Abstract
Disabling limb spasticity can result from stroke, traumatic brain injury or other disorders causing upper motor neuron lesions such as multiple sclerosis. Clinical studies have shown that abobotulinumtoxinA (AboBoNT-A) therapy reduces upper and lower limb spasticity in adults. However, physicians may administer potentially inadequate doses, given the lack of consensus on adjusting dose according to muscle volume, the wide dose ranges in the summary of product characteristics or cited in the published literature, and/or the high quantity of toxin available for injection. Against this background, a systematic literature review based on searches of MEDLINE and Embase (via Ovid SP) and three relevant conferences (2018 to 2020) was conducted in November 2020 to examine AboBoNT-A doses given to adults for upper or lower limb muscles affected by spasticity of any etiology in clinical and real-world evidence studies. From the 1781 unique records identified from the electronic databases and conference proceedings screened, 49 unique studies represented across 56 publications (53 full-text articles, 3 conference abstracts) were eligible for inclusion. Evidence from these studies suggested that AboBoNT-A dose given per muscle in clinical practice varies considerably, with only a slight trend toward a relationship between dose and muscle volume. Expert-based consensus is needed to inform recommendations for standardizing AboBoNT-A treatment initiation doses based on muscle volume.
Keywords:
botulinum toxins; muscle hypertonia; muscle spasticity; injections; intramuscular; central nervous system diseases Key Contribution:
This research is the first systematic review on AboBoNT-A doses injected in upper and lower limb muscles to treat adults with spasticity and has highlighted wide variation in such practice; the results could prompt the development of standardization of AboBoNT-A treatment based on muscle volume.
Plain Language Summary: People with specific diseases or injuries of their nervous system may develop permanent stiffening of muscles in their arms and/or legs, known as spasticity; this can follow, for example, a stroke, brain damage from head injuries, or certain neurological diseases and impact mobility. Spasticity can be reduced by periodic injections of a drug called abobotulinumtoxinA (AboBoNT-A) into affected muscles; this treatment reduces muscles’ ability to contract, thereby lessening the stiffening. However, there are concerns physicians may give insufficient doses of AboBoNT-A through fears about excessive dosing; this wariness probably reflects the lack of both agreement among medical experts and clear guidance in the product literature about how to adjust doses according to the volume (i.e., bulk) of different muscles. Given this uncertainty, we carried out a systematic review to identify and analyze published information on the doses of AboBoNT-A used to inject different muscles. Specifically, we searched standard databases and websites of three scientific conferences for research on patients with spasticity (from any cause) treated with AboBoNT-A, either as part of a clinical trial or during everyday medical care. Thus, 49 relevant studies were identified for inclusion in the review. Evidence from these studies suggested that the AboBoNT-A dose given per muscle in clinical practice varies greatly, with little or no link between dose and muscle volume. Thus, there is a need for agreement between experts so that clear recommendations can then be drawn up on how best to choose the appropriate starting dose of AboBoNT-A for a particular muscle volume.
1. Introduction
Limb spasticity is a disabling condition characterized by muscle stiffness, pain and occasionally sudden uncontrollable movements (muscle spasms) of the upper or lower limbs [1,2]. Here, spasticity is used as a standard term to refer to the three components of muscle hypertonia: spasticity, spastic dystonia and spastic co-contractions; it develops in the lower limbs of almost half of adults who experience a stroke and can also occur following traumatic brain injury, cerebral palsy or as part of progressive diseases causing upper motor neuron lesions such as multiple sclerosis [1,2]. Clinical studies have shown that treatment with abobotulinumtoxinA (AboBoNT-A), a neurotoxin that causes muscle weakness by blocking the release of acetylcholine at the neuromuscular junction, reduces both upper and lower limb spasticity in adults, with a good tolerance profile [3,4]. However, there are concerns around AboBoNT-A treatment initiation that can prompt clinicians to be over-cautious in using the therapy, so resulting in the administration of doses that are inadequate for patients’ needs; this situation is likely due to the wide dose ranges per muscle described in the summary of product characteristics [5] or cited in the literature, the lack of consensus on adjusting these doses according to several factors (e.g., muscle volume, etiology and severity of spasticity, muscle structure), and/or the high quantity of toxin available for injection; it has been demonstrated that at maximal dose per label, higher toxin quantity (2 to 3 fold) could be injected over a single session with AboBoNT-A in adults, compared with other formulations, allowing treatment of a greater number of target muscles [6]. Current French clinical guidelines for the treatment of spasticity did not provide recommendations about AboBoNT-A dose to be injected per specific muscle [7], while clinical guidelines from the Royal College of Physicians in the United Kingdom reported muscle-specific recommendations with large dose ranges for several muscles (e.g., biceps brachii: 100–300 U) [8]. Recently, consensus guidelines for botulinum toxin therapy from the Interdisciplinary Working Group for Movement Disorders (IAB) did not consider AboBoNT-A because this drug was said to have different potency labeling compared with the other two main botulinum toxins A (onabotulinumtoxinA and incobotulinumtoxinA) [9]; this is keeping with a general acceptance that none of these toxins can be compared directly since they each contain a different quantity of neuroactive toxin and dose units are not interchangeable between them [6].
Given the uncertainties around current clinical practice, this study aimed to gather evidence on intramuscular dosages of AboBoNT-A used by healthcare professionals. Specifically, it involved conducting a systematic review to explore data from published interventional and observational studies of such treatment in adults with upper or lower limb spasticity regardless of the etiology of this condition.
2. Results
2.1. Study Selection
The literature searches identified 1781 unique records from the electronic databases. Of these, 349 abstracts met the criteria for full-text review, which determined that 53 of the publications were eligible for inclusion in the systematic review. In addition, 3 eligible conference abstracts were identified from the grey literature searches of conference proceedings, so resulting in a total of 56 publications (see Figure 1). Most of these (49 of 56) were the primary publications for unique studies, with the rest (7 of 56) being deemed related publications because of a clear overlap with population/patient samples reported in some of the primary publications, based on details of the trial/cohort name, and enrollment years.
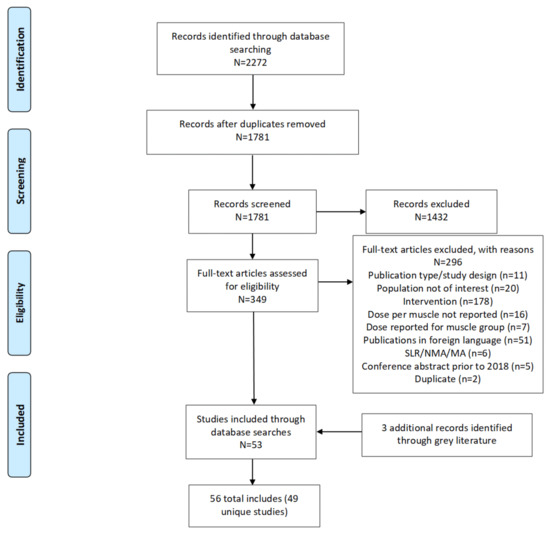
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow diagram. Legend: MA = meta-analysis; N = total number of records in the identified box; n = number of records in each category; NMA = network meta-analysis; SLR = systematic literature review.
2.2. Study Characteristics
Most of the 49 primary studies included in the systematic review were conducted in Europe (n = 30), with the rest being international studies or from the Middle East/Asia (n = 7 each), Oceania (n = 3), Africa (Tunisia; n = 1), and South America (Brazil; n = 1). About half of the studies were randomized controlled trials (RCTs; n = 24), with the rest being observational real-world studies (n = 18), single-arm trials (n = 6) or non-randomized trials (n = 1). Sample sizes across studies ranged from nine to 456 patients, with most studies (36/49; 73%) enrolling fewer than 100 patients each. Most studies (31/49; 63%) reported on patients with upper limb spasticity, while 4 studies reported on patients with upper or lower limb spasticity. Overall study characteristics of the included studies are shown in Table 1.

Table 1.
Characteristics of the included studies.
The mean age of patients varied between 41.6 and 69 years. Information on the underlying etiology of spasticity was available for 44 studies. Most included patients with limb spasticity due to stroke or brain injury (38 studies), five studies included patients with multiple sclerosis or other disorders causing upper motor neuron lesions (e.g., degenerative myelopathy, Strümpell–Lorrain disease), and one study included a population with head or spinal cord injuries, or those who had undergone neurosurgery.
2.3. Risk of Bias
The 46 full-text studies included in the systematic review included 23 RCTs, seven quantitative non-randomized studies, and 16 quantitative descriptive studies (Appendix A). The risk-of-bias assessment indicated no concerns regarding study quality across the 23 RCTs, but not all assessment questions could be fully answered for the non-randomized and quantitative descriptive studies. However, these data were not considered to have a material bearing on the findings of the systematic review because the primary focus of the quality-assessment tool was the impact of study quality on treatment outcomes, rather than on assigned treatment dosing (the focus of the review).
2.4. Treatment Information Available from Included Studies
Studies were selected for inclusion in the systematic review on the basis that they reported a mean/median AboBoNT-A dose, a fixed dose (i.e., patients received a specific dose for a specific muscle) or a dose range for a specific muscle. Although some studies also included other treatment arms (e.g., placebo/control or another botulinum toxin A treatment), only data relating to AboBoNT-A were extracted. The data on the administration of AboBoNT-A derived from individual studies for analysis in the systematic review are presented in Appendix B.
The range of concomitant treatments used with AboBoNT-A across the studies included other medications, rehabilitation programs (e.g., physiotherapy and occupational therapy), and electrical stimulation, and one study used robot-assisted gait training to improve patient walking ability [48].
2.5. Dose per Muscle Volume Analysis
The 49 unique clinical trials and real-world practice studies collectively reported AboBoNT-A dose information across 50 specific muscles of both limbs. The relationship between muscle volume and AboBoNT-A dose given in these studies was explored through scatter plots. For these plots, the specific muscles injected in each study were assumed to have the average muscle volume in cm3 that was reported for upper-limb muscles in Holzbaur et al., 2007 [59] and lower-limb muscles in Handsfield et al., 2014 [60]. Accordingly, dose values were plotted only for those muscles for which the muscle volume was available. For example, no information on the volume of the adductor pollicis muscle was available, and therefore AboBoNT-A dose values reported for this muscle were not included in the upper-limb scatter plot. Based on muscle-volume clusters on the volume-dose plots, muscles were grouped into three volume categories (small, medium, and large). In the upper limb, large-, medium-, and small-volume muscles had a volume of ≥100 cm3, 20–99 cm3, and <20 cm3, respectively. In the lower limb, the respective volumes were ≥400 cm3, 100–399 cm3, and <100 cm3.
2.5.1. Upper Limb
In the upper limb, mean, median, or fixed doses were most commonly reported for the flexor digitorum profundus (23 studies), biceps brachii (20), flexor carpi ulnaris (20), flexor digitorum superficialis (20), flexor carpi radialis (19), brachioradialis (15), and pectoralis major (14).
Wide dose ranges were found across studies, even when accounting for average muscle volume. In the small-volume muscle group, AboBoNT-A mean and median doses ranged from 47 U to 150 U, and 25 U to 200 U, respectively (Table 2). In the medium-volume group, mean and median doses ranged from 62.5 U to 200 U, and 50 U to 300 U, respectively. In the large-volume muscle group, mean and median doses ranged from 50 U to 400 U, and 75 U to 300 U, respectively.

Table 2.
Range of mean and median doses by muscle-volume categories across the included studies.
A positive correlation between AboBoNT-A dose and average muscle volume was more clearly identified when including only studies that reported the number of patients injected with AboBoNT-A into a specific muscle (Figure 2). The mean/median dose generally ranged from 100 U to 200 U for small- and medium-volume muscles, when considering only values for 50 or more treated patients. A similar trend was observed for the large-volume muscle group, although the mean/median AboBoNT-A dose was more likely to be around 200 U to 250 U, particularly in larger muscles with an average volume of 250 cm3 or more. These findings should, however, be interpreted with caution as some studies reporting on upper limb muscles (6 of 34) were not included in the plot as they did not report the number of patients receiving AboBoNT-A treatment per muscle. Of note, the plots did not provide any evidence to suggest differences between interventional and RWE studies in the relationship between muscle volume and dose.
