PTX Instructs the Development of Lung-Resident Memory T Cells in Bordetella pertussis Infected Mice
Abstract
1. Introduction
2. Results
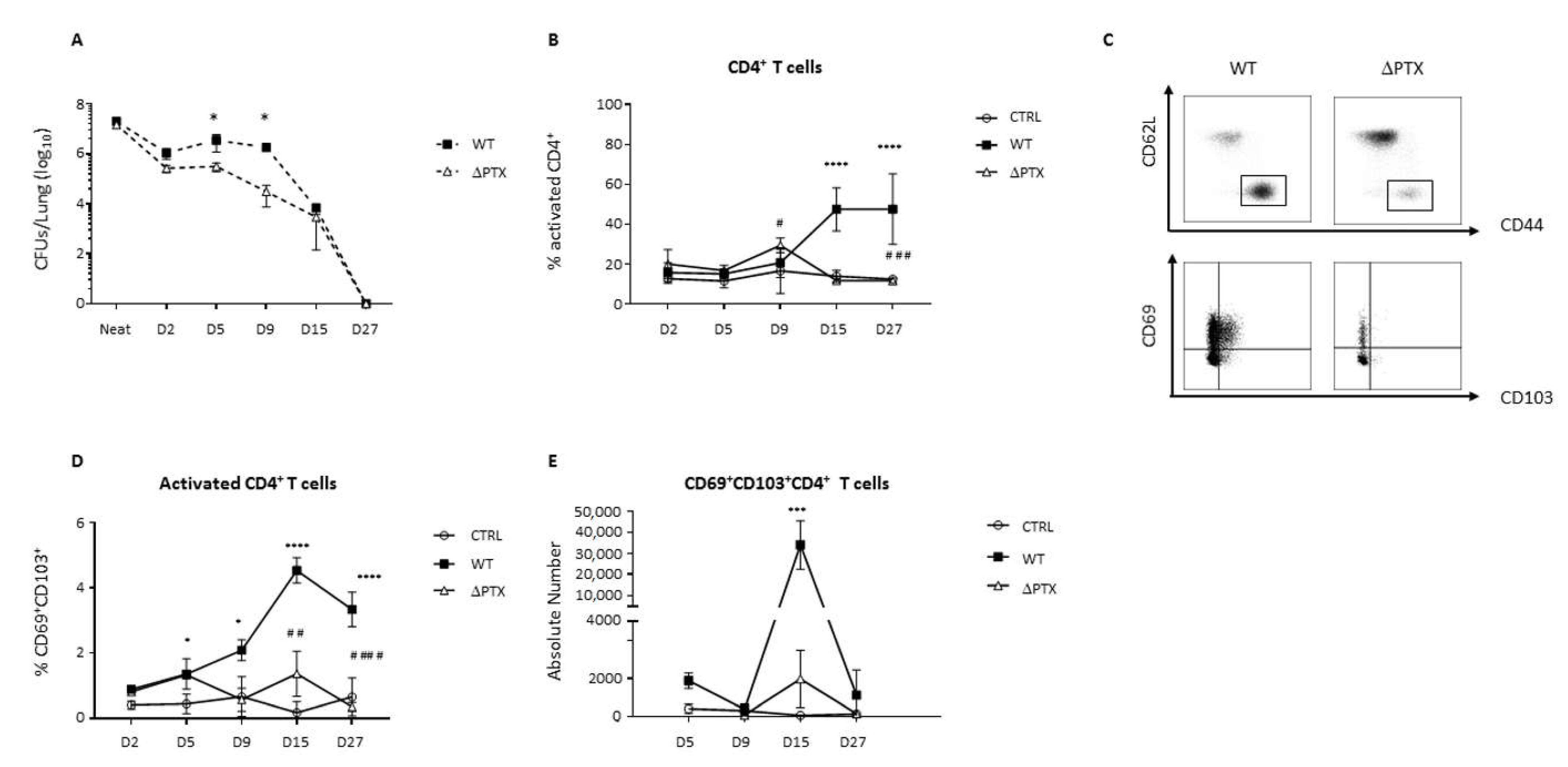
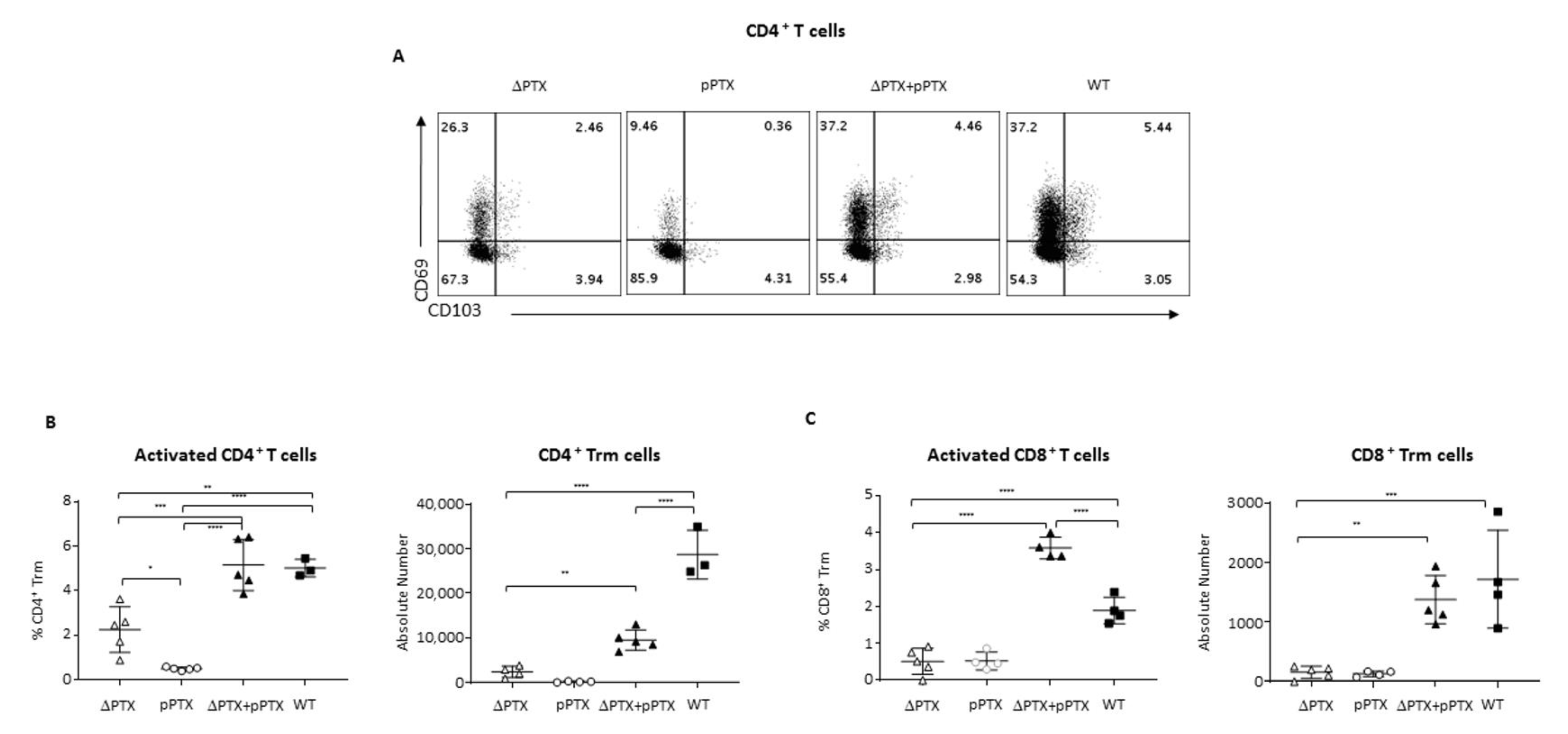
2.1. PTX Is Required to Generate CD69+CD103+ T Cell Populations
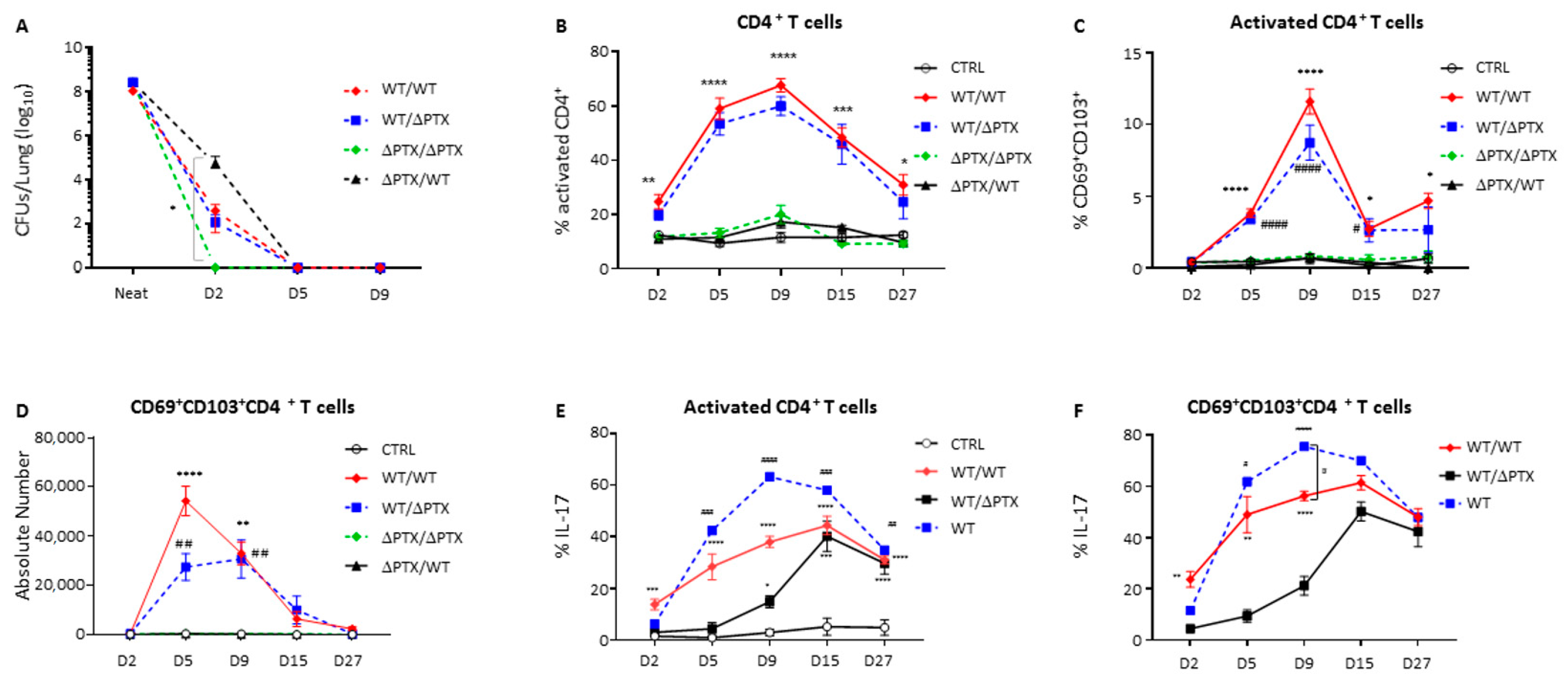
2.2. CD69+CD103+ T Cell Populations Expand upon New Bacteria Challenge
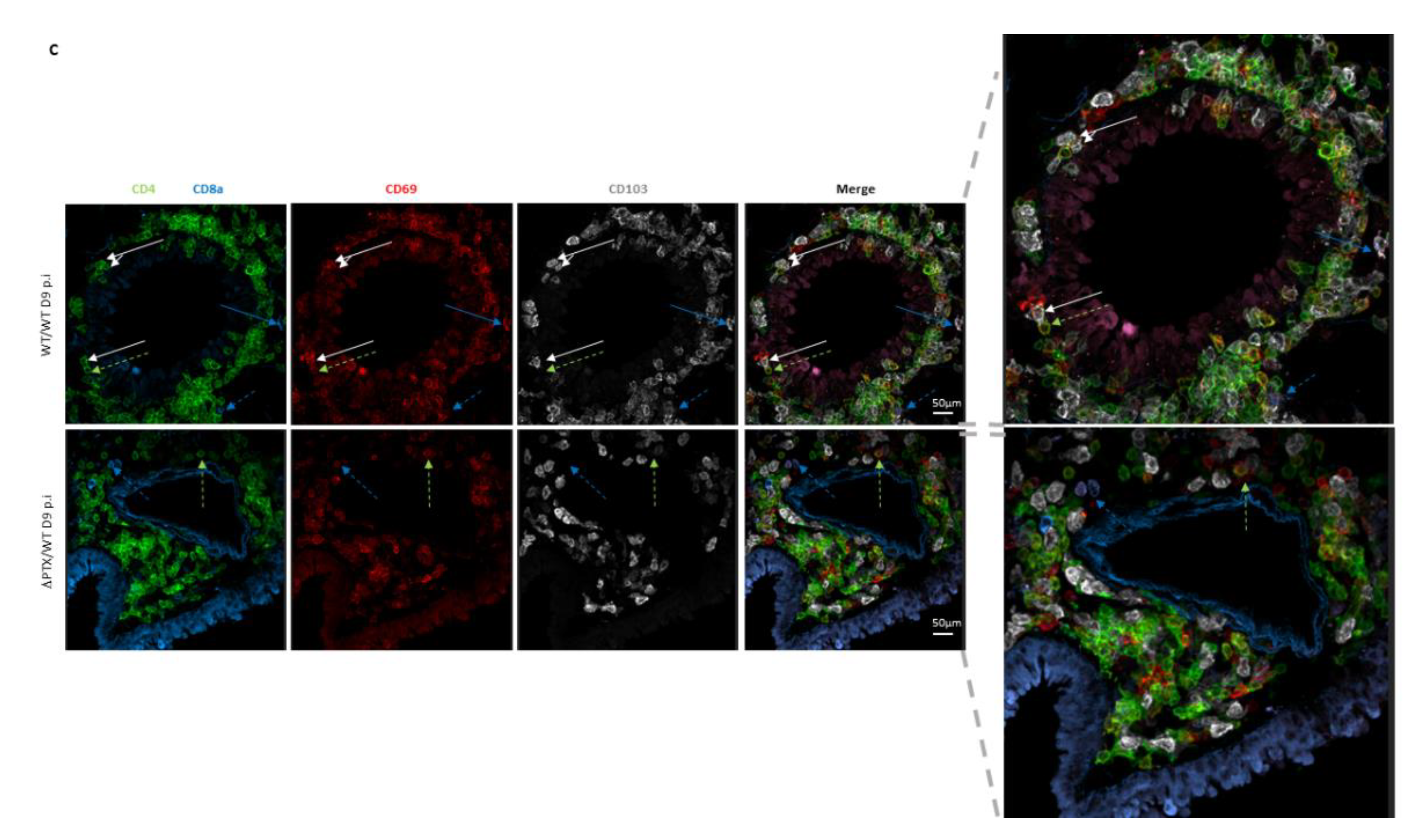
2.3. Trm Are Localized at the Front Line of Defense
2.4. Trm Favor a Th17 Environment in the Lung of Infected Mice
2.5. PTx Is Critical to Generate Trm
2.6. Trm Are Sufficient to Protect against a New Infection
3. Discussion
4. Materials and Methods
4.1. Bordetella Pertussis Strains and Growth Conditions
4.2. Mice
4.3. Infection Model
4.4. Fingolimod Treatment
4.5. BrdU Pulse Chase Experiment
4.6. Pertussis Toxin Complementation
4.7. Isolation of Lymphocytes
4.7.1. Lungs
4.7.2. Mediastinal Lymph Nodes (mLN) and Lymph Nodes (LN)
4.7.3. Bronchoaveolar Lavage Fluids (BALf)
4.7.4. Spleen
4.7.5. Blood
4.7.6. Intracellular Staining
4.8. FACS Analyses
4.9. Cell Sorting and Adoptive Transfer
4.10. Immunostaining and FISH
4.11. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeung, K.H.T.; Duclos, P.; Nelson, E.A.S.; Hutubessy, R.C.W. An update of the global burden of pertussis in children younger than 5 years: A modelling study. Lancet Infect. Dis. 2017, 17, 974–980. [Google Scholar] [CrossRef]
- Connelly, C.E.; Sun, Y.; Carbonetti, N.H. Pertussis Toxin Exacerbates and Prolongs Airway Inflammatory Responses during Bordetella pertussis Infection. Infect. Immun. 2012, 80, 4317–4332. [Google Scholar] [CrossRef]
