Abstract
Oromandibular dystonia (OMD) induces severe motor impairments, such as masticatory disturbances, dysphagia, and dysarthria, resulting in a serious decline in quality of life. Non-invasive brain-imaging techniques such as electroencephalography (EEG) and magnetoencephalography (MEG) are powerful approaches that can elucidate human cortical activity with high temporal resolution. Previous studies with EEG and MEG have revealed that movements in the stomatognathic system are regulated by the bilateral central cortex. Recently, in addition to the standard therapy of botulinum neurotoxin (BoNT) injection into the affected muscles, bilateral deep brain stimulation (DBS) has been applied for the treatment of OMD. However, some patients’ OMD symptoms do not improve sufficiently after DBS, and they require additional BoNT therapy. In this review, we provide an overview of the unique central spatiotemporal processing mechanisms in these regions in the bilateral cortex using EEG and MEG, as they relate to the sensorimotor functions of the stomatognathic system. Increased knowledge regarding the neurophysiological underpinnings of the stomatognathic system will improve our understanding of OMD and other movement disorders, as well as aid the development of potential novel approaches such as combination treatment with BoNT injection and DBS or non-invasive cortical current stimulation therapies.
Keywords:
oromandibular dystonia; botulinum neurotoxin; stomatognathic function; deep brain stimulation; sensorimotor function; magnetoencephalography; electroencephalography; motor function; globus pallidus Key Contribution:
The uncertain effects of deep brain stimulation (DBS) on oromandibular dystonia may, in part, be explained by the unique characteristics of the bilateral central regulation of sensorimotor functions in the stomatognathic system. An improved understanding of the central mechanisms related to stomatognathic functions will promote the development of new approaches, such as combination treatment with botulinum neurotoxin injection and DBS or non-invasive cortical current stimulation therapies.
1. Introduction
The stomatognathic system is an anatomic and functional unit comprising the mandible, maxilla, dental arches, teeth, temporomandibular joint, masticatory muscles, surrounding nerves and vessels, and salivary glands. The stomatognathic system plays important roles in a variety of critical motor functions, including mastication, swallowing, speech production, and respiration. Focal dystonia in the stomatognathic system induces severe motor dysfunction that negatively affects quality of life via factors such as masticatory disturbances, limited mouth opening, dysphagia, and dysarthria caused by involuntary movements in the stomatognathic system [1,2,3].
Dystonia is characterized by sustained or intermittent muscle contractions that produce involuntary, unwanted, and often repetitive movements, postural changes, or both [4]. Focal dystonia includes blepharospasm (eyelids), cervical dystonia (neck), writer’s cramp, musician’s cramp (hand and arm), and oromandibular dystonia (OMD) [5]. OMD is a focal type of dystonia that involves the masticatory, lower facial, lingual, or orbicularis oris muscles [1,2,3,6]. Clinically, OMD presents as dystonia during jaw opening or closing, jaw deviation, jaw protrusion, lingual dystonia, lip dystonia, or a combination of these abnormal movements [7].
When OMD occurs in conjunction with blepharospasm, it is usually referred to as Meige syndrome. Meige syndrome, which was first described by Henri Meige in 1910, is cranial dystonia characterized by the combination of upper and lower cranial involvement and including blepharospasm and OMD [8]. In Meige’s syndrome, there is progressive worsening of blepharospasm with the spread of symptoms to involve the oromandibular, cervical, and limb muscles; however, the symptoms spread earliest and greatest in the oromandibular muscles [9,10,11].
A recent multicenter study conducted among Institutions across seven countries found that OMD had a prevalence of 8.7% among all types of focal dystonia, making it one of the less prevalent subtypes of this neurological disorder [12]. The overall prevalence of primary dystonia was calculated as 16.4 per 100,000 cases in a meta-analysis [13], and that of OMD was estimated at 6.8 per 100,000 cases [14]. Another study reported an estimated OMD prevalence rate of 0.33 per 100,000 cases [15]. However, most of the studies evaluated relatively few patients with OMD. A recent epidemiologic study of 144 patients with OMD reported an estimated prevalence of 9.8 per 100,000 cases, suggesting that OMD is as common as cervical dystonia or blepharospasm [16].
Botulinum neurotoxin (BoNT) injection represents a standard therapy for focal dystonia, including cases of OMD [3,17]. BoNT is injected into the target muscles exhibiting disordered movements where it blocks acetylcholine release at the neuromuscular junction (NMJ). Proper muscle identification, dose selection, and management of patient expectations are required to ensure that BoNT injection is both safe and effective in patients with OMD [3], since stomatognathic functions are performed by various bilateral muscles in the stomatognathic system that differ in size and tension. Moreover, since each muscle of the stomatognathic system is controlled by the bilateral cortex through the corticobulbar tract and certain cranial nerves (CNs (CN V, VII, X, XII)), identifying the neurophysiological and neuroanatomical basis of sensorimotor functions in the stomatognathic system remains essential.
In addition to BoNT injection, deep brain stimulation (DBS) represents an optional therapy for focal dystonia in the limbs [18]. The main target of conventional DBS is the internal segment of the unilateral globus pallidus (GPi). DBS of the unilateral GPi improves symptoms of disordered movement on the contralateral side, based on the principle that unilateral movements in the limbs are regulated by the contralateral sides of the central regions via the corticospinal tract (Figure 1). However, the application of DBS for OMD has been limited, and evidence regarding its clinical utility in these patients is still considered preliminary.
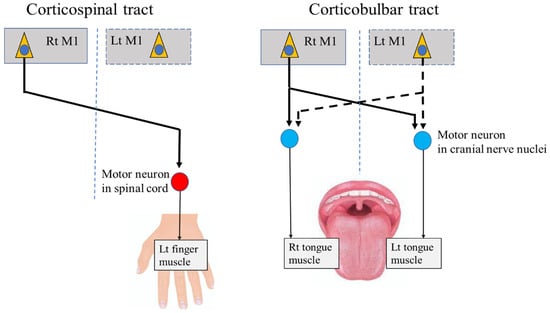
Figure 1.
Corticospinal tract and corticobulbar tract. M1: primary motor cortex, Rt: right, Lt: left.
Several case reports from the past two decades have described the use of DBS of the bilateral Gpi for the treatment of lingual dystonia [19,20,21,22]. This bilateral approach is based on the neurophysiological principle that tongue movements are regulated by the bilateral central regions through the corticobulbar tract, which differs from the contralateral regulation of the limbs (Figure 1). The uncertain effects of DBS on OMD may, in part, be explained by the unique characteristics of the bilateral central regulation of sensorimotor functions in the stomatognathic system. To establish effective treatments targeting the central regions, including DBS, further research is required to elucidate the neurophysiological basis and central mechanisms related to stomatognathic functions.
Recent advances in non-invasive electromagnetic physiological techniques, such as electroencephalography (EEG) and magnetoencephalography (MEG), have enabled the investigation of cortical mechanisms in humans with high temporal resolution. In particular, MEG is advantageous owing to its high spatial resolution given that the magnetic permeability of biological tissues is nearly identical to that of empty space; thus, the magnetic field is not distorted by the scalp or skull [23].
