Abstract
Aflatoxin B1 (AFB1), the most potent and widespread mycotoxin produced by Aspergillus flavus and Aspergillus parasiticus, poses a significant global threat to food safety and human health, with chronic exposure strongly linked to hepatocellular carcinoma (HCC). While physical and chemical detoxification approaches exist, their limitations have led to an increased interest in biological strategies, particularly probiotic interventions. In this review, we synthesize current in vivo and clinical evidence on the ability of probiotic lactic acid bacteria—including Lactobacillus casei Shirota, Lactobacillus rhamnosus GG, Lactobacillus rhamnosus LC705, Lactococcus lactis, and selected Bifidobacterium species—to reduce AFB1 absorption and toxicity. We summarize mechanistic insights into cell wall adsorption, gut microbiota modulation, intestinal barrier protection, and antioxidant enhancement. Clinical trials have shown reductions in AFB1 biomarkers following probiotic supplementation, supporting their translational potential for human health. However, clinical evidence remains limited by small sample sizes, short intervention periods, and variability in endpoints. Collectively, this review consolidates mechanistic, preclinical, and clinical findings to position probiotic lactic acid bacteria as promising biological countermeasures against AFB1-induced hepatocellular carcinoma.
Keywords:
aflatoxins; probiotic lactic acid bacteria (LAB); anti-carcinogenic effect; hepatocellular carcinoma; bio-adsorbent Key Contribution:
This review synthesizes in vivo evidence demonstrating that probiotic lactic acid bacteria can detoxify aflatoxin B1, reduce its absorption, and serve as practical dietary strategies to lower hepatocellular carcinoma risk.
1. Introduction
Filamentous fungi, including Aspergillus, Penicillium, and Fusarium, are prevalent in soils, farms, and other agricultural environments. Many species produce mycotoxins, which were defined by Bennett [1] as “natural products produced by fungi that evoke a toxic response when introduced in low concentration to higher vertebrates and other animals by a natural route.” As low-molecular-weight bioactive molecules, mycotoxins frequently infiltrate human food systems and animal feed supplies, establishing themselves as persistent threats to global health security [2]. The magnitude of mycotoxin contamination across global food systems represents a substantial crisis. Earlier estimates by the United Nations Food and Agriculture Organization (UN FAO) and World Health Organization (WHO) placed global mycotoxin contamination in crops at around 25%, but more recent assessment by Eskola et al. [3] suggests that the actual prevalence is considerably higher across food crops worldwide [3,4]. While the scientific literature documents nearly 400 distinct mycotoxin compounds, only a select group demonstrates the combination of extreme toxicity, environmental persistence, and widespread distribution that creates significant public health concerns. This critical subset encompasses aflatoxins (AF), ochratoxins (OT), zearalenone (ZEA), T-2 toxin, fumonisins (FB), deoxynivalenol (DON), and related trichothecene compounds [5]. The inherent chemical and thermal stability of these molecules renders conventional food processing techniques largely ineffective for their elimination [6,7].
The discovery of AF emerged from tragedy when an enigmatic illness, subsequently termed “Turkey X disease,” devastated British poultry in 1960, claiming approximately 100,000 turkey lives [8]. AF-producing fungi, including Aspergillus flavus and Aspergillus parasiticus, are common in crops including maize, corn, wheat, and sorghum [9,10,11,12]. Contemporary risk assessments indicate that between 4.5 and 5.5 billion individuals globally face ongoing AF exposure through dietary consumption [10,13]. Within the AF family, aflatoxin B1 (AFB1) stands as the most biologically potent member, capable of inducing genotoxicity, oncogenesis, and immunological dysfunction [14,15]. Therefore, the U.S. Food and Drug Administration (FDA) imposes an action level of 20 parts per billion (ppb) for total AF in foods. The carcinogenic potential of AFB1 has earned it a classification as a Group I carcinogen by the International Agency for Research on Cancer (IARC), with hepatic malignancy representing its primary oncological consequence [16,17]. Upon consumption, AFB1 undergoes hepatic biotransformation through cytochrome P450 enzymatic pathways, specifically involving CYP1A2 and CYP3A4 isoforms, resulting in the formation of aflatoxin-8,9-exo-epoxide, a highly reactive intermediate [17,18,19]. This metabolic intermediate demonstrates high affinity with DNA, RNA, and protein molecules [20,21]. The predominant DNA lesion involves adduct formation at guanine N7 positions, which is recognized as a biomarker for hepatocarcinogenic risk assessment [19,22]. These DNA modifications frequently compromise p53 tumor suppressor function through characteristic G→T transversion mutations occurring at codon 249, establishing conditions conducive to hepatocellular carcinoma (HCC) development (see Figure 1) [11,20,23,24,25]. Numerous studies have conclusively established chronic AF exposure as a principal etiological factor in HCC development [26,27,28,29,30,31]. Beyond direct genotoxic effects, aflatoxicosis initiates inflammation by damaging the liver tissue and results in elevated levels of apoptosis and carcinogenic activities [32,33,34].
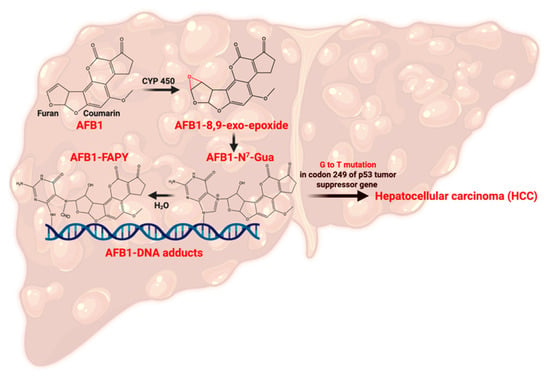
Figure 1.
Pathway of AFB1 metabolism leading to hepatocellular carcinoma (HCC). Created with Biorender.com.
HCC represents a central global malignancy, ranking fifth among all cancer types and third in cancer-associated mortality worldwide [35]. Current estimates attribute 4.6–28.2% of the global liver cancer burden directly to AF exposure [36,37]. Therefore, scientists are actively looking for strategies to mitigate the impact of AF on human health. In recent years, the human gut microbiome and probiotics have been extensively studied for potential anticarcinogenic functions, and interest has been raised in modifying the gut microbiome to reduce AFB1 bioavailability and toxicity [38]. The relationship between gut microbiota and AFB1 uptake has attracted considerable scientific attention and has been widely investigated. This review provides a critical synthesis of mechanistic, preclinical, and clinical data on probiotic interventions for AFB1 toxicity. It specifically evaluates the molecular mechanisms of action, summarizes key findings from animal models and human intervention studies, and discusses both the therapeutic potential and current limitations of probiotic strategies for AFB1 detoxification.
