Neurotoxic Sleight of Fang: Differential Antivenom Efficacy Against Mamba (Dendroaspis spp.) Venom Spastic-Paralysis Presynaptic/Synaptic vs. Flaccid-Paralysis Postsynaptic Effects
Abstract
1. Introduction
2. Results
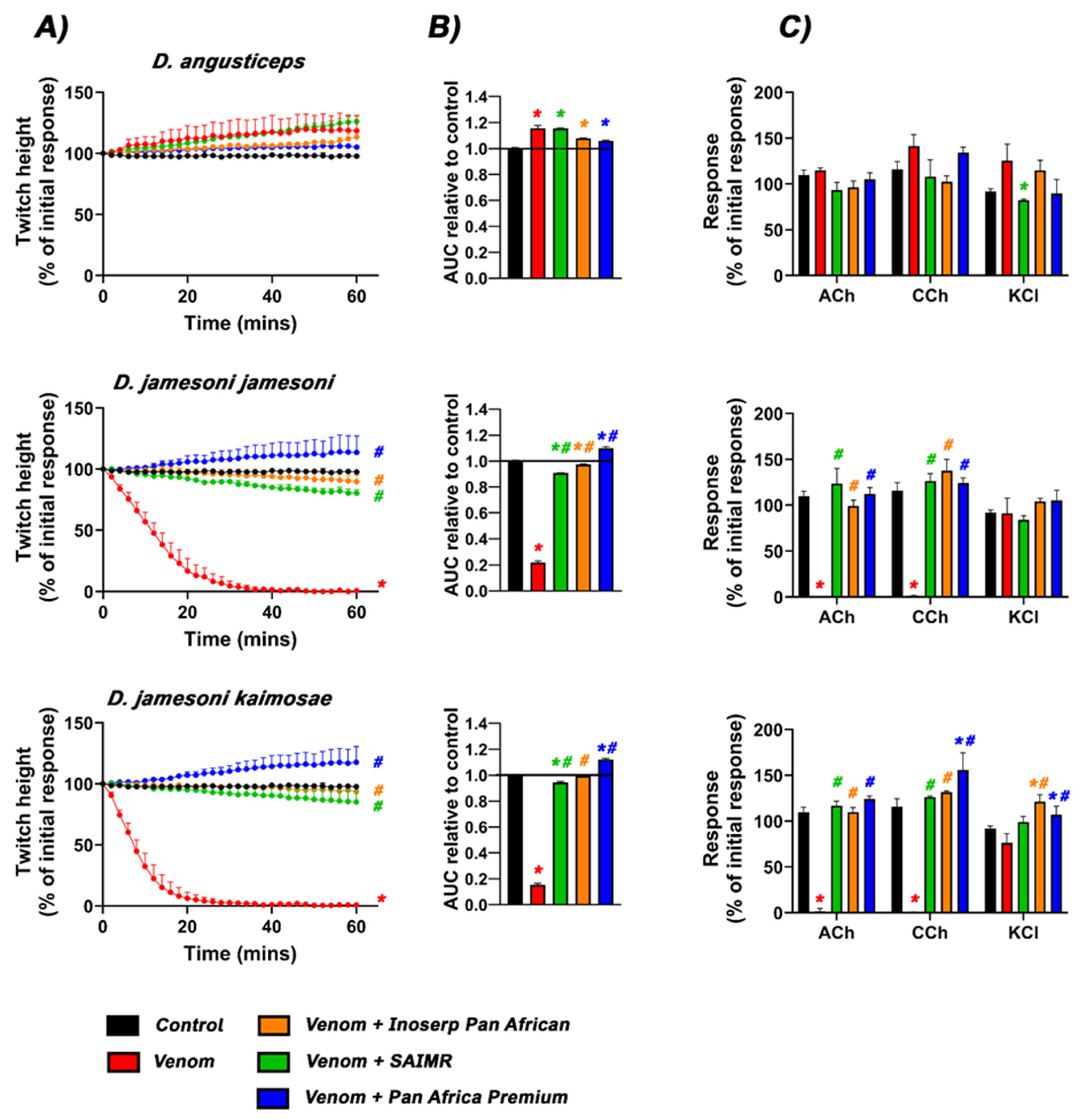
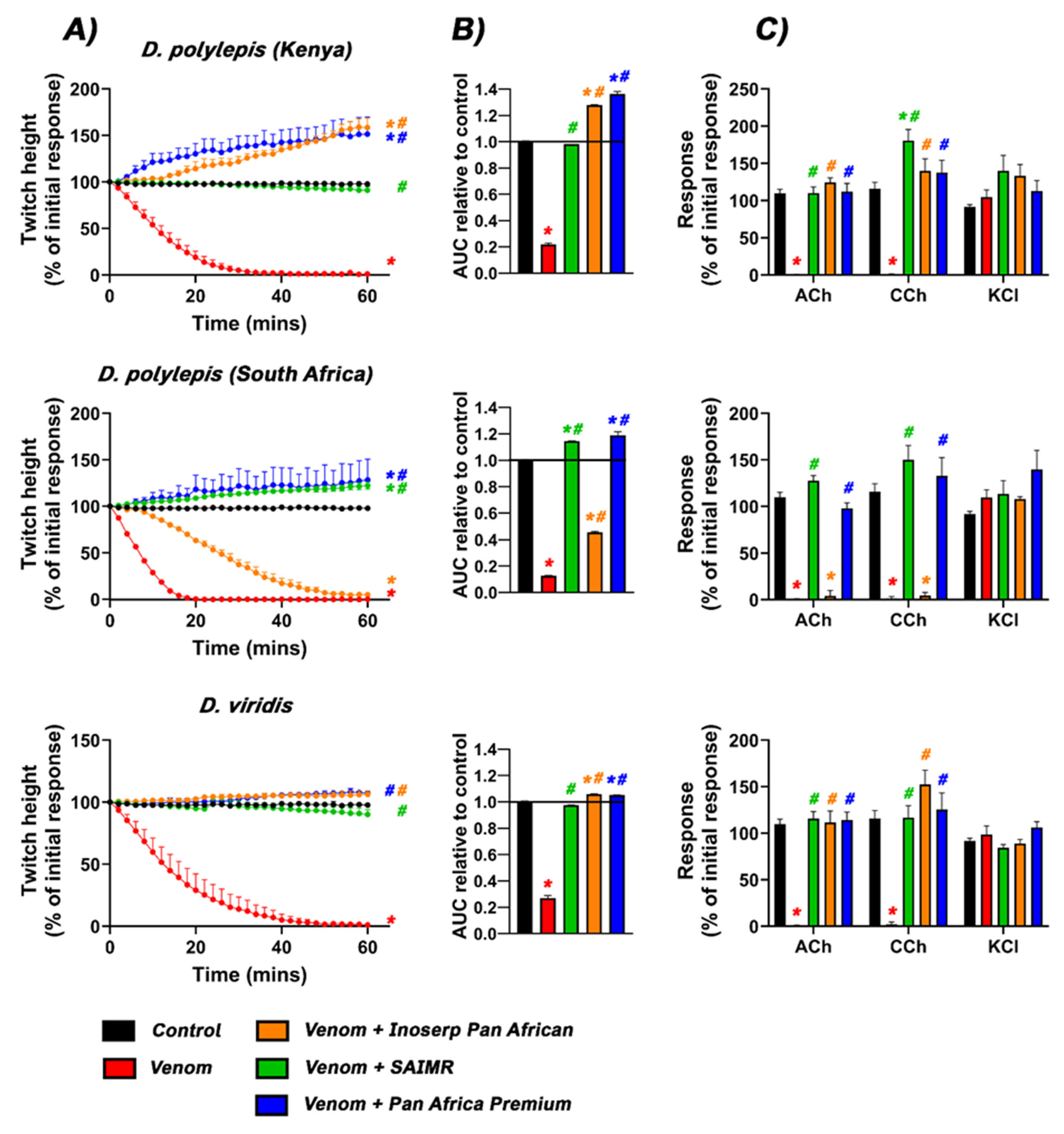
2.1. Neurotoxicity and Antivenom Testing
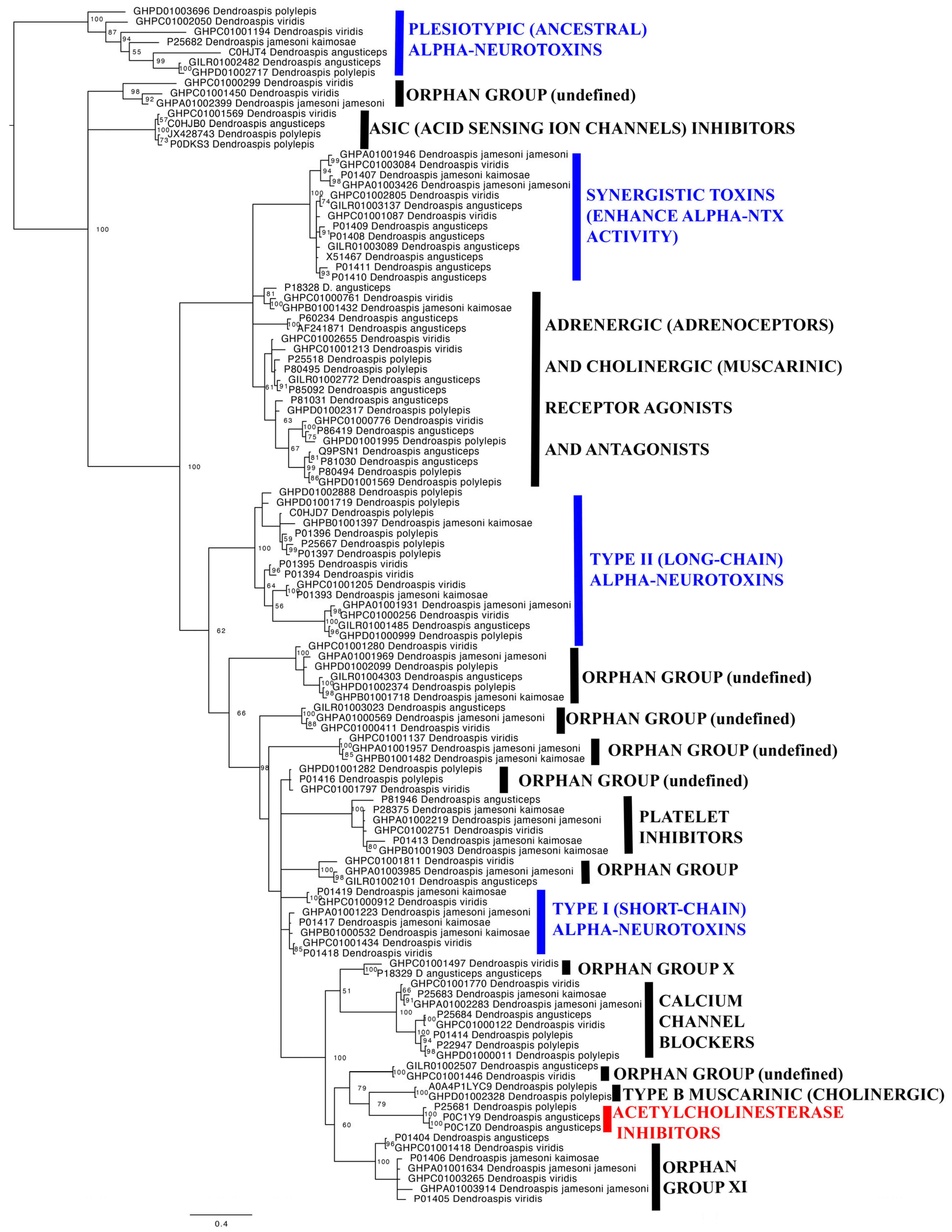
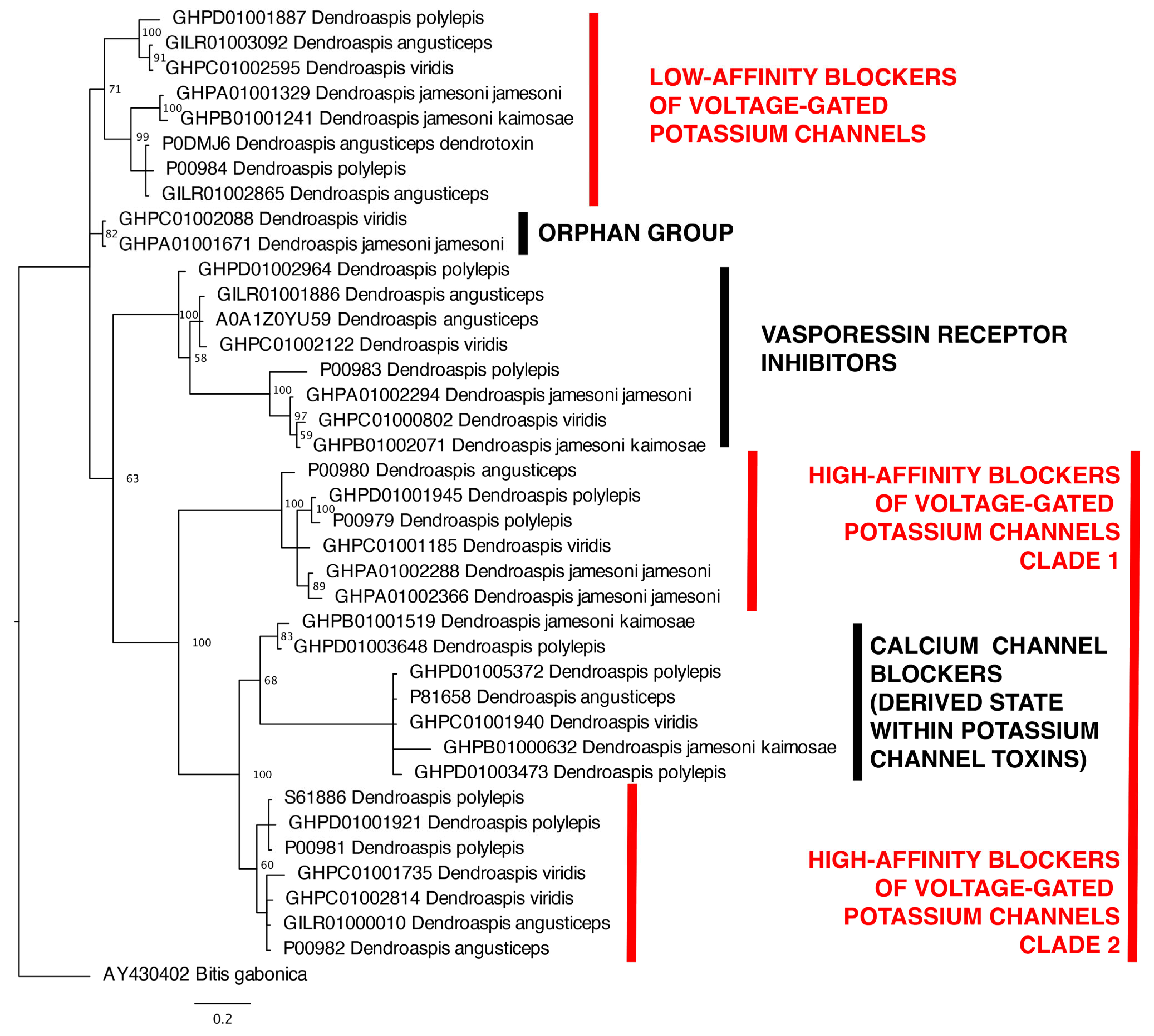
2.2. Molecular Phylogenetics and Evolution
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venoms and Antivenoms
5.2. Drugs and Reagents
5.3. Chick Biventer Cervicis Nerve-Muscle Preparation
5.4. Computational Molecular Evolution
5.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chippaux, J.-P. Estimate of the burden of snakebites in sub-Saharan Africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Tolley, K.A.; Alexander, G.J.; Winder, I.C.; Dobson, C.; Hall, C.; Barlow, A.; McBride, E.; Reissig, J.; Trape, J.-F.; Nagy, Z.T. Phylogeny and species delimitation in an iconic snake genus: The African mambas. Zool. J. Linn. Soc. 2025, 3, zlaf062. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switerland, 2010; Volume 134. [Google Scholar]
- Warrell, D.A. Clinical toxicology of snakebite in Africa and the Middle East/Arabian Peninsula. In Handbook of Clinical Toxicology of Animal Venoms and Poisons; CRC Press: Boca Raton, FL, USA, 2017; pp. 433–492. [Google Scholar]
- Hodgson, P.S.; Davidson, T.M. Biology and treatment of the mamba snakebite. Wilderness Environ. Med. 1996, 7, 133–145. [Google Scholar] [CrossRef]
- Hilligan, R. Black mamba bites-a report of 2 cases. S. Afr. Med. J. 1987, 72, 220–221. [Google Scholar]