- Aricò, B.; Gross, R.; Smida, J.; Rappuoli, R. Evolutionary relationships in the genus Bordetella. Mol. Microbiol. 1987, 1, 301–308. [Google Scholar] [CrossRef]
- Mangmool, S.; Kurose, H. Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX). Toxins 2011, 3, 884–899. [Google Scholar] [CrossRef]
- Locht, C. A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit. Biochimie 1995, 77, 333–340. [Google Scholar] [CrossRef]
- Mills, K.H.; Barnard, A.; Watkins, J.; Redhead, K. Cell-mediated immunity to Bordetella pertussis: Role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 1993, 61, 399–410. [Google Scholar] [CrossRef]
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.; McLoughlin, R.; Mills, K.H.G. Relative Contribution of Th1 and Th17 Cells in Adaptive Immunity to Bordetella pertussis: Towards the Rational Design of an Improved Acellular Pertussis Vaccine. PLoS Pathog. 2013, 9, e1003264. [Google Scholar] [CrossRef]
- Raeven, R.H.M.; Brummelman, J.; Pennings, J.L.; Nijst, O.E.M.; Kuipers, B.; Blok, L.; Helm, K.; Van Riet, E.; Jiskoot, W.; Van Els, C.A.C.M.; et al. Molecular Signatures of the Evolving Immune Response in Mice following a Bordetella pertussis Infection. PLoS ONE 2014, 9, e104548. [Google Scholar] [CrossRef]
- Turner, D.L.; Farber, D.L. Mucosal Resident Memory CD4 T Cells in Protection and Immunopathology. Front. Immunol. 2014, 5, 331. [Google Scholar] [CrossRef]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.M.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef]