2. AFB1-Induced Alterations of Gut Microbiota
2.1. Adsorption of AFB1 in the GI Tract
The gastrointestinal tract (GI) serves as a crucial barrier, offering multifaceted defense mechanisms against pathogenic microorganisms, environmental toxins, and harmful xenobiotics. However, exposure to AFB1 disrupts this protective interface. Studies demonstrate that AFB1 exposure leads to a dose-dependent increase in intestinal crypt depth, even when villus length remains unchanged, indicating structural alterations that undermine normal mucosal function. These morphological changes, combined with reductions in intestinal weight that impaired nutrient absorption and diminished mucosal health, facilitated greater susceptibility to toxins like AFB1 [39,40]. Moreover, in vivo studies have shown that AFB1 exposure increases the plasma lactulose-to-rhamnose ratio, a marker of intestinal permeability since lactulose absorption is enhanced when tight junction integrity is compromised [41]. This disruption is accompanied by leukocyte and lymphocyte infiltration into the lamina propria [2], suggesting acute inflammation and impaired gut–liver axis function. The cytotoxic effects of AFB1 are primarily attributed to the excessive generation of intracellular reactive oxygen species (ROS), which triggers the release of high levels of lactate dehydrogenase (LDH) and consequently damages cell membranes and DNA integrity [42]. Within the GI tract, AFB1 has been linked to intestinal barrier disruption, altered cell proliferation, and increased apoptosis [11]. While detoxification of AFB1 primarily occurs in the large intestine through microbial biotransformation into less toxic derivatives, more than 80% of the toxin is rapidly absorbed in the duodenum by passive diffusion [11,42]. Therefore, restoration of barrier function and protection against these adverse effects can be achieved by modulating the gut microbiota, highlighting its critical role in maintaining intestinal health.
2.2. Modulation of Gut-Health-Induced Microbiota
Exposure to AFB1 induces significant changes in gut microbiota composition, and how microbiota reacts to AFB1 depends on the exposure level, as described in Table 1. A consistent observation across multiple studies is that AFB1 exposure modulates gut microbiota composition, characterized by a reduction in Bacteroidetes and a concomitant increase in Firmicutes [43,44,45,46]. These two phyla represent the dominant constituents of the gut microbiome, and their imbalance reflects a significant shift in microbial homeostasis [44]. This shift suggests that members of Firmicutes may possess greater tolerance to AFB1, enabling them to outcompete other taxa under toxin stress. Firmicutes comprise several Gram-positive genera, including Lactobacillus and Streptococcus, which belong to the LAB group. LAB can remove AFB1 through binding to cell wall structures [47,48]. Nevertheless, the impact of AFB1 on specific Firmicutes taxa remains inconsistent across studies. For instance, Streptococcus spp. and Lactococcus spp. showed pronounced declines at AFB1 concentrations ranging from 5 to 75 ppb [49], and Lactobacillus spp. abundance decreased by 50.5% (p < 0.05) in piglets when exposed to 320 ppb of AFB1 [50]. Conversely, other investigations have reported increased total LAB populations at 1500–2000 ppb AFB1 (p < 0.05), while a significant reduction was observed in broilers exposed to 1000 ppb [51]. Such discrepancies suggest that LAB responses to AFB1 are highly dose-dependent and possibly influenced by host species and diet. In addition to compositional changes, exposure to AFB1 affects microbial metabolism. At 2500 ppb AFB1, reductions in short-chain fatty acids (SCFAs) were observed, alongside depletion of SCFA-producing LAB strains [3,52]. This decline in SCFAs, critical for maintaining intestinal homeostasis, highlights the potential for AFB1 to disrupt gut metabolic activity. Interestingly, some taxa appear to proliferate under high toxin exposure, such as Bifidobacterium spp. abundance increased significantly at 10,000 ppb AFB1 (p = 0.001), accompanied by elevated xylanase (p = 0.005) and cellulase (p = 0.002) activities, suggesting an enzymatic adaptation to counter intestinal microecological imbalance [53]. Collectively, these findings indicate that AFB1 exerts profound, dependent effects on gut microbiota composition, toxin’s exposure level and metabolism. Shifts in the LAB populations highlight the dynamic interactions between probiotics and AFB1 stress, offering new insights into the role of beneficial microbes in AFB1 detoxification.

Table 1.
In vivo experiments: Gut-health induced microbiota alteration caused by AFB1.
3. Methods for Detoxifying AFB1 in the GI Tract
A variety of physical, chemical, and biological approaches have been explored to inactivate and detoxify AFB1 in food and feed [54,55,56,57]. For example, clay minerals are widely used to selectively or nonspecifically adsorb mycotoxins in the GI tract [55,58]. Inorganic adsorbents, such as aluminosilicates, have also demonstrated a strong binding capacity for AFB1 in animal studies [59,60,61,62,63]. While they are generally recognized as safe (GRAS) for dietary inclusion, no adsorbent has yet been approved by the U.S. Food and Drug Administration (FDA) for clinical treatment of aflatoxicosis. Moreover, these strategies are often costly and impractical for widespread application. Despite the benefits of existing physical and chemical methods, there remains an urgent need for more effective, safe, and affordable detoxification strategies. In recent years, biological processes, particularly probiotic interventions, have gained increasing attention as promising alternatives. These approaches utilize the natural binding and detoxifying capacities of the gut microbiota to mitigate AFB1 toxicity in the gastrointestinal tract.