In this review, we present an overview of the unique central mechanisms of bilateral spatiotemporal processing associated with the sensorimotor functions of the stomatognathic system. These mechanisms have been demonstrated in MEG/EEG studies using multiple parameters for analysis, including somatosensory evoked fields/potentials (SEFs/SEPs), movement-related cortical fields/potentials (MRCFs/MRCPs), and cortico-muscular/cortico-kinematic coherence (CMC/CKC). We also discuss the pathophysiological characteristics of typical cases of hand dystonia, such as writer’s cramp and OMD, as demonstrated using EEG and MEG. Finally, we identify current problems and future directions in OMD treatment.
2. Neural Pathways from the Primary Motor Cortex (M1) to Stomatognathic Systems
In the human primary motor cortex (M1), the cortical representations of various body parts are organized in the form of the “classical homunculus” [24]. In general, the homunculus resembles an upside-down map of each body part, in which the stomatognathic systems are closer to the lateral sulcus than the upper and lower extremities. The area of the M1 that represents the stomatognathic system is widely distributed relative to its actual size in the body [24], suggesting a rich innervation of these areas.
Anatomically, each muscle of the stomatognathic system receives innervation from the bilateral M1 through the corticobulbar tract, which terminates in motor neurons within the CN nuclei (Figure 1). In contrast, the muscles of the upper and lower limbs are predominantly innervated by the contralateral cortex through the corticospinal tract, which terminates in motor neurons within the spinal cord (Figure 1). Indeed, a previous study in patients with epilepsy showed that direct unilateral cortical stimulation of the M1 areas for the oral and upper limb muscles induced bilateral oral movements but unilateral limb movements [24].
The stomatognathic system is composed of various muscles, including the tongue muscles, orbicularis oris, and masticatory muscles responsible for jaw closing (masseter, temporalis, and medial pterygoid) and opening (inferior head of lateral pterygoid, anterior belly of digastric, geniohyoid, and mylohyoid). All the muscles of the tongue are innervated by the hypoglossal nerve (CN XII), except for the palatoglossal muscles, which are innervated by the vagus nerve (CN X). Masticatory muscles are innervated by the trigeminal nerve (CN V), except for the posterior bellies of the digastric muscles, which are innervated by the facial nerve (CN VII). The orbicularis oris muscle is innervated by the facial nerve (CN VII).
Moreover, bilateral innervation contributes to somatosensory sensations in the stomatognathic system. Somatosensory information from the tongue is transmitted by the mandibular branch of the trigeminal nerve (CN V) (anterior two-thirds of the tongue), glossopharyngeal nerve (CN IX) (posterior one-third of the tongue), and vagus nerve (posterior part of the tongue root) (CN X). Somatosensory information from the soft palate, teeth, gingiva, face, and lips is transmitted by the mandibular or maxillary branches of the trigeminal nerve (CN V). Proprioceptive sensations from the tongue muscle are transmitted by the hypoglossal nerve (CN XII) and its rich innervation of the related muscle spindles. Proprioceptive sensations from the masticatory muscles are mainly transmitted by the trigeminal nerve (CN V) for jaw-closing muscles, as the jaw-closing muscles have many more muscle spindles than the jaw-opening muscles [25].
3. Bilateral Brain Activation Related to Stomatognathic Functions
A previous study involving non-invasive, whole-head MEG successfully identified the classical homunculus in the primary somatosensory cortex following tactile stimulation of multiple points on the human body [26]. Cortical representations of oromandibular areas are widely distributed in the primary sensorimotor cortex relative to their actual size in the human body [26]. In this section, we describe in detail the unique central mechanisms of bilateral spatiotemporal information processing involved in the sensorimotor functions of the stomatognathic system, as demonstrated using MEG/EEG.
3.1. Movement-Related Cortical Fields and Potentials
In early studies, researchers observed slowly increasing cortical fields over the contralateral hemisphere before the onset of voluntary unilateral movements of a finger, which they termed MRCFs [27,28]. Cheyne et al. [29] first reported MRCFs associated with repetitive tongue protrusion in a patient using a seven-channel MEG system to record from the left hemisphere. Nakasato et al. [30] demonstrated whole-head MRCFs for tongue protrusion in five healthy volunteers, using a trigger signal that was detected when the tip of the tongue reached the anterior region of the palate. They reported that the peak magnitude of the MRCFs was derived from the areas of the bilateral M1 region representing the tongue. A recent MEG study investigated the characteristics of bilateral MRCFs before and after voluntary self-paced tongue movements, using surface electromyography (EMG) from the tongue [31]. As reported in previous MRCF studies of finger movement [27,28,32], three components were detected in response to tongue movement: the readiness field (RF), motor field (MF), and movement-evoked field (MEF). Slowly increasing RF components were observed bilaterally before the onset of movement, peaking in MFs around the onset of movement. The MF component appeared after the pre-movement RF component and originated from part of the primary motor area (M1) representing the tongue. The MEF component, which appears after movement onset and originates from the bilateral primary somatosensory area (S1), may reflect proprioceptive feedback from the tongue during voluntary tongue movement. These results suggest that the bilateral M1 and S1 are involved in the preparation and execution of tongue movements [31].
MRCPs are slowly increasing cortical shifts in negative potentials that occur prior to voluntary movements, as demonstrated using EEG [33]. In an EEG study of MRCPs related to jaw movement, a previous study [33] reported a slowly increasing negative potential originating 1.5–2 s before EMG onset, known as the Bereitschaftspotential (BP) [34,35]. The BP, also called the readiness potential, is a measure of brain activity in the motor cortex and supplementary motor area leading up to voluntary muscle movement. The maximum BP occurred over the vertex region, and the negative slope (NS’) occurred approximately 300–700 ms before EMG onset. The authors reported that BP/NS’ amplitudes at the onset of movement differed significantly among open, closed, and lateral movements. The MRCPs at mouth opening and closing were symmetrically distributed, whereas those at lateral movements were predominant over the hemisphere ipsilateral to the direction of movement.
In an MEG study of jaw movement in five healthy volunteers [36], RFs were observed bilaterally, starting around 860 and 600 ms prior to the onset of masseter and digastric EMGs, respectively, and gradually increasing in magnitude until peaking within 100 ms before EMG onset. In all participants, the equivalent current dipoles generating the RFs accompanying jaw-opening movements were located in the bilateral M1 areas representing the jaw. These results suggest that MRCF/MRCP recordings provide a means for exploring the spatiotemporal characteristics of the sensorimotor cortex before and after voluntary movements in the stomatognathic system.
3.2. Cortico-Muscular Coherence and Cortico-Kinematic Coherence during Voluntary Movements
CMC analyses can aid in evaluating oscillatory functional connections between the motor cortex and corresponding peripheral muscles during sustained muscle contraction [37,38]. The CMC in the β-frequency band (β-CMC) during the sustained unilateral movements of a finger mainly represents the descending motor commands from the M1 to the contralateral finger muscle through corticospinal pathways [38]. In our previous MEG study [39], the CMC of the tongue was detected at two different frequency bands (the β-band and a low-frequency band at 2–10 Hz) over both hemispheres for each side of the tongue during isometric tongue protrusion [39,40]. The β-CMC for the tongue mainly reflects the descending motor commands from each side of the M1 to both sides of the tongue, with contralateral dominance through hypoglossal motoneuron pools (Figure 2). Moreover, the somatotopic organization of the tongue and finger regions in the M1 allows for differentiation of the cortical source locations of the β-CMC for the tongue and finger [39]. In contrast to β-CMC, CMC in the low-frequency band (low-CMC) reflects oscillatory coupling related to proprioceptive feedback from the tongue muscles to the bilateral S1 [40]. This pattern of central regulation with oscillatory activity in two frequency bands is a unique characteristic of stomatognathic functions, since a stable low-CMC is not detected during finger movements.