- Mackay, L.; Rahimpour, A.; Ma, J.; Collins, N.C.; Stock, A.T.; Hafon, M.-L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.; Stefanovic, T.; et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef]
- Mackay, L.K.; Kallies, A. Transcriptional Regulation of Tissue-Resident Lymphocytes. Trends Immunol. 2017, 38, 94–103. [Google Scholar] [CrossRef]
- Rosato, P.C.; Beura, L.K.; Masopust, D. Tissue resident memory T cells and viral immunity. Curr. Opin. Virol. 2017, 22, 44–50. [Google Scholar] [CrossRef]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef]
- Gebhardt, T.; Whitney, P.; Zaid, A.; Mackay, L.; Brooks, A.; Heath, W.; Carbone, F.R.; Mueller, S. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nat. Cell Biol. 2011, 477, 216–219. [Google Scholar] [CrossRef]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J. Immunol. 2015, 194, 2059–2063. Available online: https://pubmed.ncbi.nlm.nih.gov/25624457/ (accessed on 13 August 2021). [CrossRef] [PubMed]
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243. [Google Scholar] [CrossRef]
- Romagnoli, P.; Sheridan, B.S.; Pham, Q.-M.; Lefrançois, L.; Khanna, K.M. IL-17A–producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 2016, 113, 8502–8507. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Pham, Q.-M.; Lee, Y.-T.; Cauley, L.S.; Puddington, L.; Lefrançois, L. Oral Infection Drives a Distinct Population of Intestinal Resident Memory CD8+ T Cells with Enhanced Protective Function. Immunity 2014, 40, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Bartlett, J.; Rowhani-Rahbar, A.; Fireman, B.; Baxter, R. Waning Protection after Fifth Dose of Acellular Pertussis Vaccine in Children. N. Engl. J. Med. 2012, 367, 1012–1019. [Google Scholar] [CrossRef]
