Abstract
The present study aimed to evaluate the acute and subacute toxicity profiles of Erodium guttatum extracts in mice using the methods described in the guidelines of the OECD. In the acute toxicity study, the LD50 value was greater than 2000 mg/kg. The subacute toxicity study of E. guttatum extracts showed no significant changes in body or organ weights. The administration of E. guttatum extracts to mice at a dose of 200 mg/kg led to an increase in white blood cells, platelets and hemoglobin. Moreover, the aqueous extract of E. guttatum only decreased liver aspartate aminotransferase (ASAT) levels at a dose of 200 mg/kg, and creatinine and urea levels did not show any significant alterations compared to the control group. Our results showed that the extracts of E. guttatum caused a slight increase in alanine aminotransferase (ALAT) and triglycerides. The histological study showed that mice treated with E. guttatum extracts experienced some histopathological changes in the liver, particularly with the methanolic extract, and slight changes in the kidneys and pancreas. Regarding the renal profile, no toxicity was observed. These results provide basic information on the toxicological profile of E. guttatum used in traditional medicine.
Key Contribution:
Acute toxicity was examined after oral administration of a single dose of Erodium guttatum extracts was given to mice for 14 days, and subacute oral toxicity was assessed after mice received daily treatment for 28 days.
1. Introduction
For a long time, natural resources have been used as remedies to prevent or cure various diseases due to their powerful therapeutic effects and low costs. People consume natural products because they think they are safe and do not have harmful effects [1]. Medicinal plants can contain many secondary metabolites, some of which are very complex. Nevertheless, herbs used to treat certain diseases are generally used without any scientific knowledge or evidence of toxicological effects [2,3].
The acute and subacute toxicity test is a method based on an evaluation of the harmlessness and safety of chemicals, as well as an analysis of their mode of action. Acute and subacute systemic toxicity studies are used for hazard disclosure and risk management in the context of the production, handling and use of chemicals [4]. Acute toxicity is a single-dose test that identifies symptoms and the extent to which toxicity affects animals [5]. Subacute toxicity studies at repeated doses can be carried out after obtaining preliminary information from acute toxicity tests, which provide information about the animal’s target tissue or organ [6].
The genus Erodium of the Geraniaceae family includes more than 70 species distributed in all continents, of which 63 are concentrated in the Mediterranean regions [7,8]. Indeed, only seven species do not occur in Europe or the Mediterranean region. Of these, one is in Central Asia, one in East Asia, one in South Africa, two in western North America, one in South America and one in Australia [9]. In addition, 16 of them have been reported as endemic to Turkey [10]. Erodium species are traditionally used to prepare astringent and antiseptic teas. Decoctions of the aerial parts are used as a remedy for dysentery, fever, wounds and worm infections. The leaves have been used in the kitchen for the preparation of salads, omelets, sandwiches, sauces and soups and certain food products [11,12]. Erodium is used to treat and/or prevent dermatological and gastrointestinal pathologies, indigestion and inflammatory diseases, diabetes, cancer, constipation, eczema, hemorrhage, and as a carminative, astringent and antiseptic agent [13]. Erodium is characterized by five fertile and five infertile flowers, which are regular or zygomorphic and glandular. The leaves are mostly pinnate or undivided (pinnatifid/pinnatisect). Leaf size and shape can vary between populations, and the variation is usually pronounced within populations [14].
The species Erodium guttatum (Desf.) Willd. is a perennial plant found in North Africa, southern Spain and Palestine. In Morocco, it is known as "Wedmi." Pharmacological studies have indicated that it has antioxidant and antimicrobial abilities [15]. To the best of our knowledge, no chemical or toxicological studies have been performed on Erodium guttatum roots. A safety evaluation of this herb is needed. In this respect, the objective of this study was to evaluate the possible acute and subacute toxic effects of the root extracts of the species E. guttatum.
2. Results
2.1. Acute Oral Toxicity
Oral administration of aqueous, ethanolic and methanolic extracts of E. guttatum at a dose of 2 g/kg showed no mortality in treated mice. Treatment with each extract did not induce weight loss, and no behavioral disorder was recorded during the 14 days of observation. Therefore, the oral LD50 of E. guttatum is greater than 2000 mg/kg (Table 1).

Table 1.