Probiotics LAB as Potential Detoxifiers of AFB1
According to the World Health Organization (WHO), probiotics are defined as “live microorganisms that, when administered in adequate amounts, provide a health benefit to the host” [64]. Beyond their well-recognized role in gut health and microbiota restoration, probiotics have also been investigated for their ability to detoxify AF in food and the GI tract. Some strains are capable of modifying the chemical structure of AFB1, converting it into less toxic or non-toxic metabolites. However, these transformations do not always eliminate toxicity entirely, as certain products, such as aflatoxicol, may retain harmful effects [65,66]. An alternative mechanism involves direct binding of AFB1 to microbial cells, which reduces its intestinal absorption and subsequent systemic toxicity. Among probiotics, LAB have shown particularly strong binding affinities toward AFB1 [48,67,68,69]. Both viable and non-viable LAB cells can effectively adsorb AFB1, indicating that the detoxification mechanism is linked to cellular structural components rather than active metabolism [70]. Supporting this, Haskard et al. [71] demonstrated that periodate treatment significantly reduced the AFB1-binding capacity of Lactobacillus rhamnosus GG, implicating carbohydrate structures in the cell wall as the key binding sites. In contrast, treatments with proteases or lipases exerted minimal effects, suggesting that proteins and lipids play a negligible role in AFB1 adsorption [70]. LAB are Gram-positive bacteria characterized by a thick peptidoglycan cell wall, a carbohydrate-rich structure regarded as the principal component responsible for sequestering AF. This was confirmed by Lahtinen et al. [72], who found that cell wall extracts of L. rhamnosus GG retained a binding capacity of 81% for AFB1, comparable to that of intact viable cells (84%), whereas purified exopolysaccharides bound less than 1%. Similarly, Zhu et al. [73] reported that highly purified (97.75%) peptidoglycan isolated from Limosilactobacillus reuteri adsorbed 64.3–75.9% of AFB1 in vitro. These findings indicate that peptidoglycan and related polysaccharide structures are the primary contributors to AFB1 adsorption onto LAB. The formation of AFB1–LAB complexes prevents toxin absorption via paracellular diffusion, thereby reducing the risk of hepatocarcinogenesis. Among probiotic LAB, Lactobacillus, Bifidobacterium, and Lactococcus are the most widely studied, with reported AFB1-binding capacities ranging from 5.6% to 59.7% [48]. In particular, Lactobacillus spp. consistently demonstrate high efficacy in sequestering AF, highlighting their potential as promising biological strategies for AFB1 mitigation [68,69,71].
Not all LAB strains exhibit the same capacity to bind AFB1. In a screening of 20 LAB strains conducted by Peltonen, el-Nezami, Haskard, Ahokas and Salminen [48], binding efficiencies ranged widely, from 5.6% in Lactococcus lactis ssp. cremoris MK4 to 59.7% in Lactobacillus amylovorus CSCC 5160. Even within the same species, significant variation was observed: Lactobacillus rhamnosus strain Lc 1/3 bound 54.6% of AFB1, while strain E-97800 bound only 22.7%. These differences suggest that factors beyond peptidoglycan, such as cell surface components, contribute to binding efficacy. Binding is believed to occur via weak, noncovalent interactions involving hydrophobic pockets, as well as through glycopolymers such as teichoic acids embedded in the cell wall [5,48,70,71,72,74,75]. Teichoic acids, in particular, influence adsorption efficiency under varying pH conditions [5]. In addition, extrinsic parameters such as probiotic cell density, initial AF concentration, and temperature play a crucial role in determining the binding efficiency of probiotic strains [76]. Given the variability in AFB1-binding affinity across species and strains, certain LAB strains have been studied in greater detail for their probiotic value and detoxification potential. Among them, Lactobacillus rhamnosus GG and LC705 demonstrated particularly strong and stable binding to AFB1 across 12 LAB strains tested (p < 0.05). Moreover, heat and acid treatments further enhanced their binding capacity. Even after washing steps, viable L. rhamnosus GG and LC705 retained 50% and 38% of their bound AFB1, respectively [71]. Similarly, Lactobacillus casei L30 exhibited high binding affinity and stability among eight L. casei strains, maintaining AFB1 binding after washing and exposure to bile salts. Interestingly, the presence of bile salts increased the proportion of AFB1–L. casei complexes, suggesting that modifications to the bacterial cell envelope may improve binding interactions with AFB1 [75]. Due to their superior binding efficiency, strains such as L. casei and L. rhamnosus have been widely investigated for their potential role in preventing AFB1-induced hepatocarcinogenesis.
4. Anticarcinogenic Effect of Probiotics LAB on AFB1-Induced Liver Carcinogenesis
4.1. Probiotic Lactobacillus casei Shirota (Lcs)
The potential of probiotic LAB as a dietary strategy to mitigate HCC risk associated with AFB1 exposure has been demonstrated in both animal studies and human clinical trials (see Table 2). Probiotic Lactobacillus casei Shirota (Lcs) has been comprehensively studied due to its outstanding efficacy in detoxifying AFB1. Supplementation with Lcs significantly lowered systemic AFB1 levels in contaminated feed models and improved liver function biomarkers, such as alanine transaminase (ALT) and aspartate transaminase (AST), which are typically elevated during aflatoxicosis [77,78]. For example, ALT and AST levels rose to 108 U/L and 124 U/L, respectively, in AFB1-exposed animals without probiotics, but decreased to 75 U/L and 100 U/L in the Lcs-fed group [77]. Another study demonstrated that the Lcs treatment reduced AFB1 in the blood from 88 ng/mL to 50 ng/mL (p < 0.05) in AFB1-exposed rats [79]. These hepatoprotective effects were further supported by reductions in lipid peroxidation and improvements in histological outcomes [80]. In parallel, Lcs enhanced the activities of key antioxidant enzymes, including glutathione peroxidase (GPx), glutathione-S-transferase (GST), superoxide dismutase (SOD), and catalase (CAT), which counteract AFB1-induced oxidative stress [80,81,82]. Notably, GST facilitates detoxification by conjugating the reactive AFB1-8,9-epoxide intermediate with glutathione, producing the excretable metabolite AFB1-mercapturic acid in urine [81]. Together, these findings underscore the dual role of probiotics in lowering systemic toxin burden and reinforcing antioxidant defenses to preserve hepatic integrity [80,82]. Beyond enzymatic activity, bacterial cell wall structures contribute to AFB1 detoxification (see Figure 2). Serrano-Niño et al. [83] reported that teichoic acids, which constitute ~30% of the Lcs cell wall, undergo conformational changes upon AFB1 binding, as visualized by scanning electron microscope (SEM) [79]. The teichoic acid backbone, which is composed of glycerol and ribitol linked by phosphodiester bonds and decorated with glucose and D-alanine, provides hydroxyl groups capable of hydrogen bonding with AFB1 carbonyls (Figure 2) [83]. Strains deficient in teichoic acids exhibited markedly lower AFB1-binding efficiency, showing their importance in the detoxification process [5]. Other cell wall constituents also contribute; for example, β-D-glucans can capture AFB1 via hydrogen bonding and van der Waals interactions, particularly at the C(6)-hydroxyl group of glucopyranose residues [84]. Surface proteins likewise serve as binding sites, with heat-denatured Lcs showing enhanced AFB1 binding to unfolded proteins [79,85,86]. Collectively, these mechanisms demonstrate that AFB1-binding capacity is strongly strain-dependent and mediated by multiple structural components of the Lcs cell wall.