- Winter, K.; Zipprich, J.; Harriman, K.; Murray, E.L.; Gornbein, J.; Hammer, S.J.; Yeganeh, N.; Adachi, K.; Cherry, J.D. Risk Factors Associated With Infant Deaths From Pertussis: A Case-Control Study. Clin. Infect. Dis. 2015, 61, 1099–1106. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- Althouse, B.M.; Scarpino, S.V. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015, 13, 1–12. [Google Scholar] [CrossRef]
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019, 8, 169–185. [Google Scholar] [CrossRef]
- Beura, L.K.; Mitchell, J.S.; Thompson, E.A.; Schenkel, J.; Mohammed, J.; Wijeyesinghe, S.; Fonseca, R.; Burbach, B.J.; Hickman, H.; Vezys, V.; et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 2018, 19, 173–182. [Google Scholar] [CrossRef]
- Alonso, S.; Pethe, K.; Mielcarek, N.; Raze, D.; Locht, C. Role of ADP-Ribosyltransferase Activity of Pertussis Toxin in Toxin-Adhesin Redundancy with Filamentous Hemagglutinin during Bordetella pertussis Infection. Infect. Immun. 2001, 69, 6038–6043. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; van der Maas, L.; Tilstra, W.; Uittenbogaard, J.P.; Bindels, T.H.E.; Kuipers, B.; van der Ark, A.; Pennings, J.L.A.; van Riet, E.; Jiskoot, W.; et al. Immunoproteomic Profiling of Bordetella pertussis Outer Membrane Vesicle Vaccine Reveals Broad and Balanced Humoral