Effects of acute oral administration of aqueous and alcoholic extracts of E. guttatum on the body weights of Swiss mice with a dose of 2000 mg/kg.
2.2. Subacute Oral Toxicity
2.2.1. Body Weights
The groups of mice treated with the three extracts of E. guttatum at a dose of 200 mg/kg recorded no significant change (p > 0.05) in body weights compared to the control group (Table 2).

Table 2.
Effects of subacute oral administration of aqueous and alcoholic extracts of E. guttatum on the body weights of Swiss mice with a dose of 200 mg/kg.
2.2.2. Organ Weights
The resulting organ weights are shown in Table 3. Macroscopic analysis of the target organs (liver, kidney and pancreas) of mice treated with aqueous, ethanolic and methanolic extracts of E. guttatum showed there were no significant changes in color or texture compared to the control group.

Table 3.
Effect of subacute oral administration of aqueous and alcoholic extracts of E. guttatum on organ weights in Swiss albino mice.
2.2.3. Hematological Parameters
The hematological results are summarized in Table 4. The animals treated with the aqueous, ethanolic and methanolic extracts of E. guttatum showed elevated levels of white blood cells (WBC), red blood cells (RBC), platelets (PLT), lymphocytes (LYMPH), monocytes (MONO), basophils (BASO) and neutrophils (NEUT) compared to the control. Similarly, the administration of the ethanolic and methanolic extract at a dose of 200 mg/kg caused no significant difference (p > 0.05) in the levels of hematological parameters.

Table 4.
Effects of subacute oral administration of aqueous and alcoholic extracts of E. guttatum on hematological parameters in Swiss albino mice.
2.2.4. Serum Biochemical Parameters
The results of the biochemical parameters are shown in Table 5. Daily oral administration of E. guttatum extracts to mice in all groups caused a slight increase in cholesterol and low-density lipoprotein (LDL) compared with mice in the control group.

Table 5.
Effects of subacute oral administration of aqueous and alcoholic extracts of E. guttatum on biochemical parameters in Swiss mice.
Regarding the levels of aspartate aminotransferase (ASAT), there was a non-significant decrease observed in the group of mice treated with the aqueous extract compared to the control group, while there was an increase in ASAT levels in the groups of mice treated with methanolic and ethanolic extracts. Similarly, the levels of alanine aminotransferase (ALAT) in the group of control mice and the group of mice treated with aqueous extract were equal. It can be seen that the mice treated with methanolic extract had higher levels of ASAT and ALAT compared to the groups of mice treated with ethanolic and aqueous extracts.
Regarding urea levels, there was a non-significant decrease in all groups of mice treated with the three extracts of E. guttatum compared to the control mice. For creatinine levels, there was an increase in the groups of mice treated with the ethanolic and methanolic extracts and a decrease in the group of mice treated with the aqueous extract.
2.2.5. Histopathology
Normal histology of mouse kidneys (glomeruli, tubules and interstitium) was found in the control group (Figure 1a). Mice treated with an aqueous extract (200 mg/kg) of E. guttatum showed minimal interstitial inflammation (Figure 1b). These observations corroborate the results of serum analysis. The histology of kidney sections from mice treated with ethanolic extract revealed minor or mild changes, including focal interstitial inflammation and increased plasma cells (Figure 1c). Conversely, the histology of kidney sections from mice treated with the methanolic extract showed major abnormalities: glomerular injury, severe interstitial inflammation and plasma cells in the interstitium (Figure 1d).
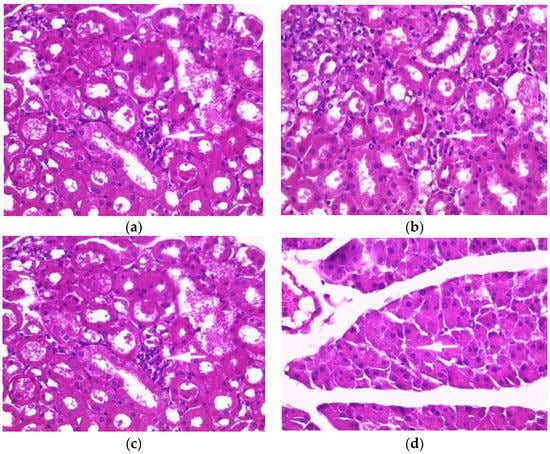
Figure 1.