- Ainsworth, S.; Petras, D.; Engmark, M.; Süssmuth, R.D.; Whiteley, G.; Albulescu, L.-O.; Kazandjian, T.D.; Wagstaff, S.C.; Rowley, P.; Wüster, W. The medical threat of mamba envenoming in sub-Saharan Africa revealed by genus-wide analysis of venom composition, toxicity and antivenomics profiling of available antivenoms. J. Proteom. 2018, 172, 173–189. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the nature of black mamba (Dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Toxicovenomics and antivenom profiling of the Eastern green mamba snake (Dendroaspis angusticeps). J. Proteom. 2016, 136, 248–261. [Google Scholar] [CrossRef]
- Petras, D.; Heiss, P.; Harrison, R.A.; Süssmuth, R.D.; Calvete, J.J. Top-down venomics of the East African green mamba, Dendroaspis angusticeps, and the black mamba, Dendroaspis polylepis, highlight the complexity of their toxin arsenals. J. Proteom. 2016, 146, 148–164. [Google Scholar] [CrossRef]
- Harvey, A.L. Twenty years of dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- Karlsson, E.; Mbugua, P.; Rodriguez-Ithurralde, D. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps. J. Physiol. 1984, 79, 232–240. [Google Scholar]
- Wang, C.-I.A.; Reeks, T.; Vetter, I.; Vergara, I.; Kovtun, O.; Lewis, R.J.; Alewood, P.F.; Durek, T. Isolation and structural and pharmacological characterization of α-elapitoxin-Dpp2d, an amidated three finger toxin from black mamba venom. Biochemistry 2014, 53, 3758–3766. [Google Scholar] [CrossRef]
- Yasuda, O.; Morimoto, S.; Jiang, B.; Kuroda, H.; Kimura, T.; Sakakibara, S.; Fukuo, K.; Chen, S.; Tamatani, M.; Ogihara, T. FS2. a mamba venom toxin, is a specific blocker of the L-type calcium channels. Artery 1994, 21, 287–302. [Google Scholar] [PubMed]
- Schweitz, H.; Heurteaux, C.; BoIs, P.; Moinier, D.; Romey, G.; Lazdunski, M. Calcicludine, a venom peptide of the kunitz-type protease inhibitor family, is a potent blocker of high-threshold Ca2+ channels with a high affinity for L-type channels in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.; Jolkkonen, M.; Satyapan, N.; Adem, A.; Kumlin, E.; Hellman, U.; Wernstedt, C. Protein toxins that bind to muscarinic acetylcholine receptors. Ann. N. Y. Acad. Sci 1994, 710, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.N. Muscarinic toxins from the green mamba. Pharmacol. Ther. 2000, 85, 87–109. [Google Scholar] [CrossRef]