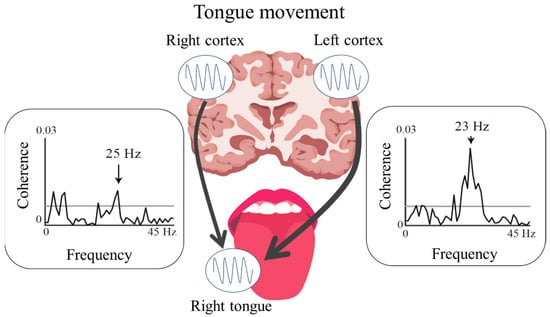
Figure 2.
Bilateral functional connections between the cortex and tongue identified using cortico-muscular coherence analysis. The figure shows contralateral (left hemispheric) dominance of the functional connection between the cortex and right side of the tongue.
CKC methods are used to quantify the coupling between MEG signals and finger kinematics, which are measured using an accelerometer during repetitive, rhythmic, or voluntary finger movements [41]. A recent MEG study has demonstrated that CKC data mainly reflect proprioceptive sensory feedback from the peripheral muscles to S1 [42]. However, applying conventional CKC to stomatognathic systems is difficult, as magnetic accelerometer devices can easily produce excessive magnetic artefacts due to the short distance between the brain and stomatognathic systems. To overcome this limitation, a recent MEG study successfully utilized a deep-learning-assisted motion capture system, in which the CKC was detected during rhythmic tongue movements at the frequency peaks of movement or its harmonics, based on signals in the bilateral primary sensorimotor cortex representing the tongue regions [43]. This combination of CKC and deep learning-assisted motion capture has the advantage of being noise-, movement-, and risk-free, particularly in the oral regions, because recording devices are not placed in the orofacial region. As the tongue muscles and those responsible for closing the jaw are rich in muscle spindles [25], the CKC approach may help to reveal the central proprioceptive mechanisms of the stomatognathic system and the pathophysiology of OMD involving impairments in proprioceptive pathways.
3.3. Somatosensory-Evoked Fields and Potentials
Since the first reports of SEFs induced by trigeminal nerve stimulation in the early 1980s [44] and 1990s [45], many MEG studies have sought to assess SEF characteristics in the tongue [45,46,47,48], lips [49,50], palate [51,52], teeth [44], gingiva [49], and lower face [53].
Following tongue stimulation, the initial SEF components occur over the bilateral hemispheres at 19 ms with electrical stimulation [47] and 15 ms with tactile stimulation [48], with an anterior current orientation. These timings are consistent with a SEP latency of 16 ms following electrical stimulation of the lip via chronically implanted subdural electrodes over the lower perirolandic area in patients undergoing epilepsy surgery [54]. However, the initial component of trigeminal SEF/SEP is not detected stably due to the low amplitude of this component and is easily masked by excessive stimulation artefacts. Thus, in clinical settings, a prominent component with a large amplitude at a middle latency ranging from 25 ms to 80 ms, with a posterior current orientation, is often adopted [45,46,49,52,53,55]. Indeed, Previous studies reported that the middle-latency component of the trigeminal SEF can be used to evaluate sensory disturbances of the tongue and lip caused by injury to the trigeminal nerve during dental surgery [50,56,57]. Source localization studies involving MEG with high spatial resolution have revealed that the initial and middle-latency components of SEFs stimulated in the stomatognathic system are derived from the bilateral S1, specifically, the posterior bank of the central sulcus [46,47,48,49,50]. This neurophysiological finding is consistent with the anatomical principle that the unilateral lingual nerve innervating the anterior part of the tongue projects to both sides of area 3b in S1 via the trigeminothalamic tract, with contralateral dominance.
4. Oromandibular Dystonia
4.1. Pathophysiology of OMD
Although the pathogenesis of primary dystonia is still a matter of debate, several probable explanations have been proposed, including basal ganglia dysfunction, hyperexcitability of motor neurons, loss of inhibition, aberrant dopamine signaling, monoaminergic dysfunction, abnormal plasticity, and abnormal sensory function [58,59,60,61]. Most previous EEG studies of focal dystonia in humans have focused on cortical sensorimotor dysfunction, especially of the primary sensorimotor cortex and premotor areas, in cases affecting the hand (e.g., writer’s cramp) [59].
MRCPs associated with hand movements consisted of at least two slow negative shifts. The early component is the BP, which is maximal at the vertex and symmetrically distributed over the scalp, whereas the latter component is called NS’, which is largest in the central area contralateral to the hand movement [62]. The BP represents the activation of supplementary motor areas (SMAs), while NS’ reflects activities in both the SMA and M1 [63,64,65]. However, the precise anatomical representations of the BP and NS’ remain controversial.
Several EEG studies have reported abnormalities in MRCPs in patients with focal dystonia [66,67,68]. Reductions in both NS’ and BP gradients have been observed in patients with symptomatic dystonia caused by lesions in the basal ganglia and anterior thalamus. Furthermore, reductions in NS’ amplitude over the contralateral central region have been observed in patients with writer’s cramp [66,67]. These results suggest impaired activation of the sensorimotor cortex contralateral to the affected hand immediately before a voluntary movement. One study also reported abnormalities in the cortical preparatory processes for voluntary muscle relaxation or motor inhibition in patients with focal hand dystonia [68]. The BP associated with voluntary muscle relaxation was reduced over the central region of the affected hemisphere in patients with focal hand dystonia, suggesting impaired activation of the inhibitory motor systems in the contralateral cortex in these patients [68]. Moreover, a functional magnetic resonance imaging (fMRI) study reported smaller activation volumes in the SMA proper and primary sensorimotor cortex during voluntary muscle contraction and relaxation of the affected (right) hand in patients with writer’s cramp than in healthy volunteers [69]. Disturbances in sensorimotor integration have also been documented as a task-specific decrease in the amplitude of contingent negative variation [70,71], abnormal pre-movement gating of somatosensory input [72], and abnormal CMC with MEG [73]. Other studies using paired-pulse transcranial magnetic stimulation have reported impairments in short-interval intracortical inhibition [74] and surround inhibition [75]. Somatotopic disorganization of the fingers has also been observed in the S1 contralateral to the affected hand [76]. These findings collectively demonstrate that focal dystonia of the hand involves impaired cortical inhibition in M1, abnormal sensorimotor integration, and disorganization of S1 [69,70].
A few EEG studies have also investigated the pathological characteristics of OMD. A study compared MRCPs associated with mandibular movements in six patients with OMD and eight healthy controls [35]. In the patient group, MRCP amplitudes over the central and parietal areas for mouth opening and lateral movements were significantly reduced compared to those observed in the control group. Moreover, in controls, the MRCPs at mouth opening and closing were symmetrically distributed, whereas those at lateral movements were predominant over the hemisphere ipsilateral to the direction of movement. This laterality was lost in the patient group. These results suggest that OMD is associated with bilateral impairments in cortical preparatory processing during jaw movement. A few studies have also investigated OMD pathology using fMRI and positron emission tomography. One fMRI study reported reduced activation of the primary sensorimotor cortex and premotor/sensory association cortices during vocalization in patients with laryngeal dystonia [77]. Analogous to the motor dysfunction observed in patients with dystonia, reduced primary somatosensory activity has previously been demonstrated [78,79], supporting the conceptualization of dystonia as a partial sensory disorder [80,81].