Effect of E. guttatum extracts on renal histology in mice. Histological sections were visualized by hematoxylin and eosin (H and E) staining and observed under a light microscope (OPTIKA Microscopes, Italy) at 40× magnification. (a) Control mice, (b) mice treated with aqueous extract of E. guttatum (200 mg/kg), (c) mice treated with ethanolic extract of E. guttatum and (d) mice treated with methanolic extract of E. guttatum.
The histology of liver sections from the control mice showed normal hepatocellular architecture as well as well-preserved liver cells, visible central veins and no histological abnormalities (Figure 2a). The liver sections from the aqueous extract (200 mg/kg)-treated groups showed some histological changes, such as intralobular inflammation around the centrilobular veins (CLVs) and cytoplasmic hydropic degeneration of hepatocytes (Figure 2b). In contrast, subacute administration of ethanolic extract caused minimal intralobular inflammation and hydropic degeneration of the cytoplasm, showing binucleated cells and a somewhat enlarged nucleus with blackish pigments (Figure 2c). The liver sections of mice treated with methanolic extract showed an intralobular mononuclear inflammatory focus around the centrilobular veins, hepatocytes with hyperchromatic nuclei, moderate binucleation, vacuolated and enlarged nucleoli, predominant nucleoli (one or two), multinucleated hepatocytes, blackish pigments, and intra sinusoidal (Figure 2d).
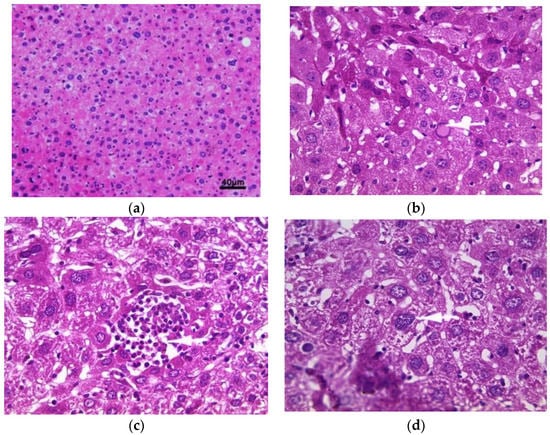
Figure 2.
Histopathology of the liver. Histological sections were visualized by hematoxylin and eosin (H and E) staining and observed under a light microscope (OPTIKA Microscopes, Italy) at 40× magnification. (a) Control mice, (b) mice treated with aqueous extract of E. guttatum (200 mg/kg), (c) mice treated with ethanolic extract of E. guttatum and (d) mice treated with methanolic extract of E. guttatum.
The pancreas of normal mice showed pancreatic acini (PA) with normal histological appearance and normal inter-acini and interlobular spaces (Figure 3a). Pancreas histology of mice treated with the aqueous extract (200 mg/kg) showed only intra-pancreatic inflammation (Figure 3b). Additionally, pancreas sections from mice treated with the ethanolic extract showed only minimal focal intralobular inflammation (Figure 3c), while the pancreas of mice treated with the methanolic extract showed intra-pancreatic inflammation and increased plasma cells (Figure 3d).
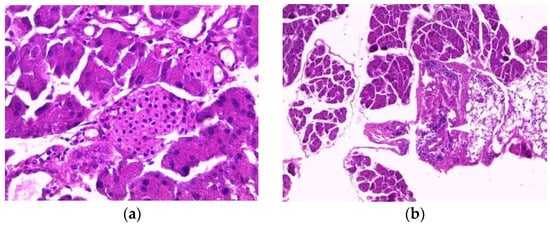
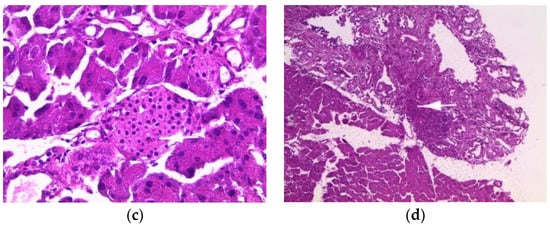
Figure 3.
Histopathology of the pancreas. Histological sections were visualized by hematoxylin and eosin (H and E) staining and observed under a light microscope (OPTIKA Microscopes, Italy) at 40× magnification. (a) Control mice, (b) mice treated with aqueous extract of E. guttatum (200 mg/kg), (c) mice treated with ethanolic extract of E. guttatum and (d) mice treated with methanolic extract of E. guttatum.
