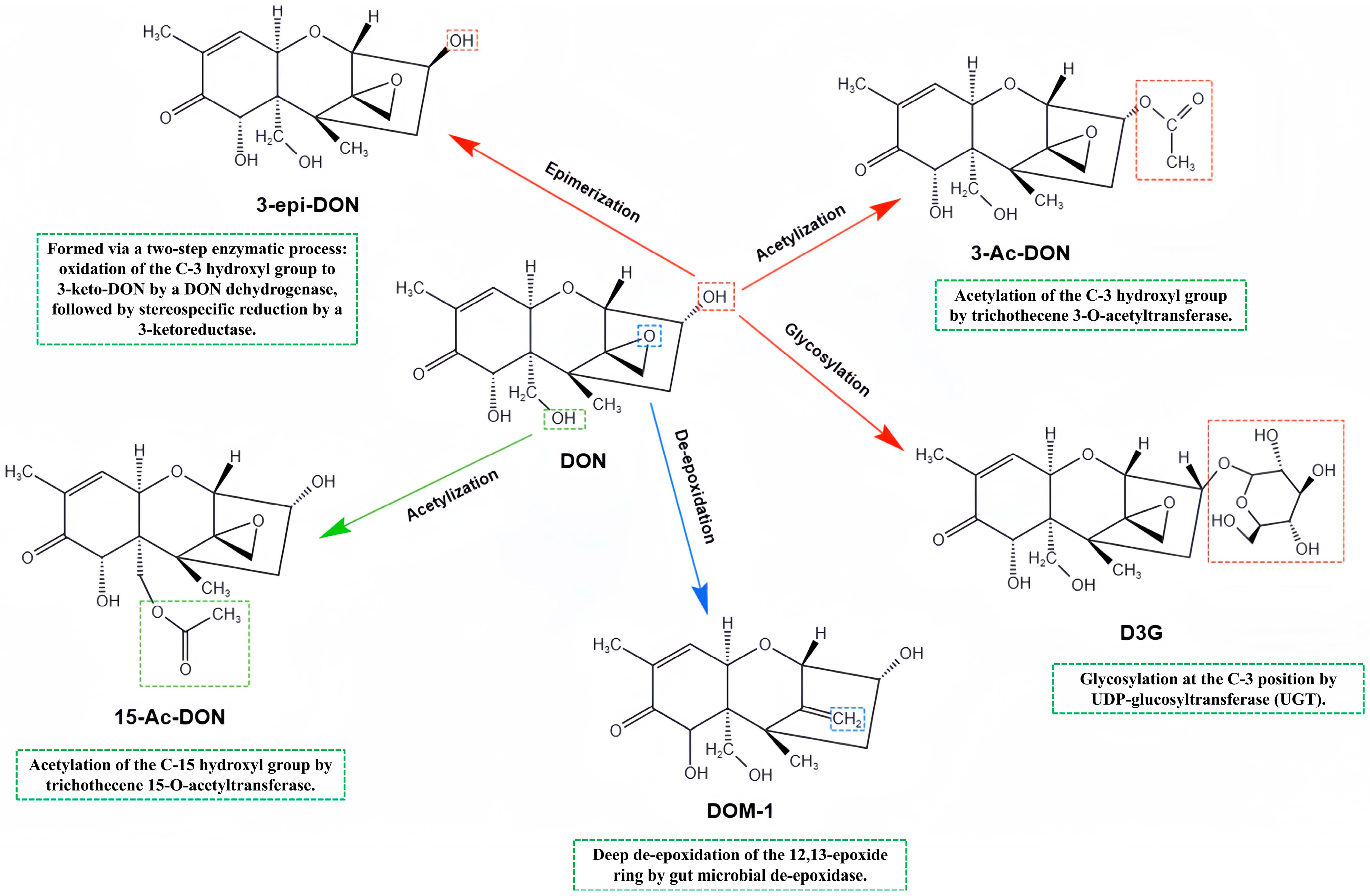
3. Biotransformation and Metabolites of DON
3.1. Secondary Metabolites of DON
3.2. Metabolism of DON in the Organism
3.2.1. Humans
3.2.2. Monogastric Animals
3.2.3. Ruminants
4. Effects of DON on the Health of the Organism
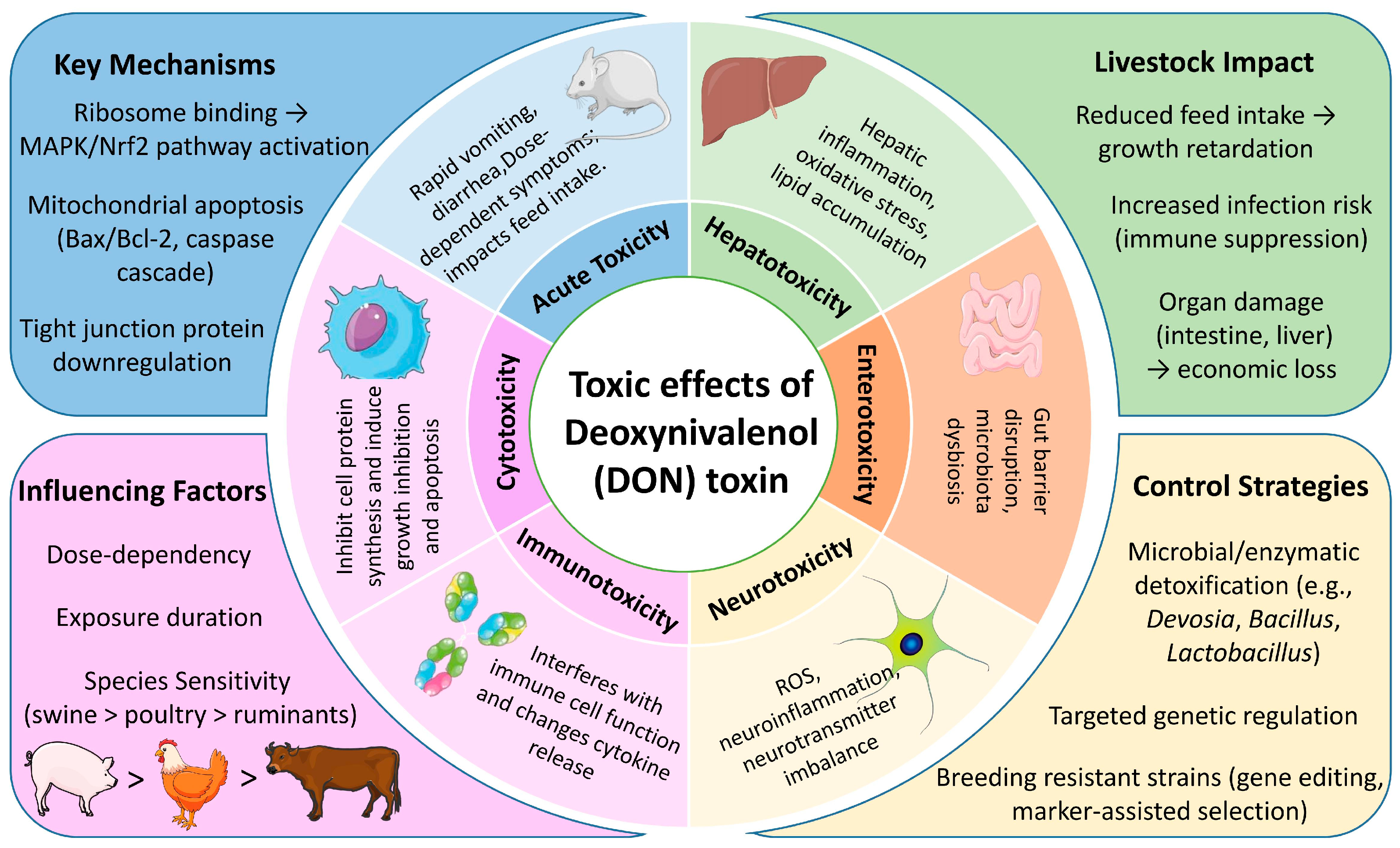
4.1. Toxic Effects of DON
4.1.1. Acute Toxicity
4.1.2. Cytotoxicity
4.1.3. Immunotoxicity
4.1.4. Neurotoxicity
4.1.5. Enterotoxicity
4.1.6. Hepatotoxicity
4.2. Effects on Biochemical Indicators
4.3. Reproductive Effects
5. Strategies for DON Contamination Control: Biodegradation and Genetic Regulation
5.1. Biodegradation Technology
5.2. Gene Regulation Technology
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Kumar Rath, S. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2019, 60, 1346–1374. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Sun, C.; Guo, X.; Yang, T.; Wang, H.; Fu, S.; Li, C.; Yang, H. A rapid Raman detection of deoxynivalenol in agricultural products. Food Chem. 2017, 221, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Duan, J.; Ren, J.; Francis, F.; Li, G. Isolation and Characterization of Two New Deoxynivalenol-Degrading Strains, Bacillus sp. HN117 and Bacillus sp. N22. Toxins 2022, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ren, Z.; Xu, C.; Wang, H.; Wu, Z.; Rehman, Z.U.; Wu, S.; Sun, M.A.; Bao, W. Chromatin Accessibility and Transcriptomic Alterations in Murine Ovarian Granulosa Cells upon Deoxynivalenol Exposure. Cells 2021, 10, 2818. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Lancova, K.; Hajslova, J.; Kostelanska, M.; Kohoutkova, J.; Nedelnik, J.; Moravcova, H.; Vanova, M. Fate of trichothecene mycotoxins during the processing: Milling and baking. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 650–659. [Google Scholar] [CrossRef]
- Wu, Q.; Kuča, K.; Humpf, H.U.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2017, 33, 79–91. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B. Fates of deoxynivalenol and deoxynivalenol-3-glucoside during bread and noodle processing. Food Control 2015, 50, 754–757. [Google Scholar] [CrossRef]
- Wolf, C.E.; Bullerman, L.B. Heat and pH alter the concentration of deoxynivalenol in an aqueous environment. J. Food Prot. 1998, 61, 365–367. [Google Scholar] [CrossRef]
- Voss, K.A.; Snook, M.E. Stability of the mycotoxin deoxynivalenol (DON) during the production of flour-based foods and wheat flake cereal. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1694–1700. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef]
- Vanhoutte, I.; De Tender, C.; Demeyere, K.; Abdallah, M.F.; Ommeslag, S.; Vermeir, P.; Saeger, S.; Debode, J.; Meyer, E.; Croubels, S.; et al. Bacterial Enrichment Cultures Biotransform the Mycotoxin Deoxynivalenol into a Novel Metabolite Toxic to Plant and Porcine Cells. Toxins 2021, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Li, Y.H.; Lin, M.F. Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens. Toxins 2017, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Qiu, Z.; Wang, L.; Luo, Y.; He, W.; Yang, J. Possible Toxic Mechanisms of Deoxynivalenol (DON) Exposure to Intestinal Barrier Damage and Dysbiosis of the Gut Microbiota in Laying Hens. Toxins 2022, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Li, K.; Zhang, C.; Tian, H.; Luo, Y. Investigation of Deoxynivalenol Contamination in Local Area and Evaluation of Its Multiple Intestinal Toxicity. Toxins 2024, 16, 353. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Gerez, J.R.; Cossalter, A.M.; Neves, M.; Laffitte, J.; Naylies, C.; Lippi, Y.; Kolf-Clauw, M.; Bracarense, A.P.L.; Pinton, P.; et al. Intestinal toxicity of the type B trichothecene mycotoxin fusarenon-X: Whole transcriptome profiling reveals new signaling pathways. Sci. Rep. 2017, 7, 7530. [Google Scholar] [CrossRef]
- Xue, R.; Li, S.; Zou, H.; Ji, D.; Lv, M.; Zhou, P.; Wei, Z.; Zhang, Z.; Cao, Y. Melatonin alleviates deoxynivalenol-induced apoptosis of human granulosa cells by reducing mutually accentuated FOXO1 and ER stress. Biol. Reprod. 2021, 105, 554–566. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.T.; Xie, W.M.; Zhang, N.Y.; Dai, J.F.; Wang, Y.; Rajput, S.A.; Qi, D.S.; et al. Individual and Combined Occurrence of Mycotoxins in Feed Ingredients and Complete Feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef]