4.2. Treatment Options for OMD
BoNT injection represents the standard therapy for patients with focal dystonia such as blepharospasm, cervical dystonia, and OMD [82,83,84]. When the target muscles, doses, and patient expectations are appropriately managed, BoNT injection is a safe and effective approach for the treatment of OMD [3]. Some studies reported the effectiveness of ultrasound-guided injections in facial muscles to avoid injuring the neural vascular structures during botulinum toxin injection [85,86].
BoNT is a microbial protein that exists in seven serotypes, designated A through G. Its ability to block acetylcholine release at the NMJ accounts for its therapeutic effects in various movement disorders associated with increased muscle tone or muscle overactivity. BoNT wields these effects by entering nerve endings at the NMJ and cleaving soluble N-ethylmaleimide-sensitive factor-attachment protein receptors, thereby preventing the vesicular release of acetylcholine from the synaptic terminal and producing the effects of muscle relaxation and flaccid paralysis [87,88].
In addition to the effect on the peripheral nervous system, BoNT may also indirectly influence the functional organization of the central nervous system associated with altered peripheral inputs [89,90]. Moreover, the central effect of BoNT injection is not limited to the cortical and subcortical regions of the treated muscles but extends beyond the neural circuits for the control of the affected body parts [89,91,92]. Further studies with non-invasive brain-imaging techniques, including MEG and EEG, may help to reveal the bilateral cortical areas affected by therapy with BoNT injection in patients with OMD.
Other treatments for focal dystonia include invasive approaches, such as DBS, targeting central regions [93,94,95]. When symptoms cannot be adequately managed through medication and rehabilitation, DBS is typically employed as a surgical intervention to treat movement disorders. The main DBS target for dystonia treatment is the internal segment of the GPi [96], which has proven efficacious in the treatment of generalized, segmental, and cervical dystonia [97,98,99]. Although evidence concerning the efficacy of DBS for other dystonia subtypes remains scarce, DBS targeting the GPi has demonstrated continued efficacy in patients with Meige syndrome [94,100,101,102]. A recent meta-analysis showed that DBS may be useful for treating refractory Meige syndrome [103]. However, other studies have demonstrated that in some patients with craniocervical and craniofacial segmental dystonia, DBS is ineffective for improving stomatognathic functions such as speech and swallowing but is effective for improving blepharospasm [95,104,105]. A recent study of DBS in 18 patients with dystonia related to KMT2B mutations reported that the greatest improvements in motor function were observed in patients with trunk and cervical dystonia, with less clinical impact observed in patients with laryngeal dystonia [106].
Currently, only three published case reports have discussed bilateral GPi-DBS for OMD, all of which were categorized as lingual dystonia. Chung et al. [20] and Asahi et al. [22] reported symptomatic improvements in lingual dystonia following bilateral GPi-DBS in two cases and one case, respectively. In patients with OMD, the unique neuroanatomical characteristics of bilateral innervation in each side of the stomatognathic system may complicate the application of conventional DBS compared to that in patients with focal dystonia of the limbs, as previous studies have reported that sufficient effects are sometimes difficult to obtain with interventions targeting the unilateral hemisphere. As contralateral dominance is involved in the control of voluntary movement in the stomatognathic system, it may be beneficial for future research to explore the most effective stimulation parameters for bilateral DBS targeting the GPi in patients with OMD. However, some patients’ OMD symptoms do not improve sufficiently even after DBS, and they require additional BoNT therapy. A highly individualized injection technique has been developed for lingual dystonia according to the direction of deviation [107]. An improved understanding of the central and peripheral mechanisms related to stomatognathic functions may promote the development of new treatments for OMD, such as the combination of BoNT injection into the affected muscles as peripheral therapy and DBS as central therapy.
Several authors have also discussed the use of unique oral appliances (e.g., oral splints) for OMD treatment [108,109,110,111,112,113]. Previous research has shown that performing certain sensory tricks (e.g., pressing the teeth or lips with the fingers; placing cigarettes, chewing gum, or other objects in the mouth; singing; and humming) may benefit a third of patients with OMD [109,114,115]. Oral appliances may be particularly useful in cases where they successfully mimic the patient’s sensory tricks [109,114], as treatment responses in such patients may be linked to sensory tricks perceived by the brain to be beneficial. Although the precise mechanism underlying this phenomenon remains unknown, the beneficial effect of splint use on OMD may be related to the modulation of hyperactive dystonic networks by altered proprioceptive feedback and antagonist activation [114]. MEG recordings with CKC and low-CMC analyses are useful for evaluating proprioceptive feedback from muscles to the cortex during voluntary movements in the stomatognathic system (see Section 3.2). These approaches may aid in revealing the effects of oral appliance therapy on abnormal cortical activity related to proprioceptive feedback in patients with OMD.
5. Future Directions
BoNT injection is the standard peripheral therapy for patients with OMD. In addition, central therapies such as bilateral DBS can be applied, providing substantial relief in patients with disordered movements. However, the introduction of stimulating electrodes deep in the brain carries significant risks, including the risk of hemorrhage [116]. Beyond DBS, recent studies have highlighted the potential of non-invasive cortical current stimulation therapies such as transcranial direct current stimulation (tDCS) [117,118] and transcranial alternating-current stimulation (tACS) [119] for patients with limb movement disorders, although their effectiveness and mechanisms of action are still unclear [120,121,122]. For example, a previous study successfully reported the non-invasive application of tACS over the M1 [119], which induced phase cancellation of the resting tremor rhythm in the upper limbs of healthy volunteers. In the future, applying tACS over the bilateral M1 in the stomatognathic system may aid in the treatment of OMD given the bilateral cortical control of this system. Indeed, in our previous study, tDCS of the bilateral M1 area representing the tongue region significantly increased the excitability of this region and improved tongue motor functions compared to the application of tDCS to the unilateral M1 of the tongue region in healthy volunteers [123].
6. Conclusions
In this review, we provide an overview of the unique central spatiotemporal processing mechanisms in the stomatognathic system. The uncertain effects of DBS on OMD may, in part, be explained by the unique characteristics of the bilateral central regulation of sensorimotor functions in the stomatognathic system. Further exploration of the neurophysiological underpinnings of stomatognathic functions will lead to an improved understanding of the etiology of OMD and may lead to innovative approaches for future symptomatic and disease-modifying treatments.
7. Methods
This literature review was conducted based on the comprehensive analysis of electronic medical literature databases (PubMed, Scopus, EMBASE, and Google scholar) prior to 1 September 2022. Search keywords included oromandibular dystonia, orofacial dystonia, mandibular dystonia, jaw dystonia, lingual dystonia, Meige syndrome, botulinum toxin, botulinum toxin therapy, deep brain stimulation, transcranial direct current stimulation (tDCS), and transcranial alternating-current stimulation (tACS) for Section 1, Section 4 and Section 5 of the manuscript. We also searched the terms trigeminal nerve, lingual nerve, tongue, palate, face, lip, oromandibular, stomatognathic system, sensory disturbance, sensory abnormality, electroencephalography (EEG), and magnetoencephalography (MEG) for Section 1, Section 2 and Section 3 and Section 5. Each search result was independently reviewed for eligibility by the author (H.M.). No restriction was placed with respect to the original text language.