3. Discussion
Currently, medicinal plants are known for their pharmacological effects. However, less is known about the potential toxicity of their biologically active substances [16,17]. A study of acute toxicity examines the adverse effects that occur in the short term after the administration of a single dose of a tested product. These tests are generally conducted on rodents and are carried out at the beginning of the development of a new substance in order to provide information on its toxicity [18]. The present study showed that aqueous, ethanolic and methanolic extracts of E. guttatum did not cause death or behavioral changes in mice at a dose of 2000 mg/kg. According to the OECD classification, with the LD50 being superior to 2 g/kg, this plant can be considered as not presenting a risk of acute toxicity.
Our study was conducted to evaluate the subacute toxicity of E. guttatum L. extracts during a 28-day experiment in mice. Subacute toxicity allows for the examination of the cumulative toxicity of a substance in the target organs or the physiological and metabolic effects of the compound by prolonged exposure to low dosage. A wide variety of adverse effects can be detected from subacute toxicity studies, and the long-term safety of a compound can be predicted. In our study, the results showed that mice treated with E. guttatum extracts at a dose of 200 mg/kg showed no signs of toxicity, and no deaths were recorded.
Changes in body weight have been used as an important indicator to assess the adverse effects of drugs and chemicals [19,20]. In our study, there was a progressive, normal increase in the mean body weights of both the treated and control groups. In addition, the difference in weight gain between the controls and the groups treated with the extracts at the dose of 200 mg/kg was statistically insignificant. According to Raina et al. [21], organ weights are markers of the state of pathological and physiological well-being of the animals. When herbal products are ingested, they can be toxic to vital organs such as the kidneys, liver and pancreas due to their various roles in the human body. Based on the organ weight results of the three treated groups compared to the control group, there were no significant weight changes in the liver, kidney or pancreas.
According to Wu et al. [22], anemia following the administration of a product may cause hemolysis and/or inhibition of hematopoiesis, as well as a decrease in hematological parameters due to the bioactive components of the extract. Blood is the main vehicle responsible for the distribution of nutrients and foreign substances in the body. Therefore, the constituents of blood, namely erythrocytes, leukocytes, platelets and hemoglobin are exposed to high doses of toxins [23,24]. Subacute administration of E. guttatum extracts resulted in an increase in white blood cells, platelets, lymphocytes, monocytes, basophils and neutrophils in all three groups. These results suggest that E. guttatum contains bioactive molecules that have an amplifying effect on the immune response by increasing the number of white blood cells [25]. The results did not show a significant change in red blood cell and hemoglobin levels, which indicates that the extracts may not contain toxic substances that can cause anemia or other abnormalities.
Biochemical analysis showed a slight, non-significant increase in total cholesterol, triglycerides, LDL and HDL levels. These results suggest that E. guttatum may induce the secretion of total cholesterol, LDL and HDL from the liver. In addition, ASAT and ALAT levels at 200 mg/kg were slightly elevated in mice treated with the methanolic and ethanolic extracts, while mice treated with the aqueous extract were able to decrease ASAT levels compared with the control, but this was not significant. An abnormal elevation of liver enzymes (ALAT and ASAT) is related to liver damage or impaired bile flow. ALAT (alanine aminotransferase) is a liver-specific enzyme that is released into the blood when a cell is damaged or injured. ASAT (aspartate aminotransferase) is an enzyme found mainly in red blood cells, heart and skeletal muscle and the kidneys [26,27]. The liver is the site of elimination or degradation of cholesterol and the main site of synthesis. The fact that a slight significant change was observed in cholesterol and triglyceride levels in this study suggests that E. guttatum may not have side effects on cholesterol metabolism in mice. The study carried out on the aerial part of E. guttatum showed that, at high doses (2000 mg/kg and 5000 mg/kg), the aqueous extract did not cause any change in the levels of biochemical parameters, such as ASAT, ALAT, urea, creatinine, cholesterol, triglycerides and blood glucose compared to the control group [28]. This confirms that the aqueous extract has hepatoprotective and nephroprotective effects.