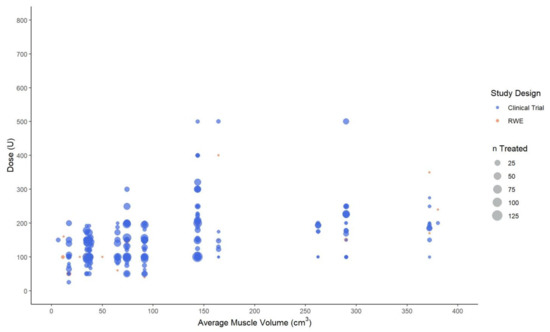
Figure 2.
Mean, median and fixed abobotulinumtoxinA dose (in units) by the average volume of upper limb muscles. Legend: U, unit; n, number of patients injected with abobotulinumtoxinA in a specific muscle at a specific dose.
2.5.2. Lower Limb
In the lower limb, mean, median, or fixed doses were most commonly reported for the tibialis posterior (10 studies), and soleus, lateral and medial gastrocnemius (8 studies each). Data for each of the remaining muscles were mostly available from one or two studies only.
In the small-volume muscle group, only two muscles were included in the scatter plot since the average volume was not available for the three other muscles reported in some studies. The mean dose for the flexor digitorum longus (average muscle volume: 30 cm3) and the flexor hallucis longus (average muscle volume: 78.8 cm3) ranged from 106 U to 233.3 U, and 94.9 U to 164 U, respectively. Relatively consistent mean-dose ranges were reported for medium-volume muscles, averaging 85 U to 372.7 U. In the large-volume muscle group, mean doses ranged from 88 U to 495.3 U (or up to 750 U if fixed doses were included).
When considering only values for groups of more than 50 patients receiving AboBoNT-A, the dose generally ranged between 100 U and 180 U for small-volume muscles, and between 100 U and 300 U for medium-volume muscles (Figure 3). Although data on larger muscles were scarce, larger studies (n > 50) tended to report a general range of 300 U to 500 U. As for the upper limb, these findings should be interpreted with caution given that some studies reporting on lower limb muscles (2 of 19) were not included on the plot as they did not report on the number of patients treated with AboBoNT-A. As for the upper limb, the plots did not provide any evidence to suggest differences between interventional and RWE studies in the relationship between muscle volume and dose.
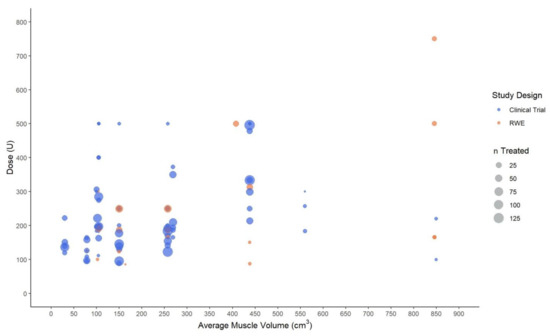
Figure 3.
Mean, median and fixed abobotulinumtoxinA dose (in units) by the average volume of lower limb muscles. Legend: U, unit; n, number of patients injected with abobotulinumtoxinA in a specific muscle at a specific dose.
3. Discussion
AboBoNT-A was approved by the United States Food and Drug Administration in 2015 for adults with upper limb spasticity, and it received label extensions for lower limb spasticity in children and adults in 2016 and 2017, respectively, and for upper limb spasticity in children in 2019. AboBoNT-A is also approved in Europe for upper and lower limb spasticity. However, the establishment of the drug as a recommended treatment option for adults with spasticity has occurred in the absence of published consensus on whether or how dosing should be adjusted in line with the volume of the target muscles, within the broad licensed dose ranges; this is the basis of concerns that treatment of such patients may be suboptimal due to the administration of inadequate doses. Although they should be the reference in terms of dosing, licensed dose ranges have been based on initial evidence from clinical trials, which helps to explain why they are wide. Against this background, the current review aimed to systematically summarize data on AboBoNT-A dose given per specific muscle of the upper and lower limb in adults with limb spasticity irrespective of underlying etiology or country in which the primary study was conducted. The results were intended to explore the extent of variability in AboBoNT-A prescribing in clinical practice, from both a clinical-trial and a real-world perspective.
Overall, there was no evidence of a strong relationship between muscle volume and AboBoNT-A dose, with wide dose ranges being reported for the same muscle or across muscles of a similar volume. For the upper limb, dose ranges were relatively consistent across small- and medium-volume muscles (mean/median 25 U to 300 U), and slightly higher doses were reported for large-volume muscles (mean/median 50 U to 400 U). Slightly higher doses and greater dose ranges were reported for the lower limb, presumably reflecting the larger volume of the muscles and the greater heterogeneity of this muscle-group category with regard to muscle volume.
This systematic review had some key strengths. To our knowledge, it is the first research to analyze potential inter-relationships between AboBoNT-A doses being used for spasticity and the volume of the injected muscles in adults, and so it targets an important gap in the literature. In a recently published systematic review and meta-analysis of clinical trials of the effects of AboBoNT-A on the Modified Ashworth Scale score in patients with stroke-related spasticity, Ojardias and colleagues reported a D50 of 491.7 U for large-volume muscles (arm muscles injected up to the elbow, and leg muscles down to the ankle) and 108 U for small-volume muscles (other muscles) [61]. Crucially, however, no further relationship analysis between muscle volume and the AboBoNT-A dose injected was reported. Other strengths of our review included its assessment of the evidence from both real-world and interventional studies, thereby ensuring the capture of relevant evidence on dosing practice across a broad range of practice settings and clinical scenarios. Moreover, the review included no limitations as to the etiology of spasticity or the specific muscles involved, to help ensure the representativeness and potential generalizability of its findings.
The review also had some limitations. First, there was considerable variability in how AboBoNT-A doses were reported across studies, and several studies did not report on the number of patients receiving an AboBoNT-A injection in a specific muscle. Furthermore, to increase the availability of AboBoNT-A dose data, this review included mean, median, and fixed values (i.e., where all patients received the same dose for the specific muscle). Mean values are, however, prone to outliers, which was evident in their considerably wider dose ranges compared to those for median values. Second, the variability of AboBoNT-A doses per muscle volume across the included studies could be explained by factors not captured by this systematic review (e.g., the severity of hypertonia, type of symptomatology, the dilution of the toxin prior to injection, pennate or fusiform muscle, and different study objectives).
4. Clinical Opinion
Despite the limitations of this systematic review, its findings indicate a pressing need for clear guidance on AboBoNT-A dosing for adults with spasticity. With this in mind and based on our practice, we propose “easy to remember “narrow AboBoNT-A dose ranges to be injected in first intention into muscles of different volume categories, as listed in Table 3. These are for first-intention AboBoNT-A treatment in botulinum toxin-naïve patients. In general, we observed that the suggested dose is 1 to 1.5 times the muscle volume (100 to 150 U for a muscle volume of 100 cm3) for both upper and lower limbs. These rather conservative dose ranges have a well-established safety profile since they are within French SmPC dose ranges (for in-label muscles) [5]. However, doses can be adjusted according to efficacy and the desired effect. Dose increases are possible in the absence of safety concerns and if there is an insufficient effect from a previous dose. These dose ranges are starting-points and the dose to be used may be adjusted based on the following factors:
- (1)
- etiology of the hypertonia;
- (2)
- type of hypertonia (i.e., spasticity vs. dystonia);
- (3)
- severity of hypertonia;
- (4)
- time post onset of spasticity;
- (5)
- structure of the muscle (i.e., smaller doses are needed to target the neuromuscular junctions in a long muscle such as the biceps brachii [neuromuscular junctions are all in the same place] whereas in bipennate muscles [e.g., rectus femoris, gastrocnemii] the junctions are much more disseminated such that greater doses may be required);
- (6)
- individual patient characteristics (e.g., size, weight, presence of fixed contractures, fibrosis);
- (7)
- whether the function associated with the muscle is impaired or not (e.g., iliac muscle for movement of the lower limb);
- (8)
- desired duration of action.

Table 3.
Proposed abobotulinumtoxinA dose ranges per muscle volume ∆.
Table 3.
Proposed abobotulinumtoxinA dose ranges per muscle volume ∆.
| Range of AboBoNT-A Doses (U) | Muscle Volume (SD) * (cm3) | Dose Ranges According to French Label (U) [5] | Muscles (Off-Label Use in Italic) |
|---|---|---|---|
| Upper Limb | |||
| 200–300 | 380.5 (157.7) | NA | Deltoideus ** |
| 372.1 (177.3) | 150–300 | Triceps brachii† | |
| 290.0 (169.0) | 100–300 | Pectoralis major | |
| 262.3 (147.2) | 150–300 | Latissimus dorsi | |
| 164.5 (63.9) | 75–300 | Subscapularis | |
| 143.7 (63.7) | 50–400 | Brachialis | |
| 143.7 (68.7) | 50–400 | Biceps brachii | |
| 100–200 | 91.6 (39.3) | 100–200 | Flexor digitorum profundus |
| 74.2 (27.4) | 100–200 | Flexor digitorum superficialis | |
| 65.1 (36.0 | 50–200 | Brachioradialis | |
| 50.0 (20.4) | NA | Supraspinatus | |
| 38.4 (17.2) | 45–200 | Pronator teres | |
| 37.1 (13.6) | 25–200 | Flexor carpi ulnaris | |
| 34.8 (17.1) | 25–200 | Flexor carpi radialis | |
| 32.7 (16.3) | NA | Teres major | |
| 28.0 (13.9) | NA | Teres minor | |
| 17.1 (6.3) | 20–200 | Flexor pollicis longus | |
| 17.0 (7.4) | NA | Extensor carpi ulnaris | |
| 25–100 | 11.9 (5.7) | NA | Abductor pollicis longus |
| 11.2 (5.8) | NA | Pronator quadratus | |
| 6.6 (3.4) | NA | Extensor pollicis longus | |
| NA | 25–50 | Thenar Eminence muscles ‡,§ | |
| NA | NA | Hypothenar Eminence muscles‡,¥ | |
| NA | NA | Dorsal and Palmar Interossei‡ | |
| Lower Limb | |||
| 200–400 | 849.0 (194.7) | 100–400 | Gluteus maximus |
| 830.9 (194.3) | NA | Vastus lateralis | |
| 559.8 (129.4) | 100–300 | Adductor magnus | |
| 438.2 (91.6) | 300–550 | Soleus | |
| 274.8 (89.9) | NA | Psoas | |
| 270.5 (56,6) | NA | Vastus intermedius | |
| 269 (64.3) | 100–400 | Rectus femoris | |
| 257.4 (61.8) | 100–450 | Medial gastrocnemius | |
| 245.4 (54.2) | NA | Semimembranosus | |
| 206.5 (48.4) | NA | Biceps femoris (long head) | |
| 150–200 | 186 (47.0) | NA | Semitendinosus |
| 176.8 (41.6) | NA | Iliacus | |
| 163.7 (41.9) | NA | Sartorius | |
| 162.1 (43.7) | 50–150 | Adductor longus | |
| 150 (42.2) | 100–450 | Lateral gastrocnemius | |
| 135.2 (27,5) | NA | Tibialis anterior | |
| 104.8 (22.3) | 100–250 | Tibialis posterior | |
| 100–150 | 104 (24.8) | 100–200 | Gracilis |
| 104 (25.8) | 50–150 | Adductor brevis | |
| 100.1 (32.0) | NA | Biceps femoris (short head) | |
| 102.3 (21.6) | NA | EDL + EHL + peroneu tertius | |
| 78.8 (23.1) | 50–200 | Flexor hallucis longus | |
| 30 (8.2) | 50–200 | Flexor digitorum longus | |
| 25–100 | NA | 50–100, 50–200 | Intrinsic muscles (abductor hallucis, flexor digitorum brevis, flexor hallucis brevis, extensor digitorum brevis) ‡ |
| NA | NA | Interossei‡ | |
∆ These proposals are intended to facilitate first intention AboBoNT-A treatment in botulinum toxin-naïve patients, not to be taken directly as clinical recommendations. * From Holzbaur et al., 2007 [59] for the upper limb and Handsfield et al., 2014 [60] for the lower limb. ** In practice, lower doses are injected in either anterior, medium or posterior deltoid. † In practice, lower doses are injected in either long or medial/lateral head. ‡ The exact volume of this muscle is unknown. Ranking is arbitrary. § Adductor pollicis, opponens pollicis, flexor pollicis brevis, abductor pollicis brevis. ¥ Opponens digiti minimi, abductor digiti minimi, flexor digiti minimi brevis, palmaris brevis. Legend: EDL, Extensor digitorum longus; EHL, Extensor hallucis longus; NA, not available; SD, standard deviation; U, unit.