- Harvey, A.L.; Kornisiuk, E.; Bradley, K.N.; Cerveñansky, C.; Durán, R.; Adrover, M.; Sánchez, G.; Jerusalinsky, D. Effects of muscarinic toxins MT1 and MT2 from green mamba on different muscarinic cholinoceptors. Neurochem. Res. 2002, 27, 1543–1554. [Google Scholar] [CrossRef]
- Jerusalinsky, D.; Harvey, A.L. Toxins from mamba venoms: Small proteins with selectivities for different subtypes of muscarinic acetylcholine receptors. Trends Pharmacol. Sci. 1994, 15, 424–430. [Google Scholar] [CrossRef]
- Jerusalinsky, D.; Kornisiuk, E.; Bernabeu, R.; Izquierdo, I.; Cerveñansky, C. Muscarinic toxins from the venom of Dendroaspis snakes with agonist-like actions. Toxicon 1995, 33, 389–397. [Google Scholar] [CrossRef]
- Karlsson, E.; Jolkkonen, M.; Mulugeta, E.; Onali, P.; Adem, A. Snake toxins with high selectivity for subtypes of muscarinic acetylcholine receptors. Biochimie 2000, 82, 793–806. [Google Scholar] [CrossRef]
- Quinton, L.; Girard, E.; Maiga, A.; Rekik, M.; Lluel, P.; Masuyer, G.; Larregola, M.; Marquer, C.; Ciolek, J.; Magnin, T. Isolation and pharmacological characterization of AdTx1, a natural peptide displaying specific insurmountable antagonism of the α1A-adrenoceptor. Br. J. Pharmacol. 2010, 159, 316–325. [Google Scholar] [CrossRef]
- Rouget, C.; Quinton, L.; Maïga, A.; Gales, C.; Masuyer, G.; Malosse, C.; Chamot-Rooke, J.; Thai, R.; Mourier, G.; De Pauw, E. Identification of a novel snake peptide toxin displaying high affinity and antagonist behaviour for the α2-adrenoceptors. Br. J. Pharmacol. 2010, 161, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Aalten, M.; Bakhuis, C.F.; Asaggau, I.; Wulfse, M.; van Binsbergen, M.F.; Arntz, E.R.; Troenokarso, M.F.; Oediet Doebe, J.L.; Mahamuud, U.; Belbachir, L. The clinical course and treatment of black mamba (Dendroaspis polylepis) envenomations: A narrative review. Clin. Toxicol. 