Renal function can be assessed by changes in creatinine, urea and glucose, and increases in these parameters indicate injury and impairment of the renal filtration mechanism [29,30]. In our study, the average creatinine level was slightly increased in mice treated with methanolic and ethanolic extracts. On the other hand, the creatinine level in mice treated with the aqueous extract remained within normal ranges. The mean values of urea and glucose were low in the three treated groups compared to the control. These results suggest that E. guttatum has no negative effect but seems to have a protective effect on the kidney. Based on these results, it is reasonable to assume that repeated administration for 28 days may cause toxicity to vital organs.
Histopathological studies serve as supportive evidence for hematological and biochemical analyses [31]. The photomicrographs of sections of the liver, kidney and pancreas of mice treated orally with extracts of E. guttatum at a dose of 200 mg/kg for 28 days showed histological changes such as an intralobular mononuclear inflammatory focus, a mononuclear inflammatory focus around the centrilobular veins (CLV), hydropic cytoplasmic degeneration of hepatocytes, binucleate cells and a nucleus, a low-bulk, dark-pigment glomerular lesion, interstitial inflammation, plasma cells in the interstitium, and intrapancreatic inflammation. Therefore, these histopathological findings corroborate the biochemical results, and a chronic study is necessary for a complete understanding of the hepatoxicity of this plant.
4. Conclusions
Our preliminary study evaluated the acute and subacute safety or toxicity of aqueous, ethanolic and methanolic extracts of E. guttatum after oral administration in mice. The acute toxicity study showed that the LD50 value was greater than 2000 mg/kg, and the extracts showed no signs of toxicity during the 14 days of the study. In the subacute toxicity study, no deaths were recorded after oral administration of 200 mg/kg of E. guttatum for 28 days. The mice showed liver toxicity, particularly those treated with the methanolic extract, as determined by hematological, serum biochemical and/or histological analyses. These results provide preliminary information on the toxicity of E. guttatum; thus, these results show the potential cytotoxic effects of methanol. Other evaluations, such as the toxicity of bioactive compounds and the neurotoxicity, reprotoxicity and genotoxicity of this plant, should be performed in future studies. Finally, it is necessary to assess the safety and toxicological aspects of all other traditionally used medicinal plants.
5. Materials and Methods
5.1. Plant Material and Extraction
The roots of E. guttatum were collected from the Oujda Region, Morocco. The specimen was identified by a botanist, Mohammed Fennane, and was deposited to the Botanical Herbarium of the Scientific Institute of Rabat under the reference number RAB 110970. The root parts were dried in the dark at room temperature. The plant material was then reduced to powder and subsequently used for the preparation of the extracts.
The plant material was prepared by utilizing two types of extraction methods (infusion and maceration). The aqueous extract was prepared using the infusion method, by which 50 g of E. guttatum powder was infused with 500 mL of distilled water for 1 h and then left to cool. The extract was filtered and evaporated at 50 °C using a rotary evaporator. Subsequently, the extract was freeze-dried and stored until use. For the ethanolic and methanolic extracts, 50 g of the root powder was macerated with 500 mL of ethanol and methanol, respectively, and stirred for 48 h at room temperature. The extract was filtered through Whatman n1 paper and evaporated at 40 °C using a rotary evaporator. The extracts obtained were stored at a temperature of 4 °C.
5.2. Experimental Animals
Female and male Swiss albino mice weighing between 25 to 35 g were used in this study. The animals were kept at an ambient temperature of 25 °C and a light/dark cycle of 12 h in cages at the Faculty of Medicine and Pharmacy in Rabat. They had free access to water and normal food throughout the experiment. The experiment was carried out according to the principles described in the "Guide to the care and use of laboratory animals," 8th edition, prepared by the National Academy of Sciences (National Research Council of the National Academies). Every effort was made to minimize animal suffering and the number of animals used for the study. Ethics approval was obtained from Mohammed V University in Rabat.
5.3. Acute Oral Toxicity
Acute oral toxicity was carried out according to the methods described in the guidelines of the Organization for Economic Co-operation and Development, No. 423 (OECD 423) [5]. The toxicity of E. guttatum extracts was evaluated in female Swiss mice. The animals were divided into four groups of six mice each.
Group I (Control): animals were administered vehicle (distilled water) orally.
Group II, III and IV: animals were administered 2000 mg/kg of their body weight with either aqueous, ethanolic or methanol extract of E. guttatum root, respectively, via oral gavage.