5. Conclusions
The AboBoNT-A doses used to treat adults with upper or lower limb spasticity reported in the literature varied considerably across muscles, having only a moderate association with muscle volume. Expert-based consensus is needed to inform recommendations for standardizing initial dose ranges of AboBoNT-A treatment based on muscle volume in such patients.
6. Materials and Methods
This systematic review was conducted in accordance with standards of established guidelines (i.e., PRISMA) [62] and the Cochrane Handbook for Systematic Reviews of Interventions [63]).
6.1. Eligibility Criteria
6.1.1. Types of Studies
Clinical trials and real-world evidence studies were of interest; however, articles indexed as case reports, reviews, letters, or news were excluded from the searches and during screening.
6.1.2. Types of Participants
Studies including only adults (age > 18 years) with upper or lower limb spasticity, regardless of etiology were considered eligible for this systematic review.
6.1.3. Types of Interventions
Studies investigating AboBoNT-A treatment and reporting a mean/median dose of AboBoNT-A or a dose range for a specific muscle were considered. Studies that reported doses only for muscle groups, rather than for specific muscles were not eligible.
6.2. Information Sources
Searches were conducted in MEDLINE, MEDLINE In-Process and Embase via Ovid SP (https://ovidsp.ovid.com, accessed on 12 November 2020). The following conferences were also searched for relevant abstracts from 2018 to 2020 meetings: (1) International Society of Physical and Rehabilitation Medicine (2018: Paris, France; 2019: Kobe, Japan; 2020: virtual); (2) World Congress for Neurorehabilitation (2018: Mumbai, India; 2020: virtual); (3) Toxin’s (International Neurotoxin Association; 2019: Copenhagen, Denmark; 2021: virtual). In addition, the bibliographies of relevant systematic reviews published in the past three years and identified during the screening of material retrieved by the searches were cross-checked as a quality-assurance step to identify any relevant studies that were not identified through the electronic database searches.
6.3. Search Strategy
Searches were based on separate search terms for upper and lower limb spasticity and AboBoNT-A as treatment. The search strategy involved a combination of Medical Subjects Headings (extremities/arm/leg/limb/muscle spasticity/muscle hypertonia/dystonia/spasticity/stroke/cerebral palsy/cerebrovascular accident/multiple sclerosis/spinal cord injury/spinal cord injuries) and the keywords “botulinum toxin A,” “dysport,” “abobotulinumtoxinA,” “abobotulinum toxin type A,” “abobotulinum toxin A,” “botulinum a toxin,” “botulinum toxin type a,” “type a botulinum toxin$,” “clostridium botulinum toxin type a,” “clostridium botulinum a toxin botulinum neurotoxin a,” “limb or arm or leg or arms or legs or extremit$,” “spastic$ or hypertonic or hypertonia$ or dystonia$ or dystonic,” “cerebral palsy/stroke or post-stroke or spinal cord injury* or multiple sclerosis” and a combination thereof. No limitations on the publication date were applied, and the searches were limited neither by language nor geography.
6.4. Selection Process
Once the literature searches had been conducted and duplicate records across the databases had been removed, each title and abstract identified was screened by two independent investigators according to the inclusion/exclusion criteria. The full-text articles of studies accepted at the abstract level were retrieved for further review. The full-text screening was conducted by two independent investigators using the same inclusion and exclusion criteria that had been applied during abstract screening. Accepted articles needed to meet all of the inclusion criteria and none of the exclusion criteria. During both rounds of screening, discrepancies were resolved through discussion between investigators, and a third, senior investigator was consulted if necessary.
6.5. Data Collection Process
Extraction of data from the included studies was performed using a Microsoft Excel®-based data extraction template. The data extraction was conducted by one investigator, and reviewed by a second, senior investigator to ensure consistency and accuracy as a validation step. Any discrepancies were resolved in discussion with a third investigator by comparing the collected data with the information provided by the full paper or abstract. Extracted items included baseline characteristics (population and disease etiology), and information related to treatment with AboBoNT-A (dose and type of value [mean, median, fixed, range], upper and/or lower limb, muscle treated). Patient and treatment characteristics were only extracted for the patient group receiving AboBoNT-A; information on comparator treatments or comparative outcome data were not extracted. Data from any study that was represented in multiple articles (including interim and/or final/complete results, post-hoc or subgroup analyses) were extracted as being from a single study.
6.6. Study Risk-of-Bias Assessment
Quality assessment of qualitative research, RCTs, non-randomized studies, quantitative descriptive studies, and/or mixed methods studies included in this systematic review was conducted by using the Mixed Methods Appraisal Tool (MMAT) version 2018, Canadian Intellectual Property Office, Industry Canada. [64]. The MMAT can be used to appraise the quality of various types of empirical studies (i.e., primary research based on experiment, observation or simulation). A single study-design category is selected for each included study and appraised with the respective questions per category. No overall score is assigned with this tool; answers to questions relevant to each category are assigned as “yes,” “no,” or “can’t tell.” Note that, in order to operate the tool, assessment of the quality of the included studies could be conducted only for the objectives and outcomes for which the studies were designed rather than specifically for the dosing data they provided for the systematic review. Conference abstracts were not quality-assessed due to the limited information available in them.
6.7. Data Analysis and Synthesis
The relationship between muscle volume and AboBoNT-A dose given in the included studies was explored through scatter plots. The specific muscles injected in each study were assumed to have the average muscle volume in cm3, as reported in Holzbaur et al., 2007 for upper-limb muscles [59] and Handsfield et al., 2014 for lower-limb muscles [60]. Based on muscle-volume clusters on the volume-dose plots, individual muscles were grouped into three volume categories (small, medium, and large). In the upper limb, large-, medium-, and small-volume muscles had a volume of ≥100 cm3, 20–99 cm3, and <20 cm3, respectively. In the lower limb, the respective volumes were ≥400 cm3, 100–399 cm3, and <100 cm3. Across studies and for muscles for which sample size was reported, average AboBoNT-A doses (mean, median or fixed-dose values, depending on data availability) were plotted against the average muscle volume to explore interrelationships between these two variables. Dose values were plotted only for muscles for which the average muscle volume was available. The dot size on the plot was weighted by sample size for each muscle injected.
Author Contributions
A.S., C.D., A.F. (Andreas Freitag), I.I., K.F., L.L., J.-Y.L., A.F. (Anne Forestier) and D.G. made substantial contributions to the conception and design, data acquisition, or analysis and interpretation of data. A.S., C.D., A.F. (Andreas Freitag), I.I., K.F., L.L., J.-Y.L., A.F. (Anne Forestier) and D.G. participated in drafting the manuscript or revising it critically for important intellectual content. All authors gave final approval of the version to be published. A.S., C.D., A.F. (Andreas Freitag), I.I., K.F., L.L., J.-Y.L., A.F. (Anne Forestier) and D.G. agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ipsen.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Simin Hua for her help in the data analysis.
Conflicts of Interest
This study was sponsored by Ipsen. AF, II, KF and LL are employees of Evidera, which received funding from Ipsen for performing the SLR and statistical analyses that informed this study. CD, JYL and AFo are employees of Ipsen. DG and AS report personal fees for consultancy from Ipsen, Allergan and Merz.
Appendix A

Table A1.
Quality assessment of quantitative randomized controlled trials.
Table A1.
Quality assessment of quantitative randomized controlled trials.
| First Author, Year | Is Randomization Appropriately Performed? | Are the Groups Comparable at Baseline? | Are There Complete Outcome Data? | Are Outcome Assessors Blinded to the Intervention Provided? | Did the Participants Adhere to the Assigned Intervention? |
|---|---|---|---|---|---|
| Bakheit, 2000 [12] | Can’t tell | Yes | Yes | Can’t tell | Yes |
| Bakheit, 2001 [13] | Yes | Yes | Yes | Yes | Yes |
| Baricich, 2008 [17] | Yes | Yes | Yes | Can’t tell | Yes |
| Bhakta, 2000 [12] | Yes | Yes | Yes | Yes | Yes |
| Burbaud, 1996 [21] | Can’t tell | Yes | Yes | Can’t tell | Yes |
| Gracies, 2017 [28] | Yes | Yes | Yes | Yes | Yes |
| Hesse, 1995 [32] | Can’t tell | Yes | Can’t tell | Can’t tell | Yes |
| Hesse, 1998 [33] | Yes | Yes | Yes | Can’t tell | Yes |
| Johnson, 2002 [35] | Yes | Yes | Yes | Can’t tell | Yes |
| Kong, 2007 [36] | Yes | Yes | Yes | Yes | Yes |
| Lam, 2012 [37] | Yes | Yes | Yes | Yes | Yes |
| Marco, 2007 [39] | Yes | Yes | Yes | Yes | Yes |
| McCrory, 2009 [40] | Yes | Yes | Yes | Yes | Yes |
| O’Dell, 2018 [43] | Can’t tell | Yes | Yes | Can’t tell | Yes |
| Picelli, 2014 [47] | Yes | Yes | Yes | Yes | Yes |
| Picelli, 2016 [48] | Yes | Yes | Yes | Yes | Yes |
| Rekand, 2019 [50] | Yes | Yes | Yes | Yes | Yes |
| Rosales, 2012 [51] | Yes | Yes | Yes | Yes | Yes |
| Shaw, 2010 [52] | Yes | Yes | Yes | Yes | Yes |
| Sun, 2010 [53] | Yes | Yes | Yes | Yes | Yes |
| Suputtitada, 2005 [54] | Yes | Yes | Yes | Yes | Yes |
| Yazdchi, 2013 [57] | Yes | Can’t tell | Yes | Can’t tell | Can’t tell |
| Yelnik, 2007 [58] | Yes | Yes | Yes | Yes | Yes |

Table A2.
Quality assessment of quantitative non-randomized studies.
Table A2.
Quality assessment of quantitative non-randomized studies.
| First Author, Year | Are the Participants Representative of the Target Population? | Are Measurements Appropriate Regarding Both the Outcome and Intervention (or Exposure)? | Are There Complete Outcome Data? | Are the Confounders Accounted for in the Design and Analysis? | During the Study Period, Is the Intervention Administered (or Exposure Occurred) as Intended? |
|---|---|---|---|---|---|
| Bakheit, 2002 [14] | Can’t tell | Yes | Yes | Can’t tell | Yes |
| Barden, 2014 [16] | Yes | Yes | Yes | Can’t tell | Yes |
| Carvalho, 2018 [23] | Yes | Yes | Yes | Can’t tell | Yes |
| de Niet, 2015 [24] | No | Yes | Yes | Yes | Yes |
| Frasson, 2005 [26] | Yes | Yes | Yes | Can’t tell | Yes |
| Ghroubi, 2020 [27] | Yes | Yes | Yes | Can’t tell | Yes |
| Turner-Stokes, 2013 [55] | Yes | Yes | Yes | Yes | Yes |

Table A3.
Quality assessment of quantitative descriptive studies.
Table A3.
Quality assessment of quantitative descriptive studies.
| First Author, Year | Is the Sampling Strategy Relevant to Address the Research Question? | Is the Sample Representative of the Target Population? | Are the Measurements Appropriate? | Is the Risk of Nonresponse Bias Low? | Is the Statistical Analysis Appropriate to Answer the Research Question? |
|---|---|---|---|---|---|
| Alvisi, 2018 [10] | Yes | No | Yes | Can’t tell | Can’t tell |
| Ashford, 2009 [11] | Yes | Yes | Yes | Can’t tell | Yes |
| Bakheit, 2004 [15] | Yes | Yes | Yes | Can’t tell | Yes |
| Beseler, 2012 [18] | Yes | Yes | Yes | Yes | Can’t tell |
| Bhakta, 1996 [19] | Yes | Yes | Yes | Can’t tell | Yes |
| Cardoso, 2007 [22] | Yes | Yes | Yes | Can’t tell | Yes |
| Finsterer, 1997 [25] | No | Yes | Yes | Yes | Yes |
| Hecht, 2008 [31] | Yes | No | Yes | Yes | No |
| Hubble, 2013 [34] | Yes | Yes | Yes | No | Can’t tell |
| Moccia, 2020 [41] | Yes | Yes | Yes | Can’t tell | Yes |
| Nott, 2014 [42] | Yes | Yes | Yes | Yes | Yes |
| Otom, 2014 [44] | Yes | Yes | Yes | Yes | Can’t tell |
| Pauri, 2000 [45] | Yes | Yes | Yes | Yes | Yes |
| Picelli, 2012 [46] | Yes | Yes | Yes | Yes | Yes |
| Picelli, 2020 [49] | Yes | Yes | Yes | Can’t tell | Can’t tell |
| Woldag, 2003 [56] | Yes | Yes | Yes | Yes | Can’t tell |
Appendix B

Table A4.