- Cai, Y.T.; McLaughlin, M.; Zhang, K. Advancing the FDA/Office of Regulatory Affairs Mycotoxin Program: New Analytical Method Approaches to Addressing Needs and Challenges. J. AOAC Int. 2020, 103, 705–709. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ’FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Teixido-Orries, I.; Molino, F.; Femenias, A.; Ramos, A.J.; Marín, S. Quantification and classification of deoxynivalenol-contaminated oat samples by near-infrared hyperspectral imaging. Food Chem. 2023, 417, 135924. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Cheng, T.X.; Xu, W.J.; Han, X.M.; Zhang, J.; Zhang, H.Y.; Wang, C.; Fanning, S.; Li, F.Q. Natural co-occurrence of multi-mycotoxins in unprocessed wheat grains from China. Food Control 2021, 130, 108321. [Google Scholar] [CrossRef]
- Xu, A.; Yu, S.; Li, Y.; Liu, H.; Yan, Z.; Wu, A.; Peng, S.; Liu, N. Total Deoxynivalenol Contamination of Wheat Products and Coarse Grains in Shanghai, China: Occurrence and Health Risk Assessment. Foods 2024, 13, 3373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. 2016, 197, 1271–1274. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Zhou, J.; Chen, J.; Li, W.; Zheng, F. Mycotoxins in wheat flour and intake assessment in Shandong province of China. Food Addit. Contam. Part B Surveill. 2016, 9, 170–175. [Google Scholar] [CrossRef]
- Birr, T.; Jensen, T.; Preusske, N.; Sonnichsen, F.D.; De Boevre, M.; De Saeger, S.; Hasler, M.; Verreet, J.A.; Klink, H. Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins 2021, 13, 110. [Google Scholar] [CrossRef]
- Twarużek, M.; Skrzydlewski, P.; Kosicki, R.; Grajewski, J. Mycotoxins survey in feed materials and feedingstuffs in years 2015-2020. Toxicon 2021, 202, 27–39. [Google Scholar] [CrossRef]
- Pack, E.D.; Weiland, S.; Musser, R.; Schmale, D.G. Survey of zearalenone and type-B trichothecene mycotoxins in swine feed in the USA. Mycotoxin Res. 2021, 37, 297–313. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Silva, M.V.; Pante, G.C.; Romoli, J.C.Z.; de Souza, A.P.M.; Rocha, G.; Ferreira, F.D.; Feijó, A.L.R.; Moscardi, S.M.P.; de Paula, K.R.; Bando, E.; et al. Occurrence and risk assessment of population exposed to deoxynivalenol in foods derived from wheat flour in Brazil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 546–554. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, L.; Chen, Y.; Gao, H.; Hua, Y.; Yuan, X.; Yang, H. Mycotoxins in Maize Silage from China in 2019. Toxins 2022, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, M.; Jara, H.T.; Suchy, S.; Drochner, W.; Müller, H.M. Fusarium toxins in wheat flour collected in an area in southwest Germany. Int. J. Food Microbiol. 2002, 72, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Trucksess, M.W.; Ready, D.W.; Pender, M.K.; Ligmond, C.A.; Wood, G.E.; Page, S.W. Determination and survey of deoxynivalenol in white flour, whole wheat flour, and bran. J. AOAC Int. 1996, 79, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Gauchi, J.P.; Sanchis, V.; Marin, S.; Ramos, A.J. Quantitative dietary exposure assessment of the Catalonian population (Spain) to the mycotoxin deoxynivalenol. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Okorski, A.; Milewska, A.; Pszczolkowska, A.; Karpiesiuk, K.; Kozera, W.; Dabrowska, J.A.; Radwinska, J. Prevalence of Fusarium fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef]
- Pei, P.; Xiong, K.; Wang, X.; Sun, B.; Zhao, Z.; Zhang, X.; Yu, J. Predictive growth kinetic parameters and modelled probabilities of deoxynivalenol production by Fusarium graminearum on wheat during simulated storing conditions. J. Appl. Microbiol. 2022, 133, 349–361. [Google Scholar] [CrossRef]
- Thammawong, M.; Okadome, H.; Shiina, T.; Nakagawa, H.; Nagashima, H.; Nakajima, T.; Kushiro, M. Distinct distribution of deoxynivalenol, nivalenol, and ergosterol in Fusarium-infected Japanese soft red winter wheat milling fractions. Mycopathologia 2011, 172, 323–330. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, T.; Shi, J.; Li, X.; Wu, K.; Xiong, Y. Identification of Deoxynivalenol and Degradation Products during Maize Germ Oil Refining Process. Foods 2022, 11, 1720. [Google Scholar] [CrossRef]