After the administration of a single dose of the different extracts of E. guttatum, the animals were observed for 14 days in order to note the behavior of the animal, the signs of possible toxicity and the number of deaths due to the extracts.
5.4. Sub-Acute Oral Toxicity
The subacute oral toxicity study was conducted in accordance with the guidelines of the Organization for Economic Co-operation and Development (OECD 407) [6]. Thirty-two animals were randomly assigned to four groups of eight animals each. Group I, control, received distilled water (vehicle). Groups II, III and IV received an oral dose of 200 mg/kg of either the aqueous, ethanolic or methanolic extracts of E. guttatum, respectively, and was administered once daily for 28 consecutive days. Body weights were recorded once a week throughout the study period.
5.4.1. Determination of Hematological and Biochemical Parameters
At the end of the experimental period, blood samples were taken from the jugular vein using tubes in heparinized tubes for hematological studies and non-heparinized tubes from which serum was isolated by centrifugation at 3000 rpm for 10 min and then used for biochemical assessments.
A complete blood count (CBC) and differential were performed on the blood samples using Sysmex KX21N, an automated 3-part differential hematology analyzer (Sysmex Corporation Kobe, Japan). Standardization, calibration of the instrument, and processing of the samples were performed according to the manufacturer’s instructions. The machine automatically dilutes whole-blood samples to 50 mL in the CBC/Differential mode and then lyses and enumerates white blood cells (WBC), red blood cells (RBC), hemoglobin concentration (Hb) and platelets. However, it does not count for eosinophils, monocytes or basophils. Therefore, a manual differential count was conducted on well-prepared thin-blood films colored using the May Grünwald Giemsa (MGG) method. The parameters studied under the optical microscope were: (1) The RBC morphology to detect possible corpuscular anomalies. (2) The leucocyte parameter was determined, double-blinded, by two operators. Each of them established the percentage of the different leucocyte populations (NEU, EOS, BAS, MONO and LYM) on 200 leucocyte elements. In case of differences of more than five cells for a leucocyte population, formulas were double-checked by two additional readings (by the same operators). The final formula was calculated from the average of both formulas. The values obtained from the NEU, EOS, BAS, LYM and MONO, expressed in 109/L, were deducted from the leucocyte numeration measured by the automation. (3) Platelets study was conducted to research morphological anomalies or platelet aggregates.
Serum levels of aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), urea, creatinine, cholesterol, triacylglycerols (TG), high-density lipoproteins (HDL) and low-density lipoproteins (LDL) were determined using an Abbott Architect c8000 model, Clinical Chemistry System, USA, auto-analyzer according to manufacturer instructions.
5.4.2. Organ Weights and Histopathology
Animals were sacrificed under mild ether anesthesia. After the sacrifice, the weights of the organs (liver, kidney and pancreas) were recorded. Vital organs were excised from the anesthetized animal and rinsed in 0.9% saline solution. Tissue pieces were fixed in 10% paraformaldehyde for paraffin histology and processed in paraffin embedding according to standard protocol. Sections of each tissue were stained with hematoxylin and eosin and observed for possible histopathological damage.
5.5. Statistical Analysis
Data were expressed as means ± SD. Statistical analyses and the comparison of means were evaluated using ANOVA (Tukey’s test). The differences were considered statistically significant at p < 0.05. Analyses were performed with GraphPad Prism 6 (San Diego, CA, USA).
Author Contributions
Conceptualization, K.B., N.S. and H.N.M.; methodology, K.B., L.C.M., K.W.G., H.M.A. and S.M.; software, K.B., L.R., R.E.B., A.D. and A.M.; investigation, K.B.; writing—original draft preparation, K.B., H.N.M. and A.B.; validation, M.M.A., A.A.A.A. and K.W.G.; review and editing, M.E.A.F. and Y.C.; supervision, K.B., H.N.M., A.B., M.E.A.F. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Ethics approval was obtained from Mohammed V University in Rabat (protocol code # UA-2021-07, date of approval 27 May 2021).