Author Contributions
Conceptualization, H.M. and K.Y.; Writing–Original Draft Preparation, H.M.; Writing–Review and Editing, K.Y. and M.H.; Funding Acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant numbers JP19K10218 and JP22H03452 to H.M.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Scorr, L.M.; Factor, S.A.; Parra, S.P.; Kaye, R.; Paniello, R.C.; Norris, S.A.; Perlmutter, J.S.; Bäumer, T.; Usnich, T.; Berman, B.D.; et al. Oromandibular dystonia: A clinical examination of 2020 cases. Front. Neurol. 2021, 12, 700714. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum toxin therapy for oromandibular dystonia and other movement disorders in the stomatognathic system. Toxins 2022, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, C.; Berardelli, A. Clinical phenomenology of dystonia. Int. Rev. Neurobiol. 2011, 98, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Manzo, N.; Ginatempo, F.; Belvisi, D.; Defazio, G.; Conte, A.; Deriu, F.; Berardelli, A. Pathophysiological mechanisms of oromandibular dystonia. Clin. Neurophysiol. 2022, 134, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Development and validation of a disease-specific oromandibular dystonia rating scale (OMDRS). Front. Neurol. 2020, 11, 583177. [Google Scholar] [CrossRef]
- Meige, H. Les convulsions de la face: Une forme clinique de convulsion faciale, bilatérale et médiane. Rev. Neurol. 1910, 10, 437–443. [Google Scholar]
- Weiss, E.M.; Hershey, T.; Karimi, M.; Racette, B.; Tabbal, S.D.; Mink, J.W.; Paniello, R.C.; Perlmutter, J.S. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov. Disord. 2006, 21, 1175–1181. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Berardelli, A.; Girlanda, P.; Marchese, R.; Martino, D.; Morgante, F.; Avanzino, L.; Colosimo, C.; Defazio, G. Long-term assessment of the risk of spread in primary late-onset focal dystonia. J. Neurol. Neurosurg. Psychiatry 2008, 79, 392–396. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, S. Meige’s syndrome: History, epidemiology, clinical features, pathogenesis and treatment. J. Neurol. Sci. 2017, 15, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Pirio Richardson, S.; Wegele, A.R.; Skipper, B.; Deligtisch, A.; Jinnah, H.A.; Dystonia Coalition Investigators. Dystonia treatment: Patterns of medication use in an international cohort. Neurology 2017, 88, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Steeves, T.D.; Day, L.; Dykeman, J.; Jette, N.; Pringsheim, T. The prevalence of primary dystonia: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Muenter, M.D.; Aronson, A.; Kurland, L.T.; Melton, L.J. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988, 3, 188–194. [Google Scholar] [CrossRef]
- Cardoso, F.; Jankovic, J. Dystonia and dyskinesia. Psychiatr. Clin. N. Am. 1997, 20, 821–838. [Google Scholar] [CrossRef]
- Yoshida, K. Prevalence and incidence of oromandibular dystonia: An oral and maxillofacial surgery service-based study. Clin. Oral Investig. 2021, 25, 5755–5764. [Google Scholar] [CrossRef]
- Hallett, M.; Benecke, R.; Blitzer, A.; Comella, C.L. Treatment of focal dystonias with botulinum neurotoxin. Toxicon 2009, 54, 628–633. [Google Scholar] [CrossRef]
- Tisch, S. Deep brain stimulation in dystonia: Factors contributing to variability in outcome in short and long term follow-up. Curr. Opin. Neurol. 2022, 35, 510–517. [Google Scholar] [CrossRef]
- Schneider, S.A.; Aggarwal, A.; Bhatt, M.; Dupont, E.; Tisch, S.; Limousin, P.; Lee, P.; Quinn, N.; Bhatia, K.P. Severe tongue protrusion dystonia: Clinical syndromes and possible treatment. Neurology 2006, 67, 940–943. [Google Scholar] [CrossRef]
- Chung, J.C.; Kim, J.P.; Chang, W.S.; Kim, H.Y.; Chang, J.W. Bilateral pallidal stimulation for “sticking-out tongue” feature in patients with primary focal tongue protrusion dystonia. Neuromodulation 2014, 17, 133–137. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Y.; Huang, P.; Wang, T.; Zhang, C.; Sun, B.; Li, D.; Li, H.; Wu, Y. Subthalamic deep brain stimulation in lingual dystonia: A case series study. Park. Relat. Disord. 2021, 88, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Asahi, T.; Ikeda, K.; Yamamoto, J.; Tsubono, H.; Muro, Y.; Sato, S. Bilateral pallidal stimulation with directional leads for primary focal lingual dystonia. Stereotact. Funct. Neurosurg. 2021, 99, 207–211. [Google Scholar] [CrossRef]
- Hari, R.; Salmelin, R. Magnetoencephalography: From SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage 2012, 61, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Kubota, K.; Masegi, T. Muscle spindle supply to the human jaw muscle. J. Dent. Res. 1977, 56, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Yamada, T.; Goto, A.; Kato, T.; Ito, K.; Abe, Y.; Kachi, T.; Kakigi, R. Somatosensory homunculus as drawn by MEG. Neuroimage 1998, 7, 377–386. [Google Scholar] [CrossRef]
- Deecke, L.; Weinberg, H.; Brickett, P. Magnetic fields of the human brain accompanying voluntary movement: Bereitschaftsmagnetfeld. Exp. Brain Res. 1982, 48, 144–148. [Google Scholar] [CrossRef]
- Hari, R.; Antervo, A.; Katila, T.; Poutanen, T.; Seppänen, M.; Tuomisto, T.; Varpula, T. Cerebral magnetic fields associated with voluntary limb movements in man. Il Nuovo Cimento D 1983, 2, 484–494. [Google Scholar] [CrossRef]
- Cheyne, D.; Kristeva, R.; Deecke, L. Homuncular organization of human motor cortex as indicated by neuromagnetic recordings. Neurosci. Lett. 1991, 122, 17–20. [Google Scholar] [CrossRef]
- Nakasato, N.; Itoh, H.; Hatanaka, K.; Nakahara, H.; Kanno, A.; Yoshimoto, T. Movement-related magnetic fields to tongue protrusion. Neuroimage 2001, 14, 924–935. [Google Scholar] [CrossRef]
- Maezawa, H.; Oguma, H.; Hirai, Y.; Hisadome, K.; Shiraishi, H.; Funahashi, M. Movement-related cortical magnetic fields associated with self-paced tongue protrusion in humans. Neurosci. Res. 2017, 117, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Kajola, M.; Salmelin, R.; Shibasaki, H.; Hari, R. Movement-related slow cortical magnetic fields and changes of spontaneous MEG- and EEG-brain rhythms. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 274–286. [Google Scholar] [CrossRef]