Results of individual studies—upper limb.
Table A4.
Results of individual studies—upper limb.
| First Author, Year | Intervention | Name of Muscle | Volume Category | Muscle Volume (cm3) | Type of AboBoNT-A Dose Measure | Dose Value (U) |
|---|---|---|---|---|---|---|
| Alvisi, 2018 [10] | AboBoNT-A | Abductor pollicis longus | Small | 11.9 | Fixed value | 160 |
| AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Range | 50–200 | |
| AboBoNT-A | Teres major | Medium | 32.7 | Fixed value | 100 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Range | 100–200 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Range | 150–200 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Range | 100–200 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Range | 100–200 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Range | 100–300 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Range | 50–350 | |
| AboBoNT-A | Triceps brachii | Large | 372.1 | Fixed value | 350 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 150–300 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Range | 100–150 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Range | 100–400 | |
| Ashford, 2009 [11] | AboBoNT-A | Subscapularis | Large | 164.5 | Fixed value | 400 |
| AboBoNT-A | Rhomboideus major | Large | NR | Fixed value | 250 | |
| AboBoNT-A | Trapezius | Large | NR | Fixed value | 100 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 150–400 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Range | 150–250 | |
| AboBoNT-A | Latissimus dorsi | Large | 262.3 | Range | 400–500 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Range | 250–500 | |
| Bakheit, 2000 [12] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Range | 75–225 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Range | 75–225 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Range | 75–225 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Range | 75–225 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 200–600 | |
| Bakheit, 2001 [13] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Fixed value | 150 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Range | NR | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 300–400 | |
| Bakheit, 2002 [14] | AboBoNT-A | Biceps brachii | Large | 143.7 | Fixed value | 500 |
| Bakheit, 2004 [15] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Fixed value | 150 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Range | 150–250 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 300–400 | |
| Barden, 2014 [16] | AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Median | 75 |
| AboBoNT-A | Pronator quadratus | Small | 11.2 | Median | 87.5 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Median | 150 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Median | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 100 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 190 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Median | 87.5 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 188 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Median | 75 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Median | 150 | |
| AboBoNT-A | Subscapularis | Large | 164.5 | Median | 150 | |
| Bhakta, 1996 [19] | AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Mean | 117.9 |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Mean | 143.2 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Mean | 134.1 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Mean | 220 | |
| Bhakta, 2000 [20] | AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 100 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Median | 100 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 200 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 300 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 300 | |
| Cardoso, 2007 [22] | AboBoNT-A | Opponens pollicis | Small | NR | Mean | 62.5 |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Mean | 187.5 | |
| AboBoNT- | Flexor carpi radialis | Medium | 34.8 | Mean | 170 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Mean | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Mean | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Mean | 150 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Mean | 150 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Mean | 225 | |
| AboBoNT-A | Deltoideus | Large | 380.5 | Mean | 200 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Mean | 250 | |
| AboBoNT-A | Triceps brachii | Large | 372.1 | Mean | 200 | |
| Carvalho, 2018 [23] | AboBoNT-A | Supraspinatus | Medium | 50.0 | Mean | 124 |
| Teres major | Medium | 32.7 | Mean | 104 | ||
| AboBoNT-A | Deltoideus | Large | 380.5 | Mean | 130 | |
| AboBoNT-A | Infraspinatus | Large | 118.6 | Mean | 50 | |
| AboBoNT-A | Latissimus dorsi | Large | 262.3 | Mean | 115 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Mean | 120 | |
| AboBoNT-A | Subscapularis | Large | 164.5 | Mean | 133 | |
| AboBoNT-A | Rhomboideus major | Large | NR | Mean | 125 | |
| AboBoNT-A | Trapezius | Large | NR | Mean | 96 | |
| Finsterer, 1997 [25] | AboBoNT-A | Brachioradialis | Medium | 65.1 | Fixed value | 60 |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 40 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 100–160 | |
| AboBoNT-A | Trapezius | Large | NR | Range | 80–100 | |
| Ghroubi, 2020 [27] | AboBoNT-A | Adductor pollicis | Small | NR | Median | 50 |
| AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Median | 50 | |
| AboBoNT-A | Pronator quadratus | Small | 11.2 | Median | 100 | |
| AboBoNT-A | Dorsal interossei (hand) | Small | NR | Median | 100 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Median | 100 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Median | 100 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 100 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 150 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Median | 100 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 200 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Median | 100 | |
| AboBoNT-A | Deltoideus | Large | 380.5 | Median | 240 | |
| AboBoNT-A | Latissimus dorsi | Large | 262.3 | Median | 200 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Median | 150 | |
| AboBoNT-A | Subscapularis | Large | 164.5 | Median | 150 | |
| AboBoNT-A | Triceps brachii | Large | 372.1 | Median | 170 | |
| Gracies, 2018 [29] | AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Mean | 106.3 |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Mean | 140.7 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Mean | 142.3 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Mean | 103.1 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Mean | 155.8 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Mean | 156.9 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Mean | 144.6 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Mean | 206.4 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Mean | 208.3 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Mean | 176.8 | |
| Gul, 2016 [30] | AboBoNT-A | Latissimus dorsi | Large | 262.3 | Range | 150–200 |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Range | 170–290 | |
| AboBoNT-A | Subscapularis | Large | 164.5 | Range | 100–175 | |
| AboBoNT-A | Triceps brachii | Large | 372.1 | Range | 150–200 | |
| Hesse, 1998 [33] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Fixed value | 125 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 125 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 125 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 125 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Fixed value | 250 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Fixed value | 250 | |
| Hubble, 2013 * [34] | AboBoNT-A in UK | Adductor pollicis | Small | NR | Mean | 291 |
| AboBoNT-A in France | Flexor pollicis longus | Small | 17.1 | Mean | 103 | |
| AboBoNT-A in Germany | Flexor pollicis longus | Small | 17.1 | Mean | 87 | |
| AboBoNT-A in Greece | Flexor pollicis longus | Small | 17.1 | Mean | 93 | |
| AboBoNT-A in Sweden | Flexor pollicis longus | Small | 17.1 | Mean | 47 | |
| AboBoNT-A in UK | Flexor pollicis longus | Small | 17.1 | Mean | 106 | |
| AboBoNT-A in France | Brachioradialis | Medium | 65.1 | Mean | 183 | |
| AboBoNT-A in Germany | Brachioradialis | Medium | 65.1 | Mean | 125 | |
| AboBoNT-A in Greece | Brachioradialis | Medium | 65.1 | Mean | 183 | |
| AboBoNT-A in Sweden | Brachioradialis | Medium | 65.1 | Mean | 183 | |
| AboBoNT-A in the UK | Brachioradialis | Medium | 65.1 | Mean | 192 | |
| AboBoNT-A in France | Flexor carpi radialis | Medium | 34.8 | Mean | 158 | |
| AboBoNT-A in Germany | Flexor carpi radialis | Medium | 34.8 | Mean | 106 | |
| AboBoNT-A in Greece | Flexor carpi radialis | Medium | 34.8 | Mean | 152 | |
| AboBoNT-A in Sweden | Flexor carpi radialis | Medium | 34.8 | Mean | 107 | |
| AboBoNT-A in UK | Flexor carpi radialis | Medium | 34.8 | Mean | 134 | |
| AboBoNT-A in France | Flexor carpi ulnaris | Medium | 37.1 | Mean | 167 | |
| AboBoNT-A in Germany | Flexor carpi ulnaris | Medium | 37.1 | Mean | 100 | |
| AboBoNT-A in Greece | Flexor carpi ulnaris | Medium | 37.1 | Mean | 127 | |
| AboBoNT-A in Sweden | Flexor carpi ulnaris | Medium | 37.1 | Mean | 80 | |
| AboBoNT-A in UK | Flexor carpi ulnaris | Medium | 37.1 | Mean | 142 | |
| AboBoNT-A in France | Flexor digitorum profundus | Medium | 91.6 | Mean | 137 | |
| AboBoNT-A in Germany | Flexor digitorum profundus | Medium | 91.6 | Mean | 127 | |
| AboBoNT-A in Greece | Flexor digitorum profundus | Medium | 91.6 | Mean | 102 | |
| AboBoNT-A in Sweden | Flexor digitorum profundus | Medium | 91.6 | Mean | 74 | |
| AboBoNT-A in the UK | Flexor digitorum profundus | Medium | 91.6 | Mean | 146 | |
| AboBoNT-A in France | Flexor digitorum superficialis | Medium | 74.2 | Mean | 145 | |
| AboBoNT-A in Germany | Flexor digitorum superficialis | Medium | 74.2 | Mean | 130 | |
| AboBoNT-A in Greece | Flexor digitorum superficialis | Medium | 74.2 | Mean | 83 | |
| AboBoNT-A in Sweden | Flexor digitorum superficialis | Medium | 74.2 | Mean | 88 | |
| AboBoNT-A in UK | Flexor digitorum superficialis | Medium | 74.2 | Mean | 218 | |
| AboBoNT-A in France | Pronator teres | Medium | 38.4 | Mean | 129 | |
| AboBoNT-A in Greece | Pronator teres | Medium | 38.4 | Mean | 96 | |
| AboBoNT-A in UK | Pronator teres | Medium | 38.4 | Mean | 136 | |
| AboBoNT-A in France | Biceps brachii | Large | 143.7 | Mean | 226 | |
| AboBoNT-A in Germany | Biceps brachii | Large | 143.7 | Mean | 188 | |
| AboBoNT-A in Greece | Biceps brachii | Large | 143.7 | Mean | 244 | |
| AboBoNT-A in Sweden | Biceps brachii | Large | 143.7 | Mean | 170 | |
| AboBoNT-A in the UK | Biceps brachii | Large | 143.7 | Mean | 364 | |
| AboBoNT-A in France | Brachialis | Large | 143.7 | Mean | 218 | |
| AboBoNT-A in Germany | Brachialis | Large | 143.7 | Mean | 112 | |
| AboBoNT-A in Greece | Brachialis | Large | 143.7 | Mean | 175 | |