- Song, X.; Qiao, L.; Chang, J.; Dou, X.; Zhang, X.; Pi, S.; Xu, C. Dietary supplementation with selenium nanoparticles-enriched Lactobacillus casei ATCC 393 alleviates intestinal barrier dysfunction of mice exposed to deoxynivalenol by regulating endoplasmic reticulum stress and gut microbiota. Ecotoxicol. Environ. Saf. 2022, 248, 114276. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Xu, S.; Meng, Z.; Li, D.; Zhou, X.; Zhang, R.; Shi, S.; Hao, L.; Liu, L.; et al. Deoxynivalenol Exposure Induced Colon Damage in Mice Independent of the Gut Microbiota. Mol. Nutr. Food Res. 2023, 67, e2300317. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Wang, L.; Li, X.; Nawaz, M.Y.; Saleemi, M.K.; Khatoon, A.; Yongping, X. Recalling the reported toxicity assessment of deoxynivalenol, mitigating strategies and its toxicity mechanisms: Comprehensive review. Chem. Biol. Interact. 2024, 387, 110799. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Luo, J.Y.; Ruan, H.N.; Wang, C.J.; Yang, M.H. Structure-toxicity relationships, toxicity mechanisms and health risk assessment of food-borne modified deoxynivalenol and zearalenone: A comprehensive review. Sci. Total Environ. 2022, 806, 151192. [Google Scholar] [CrossRef] [PubMed]
- Feizollahi, E.; Roopesh, M.S. Mechanisms of deoxynivalenol (DON) degradation during different treatments: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5903–5924. [Google Scholar] [CrossRef]
- Li, F.; Jin, J.; Rietjens, I.; Xing, F. Interindividual Differences in In Vitro Human Intestinal Microbial Conversion of 3-Acetyl-DON and 15-Acetyl-DON. Toxins 2022, 14, 199. [Google Scholar] [CrossRef]
- Li, K.; Wang, L.; Yu, D.; Yan, Z.; Liu, N.; Wu, A. Cellobiose inhibits the release of deoxynivalenol from transformed deoxynivalenol-3-glucoside from Lactiplantibacillus plantarum. Food Chem. 2022, 4, 100077. [Google Scholar] [CrossRef]
- Ruhnau, D.; Hess, C.; Doupovec, B.; Grenier, B.; Schatzmayr, D.; Hess, M.; Awad, W. Deepoxy-deoxynivalenol (DOM-1), a derivate of deoxynivalenol (DON), exhibits less toxicity on intestinal barrier function, Campylobacter jejuni colonization and translocation in broiler chickens. Gut Pathog. 2021, 13, 44. [Google Scholar] [CrossRef]
- Li, X.Z.; Hassan, Y.I.; Lepp, D.; Zhu, Y.; Zhou, T. 3-keto-DON, but Not 3-epi-DON, Retains the in Planta Toxicological Potential after the Enzymatic Biotransformation of Deoxynivalenol. Int. J. Mol. Sci. 2022, 23, 7230. [Google Scholar] [CrossRef]
- Wu, L.; Wang, B. Transformation of deoxynivalenol and its acetylated derivatives in Chinese steamed bread making, as affected by pH, yeast, and steaming time. Food Chem. 2016, 202, 149–155. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, J.; Zhang, Y.; Lu, M.B.; Sun, L.J.; Li, W.X.; Hu, X.X.; Wang, B.J. Retention of deoxynivalenol and its derivatives during storage of wheat grain and flour. Food Control 2016, 65, 177–181. [Google Scholar] [CrossRef]
- Bryla, M.; Ksieniewicz-Wozniak, E.; Waskiewicz, A.; Szymczyk, K.; Jedrzejczak, R. Co-occurrence of nivalenol, deoxynivalenol and deoxynivalenol-3-glucoside in beer samples. Food Control 2018, 92, 319–324. [Google Scholar] [CrossRef]
- Guerrero-Netro, H.M.; Barreta, M.H.; Costa, E.; Goetten, A.; Dupras, R.; Mills, L.; Koch, J.; Portela, V.M.; Price, C.A.; Chorfi, Y. Effects of the mycotoxin metabolite de-epoxy-deoxynivalenol (DOM-1) on embryo development and sperm motility in cattle. J. Appl. Toxicol. 2021, 41, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Jiang, C.; Wu, W.; Pang, B.; Lei, S.; Lian, Z.; Shao, D.; Jin, M.; Shi, J. Conversion of DON to 3-epi-DON in vitro and toxicity reduction of DON in vivo by Lactobacillus rhamnosus. Food Funct. 2019, 10, 2785–2796. [Google Scholar] [CrossRef] [PubMed]
- Springler, A.; Hessenberger, S.; Reisinger, N.; Kern, C.; Nagl, V.; Schatzmayr, G.; Mayer, E. Deoxynivalenol and its metabolite deepoxy-deoxynivalenol: Multi-parameter analysis for the evaluation of cytotoxicity and cellular effects. Mycotoxin Res. 2017, 33, 25–37. [Google Scholar] [CrossRef]
- Mengelers, M.; Zeilmaker, M.; Vidal, A.; De Boevre, M.; De Saeger, S.; Hoogenveen, R. Biomonitoring of Deoxynivalenol and Deoxynivalenol-3-glucoside in Human Volunteers: Renal Excretion Profiles. Toxins 2019, 11, 466. [Google Scholar] [CrossRef]
- Chen, L.; Yu, M.; Wu, Q.; Peng, Z.; Wang, D.; Kuča, K.; Yao, P.; Yan, H.; Nüssler, A.K.; Liu, L.; et al. Gender and geographical variability in the exposure pattern and metabolism of deoxynivalenol in humans: A review. J. Appl. Toxicol. 2017, 37, 60–70. [Google Scholar] [CrossRef]