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors would like to thank the Ministry of Education for supporting this work (Institutional Fund Projects Program, grant code IFP22UQU4310026DSR006).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ha, A.W.; Kang, H.J.; Kim, S.L.; Kim, M.H.; Kim, W.K. Acute and subacute toxicity evaluation of corn silk extract. Prev. Nutr. Food Sci. 2018, 23, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brondani, J.C.; Reginato, F.Z.; Brum, E.D.S.; Vencato, M.D.S.; Lhamas, C.L.; Viana, C.; da Rocha, M.I.U.M.; Bauermann, L.D.F.; Manfron, M.P. Evaluation of acute and subacute toxicity of hydroethanolic extract of Dolichandra unguis-cati L. leaves in rats. J. Ethnopharmacol. 2017, 202, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M.; Arbid, M.S.; El-Attar, M.A. Acute and subacute toxicity of Ammi visnaga on rats. Interdiscip. Toxicol. 2019, 12, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhuang, Y.; Tian, W.; Sun, L. In vivo acute and subacute toxicities of phenolic extract from rambutan (Nephelium lappaceum) peels by oral administration. Food Chem. 2020, 320, 126618. [Google Scholar] [CrossRef] [PubMed]
- OCDE423; Ligne Directrice de l’Ocde pour les Essais de Produits Chimiques. OCDE: Paris, France, 2001.
- OCDE407; Lignes Directrices de l’Ocde pour les Essais de Produits Chimiques. OCDE: Paris, France, 2008.
- Fiz, O.; Vargas, P.; Alarcón, M.L.; Aldasoro, J.J. Phylogenetic relationships and evolution in Erodium (Geraniaceae) based on trnL-trnF sequences. Syst. Bot. 2006, 31, 739–763. [Google Scholar] [CrossRef]
- Alarcón, M.; Vargas, P.; Sáez, L.; Molero, J.; Aldasoro, J.J. Genetic diversity of mountain plants: Two migration episodes of Mediterranean Erodium (Geraniaceae). Mol. Phylogenet. Evol. 2012, 63, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, R.L.; Venter, H.J.T. Pollen morphology of Erodium in southern Africa. South Afr. J. Bot. 1987, 53, 279–283. [Google Scholar] [CrossRef]
- Sevindik, B.; Tutuncu, M.; Izgu, T.; Tagipur, E.M.; Curuk, P. Micropropagation of Erodium olympicum Endemic to Turkey Micropropagation of Erodium olympicum Endemic to Turkey. Am. J. Plant Biol. 2017, 2, 24–27. [Google Scholar] [CrossRef]
- Fecka, I.; Cisowski, W. TLC determination of tannins and flavonoids in extracts from some erodium species using chemically modified stationary phases. J. Planar Chromatogr. Mod. TLC 2002, 15, 429–432. [Google Scholar] [CrossRef]
- Gohar, A.A.; Lahloub, M.F.; Niwa, M. Antibacterial Polyphenol from Erodium glaucophyllum. Z. Naturforsch. C 2003, 58, 670–674. [Google Scholar] [CrossRef]
- Munekata, P.E.; Alcántara, C.; Collado, M.C.; Garcia-Perez, J.V.; Saraiva, J.A.; Lopes, R.P.; Barba, F.J.; Silva, L.D.P.; Sant’Ana, A.S.; Fierro, E.M.; et al. Ethnopharmacology, phytochemistry and biological activity of Erodium species: A review. Food Res. Int. 2019, 126, 108659. [Google Scholar] [CrossRef] [PubMed]
- Venter, H.J.T.; Verhoeven, R.L. The genus Erodium in southern Africa. South Afr. J. Bot. 1990, 56, 79–92. [Google Scholar] [CrossRef]
- Hamza, G.; Emna, B.H.; Yeddes, W.; Dhouafli, Z.; Moufida, T.S.; el Akrem, H. Chemical composition, antimicrobial and antioxidant activities data of three plants from Tunisia region: Erodium glaucophyllum, Erodium hirtum and Erodium guttatum. Data Br. 2018, 19, 2352–2355. [Google Scholar] [CrossRef] [PubMed]
- Célia, O.; Hamisa-Saida, C.; Fella, H.-C.; Loubna, M.; Samira, D.; Rym, H.; Nouria, N.; Fairouz, S. Toxicité aigue et subaigue des extraits méthaloniques d’Inula viscosa L. (Dittrichia viscosa L.). Rev. Agrobiol. 2017, 7, 562–573. [Google Scholar]
- Benrahou, K.; Doudach, L.; el Guourrami, O. Acute toxicity, phenol content, antioxidant and postprandial anti-diabetic activity of Echinops spinosus extracts. Int. J. Second. Metab. 2022, 9, 91–102. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Z.; Wang, Y.; Kai, G.; Njateng, G.S.S.; Cai, S.; Cao, J.; Cheng, G. Acute and subacute toxicity evaluation of ethanol extract from aerial parts of Epigynum auritum in mice. Food Chem. Toxicol. 2019, 131, 110534. [Google Scholar] [CrossRef] [PubMed]
- Vahalia, M.K.; Thakur, K.S.; Nadkarni, S.; Sangle, V.D. Chronic Toxicity Study For Tamra Bhasma (A Generic Ayurvedic Mineral Formulation) in Laboratory Animals. Recent Res. Sci. Technol. 2011, 3, 2076–5061. Available online: https://updatepublishing.com/journal/index.php/rrst/article/view/834 (accessed on 21 April 2022).