2021, 59, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Oluoch, G.O.; Ainsworth, S.; Alsolaiss, J.; Bolton, F.; Arias, A.-S.; Gutiérrez, J.-M.; Rowley, P.; Kalya, S.; Ozwara, H. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl. Trop. Dis. 2017, 11, e0005969. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wüster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an ancient venom: Recognition of a novel family of cnidarian toxins and the common evolutionary origin of sodium and potassium neurotoxins in sea anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef]
- Barrett, J.; Harvey, A. Effects of the venom of the green mamba, Dendroaspis angusticeps on skeletal muscle and neuromuscular transmission. Br. J. Pharmacol. 1979, 67, 199. [Google Scholar] [CrossRef]
- Závada, J.; Valenta, J.; Kopecký, O.; Stach, Z.; Leden, P. Black mamba Dendroaspis polylepis bite: A case report. Prague Med. Rep. 2011, 112, 298–304. [Google Scholar]
- Erulu, V.E.; Okumu, M.O.; Ochola, F.O.; Gikunju, J.K. Revered but poorly understood: A case report of Dendroaspis polylepis (black mamba) envenomation in watamu, malindi Kenya, and a review of the literature. Trop. Med. Infect. 2018, 3, 104. [Google Scholar] [CrossRef]
- Adio, A.A.-I.; Malami, I.; Lawal, N.; Jega, A.Y.; Abubakar, B.; Bello, M.B.; Ibrahim, K.G.; Abubakar, M.B.; Abdussamad, A.; Imam, M.U. Neurotoxic snakebites in Africa: Clinical implications, therapeutic strategies, and antivenom efficacy. Toxicon 2024, 247, 107811. [Google Scholar] [CrossRef]
- Greene, S.C.; Cue, K.; Khan, R.; Gilbert, M.B.; Rahimi, J. Captive black mamba (Dendroaspis polylepis) bite leading to respiratory failure. J. Emerg. Med. 2023, 64, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Tan, N.H. Geographical venom variations of the Southeast Asian monocled cobra (Naja kaouthia): Venom-induced neuromuscular depression and antivenom neutralization. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 185, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.; Liang, Q.; Isbister, G.K.; Hodgson, W.C. In vitro efficacy of antivenom and varespladib in neutralising Chinese russell’s viper (Daboia siamensis) venom toxicity. Toxins 2023, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Parkington, H.C.; Isbister, G.K.; Konstantakopoulos, N.; Hodgson, W.C. Differential myotoxic and cytotoxic activities of pre-synaptic neurotoxins from Papuan Taipan (Oxyuranus scutellatus) and Irian Jayan Death Adder (Acanthophis rugosus) Venoms. Basic Clin. Pharmacol. Toxicol. 2013, 112, 325–334. [Google Scholar] [CrossRef]
- Lay, M.; Hodgson, W.C. A comparison of the efficacy of antivenoms and varespladib against the in vitro pre-synaptic neurotoxicity of Thai and Javanese Russell’s Viper (Daboia spp.) venoms. Toxins 2024, 16, 124. [Google Scholar] [CrossRef]
- Deshimaru, M.; Ogawa, T.; Nakashima, K.-i.; Nobuhisa, I.; Chijiwa, T.; Shimohigashi, Y.; Fukumaki, Y.; Niwa, M.; Yamashina, I.; Hattori, S. Accelerated evolution of Crotalinae snake venom gland serine proteases. FEBS Lett. 1996, 397, 83–88. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Harrison, R.A.; Renjifo, C.; Wüster, W. Domain loss facilitates accelerated evolution and neofunctionalization of duplicate snake venom metalloproteinase toxin genes. Mol. Biol. Evol. 2011, 28, 2637–2649. [Google Scholar] [CrossRef]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef]
- Suranse, V.; Jackson, T.N.; Sunagar, K. Contextual constraints: Dynamic evolution of snake venom phospholipase A2. Toxins 2022, 14, 420. [Google Scholar] [CrossRef]
- Sunagar, K.; Moran, Y. The rise and fall of an evolutionary innovation: Contrasting strategies of venom evolution in ancient and young animals. PLoS Genet. 2015, 11, e1005596. [Google Scholar] [CrossRef]
- Jackson, T.N.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef]