- Gratz, S.W.; Duncan, G.; Richardson, A.J. The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary deepoxy-deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1821–1825. [Google Scholar] [CrossRef]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef]
- Deng, C.; Li, C.; Zhou, S.; Wang, X.; Xu, H.; Wang, D.; Gong, Y.Y.; Routledge, M.N.; Zhao, Y.; Wu, Y. Risk assessment of deoxynivalenol in high-risk area of China by human biomonitoring using an improved high throughput UPLC-MS/MS method. Sci. Rep. 2018, 8, 3901. [Google Scholar] [CrossRef]
- Gerding, J.; Cramer, B.; Humpf, H.U. Determination of mycotoxin exposure in Germany using an LC-MS/MS multibiomarker approach. Mol. Nutr. Food Res. 2014, 58, 2358–2368. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Wells, L.; Hardie, L.; Williams, C.; White, K.; Liu, Y.; De Santis, B.; Debegnach, F.; Moretti, G.; Greetham, S.; Brera, C.; et al. Deoxynivalenol Biomarkers in the Urine of UK Vegetarians. Toxins 2017, 9, 196. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Miano, B.; Moretti, G.; Sonego, E.; Chiaretti, A.; Buonsenso, D.; Brera, C. Determination of Deoxynivalenol Biomarkers in Italian Urine Samples. Toxins 2019, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; De Boevre, M.; De Saeger, S.; et al. Deoxynivalenol exposure assessment through a modelling approach of food intake and biomonitoring data—A contribution to the risk assessment of an enteropathogenic mycotoxin. Food Res. Int. 2021, 140, 109863. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Döll, S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 2004, 58, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for Exposure as A Tool for Efficacy Testing of A Mycotoxin Detoxifier in Broiler Chickens and Pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef]
- Dänicke, S.; Brüssow, K.P.; Valenta, H.; Ueberschär, K.H.; Tiemann, U.; Schollenberger, M. On the effects of graded levels of Fusarium toxin contaminated wheat in diets for gilts on feed intake, growth performance and metabolism of deoxynivalenol and zearalenone. Mol. Nutr. Food Res. 2005, 49, 932–943. [Google Scholar] [CrossRef]
- Goyarts, T.; Dänicke, S. Effects of deoxynivalenol (DON) on growth performance, nutrient digestibility and DON metabolism in pigs. Mycotoxin Res. 2005, 21, 139–142. [Google Scholar] [CrossRef]
- Panisson, J.C.; Wellington, M.O.; Bosompem, M.A.; Nagl, V.; Schwartz-Zimmermann, H.E.; Columbus, D.A. Urinary and Serum Concentration of Deoxynivalenol (DON) and DON Metabolites as an Indicator of DON Contamination in Swine Diets. Toxins 2023, 15, 120. [Google Scholar] [CrossRef]
- Riahi, I.; Marquis, V.; Pérez-Vendrell, A.M.; Brufau, J.; Esteve-Garcia, E.; Ramos, A.J. Effects of Deoxynivalenol-Contaminated Diets on Metabolic and Immunological Parameters in Broiler Chickens. Animals 2021, 11, 147. [Google Scholar] [CrossRef]
- Schwartz-Zimmermann, H.E.; Fruhmann, P.; Dänicke, S.; Wiesenberger, G.; Caha, S.; Weber, J.; Berthiller, F. Metabolism of deoxynivalenol and deepoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins 2015, 7, 4706–4729. [Google Scholar] [CrossRef]
- Yunus, A.W.; Blajet-Kosicka, A.; Kosicki, R.; Khan, M.Z.; Rehman, H.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult. Sci. 2012, 91, 852–861. [Google Scholar] [CrossRef]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Debevere, S.; Cools, A.; Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity. Toxins 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.N.; Zhao, Z.K.; Wang, Z.Q.; Li, S.Z.; Zhang, Y.P.; Sun, Z.; Qin, G.X.; Zhang, X.F.; Zhao, W.; Aschalew, N.D.; et al. Impact of deoxynivalenol on rumen function, production, and health of dairy cows: Insights from metabolomics and microbiota analysis. J. Hazard. Mater. 2024, 465, 133376. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; You, L.; Wang, X.; Wu, W.; Kuca, K.; Wu, Q.; Wei, W. Deoxynivalenol: Emerging Toxic Mechanisms and Control Strategies, Current and Future Perspectives. J. Agric. Food Chem. 2023, 71, 10901–10915. [Google Scholar] [CrossRef]
- Aihara, R.; Ookawara, T.; Morimoto, A.; Iwashita, N.; Takagi, Y.; Miyasaka, A.; Kushiro, M.; Miyake, S.; Fukuyama, T. Acute and subacute oral administration of mycotoxin deoxynivalenol exacerbates the pro-inflammatory and pro-pruritic responses in a mouse model of allergic dermatitis. Arch. Toxicol. 2020, 94, 4197–4207. [Google Scholar] [CrossRef]