- Kornhuber, H.H.; Deecke, L. Changes in the brain potential in voluntary movements and passive movements in man: Readiness potential and reafferent potentials. Pflüger’s Arch. Gesamte Physiol. Menschen Tiere 1965, 284, 1–17. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Hamano, T.; Kohara, N.; Kimura, J.; Shibasaki, H.; Iizuka, T. Cortical potentials associated with voluntary mandibular movements. J. Dent. Res. 2000, 79, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Kohara, N.; Murase, N.; Ikeda, A.; Shibasaki, H.; Iizuka, T. Movement-related cortical potentials before jaw excursions in oromandibular dystonia. Mov. Disord. 2003, 18, 94–100. [Google Scholar] [CrossRef]
- Shibukawa, Y.; Shintani, M.; Kumai, T.; Suzuki, T.; Nakamura, Y. Cortical neuromagnetic fields preceding voluntary jaw movements. J. Dent. Res. 2004, 83, 572–577. [Google Scholar] [CrossRef]
- Conway, B.A.; Halliday, D.M.; Farmer, S.F.; Shahani, U.; Maas, P.; Weir, A.I.; Rosenberg, J.R. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J. Physiol. 1995, 489, 917–924. [Google Scholar] [CrossRef]
- Mima, T.; Hallett, M. Corticomuscular coherence: A review. J. Clin. Neurophysiol. 1999, 16, 501–511. [Google Scholar] [CrossRef]
- Maezawa, H.; Mima, T.; Yazawa, S.; Matsuhashi, M.; Shiraishi, H.; Hirai, Y.; Funahashi, M. Contralateral dominance of corticomuscular coherence for both sides of the tongue during human tongue protrusion: An MEG study. Neuroimage 2014, 101, 245–255. [Google Scholar] [CrossRef]
- Maezawa, H.; Mima, T.; Yazawa, S.; Matsuhashi, M.; Shiraishi, H.; Funahashi, M. Cortico-muscular synchronization by proprioceptive afferents from the tongue muscles during isometric tongue protrusion. Neuroimage 2016, 128, 284–292. [Google Scholar] [CrossRef]
- Bourguignon, M.; De Tiège, X.; Op de Beeck, M.; Pirotte, B.; Van Bogaert, P.; Goldman, S.; Hari, R.; Jousmäki, V. Functional motor-cortex mapping using corticokinematic coherence. Neuroimage 2011, 55, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, M.; Piitulainen, H.; De Tiège, X.; Jousmäki, V.; Hari, R. Corticokinematic coherence mainly reflects movement-induced proprioceptive feedback. Neuroimage 2015, 106, 382–390. [Google Scholar] [CrossRef]
- Maezawa, H.; Fujimoto, M.; Hata, Y.; Matsuhashi, M.; Hashimoto, H.; Kashioka, H.; Yanagida, T.; Hirata, M. Functional cortical localization of tongue movements using corticokinematic coherence with a deep learning-assisted motion capture system. Sci. Rep. 2022, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Kaukoranta, E.; Reinikainen, K.; Huopaniemie, T.; Mauno, J. Neuromagnetic localization of cortical activity evoked by painful dental stimulation in man. Neurosci. Lett. 1983, 42, 77–82. [Google Scholar] [CrossRef]
- Karhu, J.; Hari, R.; Lu, S.T.; Paetau, R.; Rif, J. Cerebral magnetic fields to lingual stimulation. Electroencephalogr. Clin. Neurophysiol. 1991, 80, 459–468. [Google Scholar] [CrossRef]
- Maezawa, H.; Yoshida, K.; Nagamine, T.; Matsubayashi, J.; Enatsu, R.; Bessho, K.; Fukuyama, H. Somatosensory evoked magnetic fields following electric tongue stimulation using pin electrodes. Neurosci. Res. 2008, 62, 131–139. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nakata, H.; Kakigi, R. Somatosensory-evoked magnetic fields following stimulation of the tongue in humans. Clin. Neurophysiol. 2008, 119, 1664–1673. [Google Scholar] [CrossRef]
- Tamura, Y.; Shibukawa, Y.; Shintani, M.; Kaneko, Y.; Ichinohe, T. Oral structure representation in human somatosensory cortex. Neuroimage 2008, 43, 128–135. [Google Scholar] [CrossRef]
- Nakahara, H.; Nakasato, N.; Kanno, A.; Murayama, S.; Hatanaka, K.; Itoh, H.; Yoshimoto, T. Somatosensory-evoked fields for gingiva, lip, and tongue. J. Dent. Res. 2004, 83, 307–311. [Google Scholar] [CrossRef]
- Maezawa, H.; Matsuhashi, M.; Yoshida, K.; Mima, T.; Nagamine, T.; Fukuyama, H. Evaluation of lip sensory disturbance using somatosensory evoked magnetic fields. Clin. Neurophysiol. 2014, 125, 363–369. [Google Scholar] [CrossRef]
- Yoshida, K.; Maezawa, H.; Nagamine, T.; Fukuyama, H.; Murakami, K.; Iizuka, T. Somatosensory evoked magnetic fields to air-puff stimulation on the soft palate. Neurosci. Res. 2006, 55, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, H.; Hirai, Y.; Shiraishi, H.; Funahashi, M. Somatosensory evoked magnetic fields following tongue and hard palate stimulation on the preferred chewing side. J. Neurol. Sci. 2014, 347, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kumamoto, Y.; Nakashima, T.; Yamamoto, T.; Inokuchi, A.; Komiyama, S. Magnetic sensory cortical responses evoked by tactile stimulations of the human face, oral cavity and flap reconstructions of the tongue. Eur. Arch. Otorhinolaryngol. 1999, 256 (Suppl. S1), S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Lüders, H.O.; Burgess, R.C.; Sakamoto, A.; Klem, G.H.; Morris, H.H.; Shibasaki, H. Generator locations of movement-related potentials with tongue protrusions and vocalizations: Subdural recording in human. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 310–328. [Google Scholar] [CrossRef]
- Disbrow, E.A.; Hinkley, L.B.; Roberts, T.P. Ipsilateral representation of oral structures in human anterior parietal somatosensory cortex and integration of inputs across the midline. J. Comp. Neurol. 2003, 467, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, H.; Yoshida, K.; Matsuhashi, M.; Yokoyama, Y.; Mima, T.; Bessho, K.; Fujita, S.; Nagamine, T.; Fukuyama, H. Evaluation of tongue sensory disturbance by somatosensory evoked magnetic fields following tongue stimulation. Neurosci. Res. 2011, 71, 244–250. [Google Scholar] [CrossRef]
- Maezawa, H.; Tojyo, I.; Yoshida, K.; Fujita, S. Recovery of impaired somatosensory evoked fields after improvement of tongue sensory deficits with neurosurgical reconstruction. J. Oral Maxillofac. Surg. 2016, 74, 1473–1482. [Google Scholar] [CrossRef]
- Hallett, M.; Kanchana, S. Pathophysiology of dystonia. In Clinical Diagnosis and Management of Dystonia; CRC Press: Abingdon, UK, 2007; pp. 43–51. [Google Scholar]
- Vidailhet, M.; Grabli, D.; Roze, E. Pathophysiology of dystonia. Curr. Opin. Neurol. 2009, 22, 406–413. [Google Scholar] [CrossRef]
- Skogseid, I.M. Dystonia—New advances in classification, genetics, pathophysiology and treatment. Acta Neurol. Scand. Suppl. 2014, 198, 13–19. [Google Scholar] [CrossRef]
- Brüggemann, N. Contemporary functional neuroanatomy and pathophysiology of dystonia. J. Neural Transm. 2021, 128, 499–508. [Google Scholar] [CrossRef]