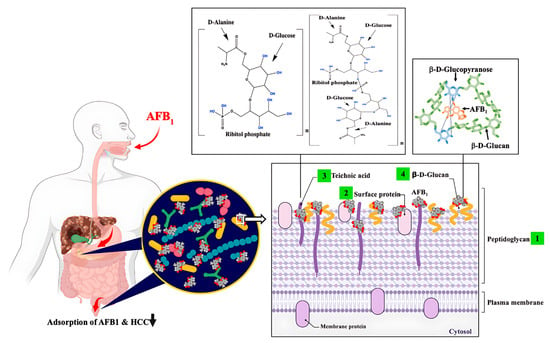
Figure 2.
Proposed interactions between AFB1 and probiotic cell wall components in the duodenum of the small intestine. This schematic (not to scale) illustrates potential mechanisms by which probiotics reduce AFB1 adsorption and thereby lower the risk of HCC. AFB1 can interact with four major bacterial cell wall constituents: (1) peptidoglycans, (2) surface proteins, (3) wall teichoic acids, and (4) β-D-glucans. Variations in teichoic acid structures are represented for Lactobacillus casei Shirota and Lactobacillus rhamnosus GG as proposed by [83]. The suggested mechanism involves hydrogen bonding between hydroxyl groups of ribitol phosphate or glucose residues in teichoic acids and carbonyl oxygens of AFB1. Although these interactions are supported by experimental observations, the precise molecular mechanisms remain to be fully elucidated. AFB1–LAB complexes have been detected in both the duodenum and feces, supporting the concept that binding to probiotic surfaces inhibits intestinal absorption and reduces the likelihood of AFB1-induced carcinogenesis. Figure created with BioRender.com; chemical structures were prepared using ChemDraw v18.0.0.231.
Building on animal studies, several clinical trials have assessed the efficacy of Lcs in reducing AFB1 absorption in humans. A prospective, randomized clinical trial recruited 71 healthy university employees with urinary aflatoxin M1 (AFM1) levels above 0.005 ng/mL, exploring whether fermented milk containing Lactobacillus casei probiotics could prevent AFB1 absorption in the GI tract [87]. According to the study, the concentrations of AFB1-lys in blood serum were reduced from 6.24 pg/mg to 5.48 pg/mg (p = 0.035) during the 4-week intervention period. Furthermore, a significant difference of 13.7% was observed between the placebo drink and milk with Lcs, at 6.35 pg/mg and 5.48 pg/mg, respectively, after 4 weeks of intervention (p = 0.005) [87]. Later, this research group recruited a broader range of participants (n = 174) from Selangor, Malaysia. After 12 weeks of intervention, the Lcs probiotics treatment group has a 23% reduction in AFM1 in excreted urine as compared to the placebo group [88]. These results provide compelling evidence that Lcs can be used as a dietary intervention to reduce AFB1 absorption and thereby lower the risk of long-term AF exposure in humans.
4.2. Probiotic Lactobacillus rhamnosus
Studies have also demonstrated that L. rhamnosus strains can substantially reduce the gastrointestinal absorption of AFB1. In chickens, duodenal uptake of AFB1 decreased by over 70% with L. rhamnosus GG (LGG) and by 37% with L. rhamnosus LC705, indicating that LGG exhibits stronger binding capacity than LC705 despite both belonging to the same species [89]. Similar findings were reported in rats: fecal excretion of AFB1 increased significantly within 24 h of LGG administration, and ALT activity was reduced, suggesting mitigation of AFB1-induced hepatotoxicity [90]. Subsequent experiments confirmed that LGG promoted the excretion of AFB1 in feces by forming stable LGG–AFB1 complexes within the GI tract, thereby reducing systemic toxicity [91]. As with other LAB strains (Section 4.1), the protective action of L. rhamnosus is also largely dependent on cell wall-mediated binding of AFB1 within the GI tract. Beyond adsorption, L. rhamnosus exhibits a distinct anti-inflammatory mechanism: it suppresses NF-κB signaling in AFB1-exposed liver tissue, thereby reducing the expression of proinflammatory cytokines (IL-1β, TNF-α, IL-6) and mitigating hepatotoxic responses [92].
The protective effects of L. rhamnosus have also been evaluated in clinical settings. In a randomized trial, El-Nezami et al. [93] showed that supplementation with L. rhamnosus LC705 significantly reduced urinary excretion of the DNA adduct AFB-N7-guanine. Levels decreased from 0.42 ng/mL to 0.27 ng/mL after 3 weeks (36% reduction) and to 0.19 ng/mL after 5 weeks (55% reduction) compared with placebo (p < 0.05). However, urinary AFB-N7-guanine levels returned to baseline after the intervention period, indicating that sustained probiotic intake may be necessary to maintain the detoxification effect [93]. All things considered, L. rhamnosus has an anti-inflammatory role and protective effects that attenuate the proinflammatory effects caused by AFB1 [90,92]. While clinical studies consistently show the potential of probiotics to reduce AFB1 absorption and related biomarkers, several limitations must be acknowledged. Many trials are restricted by relatively small sample sizes, short intervention durations (weeks rather than months), and variability in measured endpoints (e.g., urinary metabolites vs. serum adducts). These factors may limit generalizability and long-term risk assessment. Future research should focus on larger, multi-center trials with standardized protocols and extended follow-up to strengthen clinical evidence.
4.3. Mixture of Probiotic LABs
In addition to individual strains, mixtures of probiotic LABs have been investigated for their synergistic protective effects against AFB1-induced HCC. One study examined fermented milk containing a combination of L. rhamnosus GG (LGG) and L. casei Shirota (Lcs) in a 1:2 ratio. After 25 weeks of supplementation, this probiotic mixture markedly reduced both tumor incidence and tumor size in AFB1-exposed animals. At the molecular level, expression of oncogenes and proliferation-related factors including c-myc, bcl-2, cyclin D1, and ras p21 was significantly downregulated. Since these genes are central to tumor progression and ROS-mediated pathways, the findings highlight the anti-hepatocarcinogenic potential of LGG–Lcs co-administration [94]. Another investigation evaluated a broader probiotic mixture composed of L. reuteri, L. plantarum, L. pentosus, L. rhamnosus, and L. paracasei. Chickens supplemented with this formulation exhibited a significant reduction in AFB1-induced liver enlargement, measured as relative liver weight (% EBW). Moreover, dietary supplementation decreased AFB1 accumulation in liver tissue by ~58% in the low-dose group (1000 ppb) and ~50% in the high-dose group (5000 ppb) (p < 0.05). Consistently, excretion of AFB1 in feces increased by 67% and 46% at the respective dose levels compared to unsupplemented controls [95]. Mixtures of probiotics may offer a more practical dietary approach than single strains, reflecting the diversity of probiotics naturally present in fermented foods. By combining different binding capacities, enzymatic defenses, and immunomodulatory functions, such formulations could provide broader protection against AFB1 and hold promise as feasible interventions for populations with regular dietary exposure.