| AboBoNT-A in Sweden | Brachialis | Large | 143.7 | Mean | 218 | |
| AboBoNT-A in the UK | Brachialis | Large | 143.7 | Mean | 160 | |
| AboBoNT-A in France | Pectoralis major | Large | 290.0 | Mean | 186 | |
| AboBoNT-A in Germany | Pectoralis major | Large | 290.0 | Mean | 114 | |
| AboBoNT-A in Greece | Pectoralis major | Large | 290.0 | Mean | 165 | |
| AboBoNT-A in Sweden | Pectoralis major | Large | 290.0 | Mean | 233 | |
| AboBoNT-A in the UK | Pectoralis major | Large | 290.0 | Mean | 271 | |
| Kong, 2007 [36] | AboBoNT-A | Biceps brachii | Large | 143.7 | Fixed value | 250 |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Fixed value | 250 | |
| Lam, 2012 [37] | AboBoNT-A | Adductor pollicis | Small | NR | Median | 100 |
| AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Median | 100 | |
| AboBoNT-A | Flexor pollicis brevis | Small | NR | Median | 50 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 150 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 250 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Median | 150 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Median | 250 | |
| Lejeune, 2020 † [38] | AboBoNT-A/baseline | Supraspinatus | Medium | 50.0 | Median | 100 |
| AboBoNT-A/baseline | Teres minor | Medium | 28.0 | Median | 100 | |
| AboBoNT-A/baseline | Rhomboideus major | Large | NR | Median | 150 | |
| AboBoNT-A/baseline | Trapezius | Large | NR | Median | 100 | |
| AboBoNT-A/cycle 1 | Latissimus dorsi | Large | 262.3 | Mean | 200 | |
| AboBoNT-A/cycle 1 | Pectoralis major | Large | 290.0 | Mean | 168 | |
| AboBoNT-A/cycle 1 | Subscapularis | Large | 164.5 | Mean | 175 | |
| AboBoNT-A/cycle 1 | Triceps brachii | Large | 372.1 | Mean | 150 | |
| AboBoNT-A/cycle 2 | Latissimus dorsi | Large | 262.3 | Mean | 194.5 | |
| AboBoNT-A/cycle 2 | Pectoralis major | Large | 290.0 | Mean | 226 | |
| AboBoNT-A/cycle 2 | Subscapularis | Large | 164.5 | Mean | 147.6 | |
| AboBoNT-A/cycle 2 | Triceps brachii | Large | 372.1 | Mean | 184.8 | |
| AboBoNT-A/cycle 3 | Latissimus dorsi | Large | 262.3 | Mean | 193.4 | |
| AboBoNT-A/cycle 3 | Pectoralis major | Large | 290.0 | Mean | 227.7 | |
| AboBoNT-A/cycle 3 | Subscapularis | Large | 164.5 | Mean | 122.8 | |
| AboBoNT-A/cycle 3 | Triceps brachii | Large | 372.1 | Mean | 186.1 | |
| AboBoNT-A/cycle 4 | Latissimus dorsi | Large | 262.3 | Mean | 175.4 | |
| AboBoNT-A/cycle 4 | Pectoralis major | Large | 290.0 | Mean | 224 | |
| AboBoNT-A/cycle 4 | Subscapularis | Large | 164.5 | Mean | 130 | |
| AboBoNT-A/cycle 4 | Triceps brachii | Large | 372.1 | Mean | 194 | |
| Marciniak, 2017 ‡ [65] | AboBoNT-A 500U | Adductor pollicis | Small | NR | Mean | 25 |
| AboBoNT-A 1000U | Adductor pollicis | Small | NR | Mean | 50 | |
| AboBoNT-A 1000U | Extensor pollicis longus | Small | 6.6 | Mean | 150 | |
| AboBoNT-A 500U | Flexor pollicis longus | Small | 17.1 | Mean | 72.5 | |
| AboBoNT-A 500U PTMG | Brachioradialis | Medium | 65.1 | Mean | 100 | |
| AboBoNT-A 500U non-PTMG | Brachioradialis | Medium | 65.1 | Mean | 81.3 | |
| AboBoNT-A 1000U PTMG | Brachioradialis | Medium | 65.1 | Mean | 200 | |
| AboBoNT-A 1000U non-PTMG | Brachioradialis | Medium | 65.1 | Mean | 105 | |
| AboBoNT-A 500U PTMG | Flexor carpi radialis | Medium | 34.8 | Mean | 100 | |
| AboBoNT-A 500U non-PTMG | Flexor carpi radialis | Medium | 34.8 | Mean | 90.6 | |
| AboBoNT-A 1000U PTMG | Flexor carpi radialis | Medium | 34.8 | Mean | 191.7 | |
| AboBoNT-A 1000U non-PTMG | Flexor carpi radialis | Medium | 34.8 | Mean | 174.7 | |
| AboBoNT-A 500U PTMG | Flexor carpi ulnaris | Medium | 37.1 | Mean | 100 | |
| AboBoNT-A 500U non-PTMG | Flexor carpi ulnaris | Medium | 37.1 | Mean | 94.1 | |
| AboBoNT-A 1000U PTMG | Flexor carpi ulnaris | Medium | 37.1 | Mean | 191.7 | |
| AboBoNT-A 1000U non-PTMG | Flexor carpi ulnaris | Medium | 37.1 | Mean | 156.8 | |
| AboBoNT-A 500U PTMG | Flexor digitorum profundus | Medium | 91.6 | Mean | 100 | |
| AboBoNT-A 500U non-PTMG | Flexor digitorum profundus | Medium | 91.6 | Mean | 62.5 | |
| AboBoNT-A 1000U PTMG | Flexor digitorum profundus | Medium | 91.6 | Mean | 194.4 | |
| AboBoNT-A 1000U non-PTMG | Flexor digitorum profundus | Medium | 91.6 | Mean | 181.3 | |
| AboBoNT-A 500U PTMG | Flexor digitorum superficialis | Medium | 74.2 | Mean | 100 | |
| AboBoNT-A 500U non-PTMG | Flexor digitorum superficialis | Medium | 74.2 | Mean | 82.5 | |
| AboBoNT-A 1000U PTMG | Flexor digitorum superficialis | Medium | 74.2 | Mean | 200 | |
| AboBoNT-A 1000U non-PTMG | Flexor digitorum superficialis | Medium | 74.2 | Mean | 196.2 | |
| AboBoNT-A 500U | Pronator teres | Medium | 38.4 | Mean | 66.7 | |
| AboBoNT-A 1000U | Pronator teres | Medium | 38.4 | Mean | 136.7 | |
| AboBoNT-A 500U | Biceps brachii | Large | 143.7 | Mean | 103.3 | |
| AboBoNT-A 1000U | Biceps brachii | Large | 143.7 | Mean | 228.6 | |
| AboBoNT-A 500U PTMG | Brachialis | Large | 143.7 | Mean | 187.5 | |
| AboBoNT-A 500U non-PTMG | Brachialis | Large | 143.7 | Mean | 124 | |
| AboBoNT-A 1000U PTMG | Brachialis | Large | 143.7 | Mean | 400 | |
| AboBoNT-A 1000U non-PTMG | Brachialis | Large | 143.7 | Mean | 211.1 | |
| AboBoNT-A 500U | Latissimus dorsi | Large | 262.3 | Mean | 100 | |
| AboBoNT-A 1000U | Latissimus dorsi | Large | 262.3 | Mean | 100 | |
| AboBoNT-A 500U | Pectoralis major | Large | 290.0 | Mean | 100 | |
| AboBoNT-A 1000U | Pectoralis major | Large | 290.0 | Mean | 250 | |
| AboBoNT-A 500U | Subscapularis | Large | 164.5 | Mean | 100 | |
| Marco, 2007 [39] | AboBoNT-A | Pectoralis major | Large | 290.0 | Fixed value | 500 |
| McCrory, 2009 § [40] | AboBoNT-A/cycle 1 | Flexor pollicis longus | Small | 17.1 | Median | 100 |
| AboBoNT-A/cycle 2 | Extensor carpi ulnaris | Small | 17 | Median | 150 | |
| AboBoNT-A/cycle 2 | Flexor pollicis longus | Small | 17.1 | Median | 200 | |
| AboBoNT-A/cycle 1 | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A/cycle 1 | Flexor carpi radialis | Medium | 34.8 | Median | 150 | |
| AboBoNT-A/cycle 1 | Flexor carpi ulnaris | Medium | 37.1 | Median | 150 | |
| AboBoNT-A/cycle 1 | Flexor digitorum profundus | Medium | 91.6 | Median | 150 | |
| AboBoNT-A/cycle 1 | Flexor digitorum superficialis | Medium | 74.2 | Median | 200 | |
| AboBoNT-A/cycle 2 | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A/cycle 2 | Flexor carpi radialis | Medium | 34.8 | Median | 150 | |
| AboBoNT-A/cycle 2 | Flexor digitorum profundus | Medium | 91.6 | Median | 150 | |
| AboBoNT-A/cycle 2 | Flexor digitorum superficialis | Medium | 74.2 | Median | 200 | |
| AboBoNT-A/cycle 1 | Biceps brachii | Large | 143.7 | Median | 300 | |
| AboBoNT-A/cycle 1 | Brachialis | Large | 143.7 | Median | 100 | |
| AboBoNT-A/cycle 1 | Triceps brachii | Large | 372.1 | Median | 275 | |
| AboBoNT-A/cycle 2 | Biceps brachii | Large | 143.7 | Median | 300 | |
| AboBoNT-A/cycle 2 | Brachialis | Large | 143.7 | Median | 100 | |
| AboBoNT-A/cycle 2 | Triceps brachii | Large | 372.1 | Median | 250 | |
| Moccia, 2020 [41] | AboBoNT-A | Brachioradialis | Medium | 65.1 | Mean | 169.3 |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Mean | 500 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Mean | 250 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Mean | 147.1 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Mean | 153.3 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Mean | 250.7 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Mean | 75 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Mean | 193.3 | |
| AboBoNT-A | Triceps brachii | Large | 372.1 | Mean | 100 | |
| Nott, 2014 [42] | AboBoNT-A | Adductor pollicis | Small | NR | Median | 37.5 |
| AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Median | 75 | |
| AboBoNT-A | Lumbricals (hand) | Small | NR | Median | 100 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Median | 150 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Median | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 100 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 190 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Median | 87.5 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 188 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Median | 75 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Median | 150 | |
| AboBoNT-A | Subscapularis | Large | 164.5 | Median | 150 | |
| O’Dell, 2018 [43] | AboBoNT-A 500U | Adductor pollicis | Small | NR | Mean | 30 |
| AboBoNT-A 1000U | Adductor pollicis | Small | NR | Mean | 50.7 | |
| AboBoNT-A 500U | Flexor pollicis longus | Small | 17.1 | Mean | 64.4 | |
| AboBoNT-A 1000U | Flexor pollicis longus | Small | 17.1 | Mean | 139.7 | |
| AboBoNT-A 500U | Brachioradialis | Medium | 65.1 | Mean | 88.3 | |
| AboBoNT-A 1000U | Brachioradialis | Medium | 65.1 | Mean | 172.1 | |
| AboBoNT-A 500U | Flexor carpi radialis | Medium | 34.8 | Mean | 92.2 | |
| AboBoNT-A 1000U | Flexor carpi radialis | Medium | 34.8 | Mean | 178.1 | |
| AboBoNT-A 500U | Flexor carpi ulnaris | Medium | 37.1 | Mean | 89.9 | |
| AboBoNT-A 1000U | Flexor carpi ulnaris | Medium | 37.1 | Mean | 171.2 | |
| AboBoNT-A 500U | Flexor digitorum profundus | Medium | 91.6 | Mean | 93.5 | |
| AboBoNT-A 1000U | Flexor digitorum profundus | Medium | 91.6 | Mean | 195.5 | |
| AboBoNT-A 500U | Flexor digitorum superficialis | Medium | 74.2 | Mean | 95.4 | |
| AboBoNT-A 1000U | Flexor digitorum superficialis | Medium | 74.2 | Mean | 196.8 | |
| AboBoNT-A 500U | Pronator teres | Medium | 38.4 | Mean | 81.8 | |
| AboBoNT-A 1000U | Pronator teres | Medium | 38.4 | Mean | 157.3 | |
| AboBoNT-A 500U | Biceps brachii | Large | 143.7 | Mean | 106.4 | |
| AboBoNT-A 1000U | Biceps brachii | Large | 143.7 | Mean | 207.4 | |
| AboBoNT-A 500U | Brachialis | Large | 143.7 | Mean | 148.5 | |
| AboBoNT-A 1000U | Brachialis | Large | 143.7 | Mean | 321.4 | |
| AboBoNT-A 500U | Latissimus dorsi | Large | 262.3 | Mean | 100 | |
| AboBoNT-A 1000U | Latissimus dorsi | Large | 262.3 | Mean | 175 | |
| AboBoNT-A 500U | Pectoralis major | Large | 290.0 | Mean | 100 | |
| AboBoNT-A 1000U | Pectoralis major | Large | 290.0 | Mean | 200 | |
| AboBoNT-A 500U | Subscapularis | Large | 164.5 | Mean | 100 | |
| AboBoNT-A 1000U | Triceps brachii | Large | 372.1 | Mean | 100 | |
| AboBoNT-A 500U | Triceps brachii | Large | 372.1 | Mean | 200 | |
| Picelli, 2014 [47] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Fixed value | 150 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 250 | |
| Rekand, 2019 [50] | AboBoNT-A current practice | Brachioradialis | Medium | 65.1 | Range | 30–210 |
| AboBoNT-A NMJ-targeted | Brachioradialis | Medium | 65.1 | Range | 40–200 | |
| AboBoNT-A current practice | Flexor carpi radialis | Medium | 34.8 | Range | 30–210 | |
| AboBoNT-A NMJ-targeted | Flexor carpi radialis | Medium | 34.8 | Range | 40–200 | |
| AboBoNT-A current practice | Flexor carpi ulnaris | Medium | 37.1 | Range | 30–210 | |
| AboBoNT-A NMJ-targeted | Flexor carpi ulnaris | Medium | 37.1 | Range | 40–200 | |
| AboBoNT-A current practice | Biceps brachii | Large | 143.7 | Range | 30–210 | |
| AboBoNT-A NMJ-targeted | Biceps brachii | Large | 143.7 | Range | 40–200 | |