- Shibasaki, H.; Hallett, M. What is the Bereitschaftspotential? Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Neshige, R.; Lüders, H.; Shibasaki, H. Recording of movement-related potentials from scalp and cortex in man. Brain 1988, 111, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Lüders, H.O.; Burgess, R.C.; Shibasaki, H. Movement-related potentials recorded from supplementary motor area and primary motor area. Role of supplementary motor area in voluntary movements. Brain 1992, 115, 1017–1043. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, H.; Sadato, N.; Lyshkow, H.; Yonekura, Y.; Honda, M.; Nagamine, T.; Suwazono, S.; Magata, Y.; Ikeda, A.; Miyazaki, M. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain 1993, 116, 1387–1398. [Google Scholar] [CrossRef]
- Fève, A.; Bathien, N.; Rondot, P. Abnormal movement related potentials in patients with lesions of basal ganglia and anterior thalamus. J. Neurol. Neurosurg. Psychiatry 1994, 57, 100–104. [Google Scholar] [CrossRef]
- Deuschl, G.; Toro, C.; Matsumoto, J.; Hallett, M. Movement-related cortical potentials in writer’s cramp. Ann. Neurol. 1995, 38, 862–868. [Google Scholar] [CrossRef]
- Yazawa, S.; Ikeda, A.; Kaji, R.; Terada, K.; Nagamine, T.; Toma, K.; Kubori, T.; Kimura, J.; Shibasaki, H. Abnormal cortical processing of voluntary muscle relaxation in patients with focal hand dystonia studied by movement-related potentials. Brain 1999, 122, 1357–1366. [Google Scholar] [CrossRef]
- Oga, T.; Honda, M.; Toma, K.; Murase, N.; Okada, T.; Hanakawa, T.; Sawamoto, N.; Nagamine, T.; Konishi, J.; Fukuyama, H.; et al. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer’s cramp: An fMRI study. Brain 2002, 125, 895–903. [Google Scholar] [CrossRef]
- Kaji, R.; Ikeda, A.; Ikeda, T.; Kubori, T.; Mezaki, T.; Kohara, N.; Kanda, M.; Nagamine, T.; Honda, M.; Rothwell, J.C. Physiological study of cervical dystonia. Task-specific abnormality in contingent negative variation. Brain 1995, 118, 511–522. [Google Scholar] [CrossRef]
- Ikeda, A.; Shibasaki, H.; Kaji, R.; Terada, K.; Nagamine, T.; Honda, M.; Hamano, T.; Kimura, J. Abnormal sensorimotor integration in writer’s cramp: Study of contingent negative variation. Mov. Disord. 1996, 11, 683–690. [Google Scholar] [CrossRef]
- Murase, N.; Kaji, R.; Shimazu, H.; Katayama-Hirota, M.; Ikeda, A.; Kohara, N.; Kimura, J.; Shibasaki, H.; Rothwell, J.C. Abnormal premovement gating of somatosensory input in writer’s cramp. Brain 2000, 123, 1813–1829. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, F.; Melgari, J.M.; Zappasodi, F.; Porcaro, C.; Milazzo, D.; Cassetta, E.; Rossini, P.M. Sensorimotor integration in focal task-specific hand dystonia: A magnetoencephalographic assessment. Neuroscience 2008, 154, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Sheean, G.; Rothwell, J.C.; Inzelberg, R.; Kujirai, T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J. Neurol. Neurosurg. Psychiatry. 1995, 59, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.H.; Hallett, M. Disturbed surround inhibition in focal hand dystonia. Ann. Neurol. 2004, 56, 595–599. [Google Scholar] [CrossRef]
- Münte, T.F.; Altenmüller, E.; Jäncke, L. The musician’s brain as a model of neuroplasticity. Nat. Rev. Neurosci. 2002, 3, 473–478. [Google Scholar] [CrossRef]
- Haslinger, B.; Erhard, P.; Dresel, C.; Castrop, F.; Roettinger, M.; Ceballos-Baumann, A.O. ‘Silent event-related’ fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology 2005, 65, 1562–1569. [Google Scholar] [CrossRef]
- Ceballos-Baumann, A.O.; Passingham, R.E.; Warner, T.; Playford, E.D.; Marsden, C.D.; Brooks, D.J. Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann. Neurol. 1995, 37, 363–372. [Google Scholar] [CrossRef]
- Tempel, L.W.; Perlmutter, J.S. Abnormal vibration-induced cerebral blood flow responses in idiopathic dystonia. Brain 1990, 113, 691–707. [Google Scholar] [CrossRef]
- Tinazzi, M.; Rosso, T.; Fiaschi, A. Role of the somatosensory system in primary dystonia. Mov. Disord. 2003, 18, 605–622. [Google Scholar] [CrossRef]
- Hallett, M. Is dystonia a sensory disorder? Ann. Neurol. 1995, 38, 139–140. [Google Scholar] [CrossRef]
- Simpson, D.M.; Blitzer, A.; Brashear, A.; Comella, C.; Dubinsky, R.; Hallett, M.; Jankovic, J.; Karp, B.; Ludlow, C.L.; Miyasaki, J.M.; et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2018, 147, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Anandan, C.; Jankovic, J. Botulinum toxin in movement disorders: An update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.S.; Youn, K.H.; Lee, J.; Kim, H.-J. Ultrasound-guided botulinum neurotoxin type A injection for correcting asymmetrical smiles. Aesthet. Surg. J. 2018, 38, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Chang, K.V.; Chang, H.C.; Chen, L.R.; Kuan, C.H.; Kao, J.T.; Wei, L.Y.; Chen, Y.J.; Han, D.S.; Özçakar, L. Ultrasound imaging of the facial muscles and relevance with botulinum toxin injections: A pictorial essay and narrative review. Toxins 2022, 27, 101. [Google Scholar] [CrossRef]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535549. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200235. [Google Scholar] [CrossRef]
- Luvisetto, S. Botulinum neurotoxins in central nervous system: An overview from animal models to human therapy. Toxins 2021, 13, 751. [Google Scholar] [CrossRef]
- Giladi, N. The mechanism of action of botulinum toxin type A in focal dystonia is most probably through its dual effect on efferent (motor) and afferent pathways at the injected site. J. Neurol. Sci. 1997, 152, 132135. [Google Scholar] [CrossRef]
- Hok, P.; Veverka, T.; Hlustik, P.; Nevrly, M.; Kanovsky, P. The central effects of botulinum toxin in dystonia and spasticity. Toxins 2021, 13, 155. [Google Scholar] [CrossRef]
- Weise, D.; Weise, C.M.; Naumann, M. Central effects of botulinum neurotoxin-Evidence from human studies. Toxins 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Ghang, J.Y.; Lee, M.K.; Jun, S.M.; Ghang, C.G. Outcome of pallidal deep brain stimulation in Meige syndrome. J. Korean Neurosurg. Soc. 2010, 48, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.K.; Birch, B.D.; Hillman, R.A.; Boucher, O.K.; Evidente, V.G. Long-term follow-up of deep brain stimulation for Meige syndrome. Neurosurg. Focus 2010, 29, E5. [Google Scholar] [CrossRef]