- Seyed Toutounchi, N.; Braber, S.; Van’t Land, B.; Thijssen, S.; Garssen, J.; Kraneveld, A.D.; Folkerts, G.; Hogenkamp, A. Exposure to Deoxynivalenol During Pregnancy and Lactation Enhances Food Allergy and Reduces Vaccine Responsiveness in the Offspring in a Mouse Model. Front. Immunol. 2021, 12, 797152. [Google Scholar] [CrossRef]
- Gonya, S.; Kallmerten, P.; Dinapoli, P. Are Infants and Children at Risk of Adverse Health Effects from Dietary Deoxynivalenol Exposure? An Integrative Review. Int. J. Environ. Res. Public Health 2024, 21, 808. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Yue, J.; Guo, D.; Gao, X.; Wang, J.; Nepovimova, E.; Wu, W.; Kuca, K. Deoxynivalenol (Vomitoxin)-Induced Anorexia Is Induced by the Release of Intestinal Hormones in Mice. Toxins 2021, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, D.M.; Yoshizawa, T.; Morooka, N.; Tuite, J. Emetic and refusal activity of deoxynivalenol to swine. Appl. Environ. Microbiol. 1977, 34, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Lafleur Lariviere, E.; Zhu, C.; Sharma, A.; Karrow, N.A.; Huber, L.A. The effects of deoxynivalenol-contaminated corn in low-complexity diets supplemented with either an immune-modulating feed additive, or fish oil on nursery pig growth performance, immune response, small intestinal morphology, and component digestibility. Transl. Anim. Sci. 2022, 6, txac068. [Google Scholar] [CrossRef]
- Hooft, J.M.; Bureau, D.P. Deoxynivalenol: Mechanisms of action and its effects on various terrestrial and aquatic species. Food Chem. Toxicol. 2021, 157, 112616. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Zentek, J. Cytotoxicity and metabolic stress induced by deoxynivalenol in the porcine intestinal IPEC-J2 cell line. J. Anim. Physiol. Anim. Nutr. 2012, 96, 709–716. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Wu, N.; Li, J.; Xi, N.; Xu, M.; Wu, F.; Fu, Q.; Yan, G.; Liu, Y.; et al. Chlorogenic acid attenuates deoxynivalenol-induced apoptosis and pyroptosis in human keratinocytes via activating Nrf2/HO-1 and inhibiting MAPK/NF-κB/NLRP3 pathways. Biomed. Pharmacother. 2024, 170, 116003. [Google Scholar] [CrossRef]
- Singh, S.; Banerjee, S.; Chattopadhyay, P.; Borthakur, S.K.; Veer, V. Deoxynivalenol induces cytotoxicity and genotoxicity in animal primary cell culture. Toxicol. Mech. Methods 2015, 25, 184–191. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, H.R.; Pan, X.; Pestka, J.J. Comparison of Anorectic Potencies of the Trichothecenes T-2 Toxin, HT-2 Toxin and Satratoxin G to the Ipecac Alkaloid Emetine. Toxicol. Rep. 2015, 2, 238–251. [Google Scholar] [CrossRef]
- Doi, K.; Uetsuka, K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.S.; Roux, J.; Mounien, L.; Dallaporta, M.; Troadec, J.D. Advances in deoxynivalenol toxicity mechanisms: The brain as a target. Toxins 2012, 4, 1120–1138. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Li, X.; Zhao, C.; Gao, J.; Yuan, W.; Zhang, J. The Degradation of Deoxynivalenol by Using Electrochemical Oxidation with Graphite Electrodes and the Toxicity Assessment of Degradation Products. Toxins 2019, 11, 478. [Google Scholar] [CrossRef]
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Zhu, L.; Xu, W.; Chu, X.; Zhang, Y.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; et al. Deoxynivalenol Induces Caspase-8-Mediated Apoptosis through the Mitochondrial Pathway in Hippocampal Nerve Cells of Piglet. Toxins 2021, 13, 73. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Cao, L.; Zhu, L.; Zhang, Y.; Chu, X.; Zhu, D.; Rahman, S.U.; Peng, C.; Feng, S.; et al. Mechanism of deoxynivalenol-induced neurotoxicity in weaned piglets is linked to lipid peroxidation, dampened neurotransmitter levels, and interference with calcium signaling. Ecotoxicol. Environ. Saf. 2020, 194, 110382. [Google Scholar] [CrossRef]
- Faeste, C.K.; Solhaug, A.; Gaborit, M.; Pierre, F.; Massotte, D. Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures. Toxins 2022, 14, 48. [Google Scholar] [CrossRef]
- Mishra, S.; Kapoor, R.; Sushma; Kanchan, S.; Jha, G.; Sharma, D.; Tomar, B.; Rath, S.K. Deoxynivalenol Induces Drp-1-Mediated Mitochondrial Dysfunction via Elevating Oxidative Stress. Chem. Res. Toxicol. 2024, 37, 1139–1154. [Google Scholar] [CrossRef]
- Jacobson, M. On the Infectious Causes of Neonatal Piglet Diarrhoea—A Review. Vet. Sci. 2022, 9, 422. [Google Scholar] [CrossRef]