- Al-Abdulla, I.; Casewell, N.R.; Landon, J. Long-term physicochemical and immunological stability of a liquid formulated intact ovine immunoglobulin-based antivenom. Toxicon 2013, 64, 38–42. [Google Scholar] [CrossRef]
- Lister, C.; Arbuckle, K.; Jackson, T.N.; Debono, J.; Zdenek, C.N.; Dashevsky, D.; Dunstan, N.; Allen, L.; Hay, C.; Bush, B. Catch a tiger snake by its tail: Differential toxicity, co-factor dependence and antivenom efficacy in a procoagulant clade of Australian venomous snakes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 202, 39–54. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Kornhauser, R.S.; Hodgson, W.C.; Isbister, G.K. An examination of the activity of expired and mistreated commercial Australian antivenoms. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Lay, M.; Neri-Castro, E.; Zarzosa, V.; Hodgson, W.C.; Lewin, M.; Fry, B.G. Breaking muscle: Neurotoxic and myotoxic effects of Central American snake venoms and the relative efficacies of antivenom and varespladib. BMC Biol. 2024, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.; Liang, Q.; Isbister, G.K.; Hodgson, W.C. In vitro toxicity of Chinese Russell’s Viper (Daboia siamensis) Venom and Neutralisation by Antivenoms. Toxins 2022, 14, 505. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Kosakovsky Pond, S.L.; Scheffler, K. FUBAR: A fast, unconstrained bayesian approximation for inferring selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef]
- Murrell, B.; Weaver, S.; Smith, M.D.; Wertheim, J.O.; Murrell, S.; Aylward, A.; Eren, K.; Pollner, T.; Martin, D.P.; Smith, D.M. Gene-wide identification of episodic selection. Mol. Biol. Evol. 2015, 32, 1365–1371. [Google Scholar] [CrossRef]
| Species | t90 (min) |
|---|---|
| D. angusticeps | Not applicable |
| D. jamesoni jamesoni | 24 ± 4 |
| D. jamesoni kaimosae | 17 ± 3 |
| D. polylepis (Kenya) | 24 ± 3 |
| D. polylepis (South Africa) | 15 ± 1 |