Immunogenicity. J. Proteome Res. 2015, 14, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, N.H.; Artamonova, G.V.; Mays, R.M.; Worthington, Z.E.V. Pertussis Toxin Plays an Early Role in Respiratory Tract Colonization by Bordetella pertussis. Infect. Immun. 2003, 71, 6358–6366. [Google Scholar] [CrossRef] [PubMed]
- Ayala, V.I.; Teijaro, J.R.; Farber, D.L.; Dorsey, S.G.; Carbonetti, N.H. Bordetella pertussis Infection Exacerbates Influenza Virus Infection through Pertussis Toxin-Mediated Suppression of Innate Immunity. PLoS ONE 2011, 6, e19016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrançois, L.; Farber, D. Cutting Edge: Tissue-Retentive Lung Memory CD4 T Cells Mediate Optimal Protection to Respiratory Virus Infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.G.; Sung, H.; Skon, C.N.; Lefrancois, L.; Deisinger, A.; Vezys, V.; Masopust, D. Cutting Edge: Intravascular Staining Redefines Lung CD8 T Cell Responses. J. Immunol. 2012, 189, 2702–2706. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. CD8 T cells and Mycobacterium tuberculosis infection. Semin. Immunopathol. 2015, 37, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Rossey, I.; Sedeyn, K.; De Baets, S.; Schepens, B.; Saelens, X. CD8+ T cell immunity against human respiratory syncytial virus. Vaccine 2014, 32, 6130–6137. [Google Scholar] [CrossRef]
- Misiak, A.; Wilk, M.M.; Raverdeau, M.; Mills, K.H.G. IL-17–Producing Innate and Pathogen-Specific Tissue Resident Memory γδ T Cells Expand in the Lungs of Bordetella pertussis–Infected Mice. J. Immunol. 2017, 198, 363–374. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Sacchi, F.; Hill, H.R.; E Van Epps, D.; A Daynes, R. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 1985, 135, 4135–4143. [Google Scholar] [PubMed]