- Jia, B.; Lin, H.; Yu, S.; Liu, N.; Yu, D.; Wu, A. Mycotoxin deoxynivalenol-induced intestinal flora disorders, dysfunction and organ damage in broilers and pigs. J. Hazard. Mater. 2023, 451, 131172. [Google Scholar] [CrossRef] [PubMed]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Chu, X.H.; Ma, R.; Wang, Y.W.; Liu, Q.; Zhang, N.Y.; Karrow, N.A.; Sun, L.H. Effects of deoxynivalenol on the porcine growth performance and intestinal microbiota and potential remediation by a modified HSCAS binder. Food Chem Toxicol. 2020, 141, 111373. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, L.; Wang, P.; Liu, Q.; Wang, J. The Effects of Deoxynivalenol on the Ultrastructure of the Sacculus Rotundus and Vermiform Appendix, as Well as the Intestinal Microbiota of Weaned Rabbits. Toxins 2020, 12, 569. [Google Scholar] [CrossRef]
- Li, D.; Ma, H.; Ye, Y.; Ji, C.; Tang, X.; Ouyang, D.; Chen, J.; Li, Y.; Ma, Y. Deoxynivalenol induces apoptosis in mouse thymic epithelial cells through mitochondria-mediated pathway. Environ. Toxicol. Pharmacol. 2014, 38, 163–171. [Google Scholar] [CrossRef]
- Cheat, S.; Pinton, P.; Cossalter, A.M.; Cognie, J.; Vilariño, M.; Callu, P.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. The mycotoxins deoxynivalenol and nivalenol show in vivo synergism on jejunum enterocytes apoptosis. Food Chem. Toxicol. 2016, 87, 45–54. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajecka, M.; Gajecki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Moon, Y.; Kim, H.K.; Suh, H.; Chung, D.H. Toxic alterations in chick embryonic liver and spleen by acute exposure to Fusarium-producing mycotoxin deoxynivalenol. Biol. Pharm. Bull. 2007, 30, 1808–1812. [Google Scholar] [CrossRef]
- Hasuda, A.L.; Person, E.; Khoshal, A.K.; Bruel, S.; Puel, S.; Oswald, I.P.; Bracarense, A.; Pinton, P. Deoxynivalenol induces apoptosis and inflammation in the liver: Analysis using precision-cut liver slices. Food Chem. Toxicol. 2022, 163, 112930. [Google Scholar] [CrossRef]
- Bracarense, A.P.F.L.; Basso, K.M.; Da Silva, E.O.; Payros, D.; Oswald, I.P. Deoxynivalenol in the liver and lymphoid organs of rats: Effects of dose and duration on immunohistological changes. World Mycotoxin J. 2017, 10, 89–96. [Google Scholar] [CrossRef]
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A., Jr.; Bracarense, A. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon 2020, 185, 203–212. [Google Scholar] [CrossRef]
- Zielonka, L.; Gajecka, M.; Tarasiuk, M.; Gajecki, M. The effects of dietary deoxynivalenol (DON) on selected blood biochemical and hematological parameters in pre-pubertal gilts. Pol. J. Vet. Sci. 2015, 18, 223–231. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)-challenged growing pigs. BMC Vet. Res. 2015, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wang, W.; Wang, L.; Shi, J.; Cheng, J.; Zhang, J.; Li, A.; He, B.; Fan, Z. Effects of Deoxynivalenol Detoxifier on Growth Performance, Blood Biochemical Indices, and Microbiota Composition of Piglets. Int. J. Mol. Sci. 2025, 26, 2045. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Zebeli, Q.; Bohm, J. Deoxynivalenol in chicken feed alters the vaccinal immune response and clinical biochemical serum parameters but not the intestinal and carcass characteristics. J. Anim. Physiol. Anim. Nutr. 2016, 100, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Riahi, I.; Marquis, V.; Ramos, A.J.; Brufau, J.; Esteve-Garcia, E.; Perez-Vendrell, A.M. Effects of Deoxynivalenol-Contaminated Diets on Productive, Morphological, and Physiological Indicators in Broiler Chickens. Animals 2020, 10, 1795. [Google Scholar] [CrossRef]
- Bernhoft, A.; Hogasen, H.R.; Rosenlund, G.; Moldal, T.; Grove, S.; Berntssen, M.H.G.; Thoresen, S.I.; Alexander, J. Effects of dietary deoxynivalenol or ochratoxin A on performance and selected health indices in Atlantic salmon (Salmo salar). Food Chem. Toxicol. 2018, 121, 374–386. [Google Scholar] [CrossRef]
- Tola, S.; Bureau, D.P.; Hooft, J.M.; Beamish, F.W.; Sulyok, M.; Krska, R.; Encarnacao, P.; Petkam, R. Effects of Wheat Naturally Contaminated with Fusarium Mycotoxins on Growth Performance and Selected Health Indices of Red Tilapia (Oreochromis niloticus × O. mossambicus). Toxins 2015, 7, 1929–1944. [Google Scholar] [CrossRef]