- Ostrem, J.L.; Marks, W.J.; Volz, M.M.; Heath, S.L.; Starr, P.A. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome). Mov. Disord. 2007, 22, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; Kalia, S.K.; Shah, B.B.; Jimenez-Shahed, J.; Prashanth, L.K.; Moro, E. Surgical treatment of dystonia. Expert Rev. Neurother. 2018, 18, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Kupsch, A.; Benecke, R.; Müller, J.; Trottenberg, T.; Schneider, G.H.; Poewe, W.; Eisner, W.; Wolters, A.; Müller, J.U.; Deuschl, G.; et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med. 2006, 355, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Asmus, F.; Bhatia, K.P.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur. J. Neurol. 2011, 18, 5–18. [Google Scholar] [CrossRef]
- Tagliati, M.; Krack, P.; Volkmann, J.; Aziz, T.; Krauss, J.K.; Kupsch, A.; Vidailhet, A.M. Long-term management of DBS in dystonia: Response to stimulation, adverse events, battery changes, and special considerations. Mov. Disord. 2011, 26 (Suppl. S1), S54–S62. [Google Scholar] [CrossRef]
- Horisawa, S.; Ochiai, T.; Goto, S.; Nakajima, T.; Takeda, N.; Kawamata, T.; Taira, T. Long-term outcome of pallidal stimulation for Meige syndrome. J. Neurosurg. 2018, 130, 84–89. [Google Scholar] [CrossRef]
- Sako, W.; Morigaki, R.; Mizobuchi, Y.; Tsuzuki, T.; Ima, H.; Ushio, Y.; Nagahiro, S.; Kaji, R.; Goto, S. Bilateral pallidal deep brain stimulation in primary Meige syndrome. Parkinsonism Relat. Disord. 2011, 17, 123–125. [Google Scholar] [CrossRef]
- Inoue, N.; Nagahiro, S.; Kaji, R.; Goto, S. Long-term suppression of Meige syndrome after pallidal stimulation: A 10-year follow-up study. Mov. Disord. 2010, 25, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Mao, Z.; Yu, X. Deep brain stimulation for Meige syndrome: A meta-analysis with individual patient data. J. Neurol. 2019, 266, 2646–2656. [Google Scholar] [CrossRef]
- Limotai, N.; Go, C.; Oyama, G.; Hwynn, N.; Zesiewicz, T.; Foote, K.; Bhidayasiri, R.; Malaty, I.; Zeilman, P.; Rodriguez, R.; et al. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. J. Neurol. 2011, 258, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Vidailhet, M.; Jutras, M.F.; Grabli, D.; Roze, E. Deep brain stimulation for dystonia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Cif, L.; Demailly, D.; Lin, J.P.; Barwick, K.E.; Sa, M.; Abela, L.; Malhotra, S.; Chong, W.K.; Steel, D.; Sanchis-Juan, A.; et al. KMT2B-related disorders: Expansion of the phenotypic spectrum and long-term efficacy of deep brain stimulation. Brain 2020, 143, 3242–3261. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum neurotoxin therapy for lingual dystonia using an individualized injection method based on clinical features. Toxins 2019, 11, 51. [Google Scholar] [CrossRef]
- Frucht, S.; Fahn, S.; Ford, B.; Gelb, M. A geste antagoniste device to treat jaw-closing dystonia. Mov. Disord. 1999, 14, 883–886. [Google Scholar] [CrossRef]
- Lo, S.E.; Gelb, M.; Frucht, S.J. Geste antagonistes in idiopathic lower cranial dystonia. Mov. Disord. 2007, 22, 1012–1017. [Google Scholar] [CrossRef]
- Satoh, M.; Narita, M.; Tomimoto, H. Three cases of focal embouchure dystonia: Classifications and successful therapy using a dental splint. Eur. Neurol. 2011, 66, 85–90. [Google Scholar] [CrossRef]
- Yoshida, K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J. Prosthodont. Res. 2018, 62, 239–244. [Google Scholar] [CrossRef]
- De Meyer, M.; Vereecke, L.; Bottenberg, P.; Jacquet, W.; Sims, A.B.; Santens, P. Oral appliances in the treatment of oromandibular dystonia: A systematic review. Acta Neurol. Belg. 2020, 120, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alegre, P.; Schneider, R.L.; Hoffman, H. Clinical, etiological, and therapeutic features of jaw-opening and jaw-closing oromandibular dystonias: A decade of experience at a single treatment center. Tremor Other Hyperkinet. Mov. 2014, 4, 231. [Google Scholar] [CrossRef]
- Schramm, A.; Classen, J.; Reiners, K.; Naumann, M. Characteristics of sensory trick-like manoeuvres in jaw-opening dystonia. Mov. Disord. 2007, 22, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, C.; Lai, E.C.; Jankovic, J. Peripherally induced oromandibular dystonia. J. Neurol. Neurosurg. Psychiatry 1998, 65, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, T.K.; Matsumoto, J.Y.; Lee, K.H. Moving forward: Advances in the treatment of movement disorders with deep brain stimulation. Front. Integr. Neurosci. 2011, 5, 69. [Google Scholar] [CrossRef]
- Marceglia, S.; Mrakic-Sposta, S.; Fumagalli, M.; Ferrucci, R.; Mameli, F.; Vergari, M.; Barbieri, S.; Priori, A. Cathodal transcranial direct current stimulation improves focal hand dystonia in musicians: A two-case study. Front. Neurosci. 2017, 11, 508. [Google Scholar] [CrossRef]
- Young, S.J.; Bertucco, M.; Sheehan-Stross, R.; Sanger, T.D. Cathodal transcranial direct current stimulation in children with dystonia: A pilot open-label trial. J. Child Neurol. 2013, 28, 1238–1244. [Google Scholar] [CrossRef]
- Brittain, J.S.; Probert-Smith, P.; Aziz, T.Z.; Brown, P. Tremor suppression by rhythmic transcranial current stimulation. Curr. Biol. 2013, 23, 436–440. [Google Scholar] [CrossRef]
- Buttkus, F.; Weidenmüller, M.; Schneider, S.; Jabusch, H.C.; Nitsche, M.A.; Paulus, W.; Altenmüller, E. Failure of cathodal direct current stimulation to improve fine motor control in musician’s dystonia. Mov. Disord. 2010, 25, 389–394. [Google Scholar] [CrossRef]
- Benninger, D.H.; Lomarev, M.; Lopez, G.; Pal, N.; Luckenbaugh, D.A.; Hallett, M. Transcranial direct current stimulation for the treatment of focal hand dystonia. Mov. Disord. 2011, 26, 1698–1702. [Google Scholar] [CrossRef]
- Bhanpuri, N.H.; Bertucco, M.; Young, S.J.; Lee, A.A.; Sanger, T.D. Multiday transcranial direct current stimulation causes clinically insignificant changes in childhood dystonia: A pilot study. J. Child Neurol. 2015, 30, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, H.; Vicario, C.M.; Kuo, M.F.; Hirata, M.; Mima, T.; Nitsche, M.A. Effects of bilateral anodal transcranial direct current stimulation over the tongue primary motor cortex on cortical excitability of the tongue and tongue motor functions. Brain Stimul. 2020, 13, 270–272. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).