| D. viridis | 29 ± 6 |
| Species | Antivenom | |||||
|---|---|---|---|---|---|---|
| Neutralisation by: | SAIMR | Inoserp Pan African | Pan Africa Premium | |||
| Against Spastic- Paralysis | Against Flaccid- Paralysis | Against Spastic- Paralysis | Against Flaccid- Paralysis | Against Spastic- Paralysis | Against Flaccid- Paralysis | |
| D. angusticeps | × | NA | Delayed | NA | × | NA |
| D. jamesoni jamesoni | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| D. jamesoni kaimosae | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| D. polylepis Kenya | ✓ | ✓ | × | ✓ | × | ✓ |
| D. polylepis South Africa | × | ✓ | NA | Partial | × | ✓ |
| D. viridis | - | ✓ | - | ✓ | - | ✓ |
| Three-Finger Toxins (3FTx) | |||||
|---|---|---|---|---|---|
| Clade | BUSTED | FUBAR (ω < 1) | FUBAR (ω > 1) | MEME | FUBAR (ω > 1) + MEME |
| Plesiotypic | 14 | 0 | 2 | 9 | 1 |
| Synergistic | 6 | 1 | 0 | 2 | 0 |
| Adrenergic + Cholinergic | 10 | 0 | 1 | 7 | 0 |
| Long chain | 10 | 1 | 2 | 7 | 1 |
| Orphan 1 | 15 | 0 | 0 | 2 | 0 |
| Orphan 2 | 15 | 0 | 1 | 2 | 1 |
| Kunitz-type toxins | |||||
| Clade | BUSTED | FUBAR (ω < 1) | FUBAR (ω > 1) | MEME | FUBAR (ω > 1) + MEME |
| Potassium channel blockers (high affinity) | 9 | 0 | 2 | 4 | 0 |
| Potassium channel blockers (low affinity) | 1 | 1 | 1 | 2 | 1 |
| Vasopressin | 7 | 0 | 0 | 2 | 0 |
| Antivenom | Manufacturer | Immunising Mixture |
|---|---|---|
| South African polyvalent | South African Institute for Medical Research (SAIMR) | Bitis arietans |
| Bitis gabonica | ||
| Dendroaspis angusticeps | ||
| Dendroaspis jamesoni | ||
| Dendroaspis polylepis | ||
| Haemachetus haemachetus | ||
| Naja annulifera | ||
| Naja melanoleuca | ||
| Naja mossambica | ||
| Naja nivea | ||
| Inoserp Pan African | Inosan Biopharma | Bitis arietans |
| Bitis gabonica | ||
| Dendroaspis jamesoni | ||
| Dendroaspis polylepis | ||
| Echis leucogaster | ||
| Echis ocellatus | ||
| Echis pyramidum | ||