- Matejova, I.; Modra, H.; Blahova, J.; Franc, A.; Fictum, P.; Sevcikova, M.; Svobodova, Z. The effect of mycotoxin deoxynivalenol on haematological and biochemical indicators and histopathological changes in rainbow trout (Oncorhynchus mykiss). Biomed. Res. Int. 2014, 2014, 310680. [Google Scholar] [CrossRef]
- Sprando, R.L.; Collins, T.F.; Black, T.N.; Olejnik, N.; Rorie, J.I.; Eppley, R.M.; Ruggles, D.I. Characterization of the effect of deoxynivalenol on selected male reproductive endpoints. Food Chem. Toxicol. 2005, 43, 623–635. [Google Scholar] [CrossRef]
- Ruan, Y.B.; Liu, X.H.; Jiang, J.Z.; Nie, T.; Ma, J. Leydig cells pyroptosis in testis mediates deoxynivalenol-induced male reproductive toxicity in mice. Sci. Total Environ. 2024, 954, 176432. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, W.; Sun, Y.; Li, Y. Deoxynivalenol induced spermatogenesis disorder by blood-testis barrier disruption associated with testosterone deficiency and inflammation in mice. Environ. Pollut. 2020, 264, 114748. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R.; Desto, S.S.; Bracarense, A. Deoxynivalenol induces toxic effects in the ovaries of pigs: An ex vivo approach. Theriogenology 2017, 90, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lemos, G.A.A.; Gerez, J.R.; Costa, J.B.; Venâncio, E.J.; Souza, M.; Favaron, P.O.; Greghi, J.R.; Gloria, E.M.; Staurengo-Ferrari, L.; Verri, W.A.; et al. Deoxynivalenol induces ovarian damage and uterine changes in prepubertal and adult mice. Toxicon 2024, 251, 108123. [Google Scholar] [CrossRef]
- Toutounchi, N.S.; Braber, S.; Land, B.V.; Thijssen, S.; Garssen, J.; Folkerts, G.; Hogenkamp, A. Deoxynivalenol exposure during pregnancy has adverse effects on placental structure and immunity in mice model. Reprod. Toxicol. 2022, 112, 109–118. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Ji, F.; Xu, L.; Yu, M.; Shi, J.; Xu, J. Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 2019, 276, 436–442. [Google Scholar] [CrossRef]
- Yao, J.; Chen, S.; Li, Y.; Liao, C.; Shang, K.; Guo, R.; Chen, J.; Wang, L.; Xia, X.; Yu, Z.; et al. Unveiling a Novel Antidote for Deoxynivalenol Contamination: Isolation, Identification, Whole Genome Analysis and In Vivo Safety Evaluation of Lactobacillus rhamnosus MY-1. Foods 2024, 13, 2057. [Google Scholar] [CrossRef]
- He, C.; Fan, Y.; Liu, G.; Zhang, H. Isolation and identification of a strain of Aspergillus tubingensis with deoxynivalenol biotransformation capability. Int. J. Mol. Sci. 2008, 9, 2366–2375. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Pinton, P.; Hupé, J.F.; Neves, M.; Lippi, Y.; Combes, S.; Castex, M.; Oswald, I.P. Saccharomyces cerevisiae Boulardii Reduces the Deoxynivalenol-Induced Alteration of the Intestinal Transcriptome. Toxins 2018, 10, 199. [Google Scholar] [CrossRef]
- Hassan, Y.I.; He, J.W.; Perilla, N.; Tang, K.; Karlovsky, P.; Zhou, T. The enzymatic epimerization of deoxynivalenol by Devosia mutans proceeds through the formation of 3-keto-DON intermediate. Sci. Rep. 2017, 7, 6929. [Google Scholar] [CrossRef]
- He, W.J.; Zhang, L.; Yi, S.Y.; Tang, X.L.; Yuan, Q.S.; Guo, M.W.; Wu, A.B.; Qu, B.; Li, H.P.; Liao, Y.C. An aldo-keto reductase is responsible for Fusarium toxin-degrading activity in a soil Sphingomonas strain. Sci. Rep. 2017, 7, 9549. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yu, X.; Liu, H.; Ding, M.; Wang, Z.; Xu, J.R.; Jiang, C. Regulation of TRI5 expression and deoxynivalenol biosynthesis by a long non-coding RNA in Fusarium graminearum. Nat. Commun. 2024, 15, 1216. [Google Scholar] [CrossRef]
- Sun, S.; Yu, D.; Guo, M.; Tang, M.; Yan, Z.; Sun, W.; Wu, A. The transcription factor FgSfp1 orchestrates mycotoxin deoxynivalenol biosynthesis in Fusarium graminearum. Commun. Biol. 2024, 7, 1584. [Google Scholar] [CrossRef]
- Shi, W.T.; Yao, C.P.; Liu, W.H.; Cao, W.Y.; Shao, W.; Liao, S.Q.; Yu, T.; Zhu, Q.F.; Chen, Z.; Zang, Y.J.; et al. An fusaric acid-based CRISPR library screen identifies MDH2 as a broad-spectrum regulator of Fusarium toxin-induced cell death. J. Hazard. Mater. 2024, 480, 135937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gangola, M.P.; Huang, C.; Kutcher, H.R.; Ganeshan, S.; Chibbar, R.N. Single Nucleotide Polymorphisms in B-Genome Specific UDP-Glucosyl Transferases Associated with Fusarium Head Blight Resistance and Reduced Deoxynivalenol Accumulation in Wheat Grain. Phytopathology 2018, 108, 124–132. [Google Scholar] [CrossRef] [PubMed]
| Categories | Information | References |