- el Hilaly, J.; Israili, Z.H.; Lyoussi, B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 2004, 91, 43–50. [Google Scholar] [CrossRef]
- Raina, P.; Chandrasekaran, C.V.; Deepak, M.; Agarwal, A.; Ruchika, K.G. Evaluation of subacute toxicity of methanolic/aqueous preparation of aerial parts of O. sanctum in Wistar rats: Clinical, haematological, biochemical and histopathological studies. J. Ethnopharmacol. 2015, 175, 509–517. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Zhao, L.; Cai, S.; Cheng, G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. 2018, 119, 14–23. [Google Scholar] [CrossRef]
- Mukinda, J.T.; Syce, J.A. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol. 2007, 112, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, Y.; Wang, L.; Li, Y.; Shi, Y.; Cui, Y.; Xue, M. Acute and subacute toxicity of ethanol extracts from Salvia przewalskii Maxim in rodents. J. Ethnopharmacol. 2010, 131, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Atsamo, A.D.; Nguelefack, T.B.; Datté, J.Y.; Kamanyi, A. Acute and subchronic oral toxicity assessment of the aqueous extract from the stem bark of Erythrina senegalensis DC (Fabaceae) in rodents. J. Ethnopharmacol. 2011, 134, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.B.; Onasanya, A.; Anadozie, S.O.; Abu, M.F.; Akintan, I.A.; Ogbole, C.J.; Olayide, I.I.; Afolabi, O.B.; Jaiyesimi, K.F.; Ajiboye, B.O.; et al. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J. Ethnopharmacol. 2016, 188, 153–158. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Doudach, L.; Kachmar, M.R.; Ed-Dra, A.; Khalil, Z.; Mrabti, N.N.; Benrahou, K.; Harraqui, K.; Zengin, G.; Bouyahya, A.; et al. Phenolic content, antibacterial, antioxidant, and toxicological investigations of Erodium guttatum (Geraniaceae) collected from the Northeast of Morocco. Turk. J. Botany 2021, 45, 739–749. [Google Scholar] [CrossRef]
- Wasan, K.M.; Najafi, S.; Wong, J.; Kwong, M.; Pritchard, P.H. Assessing plasma lipid levels, body weight, and hepatic and renal toxicity following chronic oral administration of a water soluble phytostanol compound, FM-VP4, to gerbils. J. Pharm. Pharm. Sci. 2001, 4, 228–234. [Google Scholar]
- Taghizadeh, M.; Ostad, S.N.; Asemi, Z.; Mahboubi, M.; Hejazi, S.; Sharafati-Chaleshtori, R.; Rashidi, A.; Akbari, H.; Sharifi, N. Sub-chronic oral toxicity of Cuminum cyminum L.’s essential oil in female Wistar rats. Regul. Toxicol. Pharmacol. 2017, 88, 138–143. [Google Scholar] [CrossRef]
- Traesel, G.K.; Menegati, S.E.L.T.; dos Santos, A.C.; Souza, R.I.C.; Boas, G.R.V.; Justi, P.N.; Kassuya, C.A.L.; Argandoña, E.J.S.; Oesterreich, S.A. Oral acute and subchronic toxicity studies of the oil extracted from pequi (Caryocar brasiliense, Camb.) pulp in rats. Food Chem. Toxicol. 2016, 97, 224–231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).