| Naja haje | ||
| Naja melanoleuca | ||
| Naja nigricollis | ||
| Naja pallida | ||
| Pan African Premium | Premium Serums and Vaccines | Bitis arietans |
| Bitis gabonica | ||
| Bitis nasicornis | ||
| Bitis rhinoceros | ||
| Echis carianatus | ||
| Echis leucogaster | ||
| Echis ocellatus | ||
| Dendroaspis angusticeps | ||
| Dendroaspis jamesoni | ||
| Dendroaspis polylepis | ||
| Dendroaspis viridis | ||
| Naja haje | ||
| Naja melanoleuca | ||
| Naja nigricollis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.; Lay, M.; Seneci, L.; Hodgson, W.C.; Koludarov, I.; Senoner, T.; Soria, R.; Fry, B.G. Neurotoxic Sleight of Fang: Differential Antivenom Efficacy Against Mamba (Dendroaspis spp.) Venom Spastic-Paralysis Presynaptic/Synaptic vs. Flaccid-Paralysis Postsynaptic Effects. Toxins 2025, 17, 481. https://doi.org/10.3390/toxins17100481
Jones L, Lay M, Seneci L, Hodgson WC, Koludarov I, Senoner T, Soria R, Fry BG. Neurotoxic Sleight of Fang: Differential Antivenom Efficacy Against Mamba (Dendroaspis spp.) Venom Spastic-Paralysis Presynaptic/Synaptic vs. Flaccid-Paralysis Postsynaptic Effects. Toxins. 2025; 17(10):481. https://doi.org/10.3390/toxins17100481
Chicago/Turabian StyleJones, Lee, Mimi Lay, Lorenzo Seneci, Wayne C. Hodgson, Ivan Koludarov, Tobias Senoner, Raul Soria, and Bryan G. Fry. 2025. "Neurotoxic Sleight of Fang: Differential Antivenom Efficacy Against Mamba (Dendroaspis spp.) Venom Spastic-Paralysis Presynaptic/Synaptic vs. Flaccid-Paralysis Postsynaptic Effects" Toxins 17, no. 10: 481. https://doi.org/10.3390/toxins17100481
APA StyleJones, L., Lay, M., Seneci, L., Hodgson, W. C., Koludarov, I., Senoner, T., Soria, R., & Fry, B. G. (2025). Neurotoxic Sleight of Fang: Differential Antivenom Efficacy Against Mamba (Dendroaspis spp.) Venom Spastic-Paralysis Presynaptic/Synaptic vs. Flaccid-Paralysis Postsynaptic Effects. Toxins, 17(10), 481. https://doi.org/10.3390/toxins17100481