|---|---|---|
| IUPAC Naming | 12,13-epoxy-3α,7α,15-trihydroxytrichothec-9-en-8-one | [5] |
| Physical state | Colorless fine needles | |
| Molecular formula | C15H20O6 | |
| Solubility | It is soluble in polar organic solvents (such as methanol, ethanol, chloroform, acetonitrile, and ethyl acetate) and water, but insoluble in n-hexane, butanol, and petroleum ether. | |
| Flash point | 206.9 ± 2.5 °C | |
| Melting point | 151–153 °C | |
| Molecular mass | 296.32 g/mol | |
| Boiling point | 500–550 °C | |
| Thermal stability in food processing | Under conditions of baking at 210 °C, frying at 140 °C, or boiling, the degradation rate of DON is only approximately 50%. | [6,7,8] |
| Stability in response to temperature, duration, and pH | 1. At pH 4, the chemical structure of DON remained intact after heating at 100 °C and 120 °C for 60 min, with only minor degradation occurring at 170 °C for 60 min. 2. At pH 7, it remained highly stable at 100 °C and 120 °C for 60 min, while partial degradation was observed at 170 °C for 15 min. 3. At pH 10, partial degradation occurred at 100 °C after 60 min; complete destruction was achieved after exposure to 120 °C for 30 min or 170 °C for 15 min. | [9] |
| Long-term storage stability | DON exhibits extremely high stability in cereals and their products and can retain its toxicity for an extended period. | [10,11] |
| Country/Region | Commodity | Maximum Limits (μg/kg) | References | |
|---|---|---|---|---|
| China | Food | Corn, cornmeal (residue, flakes) | 1000 | [17,18] |
| Barley, wheat, muesli, wheat flour | 1000 | |||
| Feed | Calves, Lambs, Lactation Concentrate Supplements | 1000 | ||
| Pig compound feed | 1000 | |||
| Plant-based feed ingredients | 5000 | |||
| USA | Food | Finished wheat products (flour, bread) | 1000 | [19] |
| Feed | Grains and grain byproducts for swine | 5000 | ||
| Grains and grain byproducts for chickens | 10,000 | |||
| Young animals (e.g., piglets) | 1000 | |||
| Cattle and poultry feed | 5000 | |||
| Japan | Food | Wheat | 1100 | [20] |
| EU | Food | Unprocessed durum wheat and oats | 1750 | [21] |
| Processed grains (flour) | 750 | |||
| Cereals for infants and young children | 200 | |||
| Bread, pastries, biscuits, cereal snacks, and breakfast cereals | 500 | |||
| Feed | Compound feed for calves, lambs | 2000 | ||
| Compound feed | 5000 | |||
| Compound feed for pigs | 900 | |||
| Feed materials: maize byproducts | 12,000 | |||
| Canada | Food | Unprocessed wheat, barley, corn | 2000 | [20] |
| Soft wheat flour (baby food) | 200 | |||
| Feed | Pigs (full-price feed) | 1000 | ||
| States | Ingredient | Number of Positives/Samples | Contamination Rate | References |
|---|---|---|---|---|
| China | Green fodder | 199/200 | 99.5% | [32] |
| Whole pig feed | 128/129 | 99.2% | [18] | |
| Wheat flours | 349/359 | 97.2% | [26] | |
| Germany | Forage corn | 120/120 | 100% | [27] |
| White wheat flours | 28/28 | 100% | [33] | |
| Whole-grain wheat flours | 19/19 | 100% | ||
| USA | Piglet feed | 143/144 | 99.3% | [29] |
| Corn silage | 1117/1266 | 88.2% | [30] | |
| White flours | 141/272 | 51.8% | [34] | |
| Whole wheat flours | 36/90 | 40.0% | ||
| Poland | Complete feed | 2005/2013 | 99.6% | [28] |
| Brazil | Barley | 72/76 | 94.7% | [31] |
| Spain | Cereal for infants and toddlers | 12/30 | 40.0% | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Wang, R.; Liu, S.; Zhao, H.; Wen, B.; Chen, S.; Guo, R.; Wang, L.; Xia, X.; Xu, Y.; et al. Deoxynivalenol and Its Metabolites: Contamination, Metabolism, and Toxicity. Toxins 2025, 17, 555. https://doi.org/10.3390/toxins17110555
Lin Y, Wang R, Liu S, Zhao H, Wen B, Chen S, Guo R, Wang L, Xia X, Xu Y, et al. Deoxynivalenol and Its Metabolites: Contamination, Metabolism, and Toxicity. Toxins. 2025; 17(11):555. https://doi.org/10.3390/toxins17110555
Chicago/Turabian StyleLin, Yukai, Ruibiao Wang, Suxian Liu, Hanqing Zhao, Bo Wen, Songbiao Chen, Rongxian Guo, Lei Wang, Xiaojing Xia, Yanzhao Xu, and et al. 2025. "Deoxynivalenol and Its Metabolites: Contamination, Metabolism, and Toxicity" Toxins 17, no. 11: 555. https://doi.org/10.3390/toxins17110555
APA StyleLin, Y., Wang, R., Liu, S., Zhao, H., Wen, B., Chen, S., Guo, R., Wang, L., Xia, X., Xu, Y., & Ding, K. (2025). Deoxynivalenol and Its Metabolites: Contamination, Metabolism, and Toxicity. Toxins, 17(11), 555. https://doi.org/10.3390/toxins17110555