| AboBoNT-A current practice | Brachialis | Large | 143.7 | Range | 30–210 | |
| AboBoNT-A NMJ-targeted | Brachialis | Large | 143.7 | Range | 40–200 | |
| Rosales, 2012 [51] | AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Median | 25 |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Median | 100 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Median | 100 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 50 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 50 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 200 | |
| Shaw, 2010 ¥ [52] | AboBoNT-A/3, 6, 9 months | Flexor pollicis longus | Small | 17.1 | Median | 50 |
| AboBoNT-A/baseline | Flexor pollicis longus | Small | 17.1 | Median | 100 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 100 | |
| AboBoNT-A/3, 6, 9 months | Flexor carpi radialis | Medium | 34.8 | Median | 100 | |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Median | 100 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 100 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 100 | |
| AboBoNT-A/baseline | Flexor carpi radialis | Medium | 34.8 | Median | 50 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 100 | |
| AboBoNT-A/baseline and 3 months | Pectoralis major | Large | 290.0 | Median | 100 | |
| AboBoNT-A/6 months | Pectoralis major | Large | 290.0 | Median | 200 | |
| AboBoNT-A/9 months | Pectoralis major | Large | 290.0 | Median | 150 | |
| Shaw, 2010 [52] | AboBoNT-A | Pronator teres | Medium | 38.4 | Median | 100 |
| Sun, 2010 [53] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Fixed value | 150 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 150 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Fixed value | 400 | |
| Suputtitada, 2005 [54] | AboBoNT-A 350U | Flexor carpi radialis | Medium | 34.8 | Fixed value | 50 |
| AboBoNT-A 500U | Flexor carpi radialis | Medium | 34.8 | Fixed value | 75 | |
| AboBoNT-A 1000U | Flexor carpi radialis | Medium | 34.8 | Fixed value | 150 | |
| AboBoNT-A 350U | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 50 | |
| AboBoNT-A 500U | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 75 | |
| AboBoNT-A 1000U | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 150 | |
| AboBoNT-A 350U | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 50 | |
| AboBoNT-A 500U | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 75 | |
| AboBoNT-A 1000U | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 150 | |
| AboBoNT-A 350U | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 50 | |
| AboBoNT-A 500U | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 75 | |
| AboBoNT-A 1000U | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 150 | |
| AboBoNT-A 350U | Biceps brachii | Large | 143.7 | Fixed value | 150 | |
| AboBoNT-A 500U | Biceps brachii | Large | 143.7 | Fixed value | 200 | |
| AboBoNT-A 1000U | Biceps brachii | Large | 143.7 | Fixed value | 400 | |
| Turner-Stokes, 2013 [55] | AboBoNT-A | Adductor pollicis | Small | NR | Median | 50 |
| AboBoNT-A | Dorsal interossei (hand) | Small | NR | Median | 150 | |
| AboBoNT-A | Flexor pollicis brevis | Small | NR | Median | 50 | |
| AboBoNT-A | Lumbricals (hand) | Small | NR | Median | 100 | |
| AboBoNT-A | Opponens pollicis | Small | NR | Median | 50 | |
| AboBoNT-A | Flexor pollicis longus | Small | 17.1 | Median | 100 | |
| AboBoNT-A | Brachioradialis | Medium | 65.1 | Median | 112.5 | |
| AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Median | 125 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Median | 150 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Median | 150 | |
| AboBoNT-A | Pronator teres | Medium | 38.4 | Median | 100 | |
| AboBoNT-A | Teres major | Medium | 32.7 | Median | 75 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Median | 200 | |
| AboBoNT-A | Brachialis | Large | 143.7 | Median | 150 | |
| AboBoNT-A | Deltoideus | Large | 380.5 | Median | 100 | |
| AboBoNT-A | Latissimus dorsi | Large | 262.3 | Median | 120 | |
| AboBoNT-A | Pectoralis major | Large | 290.0 | Median | 200 | |
| AboBoNT-A | Subscapularis | Large | 164.5 | Median | 200 | |
| AboBoNT-A | Triceps brachii | Large | 372.1 | Median | 175 | |
| Woldag, 2003 [56] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Fixed value | 120 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Fixed value | 120 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Fixed value | 120 | |
| AboBoNT-A | Flexor digitorum superficialis | Medium | 74.2 | Fixed value | 120 | |
| Yazdchi, 2013 [57] | AboBoNT-A | Flexor carpi radialis | Medium | 34.8 | Range | 50–100 |
| AboBoNT-A | Flexor carpi ulnaris | Medium | 37.1 | Range | 50–100 | |
| AboBoNT-A | Flexor digitorum profundus | Medium | 91.6 | Range | 100–150 | |
| AboBoNT-A | Biceps brachii | Large | 143.7 | Range | 150–200 | |
| Yelnik, 2007 [58] | AboBoNT-A | Subscapularis | Large | 164.5 | Fixed value | 500 |
* Survey of AboBoNT-A use by physicians in five European countries. † Study in which patients received up to 4 additional AboBoNT-A treatment cycles at least 12 weeks apart over 1 year. ‡ Study in which doses of AboBoNT-A were reported or not reported for each muscle as part of the most hypertonic muscle group among the elbow, wrist, or finger flexors (primary target muscle group [PTMG]). § Study in which patients received 2 cycles of treatment, 12 weeks apart. ¥ Study in which participants in the intervention group received AboBoNT-A injections to the upper limb immediately following study entry, plus repeat injections at 3, 6 and 9 months if clinically indicated. Legend: AboBoNT-A, abobotulinumtoxinA; NMJ, neuromuscular junction; NR, not reported; PTMG, primary target muscle group; U, unit; UK, United Kingdom.

Table A5.
Results of individual studies—lower limb.
Table A5.
Results of individual studies—lower limb.
| First Author, Year | Intervention | Name of Muscle | Volume Category | Muscle Volume (cm3) | Type of AboBoNT-A Dose Measure | Dose Value (U) |
|---|---|---|---|---|---|---|
| Baricich, 2008 [17] | AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Range | 150–250 |
| AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150.0 | Range | 150–250 | |
| Beseler, 2012 [18] | AboBoNT-A | Flexor hallucis longus | Small | 78.8 | Mean | 125 |
| AboBoNT-A | Flexor digitorum brevis | Small | NR | Mean | 85 | |
| AboBoNT-A | Extensor hallucis longus | Medium | 102.3 | Mean | 100 | |
| AboBoNT-A | Sartorius | Medium | 163.7 | Mean | 85 | |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Mean | 200 | |
| AboBoNT-A | Soleus | Large | 438.2 | Mean | 150 | |
| AboBoNT-A | Triceps surae | Large | 845.6 | Mean | 166 | |
| Burbaud, 1996 [21] | AboBoNT-A | Flexor digitorum longus | Small | 30.0 | Range | 150–300 |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Range | 200–350 | |
| AboBoNT-A | Triceps surae | Large | 845.6 | Range | 500–1000 | |
| AboBoNT-A | Soleus | Large | 438.2 | Range | 200–400 | |
| de Niet, 2015 [24] | AboBoNT-A | Triceps surae | Large | 845.6 | Fixed value | 500 |
| AboBoNT-A | Triceps surae | Large | 845.6 | Fixed value | 750 | |
| Esquenazi, 2020 [66] | AboBoNT-A 1000U | Flexor digitorum longus | Small | 30.0 | Mean | 139.1 |
| AboBoNT-A 1500U | Flexor digitorum longus | Small | 30.0 | Mean | 221.7 | |
| AboBoNT-A 1000U | Flexor digitorum brevis | Small | NR | Mean | 77.3 | |
| AboBoNT-A 1500U | Flexor digitorum brevis | Small | NR | Mean | 137.5 | |
| AboBoNT-A 1000U | Flexor hallucis longus | Small | 78.8 | Mean | 94.9 | |
| AboBoNT-A 1500U | Flexor hallucis longus | Small | 78.8 | Mean | 164 | |
| AboBoNT-A 1000U | Flexor hallucis brevis | Small | NR | Mean | 111.1 | |
| AboBoNT-A 1500U | Flexor hallucis brevis | Small | NR | Mean | 160 | |
| AboBoNT-A 1000U | Biceps femoris | Medium | 100.1 | Mean | 183.3 | |
| AboBoNT-A 1500U | Biceps femoris | Medium | 100.1 | Mean | 300 | |
| AboBoNT-A 1000U | Gastrocnemius (lateralis) | Medium | 150.0 | Mean | 88.8 | |
| AboBoNT-A 1000U | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 141.6 | |
| AboBoNT-A 1500U | Gastrocnemius (lateralis) | Medium | 150.0 | Mean | 128.8 | |
| AboBoNT-A 1500U | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 169.6 | |
| AboBoNT-A 1000U | Rectus femoris | Medium | 269.0 | Mean | 186.9 | |
| AboBoNT-A 1500U | Rectus femoris | Medium | 269.0 | Mean | 372.7 | |
| AboBoNT-A 1000U | Tibialis posterior | Medium | 104.8 | Mean | 190 | |
| AboBoNT-A 1500U | Tibialis posterior | Medium | 104.8 | Mean | 274.7 | |
| AboBoNT-A 1500U | Adductor magnus | Large | 559.8 | Mean | 300 | |
| AboBoNT-A 1000U | Soleus | Large | 438.2 | Mean | 333.3 | |
| AboBoNT-A 1500U | Soleus | Large | 438.2 | Mean | 478.6 | |
| Finsterer, 1997 [25] | AboBoNT-A | Rectus femoris | Medium | 269.0 | Range | 40–80 |
| AboBoNT-A | Adductor magnus | Large | 559.8 | Range | 60–240 | |
| AboBoNT-A | Gastrocnemius (combined) | Large | 407.4 | Range | 60–120 | |
| Frasson, 2005 [26] | AboBoNT-A | Extensor digitorum brevis | Small | NR | Fixed value | 50 |
| Gracies, 2017 [28] | AboBoNT-A 1000U | Flexor digitorum brevis | Small | NR | Mean | 89.4 |
| AboBoNT-A 1500U | Flexor digitorum brevis | Small | NR | Mean | 140.8 | |
| AboBoNT-A 1000U | Flexor hallucis brevis | Small | NR | Mean | 93.3 | |
| AboBoNT-A 1500U | Flexor hallucis brevis | Small | NR | Mean | 107.9 | |
| AboBoNT-A 1000U | Flexor digitorum longus | Small | 30.0 | Mean | 136.7 | |
| AboBoNT-A 1000U | Flexor hallucis longus | Small | 78.8 | Mean | 96.4 | |
| AboBoNT-A 1500U | Flexor hallucis longus | Small | 78.8 | Mean | 158.6 | |
| AboBoNT-A 1000U | Biceps femoris | Medium | 100.1 | Mean | 195.8 | |
| AboBoNT-A 1500U | Biceps femoris | Medium | 100.1 | Mean | 306.3 | |
| AboBoNT-A 1500U | Extensor digitorum longus | Medium | 102.3 | Mean | 220.9 | |
| AboBoNT-A 1000U | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 122.5 | |
| AboBoNT-A 1500U | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 183.5 | |
| AboBoNT-A 1000U | Gastrocnemius (lateralis) | Medium | 150.0 | Mean | 95.2 | |
| AboBoNT-A 1500U | Gastrocnemius (lateralis) | Medium | 150.0 | Mean | 145.6 | |
| AboBoNT-A 1000U | Gracilis | Medium | 104.0 | Mean | 111.1 | |
| AboBoNT-A 1500U | Gracilis | Medium | 104.0 | Mean | 183.3 | |
| AboBoNT-A 1000U | Rectus femoris | Medium | 269.0 | Mean | 210.1 | |
| AboBoNT-A 1500U | Rectus femoris | Medium | 269.0 | Mean | 350 | |
| AboBoNT-A 1000U | Tibialis posterior | Medium | 104.8 | Mean | 196.8 | |
| AboBoNT-A 1500U | Tibialis posterior | Medium | 104.8 | Mean | 284.3 | |
| AboBoNT-A 1000U | Adductor magnus | Large | 559.8 | Mean | 183.3 | |
| AboBoNT-A 1500U | Adductor magnus | Large | 559.8 | Mean | 257.1 | |
| AboBoNT-A 1000U | Gluteus maximus | Large | 849.0 | Mean | 100 | |
| AboBoNT-A 1500U | Gluteus maximus | Large | 849.0 | Mean | 220 | |
| AboBoNT-A 1000U | Soleus | Large | 438.2 | Mean | 333.3 | |
| AboBoNT-A 1500U | Soleus | Large | 438.2 | Mean | 495.3 | |
| Gracies, 2018 [29] | AboBoNT-A | Flexor digitorum longus | Small | 30.0 | Mean | 151.1 |
| AboBoNT-A | Flexor hallucis longus | Small | 78.8 | Mean | 125.7 | |
| AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 192.1 | |
| AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150.0 | Mean | 177.1 | |
| AboBoNT-A | Rectus femoris | Medium | 269.0 | Mean | 194.4 | |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Mean | 196 | |
| AboBoNT-A | Soleus | Large | 438.2 | Mean | 299.2 | |
| Hecht, 2008 [31] | AboBoNT-A | Tibialis posterior | Medium | 104.8 | Range | 240–480 |
| AboBoNT-A | Gastrocnemius (combined) | Large | 407.4 | Range | 150–500 | |
| Hesse, 1995 [32] | AboBoNT-A 2000U | Gastrocnemius (medialis) | Medium | 257.4 | Fixed value | 500 |
| AboBoNT-A 2000U | Gastrocnemius (lateralis) | Medium | 150.0 | Fixed value | 500 | |
| AboBoNT-A 1500U | Gastrocnemius (medialis) | Medium | 257.4 | Fixed value | 250 | |
| AboBoNT-A 1500U | Gastrocnemius (lateralis) | Medium | 150.0 | Fixed value | 250 | |
| AboBoNT-A 2000U | Tibialis posterior | Medium | 104.8 | Fixed value | 500 | |
| AboBoNT-A 1500U | Tibialis posterior | Medium | 104.8 | Fixed value | 500 | |
| AboBoNT-A 2000U | Soleus | Large | 438.2 | Fixed value | 500 | |
| AboBoNT-A 1500U | Soleus | Large | 438.2 | Fixed value | 500 | |
| Hubble, 2013* [34] | AboBoNT-A in France | Flexor digitorum longus | Small | 30.0 | Mean | 150 |
| AboBoNT-A in German | Flexor digitorum longus | Small | 30.0 | Mean | 106 | |
| AboBoNT-A in Sweden | Flexor digitorum longus | Small | 30.0 | Mean | 167 | |
| AboBoNT-A in UK | Flexor digitorum longus | Small | 30.0 | Mean | 212 | |
| AboBoNT-A in France | Tibialis posterior | Medium | 104.8 | Mean | 244 | |
| AboBoNT-A in Germany | Tibialis posterior | Medium | 104.8 | Mean | 200 | |
| AboBoNT-A in Greece | Tibialis posterior | Medium | 104.8 | Mean | 185 | |
| AboBoNT-A in Sweden | Tibialis posterior | Medium | 104.8 | Mean | 161 | |
| AboBoNT-A in UK | Tibialis posterior | Medium | 104.8 | Mean | 306 | |
| AboBoNT-A in France | Adductor magnus | Large | 559.8 | Mean | 287 | |
| AboBoNT-A in Germany | Adductor magnus | Large | 559.8 | Mean | 243 | |
| AboBoNT-A in Greece | Adductor magnus | Large | 559.8 | Mean | 385 | |
| AboBoNT-A in Sweden | Adductor magnus | Large | 559.8 | Mean | 264 | |
| AboBoNT-A in UK | Adductor magnus | Large | 559.8 | Mean | 416 | |
| AboBoNT-A in France | Gastrocnemius (combined) | Large | 407.4 | Mean | 277 | |
| AboBoNT-A in Germany | Gastrocnemius (combined) | Large | 407.4 | Mean | 336 | |
| AboBoNT-A in Greece | Gastrocnemius (combined) | Large | 407.4 | Mean | 150 | |
| AboBoNT-A in Sweden | Gastrocnemius (combined) | Large | 407.4 | Mean | 168 | |
| AboBoNT-A in UK | Gastrocnemius (combined) | Large | 407.4 | Mean | 367 | |
| AboBoNT-A in UK | Quadriceps femoris | Large | 1803.0 | Mean | 437 | |
| AboBoNT-A in France | Soleus | Large | 438.2 | Mean | 259 | |
| AboBoNT-A in Germany | Soleus | Large | 438.2 | Mean | 275 | |
| AboBoNT-A in Greece | Soleus | Large | 438.2 | Mean | 100 | |
| AboBoNT-A in Sweden | Soleus | Large | 438.2 | Mean | 141 | |
| AboBoNT-A in UK | Soleus | Large | 438.2 | Mean | 267 | |
| Johnson, 2002 [35] | AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150.0 | Fixed value | 200 |
| AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Fixed value | 200 | |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Fixed value | 400 | |
| Moccia, 2020 [41] | AboBoNT-A | Flexor digitorum longus | Small | 30.0 | Mean | 233.3 |
| AboBoNT-A | Adductor longus | Medium | 162.1 | Mean | 323.5 | |
| AboBoNT-A | Extensor hallucis longus | Medium | 102.3 | Mean | 115.6 | |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Mean | 271.2 | |
| AboBoNT-A | Iliopsoas | Large | 451.6 | Mean | 220 | |
| AboBoNT-A | Quadriceps femoris | Large | 1803 | Mean | 310.4 | |
| AboBoNT-A | Triceps surae | Large | 845.6 | Mean | 411.3 | |
| Otom, 2014 [44] | AboBoNT-A | Gastrocnemius (combined) | Large | 407.4 | Fixed value | 500 |
| Pauri, 2000 [45] | AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150.0 | Mean | 123.3 |
| AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 165.9 | |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Mean | 300 | |
| AboBoNT-A | Soleus | Large | 438.2 | Mean | 88 | |
| Picelli, 2012 [46] | AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Fixed value | 250 |
| AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150.0 | Fixed value | 250 | |
| Picelli, 2016 † [48] | AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Fixed value | 250 |
| AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150.0 | Fixed value | 250 | |
| AboBoNT-A | Soleus | Large | 438.2 | Fixed value | 250 | |
| Picelli, 2020 [49] | AboBoNT-A | Gastrocnemius (medialis) | Medium | 257.4 | Mean | 188 |
| AboBoNT-A | Gastrocnemius (lateralis) | Medium | 150 | Mean | 187 | |
| AboBoNT-A | Tibialis posterior | Medium | 104.8 | Mean | 191 | |
| AboBoNT-A | Soleus | Large | 438.2 | Mean | 313 |
* Survey of AboBoNT-A use by physicians in five European countries. † Study in which patients received robot-assisted gait training (RAGT). Legend: AboBoNT-A, abobotulinumtoxinA; NR, not reported; U, unit; UK, United Kingdom.
References
- Javed, M.; Ali, M. Epidemiological Burden of Lower Limb Spasticity in Adults: A Systematic Review. J. Med. Res. Innov. 2019, 4, e000195. [Google Scholar] [CrossRef]
- Martin, A.; Abogunrin, S.; Kurth, H.; Dinet, J. Epidemiological, humanistic, and economic burden of illness of lower limb spasticity in adults: A systematic review. Neuropsychiatr. Dis. Treat. 2014, 10, 111–122. [Google Scholar] [CrossRef]
- Dashtipour, K.; Chen, J.J.; Walker, H.W.; Lee, M.Y. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am. J. Phys. Med. Rehabil. 2015, 94, 229–238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dashtipour, K.; Chen, J.J.; Walker, H.W.; Lee, M.Y. Systematic Literature Review of AbobotulinumtoxinA in Clinical Trials for Lower Limb Spasticity. Medicine 2016, 95, e2468. [Google Scholar] [CrossRef] [PubMed]
- Agence nationale de sécurité du médicament et des produits de santé (ANSM)DYSPORT. Available online: http://agence-prd.ansm.sante.fr/php/ecodex/frames.php?specid=60242321&typedoc=N&ref=N0255634.htm (accessed on 12 December 2021).
- Field, M.; Splevins, A.; Picaut, P.; van der Schans, M.; Langenberg, J.; Noort, D.; Snyder, D.; Foster, K. AbobotulinumtoxinA (Dysport((R))), OnabotulinumtoxinA (Botox((R))), and IncobotulinumtoxinA (Xeomin((R))) Neurotoxin Content and Potential Implications for Duration of Response in Patients. Toxins 2018, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Afssaps (French Health Products Safety Agency). Recommandations de Bonne Pratique. Traitements Medicamenteux de la SpasticiteRecommandations de Bonne Pratique. Traitements Medicamenteux de la Spasticite. Available online: https://archiveansm.integra.fr/var/ansm_site/storage/original/application/9771c86bf98d7af854c30b202846ab35.pdf (accessed on 12 December 2021).
- Royal College of Physicians; British Society of Rehabilitation Medicine; The Chartered Society of Physiotherapy; Association of Chartered Physiotherapists in Neurology; the Royal College of Occupational Therapists. Spasticity in Adults: Man-Agement Using Botulinum Toxin. National Guidelines; RCP: London, UK, 2018. [Google Scholar]
- Dressler, D.; Altavista, M.C.; Altenmueller, E.; Bhidayasiri, R.; Bohlega, S.; Chana, P.; Chung, T.M.; Colosimo, C.; Fheodoroff, K.; Garcia-Ruiz, P.J.; et al. Consensus guidelines for botulinum toxin therapy: General algorithms and dosing tables for dystonia and spasticity. J. Neural. Transm. 2021, 128, 321–335. [Google Scholar] [CrossRef]
- Alvisi, E.; Serrao, M.; Conte, C.; Alfonsi, E.; Tassorelli, C.; Prunetti, P.; Cristina, S.; Perrotta, A.; Pierelli, F.; Sandrini, G. Botulinum toxin A modifies nociceptive withdrawal reflex in subacute stroke patients. Brain Behav. 2018, 8, e01069. [Google Scholar] [CrossRef]
- Ashford, S.; Turner-Stokes, Lynne. Management of shoulder and proximal upper limb spasticity using botulinum toxin and concurrent therapy interventions: A preliminary analysis of goals and outcomes. Disabil. Rehabil. 2009, 31, 220–226. [Google Scholar] [CrossRef]
- Bakheit, A.M.O.; Thilmann, A.F.; Ward, A.B.; Poewe, W.; Wissel, J.; Muller, J.; Benecke, R.; Collin, C.; Muller, F.; Ward, C.D.; et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke 2000, 31, 2402–2406. [Google Scholar] [CrossRef]
- Bakheit, A.M.O.; Pittock, S.; Moore, A.P.; Wurker, M.; Otto, S.; Erbguth, F.; Coxon, L. A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur. J. Neurol. 2001, 8, 559–565. [Google Scholar] [CrossRef]
- Bakheit, A.M.O.; Sawyer, J. The effects of botulinum toxin treatment on associated reactions of the upper limb on hemiplegic gait-A pilot study. Disabil. Rehabil. 2002, 24, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.M.O.; Fedorova, N.V.; Skoromets, A.A.; Timerbaeva, S.L.; Bhakta, B.B.; Coxon, L. The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Barden, H.L.H.; Baguley, I.J.; Nott, M.T.; Chapparo, C. Measuring spasticity and fine motor control (Pinch) change in the hand after botulinum toxin-a injection using dynamic computerized hand dynamometry. Arch. Phys. Med. Rehabil. 2014, 95, 2402–2409. [Google Scholar] [CrossRef]
- Baricich, A.; Carda, S.; Bertoni, M.; Maderna, L.; Cisari, C. A single-blinded, randomized pilot study of botulinum toxin type a combined with non-pharmacological treatment for spastic foot. J. Rehabil. Med. 2008, 40, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Beseler, M.R.; Grao, C.M.; Gil, A.; Martinez Lozano, M.D. Walking assessment with instrumented insoles in patients with lower limb spasticity after botulinum toxin infiltration. Neurologia 2012, 27, 519–530. [Google Scholar] [CrossRef]
- Bhakta, B.B.; Cozens, J.A.; Bamford, J.M.; Chamberlain, M.A. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J. Neurol. Neurosurg. Psychiatry 1996, 61, 30–35. [Google Scholar] [CrossRef]
- Bhakta, B.B.; Cozens, J.A.; Chamberlain, M.A.; Bamford, J.M. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: A randomised double blind placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2000, 69, 217–221. [Google Scholar] [CrossRef]
- Burbaud, P.; Wiart, L.; Dubos, J.L.; Gaujard, E.; Debelleix, X.; Joseph, P.A.; Mazaux, J.M.; Bioulac, B.; Barat, M.; Lagueny, A. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. J. Neurol. Neurosurg. Psychiatry 1996, 61, 265–269. [Google Scholar] [CrossRef]
- Cardoso, E.; Pedreira, G.; Prazeres, A.; Ribeiro, N.; Melo, A. Does botulinum toxin improve the function of the patient with spasticity after stroke? Arq. Neuro-Psiquiatr. 2007, 65, 592–595. [Google Scholar] [CrossRef][Green Version]
- Carvalho, M.P.D.; Pinto, D.; Gorayeb, M.; Jacinto, J. Analysis of a 15-years’ experience in including shoulder muscles, when treating upper-limb spasticity post-stroke with botulinum toxin type A. Top. Stroke Rehabil. 2018, 25, 194–202. [Google Scholar] [CrossRef]