Table 2.
In vivo experiments: Anticarcinogenic effect of probiotic LABs on AFB1-induced liver carcinogenesis.
Table 2.
In vivo experiments: Anticarcinogenic effect of probiotic LABs on AFB1-induced liver carcinogenesis.
| Subjects | Dose of AFB1 | Treatment Period | Anti-Hepatocarcinogenic Functions | LAB Strains | Ref |
|---|---|---|---|---|---|
| Male Sprague Dawley rats (7–8 weeks old) | 25 ppb | Daily for 20 days | ALT & AST ↓ Serum AFB1 ↓ | L. casei Shirota | [77] |
| Male Sprague Dawley rats (7–8 weeks old) | 25 ppb | Daily for 5 days | Serum AFB1 ↓ | L. casei Shirota | [79] |
| Male Wistar rats (4 weeks old) | 450 ppb | Twice/week for 6 weeks | TBARS ↓ Antioxidant enzymes ↑ | L. casei Shirota L. rhamnosus GG | [80] |
| 71 employees in UPM | Urinary AFM1 > 0.005 ppb | 4 weeks of intervention | Serum AFB1 ↓ | L. casei Shirota | [87] |
| Broiler chickens (1 week old) | 3000 ppb | Single injection | AFB1 in duodenal tissue & luminal fluid ↓ | L. rhamnosus LC705 L. rhamnosus GG | [89] |
| Han-Wistar rats (5 weeks old) | 1500 ppb | Daily for 3 days | ALT ↓ AFB1 in feces ↑ | L. rhamnosus GG | [90] |
| Male Holstein calves (120 days old) | 38 ppb | Single oral | AFB1 in feces ↑ | L. rhamnosus GG | [91] |
| Male Kunming mice (5 weeks old) | 300 ppb | Twice/day for 8 weeks | Inflammatory factors ↓ ALT & AST ↓ | L. rhamnosus | [92] |
| 90 male students at Sun Yat-Sen University | Urinary AFM1 > 0.008 ppb | Twice/day for 5 weeks of intervention | Urinary AFB-N7-guanine ↓ | L. rhamnosus LC705 | [93] |
| Male Wistar rats (4 weeks old) | 450 ppb | Twice/week for 25 weeks | c-myc, bcl-2, cyclin D1 & rasp-21 ↓ Tumor incidence ↓ | Mixture of L. casei Shirota & L. rhamnosus GG | [94] |
| Male Ross broiler chicks (1 day old) | Low (1000 ppb) High (5000 ppb) | Daily for 35 days | Liver EBW ↓ AFB1 in liver tissue ↓ AFB1 in excreta ↑ | Mixture of LAB | [95] |
ALT: alanine transaminase; AST: aspartate transaminase; EBW (empty body weight) = body weight before sacrifice—weight of alimentary tract filled with chime; ppb: part per billion (1 ppb = μg/kg), ↑: indicates increase, ↓: indicates decrease.
5. Conclusions
AFB1 remains a significant global challenge to food safety and public health, with chronic dietary exposure strongly associated with hepatocellular carcinoma (HCC). Probiotic lactic acid bacteria (LAB) have emerged as promising biological countermeasures, supported by growing evidence from mechanistic studies, animal experiments, and human clinical trials. These probiotics, including Lactobacillus casei Shirota, Lactobacillus rhamnosus GG, Lactobacillus rhamnosus LC705, Lactococcus lactis, and selected Bifidobacterium species, mitigate AFB1 toxicity through multiple complementary mechanisms. The most compelling evidence comes from clinical intervention trials, which consistently demonstrate reductions in urinary AFM1 and DNA adducts following probiotic supplementation, highlighting clear translational potential. Moreover, findings from mechanistic and animal studies—such as enhanced antioxidant activity, NF-κB pathway modulation, intestinal barrier restoration, and shifts in gut microbiota—provide valuable insight but still require confirmation in long-term human populations. Looking ahead, approaches such as engineered probiotics, probiotic–prebiotic combinations, and standardized multi-strain formulations represent exciting future directions, though they remain largely hypothetical and demand rigorous evaluation. At the molecular level, probiotic protection is largely mediated by cell wall components such as peptidoglycans, teichoic acids, β-D-glucans, and surface proteins, which adsorb AFB1 and limit its intestinal absorption. These interactions emphasize the importance of strain selection and mechanistic characterization, particularly given the variability in binding efficiency across strains. To advance translation, further biochemical studies are needed to clarify structural mechanisms, and large-scale randomized controlled trials in high-risk populations are required to establish efficacy, optimize dosing regimens, and evaluate the synergistic effects of probiotic mixtures. From a practical perspective, probiotics are generally safe, affordable, and widely accepted within dietary contexts, making them attractive candidates for large-scale food safety interventions. Nonetheless, regulatory approval, product standardization, and quality control can remain critical challenges to their widespread application. In summary, probiotic LABs represent safe, cost-effective, and scalable interventions for reducing AFB1 exposure and preventing HCC. Their multifaceted mechanisms of action highlight their promise as a cornerstone of global mycotoxin management, provided that ongoing mechanistic research and clinical validation bridge the gap between laboratory potential and real-world implementation.