- Poland, G.A.; Fleming, D.M.; Treanor, J.J.; Maraskovsky, E.; Luke, T.C.; Ball, E.M.; Poland, C.M. New Wisdom to Defy an Old Enemy. In Proceedings of the 4th Influenza Vaccines for the World (IVW) 2012 Congress, Valencia, Spain, 11 October 2012; pp. A1–A20. [Google Scholar] [CrossRef]
- Greer, A.L.; Fisman, D.N. Use of Models to Identify Cost-effective Interventions: Pertussis Vaccination for Pediatric Health Care Workers. Pediatrics 2011, 128, e591–e599. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y. Update on pertussis and pertussis immunization. Korean J. Pediatr. 2010, 53, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Mackay, L. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef]
- Hombrink, P.; Helbig, C.; Backer, R.A.; Piet, B.; Oja, A.E.; Stark, R.; Brasser, G.; Jongejan, A.; Jonkers, R.E.; Nota, B.; et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol. 2016, 17, 1467–1478. [Google Scholar] [CrossRef]
- Higgs, R.; Higgins, S.C.; Ross, P.J.; Mills, K.H.G. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012, 5, 485–500. [Google Scholar] [CrossRef]
- Coleman, M.; Finlay, C.; Moran, B.; Keane, J.; Dunne, P.J.; Mills, K. The immunoregulatory role of CD4+FoxP3+CD25−regulatory T cells in lungs of mice infected with Bordetella pertussis. FEMS Immunol. Med Microbiol. 