- De Niet, M.; de Bot, S.T.; van de Warrenburg, B.P.; Weerdesteyn, V.; Geurts, A.C. Functional effects of botulinum toxin type-A treatment and subsequent stretching of spastic calf muscles: A study in patients with hereditary spastic paraplegia. J. Rehabil. Med. 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Finsterer, J.; Fuchs, I.; Mamoli, B. Automatic EMG-guided botulinum toxin treatment of spasticity. Clin. Neuropharmacol. 1997, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Frasson, E.; Priori, A.; Ruzzante, B.; Didone, G.; Bertolasi, L. Nerve stimulation boosts botulinum toxin action in spasticity. Mov. Disord. 2005, 20, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Ghroubi, S.; Alila, S.; Elleuch, W.; Ayed, H.B.; Mhiri, C.; Elleuch, M.H. Efficacy of botulinum toxin a for the treatment of hemiparesis in adults upper limb spasticity. Pan Afr. Med. J. 2020, 35, 55. [Google Scholar] [CrossRef]
- Gracies, J.M.; Esquenazi, A.; Brashear, A.; Banach, M.; Kocer, S.; Jech, R.; Khatkova, S.; Benetin, J.; Vecchio, M.; McAllister, P.; et al. Efficacy and safety of abobotulinumtoxinA in spastic lower limb: Randomized trial and extension. Neurology 2017, 89, 2245–2253. [Google Scholar] [PubMed]
- Gracies, J.M.; Francisco, G.E.; Jech, R.; Boyer, F.C.; Balcaitiene, J.; Maisonobe, P. ISPR8-2514: Simultaneous upper and lower limb abobotulinumtoxina injections and guided self-rehabilitation contracts in spastic hemiparesis: Baseline data from the engage study. J. Int. Soc. Phys. Rehabil. Med. 2018, 1, S365–S366. [Google Scholar]
- Gul, F.; O’Dell, M.; Jech, R.; Banach, M.; Vilain, C.; Grandoulier, A.-S.A.; Germain, J.-M.; Gracies, J.-M. Poster 292 Improvement of Spasticity Following AbobotulinumtoxinA (Dysport R) Injections in Shoulder Muscles in Hemiparetic Patients with Upper Limb Spasticity-Sub-Analysis of a Prospective, Long-Term, Open-Label Study with Single and Repeated Injection Cycles. PM R J. Inj. Funct. Rehabil. 2016, 8, S255. [Google Scholar]
- Hecht, M.J.; Stolze, H.; Auf Dem Brinke, M.; Giess, R.; Treig, T.; Winterholler, M.; Wissel, J. Botulinum neurotoxin type A injections reduce spasticity in mild to moderate hereditary spastic paraplegia-Report of 19 cases. Mov. Disord. 2008, 23, 228–233. [Google Scholar] [CrossRef]
- Hesse, S.; Jahnke, M.T.; Luecke, D.; Mauritz, K.H. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci. Lett. 1995, 201, 37–40. [Google Scholar] [CrossRef]
- Hesse, S.; Reiter, F.; Konrad, M.; Jahnke, M.T. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: A randomized, double-blind, placebo-controlled trial. Clin. Rehabil. 1998, 12, 381–388. [Google Scholar]
- Hubble, J.; Schwab, J.; Hubert, C.; Abbott, C.C. Dysport (botulinum toxin type A) in routine therapeutic usage: A telephone needs assessment survey of european physicians to evaluate current awareness and adherence to product labeling changes. Clin. Neuropharmacol. 2013, 36, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Wood, D.E.; Swain, I.D.; Tromans, A.M.; Strike, P.; Burridge, J.H. A pilot study to investigate the combined use of botulinum neurotoxin type a and functional electrical stimulation, with physiotherapy, in the treatment of spastic dropped foot in subacute stroke. Artif. Organs 2002, 26, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Neo, J.J.; Chua, K.S.G. A randomized controlled study of botulinum toxin A in the treatment of hemiplegic shoulder pain associated with spasticity. Clin. Rehabil. 2007, 21, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Lau, K.K.; So, K.K.; Tam, C.K.; Wu, Y.M.; Cheung, G.; Liang, K.S.; Yeung, K.M.; Lam, K.Y.; Yui, S.; et al. Can Botulinum Toxin Decrease Carer Burden in Long Term Care Residents With Upper Limb Spasticity? A Randomized Controlled Study. J. Am. Med. Dir. Assoc. 2012, 13, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, T.; Khatkova, S.; Turner-Stokes, L.; Picaut, P.; Maisonobe, P.; Balcaitiene, J.; Boyer, F.C. Abobotulinumtoxina injections in shoulder muscles to improve adult upper limb spasticity: Results from a phase 4 real-world study and a phase 3 open-label trial. J. Rehabil. Med. 2020, 52, jrm00068. [Google Scholar] [CrossRef]
- Marco, E.; Duarte, E.; Vila, J.; Tejero, M.; Guillen, A.; Boza, R.; Escalada, F.; Espadaler, J.M. Is botulinum toxin type A effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J. Rehabil. Med. 2007, 39, 440–447. [Google Scholar] [CrossRef]
- McCrory, P.; Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Sandanam, J.; Davies, L.; Munns, M.; Hughes, A. Botulinum toxin a for treatment of upper limb spasticity following stroke: A multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J. Rehabil. Med. 2009, 41, 536–544. [Google Scholar] [CrossRef]
- Moccia, M.; Frau, J.; Carotenuto, A.; Butera, C.; Coghe, G.; Barbero, P.; Frontoni, M.; Groppo, E.; Giovannelli, M.; Del Carro, U.; et al. Botulinum toxin for the management of spasticity in multiple sclerosis: The Italian botulinum toxin network study. Neurol. Sci. 2020, 41, 2781–2792. [Google Scholar] [CrossRef]
- Nott, M.T.; Barden, H.L.; Baguley, I.J. Goal attainment following upper-limb botulinum toxin-A injections: Are we facilitating achievement of client-centred goals? J. Rehabil. Med. 2014, 46, 864–868. [Google Scholar] [CrossRef]
- O’Dell, M.W.; Brashear, A.; Jech, R.; Lejeune, T.; Marque, P.; Bensmail, D.; Ayyoub, Z.; Simpson, D.M.; Volteau, M.; Vilain, C.; et al. Dose-Dependent Effects of AbobotulinumtoxinA (Dysport) on Spasticity and Active Movements in Adults With Upper Limb Spasticity: Secondary Analysis of a Phase 3 Study. PM R 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Otom, A.H.; Al-Khawaja, I.M.; Al-Quliti, K.W. Botulinum toxin type-A in the management of spastic equinovarus deformity after stroke. Neurosciences 2014, 19, 199–202. [Google Scholar]
- Pauri, F.; Boffa, L.; Cassetta, E.; Pasqualetti, P.; Rossini, P.M. Botulinum toxin type-A treatment in spastic paraparesis: A neurophysiological study. J. Neurol. Sci. 2000, 181, 89–97. [Google Scholar] [CrossRef]
- Picelli, A.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Girardi, P.; Manca, M.; Gimigliano, R.; Smania, N. Is spastic muscle echo intensity related to the response to botulinum toxin type A in patients with stroke? A cohort study. Arch. Phys. Med. Rehabil. 2012, 93, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Lobba, D.; Midiri, A.; Prandi, P.; Melotti, C.; Baldessarelli, S.; Smania, N. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: A randomized controlled trial comparing three injection techniques. Clin. Rehabil. 2014, 28, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Bacciga, M.; Melotti, C.; La Marchina, E.; Verzini, E.; Ferrari, F.; Pontillo, A.; Corradi, J.; Tamburin, S.; Saltuari, L.; et al. Combined effects of robot-assisted gait training and botulinum toxin type A on spastic equinus foot in patients with chronic stroke: A pilot, single blind, randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 759–766. [Google Scholar]
- Picelli, A.; Battistuzzi, E.; Filippetti, M.; Modenese, A.; Gandolfi, M.; Munari, D.; Smania, N. Diagnostic nerve block in prediction of outcome of botulinum toxin treatment for spastic equinovarus foot after stroke: A retrospective observational study. J. Rehabil. Med. 2020, 52, jrm00069. [Google Scholar] [CrossRef]
- Rekand, T.; Biering-Sorensen, B.; He, J.; Vilholm, O.J.; Christensen, P.B.; Ulfarsson, T.; Belusa, R.; Strom, T.; Myrenfors, P.; Maisonobe, P.; et al. Botulinum toxin treatment of spasticity targeted to muscle endplates: An international, randomised, evaluator-blinded study comparing two different botulinum toxin injection strategies for the treatment of upper limb spasticity. BMJ Open 2019, 9, e024340. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.T.; Delgado-De Los Santos, M.M.; Chua, K.S.G.; Abdullah, S.J.B.F.; Zakine, B.; Maisonobe, P.; et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: A randomized controlled trial. Neurorehabilit. Neural Repair 2012, 26, 812–821. [Google Scholar] [CrossRef]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomized controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef]
- Sun, S.F.; Hsu, C.W.; Sun, H.P.; Hwang, C.W.; Yang, C.L.; Wang, J.L. Combined botulinum toxin type A with modified constraint-induced movement therapy for chronic stroke patients with upper extremity spasticity: A randomized controlled study. Neurorehabilit. Neural Repair 2010, 24, 34–41. [Google Scholar] [CrossRef]
- Suputtitada, S.; Suwanwela, N.C. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil. Rehabil. 2005, 27, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P. Results from the Upper Limb International Spasticity Study-II (ULIS-II): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin a in real-life clinical management. BMJ Open 2013, 3, e002771. [Google Scholar] [CrossRef]
- Woldag, H.; Hummelsheim, H. Is the reduction of spasticity by botulinum toxin A beneficial for the recovery of motor function of arm and hand in stroke patients? Eur. Neurol. 2003, 50, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yazdchi, M.; Ghasemi, Z.; Moshayedi, H.; Rikhtegar, R.; Mostafayi, S.; Mikailee, H.; Najmi, S. Comparing the efficacy of botulinum toxin with tizanidine in upper limb post stroke spasticity. Iran. J. Neurol. 2013, 12, 47–50. [Google Scholar] [PubMed]
- Yelnik, A.P.; Colle, F.M.; Bonan, I.V.; Vicaut, E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: A randomised, double blind, placebo controlled study of botulinum toxin A. J. Neurol. Neurosurg. Psychiatry 2007, 78, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Holzbaur, K.R.; Murray, W.M.; Gold, G.E.; Delp, S.L. Upper limb muscle volumes in adult subjects. J. Biomech. 2007, 40, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Handsfield, G.G.; Meyer, C.H.; Hart, J.M.; Abel, M.F.; Blemker, S.S. Relationships of 35 lower limb muscles to height and body mass quantified using MRI. J. Biomech. 2014, 47, 631–638. [Google Scholar] [CrossRef]
- Ojardias, E.; Ollier, E.; Lafaie, L.; Celarier, T.; Giraux, P.; Bertoletti, L. Time course response after single injection of botulinum toxin to treat spasticity after stroke: Systematic review with pharmacodynamic model-based meta-analysis. Ann. Phys. Rehabil. Med. 2021, 65, 101579. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions (June 2017: Handbook Editors’ Update). Available online: http://handbook.cochrane.org/ (accessed on 19 May 2021).
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 1–7. [Google Scholar] [CrossRef]
- Marciniak, C.; Vilain, C.; Picaut, P.; Grandoulier, A.S.; Ayyoub, Z.; Banach, M.; Bensmail, D.; Bentivoglio, A.R.; Boyer, F.C.; Brashear, A.; et al. Efficacy and Safety of AbobotulinumtoxinA (Dysport) for the Treatment of Hemiparesis in Adults With Upper Limb Spasticity Previously Treated With Botulinum Toxin: Subanalysis From a Phase 3 Randomized Controlled Trial. PM R 2017, 9, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Stoquart, G.; Hedera, P.; Jacinto, L.J.; Dimanico, U.; Constant-Boyer, F.; Brashear, A.; Grandoulier, A.S.; Vilain, C.; Picaut, P.; et al. Efficacy and Safety of AbobotulinumtoxinA for the Treatment of Hemiparesis in Adults with Lower Limb Spasticity Previously Treated With Other Botulinum Toxins: A Secondary Analysis of a Randomized Controlled Trial. PM R 2020, 12, 853–860. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).