Author Contributions
Conceptualization: D.C.; Investigation: D.C., and X.F.; Writing—Original Draft: D.C.; Writing-Review & Editing: D.C.; X.F., and J.-H.Y.; Funding Acquisition: J.-H.Y.; Supervision: J.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Hatch Project (No. 7000326) from the National Institute of Food and Agriculture, U.S. Department of Agriculture, awarded to J-H.Y., and by the Food Research Institute at the University of Wisconsin–Madison.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bennett, J.W. Mycotoxins, mycotoxicoses, mycotoxicology and Mycopathologia. Mycopathologia 1987, 100, 3–5. [Google Scholar] [CrossRef]
- Akinrinmade, F.J.; Akinrinde, A.S.; Amid, A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: Modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Res. 2016, 32, 53–60. [Google Scholar] [CrossRef]
- Mannon, J.; Johnson, E. Fungi down on the farm. New Sci. 1985, 1445, 12–16. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Guzman-de-Peña, D.; Garcia, H.S. Key role of teichoic acids on aflatoxin B binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I. Toxigenic fungi: Which are important? Med. Mycol. 2000, 38 (Suppl. S1), 17–22. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef]
- Makun Hussaini, A.; Dutton Michael, F.; Njobeh Patrick, B.; Gbodi Timothy, A.; Ogbadu Godwin, H. Aflatoxin Contamination in Foods and Feeds: A Special Focus on Africa. In Trends in Vital Food and Control Engineering; Ayman Hafiz Amer, E., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. 24919. [Google Scholar]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef]
- Liew, W.P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, A.; Mahato, D.K.; Pandhi, S.; Pandey, A.K.; Kargwal, R.; Mishra, S.; Suhag, R.; Sharma, N.; Saurabh, V.; et al. Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies. Toxins 2022, 14, 687. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Khlangwiset, P.; Shephard, G.S.; Wu, F. Aflatoxins and growth impairment: A review. Crit. Rev. Toxicol. 2011, 41, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Fetaih, H.A.; Dessouki, A.A.; Hassanin, A.A.; Tahan, A.S. Toxopathological and cytogenetic effects of aflatoxin B1 (AFB1) on pregnant rats. Pathol. Res. Pract. 2014, 210, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on Evaluation of Carcinogenic Risk to Humans: Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; International Agency for Research on Cancer: Lyon, France, 1993; Volume 56. [Google Scholar]
- Muhammad, I.; Sun, X.; Wang, H.; Li, W.; Wang, X.; Cheng, P.; Li, S.; Zhang, X.; Hamid, S. Curcumin successfully inhibited the computationally identified cyp2a6 enzyme-mediated bioactivation of aflatoxin b1 in arbor acres broiler. Front. Pharmacol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Lewis, C.W.; Smith, J.E.; Anderson, J.G.; Freshney, R.I. Increased cytotoxicity of food-borne mycotoxins toward human cell lines in vitro via enhanced cytochrome p450 expression using the MTT bioassay. Mycopathologia 1999, 148, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroli, C.; Pulliero, A.; Geretto, M.; Izzotti, A. Molecular fingerprints of environmental carcinogens in human cancer. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 188–228. [Google Scholar] [CrossRef]
- Qi, L.N.; Bai, T.; Chen, Z.S.; Wu, F.X.; Chen, Y.Y.; De Xiang, B.; Peng, T.; Han, Z.G.; Li, L.Q. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi, China: Role of chronic hepatitis B virus infection and aflatoxin B1 exposure. Liver Int. 2015, 35, 999–1009. [Google Scholar] [CrossRef]
- Bressac, B.; Kew, M.; Wands, J.; Ozturk, M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature 1991, 350, 429–431. [Google Scholar] [CrossRef]
- Wild, C.P.; Turner, P.C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481. [Google Scholar] [CrossRef]
- Smela, M.E.; Hamm, M.L.; Henderson, P.T.; Harris, C.M.; Harris, T.M.; Essigmann, J.M. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2002, 99, 6655–6660. [Google Scholar] [CrossRef]
- Hussain, S.P.; Schwank, J.; Staib, F.; Wang, X.W.; Harris, C.C. TP53 mutations and hepatocellular carcinoma: Insights into the etiology and pathogenesis of liver cancer. Oncogene 2007, 26, 2166–2176. [Google Scholar] [CrossRef]
- Diamantis, I.D.; McGandy, C.; Chen, T.J.; Liaw, Y.F.; Gudat, F.; Bianchi, L. A new mutational hot-spot in the p53 gene in human hepatocellular carcinoma. J. Hepatol. 1994, 20, 553–556. [Google Scholar] [CrossRef]
- Chen, J.G.; Egner, P.A.; Ng, D.; Jacobson, L.P.; Muñoz, A.; Zhu, Y.R.; Qian, G.S.; Wu, F.; Yuan, J.M.; Groopman, J.D.; et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev. Res. 2013, 6, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Mo, X.; Yang, Y.; He, K.; Xiao, J.; Liu, C.; Chen, J.; Lin, Y. Association between aflatoxin B1 occupational airway exposure and risk of hepatocellular carcinoma: A case-control study. Tumour Biol. 2014, 35, 9577–9584. [Google Scholar] [CrossRef]
- Qian, G.S.; Ross, R.K.; Yu, M.C.; Yuan, J.M.; Gao, Y.T.; Henderson, B.E.; Wogan, G.N.; Groopman, J.D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 1994, 3, 3–10. [Google Scholar]
- Yard, E.E.; Daniel, J.H.; Lewis, L.S.; Rybak, M.E.; Paliakov, E.M.; Kim, A.A.; Montgomery, J.M.; Bunnell, R.; Abudo, M.U.; Akhwale, W.; et al. Human aflatoxin exposure in Kenya, 2007: A cross-sectional study. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1322–1331. [Google Scholar] [CrossRef]
- Asim, M.; Sarma, M.P.; Thayumanavan, L.; Kar, P. Role of aflatoxin B1 as a risk for primary liver cancer in north Indian population. Clin. Biochem. 2011, 44, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Monson, M.S.; Settlage, R.E.; McMahon, K.W.; Mendoza, K.M.; Rawal, S.; El-Nezami, H.S.; Coulombe, R.A.; Reed, K.M. Response of the hepatic transcriptome to aflatoxin B1 in domestic turkey (Meleagris gallopavo). PLoS ONE 2014, 9, e100930. [Google Scholar] [CrossRef]