2012, 64, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Lelouard, H.; Fallet, M.; de Bovis, B.; Meresse, S.; Gorvel, J.-P. Peyer’s Patch Dendritic Cells Sample Antigens by Extending Dendrites Through M Cell-Specific Transcellular Pores. Gastroenterology 2012, 142, 592–601.e3. [Google Scholar] [CrossRef] [PubMed]
- Pédron, T.; Mulet, C.; Dauga, C.; Frangeul, L.; Chervaux, C.; Grompone, G.; Sansonetti, P.J. A Crypt-Specific Core Microbiota Resides in the Mouse Colon. mBio 2012, 3, e00116-12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomas, J.; Koo, Y.; Popoff, D.; Arce-Gorvel, V.; Hanniffy, S.; Gorvel, J.-P.; Mionnet, C. PTX Instructs the Development of Lung-Resident Memory T Cells in Bordetella pertussis Infected Mice. Toxins 2021, 13, 632. https://doi.org/10.3390/toxins13090632
Tomas J, Koo Y, Popoff D, Arce-Gorvel V, Hanniffy S, Gorvel J-P, Mionnet C. PTX Instructs the Development of Lung-Resident Memory T Cells in Bordetella pertussis Infected Mice. Toxins. 2021; 13(9):632. https://doi.org/10.3390/toxins13090632
Chicago/Turabian StyleTomas, Julie, Yoon Koo, Dimitri Popoff, Vilma Arce-Gorvel, Sean Hanniffy, Jean-Pierre Gorvel, and Cyrille Mionnet. 2021. "PTX Instructs the Development of Lung-Resident Memory T Cells in Bordetella pertussis Infected Mice" Toxins 13, no. 9: 632. https://doi.org/10.3390/toxins13090632
APA StyleTomas, J., Koo, Y., Popoff, D., Arce-Gorvel, V., Hanniffy, S., Gorvel, J.-P., & Mionnet, C. (2021). PTX Instructs the Development of Lung-Resident Memory T Cells in Bordetella pertussis Infected Mice. Toxins, 13(9), 632. https://doi.org/10.3390/toxins13090632