- Rawal, S.; Bauer, M.M.; Mendoza, K.M.; El-Nezami, H.; Hall, J.R.; Kim, J.E.; Stevens, J.R.; Reed, K.M.; Coulombe, R.A., Jr. Aflatoxicosis chemoprevention by probiotic Lactobacillius and lack of effect on the major histocompatibility complex. Res. Vet. Sci. 2014, 97, 274–281. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, C.-C.H.; Marsh, G.M.; Wu, F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Tillett, T. Carcinogenic crops: Analyzing the effect of aflatoxin on global liver cancer rates. Environ. Health Perspect. 2010, 118, A258. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; El-Nezami, H. Targeting gut microbiota in hepatocellular carcinoma: Probiotics as a novel therapy. Hepatobiliary Surg. Nutr. 2018, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Applegate, T.J.; Schatzmayr, G.; Prickel, K.; Troche, C.; Jiang, Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult. Sci. 2009, 88, 1235–1241. [Google Scholar] [CrossRef]
- Yunus, A.W.; Ghareeb, K.; Abd-El-Fattah, A.A.; Twaruzek, M.; Böhm, J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult. Sci. 2011, 90, 1683–1689. [Google Scholar] [CrossRef]
- Chen, X.; Naehrer, K.; Applegate, T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef]
- Cao, Q.-Q.; Lin, L.-X.; Xu, T.-T.; Lu, Y.; Zhang, C.-D.; Yue, K.; Huang, S.-C.; Dong, H.-J.; Jian, F.-C. Aflatoxin B1 alters meat quality associated with oxidative stress, inflammation, and gut-microbiota in sheep. Ecotoxicol. Environ. Saf. 2021, 225, 112754. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, H.; Tsim, K.W.K.; Shen, X.; Li, X.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 induces inflammatory liver injury via gut microbiota in mice. J. Agric. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef]
- Sui, Y.; Lu, Y.; Zuo, S.; Wang, H.; Bian, X.; Chen, G.; Huang, S.; Dai, H.; Liu, F.; Dong, H. Aflatoxin B1 exposure in sheep: Insights into hepatotoxicity based on oxidative stress, inflammatory injury, apoptosis, and gut microbiota analysis. Toxins 2022, 14, 840. [Google Scholar] [CrossRef]
- Chen, H.; Ye, L.; Wang, Y.; Chen, J.; Wang, J.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 exposure causes splenic pyroptosis by disturbing the gut microbiota-immune axis. Food Funct. 2024, 15, 3615–3628. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface binding of toxins and heavy metals by probiotics. Mini Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef]
- Peltonen, K.; el-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy. Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, L.; Glenn, T.C.; Wang, J.S. Aflatoxin B1 induced compositional changes in gut microbial communities of male F344 rats. Toxicol. Sci. 2016, 150, 54–63. [Google Scholar] [CrossRef]
- Grosu, I.A.; Pistol, G.C.; Taranu, I.; Marin, D.E. The impact of dietary grape seed meal on healthy and aflatoxin b1 afflicted microbiota of pigs after weaning. Toxins 2019, 11, 25. [Google Scholar] [CrossRef]
- Galarza-Seeber, R.; Latorre, J.D.; Bielke, L.R.; Kuttappan, V.A.; Wolfenden, A.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Vicente, J.L.; Donoghue, A.; Cross, D.; et al. Leaky gut and mycotoxins: Aflatoxin b1 does not increase gut permeability in broiler chickens. Front. Vet. Sci. 2016, 3, 10. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Wang, J.; Wang, J.S. Aflatoxin B1 disrupts gut-microbial metabolisms of short-chain fatty acids, long-chain fatty acids, and bile acids in male F344 rats. Toxicol. Sci. 2018, 164, 453–464. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Guo, Y.; Xiao, N.; Tan, Z. Influences of Aflatoxin B1 on main intestinal bacteria communities and enzyme activities in mice. Toxin Rev. 2019, 38, 121–126. [Google Scholar] [CrossRef]
- García-Cela, E.; Ramos, A.J.; Sanchis, V.; Marin, S. Emerging risk management metrics in food safety: FSO, PO. How do they apply to the mycotoxin hazard? Food Control 2012, 25, 797–808. [Google Scholar] [CrossRef]
- Yazdanpanah, H.; Eslamizad, S. Aflatoxins and Their Management. In Biological Toxins and Bioterrorism; Gopalakrishnakone, P., Balali-Mood, M., Llewellyn, L., Singh, B.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 103–120. [Google Scholar]
- Gorran, A.; Farzaneh, M.; Shivazad, M.; Rezaeian, M.; Ghassempour, A. Aflatoxin B1-reduction of Aspergillus flavus by three medicinal plants (Lamiaceae). Food Control 2013, 31, 218–223. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.; Hell, K.; Adjovi, Y.; Fandohan, P.; Koudande, D.O.; Mensah, G.A.; Sanni, A.; Brimer, L. A review on aflatoxin contamination and its implications in the developing world: A sub-Saharan African perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 349–365. [Google Scholar] [CrossRef]
- Dixon, J.B.; Kannewischer, I.; Tenorio Arvide, M.G.; Barrientos Velazquez, A.L. Aflatoxin sequestration in animal feeds by quality-labeled smectite clays: An introductory plan. Appl. Clay Sci. 2008, 40, 201–208. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Zartman, R.E.; Hudnall, W.H. Aflatoxin B1 adsorption by clays from water and corn meal. Appl. Clay Sci. 2007, 36, 197–205. [Google Scholar] [CrossRef]
- Papaioannou, D.; Katsoulos, P.D.; Panousis, N.; Karatzias, H. The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: A review. Microporous Mesoporous Mater. 2005, 84, 161–170. [Google Scholar] [CrossRef]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Fowler, J.; Li, W.; Bailey, C. Effects of a calcium bentonite clay in diets containing aflatoxin when measuring liver residues of aflatoxin b1 in starter broiler chicks. Toxins 2015, 7, 3455–3464. [Google Scholar] [CrossRef]
- World Health Organization Food & Agriculture Organization of the United. Nations Food Safety Risk Analysis: A Guide for National Food Safety Authorities; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Verheecke, C.; Liboz, T.; Mathieu, F. Microbial degradation of aflatoxin B1: Current status and future advances. Int. J. Food Microbiol. 2016, 237, 1–9. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Nwinyi, O.C.; Mavumengwana, V. Review on microbial degradation of aflatoxins. Crit. Rev. Food Sci. Nutr. 2017, 57, 3208–3217. [Google Scholar] [CrossRef]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef]
- Bueno, D.J.; Casale, C.H.; Pizzolitto, R.P.; Salvano, M.A.; Oliver, G. Physical adsorption of aflatoxin B1 by lactic acid bacteria and Saccharomyces cerevisiae: A theoretical model. J. Food Prot. 2007, 70, 2148–2154. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Guzman-De-Peña, D.; González-Córdova, A.F.; Vallejo-Córdoba, B.; Garcia, H.S. In vivo assessment of the potential protective effect of Lactobacillus casei Shirota against aflatoxin B1. Dairy. Sci. Technol. 2010, 90, 729–740. [Google Scholar] [CrossRef]
- Haskard, C.; Binnion, C.; Ahokas, J. Factors affecting the sequestration of aflatoxin by Lactobacillusrhamnosus strain GG. Chem.-Biol. Interact. 2000, 128, 39–49. [Google Scholar] [CrossRef]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Zhu, F.H.; Chen, X.Y.; Hou, L.L.; Dong, J.H.; Liu, H.W.; Zhu, L.Q.; Chen, F. Limosilactobacillus reuteri peptidoglycan alleviates aflatoxin B1-induced toxicity through adsorbing toxins and improving growth, antioxidant status, immunity and liver pathological changes in chicks. Br. Poult. Sci. 2024, 65, 352–360. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Nasiraii, L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Garcia, H.S.; Steele, J.L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009, 47, 1064–1068. [Google Scholar] [CrossRef]
- Macit, A.; Sevim, S.; Kizil, M. Aflatoxin B1 and M1 detoxification in foodstuffs: Examining the efficacy of probiotics with and without prebiotics—A systematic review. Food Biosci. 2024, 58, 103724. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, E.; Jamaluddin, R.; Abdul Mutalib, M.S.; Khaza’ai, H.; Khalesi, S.; Mohd Redzwan, S. Reduction of aflatoxin level in aflatoxin-induced rats by the activity of probiotic Lactobacillus casei strain Shirota. J. Appl. Microbiol. 2013, 114, 1507–1515. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.; Kholif, A. Mycotoxins in animal feeds and prevention strategies: A review. Asian J. Anim. Sci. 2010, 4, 113–131. [Google Scholar] [CrossRef]
- Liew, W.P.; Nurul-Adilah, Z.; Than, L.T.L.; Mohd-Redzwan, S. The binding efficiency and interaction of lactobacillus casei shirota toward aflatoxin b1. Front. Microbiol. 2018, 9, 1503. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, V.; Nagpal, R.; Kumar, A.; Behare, P.V.; Singh, B.; Aggarwal, P.K. Anticarcinogenic effect of probiotic fermented milk and chlorophyllin on aflatoxin-B1-induced liver carcinogenesis in rats. Br. J. Nutr. 2012, 107, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Neal, G.E.; Green, J.A. The requirement for glutathione S-transferase in the conjugation of activated aflatoxin B1 during aflatoxin hepatocarcinogenesis in the rat. Chem. Biol. Interact. 1983, 45, 259–275. [Google Scholar] [CrossRef]
- Zuo, R.Y.; Chang, J.; Yin, Q.Q.; Wang, P.; Yang, Y.R.; Wang, X.; Wang, G.Q.; Zheng, Q.H. Effect of the combined probiotics with aflatoxin B1-degrading enzyme on aflatoxin detoxification, broiler production performance and hepatic enzyme gene expression. Food Chem. Toxicol. 2013, 59, 470–475. [Google Scholar] [CrossRef]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; Cantú-Cornelio, F.; González-Córdova, A.F.; Vallejo-Córdoba, B.; Hernández-Mendoza, A.; García, H.S. In vitro reduced availability of aflatoxin B1 and acrylamide by bonding interactions with teichoic acids from lactobacillus strains. LWT Food Sci. Technol. 2015, 64, 1334–1341. [Google Scholar] [CrossRef]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.G.; Jeminet, G.; Bertin, G.; Jouany, J.P. Chemical and conformational study of the interactions involved in mycotoxin complexation with beta-D-glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zhang, L.; He, X.; Zhang, J.Z.H. Computational search for aflatoxin binding proteins. Chem. Phys. Lett. 2017, 685, 1–8. [Google Scholar] [CrossRef]
- Assaf, J.C.; Atoui, A.; Khoury, A.E.; Chokr, A.; Louka, N. A comparative study of procedures for binding of aflatoxin M1 to Lactobacillus rhamnosus GG. Braz. J. Microbiol. 2018, 49, 120–127. [Google Scholar] [CrossRef]
- Mohd Redzwan, S.; Abd Mutalib, M.S.; Wang, J.S.; Ahmad, Z.; Kang, M.S.; Abdul Rahman, N.; Nikbakht Nasrabadi, E.; Jamaluddin, R. Effect of supplementation of fermented milk drink containing probiotic Lactobacillus casei Shirota on the concentrations of aflatoxin biomarkers among employees of Universiti Putra Malaysia: A randomised, double-blind, cross-over, placebo-controlled study. Br. J. Nutr. 2016, 115, 39–54. [Google Scholar] [CrossRef]
- Chang, W.L.; Akiyama, T.; Wang, J.-S.; Yong, H.Y.; Hassan, F.; Abu Saad, H.; Jamaluddin, R.; Sabran, M.R. Impact of probiotic Lacticaseibacillus paracasei strain shirota (lcs) on aflatoxin exposure among healthy Malaysian adults: A randomized, double-blind, placebo-controlled intervention study. J. Nutr. 2025, 155, 2110–2121. [Google Scholar] [CrossRef]
- El-Nezami, H.; Mykkänen, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B, from the chicken duodenum. J. Food Prot. 2000, 63, 549–552. [Google Scholar] [CrossRef]
- Gratz, S.; Täubel, M.; Juvonen, R.O.; Viluksela, M.; Turner, P.C.; Mykkänen, H.; El-Nezami, H. Lactobacillus rhamnosus strain GG modulates intestinal absorption, fecal excretion, and toxicity of aflatoxin B1 in rats. Appl. Environ. Microbiol. 2006, 72, 7398–7400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Liu, S.; Zhao, X.J.; Wang, N.; Jiang, X.; Xin, H.S.; Zhang, Y.G. Lactobacillus rhamnosus GG modulates gastrointestinal absorption, excretion patterns, and toxicity in Holstein calves fed a single dose of aflatoxin B1. J. Dairy. Sci. 2019, 102, 1330–1340. [Google Scholar] [CrossRef]
- Chen, Y.; Li, R.; Chang, Q.; Dong, Z.; Yang, H.; Xu, C. Lactobacillus bulgaricus or Lactobacillus rhamnosus Suppresses NF-κB Signaling Pathway and Protects against AFB1-Induced Hepatitis: A Novel Potential Preventive Strategy for Aflatoxicosis? Toxins 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.S.; Polychronaki, N.N.; Ma, J.; Zhu, H.; Ling, W.; Salminen, E.K.; Juvonen, R.O.; Salminen, S.J.; Poussa, T.; Mykkänen, H.M. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. Am. J. Clin. Nutr. 2006, 83, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, V.; Nagpal, R.; Kumar, A.; Gautam, S.K.; Behare, P.V.; Grover, C.R.; Aggarwal, P.K. Effect of probiotic fermented milk and chlorophyllin on gene expressions and genotoxicity during AFB1-induced hepatocellular carcinoma. Gene 2011, 490, 54–59. [Google Scholar] [CrossRef]
- Śliżewska, K.; Cukrowska, B.; Smulikowska, S.; Cielecka-Kuszyk, J. The Effect of Probiotic Supplementation on Performance and the Histopathological Changes in Liver and Kidneys in Broiler Chickens Fed Diets with Aflatoxin B1. Toxins 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).